Mechanism of catalytic ozonation in different surface acid sites of oxide aqueous suspensions†
Abstract
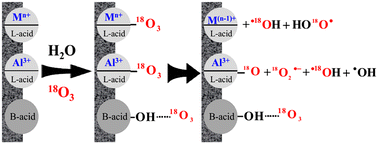
The behaviour of ozone on the surface of different oxides, including MCM-41, γ-Al2O3, α-Fe2O3, α-FeOOH, Fe3O4, α-MnO2 and β-MnO2, was investigated for the degradation of ibuprofen, acyclovir and humic acid in water. According to the temperature-programmed desorption of NH3, Fourier transform infrared (FTIR) spectroscopy of adsorbed pyridine and in situ attenuated total reflection FTIR (ATR-FTIR) spectroscopy measurements, Brønsted acid sites from the isolated hydroxyl and hydrogen-bonded hydroxyl groups were predominant on MCM-41 and α-Fe2O3, both Brønsted and Lewis acid sites existed on Fe3O4 and β-MnO2 and Lewis acid sites were predominant on γ-Al2O3, α-FeOOH and α-MnO2. In situ ATR-FTIR, in situ Raman and electron spin resonance spectroscopy using isotopic ozone revealed that ozone was electrostatically adsorbed on the Brønsted acid sites and hardly decomposed; thus, ozone served as a direct oxidant for pollutant degradation over MCM-41 and α-Fe2O3 suspensions. However, ozone was adsorbed on the surface Lewis acid sites, competing with water, and decomposed into surface atomic oxygen species and ˙OH and O2˙− radicals in the γ-Al2O3 suspension. Thus, more ˙OH and O2˙− radicals were generated on the surface of multivalent oxides (α-FeOOH, Fe3O4, α-MnO2 and β-MnO2) due to their redox activity, which was enhanced by the formation of complexes of pollutants and by-products with multivalent metals. On the basis of the experimental results, a mechanism for the transformation of ozone on the different surface acid sites is proposed.
- This article is part of the themed collections: Environmental Science: Nano Recent HOT Articles, Nanomaterial applications in water and International Day for the Preservation of the Ozone Layer


 Please wait while we load your content...
Please wait while we load your content...