Synthetic (bio)degradable polymers – when does recycling fail?
Beatriz
Agostinho
 a,
Armando J. D.
Silvestre
a,
Armando J. D.
Silvestre
 a,
João A. P.
Coutinho
a,
João A. P.
Coutinho
 a and
Andreia F.
Sousa
a and
Andreia F.
Sousa
 *ab
*ab
aCICECO—Aveiro Institute of Materials, Department of Chemistry, University of Aveiro, 3810-193 Aveiro, Portugal. E-mail: andreiafs@ua.pt
bCentre for Mechanical Engineering, Materials and Processes, Department of Chemical Engineering, University of Coimbra Rua Sílvio Lima – Polo II, 3030-790 Coimbra, Portugal
First published on 21st October 2022
Abstract
Given the current environmental crisis caused by polymers, we have seen that it is mandatory to make changes in the way we produce, consume, and, at the downstream, dispose, and manage polymeric materials after use. For applications where recycling might not be the adequate answer, or landfilling is highly likely to occur, (bio)degradable polymers can play an important role. This appraisal acknowledges the most important synthetic (bio)degradable polymers, noticeably polyesters, and their potential for substitution of traditional persistent ones, in terms of thermal and mechanical properties and more adequate fate in controlled compostable media or instead in natural environments. Special focus is given to bio-based poly(lactic acid), poly(butylene succinate) and furan-based polyesters. Alternatively, the most recent progress and contributions to rendering fossil-based polymers such as poly(caprolactone) and poly(glycolic acid) greener are discussed.
Introduction
Polymers are a vast and versatile family of macromolecular organic compounds that are lightweight and durable and exhibit a vast array of physical and chemical properties allowing them to fit the purposes of countless applications, spanning from packaging and textiles to more sophisticated purposes within electronics and medicine.In this vein, our dependency on polymeric materials is not surprising, as clearly demonstrated by their annual production, which reached 367 Mt in 2020.1 The polymers with higher productions are polyolefins, namely poly(ethylene) (PE), including linear low-density (LLDPE) and high-density PE (HDPE), and poly(propylene) (PP).1 The polyester poly(ethylene terephthalate) (PET) is also among the most produced ones.1 All these polymers find their main applications among packaging, which represents the biggest market demand, while other sectors, such as textiles and agriculture absorb the rest of the production.1 Despite their commercial relevance, the synthesis of these polymers relies on petroleum-based monomers, which are associated with greenhouse gas (GHG) emissions,2 besides the fact that they do not readily (bio)degrade under common environmental conditions.3 Indeed, the degradation of these polymers by microorganisms is an extremely slow process,4 with PET plastic bottles having an estimated minimum half-life of over 2500 years, whilst this value could reach 5000 years for conventional HDPE pipes.3
Recycling is often the advocated solution to manage polymers’ End-of-Life (EoL), playing an unarguable role.5,6 However, despite this, only around 35% of polymer waste is being recycled in the EU,1 and an even smaller amount in the USA with only 9% in 2018.7 Furthermore, this option is mainly applied for packaging materials,8 leaving out other polymeric residues, such as textile fibres. Additionally, mechanical recycling (the most commonly used and developed approach)9 fails to meet all the requirements due to polymers’ molecular weight decay over repeating recycling cycles and/or the presence of additives and/or other polymer contaminations usually leading to devaluation.10 Additionally, mechanical recycling is not a valid option for multilayer packaging materials where multiple polymers are present and separation is not always viable. The approach is also not an option for thermosets, which are out of the scope of this appraisal dedicated to thermoplastics.
Another topic that must not be neglected is the fact that the possibility of having efficient waste collection and appropriate recycling facilities is far from being reached globally, in particular in developing countries.11,12
Therefore, it is not surprising that the provisional data for 2020 show that over 23% of polymer waste was still being disposed in landfills in Europe, and around 75% in the USA.1,7 Additionally, 42% of polymer waste was incinerated for energy recovery in Europe, while the amount in the USA was again much lower (15%). This intricate polymer scene with, on the one hand, their typical durability, and, on the other hand, recycling failing to promptly provide a solution to polymer waste management, urges for adequate answers.
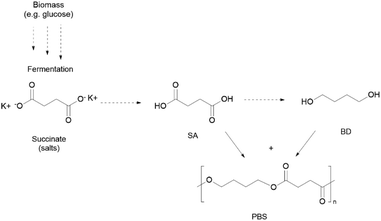
Some synthetic polymers, able to be assimilated by the environment, or at least to be industrially compostable (ISO 17088, ISO 14855),13,14 have the potential to be part of the solution. It is expected that around 5.3 Mt of (bio)degradable polymers will be produced in 2026, mainly due to the strong developments of bio-based thermoplastic polyesters, notably poly(lactic acid) (PLA), poly(butylene succinate) (PBS), and/or partially renewable poly(butylene adipate terephthalate) (PBAT).15 All these polymers are polyesters that possess in their backbone liable ester bonds prone to, for example, hydrolysis, thus triggering depolymerisation, although other polycondensates such as poly(carbonates) (PC) can also be considered due to their hydrolysable functional groups. Investigation on their use for biomedical applications has been extensively overviewed throughout the years, but these polymers have also gained both academic and commercial relevance among single use (SU) packaging, and have been applied in films in agriculture or in textile fibres.
Specifically, PLA is already among the most used polymers in the textile industry, while PBS also shows high growth potential.16 Their contribution, as fibres17 or as coatings and finishings,16 is unequivocable for supporting an expanding textile industry, which doubled the production of synthetic polymers in the last two decades, but still more than two-thirds are going to be disposed in landfills.18,19
PBAT is a promising alternative for SU flexible polymers often used in packaging,15 and for mulch films in agriculture.20–22 For this application, degradability can circumvent additional costs and procedures often incurred for removing these agricultural films from the fields after use and, thus, can mitigate their environmental impact.20
These ongoing developments are motivated and supported by large-scale initiatives within the European Green Deal or the United Nations Sustainable Development Goals, which continue to contribute to a more sustainable future.23,24 Furthermore, many studies focusing on these (bio)degradable polymers advantageously follow some of the Green Chemistry principles, notably renewable origin, eco-friendly synthesis, and above all proving a more sustainable fate.
The scope
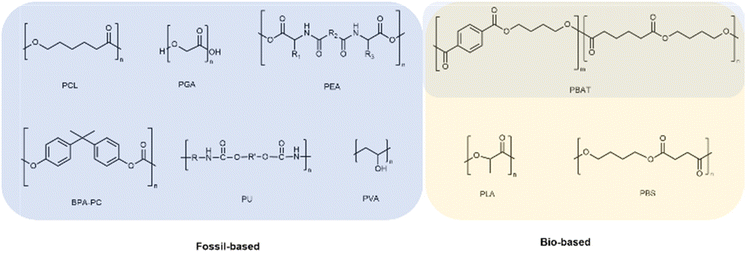
This appraisal intends to shine a light on the ongoing research and development activities on the synthesis of (bio)degradable polymers, mainly polyesters, either fossil-based or instead renewable-based, in a quest for more sustainable alternatives.25,26 Greener synthetic pathways will be preferentially overviewed. Together, characterisation and related applications are also addressed. All polymers considered for this review can be overviewed in Scheme 1.Although naturally occurring polymers, such as thermoplastic starch and cellulose, among many others, play an important role among (bio)degradable polymers, they have been broadly overviewed before.27,28 This review focuses, instead, on synthetic polymers due to their known reproducibility, versatility to fulfil specific applications’ requirements,29 and in many cases broad market implantation.
It should be noted, however, that the IUPAC definition is not restricted to (bio)degradation in a natural environment, as marine or terrestrial ones, but instead the scope is broader and often leads to erroneous assumptions of safe disposal and an adequate EoL of polymers such as PLA that will not biodegrade ‘easily’ in the environment.33 Therefore, in this review, for clarity reasons, whenever a polymer can be completely (bio)degraded under natural conditions, this will be unambiguously stated.
Also, for objectivity purposes, whenever a polymer (bio)degrades under very specific composting conditions, according to the international standards (e.g., EN 13432, ISO 14855, ISO 17088, ASTM D6400),13,14,34,35 usually performed industrially in an aerobic or anaerobic environment or under home-composting conditions, this polymer will be referred hereinafter as compostable (according to a specific standard).
Both definitions (biodegradable and compostable polymers) lie under the more general umbrella of degradable polymers, defined, according to the IUPAC, as ‘polymers in which macromolecules are able to undergo chain scissions, resulting in a decrease of molar mass’.31 Degradation can be prompted by the action of different factors, such as water (hydrolytic degradation) and UV/Vis light (photodegradation), among others. Nevertheless, the relevance of this definition, without a clear indication of the (bio)degradation conditions, medium and timescale remains vague. In a circular economy approach, (bio)degradable polymers can play an effective role in efficient waste management,36 in very specific situations and with adequate labelling, as already pointed out previously in this appraisal. Herein, we will disclose their uncovered potential in packaging, textiles, and film applications, thus beyond the extensively reviewed biomedical applications.
(Bio)degradable polymers
Overview of the main commercially available fossil-based polymers
Although PCL is derived from fossil resources, efforts to synthesise ε-caprolactone (ε-CL) (or similar monomers) from biomass sugars via the platform chemical 5-hydroxymethylfurfural (5-HMF) root (Scheme 2) or from vegetable oils have been reported.40,41 However, to the best of our knowledge, none of these alternatives have reached high maturity Technology Readiness Levels (TRL).
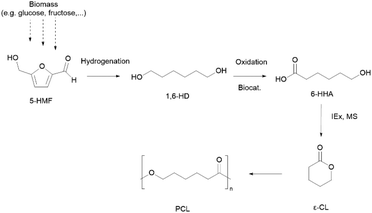 | ||
| Scheme 2 An integrated microbial-chemical route to produce bio-based ε-CL via 5-hydroxymethylfurfural (5-HMF), 1,6-hexanediol (1,6-HD) and 6-hydroxyhexanoic acid (6-HHA). Biocat: biocatalyst; IEx: ion exchange catalyst.40 | ||
In terms of synthesis, PCL is generally prepared through the ring-opening polymerisation (ROP) of ε-CL, under relatively mild conditions leading to PCL with a low polydispersity (1.06 to 1.35).42,43 Usually, metal-based catalysts, such as tin(II) 2-ethylhexanoate, are used, although there have been efforts to promote the development of greener processes using, for example, enzymes.42
PCL is widely known for being semicrystalline, hydrophobic and soluble in most non-polar organic solvents (e.g., chloroform, benzene, toluene, etc.) at room temperature, and for its moderate thermal features, viz low melting and glass transition temperatures (60–65 °C and −60 °C, respectively).44,45
PCL also showcases enhanced barrier properties, with an oxygen (O2) permeability coefficient of 0.88, and great flexibility with a maximum elongation at break of around 1000% (LDPE has only max. elongation of 600%). When it comes to tensile strength, it also shows improvements in relation to LDPE, with maximum 785 MPa for PCL and only max. 35 MPa for LDPE. Nevertheless, some of these features are still moderate compared to its aromatic counterparts (Table 1),46 although these can also be viewed as an advantage in terms of processing energy and associated costs.
| Category | Polymer | Mechanical properties | Thermal properties | Barrier properties | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Elongation at break (%) | Elongation at yield (%) | Young's modulus/GPa | Tensile strength/MPa | T g/°C | T m /°C | T d/°C | O2 permeability coefficient | CO2 permeability coefficient | |||
| Low density poly(ethylene) (LDPE), poly(ethylene terephthalate) (PET), poly(caprolactone) (PCL), poly(glycolic acid) (PGA), poly(butylene adipate terephthalate) (PBAT), poly(vinyl alcohol) (PVA), poly(carbonate) (PC), poly(urethane) (PUR), poly(lactic acid) (PLA), poly(ethylene 2,5-furandicarboxylate-co-poly(L-lactic acid) (PEFL), poly(butylene adipate-co-butylene 2,5-furandicarboxylate) (PBAF), poly(butylene succinate) (PBS), Mater-Bi®(starch/PCL blend). | |||||||||||
| Fossil-based persistent | LDPE | 100–1000 | 5–425 | 100–200 | 7–35 | −100 | 98–218 | 49–51 | |||
| PET | 30–300 | 4–6 | 1700 | 48–72 | 69–125 | 245–280 | 0.03–0.04 | 0.12–0.23 | 46 and 51 | ||
| Fossil-based (bio)degradable | PCL | 20–1000 | 0.2–0.4 | 4–785 | −65 to −60) | 58–64 | 350 | 0.88 | 14.3 | 51 and 52 | |
| PGA | 15–35 | 339–394 | 36–45 | 210–230 | 254 | 0.01 | 0.05 | 52 | |||
| PBAT | 500–800 | 21–40 | −30 | 110–125 | 51 and 53 | ||||||
| PVA | 225–445 | 37–45 | 24–110 | 58–85 | 180–265 | 0.02 | 0.09 | 51, 52 and 54 | |||
| PC | 10–138 | 3–50 | 62 | 150 | 250–343 | 1.05 | 6 | 50 and 52 | |||
| PUR | 2–950 | 11–540 | 8–39 | −34 to −15 | 46–48 | 108–160 | 55 and 56 | ||||
| Bio-based (bio)degradable | PLA | 2–240 | 1.8–3.7 | 3.98 | 28–53 | 40–70 | 130–180 | 235–255 | 51, 52 and 57 | ||
| PEFL | 25–76 | 120 | 289–384 | 52 | |||||||
| PBAF | 307–748 | 0.02–0.12 | 9–42 | −53–23 | 27–156 | 417–430 | 58 | ||||
| PBS | 379 | 320 | 27 | −44 | 109 | 400 | 59 | ||||
| Blends (bio)degradable | Mater-Bi® | 780–886 | 180–185 | 25–30 | 160 | 49 | |||||
Although PCL's fate is not desirable to occur in natural environments, notably aquatic media, and particularly marine salty ones, if it happens it is quite important to have data beforehand on degradation in these media. In this regard, Narancic et al.47 reported that PCL reached 80% degradation in the marine environment and a maximum 50% degradation in fresh water in 8 weeks. Also, Tsuji et al.48 studied the behaviour of PCL and several other (bio)degradable aliphatic polyester films in static sea water (10 weeks at 25 °C), showing that the degradability of the aliphatic polyesters in controlled seawater decreased in the order PCL > poly[(R)-3-hydroxybutyrate] > poly(L-lactide) (PLLA).
Enzymatic pathways have also been explored as an effective way to degrade PCL. In this vein, Su and Wang60 reported the use of Candida antarctica lipase B (CALB) for degrading PCL with different starting weight-average molecular weights (Mw). The study demonstrated the rapid degradation of PCL in the first 12 h with around 85% of mass loss (phosphate-buffered saline medium at 45 °C), without the influence of the initial Mw. Another study reported the processing of PCL into microparticles and their enzymatic degradation with lipases (although the authors have not specified which lipase was used).61
Besides, in 2012, a PCL-degrading Bacillus strain, namely B. pumilus was reported.62 In this study, the ability of the microorganism to degrade PCL films during soil burial tests (35% moisture and 40 °C) was studied and it was demonstrated that degradation can be seen on the surface of PCL films in just 20 days of incubation.
One of PCL's most highlighted properties is undoubtably its compostability, especially when recycling is not adopted. Narancic et al.47 evaluated PCL degradation under industrial (ISO 14855)14 and home composting conditions through net gaseous CO2 production measurements. The authors found that for the former, complete degradation was achieved in just 45 days, whereas in home composting (28 °C) it took 88 days to reach full degradation. Rutkowska et al.63 compared PCL's degradation in different environments and under different conditions, namely in compost with activated sludge. PCL's degradation was the quickest with the activated sludge (compared to liquid medium), with complete disintegration observed just after 7 weeks of incubation.
Blending and copolymerisation have both been used to circumvent the described PCL's moderate thermal and mechanical properties, with the additional possibility of incorporating bio-based materials, and therefore increasing the renewable content.64,65 In this vein, Mater-Bi®, commercialised by Novamont (Italy), is a very interesting example of a blend of PCL and starch.66 This blend is certified compostable according to the European standard EN 1343235 and is mostly used to produce carrier bags, cutlery, and food packaging, among others.20
In some studies, the addition of starch to improve PCL's Young's modulus is reported in order to make the films less ductile, which also means a decrease in tensile strength and elongation at break.65 PCL/starch blends have comparable mechanical performance to well established LDPE. They exhibit greater elongation at break (780–886% vs. 150–600% for LDPE) and comparable Young's modulus and tensile strength (Table 1).49,66
PCL films modified with a 10% ethylene butyl acrylate copolymer were studied for their degradation in aqueous environments, revealing that the seawater medium gave improved results with complete degradation after 7 weeks.63 Along the same line, PLA and PCL blends and copolymers have been reported in the literature,67–72 showing that PCL enhances PLA's degradability, but this topic will be discussed in detail in a later section of this review. Additionally, due to the non-degradability of commonly used PET, a PET/PCL reactive blend has been used to attempt accelerating degradability.73–75
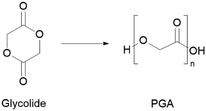
Currently, glycolide is predominantly produced from the carbonylation of formaldehyde, a toxic compound, requiring high temperature and pressure.77 This monomer can also be produced via hydrolysis of chloroacetic acid (also known to be toxic and corrosive).77 Partially greener alternatives include formaldehyde conversion into glycolonitrile, which is then converted into high-purity glycolide via chemoenzymatic processes.78,79 More recently, there have also been attempts to synthesise glycolic acid from biomass derived sugars.80 As for PGA production, it is mainly synthesised by ROP using glycolide lactone (Scheme 3).
When it comes to its general properties, in Table 1, it is possible to note PGA's high melting point (Tm = 220–230 °C), especially when compared to PCL, associated with its linear close packing aliphatic chains, and a high degree of crystallinity (45–55%). Additionally, it also has excellent barrier properties of extreme importance for food and beverage packaging, with an O2 and CO2 permeability coefficient of 0.014 and 0.052, respectively.77
Typically, PGA easily undergoes hydrolytic degradation, even in the absence of enzymes.77 Shawe et al.81 studied the degradation of PGA films with different thicknesses (at 37 °C in phosphate-buffered saline) and their weight loss variation throughout 24 days. By the end of this study, the thinner film (0.06 mm) had 60% mass loss compared to 35% weight loss for the thicker ones (0.3 mm).
Despite these features, PGA's high hydrophilicity, rapid degradation, and brittleness have limited its broader practical application, yet they gave its copolymers and blends a lot of importance. Glycolic acid has been often copolymerised with lactic acid forming the well-known poly(lactic-co-glycolic acid) (PLGA)82 or with ε-caprolactone to produce poly(glycolic acid-co-ε-caprolactone) (PGC).83,84 Cai et al.83 performed tensile mechanical tests indicating that PGC could withstand an extension of 250% without cracking, which was much higher than that previously reported for PLGA (10–15%) or PGA (15–35%). Degradation studies (in phosphate-buffered saline at 37 °C) showed good degradability, with PGC having 50% mass loss in just 6 weeks.
With regard to other blends, Vieira et al.68 studied the degradation of some aliphatic polyesters processed as fibres, namely PGA and a PGA/PCL blend. The fibres obtained from the PGA/PCL blend were the most prone to degradation after 12 weeks with over 80% weight loss (in phosphate-buffered saline at 37 °C). Although PCL has a lower degradation rate than PGA, the combination of the two results in less crystalline copolyesters accelerated the degradation process.
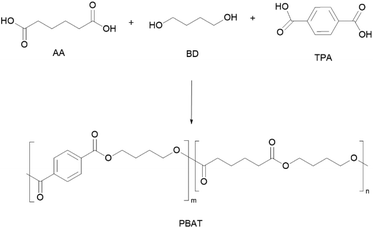
PBAT is one of the most produced polyesters nowadays with almost 20% of global production capacity of bio-based and (bio)degradable polymers.15 It is typically synthesised by the conventional two-stage bulk polyesterification86 of fossil-based terephthalic acid (TPA), adipic acid (AA) and 1,4-butanediol (BD) (Scheme 4), although there are several reports on the synthesis of bio-based AA.87,88 In this regard, bio-based AA can be obtained from the direct fermentation of biomass into AA or fermentation to muconic or glucaric acids, followed by chemical hydrogenation.89
PBAT conventional synthesis requires harsh conditions, such as high temperature, vacuum, long reaction times, and metal-based catalysts.53 To our knowledge, efforts to render this synthesis greener have not yet been a focus of scientific interest.
Although PBAT's general mechanical properties depend on the TPA/AA monomer composition and polymers’ molecular weight, they are quite similar to LDPE53 which makes PBAT a promising partially bio-based and greener alternative. Also, typically, the Tm varies between 110 and 125 °C and the Tg is around −30 °C;90 while the elongation at break is around 500–800% and the tensile strength is in the range of 21–40 MPa (compared to LDPE 7–35 MPa, Table 1).53
When it comes to PBAT's copolyester degradation, Witt et al.91,92 published a couple of studies on this topic. First, in 1995 they reported the influence of TPA and AA ratios in the degradation process (in aerated mineral liquid medium at 30 °C and soil burial), concluding that for a TPA molar fraction >0.3 (including neat PBT), no degradation could be observed in liquid medium. However, for soil burial tests, for copolyesters with 0.39 and 0.43 (mol/mol) of TPA units a relevant weight loss (around 20%) was observed at the end of 3 months.91,92
More recently, Kijchavengkul et al.93 studied the composting degradation (ASTM D5338 at 58 °C)94 of PBAT films, by monitoring their conversion into CO2. The authors observed a total degradation of films in manure compost after 45 days.
Another important sustainability aspect approached by Witt et al.95 is the ecotoxicity of degradation intermediates. They found that no toxic effects from composting PBAT could be detected, which can be considered a great advantage for its fate after use.95 When it comes to blending, there are several reports on PBAT blends with bio-based PLA in order to improve PLA's degradability, but that will be discussed in the PLA section below.96–98
PEAs are mainly composed of fossil-based monomers, such as the above-mentioned HMDA or caprolactam (CLM), although there is currently a trend to replace them with their renewable counterparts, such as, for example, biobased HMDA obtained from HMF or adiponitrile by hydrogenation.101 In this regard, some progress has been made with PEAs derived from BD and AA,102 amino acids103,104 or sebacic acid,105 or other natural products such as carbohydrates.106
PEAs can be synthesised via polycondensation of linear monomers (Scheme 5, top), usually performed under reduced pressure, high temperatures and using transesterification catalysts.102,107 Alternatively, greener strategies based on the ROP of cyclic monomers have also been used (Scheme 5, bottom), which can be associated with high Mw PEAs, low dispersity108 and without any volatile by-products. This approach is also associated with increased atom economy.
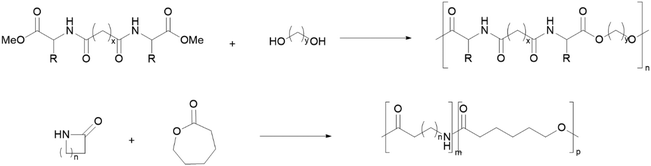 | ||
| Scheme 5 Examples of PEA synthesis through polycondensation of a diamine diester and an alkyldiol (top) and ROP of lactams and lactones (bottom).102,107,108 | ||
In terms of properties, PEAs are a very adaptable family of polymers prompted by varying the structural nature of the starting monomers and of course their relative proportion.109 As an example, Ge et al.110 studied PEAs with the incorporation of 11-aminoundecanoic acid into the PLA chain to obtain improved thermal properties. The ensuing polymer showed enhanced thermal degradation compared to neat PLA (252 to 214 °C, respectively).
When it comes to PEA degradability, Gonsalves et al.111 and Chen et al.112 reported the hydrolytic degradation of two types of PEA copolymers, one based on ε-caprolactone and ε-caprolactam and the other on 1,6-hexanediol, HMDA and adipoyl chloride. In aqueous media, these copolymers had a maximum weight loss of 59% in 19 days, under mild conditions (phosphate-buffered saline at 37 °C). However, only the hydrolysis of ester groups and no breakdown of amide bonds were detected, which is expected based on the nature of each group.
Similar observations were made by Zhiyong et al.113 with PEAs based on lactic and 1,10-aminoundecanoic acids. The lowest hydrolytic degradation rate was associated with the highest amide content and crystallinity (phosphate-buffered saline at 37 °C).
Some more PEA degradation studies were performed in basic and acidic aqueous media.114–116 Across the literature, it is clear that PEAs are more prone to alkaline hydrolysis than neutral hydrolysis, with the degradation mechanism occurring primarily on ester bonds. Some authors also pointed out the inability of lipase from C. cylindracea to prompt enzymatic degradation compared with proteases.117
More recently, Novotný et al.118 studied the performance of the bacterium Pseudomonas aeruginosa, yeast C. guilliermondii and micromycete Aspergillus fumigatus in a six week degradation trial at 28 °C of PEA fibres based on ε-caprolactone and ε-caprolactam (Fig. 1).
 | ||
| Fig. 1 SEM images of PEA fibres based on ε-caprolactone and ε-caprolactam after degradation with: Aspergillus fumigatus (left), C. guilliermondii (middle), and the abiotic control (right). Adapted with permission from ref. 118 Copyright 2014, with permission from Elsevier. | ||
The morphological changes observed in SEM images lead to mass losses between 5 and 10% in both biotic experiments and an abiotic control, demonstrating that the microorganisms’ contributions are not relevant. However, the culture supernatants that contained polymer degradation products exhibited significant biological toxicity. This topic needs to be further investigated to provide adequate EoL management.118
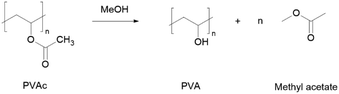
The repeating unit of PVA is vinyl alcohol, which is, in principle, unstable and cannot be isolated or obtained in high concentrations.124 Because of that, the most common method to synthesize PVA is through the alcoholysis or hydrolysis of polyvinyl acetate (PVAc) (Scheme 6). To the best of our knowledge, there are no reports of efforts on PVA green synthesis.
Although its properties depend on the degree of alcoholysis (and thus Mw), PVA is resistant to oil, grease, and organic solvents,124 and has excellent barrier properties, such as a low O2 permeability (0.02 vs. 0.03–0.04 for PET, Table 1) due to its relatively high degree of crystallinity and strong intermolecular forces arising from the hydroxyl groups in its repeating unit.125
Regarding degradability, Chiellini's group performed several studies on PVA degradation.126–129 In a comparative study, through composting, soil burial and aquatic degradation tests, they managed to identify an active PVA-degrading bacterial mixed culture starting from sewage sludge of a paper mill as an inoculum.126 They found the most significant degradation level within a short incubation time in liquid cultures (13% degradation in 21 days) in comparison with soil or compost samples where they found limited mineralisation. Additionally, PVA with different molecular weights was studied and no influence of molecular weight on bacterial degradation was observed.128
In an effort to endow PVA based materials with an increased renewable content, and also to modulate their properties, some authors followed a trend already observed for previously discussed polymers, that is to prepare blends with natural polymers and other biobased mixtures/compounds, such as protein wastes, collagen hydrolysate, and glycerol.130 This latter PVA blend was degraded by a mixed culture from water treatment after an approximately 10-day lag phase. This combination was proved to be a good option since the added protein hydrolysate and glycerol in PVA increased the (bio)degradability (activated sludge at 25 °C) more than expected from the proportional representation of the individual components, with increased CO2 production (up to approx. 94%).
Azahari et al.131 reported the addition of corn-starch to a PVA matrix in an attempt to increase its degradation rate. In this case, a 30/70 (in %) PVA/starch blend had a 92.5% weight loss for enzymatic degradation after just 60 hours, and up to 85% in both the soil and compost for 8 weeks (while pure PVA had only 60 and 20% degradation, respectively). However, the results from mechanical testing showed that as the starch content increased, the tensile and elongation at break decreased, whereas the modulus increased (Fig. 2).
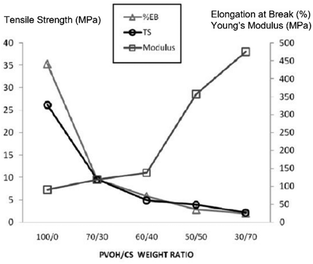 | ||
| Fig. 2 Effect of the variation of PVA/starch blend content on the tensile strength, elongation at break and Young's modulus. Adapted from ref. 130 Copyright 2011, with permission from USM Press. | ||
To overcome these disadvantages, Guohua et al.132 reported the preparation of PVA blends with methylated corn-starch (MCS). The obtained films had higher water resistance and both tensile strength and percent elongation at break of the MCS/PVA film were improved as the degree of substitution of MCS increased. In soil burial experiments, the MCS/PVA film showed rapid degradation in the initial 45 days (around 60% weight loss) followed by a slow degradation until the end of the experiment (maximum 70% in 180 days). Still, the blend showed improved degradation when compared to pure PVA, that could only reach a maximum of 20% weight loss.
PCs are conventionally synthesized by polycondensation of either highly toxic phosgene or harmful diphenyl carbonate, and with the health and environmental hazardous bisphenol A (BPA). Indeed, this compound has been identified as an endocrine disruptor with acute toxicity in the range of 1–10 μg ml−1 for aquatic species.133 Despite that, BPA-PCs (PCs containing BPA) are sold under a number of trade names, including the above cited Cyrolon (Acrylite)134 or Markrolon (Bayer).135 Furthermore, they have been mainly used in plastic lenses for eyewear, protective gear, and even to manufacture baby bottles.
This success is clearly related to their outstanding properties. In this regard these polymers have superior mechanical properties, such as great impact resistance (850 vs. 90 J m−1 in comparison with PET)52 and also toughness and flexibility at room temperature. Besides they present heat resistance and superior optical properties, such as transparency.
When it comes to BPA-PC degradation, Artham et al.136 performed relevant studies in seawater medium. BPA-PC was able to show only 9% weight loss and 5% Mn reduction after 1 year of incubation under laboratory conditions, proving its slow degradation in the marine environment. Despite this result, the same authors, on another study, reported 9 μg ml−1 of BPA and the respective oxidized products in the supernatant for seawater degradation studies.137 Further investigation showed that, in fact, the amount of released BPA in 1 year was higher than the half-maximal effective concentration (EC50) for marine species. In 2003, Sajiki et al.138 also studied the release of BPA but in different aquatic media (10 ml of water, river water, seawater at 20 °C) and BPA's behaviour afterwards under laboratory conditions. Fig. 3 shows BPA's concentration along time in these different media and at different temperatures.
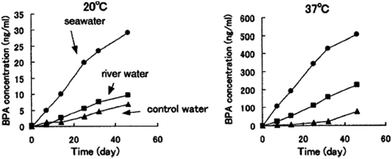 | ||
| Fig. 3 Variation of the time of PBA concentration in water (20 and 37 °C) from PC's degradation at 20 °C (left) and 37 °C (right). Reprinted with permission from ref. 138 Copyright 2002, with permission from Elsevier. | ||
They found that BPA's leaching rate in seawater was the fastest for both tested temperatures, with BPA's rapid increase at 11 ng day−1 at 37 °C. This resulted in the highest concentration of BPA, around 500 ng ml−1, after 45 days. This value corresponds to approximately half of the minimum concentration for acute toxicity for aquatic species.133
Nevertheless, BPA-free and more sustainable PCs are being increasingly studied,139,140 with some alternatives in the market such as Kostrate® PC by the Plastic Selection Group.141 Other alternatives to prepare BPA-free PCs can be seen in Scheme 7, with for example PCs synthesised from creosol-derivatives, which are simultaneously non-estrogenic and bio-based.139
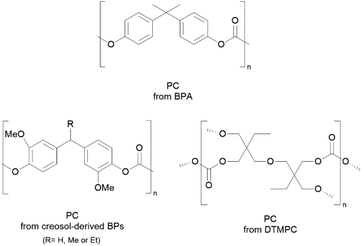 | ||
| Scheme 7 Chemical structures of PCs derived from BPA and some alternatives derived from creosol and DTMPC.138,141 Reproduced from ref. 138, 141 with permission from the Royal Society of Chemistry, copyright 2021 and 2018. | ||
These creosol-derived BPA-free PCs show a good thermal performance with high thermal stabilities, with a decomposition temperature (Td5%) up to 383 °C.139 In that work no degradability tests were carried out, despite their relevance for anticipating future behaviour both in natural environments and under controlled composting conditions.
Other studies suggest the synthesis of BPA-free PCs from di-trimethylolpropane dicyclic carbonate (DTMPC) by greener ROP homopolymerisation (Scheme 1) with a metal-free organic-based catalyst system.142 The ensuing PCs have shown to be highly transparent materials when compared to commercial PC sheets (Fig. 4).
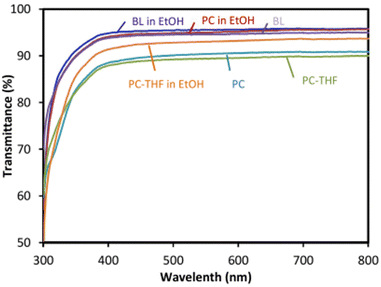 | ||
| Fig. 4 Optical transparency (% transmittance) properties of PBA-free PCs, prepared from DTMPC. BL: blank; BL in EtOH: blank in ethanol; PC in EtOH: PC in ethanol; PC-THF: PC after being submerged in THF for 15 min; PC-THF in EtOH: PC after being submerged in THF for 15 min in ethanol. Reproduced from ref. 141 with permission from the Royal Society of Chemistry, copyright 2018. | ||
The high optical transparency (with all BPA-free PCs above 85%) makes these new PCs excellent candidates for a variety of applications, such as food contacting materials in packaging. Despite the initial stages of development of these BPA-free alternatives, the existing BPA-PCs’ degradation studies are of extreme importance to understand the persistence (or not) of these polymers in the environment.
However, linear PURs are typically synthesised from hazardous and fossil-based diisocyanates with polyols. These different building blocks can be made of aromatic or aliphatic isocyanates and several types of polyols, such as polyester-, polyether-, and PC-derivatives, among others.
There is great interest today in a greener transition, and, hence, in switching to bio-based PURs, to reduce their fossil resource dependency. There are many bio-based polyols available,145 mainly derived from vegetable oils, fatty acids, glycerol, crop residues and protein-based feedstocks.146 PURs produced with bio-based polyols and fossil-based isocyanates have been reported.147
In order to obtain a completely bio-based PUR, alternatives for isocyanate substitution have also been addressed. For example, Hojabri et al.148 reported the synthesis and characterisation of unsaturated diisocyanate from oleic acids and its incorporation into a bio-based PUR with canola diol and canola polyol.
Another issue regarding the quest for more sustainable PURs is the fact that typically diisocyanates have high toxicity for humans. In this regard, in 2020, the EU agency of Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) published restrictions on the use of diisocyanates.149 Additionally, the release of diisocyanates in the EoL of PURs is of serious concern and brings about an enormous disadvantage for their use. Nevertheless, as reported by Kim et al.150 PURs do suffer from composting degradation, the extent of which depends on the different chemical structures. In this work, the diisocyanates used were 4,4′-diphenylmethane diisocyanate and 1,6-hexane diisocyanate with a variety of polyols. PURs’ weight losses ranged from 5 to 43% at the end of 45 days of composting (ASTM D 5338).94 When the polyol used was poly(1,6-hexamethylene adipate)diol or PCL-diol, the maximum degradation was achieved when compared to poly(1,10-decamethylene adipate) diol or poly(ethylene adipate)diol, for example.
Therefore, diisocyanate substitution is of extreme relevance when it comes to the EoL, with extreme priority for non-toxic alternatives. In that perspective, there are precisely several efforts being made to produce alternative isocyanate-free PURs.151–153 Besse et al.154 reported the use of isosorbide dicyclocarbonates, instead of diisocyanates, in the preparation of biobased PURs (Scheme 8). The above-mentioned alternative isosorbide-based PUR154 had no alcohol or alkene by-products being formed during thermal degradation (T < 500 °C), and the only degradation products were CO2 and secondary amines. Further hydrolytic degradation studies, but specially composting tests due to their relevance to waste management, should be performed to understand their environmental impact in-depth.
 | ||
| Scheme 8 Example of an isocyanate-free PUR synthesized from a carbonated oligoisosorbide and 1,10-diaminodecane.154 | ||
Overview of the main commercially available bio-based polymers
In the last two decades an unprecedented quest for renewable-based chemicals, monomers and polymers thereof started,155–157 mainly motivated by expert predictions about the depletion of fossil reserves, and the prevailing worrisome concern about the geopolitical situation of these dwindling resources.158Research and innovation initiatives have been implemented ubiquitously both by academia and industrial sectors. Biomass became an inexhaustible source of building-block monomers suitable for increasing the renewable content of polymers such as the case of PBAT, mentioned previously, or instead to prepare a variety of fully bio-based thermoplastic polyesters, described subsequently, a few of which are already at the market (e.g., PLA, PBS).
PLA can be obtained from the lactic acid (LA) monomer, although the related lactide dimer is more commonly used via ROP (Scheme 9). Because of the chirality of the lactyl unit, lactide exists in three diastereoisomeric forms: L-lactide, D-lactide and meso-lactide (Scheme 9).163 The polymer thereof can be obtained as the enantiomerically pure form poly(D-lactide) (PDLA) and poly(L-lactide) (PLLA), or as the racemic poly-(DL-lactide) (PDLLA) form.
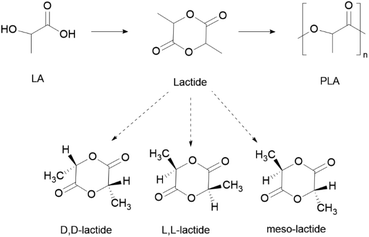 | ||
| Scheme 9 General process of PLA synthesis, through ROP, and respective stereoisomeric forms of the lactide monomer. | ||
PLA's renewable origin is, without a doubt, one of the most attractive features. However, its mechanical properties, when compared to, for example, fossil-based PCL can limit its widespread use (lower tensile strength, elongation at break and yield, as observed in Table 1. PLA possesses an intrinsic low crystallisation rate and limited barrier properties compared to its oil-based counterparts, like PET (3.5–15 vs. 1–5 O2 permeability coefficient).164 As can be seen in Fig. 5, diffusivity decreases as the crystallinity increases.164 Furthermore, PLA samples annealed at 125 °C have a higher diffusivity than those annealed at a lower temperature, but with the same level of crystallinity.
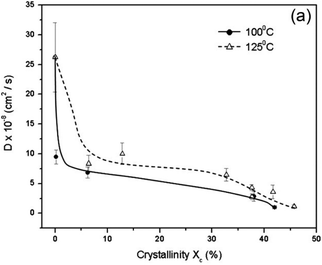 | ||
| Fig. 5 Effect of crystallinity on the O2 diffusion coefficient for PLA samples annealed at 100 and 125 °C. Reprinted with permission from ref. 164. Copyright © 2009 Wiley Periodicals, Inc. | ||
Studies on PLA hydrolytic degradation in aquatic environments, where temperatures are usually very low, revealed low degradation under these media. In this vein, Bagheri et al.165 performed a comparative degradation study of PLA with several (bio)degradable polymers, namely PCL, in fresh water and seawater. PLA showed no significant weight variation for one year period in both aquatic media, and the same result was obtained for PCL. In static seawater (25 °C), Tsuji et al.48 also studied the comparative degradation of PLLA and PCL. While the latter showed a 25% weight loss at the end of 10 weeks, PLLA showed no significant changes during that period. The relevant work of the Scott and Suh group highlights precisely the same trend, even showing that the specific surface degradation rate of PLA and the well-established non-degradable HDPE are similar in the marine environment.3
Despite the non-degradability of PLA in aquatic media, and the probable persistence in the case of ending up in these environments, if suitable composting facilities are instead used, following well-defined international standards (e.g., EN 13432, ISO 14855, ISO 17088, ASTM D6400),13,14,34,35 PLA will have an adequate EoL. As an example, Kale et al.166 studied the industrial composting (60 °C) of PLA bottles reporting complete degradation in just 30 days. Despite that, PLA's degradation at ambient temperatures (e.g., home composting conditions) becomes much slower due to the lower temperatures and most probable different biota. Rudnik et al.33 reported the degradation of PLA fibres and films in soil and compost at ambient temperatures. The first significant changes started to occur only after 7 months, and sample disintegration could only be clearly observed at the end of 11 months.
PLA's previously mentioned poor mechanical properties can be circumvented, as pointed out for other polymers, by copolymerisation or by blending PLA and polymers with enhanced mechanical properties like PCL.47 In fact, blending PLA with a relatively low loading of PCL (<20% w/w) leads to a substantial reduction of the Young's modulus and increase of the elongation at break when compared to pristine PLA, resulting, thus, in more flexible materials (Fig. 6).47
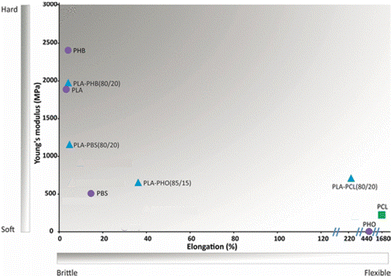 | ||
| Fig. 6 Mechanical properties of biobased polymers (purple circles) and fossil-based PCL (green square) and their blends (blue triangles). Adapted with permission from ref. 47. Copyright 2018 American Chemical Society. | ||
The same study reported other PLA blends (Fig. 6), showcasing for example, the incorporation of poly(hydroxyoctanoate) (PHO) to improve PLA's flexibility, with the PLA/PHO (85/15) blend having 35% elongation at break.
Logically, PLA's blends and copolymers have also been studied in the attempt to improve its degradation under mild conditions. When PLA is blended with fossil-based PCL, besides improving mechanical properties, it also becomes home compostable (180 days at ambient temperature), which is of extreme importance.47 In this study a PLA/PCL blend with a ratio of 80/20 was able to degrade under simulated home composting conditions (ISO 14855 at 28 °C)14 in 260 days, unlike PLA alone.47 However, this PLA/PCL blend was not able to pass other degradation tests according to the ISO and ASTM standards, so it would not be able to degrade in marine, fresh water, or soil environments.
Following this trend, Fukushima et al.71 also tested a blend of poly(DL-lactide) and PCL (PDLA/PCL = 80/20 (in %)). The addition of PCL also showed improvements in PDLA's composting behaviour that was generally higher in compost after just 84 days at 37 °C. Other attempts for improving PLA's degradation with alternative blends, such as PLA/PBAT, can also be found in the specialised literature.96,97 However, it was found that the expectation of accelerating PLA degradation in soil by blending with PBAT was not achieved, since PLA and PBAT individual controls degraded faster than the blend thereof.
When it comes to copolymers, the incorporation of the glycolic moiety is able to enhance the degradation, with the copolymer poly(lactic-co-glycolic acid) (PLGA) (lactide/glycolide molar feed ratio of 50/50), known to be the most common bioresorbable copolymer, showing the best results of Bagheri's seawater study (25 °C) with complete degradation after 270 days.165
PBS is a highly crystalline polyester with a melting point at around 115 °C.170 When compared to well-established PLA (Table 1), it has a lower Tm (115 °C vs. 160 °C), but it reveals a higher flexibility, with an elongation at break of 379% (maximum 240% for PLA). These characteristics make PBS a viable option to replace, for example, fossil-based PP in fishing gear.
In this vein, Deroiné et al.171 reported a resistant monofilament based on PBS as an alternative for fishing gear. The monofilaments were prepared by melt-processing using a single-screw extruder and by controlling the processing temperature they managed to improve the tensile behaviour of PBS monofilaments (Fig. 7).
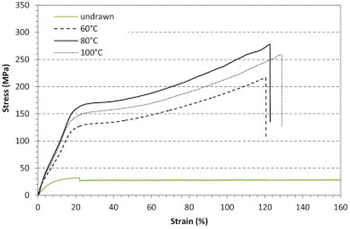 | ||
| Fig. 7 Evolution of the tensile behaviour of PBS monofilaments drawn at different temperatures. Adapted with permission from ref. 171. Copyright 2018, with permission from Elsevier. | ||
The monofilaments’ tensile behaviour was improved in comparison with undrawn PBS. However, in this study degradation studies in aquatic environments (at least hydrolytic degradation) of PBS monofilaments are missing, despite their particular relevance for target fishing gear application. Nevertheless, other authors studied the hydrolytic degradation of PBS pieces (5 × 6 × 0.2 mm3) at ambient temperatures in phosphate-buffered saline.172 The samples started losing weight slowly, with only 10% after 9 weeks, but they reached 65% weight loss at the end of 15 weeks. When it comes to microbial degradation, Zhao et al.173 isolated and identified different microbial strains from the compost of PBS degradation, and Aspergillus versicolor revealed to be the best PBS-degrading microorganism.
When it comes to controlled composting studies (ISO 14855, 58 °C),14 Zhao et al.173 studied PBS degradation in different forms (powder, granulate, and films). The maximum composting degradation was achieved, as one could anticipate, with powdered samples with maximum 72% after 90 days. The SEM analysis allows the observation of the changes in the PBS surface, thus confirming its degradation in compost (Fig. 8).
 | ||
| Fig. 8 SEM images of the surface changes of PBS films before composting (left) and after composting (right) for 90 days. Adapted with permission from ref. 173. Copyright © 2005 Wiley Periodicals, Inc. | ||
Some copolymers and blends with PBS have also been reported as a strategy to tailor degradation. In a comparative study, Tsutsumi et al.174 studied both the enzymatic (lipases in phosphate-buffered solution at 37 °C) and oxidative degradation (sodium hydroxide solution at 37 °C) of PBSA and a PBS/PCL blend and compared them with PBS. They found that PBSA was readily degraded by various lipases, with a maximum weight loss of 96% after just 10 days by AK lipase from Pseudomonas sp. The degradation rate of PBSA by the same lipase was 16 times faster than that by the tested oxidative degradation. Also, for the same enzyme, the PBS/PCL blend reached a ca. 56% weight loss while PBS reached only 14%.
Zhu et al.175 described the microbial degradation of poly(butylene succinate-co-adipate) (PBSA) and poly(butylene-co-hexylene succinate) (PBHS) copolymers carried out with Penicillium chrysogenum (37 °C), whereas Wang and co-workers176 used Aspergillus versicolor fungi (30 °C) instead. For the former, the copolyesters reached over the course of 52 days, a maximum weight loss of only ca. 7% (PBSA), although this result is higher than that observed for pristine PBS. Hence, in the case of Penicillium chrysogenum-mediated degradation (as well as soil burial tests) the copolymerisation strategy was not enough to make a noticeable impact on the overall degradation process. Oppositely, in the case of Aspergillus versicolor mediated microbial degradation of PBSA, more than 90% of the copolymer was assimilated within 25 days.176 At the early stage of PBSA microbial degradation, the decrease in molecular weight is mostly attributed to the random non-enzymatic hydrolysis of the polymer chains. Once the molecular weight decreases to a point where microbial attack can proceed, the biotic hydrolysis is accelerated.
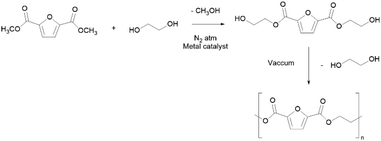
PEF is synthesized from bio-based 2,5-furandicarboxylic acid (FDCA) as opposed to fossil-based TPA, and ethylene glycol through a typical two-step melt polycondensation procedure (Scheme 11) applying high temperature (>200 °C), vacuum, and long reaction times and using metal-based catalysts.184,185 Nevertheless, greener synthesis has been proposed, either using ROP in less than 30 min,186,187 or using ionic liquids or non-metal catalysts.188,189
PEF is expected to reach several markets, with applications in packaging, mainly in the form of bottles for soft drinks (being 31 times less permeable to CO2 than PET); other potential applications comprise textiles and as films.178,190 The unique set of properties of PEF include thermal properties such as high melting (200–215 °C) and glass transition (75–80 °C) temperatures, together with high thermal stability (up to 350 °C).177 Furthermore, this polyester is a highly stiff material with a Young's modulus of approximately 2450 MPa and maximum stress equal to 35 MPa.184
In terms of composting experiments, Avantium reported the degradation of PEF under accelerated (58 °C) industrial composting conditions by Organic Waste System (OWS).182,191 In these tests, it was observed that in just 9 months PEF reached 90% degradation based on the amount of CO2 released (vs. only 10% for PET). Nevertheless, further studies under natural conditions (such as typical aquatic and soil degradation) are required to provide a more realistic scenario of PEF behaviour.
Furan-based polymers include many others, besides PEF, namely furanic–aliphatic (co)polyesters with potential enhanced degradability compared to PEF, and in this regard, with multiple EoL options. In this vein, Sousa et al.192 introduced the synthesis of this family of polymers with poly(ethylene 2,5-furandicarboxylate-co-poly(L-lactic acid))s (PEFLs). The authors reported an overall trend of improved thermal stability of PEFLs when compared with the PLLA precursor (>300 °C vs. 257 °C, respectively). Also, the Tg was higher due to an increase in stiffness prompted by the furanic moieties. The incorporation of just 8 mol% of lactyl units was enough to increase the enzymatic degradability of the copolyester in phosphate-buffered saline medium (37 °C), while not compromising the thermal properties, as can be seen in Table 1. Nevertheless, the highest weight loss was achieved with 74% lactyl units, reaching almost 60% total weight loss over the course of 12 weeks. Later, in 2015, Wu et al. complemented the previous study with PEFL degradation tests in garden soil at 35 °C, for 55 days.193 As observed for the phosphate-buffered saline incubation tests performed by Sousa et al.,192 a maximum weight loss was achieved for the copolymer incorporating 80% of lactyl units.
Efforts on this family of polymers have also turned to furanic replacements of previously described fossil-based TPA in PBAT, that is poly(butylene adipate-co-butylene 2,5-furandicarboxylate)s (PBAFs).58,194,195 These copolyesters possess the excellent thermal stability of furan-based polyesters (Td,5% > 340 °C).58 PBAFs with a lower furanic content (<50%) also exhibited high-elastic deformation and rebound resilience, but the Young's modulus ranged between 18 and 122 MPa and the tensile strength was 9–17 MPa.58 PBAF with 40% of butylene 2,5-furanoate moieties has the lowest tensile modulus and strength due to the weakest ability to crystallise (also associated with statistic factors). With increasing furanic content, the stress hardening becomes more obvious, and the tensile modulus and strength of PBAF increase, but the elongation at break follows a downward trend.
Degradation experiments in phosphate-buffered saline medium (37 °C), simulated seawater (5–10 °C) and CALB enzymatic incubation revealed PBAFs’ tendency to degrade, especially for higher percentages of adipic acid,194 as already observed for the PBAT counterparts.196
In other similar studies, PBSFs prepared from FDCA, succinic acid and 1,4-butanediol were also mentioned,58,197,198 but many others could also be included as those made of poly(ethylene glycol) segments199,200 or from long chain alkanoids and alkane diols.201,202
Criteria and opportunities for (bio)degradable polymers
(Bio)degradable polymers can play a role in polymer waste management or, at least, in mitigating their accumulation in natural environments, and, thus, prompting a more adequate EoL for polymer articles. This review shows that there is not a one size fits all solution, as would be expected for a complex problem as polymer waste, instead it requires a series of varied efforts, starting from social behavioural ones, along with adequate legal frameworks, to, at the downstream, stronger collection, sorting and better recycling technologies.203 However, it is undeniable that in certain circumstances compostable or fully (bio)degradable polymers can attenuate the persistent polymers’ environmental impact. Anticipating the need for a judicious replacement of traditional persistent polymers with compostable ones, the European Bioplastics Association (EUBP) has set forth a group of recommendation criteria for considering such substitution:204(i) alternative reusable solutions cannot be redesigned, highlighting the need to reuse polymeric objects;
(ii) contamination with food waste occurs and cleaning is not feasible;
(iii) they provide potential to reduce biowaste contamination;
(iv) only when mechanical recycling fails to effectively recycle polymers and likely they will end up in the organic waste collection, (bio)degradable options should be considered.
Besides the need to reuse before performing a substitution, polymer separation from organic waste might be challenging and cross-contamination might be a serious problem.205 It is not uncommon for polymer articles made of non-degradable polymers to coexist with food residues in a collection stream. Here, they act as cross-contaminants to each other, limiting both their EoL solutions, namely recycling and composting, respectively. Precisely, several studies indicate that approximately 4 to 5 wt% of biowaste streams are contaminated with non-(bio)degradable polymers, with the majority being plastic bags and packaging films typically made from PE and PP, or even PET.206,207 Other products that fall under similar EoL issues are, for example, tea bags and fruit stickers.204 In these situations, the most probable fate of these contaminated polymers is either landfills or incinerators.208 From a general point of view, the advantage of using compostable polymers is evident when there is a risk of cross-contamination, and recycling fails.
Furthermore, collection is often associated to several issues, being not cost-effective, rather incomplete, or even impossible due to causes as diverse as the countries’ underdeveloped collecting/separation system,209 not practiced by the users as is the case of agriculture mulch films from LDPE, and of the fishing gear's dolly rope, commercially manufactured from persistent PP. All of these open up great opportunities for compostable polymers, such as PBAT or PLA, as well as to innovative recycling technologies.
Additionally, there may be advantages of using biodegradable polymers in situations where separation is an impossible task, for example when small polymer pieces are unavoidably mixed with sewage and likely to escape to the environment.205 For example, microbeads with typically less than 1 mm in diameter present in exfoliants are small plastic pieces.210 Recently, there have been further reports on the environmental impact of microplastics in cosmetics’, stressing the urgent need for global legislation.211,212
To conclude, following the previously mentioned criteria, a series of products can be identified as potential for being produced from compostable polymeric materials, although this path also brings technological challenges. Nevertheless, Fig. 9 illustrates some of those products based on how commonly they are found in compost and how rarely they are recycled. In this regard tailor-made synthetic (bio)degradable polymers are a very promising solution for a sustainable development.
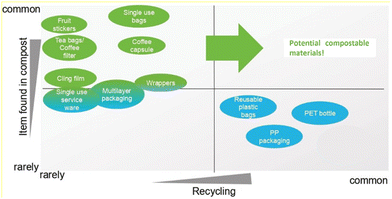 | ||
| Fig. 9 Several products and their potential compostable materials based on how often they are found in compost and their recyclability. Reproduced from ref. 203 with permission from European Bioplastics e.V., 2022. | ||
Conclusions
In summary, there are a variety of promising synthetic (bio)degradable polymers, either fossil-based or, even better, biobased ones. PCL, PGA, PBAT, PLA, PBS and furan-based polymers are some of the materials judiciously selected to be reviewed in this appraisal due to their properties and related (potential) commercial relevance for packaging, textile fibres or films. They were in-depth overviewed from three main aspects: green efforts to render their composition and/or synthesis greener, properties and applications, and (bio)degradation behaviour in natural environments and under controlled composting conditions.We have also discussed a very pertinent point for a conscientious transition from persistent polymers to (bio)degradable ones – the criteria and the specific situations where they are beneficial for a sustainable development. For example, in cases where reuse is not possible, cross-contamination and separation are not cost-effective or mostly incomplete, and recycling fails.
Author contributions
AFS – conceptualisation, supervision, and writing – original draft and review & editing. BA – data curation and writing – review & editing. AJDS and JC – writing – original draft and review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was developed within the scope of the CICECO—Aveiro Institute of Materials (UIDB/50011/2020 & UIDP/50011/2020) & LA/P/0006/2020, financed by national funds through the FCT —Fundação para a Ciência e a Tecnologia/MEC (PIDDAC). This research is also sponsored by FEDER funds through the program COMPETE—Programa Operacional Factores de Competitividade—and by national funds through the FCT under the project UID/EMS/00285/2020. The FCT is also acknowledged for the research contract under Scientific Employment Stimulus to AFS (CEECIND/02322/2020) and for a doctorate grant to BA (2020.04495.BD). This publication is supported by COST Action FUR4Sustain—European network of FURan based chemicals and materials FOR a Sustainable development, CA18220, supported by COST (European Cooperation in Science and Technology).References
- Plastics Europe - Association of Plastic Manufacturers (Organization), Plastics - the Facts 2021, 2021 Search PubMed.
- R. A. Sheldon and M. Norton, Green Chem., 2020, 22, 6310–6322 RSC.
- A. Chamas, H. Moon, J. Zheng, Y. Qiu, T. Tabassum, J. H. Jang, M. Abu-Omar, S. L. Scott and S. Suh, ACS Sustainable Chem. Eng., 2020, 8, 3511 Search PubMed.
- J. D. Gu, Int. Biodeterior. Biodegrad., 2003, 52, 69–91 CrossRef CAS.
- B. Agostinho, A. J. D. Silvestre and A. F. Sousa, Green Chem., 2022, 24, 3115–3119 RSC.
- I. Vollmer, M. J. F. Jenks, M. C. P. Roelands, R. J. White, T. van Harmelen, P. de Wild, G. P. van der Laan, F. Meirer, J. T. F. Keurentjes and B. M. Weckhuysen, Angew. Chem., Int. Ed., 2020, 59, 15402–15423 CrossRef CAS PubMed.
- United States Environmental Protection Agency, National Overview: Facts and Figures on Materials, Wastes and Recycling, https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/national-overview-facts-and-figures-materials, (accessed 10 May 2022).
- European Environment Agency, Waste recycling in Europe, https://www.eea.europa.eu/ims/waste-recycling-in-europe, (accessed 10 May 2022).
- Plastics Europe - Association of Plastic Manufacturers (Organization), Recycling technologies, https://plasticseurope.org/sustainability/circularity/recycling/recycling-technologies/, (accessed 21 June 2022).
- I. A. Ignatyev, W. Thielemans and B. Vander Beke, ChemSusChem, 2014, 7, 1579–1593 CrossRef CAS PubMed.
- United Nations Conference on Trade and Development, UN recognition of the least developed countries, https://unctad.org/topic/least-developed-countries/recognition, (accessed 19 January 2022).
- J. Jambeck, B. D. Hardesty, A. L. Brooks, T. Friend, K. Teleki, J. Fabres, Y. Beaudoin, A. Bamba, J. Francis, A. J. Ribbink, T. Baleta, H. Bouwman, J. Knox and C. Wilcox, Mar. Policy, 2018, 96, 256–263 CrossRef.
- ISO - Online Browsing Platform (OBP), ISO 17088 - Plastics - Organic recycling - Specifications for compostable plastics, https://www.iso.org/obp/ui/#iso:std:iso:17088:ed-3:v1:en, (accessed 11 April 2022).
- ISO - Online Browsing Platform (OBP), ISO 14855-2 -Determination of the ultimate aerobic biodegradability of plastic materials under controlled composting conditions - Method by analysis of evolved carbon dioxide - Part 2: Gravimetric measurement of carbon dioxide evolved in a laboratory-scale, https://www.iso.org/standard/72046.html, (accessed 11 April 2022).
- European Bioplastics e.V., Bioplastics market data, https://www.european-bioplastics.org/market/, (accessed 22 March 2022).
- T. Grethe, Tekstilec, 2021, 64, 32–46 CrossRef CAS.
- K. Twarowska-Schmidt, Fibres Text. East. Eur., 2004, 12, 17–20 CAS.
- B. Younes, J. Text. Inst., 2017, 108, 674–682 CrossRef.
- K. Shirvanimoghaddam, B. Motamed, S. Ramakrishna and M. Naebe, Sci. Total Environ., 2020, 718, 137317 CrossRef CAS PubMed.
- D. J. Sarkar, M. Barman, T. Bera, M. De and D. Chatterjee, in Encyclopedia of Polymer Applications, 2018, pp. 1–20 Search PubMed.
- S. Kasirajan and M. Ngouajio, Agron. Sustainable Dev., 2012, 32, 501–529 CrossRef CAS.
- S. Bandopadhyay, L. Martin-Closas, A. M. Pelacho and J. M. DeBruyn, Front. Microbiol., 2018, 9, 1–7 CrossRef PubMed.
- European Comission, The European Green Deal, Brussels, 2019 Search PubMed.
- United Nations Development Programme, The SDGs Goals in Action, https://www.undp.org/sustainable-development-goals, (accessed 22 March 2022).
- T. V. Shah and D. V. Vasava, e-Polymers, 2019, 19, 385–410 CAS.
- B. D. Ulery, L. S. Nair and C. T. Laurencin, J. Polym. Sci., Part B: Polym. Phys., 2011, 49, 832–864 CrossRef CAS PubMed.
- P. Béguin and J.-P. Aubert, FEMS Microbiol. Rev., 1994, 13, 25–58 CrossRef PubMed.
- A. M. Nafchi, M. Moradpour, M. Saeidi and A. K. Alias, Starch/Staerke, 2013, 65, 61–72 CrossRef.
- V. Siracusa, P. Rocculi, S. Romani and M. D. Rosa, Trends Food Sci. Technol., 2008, 19, 634–643 CrossRef CAS.
- IUPAC, Compendium of Chemical Terminology, Blackwell Scientific Publications, Oxford, 2nd edn, 1997 Search PubMed.
- M. Vert, Y. Doi, K. H. Hellwich, M. Hess, P. Hodge, P. Kubisa, M. Rinaudo and F. Schué, Pure Appl. Chem., 2012, 84, 377–410 CrossRef CAS.
- K. Horie, M. Barón, R. B. Fox, J. He, M. Hess, J. Kahovec, T. Kitayama, P. Kubisa, E. Maréchal, W. Mormann, R. F. T. Stepto, D. Tabak, J. Vohlídal, E. S. Wilks and W. J. Work, Pure Appl. Chem., 2004, 76, 889–906 CrossRef CAS.
- E. Rudnik and D. Briassoulis, Ind. Crops Prod., 2011, 33, 648–658 CrossRef CAS.
- ASTM International, Standard Specification for Labeling of End Items that Incorporate Plastics and Polymers as Coatings or Additives with Paper and Other Substrates Designed to be Aerobically Composted in Municipal or Industrial Facilities, https://www.astm.org/Standards/D6868.htm, (accessed 22 April 2021).
- Comite Europeen de Normalisation (CEN), 2005.
- J. G. Rosenboom, R. Langer and G. Traverso, Nat. Rev. Mater., 2022, 7, 117–137 CrossRef PubMed.
- Ingevity, Capa® 6500, https://www.ingevity.com/products/capa-6500/, (accessed 13 April 2022).
- U.S. Food & Drug Administration (FDA), CFR - Code of Federal Regulations Title 21, https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=175.105, (accessed 6 June 2022).
- EUR-Lex (European Union law), Commission Regulation (EU) No 10/2011, https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32011R0010, (accessed 6 June 2022).
- S. H. Pyo, J. H. Park, V. Srebny and R. Hatti-Kaul, Green Chem., 2020, 22, 4450–4455 RSC.
- G. Acik, J. Polym. Environ., 2020, 28, 668–675 CrossRef CAS.
- M. Labet and W. Thielemans, Chem. Soc. Rev., 2009, 38, 3484–3504 RSC.
- T. L. Yu, C. C. Wu, C. C. Chen, B. H. Huang, J. Wu and C. C. Lin, Polymer, 2005, 46, 5909–5917 CrossRef CAS.
- I. Vroman and L. Tighzert, Materials, 2009, 2, 307–344 CrossRef CAS.
- V. Guarino, G. Gentile, L. Sorrentino and L. Ambrosio, in Encyclopedia of Polymer Science and Technology, 2017, pp. 1–36 Search PubMed.
- A. F. Sousa, R. Patrício, Z. Terzopoulou, D. N. Bikiaris, T. Stern, J. Wenger, K. Loos, N. Lotti, V. Siracusa, A. Szymczyk, S. Paszkiewicz, K. S. Triantafyllidis, A. Zamboulis, M. S. Nikolic, P. Spasojevic, S. Thiyagarajan, D. S. Van Es and N. Guigo, Green Chem., 2021, 23, 8795–8820 RSC.
- T. Narancic, S. Verstichel, S. Reddy Chaganti, L. Morales-Gamez, S. T. Kenny, B. De Wilde, R. Babu Padamati and K. E. O'Connor, Environ. Sci. Technol., 2018, 52, 10441–10452 CrossRef CAS PubMed.
- H. Tsuji and K. Suzuyoshi, Polym. Degrad. Stab., 2002, 75, 347–355 CrossRef CAS.
- C. Bastioli, Polym. Degrad. Stab., 1998, 59, 263–272 CrossRef CAS.
- M. LLc, Matweb - Material Property Data, https://www.matweb.com/search/DataSheet.aspx?MatGUID=501acbb63cbc4f748faa7490884cdbca&ckck=1, (accessed 19 April 2022).
- P. A. M. Lips and P. J. Dijkstra, Biodegradable Polymers for Industrial Applications, Woodhead Publisihing Limited, 2005 Search PubMed.
- J. E. Mark, Polymer Data Handbook, Oxford University Press, New York, 1999 Search PubMed.
- J. Jian, Z. Xiangbin and H. Xianbo, Adv. Ind. Eng. Polym. Res., 2020, 3, 19–26 Search PubMed.
- L. K. Massey, Permeability Properties of Plastics and Elastomers - A Guide to Packaging and Barrier Materials, 2nd edn, 2003, vol. 92 Search PubMed.
- A. Eceiza, K. de la Caba, G. Kortaberria, N. Gabilondo, M. Corcuera and I. Mondragon, Polym. Eng. Sci., 2008, 48, 297–306 CrossRef CAS.
- A. Boubakri, N. Haddar, K. Elleuch and Y. Bienvenu, Mater. Des., 2010, 31, 4194–4201 CrossRef CAS.
- V. C. Pinto, T. Ramos, S. Alves, J. Xavier, P. Tavares, P. M. G. P. Moreira and R. M. Guedes, Procedia Eng., 2015, 114, 635–642 CrossRef CAS.
- B. Wu, Y. Xu, Z. Bu, L. Wu, B. G. Li and P. Dubois, Polymer, 2014, 55, 3648–3655 CrossRef CAS.
- T. M. Crizel, T. M. H. Costa, A. O. Rios and S. H. Flôres, Ind. Crops Prod., 2016, 87, 218–228 CrossRef CAS.
- Q. Ma, K. Shi, T. Su and Z. Wang, J. Polym. Environ., 2020, 28, 2947–2955 CrossRef CAS.
- D. R. Chen, J. Z. Bei and S. G. Wang, Polym. Degrad. Stab., 2000, 67, 455–459 CrossRef CAS.
- M. J. Motiwalla, P. P. Punyarthi, M. K. Mehta and V. Kelkar-mane, J. Environ. Biol., 2013, 34, 43–49 Search PubMed.
- M. Rutkowska, K. Krasowska, A. Heimowska, H. Janik, J. Haponiuk and S. Karlsson, Pol. J. Environ. Stud., 2002, 11, 413–420 CAS.
- M. E. F. César, P. D. S. C. Mariani, L. H. Innocentini-Mei and E. J. B. N. Cardoso, Polym. Test., 2009, 28, 680–687 CrossRef.
- H. Yavuz and C. Babaç, J. Polym. Environ., 2003, 11, 107–113 CrossRef CAS.
- Novamont S.p.A., Mater-Bi, https://www.novamont.com/eng/mater-bi, (accessed 28 February 2022).
- A. Ostafinska, I. Fortelny, M. Nevoralova, J. Hodan, J. Kredatusova and M. Slouf, RSC Adv., 2015, 5, 98971–98982 RSC.
- A. C. Vieira, J. C. Vieira, R. M. Guedes and A. T. Marques, Mater. Sci. Forum, 2010, 636–637, 825–832 CAS.
- N. T. T. Thao, S. Lee, G. R. Shin, Y. Kang, S. Choi and M. S. Kim, Pharmaceutics, 2021, 13, 253 CrossRef CAS PubMed.
- A. Richert and G. B. Dąbrowska, Int. J. Biol. Macromol., 2021, 176, 226–232 CrossRef CAS PubMed.
- K. Fukushima, J. L. Feijoo and M. C. Yang, Eur. Polym. J., 2013, 49, 706–717 CrossRef CAS.
- N. K. Kalita, S. M. Bhasney, C. Mudenur, A. Kalamdhad and V. Katiyar, Chemosphere, 2020, 247, 125875 CrossRef CAS PubMed.
- K. Y. Lim, B. C. Kim and K. J. Yoon, J. Appl. Polym. Sci., 2003, 88, 131–138 CrossRef CAS.
- K. Y. Lim, B. C. Kim and K. J. Yoon, J. Polym. Sci., Part B: Polym. Phys., 2002, 40, 2552–2560 CrossRef CAS.
- E. Chiellini, J. Environ. Polym. Degrad., 1996, 4, 37–50 CrossRef CAS.
- Kureha Corporation, Kuredux, https://www.kureha.co.jp/en/business/material/kuredux.html, (accessed 13 April 2022).
- P. K. Samantaray, A. Little, D. M. Haddleton, T. Mcnally, B. Tan, Z. Sun, W. Huang, Y. Ji and C. Wan, Green Chem., 2020, 22, 4055–4081 RSC.
- A. Panova, L. J. Mersinger, Q. Liu, T. Foo, D. C. Roe, W. L. Spillan, A. E. Sigmund, A. Ben-Bassat, L. W. Wagner, D. P. O’Keefe, S. Wu, K. L. Petrillo, M. S. Payne, S. T. Breske, F. G. Gallagher and R. Dicosimo, Adv. Synth. Catal., 2007, 349, 1462–1474 CrossRef CAS.
- S. Wu, A. J. Fogiel, K. L. Petrillo, R. E. Jackson, K. N. Parker, R. DiCosimo, A. Ben-Bassat, D. P. O'Keefe and M. S. Payne, Biotechnol. Bioeng., 2008, 99, 717–720 CrossRef CAS PubMed.
- C. Lachaux, C. J. R. Frazao, F. Krauβer, N. Morin, T. Walther and J. M. François, Front. Bioeng. Biotechnol., 2019, 7, 359 CrossRef PubMed.
- S. Shawe, F. Buchanan, E. Harkin-Jones and D. Farrar, J. Mater. Sci., 2006, 41, 4832–4838 CrossRef CAS.
- R. S. Bezwada, D. D. Jamiolkowski, I. Y. Lee, V. Agarwal, J. Persivale, S. Trenka-Benthin, M. Erneta, J. Suryadevara, A. Yang and S. Liu, Biomaterials, 1995, 16, 1141–1148 CrossRef CAS PubMed.
- Q. Cai, J. Bei and S. Wang, Polymer, 2002, 43, 3585–3591 CrossRef CAS.
- S. H. Lee, B. S. Kim, S. H. Kim, S. W. Choi, S. I. Jeong, I. K. Kwon, S. W. Kang, J. Nikolovski, D. J. Mooney, Y. K. Han and Y. H. Kim, J. Biomed. Mater. Res., Part A, 2003, 66, 29–37 CrossRef PubMed.
- BASF SE, Ecoflex® (PBAT): The Original since 1998 – Certified Compostable Plastic, https://plastics-rubber.basf.com/global/en/performance_polymers/products/ecoflex.html, (accessed 24 February 2022).
- Q. Zhiguo, Y. Jilei, Q. Yi, Z. Langhui, S. Jie, G. Xueshan, Z. Chaoshan, S. Yuzeng, M. Yuebo, F. Buxue, L. Wei, W. Li, X. L. Xianfeng, W. Haiqiang, C. Liang, T. Shuai, C. Jinbo, H. Yongning and Y. Chuanzhong, CN105237750A, 2015.
- S. Unlu, W. Niu, Y. Yas, Y. Demirel, S. Unlu, W. Niu and Y. Demirel, Biofuels, Bioprod. Biorefin., 2020, 14, 794–807 CrossRef CAS.
- T. Beardslee and S. Picataggio, Lipid Technol., 2012, 24, 223–225 CrossRef CAS.
- E. Skoog, J. H. Shin, V. Saez-Jimenez, V. Mapelli and L. Olsson, Biotechnol. Adv., 2018, 36, 2248–2263 CrossRef CAS PubMed.
- Y. Deng, C. Yu, P. Wongwiwattana and N. L. Thomas, J. Polym. Environ., 2018, 26, 3802–3816 CrossRef CAS.
- U. Witt, R.-J. Müller and W.-D. Deckwer, Chem. Ing. Tech., 1995, 67, 904–907 CrossRef CAS.
- U. Witt, R. J. Müller and W.-D. Deckwer, J. Macromol. Sci., Part A: Pure Appl.Chem., 1995, 32, 851–856 CrossRef.
- T. Kijchavengkul, R. Auras, M. Rubino, S. Selke, M. Ngouajio and R. T. Fernandez, Polym. Degrad. Stab., 2010, 95, 2641–2647 CrossRef CAS.
- ASTM International, Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials Under Controlled Composting Conditions, Incorporating Thermophilic Temperatures, https://www.astm.org/d5338-15r21.html, (accessed 12 July 2022).
- U. Witt, T. Einig, M. Yamamoto, I. Kleeberg, W. D. Deckwer and R. J. Müller, Chemosphere, 2001, 44, 289–299 CrossRef CAS PubMed.
- P. A. Palsikowski, C. N. Kuchnier, I. F. Pinheiro and A. R. Morales, J. Polym. Environ., 2018, 26, 330–341 CrossRef CAS.
- Y. X. Weng, Y. J. Jin, Q. Y. Meng, L. Wang, M. Zhang and Y. Z. Wang, Polym. Test., 2013, 32, 918–926 CrossRef CAS.
- Y. Han, J. Shi, L. Mao, Z. Wang and L. Zhang, Ind. Eng. Chem. Res., 2020, 59, 21779–21790 CrossRef CAS.
- E. Grigat, R. Koch and R. Timmermann, Polym. Degrad. Stab., 1998, 59, 223–226 CrossRef CAS.
- S. Mecking, Angew. Chem., Int. Ed., 2004, 43, 1078–1085 CrossRef CAS PubMed.
- J. Lee, Y. Lee, S. Kim, E. E. Kwon and K. Y. A. Lin, Korean J. Chem. Eng., 2021, 38, 1079–1086 CrossRef CAS.
- M. Winnacker and B. Rieger, Polym. Chem., 2016, 7, 7039–7046 RSC.
- H. Sun, F. Meng, A. A. Dias, M. Hendriks, J. Feijen and Z. Zhong, Biomacromolecules, 2011, 12, 1937–1955 CrossRef CAS PubMed.
- C. C. Chu, J. Fiber Bioeng. Inform., 2012, 5, 1–31 CrossRef.
- L. J. del Valle, M. Roa, A. Díaz, M. T. Casas, J. Puiggalí and A. Rodríguez-Galán, J. Polym. Res., 2012, 19, 9792 CrossRef.
- M. B. Martínez, I. M. Pinilla, F. Z. Mata and J. A. G. Férez, Macromolecules, 1997, 30, 3197–3203 CrossRef.
- A. Rodriguez-Galan, L. Franco and J. Puiggali, Polymers, 2011, 3, 65–99 CrossRef CAS.
- L. Wen, S. Zhang, Y. Xiao, J. He, S. Zhu, J. Zhang, Z. Wu and M. Lang, Macromolecules, 2020, 53, 5096–5104 CrossRef CAS.
- K. Guo and C. C. Chu, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 1595–1606 CrossRef CAS.
- Y. P. Ge, D. Yuan, Z. L. Luo and B. B. Wang, eXPRESS Polym. Lett., 2014, 8, 50–54 CrossRef.
- K. E. Gonsalves, X. Chen and J. A. Cameron, Macromolecules, 1992, 25, 3309–3312 CrossRef CAS.
- X. Chen, K. E. Gonsalves and J. A. Cameron, J. Appl. Polym. Sci., 1993, 50, 1999–2006 CrossRef CAS.
- Q. Zhiyong, L. Sai, Z. Hailian and L. Xiaobo, Colloid Polym. Sci., 2003, 281, 869–875 CrossRef.
- Z. Qian, S. Li, Y. He, H. Zhang and X. Liu, Biomaterials, 2004, 25, 1975–1981 CrossRef CAS PubMed.
- H. Zhang, Y. He, S. Li and X. Liu, Polym. Degrad. Stab., 2005, 88, 309–316 CrossRef CAS.
- Y. He, Z. Qian, H. Zhang and X. Liu, Colloid Polym. Sci., 2004, 282, 972–978 CrossRef CAS.
- Y. Fan, M. Kobayashi and H. Kise, J. Polym. Sci., Part A: Polym. Chem., 2001, 39, 1318–1328 CrossRef CAS.
- Č. Novotný, P. Erbanová, H. Sezimová, K. Malachová, Z. Rybková, L. Malinová, I. Prokopová and J. Brožek, Int. Biodeterior. Biodegrad., 2015, 97, 25–30 CrossRef.
- R. A. Gross and B. Kalra, Science, 2002, 297, 803–807 CrossRef CAS PubMed.
- N. B. Halima, RSC Adv., 2016, 6, 39823–39832 RSC.
- Kuraray Europe GmbH, Polyvinyl alcohol, PVA, PVOH - Kuraray Poval™, https://www.kuraray-poval.com/, (accessed 21 June 2022).
- Sekisui Specialty Chemicals, Selvol™ Polyvinyl Alcohol Product Information, https://www.sekisui-sc.com/products/polyvinyl-alcohol/, (accessed 21 June 2022).
- P. Alexy, D. Káchová, M. Kršiak, D. Bakoš and B. Šimková, Polym. Degrad. Stab., 2002, 78, 413–421 CrossRef CAS.
- O. Olabisi and K. Adewale, Handbook of Thermoplastics, Taylor & Francis Group, LLC, 2nd edn, 2016 Search PubMed.
- M. Lim, H. Kwon, D. Kim, J. Seo, H. Han and S. B. Khan, Prog. Org. Coat., 2015, 85, 68–75 CrossRef CAS.
- E. Chiellini, A. Corti and R. Solaro, Polym. Degrad. Stab., 1999, 64, 305–312 CrossRef CAS.
- R. Solaro, A. Corti and E. Chiellini, Polym. Adv. Technol., 2000, 11, 873–878 CrossRef CAS.
- A. Corti, R. Solaro and E. Chiellini, Polym. Degrad. Stab., 2002, 75, 447–458 CrossRef CAS.
- E. Chiellini, A. Corti, G. Del Sarto and S. D'Antone, Polym. Degrad. Stab., 2006, 91, 3397–3406 CrossRef CAS.
- J. Hoffmann, I. Řezníčková, J. Kozáková, J. Růžička, P. Alexy, D. Bakoš and L. Precnerová, Polym. Degrad. Stab., 2003, 79, 511–519 CrossRef CAS.
- N. A. Azahari, N. Othman and H. Ismail, J. Phys. Sci., 2011, 22, 15–31 CAS.
- Z. Guohua, L. Ya, F. Cuilan, Z. Min, Z. Caiqiong and C. Zongdao, Polym. Degrad. Stab., 2006, 91, 703–711 CrossRef.
- H. C. Alexander, D. C. Dill, L. W. Smith, P. D. Guiney and P. Dorn, Environ. Toxicol., 1988, 7, 19–26 CrossRef CAS.
- Acrylite, Cyrolon® Polycarbonate, https://www.acrylite.co/products/our-brands/cyrolon/polycarbonate, (accessed 22 June 2022).
- Covestro, Makrolon® resists heat and attracts innovators, https://solutions.covestro.com/en/brands/makrolon, (accessed 22 June 2022).
- T. Artham and M. Doble, J. Polym. Environ., 2009, 17, 170–180 CrossRef CAS.
- T. Artham and M. Doble, Environ. Chem. Lett., 2012, 10, 29–34 CrossRef CAS.
- J. Sajiki and J. Yonekubo, Chemosphere, 2003, 51, 55–62 CrossRef CAS PubMed.
- M. D. Garrison, P. J. Storch, W. S. Eck, V. H. Adams, P. W. Fedick and B. G. Harvey, Green Chem., 2021, 23, 8016–8029 RSC.
- P. Wang, J. H. Park, M. Sayed, T.-S. Chang, A. Moran, S. Chen and S.-H. Pyo, Polym. Chem., 2018, 9, 3798–3807 RSC.
- Plastic Selection Group Inc., Plastic Selection Group Products, https://www.go2psg.com/clear-thermoplastic-resin-products, (accessed 4 March 2022).
- P. Wang, J. H. Park, M. Sayed, T. S. Chang, A. Moran, S. Chen and S. H. Pyo, Polym. Chem., 2018, 9, 3798–3807 RSC.
- V. S. Bonab and I. Manas-Zloczower, J. Polym. Sci., Part B: Polym. Phys., 2017, 55, 1553–1564 CrossRef.
- BASF SE, Think, create, Elastollan® (TPU), https://plastics-rubber.basf.com/emea/en/performance_polymers/products/elastollan.html?at_medium=sl&at_campaign=PM_BAW_EMEA_EN_Elastollan_TRA_CROSS&at_term=tpuplastic&at_creation=Search_Google_SERP_Elastollan-General-EMEA-EN&at_platform=google&at_variant, (accessed 23 June 2022).
- I. Singh, S. K. Samal, S. Mohanty and S. K. Nayak, Eur. J. Lipid Sci. Technol., 2020, 122, 1900225 CrossRef CAS.
- Y. Li, X. Luo and S. Hu, Bio-based Polyols and Polyurethanes, Springer International Publishing, Cham, 2015 Search PubMed.
- R. Mort, K. Vorst, G. Curtzwiler and S. Jiang, RSC Adv., 2021, 11, 4375–4394 RSC.
- L. Hojabri, X. Kong and S. S. Narine, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 3302–3310 CrossRef CAS.
- Isopa (European Diisocyanate & Polyol Producers Association), A guide to the REACH Restriction applying to diisocyanates, 2021 Search PubMed.
- Y. D. Kim and S. C. Kim, Polym. Degrad. Stab., 1998, 62, 343–352 CrossRef CAS.
- B. Quienne, N. Kasmi, R. Dieden, S. Caillol and Y. Habibi, Biomacromolecules, 2020, 21, 1943–1951 CrossRef CAS PubMed.
- M. Włoch and K. Błazek, ACS Symp. Ser., 2021, 1380, 107–166 CrossRef.
- L. Maisonneuve, O. Lamarzelle, E. Rix, E. Grau and H. Cramail, Chem. Rev., 2015, 115, 12407–12439 CrossRef CAS PubMed.
- V. Besse, R. Auvergne, S. Carlotti, G. Boutevin, B. Otazaghine, S. Caillol, J. P. Pascault and B. Boutevin, React. Funct. Polym., 2013, 73, 588–594 CrossRef CAS.
- A. F. Sousa, C. Vilela, A. C. Fonseca, M. Matos, C. S. R. Freire, G. J. M. Gruter, J. F. J. Coelho and A. J. D. Silvestre, Polym. Chem., 2015, 6, 5961–5983 RSC.
- A. F. Sousa and A. J. D. Silvestre, Curr. Opin. Green Sustain. Chem., 2022, 33, 100557 CrossRef CAS.
- M. Brodin, M. Vallejos, M. T. Opedal, M. C. Area and G. Chinga-Carrasco, J. Cleaner Prod., 2017, 162, 646–664 CrossRef CAS.
- I. Capellán-Pérez, I. Arto, J. M. Polanco-Martínez, M. González-Eguino and M. B. Neumann, Energy Environ. Sci., 2016, 9, 2482–2496 RSC.
- R. Datta and M. Henry, J. Chem. Technol. Biotechnol., 2006, 81, 1119–1129 CrossRef CAS.
- NatureWorks, Ingeo, https://www.natureworksllc.com/, (accessed 12 April 2022).
- Corbion, PURAC®, https://www.corbion.com/biochemicals/pharma/brands/purac, (accessed 12 April 2022).
- Futerro, Renew, https://www.futerro.com/, (accessed 12 April 2022).
- J. Ahmed, J.-X. Zhang, Z. Song and S. K. Varshney, J. Therm. Anal. Calorim., 2009, 95, 957–964 CrossRef CAS.
- M. Drieskens, R. Peeters, J. Mullens, D. Franco, P. J. Lemstra and D. G. Hristova-Bogaerds, J. Polym. Sci., Part B: Polym. Phys., 2009, 47, 2247–2258 CrossRef CAS.
- A. R. Bagheri, C. Laforsch, A. Greiner and S. Agarwal, Global Challenges, 2017, 1, 1700048 CrossRef PubMed.
- G. Kale, R. Auras, S. P. Singh and R. Narayan, Polym. Test., 2007, 26, 1049–1061 CrossRef CAS.
- H. Song and S. Y. Lee, Enzyme Microb. Technol., 2006, 39, 352–361 CrossRef CAS.
- C. Delhomme, D. Weuster-Botz and F. E. Kühn, Green Chem., 2009, 11, 13–26 RSC.
- Mitsubishi Chemical Corporation, BioPBS™, https://www.mcpp-global.com/en/mcpp-europe/products/brand/biopbsTM/, (accessed 12 April 2022).
- J. Xu and B. H. Guo, Biotechnol. J., 2010, 5, 1149–1163 CrossRef CAS PubMed.
- M. Deroiné, I. Pillin, G. Le Maguer, M. Chauvel and Y. Grohens, Polym. Test., 2019, 74, 163–169 CrossRef.
- H. Li, J. Chang, A. Cao and J. Wang, Macromol. Biosci., 2005, 5, 433–440 CrossRef CAS PubMed.
- J. H. Zhao, X. Q. Wang, J. Zeng, G. Yang, F. H. Shi and Q. Yan, J. Appl. Polym. Sci., 2005, 97, 2273–2278 CrossRef CAS.
- C. Tsutsumi, N. Hayase, K. Nakagawa, S. Tanaka and Y. Miyahara, Macromol. Symp., 2003, 197, 431–442 CrossRef CAS.
- C. Zhu, Z. Zhang, Q. Liu, Z. Wang and J. Jin, J. Appl. Polym. Sci., 2003, 90, 982–990 CrossRef CAS.
- J. H. Zhao, X. Q. Wang, J. Zeng, G. Yang, F. H. Shi and Q. Yan, Polym. Degrad. Stab., 2005, 90, 173–179 CrossRef CAS.
- K. Loos, R. Zhang, I. Pereira, B. Agostinho, H. Hu, D. Maniar, N. Sbirrazzuoli, A. J. D. Silvestre, N. Guigo and A. F. Sousa, Front. Chem., 2020, 8, 1–18 CrossRef PubMed.
- S. K. Burgess, R. M. Kriegel and W. J. Koros, Macromolecules, 2015, 48, 2184–2193 CrossRef CAS.
- S. K. Burgess, O. Karvan, J. R. Johnson, R. M. Kriegel and W. J. Koros, Polymer, 2014, 55, 4748–4756 CrossRef CAS.
- S. K. Burgess, R. M. Kriegel and W. J. Koros, Macromolecules, 2015, 48, 2184–2193 CrossRef CAS.
- X. Fei, X. Fei, X. Fei, J. Wang, J. Wang, J. Zhu, J. Zhu, X. Wang, X. Liu and X. Liu, ACS Sustainable Chem. Eng., 2020, 8, 8471–8485 CrossRef CAS.
- G. Gruter, Technology & Markets Day Path to the Future, https://www.avantium.com/wp-content/uploads/2019/06/20190606-Technology-Day_CTO_Gert-Jan_Gruter_breakout_final_.pdf Search PubMed, (accessed 29 November 2020).
- Avantium, Avantium and Carlsberg sign offtake agreement on PEF, https://www.avantium.com/press-releases/avantium-and-carlsberg-sign-offtake-agreement-on-pef/, (accessed 13 July 2022).
- R. J. I. Knoop, W. Vogelzang, J. Van Haveren and D. S. Van Es, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 4191–4199 CrossRef CAS.
- L. Papadopoulos, A. Zamboulis, N. Kasmi, M. Wahbi, C. Nannou, D. A. Lambropoulou, M. Kostoglou, G. Z. Papageorgiou and D. N. Bikiaris, Green Chem., 2021, 23, 2507–2524 RSC.
- J. G. Rosenboom, D. K. Hohl, P. Fleckenstein, G. Storti and M. Morbidelli, Nat. Commun., 2018, 9, 1–7 CrossRef CAS PubMed.
- J. C. Morales-Huerta, A. M. Ilarduya and S. Muñoz-Guerra, Polymer, 2016, 87, 148–158 CrossRef.
- X. L. Qu, M. Jiang, B. Wang, J. Deng, R. Wang, Q. Zhang, G.-Y. Zhou and J. Tang, ChemSusChem, 2019, 12, 4927–4935 CrossRef CAS PubMed.
- J. Wu, H. Xie, L. Wu, B. G. Li and P. Dubois, RSC Adv., 2016, 6, 101578–101586 RSC.
- V. Haas, J. Wenger, L. Ranacher, N. Guigo, A. Sousa and T. Stern, Sustain. Prod. Consum., 2022, 31, 370–383 CrossRef.
- E. de Jong, H. A. Visser, A. S. Dias, C. Harvey and G.-J. M. Gruter, Polymers, 2022, 14, 943 CrossRef CAS PubMed.
- M. Matos, A. F. Sousa, A. C. Fonseca, C. S. R. Freire, J. F. J. Coelho and A. J. D. Silvestre, Macromol. Chem. Phys., 2014, 215, 2175–2184 CrossRef CAS.
- H. Wu, B. Wen, H. Zhou, J. Zhou, Z. Yu, L. Cui, T. Huang and F. Cao, Polym. Degrad. Stab., 2015, 121, 100–104 CrossRef CAS.
- H. Hu, J. Li, S. Luo, Y. Tian, J. Wang, Y. L. Zhao, R. Zhang and J. Zhu, J. Hazard. Mater., 2022, 425, 127752 CrossRef CAS PubMed.
- A. K. Moeller, K. Molawi and M. Yamamot, US010189989B2, 2019, 2.
- U. Witt, R. J. Müller and W. D. Deckwer, J. Environ. Polym. Degrad., 1996, 4, 9–20 CrossRef CAS.
- S. Peng, L. Wu, B. G. Li and P. Dubois, Polym. Degrad. Stab., 2017, 146, 223–228 CrossRef CAS.
- N. Jacquel, R. Saint-Loup, J. P. Pascault, A. Rousseau and F. Fenouillot, Polymer, 2015, 59, 234–242 CrossRef CAS.
- H. Hu, R. Zhang, Z. Kong, K. Wang, W. B. Ying, J. Wang and J. Zhu, Adv. Ind. Eng. Polym. Res., 2019, 2, 167–177 Search PubMed.
- A. F. Sousa, J. F. J. Coelho and A. J. D. Silvestre, Polymer, 2016, 98, 129–135 CrossRef CAS.
- Z. Jia, J. Wang, L. Sun, J. Zhu and X. Liu, J. Appl. Polym. Sci., 2018, 135, 1–11 Search PubMed.
- M. J. Soares, P. K. Dannecker, C. Vilela, J. Bastos, M. A. R. Meier and A. F. Sousa, Eur. Polym. J., 2017, 90, 301–311 CrossRef CAS.
- L. Filiciotto and G. Rothenberg, ChemSusChem, 2021, 14, 56–72 CrossRef CAS PubMed.
- European Bioplastics e.V., EUBP proposes criteria and product examples for preferable use of compostable plastics, https://www.european-bioplastics.org/eubp-proposes-criteria-and-product-examples-for-preferable-use-of-compostable-plastics/, (accessed 18 April 2022).
- Science Advice for Policy by European Academies, Biodegradability of Plastics in the Open Environment, 2020 Search PubMed.
- Environmental Protection Agency, Household Waste Characterisation Campaign Final Report, 2018 Search PubMed.
- CIC - Italian Composting and Biogas Association, Annual Report on Biowaste Recycling November, Rome, 2017 Search PubMed.
- F. D. Innocenti and T. Breton, ACS Sustainable Chem. Eng., 2020, 8, 9239–9249 CrossRef.
- O. O. Ayeleru, S. Dlova, O. J. Akinribide, F. Ntuli, W. K. Kupolati, P. F. Marina, A. Blencowe and P. A. Olubambi, Waste Manage., 2020, 110, 24–42 CrossRef PubMed.
- H. A. Leslie, Review of Microplastics in Cosmetics – Scientific background on a potential source of plastic particulate marine litter to support decision-making, 2014, vol. 476 Search PubMed.
- C. Guerranti, T. Martellini, G. Perra, C. Scopetani and A. Cincinelli, Environ. Toxicol. Pharmacol., 2019, 68, 75–79 CrossRef CAS PubMed.
- L. Anagnosti, A. Varvaresou, P. Pavlou, E. Protopapa and V. Carayanni, Mar. Pollut. Bull., 2021, 162, 111883 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |