 Open Access Article
Open Access ArticleVault, viral, and virus-like nanoparticles for targeted cancer therapy
Siavash
Iravani
 *a and
Rajender S.
Varma
*a and
Rajender S.
Varma
 b
b
aFaculty of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, 81746-73461, Isfahan, Iran. E-mail: siavashira@gmail.com
bInstitute for Nanomaterials, Advanced Technologies and Innovation (CxI), Technical University of Liberec (TUL), Studentská 1402/2, Liberec 1 461 17, Czech Republic
First published on 9th June 2023
Abstract
Despite different strategies routinely applied for cancer therapy such as chemotherapy, surgery, and radiotherapy or a combination of them, they are still beleaguered by poor long-term survival rates, possible toxic/side effects, low therapeutic efficiency, and poor targeting attributes. Nanotechnology can help to develop advanced and selective tactics and nanosystems with fewer side effects, enhanced biosafety, improved targeting properties, and high therapeutic efficiency; assorted advanced nanosystems have been introduced to overcome drug resistance, showing improved rate of survival. In this context, vault, viral, and virus-like nanoparticles (NPs) with unique properties and structures can be deployed for smart targeted cancer therapy. Vaults are interesting examples of naturally occurring NPs mimicking diverse features of viral delivery tools without the significant risk of an immunological response, as they are an endogenous cellular component. The modifications of their primary sequences can enhance their capability for specific targeting properties and efficient encapsulation of bioactive molecules. In addition, virus-like particles have been studied for targeted anticancer drug delivery as well as cancer immunotherapy/vaccination and antitumor immunity. Thus, viral and virus-like NPs have excellent potential for targeted cancer therapy, with promise for multivalent antigen presentation and drug delivery. However, comprehensive clinical trials/studies are still required for ensuring their biosafety and efficacy. This highlight deliberates the current state-of-the-knowledge on the topic focusing on recent advances pertaining to the applications of vault, viral, and virus-like NPs for targeted cancer therapy.
1. Introduction
Nanoparticles (NPs) and nanoarchitectures have been widely exploited in the field of bio- and nanomedicine, especially for gene/drug delivery, tissue engineering, regenerative medicine, cancer theranostics, and imaging/diagnosis.1–5 To treat or manage cancer, various chemotherapy strategies are routinely applied, but concerns/challenges are still faced due to the possible drug resistance and low targeting properties,6 along with the probable side effects/toxicity. Thus, there is a vital need for developing efficient and safer drug carriers with enhanced therapeutic efficacy and controlled release behavior.7,8 In this context, advanced organic, inorganic, and organic–inorganic nanosystems are designed to offer targeted anticancer drug delivery options with high therapeutic efficiency.9–14 Indeed, a wide variety of nanosystems and nanoformulations have been introduced for enhancing the bioavailability, biosafety, and pharmacokinetics of drugs/therapeutic agents, as well as reducing their side effects and toxicity, thus leading to improved therapeutic efficacy and biological compatibility.15–17 Despite numerous advancements, crucial challenges/limitations still subsist regarding the low targeting properties (e.g., selectivity/specificity), insufficient therapeutic efficacy, and poor drug release behavior, which restricts the clinical potential of current nano-based strategies for the removal of tumors/malignancies along with the management and treatment of cancers/metastasis.18 Protein-based NPs like protein cages (as self-assembled supramolecular structures) or virus-like particles (VLPs) (which are specifically assembled from the coat proteins of the native viruses) can be considered as novel drug nanocarriers for cancer therapy.19Viral NPs as virus-originated nanoformulations are protein–polynucleotide hybridized supramolecular assemblies, which are deployable as building blocks or templates with unique architectures.20 In this context, plant virus NPs with multifunctionality and a lack of infection-related drawbacks can be considered as next-generation candidates for cancer therapy and immunity;21,22 the loading of anticancer drugs to viral NPs is attainable physically or via chemical linkage. Additionally, they can be modified using chemical or genetic alteration of their inner cavities and outer-surfaces, offering proper sites for coupling markers or therapeutic agents for cancer treatment and imaging purposes.23 For instance, after the conjugation of plant mosaic virus with folic acid, the designed nanocarrier could be deployed for specifically transferring doxorubicin. This complex exhibited sustained drug release behavior in tumor sites as well as improved drug uptake in breast cancer cells, thus prompting efficient antitumor/anticancer effects and reduced growth of tumors.24
Virus-like particles (VLPs) have been studied for the delivery of different biologics and synthetic cargo, as well as cancer vaccination and immunotherapy.25,26 There are several tactics available for transporting cargo using VLPs, comprising self-assembly around cargo, infusion of cargo, genetic engineering methods (using genetically-conjugated scaffolding proteins for drug encapsulation), and bio-conjugation techniques (using exterior surface-exposed residues).27–29 For instance, cowpea chlorotic mottle-derived VLPs have been developed for improving the antitumor efficacy of CpG oligonucleotides (ODN).30 The injection of ODN1826 VLPs into tumors could induce efficient antitumor effects by enhancing the phagocytic function of tumor-associated macrophages in the tumor microenvironment. Remarkably, these particles could substantially enhance the efficacy of ODN1826 compared to the free drug, with reduction in the growth of tumors and enhancing the survival rate for the examined mice with colon cancer and melanoma.30
Vaults, as the largest known naturally occurring ribonucleic protein complex (13 MDa) in eukaryotic organisms, are particles with hollow and barrel-shaped structures composed of 78 copies of the major vault protein, these particles being initially identified using electron microscopy in liver cell preparations.31 Vaults have been contemplated as natural nanoparticle delivery systems for the targeted delivery of proteins, nucleic acids, and small molecules to tumors (Fig. 1(A) and (B)).32–34 Notably, the capability of engineering vault NPs with intended features and functionalities can be considered as an important strategy for developing biocompatible nanocapsules. Vault NPs with good stability, monodispersity, non-immunogenicity, and biocompatibility have shown attractive potential for the delivery of drugs/probes as well as the encapsulation of proteins/peptides, and siRNA/mRNA.32,35,36 For instance, recombinant human vault NPs have been chemically conjugated with antiretroviral drugs for targeted delivery applications against human immunodeficiency virus (HIV) type 1 infection.37 Herein, the recent advancements pertaining to the applications of vault, viral, and virus-like NPs in smart anticancer drug delivery and cancer therapy are cogitated, with a focus on crucial challenges and future outlooks.
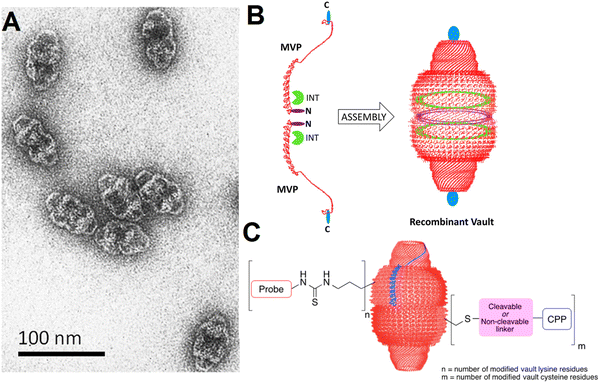 | ||
| Fig. 1 (A) Transmission electron microscopy (TEM) of vault particles purified from rat liver. (B) The engineering of vaults: two single major vault protein (MVP) chains (the left side, red color) with the locations specified where additional amino acids can be added at the N-terminus (purple color) and C-terminus (blue color). Reproduced with permission from ref. 32 Copyright 2012 American Chemical Society. (C) The covalent attachment of fluorescent probes and cell penetrating peptides (CPP) to vault NPs, offering enhanced delivery and imaging opportunities. Reproduced with permission from ref. 35 Copyright 2016 American Chemical Society. | ||
2. Vault NPs in cancer therapy
Biologically engineered recombinant vault NPs coupled with glioma-associated antigens (e.g., NY-ESO-1 peptide) can induce an antitumor response as they stimulate an immune response to glioblastoma in a mouse model after injection (in vivo).38 In addition, a cell penetration peptide (TAT) can be augmented to obtain recombinant vaults with enhanced cellular delivery potential (in vitro), thus displaying highly improved binding and uptake efficiency. The introduced TAT-vault NPs have been considered as promising delivery systems with enhanced cellular uptake for targeted cancer therapy.39 Vault NPs can efficiently deliver CCL21 (chemokine (C–C motif) ligand 21) into lung cancer tumors, leading to efficient antitumor activity and lung cancer inhibition. This strategy for the treatment of oncology malignancies using vault NPs is based on the modulation of the immune system, thus directing the cells of the immune system to the tumor sites for slowing or stopping the tumor development.40 Similarly, the injection of CCL21-packaged vault NPs has efficiently reduced the volume of tumors (in vivo).41 However, additional explorations are still necessitated on intracranial orthotopic tumor models to assess the application of these vault NPs as a promising tactic to bypass the blood brain barrier, thus enhancing the intracranial immune performance and improving the intracranial tumor control and survival rate.41Benner et al.35 have discussed the chemical modifications of vault NPs for imaging and delivery functions. These particles with their unique ability to encapsulate various cargos have been deployed for a variety of therapeutic delivery applications. Endogenous vaults and bioengineered vault NPs were deployed for targeted tumor or cancer therapy, as exemplified in the case of glioblastomas.42 Remarkably, with suitable chemical modification techniques, the properties of vault NPs like cellular uptake could be improved for imaging and targeted delivery applications.35 For instance, vault lysine and cysteine residues were chemically substituted by applying nucleophilic substitutions and disulfide exchange reactions and Michael additions, consequently offering enhanced cellular uptake for imaging purposes (Fig. 1(C)).35
3. Viral and virus-like NPs in cancer therapy
Virus-like NPs (VLNs) as non-infectious and nontoxic protein structures have been considered for targeted drug delivery, immunotherapy, and cancer theranostics.43,44 In one study, after the modification of bacteriophage MS2 VLPs with a targeting peptide (SP94), they were exploited for specific delivery of doxorubicin, cisplatin and 5-fluorouracil to human hepatocellular carcinoma cells, revealing selective cytotoxicity (in vitro) and high avidity for human hepatocellular carcinoma. Accordingly, the half maximal inhibitory concentration (IC50) for doxorubicin-loaded VLPs was ∼10–15 nM, much better (∼20 times) than the free doxorubicin.45 In addition, red clover necrotic mosaic virus-derived plant viral NPs were studied for controlled delivery of doxorubicin.46 Notably, the controlled release behavior from these NPs could be realized by adjusting the net charge of the outer surfaces of these viruses and the accessibility of their interior cavity, along with the control of drug loading capacity and binding sites with the viral capsids. The rate and amount of doxorubicin release may well be enhanced by increasing the environmental pH or by decreasing the concentration of divalent cations; this pH-responsive release behavior was dependent on the location of the bound or loaded active molecules.46 In another study, doxorubicin-loaded VLNs of MS2 spheres (∼27 nm), tobacco mosaic virus disks (18 × 5 nm), and nanophage filamentous rods (50 × 6 nm) were introduced to evaluate the effects of NP morphology on drug delivery.47 As a result, these VLNs exhibited suitable drug delivery potential as well as cell uptake ability (in vitro); though, the survival rate was enhanced in only U87-Luc glioblastoma-bearing mice treated by convection-enhanced delivery of doxorubicin-conjugated MS2 spheres and tobacco mosaic virus disks.47Different bacteriophages and plant viruses have been exploited for displaying and transferring therapeutic agents (e.g., proteins/peptides or anticancer drugs).44 For instance, to overcome drug resistance, tobacco mosaic virus was deployed for the delivery of cisplatin to treat platinum (Pt)-resistant ovarian cancer cells (Fig. 2).48 Compared to free cisplatin, the designed nanosystem exhibited superb cytotoxic effects and DNA double strand breakage in cancer cells. Such plant virus-based nanocarriers have been deemed as promising candidates to reduce the effective dosage of anticancer drugs (like cisplatin) in the treatment of cancers/malignancies, along with the enhancement in their potency for refractory diseases.48 To reduce the severe cardiac side effects of mitoxantrone (a topoisomerase II inhibitor for cancer chemotherapy), Lin et al.49 introduced tobacco mosaic virus as a nanocarrier for targeted drug delivery. The intratumoral injection of mitoxantrone-tobacco mosaic virus was performed when the tumors reached 100 mm3. This nanocarrier exhibited superior efficacy against triple negative breast cancer (in vivo). Besides, the targeted delivery of mitoxantrone was performed using cowpea mosaic virus for the treatment of glioblastomas, exhibiting enhanced cytotoxicity (in vitro) against glioma cells.50
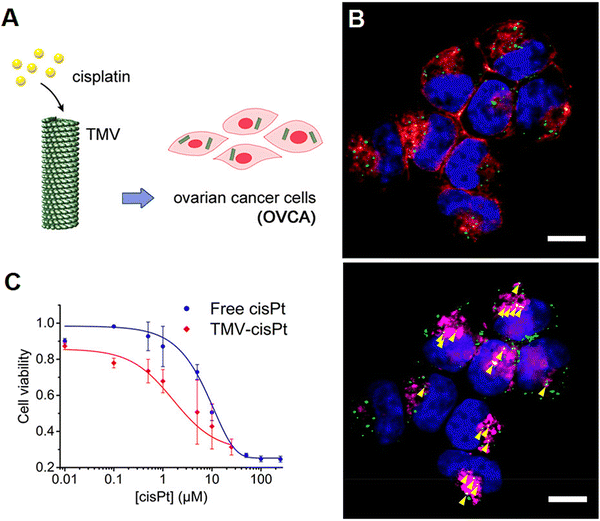 | ||
| Fig. 2 (A) Tobacco mosaic virus for the targeted delivery of cisplatin in Pt-resistant ovarian cancer cells, paving the way to overcoming drug resistance. (B) Tobacco mosaic virus and its cellular interactions: representative fluorescent images showing the internalization of the virus (green color) in A2780 cells (scale bar = 10 μm). The incubation of cells was performed for 24 h with 2.5 × 106 particles per cell and stained for tobacco mosaic virus (green color), cell membrane (red color, shown in the top panel), endolysosomes (magenta, shown in the bottom panel), and nuclei (blue color). (C) An MTT (2,5-diphenyl-2H-tetrazolium bromide) assay was completed on Pt-resistant ovarian cells, showing high treatment efficacy at all tested concentrations of cisplatin (cis-[Pt(NH3)2Cl2], cisPt). Reproduced with permission from ref. 48 Copyright 2017 American Chemical Society. | ||
For targeting non-Hodgkin's lymphoma, valine-citrulline monomethyl auristatin E (an antimitotic drug) was loaded onto the exterior surface of tobacco mosaic virus.51 The nucleoprotein components of tobacco mosaic virus have been applied as nanocarriers with effectual cytotoxic effects towards the non-Hodgkin's lymphoma cell lines (in vitro), with an IC50 of ∼250 nM.51 In addition, viral NPs based on potato virus X were deployed as a nanocarrier for specific delivery of Herceptin (a chemotherapeutic drug) against breast cancer.52 Accordingly, this nanosystem could successfully induce apoptosis in SK-OV-3 and SK-BR-3 cells (HER2 positive cell lines); the conjugation between potato virus X and Herceptin vastly improved the therapeutic potential of the drug.52 To improve the potency and in vivo efficacy of phenanthriplatin as a cationic mono-functional DNA-binding Pt(II) anticancer drug, tobacco mosaic virus was applied for its targeted delivery prowess (Fig. 3).53 As a result, in vivo tumor delivery and efficacy were successfully improved, and the size of the tumor was efficiently reduced, due to the enhanced accumulation of the drug within the tumor tissues.53
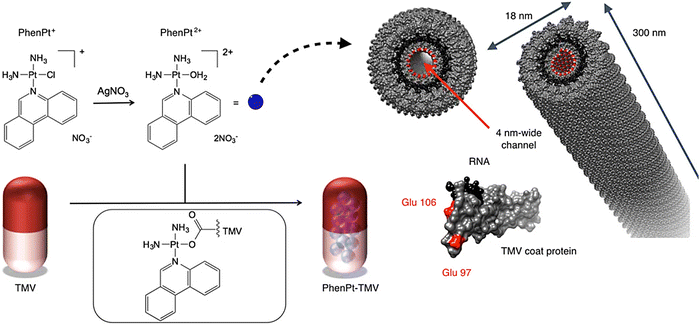 | ||
| Fig. 3 The preparative process for phenanthriplatin (PhenPt)-tobacco mosaic virus (TMV) using the nano-encapsulation technique. The structure of TMV is shown; the coat protein is depicted in gray, the RNA in black, and interior glutamic acids Glu 97 and Glu 106 are shown in red. Reproduced with permission from ref. 53 Copyright 2016 American Chemical Society. | ||
Peptide drugs have been intracellularly delivered using viral NPs of bacteriophage P22.54 These covalently-loaded peptides exhibited synergistic cytotoxicity and adaptable release stimulated by Cathepsin B (a highly over-expressed protease in different tumors). The designed nanosystem could efficiently deliver peptides against MDA-MB-231 tumor cells, hence revealing promising multifunctional theranostic applicability.54 Potato virus X was employed as a potential nanocarrier for targeted delivery of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL); this protein drug could stimulate apoptosis in cancerous cells but not normal cells.55 Accordingly, potato virus X-delivered TRAIL efficiently activated caspase-mediated apoptosis, displaying reduction in the growth of tumors in examined mouse models bearing human triple-negative breast cancer xenografts (Fig. 4).55 Such filamentous plant virus NPs have been considered as potential nanocarriers for targeted delivery of protein drugs in cancer therapy, offering suitable platforms for transferring nano-contrast or therapeutic agents after suitable chemical and genetic modifications. A study performed by Le et al.56 demonstrated the design of a nanocarrier based on potato virus X for targeted delivery of doxorubicin. This nanosystem efficiently functioned against the ovarian, breast, and cervical cancer cell lines and effectively reduced the tumor growth in mice models bearing human MDA-MB-231 breast cancer xenografts.56
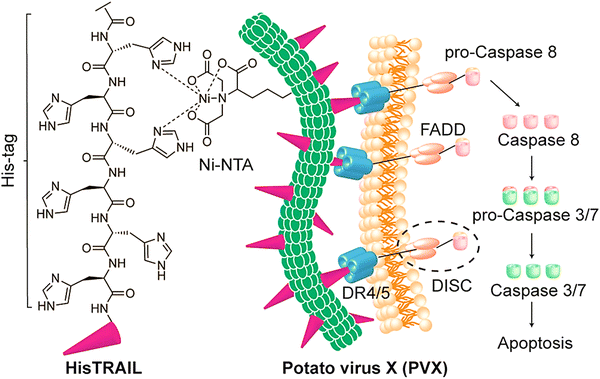 | ||
| Fig. 4 The preparative process for potato virus X (PVX)-HisTRAIL by coordinating the bond between a Ni-nitrilotriacetic (NTA) group on the virus; the His-tag at the N-terminus of HisTRAIL is shown with a purple triangle. Multivalent display of HisTRAIL on the elongated PVX particle permits proper binding on death receptors DR4/5 (the trimers with blue color) for activating the caspase-dependent apoptosis in cancerous cells. FADD: FAS-associated protein with death domain; DISC: death-inducing signaling complex. Reproduced with permission from ref. 55 Copyright 2019 American Chemical Society. | ||
For cancer immunotherapy and vaccination, VLNs have displayed excellent potentials, but most of them are still in preclinical stages (Table 1);30,57 viral capsids as scaffolds have been employed for multi-purpose applications after suitable modifications.58 In one study, the inhalation of VLNs from cowpea mosaic virus was exploited to reduce lung melanoma (in situ), disclosing potent systemic antitumor immunity against poorly immunogenic B16F10 in the skin.59 These inhaled NPs, endowed with stability and nontoxicity, could be quickly taken up by activated neutrophils in the tumor microenvironment as a crucial part of the antitumor immune reaction. The potential of these VLNs should be further scrutinized for immunotherapy against metastatic cancers. More explorations are still required for designing suitable VLP-based candidate vaccines. T lymphocyte-mediated immune responses with robust cytotoxic effects against tumor cells have been induced by applying VLPs for transferring antigens to the cytosol and activating the major histocompatibility class I pathway.60
| Viral NPs/VLPs | Cancer | Remarks | Ref. |
|---|---|---|---|
| Bacteriophage MS2 VLPs | Metastatic breast cancer | • Robust antibody responses against xCT protein. | 61 |
| • The function of xCT was inhibited (in vitro); the formation of metastases was reduced (in vivo) | |||
| Murine Trop2 (mTrop2) VLPs | Pancreatic cancer | • The reduction in growth of tumors due to the activation and tumor infiltration of T cells (CD4+/CD8+), and natural killer T cells. | 62 |
| • VLP immunization combined with gemcitabine could improve the efficacy and enhance the survival of tumor bearing mice. | |||
| Simian/human immunodeficiency virus (SHIV) VLPs | Pancreatic cancer/tumor cells | • The significant reduction in tumor growth and volume; ∼60% of the treated mice could survive. | 63 |
• Mesothelin VLPs could highly promote the formation of antitumor antibodies as well as CD8+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) T cell immunity. T cell immunity. |
|||
| Bacteriophage MS2 VLPs | Cervical cancer | • Broad protection against multiple human papillomaviruses (HPV) types. | 64 |
| • VLPs displayed a tandem HPV L2 peptide, offering similar protection in mice to Gardasil-9 (an HPV vaccine). | |||
| Cowpea mosaic virus | Malignancies with highly immunogenic cancer testis antigen NY-ESO-1 | • Stimulation of a potent CD8+ T cell response in transgenic human HLA-A2 expressing mice. | 65 |
| • Efficient cancer cell cytotoxicity. | |||
| Cucumber-mosaic virus (NPs combined with micro-adjuvants) | Melanoma | • The formulated CuMVTT-p33 vaccine with the micron-sized microcrystalline tyrosine adjuvant could enhance the specific T cell response against melanoma (a preclinical study). | 66 |
| Cowpea mosaic virus (in situ vaccine delivery) | Ovarian cancer | • Enhanced antitumor efficacy. | 67 |
| • Viral-dendrimer hybrids for delayed release applications. |
A human epidermal growth factor receptor-2 (HER2)-VLP vaccine was introduced as a cost-effective strategy for preventing and treating HER2-positive cancer, by inducing therapeutically potent anti-HER2 auto-antibody responses.68 The prophylactic vaccination reduced the impulsive progress of mammary carcinomas by 50%–100% in human HER2 transgenic mice, and could also inhibit the growth of HER2-positive tumors implanted in wild-type mice. Such VLP-based platforms have been contemplated as promising tools for developing vaccines against cancers;68 their utilization as adjuvants successfully promotes the robust immune responses. The innate and adaptive immune systems can be stimulated using proper VLPs; the designed vaccines using such NPs with no additional use of any adjuvants could every so often promote the humoral and cellular immunity via the major histocompatibility complex class I and II pathways.60 Liu et al.69 introduced VLNs with core–shell structures for the targeted co-delivery of small molecule drugs and clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 systems, hence offering a suitable nanoplatform for cancer immunotherapy and malignant cancer treatment (Fig. 5). To design VLNs, small molecule drugs were loaded into the pores of surface-thiolated mesoporous silica NPs (MSN-SH); after that, the pores were locked via the conjugation of ribonucleoprotein (RNP) to MSN-SH by disulfide bonds (RMSN). The NPs could release the CRISPR/Cas9 system and small molecule drugs in response to the reductive microenvironment, leading to the synergistic adjustment of multiple cancer-associated paths. The introduced VLNs disrupted multiple immunosuppressive paths and suppressed the growth of melanoma (in vivo) by loading a single guide RNA (sgRNA) targeting programmed death-ligand 1 and axitinib.69
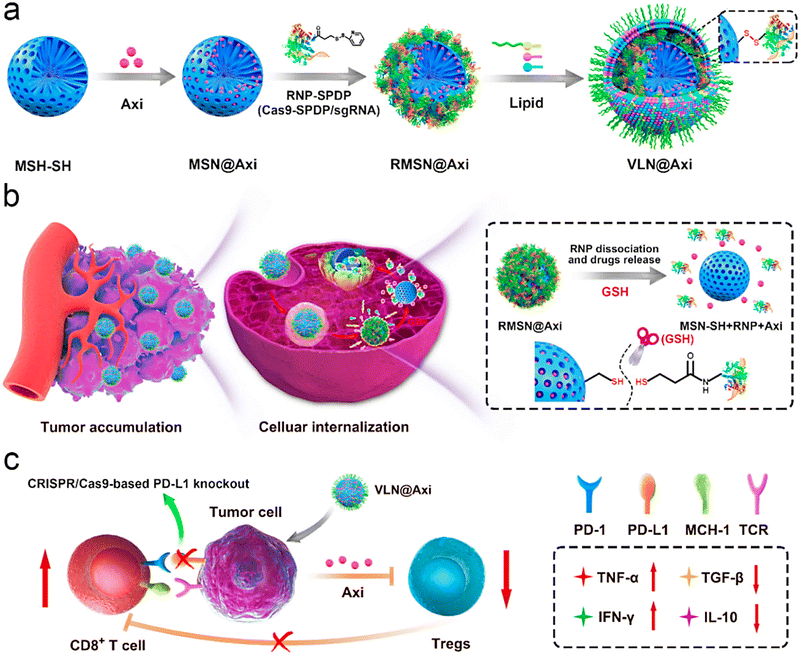 | ||
| Fig. 5 (a) The preparative process for the VLN@axitinib (Axi) system, and (b) its delivery after intravenous injection (tumor accumulation and cellular internalization). (c) The employment of VLN@Axi for returning the exhaustion of CD8+ T cells and suppressing the regulatory T cells (Tregs), providing an efficient system for cancer immunotherapy. PD-L1 encoding gene (sgPD-L1); N-succinimidyl-3-(2-pyridyldithiol) propionate (SPDP); glutathione (GSH); tumor necrosis factor (TNF); transforming growth factor (TGF); interferon (IFN); interleukin (IL). Reproduced with permission from ref. 69 Copyright 2020 Elsevier. | ||
4. Challenges and perspectives
Vault NPs, as a class of naturally-occurring NPs, have been studied as a drug delivery system for cancer therapy with some advantages, comprising multifunctionality, uniformity/monodispersity, biocompatibility, and improved drug biodistribution.39 The application of nanocarriers like vault NPs can improve the drug biodistribution to specifically target active molecules to diseased cells/tissues while protecting the healthy tissue.33 Vault NPs have been modified with cell penetrating peptides, which are short chains of amino acids that can penetrate the cell membrane and deliver the NPs to the cytoplasm.35 Studies have shown that the attachment of octaarginine cell penetrating peptides to vault NPs enhances their cellular uptake.35 In addition, vault NPs can be engineered to improve their escape from the endosomal compartment through the fusion of a membrane lytic peptide originated from adenovirus protein VI to the N-terminus of the major vault protein. This allows the NPs to escape the endosome and deliver their payload to the cytoplasm.34 Overall, the mechanism by which vault NPs target the diseased cells depends on the applied specific modifications to them. By targeting specific cell surface receptors or penetrating the cell membrane, these NPs transfer drugs/biomolecules to the cytoplasm of targeted cells, potentially improving the efficacy of cancer therapy.70,71 However, there are several challenges associated with the deployment of vault NPs for cancer therapy, namely the lack of clinical trials, limited in vitro/in vivo studies, biological barriers, specificity, and regulation. Vault NPs must be engineered to target cancer cells specifically, while avoiding healthy cells. On the other hand, a series of biological barriers may interfere with the transport of NPs to target tissues, restricting the delivery efficiency.72Plant virus-based NPs are recognized as highly suitable systems for the specific delivery of drugs to tissues affected by cancer.73,74 The nonpathogenic nature of plant viral NPs makes them an ideal choice for targeted cancer therapy. In addition, the permeability of plant virus NPs can be modified by adjusting the pH and ionic strength, which is a tremendous advantage for their application as drug delivery platforms. Plant viral NPs with their nontoxicity are inherently stable and capable of being mass-produced as well as being altered with antigens/drugs.74 Notably, VLPs have several advantages for cancer therapy, encompassing biocompatibility, biodegradability, uniform structure and controllable assembly as well as the capability of genetic/chemical modifications and enhanced permeability and retention.5,60 However, there are obstacles in the journey towards clinical translation, involving toxicity, stability, and efficacy.44,75 Clinical studies of viral and virus-like NPs in cancer therapy should be conducted to explore the potential of nanotechnology in reducing the drawbacks of chemotherapy and improving the efficiency of cancer treatment approaches.76 In this context, VLPs as non-infectious viral capsids with the benefits of particle stability, significant loading capacity, and structural uniformity have excellent potential for targeted anticancer drug delivery and therapeutic protein encapsulation applications.77 For instance, the engineered hepatitis B VLP delivery platform exhibited improved uptake and toxicity in epidermal growth factor receptor-overexpressing triple negative breast cancer cells in contrast to non-malignant breast epithelial cells.78 Despite significant advancements in cancer therapy using viral NPs, there are still challenges pertaining to the multi-drug resistance, complexity and heterogeneity of tumor biology, incomplete understanding of nano-bio interactions, immunogenicity, and biological instability.79–81 However, these NPs continue to be an area of active research in cancer therapy as exemplified by multifunctional plant virus NPs, which show exceptional promise in targeting breast cancer tumors.82
5. Conclusion
A variety of protocols have been introduced for the treatment of cancer and the removal of tumors. Despite their advantages, several major challenges/limitations regarding their targeting properties and toxicity/biosafety issues as well as their low therapeutic efficiency remain unattended. Vault, viral and virus-like NPs can be deployed to encapsulate therapeutic agents (such as nucleic acids, proteins, peptides, and drugs) for targeted anticancer drug delivery, thus reducing the systemic side effects through the alteration of pharmacokinetics of these encapsulated compounds. Numerous studies have focused on cancer immunotherapy and vaccination using VLPs with nontoxicity and cost-effectiveness as the primary benefits. Despite the unique structures and attractive properties of viral NPs, their immunogenicity and biosafety issues ought to be systematically evaluated; most of the reported studies are still in preclinical stages, and hence future explorations should be directed towards clinical translational studies and clinical trials.Since VLP-based immunization in combination with chemotherapy drugs (e.g., gemcitabine) has shown improved efficacy and enhanced survival of tumor bearing mice models, future studies need to focus on combinational cancer therapy using viral NPs. However, there are still challenges pertaining to the resistance, disease recurrence, toxicity drawbacks, and possible autoimmunity concerns. Remarkably, since medical applications of mammalian viruses have raised the concerns of possible mutations and unwanted reactions or virulent forms of viruses, plant viruses can be considered as ideal candidates with the benefit of biocompatibility, biodegradability, and lacking the infection-related concerns (non-infectious in mammals), thus providing excellent (nano)platforms/nanocarriers for cancer therapies and antitumor immunity.
Conflicts of interest
The author(s) declare no competing interest.References
- A. D. Friedman, S. E. Claypool and R. Liu, Curr. Pharm. Des., 2013, 19, 6315–6329 CrossRef CAS PubMed
.
- A. Wicki, D. Witzigmann, V. Balasubramanian and J. Huwyler, J. Controlled Release, 2015, 200, 138–157 CrossRef CAS PubMed
.
- S. Iravani and R. Varma, ACS Sustainable Chem. Eng., 2019, 7, 8055–8069 CrossRef CAS
.
- S. Iravani and R. S. Varma, Mar. Drugs, 2022, 20, 598 CrossRef CAS PubMed
.
- K. R. Kim, A. S. Lee, S. M. Kim, H. R. Heo and C. S. Kim, Front. Bioeng. Biotechnol., 2023, 10, 1106767 CrossRef PubMed
.
- F. M. Kievit and M. Zhang, Adv. Mater., 2011, 23, H217–H247 CrossRef CAS PubMed
.
- J. Shi, P. W. Kantoff, R. Wooster and O. C. Farokhzad, Nat. Rev. Cancer, 2017, 17, 20–37 CrossRef CAS PubMed
.
- F. S. Hussain, N. Q. Abro, N. Ahmed, S. Q. Memon and N. Memon, Front. Nanotechnol., 2022, 4, 1064615 CrossRef
.
- S. Iravani and R. S. Varma, ACS Biomater. Sci. Eng., 2021, 7, 1900–1913 CrossRef CAS PubMed
.
- S. Iravani and R. S. Varma, Nanomaterials, 2022, 12, 3360 CrossRef CAS PubMed
.
- S. Iravani and R. S. Varma, Nanomaterials, 2022, 12, 2440 CrossRef CAS PubMed
.
- V. P. Jain, S. Chaudhary, D. Sharma, N. Dabas, R. S. Kumar Lalji, B. K. Singh and G. Jaiswar, Eur. Polym. J., 2021, 142, 110124 CrossRef CAS
.
- Y. Li, X. Zheng and Q. Chu, Nano Today, 2021, 38, 101134 CrossRef CAS
.
- C. Murugan, V. Sharma, R. K. Murugan, G. Malaimegu and A. Sundaramurthy, J. Controlled Release, 2019, 299, 1–20 CrossRef CAS PubMed
.
- A. Casañas, P. Guerra, I. Fita and N. Verdaguer, Curr. Opin. Biotechnol, 2012, 23, 972–977 CrossRef PubMed
.
- C. C. Carrion, M. Nasrollahzadeh, M. Sajjadi, B. Jaleh, G. Jamalipour Soufi and S. Iravani, Int. J. Biol. Macromol., 2021, 178, 193–228 CrossRef CAS PubMed
.
- N. Olov, S. Bagheri-Khoulenjani and H. Mirzadeh, J. Biomed. Mater. Res., Part A, 2018, 106, 2272–2283 CrossRef CAS PubMed
.
- X. Kong, Y. Qi, X. Wang, R. Jiang, J. Wang, Y. Fang, J. Gao and K. C. Hwang, Prog. Mater. Sci., 2023, 101070, DOI:10.1016/j.pmatsci.102023.101070
.
- M. Perotti and L. Perez, Viruses, 2020, 12, 35 CrossRef CAS PubMed
.
- X. Y. Ma, B. D. Hill, T. Hoang and F. Wen, Semin. Cancer Biol., 2022, 86, 1143–1157 CrossRef CAS PubMed
.
- M. Shahgolzari, H. Dianat-Moghadam and S. Fiering, Semin. Cancer Biol., 2022, 86, 1076–1085 CrossRef CAS PubMed
.
- R.-H. Li, X.-Y. Feng, J. Zhou, F. Yi, Z.-Q. Zhou, D. Men and Y. Sun, Inorg. Chem., 2021, 60, 431–437 CrossRef CAS PubMed
.
- E. Alemzadeh, A. Dehshahri, K. Izadpanah and F. Ahmadi, Colloids Surf., B, 2018, 167, 20–27 CrossRef CAS PubMed
.
- E. Alemzadeh, A. Dehshahri, A. R. Dehghanian, A. Afsharifar, A. A. Behjatnia, K. Izadpanah and F. Ahmadi, Colloids Surf., B, 2019, 174, 80–86 CrossRef CAS PubMed
.
- N. Alvandi, M. Rajabnejad, Z. Taghvaei and N. Esfandiari, J. Drug Targeting, 2022, 30, 151–165 CrossRef CAS PubMed
.
- A. Shahrivarkevishahi, L. M. Hagge, O. R. Brohlin, S. Kumari, R. Ehrman, C. Benjamin and J. J. Gassensmith, Mater. Today Chem., 2022, 24, 100808 CrossRef CAS
.
- I. L. Aanei, J. E. Glasgow, S. L. Capehart and M. B. Francis, Methods Mol. Biol., 2018, 1776, 303–317 CrossRef CAS PubMed
.
- Y. Ren, S. M. Wong and L.-Y. Lim, Bioconjugate Chem., 2007, 18, 836–843 CrossRef CAS PubMed
.
- M. V. de Ruiter, R. M. van der Hee, A. J. M. Driessen, E. D. Keurhorst, M. Hamid and J. J. L. M. Cornelissen, J. Controlled Release, 2019, 307, 342–354 CrossRef CAS PubMed
.
- H. Cai, S. Shukla and N. F. Steinmetz, Adv. Funct. Mater., 2020, 30, 1908743 CrossRef CAS PubMed
.
- G. Frascotti, E. Galbiati, M. Mazzucchelli, M. Pozzi, L. Salvioni, J. Vertemara and P. Tortora, Cancers, 2021, 13, 707 CrossRef CAS PubMed
.
- L. H. Rome and V. A. Kickhoefer, ACS Nano, 2013, 7, 889–902 CrossRef CAS PubMed
.
- A. Muñoz-Juan, A. Carreño, R. Mendoza and J. L. Corchero, Pharmaceutics, 2019, 11, 300 CrossRef PubMed
.
- M. Han, V. A. Kickhoefer, G. R. Nemerow and L. H. Rome, ACS Nano, 2011, 5, 6128–6137 CrossRef CAS PubMed
.
- N. L. Benner, X. Zang, D. C. Buehler, V. A. Kickhoefer, M. E. Rome, L. H. Rome and P. A. Wender, ACS Nano, 2017, 11, 872–881 CrossRef CAS PubMed
.
- D. C. Buehler, M. D. Marsden, S. Shen, D. B. Toso, X. Wu, J. A. Loo, Z. H. Zhou, V. A. Kickhoefer, P. A. Wender, J. A. Zack and L. H. Rome, ACS Nano, 2014, 8, 7723–7732 CrossRef CAS PubMed
.
- J. A. Fulcher, K. Tamshen, A. L. Wollenberg, V. A. Kickhoefer, J. Mrazek, J. Elliott, F. J. Ibarrondo, P. A. Anton, L. H. Rome, H. D. Maynard, T. Deming and O. O. Yang, Bioconjugate Chem., 2019, 30, 2216–2227 CrossRef CAS PubMed
.
- D. T. Nagasawa, J. Yang, P. Romiyo, C. Lagman, L. K. Chung, B. L. Voth, C. Duong, V. A. Kickhoefer, L. H. Rome and I. Yang, J. Neuro-Oncol., 2020, 148, 1–7 CrossRef CAS PubMed
.
- J. Yang, A. Srinivasan, Y. Sun, J. Mrazek, Z. Shu, V. A. Kickhoefer and L. H. Rome, Integr. Biol., 2013, 5, 151–158 CrossRef CAS PubMed
.
- U. K. Kar, M. K. Srivastava, A. Andersson, F. Baratelli, M. Huang, V. A. Kickhoefer, S. M. Dubinett, L. H. Rome and S. Sharma, PLoS One, 2011, 6, e18758 CrossRef CAS PubMed
.
- B. L. Voth, P. E. Pelargos, N. E. Barnette, N. S. Bhatt, C. H. Jacky Chen, C. Lagman, L. K. Chung, T. Nguyen, J. P. Sheppard, P. Romiyo, S. Mareninov, V. A. Kickhoefer, W. H. Yong, L. H. Rome and I. Yang, J. Neurooncol., 2020, 147, 599–605 CrossRef CAS PubMed
.
- J. Yang, D. T. Nagasawa, M. Spasic, M. Amolis, W. Choy, H. M. Garcia, R. M. Prins, L. M. Liau and I. Yang, Neurosurg. Clin. N. Am., 2012, 23, 451–458 CrossRef PubMed
.
- P. H. Beatty and J. D. Lewis, Adv. Drug Delivery Rev., 2019, 145, 130–144 CrossRef CAS PubMed
.
- Y. H. Chung, H. Cai and N. F. Steinmetz, Adv. Drug Delivery Rev., 2020, 156, 214–235 CrossRef CAS PubMed
.
- C. E. Ashley, E. C. Carnes, G. K. Phillips, P. N. Durfee, M. D. Buley, C. A. Lino, D. P. Padilla, B. Phillips, M. B. Carter, C. L. Willman, C. J. Brinker, J. D. C. Caldeira, B. Chackerian, W. Wharton and D. S. Peabody, ACS Nano, 2011, 5, 5729–5745 CrossRef CAS PubMed
.
- J. Cao, R. H. Guenther, T. L. Sit, C. H. Opperman, S. A. Lommel and J. A. Willoughby, Small, 2014, 10, 5126–5136 CAS
.
- J. A. Finbloom, I. L. Aanei, J. M. Bernard, S. H. Klass, S. K. Elledge, K. Han, T. Ozawa, T. P. Nicolaides, M. S. Berger and M. B. Francis, Nanomaterials, 2018, 8, 1007, DOI:10.3390/nano8121007
.
- C. E. Franke, A. E. Czapar, R. B. Patel and N. F. Steinmetz, Mol. Pharmaceutics, 2018, 15, 2922–2931 CrossRef CAS PubMed
.
- R. D. Lin and N. F. Steinmetz, Nanoscale, 2018, 10, 16307–16313 RSC
.
- P. Lam, R. Lin and N. Steinmetz, J. Mater. Chem. B, 2018, 6, 5888–5895 RSC
.
- D. L. Kernan, A. M. Wen, A. S. Pitek and N. F. Steinmetz, Exp. Biol. Med., 2017, 242, 1405–1411 CrossRef CAS PubMed
.
- N. Esfandiari, M. K. Arzanani, M. Soleimani, M. Kohi-Habibi and W. E. Svendsen, Tumor Biol., 2016, 37, 1229–1236 CrossRef CAS PubMed
.
- A. E. Czapar, Y.-R. Zheng, I. A. Riddell, S. Shukla, S. G. Awuah, S. J. Lippard and N. F. Steinmetz, ACS Nano, 2016, 10, 4119–4126 CrossRef CAS PubMed
.
- J. Wang, T. Fang, M. Li, W. Zhang, Z.-P. Zhang, X.-E. Zhang and F. Li, J. Mater. Chem. B, 2018, 6, 3716–3726 RSC
.
- D. H. T. Le, U. Commandeur and N. F. Steinmetz, ACS Nano, 2019, 13, 2501–2510 CrossRef CAS PubMed
.
- D. H. Le, K. L. Lee, S. Shukla, U. Commandeur and N. F. Steinmetz, Nanoscale, 2017, 9, 2348–2357 RSC
.
- A.-G. Niculescu and A. M. Grumezescu, Int. J. Mol. Sci., 2022, 23, 5253 CrossRef CAS PubMed
.
- A. E. Czapar and N. F. Steinmetz, Curr. Opin. Chem. Biol., 2017, 38, 108–116 CrossRef CAS PubMed
.
- P. H. Lizotte, A. M. Wen, M. R. Sheen, J. Fields, P. Rojanasopondist, N. F. Steinmetz and S. Fiering, Nat. Nanotechnol., 2016, 11, 295–303 CrossRef CAS PubMed
.
- S. Nooraei, H. Bahrulolum, Z. S. Hoseini, C. Katalani, A. Hajizade, A. J. Easton and G. Ahmadian, J. Nanobiotechnol., 2021, 19, 59 CrossRef CAS PubMed
.
- V. Rolih, J. Caldeira, E. Bolli, A. Salameh, L. Conti, G. Barutello, F. Riccardo, J. Magri, A. Lamolinara, K. Parra, P. Valenzuela, G. Francia, M. Iezzi, F. Pericle and F. Cavallo, Cancers, 2020, 12, 1492 CrossRef CAS PubMed
.
- R. Cubas, S. Zhang, M. Li, C. Chen and Q. Yao, J. Immunother., 2011, 34, 251–263 CrossRef CAS PubMed
.
- S. Zhang, L.-K. Yong, D. Li, R. Cubas, C. Chen and Q. Yao, PLoS One, 2013, 8, e68303 CrossRef CAS PubMed
.
- L. Zhai, J. Peabody, Y. S. Pang, J. Schiller, B. Chackerian and E. Tumban, Antiviral Res., 2017, 147, 116–123 CrossRef CAS PubMed
.
- B. K. Patel, C. Wang, B. Lorens, A. D. Levine, N. F. Steinmetz and S. Shukla, ACS Appl. Bio Mater., 2020, 3, 4179–4187 CrossRef CAS PubMed
.
- M. O. Mohsen, M. D. Heath, G. Cabral-Miranda, C. Lipp, A. Zeltins, M. Sande, J. V. Stein, C. Riether, E. Roesti, L. Zha, P. Engeroff, A. El-Turabi, T. M. Kundig, M. Vogel, M. A. Skinner, D. E. Speiser, A. Knuth, M. F. Kramer and M. F. Bachmann, J. ImmunoTher. Cancer., 2019, 7, 114 CrossRef PubMed
.
- A. E. Czapar, B. D. B. Tiu, F. A. Veliz, J. K. Pokorski and N. F. Steinmetz, Adv. Sci., 2018, 5, 1700991 CrossRef PubMed
.
- A. Palladini, S. Thrane, C. M. Janitzek, J. Pihl, S. B. Clemmensen, W. Adriaan de Jongh, T. M. Clausen, G. Nicoletti, L. Landuzzi, M. L. Penichet, T. Balboni, M. L. Ianzano, V. Giusti, T. G. Theander, M. A. Nielsen, A. Salanti, P.-L. Lollini, P. Nanni and A. F. Sander, Oncoimmunology, 2018, 7, e1408749 CrossRef PubMed
.
- Q. Liu, C. Wang, Y. Zheng, Y. Zhao, Y. Wang, J. Hao, X. Zhao, K. Yi, L. Shi, C. Kang and Y. Liu, Biomaterials, 2020, 258, 120275 CrossRef CAS PubMed
.
- G. Tomaino, C. Pantaleoni, D. Ami, F. Pellecchia, A. Dutriaux, L. Barbieri, S. Garbujo, A. Natalello, P. Tortora and G. Frascotti, Int. J. Mol. Sci., 2023, 24, 4214 CrossRef CAS PubMed
.
- F. Rodríguez, P. Caruana, N. De la Fuente, P. Español, M. Gámez, J. Balart, E. Llurba, R. Rovira, R. Ruiz, C. Martín-Lorente, J. L. Corchero and M. V. Céspedes, Biomolecules, 2022, 12, 784 CrossRef PubMed
.
- J. Wang, Y. Li and G. Nie, Nat. Rev. Mater., 2021, 6, 766–783 CrossRef CAS PubMed
.
- E. Shoeb and K. Hefferon, Future Sci. OA, 2019, 5, FSO401 CrossRef CAS PubMed
.
- S. Venkataraman, P. Apka, E. Shoeb, U. Badar and K. Hefferon, Front. Bioeng. Biotechnol., 2021, 9, 642794 CrossRef PubMed
.
- D. Mundekkad and W. C. Cho, Int. J. Mol. Sci., 2022, 23, 1685 CrossRef CAS PubMed
.
- Z. Cheng, M. Li, R. Dey and Y. Chen, J. Hematol. Oncol., 2021, 14, 85 CrossRef PubMed
.
- B. Yuan, Y. Liu, M. Lv, Y. Sui, S. Hou, T. Yang, Z. Belhadj, Y. Zhou, N. Chang, Y. Ren and C. Sun, J. Drug Targeting, 2023, 31, 433–455 CrossRef CAS PubMed
.
- D. Yur, M. O. Sullivan and W. Chen, J. Mater. Chem. B, 2023, 11, 3985–3993 RSC
.
- M. E. Karim, S. T. Haque, H. Al-Busaidi, A. Bakhtiar, K. K. Tha, M. M. Banaszak Holl and E. H. Chowdhury, Arch. Pharmacal Res., 2022, 45, 865–893 CrossRef CAS PubMed
.
- J. Shi, P. W. Kantoff, R. Wooster and O. C. Farokhzad, Nat. Rev. Cancer, 2017, 17, 20–37 CrossRef CAS PubMed
.
- Y. Wu, J. Li and H.-J. Shin, Biotechnol. Bioprocess Eng., 2021, 26, 25–38 CrossRef CAS PubMed
.
- M. Shahgolzari, H. Dianat-Moghadam, A. Yavari, S. N. Fiering and K. Hefferon, Vaccines, 2022, 10, 1431 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2023 |
