A tertiary phosphine oxide ligand-based recyclable system for the Suzuki–Miyaura and Negishi reactions: evidence for pseudo-homogeneous catalysis†
Andrew K.
King‡
ab,
Aneelman
Brar‡
ab and
Michael
Findlater
 *ab
*ab
aDepartment of Chemistry & Biochemistry, Texas Tech University, Lubbock, TX 79409, USA
bDepartment of Chemistry & Chemical Biology, University of California, 5200 N. Lake Road, Merced, CA 95343, USA. E-mail: michaelfindlater@ucmerced.edu
First published on 2nd January 2023
Abstract
A recoverable catalyst system has been developed and its application in both Suzuki–Miyaura and Negishi coupling reactions has been demonstrated. We have also investigated the nature of the active catalyst in solution – a controversial topic as recent reports describe ‘metal-free’ coupling chemistry and whether heterogeneous reactions are actually homogeneous. We classify our catalytic system as ‘pseudo-homogeneous’ and employ Pd(OAc)2 and a commercially available cyclohexyldiphenyl phosphine oxide ligand. The system can be reused in up to 10 cycles while retaining good to excellent isolated yields.
Palladium-catalysed cross-coupling reactions between an aryl halide electrophile and an organoboron nucleophile are a clean and efficient strategy for the formation of biaryls; which facilitates a myriad of applications in the synthesis of pharmaceutical precursors, agrochemicals, and high-tech materials.1–4 The cross-coupling reaction using boronic acid/esters, known as the Suzuki–Miyaura5 reaction has been investigated extensively because of the broad availability and relative air and moisture stability of boronic acids and their derivatives.6 In parallel, the development of other cross-coupling methodology has resulted in a number of different strategies. For example, the Negishi coupling is a valuable alternative due to the fast transmetalation of organozinc reagents to palladium in comparison with boronic acids.7 Generally, the Suzuki–Miyaura coupling reaction is performed using palladium catalysts in the presence of ancillary ligands, most often N-heterocyclic carbenes or tertiary phosphines.8 Phosphine oxides have also seen widespread application as ligands for cross-coupling reactions arising from their pentavalent nature, which provides air and moisture stability.9–11 Denmark and co-workers employed triphenyl phosphine oxide as a ligand in the cross-coupling of arylsilanolates with aryl bromides; where they described phosphine oxide as a ‘buffering ligand’ that stabilises the highly active palladium nanoparticles formed in situ which govern catalysis.12 Later, Ackermann reported the first use of secondary phosphine oxides in the arylation of α C–H acidic compounds.13
The dominant role palladium plays in cross-coupling reactions presents a sustainability issue as palladium is a non-abundant metal. In developing more sustainable approaches to chemical synthesis, it would be desirable to either replace or, at least, recycle palladium catalysts over multiple catalytic runs.14,15 Different approaches to catalyst reuse have previously been reported, including work by Fan and co-workers where a palladium/phosphine dendrimer system was successfully re-used up to 9 times in Suzuki–Miyaura cross-coupling reactions.16 Impressively, only 0.2 mol% of Pd catalyst is required to reach full conversion, however reaction conditions are rather forcing – heating at reflux in dioxane. A report by Afewerki et al. described the use of a Pd heterogeneous catalyst derived from rice husk waste which could be reused in up to 6 cycles in Suzuki–Miyaura chemistry.17 Ye and co-workers reported the recyclability of a Pd-based electride material Y3Pd2; which was recycled 20 times. The catalyst recyclability is excellent but 40 mol% of catalyst was required!18 In any attempt to make a more cost-effective and sustainable catalytic system the importance of palladium recovery and reuse is integral to newly developed methods.19,20
Recent work by Ananikov and co-workers introduced the concept of “cocktail” catalysis.21–26 The Ananikov group demonstrated that rather than being homogenous or heterogenous the nature of the metal catalyst can be mixed, with a “cocktail” of metal species responsible for driving reactions. Hence a dynamic metal catalyst system can contain multiple species which are key to catalytic activity with nanoparticle and molecular catalysts both present. Leaching of heterogenous species can occur giving the same phenomena observed with pseudo-homogenous catalysis. These systems then comprise potentially reusable and recyclable catalysts.
Thus, we sought a simple approach to palladium recycling which employs inexpensive and readily available ligands under ambient conditions; we investigated the use of tertiary phosphine oxides as ligands in palladium-catalysed Suzuki–Miyaura and Negishi coupling reactions.
Suzuki–Miyaura coupling
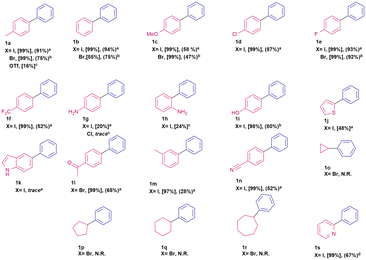
The coupling reaction between iodotoluene and phenylboronic acid (Scheme 1) was chosen as a model reaction with which to study the effectiveness of our catalytic system. Different commercially available phosphine oxide ligands and solvents were screened (Table S1, ESI†) and the use of cyclohexyl diphenylphosphine oxide as ligand and THF as solvent were found to provide optimal reaction conditions. Although the goal of our work was to investigate recyclable palladium catalysts, we considered it important to demonstrate that a robust Suzuki–Miyaura catalyst had been achieved. Thus, we explored a limited scope of substrates (Table 1).| a Pd(OAc)2 (0.015 mmol), ligand (0.03 mmol), aryl halide (0.3 mmol), phenylboronic acid (0.6 mmol), potassium tert-butoxide (0.6 mmol), THF (2 mL), reaction time = 24 hours. Conversion calculated by Gas Chromatography coupled with Mass Spectrometry (GC-MS), isolated yields []. b Temperature = 70 °C. c Solvent = toluene, temperature = 100 °C. d Solvent = EtOH:H2O. |
|---|
|
To demonstrate the further utility of this system we ran a series of coupling reactions between aryl halides or aryl triflates and phenyl boronic acid. As summarized in Table 1, our catalytic system demonstrated a respectable reactivity profile and good functional group tolerance under mild reaction conditions. Most functional groups investigated were well tolerated, and isolated biphenyls were obtained in good to excellent yields. An unsuccessful attempt was made to effect sp2–sp3 couplings with cyclic alkyl bromides. Additional experiments were carried out with 4-iodopyridine and 2-iodopyridine, spectroscopic conversion of the starting materials proved to be poor (26% and 13% respectively). We hypothesize that this is due to the coordination of the pyridine moiety to the Pd nanoparticles thus hindering catalytic turnover. When the solvent system is changed to a mixture of ethanol and water the reaction exhibits full conversion and 2-phenylpyridine (1s) was isolated in good yield.
Recycling studies
We investigated the recyclability of Pd(OAc)2 with cyclohexyl diphenylphosphine oxide for Suzuki–Miyaura coupling. As illustrated in Table 2, the catalyst system could be recycled through 10 consecutive coupling reactions without any loss of activity and good yields of the coupled product in each run were obtained (see ESI† for description of recycling method). Catalyst and product were readily separable in hexane, and the recovered (precipitated) catalyst was re-used directly in subsequent catalytic cycles. Separation was achieved via a simple filtration operation akin to a heterogeneous process.| Cyclea | Temperature (°C) | Yield (%) (spectroscopic (isolated)) | Selectivity (1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a′) 1a′) |
|---|---|---|---|
Conditions: recyclability studies w/ O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PCyPh2, Pd(OAc)2.a Pd(OAc)2 (0.015 mmol), ligand (0.03 mmol), iodotoluene (0.3 mmol), phenylboronic acid (0.6 mmol), potassium tert-butoxide (0.6 mmol), solvent = 2 mL, reaction time = 24 h. Spectroscopic yield calculated with tetramethylsilane as an internal standard. Ratio of heterocoupled product 4-methyl-1,1-biphenyl (1a) to homocoupled product 4,4-dimethyl-1,1-biphenyl (1a′). PCyPh2, Pd(OAc)2.a Pd(OAc)2 (0.015 mmol), ligand (0.03 mmol), iodotoluene (0.3 mmol), phenylboronic acid (0.6 mmol), potassium tert-butoxide (0.6 mmol), solvent = 2 mL, reaction time = 24 h. Spectroscopic yield calculated with tetramethylsilane as an internal standard. Ratio of heterocoupled product 4-methyl-1,1-biphenyl (1a) to homocoupled product 4,4-dimethyl-1,1-biphenyl (1a′). |
|||
| 1 | R.T. | >99 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | R.T. | >99 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | R.T. | >99 (96) | 105![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | R.T. | >99 (90) | 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | R.T. | >99 | >400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | R.T. | >99 | 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | R.T. | >99 | 280![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | R.T. | >99 | 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | R.T. | >99 | 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | R.T. | >99 | 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Negishi coupling
To further explore potential applications of our catalytic system, we decided to investigate the Negishi coupling. The coupling reaction between iodotoluene and tolylzinc iodide (Scheme 2) was performed and was found to proceed cleanly and in good to excellent yield.Substrate scope and recyclability were subsequently explored in a manner consistent with earlier Suzuki–Miyaura studies (see ESI,† Tables S2 and S3). Once more, the catalyst and product were readily separable via hexane addition to spent reaction mixture; with simple filtration allowing reclamation of the catalyst. The recovered catalyst was re-used in subsequent catalytic cycles after readdition of the reagents. The palladium catalyst could be re-used up to ten times without significant loss of yields (see ESI†). The scope of our Negishi coupling chemistry was explored using phenyl zinc bromide and a total of eight substrates and isolated yields of up to 85% were obtained (see ESI†).
Homogenous, heterogenous and pseudo-homogenous catalysis
To determine the nature of the active catalytic species present in solution, several test reactions were carried out. In testing catalyst homogeneity a mercury drop test is typically employed, however this can often lead to inconclusive results due to the fact that mercury only forms amalgams with certain metals.27 A Suzuki–Miyaura reaction between 4-iodotoluene and phenylboronic acid was conducted under standard conditions and allowed to stir for three hours, the reaction was then filtered and allowed to stir for a further 21 hours (24 hours total).28 From the precipitate, black palladium species were observable suggesting the formation of nanocomposites, surprisingly the reaction still proceeded to completion suggesting that even after filtration catalytically active Pd species remain present in solution (i.e. homogenous reactivity).16,29Recent reports suggest that many supposed heterogenous systems actually operate in a pseudo-homogenous regime in which the heterogeneous species acts as a “well” of active Pd species which can ‘break off’ and be catalytically active in solution.30,31 To probe for such behaviour, further tests were then performed in an effort to “trap” nanocomposite palladium structures. Thus, poly(vinylpyridine) was added to a standard Suzuki–Miyaura reaction (7.5 mol% polymer); the polymer acts to suppress heterogeneous catalysis by nanocomposites. The reaction mixture was allowed to stir at room temperature for 24 hours. The reaction proceeded to completion, further suggesting that the catalytically active Pd species are homogeneous in nature. Analogous tests were carried out for Negishi coupling reactions (see ESI†). Previous reports have demonstrated the use of heterogenous catalysts in palladium-catalysed Negishi couplings.32,33
To further characterise the palladium nanostructures formed during the reaction, transmission electron microscope (TEM) imaging was used to explore heterogeneous structural details. As evidenced by TEM imaging the recovered Pd catalyst is nanoparticulate in nature (Fig. 1). Given the evidence, we propose that the active palladium species responsible for Suzuki–Miyaura coupling are homogenous in nature but are sourced from a heterogeneous palladium nanocomposite; our catalyst is pseudo-homogenous.
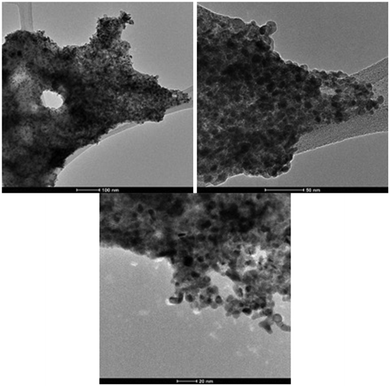 | ||
| Fig. 1 TEM images of Pd nanocomposites generated in catalytic Suzuki–Miyaura reactions, at 100, 50 and, 20 nm magnification. | ||
To further interrogate the nature of the catalytically relevant species, 31P NMR was carried out in both the solid-state (SSNMR) and in the solution state. Analysis of the catalyst both before and after precipitation from solution affords a signal in the 31P NMR at 35.9 ppm (see ESI†), consistent with the phosphine oxide being present in both solution and the solid-state.
In conclusion, we have developed a highly recyclable catalyst system from commercially available cyclohexyl diphenyl phosphine oxide and Pd(OAc)2 capable of effecting both Suzuki–Miyaura and Negishi couplings. The robust nature of this system is demonstrated via recycling experiments which deliver high yields in up to 10 catalytic cycles. Finally, investigation into the nature of the active catalyst species via TEM, poisoning experiments and both solution and solid-state NMR analysis strongly supports a pseudo-homogenous catalytic system.
Author contributions
AKK, AB and MF designed the experimental studies, which AKK and AB performed. AKK, AB and MF wrote the manuscript.Conflicts of interest
There are no conflict of interests to declare.Acknowledgements
The financial support of the Welch Foundation (Grant No. D-1807) and The University of California Merced is gratefully acknowledged.Notes and references
- F. Diederich and P. J. Stang, Metal-catalyzed cross-coupling reactions, John Wiley & Sons, 2008 Search PubMed.
- D. Shen, Y. Xu and S.-L. Shi, J. Am. Chem. Soc., 2019, 141, 14938–14945 CrossRef PubMed.
- N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef.
- E.-i. Negishi and A. De Meijere, Handbook of organopalladium chemistry for organic synthesis, John Wiley & Sons, 2003 Search PubMed.
- N. Miyaura, K. Yamada and A. Suzuki, Tetrahedron Lett., 1979, 20, 3437–3440 CrossRef.
- D. G. Hall, in Boronic Acids, 2005, pp. 1–99 Search PubMed.
- E. Negishi, A. O. King and N. Okukado, J. Org. Chem., 1977, 42, 1821–1823 CrossRef CAS.
- S. Kotha, K. Lahiri and D. Kashinath, Tetrahedron, 2002, 58, 9633–9695 CrossRef CAS.
- V. V. Grushin, Chem. Rev., 2004, 104, 1629–1662 CrossRef CAS.
- K. L. Billingsley and S. L. Buchwald, Angew. Chem., Int. Ed., 2008, 47, 4695–4698 CrossRef CAS PubMed.
- P. Das, U. Bora, A. Tairai and C. Sharma, Tetrahedron Lett., 2010, 51, 1479–1482 CrossRef CAS.
- S. E. Denmark, R. C. Smith and S. A. Tymonko, Tetrahedron, 2007, 63, 5730–5738 CrossRef CAS PubMed.
- L. Ackermann, R. Vicente and N. Hofmann, Org. Lett., 2009, 11, 4274–4276 CrossRef CAS PubMed.
- A. D. Genkin and T. L. Evstigneeva, Econ. Geol., 1986, 81, 1203–1212 CrossRef CAS.
- H. Renner, G. Schlamp, I. Kleinwächter, E. Drost, H. M. Lüschow, P. Tews, P. Panster, M. Diehl, J. Lang, T. Kreuzer, A. Knödler, K. A. Starz, K. Dermann, J. Rothaut, R. Drieselmann, C. Peter, R. Schiele, J. Coombes, M. Hosford and D. F. Lupton, in Ullmann's Encyclopedia of Industrial Chemistry, 2018, pp. 1–73 Search PubMed.
- L. Wu, B.-L. Li, Y.-Y. Huang, H.-F. Zhou, Y.-M. He and Q.-H. Fan, Org. Lett., 2006, 8, 3605–3608 CrossRef CAS PubMed.
- S. Afewerki, A. Franco, A. M. Balu, C.-W. Tai, R. Luque and A. Córdova, Sci. Rep., 2020, 10, 6407 CrossRef CAS PubMed.
- T.-N. Ye, Y. Lu, Z. Xiao, J. Li, T. Nakao, H. Abe, Y. Niwa, M. Kitano, T. Tada and H. Hosono, Nat. Commun., 2019, 10, 5653 CrossRef PubMed.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998 Search PubMed.
- Á. Molnár, Chem. Rev., 2011, 111, 2251–2320 CrossRef PubMed.
- V. P. Ananikov and I. P. Beletskaya, Organometallics, 2012, 31, 1595–1604 CrossRef CAS.
- A. S. Kashin and V. P. Ananikov, J. Org. Chem., 2013, 78, 11117–11125 CrossRef CAS PubMed.
- D. B. Eremin and V. P. Ananikov, Coord. Chem. Rev., 2017, 346, 2–19 CrossRef CAS.
- M. V. Polynski and V. P. Ananikov, ACS Catal., 2019, 9, 3991–4005 CrossRef CAS.
- D. O. Prima, N. S. Kulikovskaya, A. S. Galushko, R. M. Mironenko and V. P. Ananikov, Curr. Opin. Green Sustainable Chem., 2021, 31, 100502 CrossRef CAS.
- V. M. Chernyshev and V. P. Ananikov, ACS Catal., 2022, 12, 1180–1200 CrossRef CAS.
- O. N. Gorunova, I. M. Novitskiy, Y. K. Grishin, I. P. Gloriozov, V. A. Roznyatovsky, V. N. Khrustalev, K. A. Kochetkov and V. V. Dunina, Organometallics, 2018, 37, 2842–2858 CrossRef CAS.
- E. Bulatov, E. Lahtinen, L. Kivijärvi, E. Hey-Hawkins and M. Haukka, ChemCatChem, 2020, 12, 4831–4838 CrossRef CAS.
- L. Wu, Z.-W. Li, F. Zhang, Y.-M. He and Q.-H. Fan, Adv. Synth. Catal., 2008, 350, 846–862 CrossRef CAS.
- N. T. S. Phan, M. Van Der Sluys and C. W. Jones, Adv. Synth. Catal., 2006, 348, 609–679 CrossRef.
- B. Sun, L. Ning and H. C. Zeng, J. Am. Chem. Soc., 2020, 142, 13823–13832 CrossRef PubMed.
- W.-Y. Wu, T.-C. Lin, T. Takahashi, F.-Y. Tsai and C.-Y. Mou, ChemCatChem, 2013, 5, 1011–1019 CrossRef.
- L. R. Moore and D. A. Vicic, Chem. – Asian J., 2008, 3, 1046–1049 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: General procedures and characterisation details including NMR spectra. See DOI: https://doi.org/10.1039/d2cy01734b |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2023 |



