Intra-cluster energy transfer editing in a dual-emitting system to tap into lifetime thermometry†
Claudia Manuela Santos
Calado
,
Diogo Alves
Gálico
and
Muralee
Murugesu
 *
*
Department of Chemistry and Biomolecular Sciences, University of Ottawa, 10 Marie Curie, Ottawa, Ontario K1N 6N5, Canada. E-mail: m.murugesu@uottawa.ca
First published on 31st October 2023
Abstract
The impact of composition control and energy transfer on luminescence thermometry was investigated in a TbIII/EuIII dual-emitting molecular cluster-aggregate, known as {Ln20}. The study of lifetime dynamics sheds new light on how one can take advantage of rational planning to enhance thermometric performance and gaining insights into intriguing optical properties.
Over the past decade, luminescence thermometry has emerged as a valuable tool for advancing remote temperature sensing. With its remarkable spatial resolution (<10 μm), high relative thermal sensitivity (>1% K−1) and rapid acquisition times, its potential applications span from biomedicine to photonics and nanoelectronics.1 This technique relies on the correlation between temperature and the luminescence properties of a material, including the intensity and spectral position of emission bands, as well as the lifetime (τ) of emitting states. By analysing this thermally dependent signal, a thermometric parameter (Δ) is obtained, and its performance can be assessed using the thermal relative sensitivity (SR), as expressed in eqn (1).2
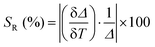 | (1) |
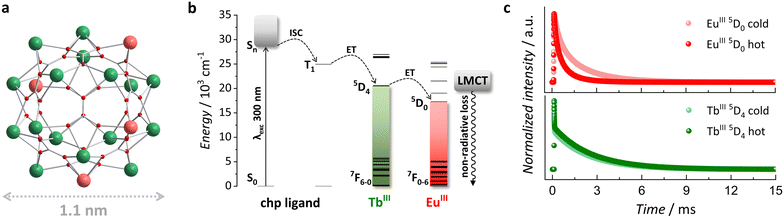
Lanthanide (LnIII)-doped materials are highly sought after in luminescence thermometry due to the distinctive optical features of 4f elements.3,4 The 4f–4f electronic transitions give rise to unique emission profiles with narrow bands, displaying characteristic energy positions and long lifetimes, making them reliable thermometric parameters.1 In addition, the control of luminescent processes through chemical composition and the use of established models to understand thermally induced spectral variations offer significant advantages for practical applications.5–7 Among the various platforms available for designing LnIII-based luminescent thermometers, molecular cluster-aggregates (MCAs) are particularly distinguished for their tuneable architectures and outstanding luminescent performances.8 Owing to their crystalline nature, MCAs possess a rigid metal core with high-nuclearity, precise coordination environment, and uniformity within a single unit. They also allow control over energy transfer (ET) processes and their influence on optical output via composition control. We have previously reported a highly tuneable icosanuclear MCA with the general formula [Ln20(chp)30(CO3)12(NO3)6(H2O)6]·H2O, {Ln20}, where chp = deprotonated 6-chloro-2-pyridinol (Fig. 1a and Fig. S1, ESI†). Through composition and ET modulation, we demonstrated fine-tuning of optical properties, including downshifting and upconversion luminescence, adjustment of colour output, and their impact on promising applications such as optical barcoding and luminescence intensity ratio thermometry.7,9,10
With these studies in mind, herein we report a series of {Ln20} MCAs, namely {Eu1Tb19} (1), {Eu2Tb18} (2), {Eu3Tb17} (3) and {Eu4Tb16} (4), and investigate these molecular materials as τ-based luminescent thermometers. By carefully varying the amount of EuIII and TbIII in the composition, we achieve precise control over intra-cluster ET processes. Our main focus is to understand how these processes impact the temperature-dependent luminescence behaviour. Specifically, we aim to provide control over the decay of EuIII and TbIII emitting states to enhance the potential of our MCAs as τ-based luminescent thermometers. Using τ as a thermometric parameter offers significant advantages for data acquisition and treatment, including insensitivity to fluctuations in excitation power, light scattering, and reflections.1,11 To improve the SR performance, we explore a ratiometric approach based on the ratio between EuIII 5D0 and TbIII 5D4τ (τEu and τTb, respectively). Our dual-emitting centre MCA, with two optically active ions in the metal core, helps develop a rational understanding of τ-based thermometry and thermal sensitivity in a molecular system. Our focus is on optimizing these features by considering variations in composition and data treatment.
We prepared MCAs 1, 2, 3 and 4 according to the previously reported procedure (Table S1, ESI†).9 Powder X-ray diffractograms (PXRD) confirm that the compounds are isostructural to the icosanuclear {Ln20}, which crystallizes in the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) space group (Fig. S2, ESI†). Fourier transform infrared (FTIR) spectra show the expected molecular vibrations (Fig. S3, ESI†), while energy-dispersive X-ray spectroscopy (EDS) data qualitatively confirm the presence of LnIII ions in each MCA (Fig. S4, ESI†). ICP-OES supports the nominal chemical composition, with a good agreement between theoretical and experimental LnIII ratio. Photoluminescent studies were carried out in acetonitrile (0.1 mg mL−1), as the stability of the {Ln20} MCA in this solvent has been demonstrated by NMR spectroscopy in previous works.12 Further experimental details are available in the ESI.†
space group (Fig. S2, ESI†). Fourier transform infrared (FTIR) spectra show the expected molecular vibrations (Fig. S3, ESI†), while energy-dispersive X-ray spectroscopy (EDS) data qualitatively confirm the presence of LnIII ions in each MCA (Fig. S4, ESI†). ICP-OES supports the nominal chemical composition, with a good agreement between theoretical and experimental LnIII ratio. Photoluminescent studies were carried out in acetonitrile (0.1 mg mL−1), as the stability of the {Ln20} MCA in this solvent has been demonstrated by NMR spectroscopy in previous works.12 Further experimental details are available in the ESI.†
To enhance the absorption coefficient of LnIII ions, molecular systems often utilize organic ligands as “antennas” to absorb photons. Subsequently, the ligands transfer the absorbed energy to the LnIII emitting centre, populating its 4f excited states. In the {Ln20} MCA, the chp ligand plays the role of a sensitizing antenna, with triplet state (T1) energy reported at 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 961 cm−1. From that level, the luminescent processes expected for the EuIII/TbIII pair are well-known (Fig. 1b).9 Due to the favourable position of TbIII 5D4 excited state (≈20
961 cm−1. From that level, the luminescent processes expected for the EuIII/TbIII pair are well-known (Fig. 1b).9 Due to the favourable position of TbIII 5D4 excited state (≈20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cm−1) relative to chp T1, an efficient T1 → TbIII ET takes place, resulting in emission bands within the visible range characteristic of TbIII. However, direct sensitization of EuIII 5D0 excited state (≈17
500 cm−1) relative to chp T1, an efficient T1 → TbIII ET takes place, resulting in emission bands within the visible range characteristic of TbIII. However, direct sensitization of EuIII 5D0 excited state (≈17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cm−1) by chp T1 is not efficient, leading to non-radiative deactivation through an Eu-based ligand-to-metal charge transfer (LMCT) state at 20
500 cm−1) by chp T1 is not efficient, leading to non-radiative deactivation through an Eu-based ligand-to-metal charge transfer (LMCT) state at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 492 cm−1.12 The characteristic EuIII emission bands are only observed due to an efficient TbIII → EuIII ET, which leads to the population of EuIII 5D0. Thus, TbIII acts as a bridging pathway that allows the electronic population to reach EuIII emitting state.
492 cm−1.12 The characteristic EuIII emission bands are only observed due to an efficient TbIII → EuIII ET, which leads to the population of EuIII 5D0. Thus, TbIII acts as a bridging pathway that allows the electronic population to reach EuIII emitting state.
By changing the TbIII/EuIII composition in our MCAs series, we can assess the influence of TbIII → EuIII ET and the efficiency of this process, along with side non-radiative deactivation pathways (such as the LMCT state), on the population of both TbIII 5D4 and EuIII 5D0 emitting states targeted for τ thermometry (Fig. 1c). The observed τ reflects the contribution of all radiative (emission of light) and non-radiative processes (vibration, energy transfer, charge-transfer state, etc.) leading to the deactivation of an emitting state. Therefore, accounting for these processes is critical for understanding the emission dynamics, τ behaviour with temperature, and the impact on thermometric performance.
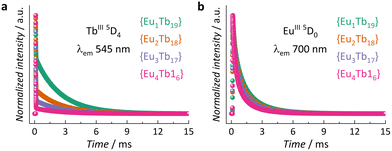
The excitation and emission spectra of all MCAs were collected at 20 °C. The presence of an intense broad band centred at 300 nm, assigned to the chp excited singlet state (S1), and the absence of noticeable 4f–4f transition bands indicate an efficient antenna effect (Fig. S5, ESI†). Under 300 nm excitation, MCAs exhibit characteristic sharp bands assigned to TbIII (5D4 → 7FJ, J = 6–0) and EuIII (5D0 → 7FJ, J = 0–4) electronic transitions (Fig. S6, ESI†). While 1 shows a higher intensity of TbIII emission bands, the rest of the series displays a higher intensity of EuIII components. This impacts the colour output of each MCA. MCA 1 displays a yellow-greenish output, while 4 exhibits an orange-reddish emission (Fig. S7, ESI†), which agrees with the change in TbIII/EuIII ion ratio in the composition. Emission decay curves of TbIII 5D4 and EuIII 5D0 emitting states collected at 20 °C (Fig. 2a and b) show a multiexponential decay profile due to the presence of four different crystallographic sites occupied by LnIII ions within the {Ln20} asymmetric unit. Average τ (〈τ〉) values were calculated from decay curves as expressed by eqn (2), where t0 is the time at which the curve hits its maximum intensity (I), and t1 is the time when it reaches the background.13
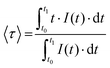 | (2) |
At a constant temperature (20 °C), the τ of both emitting centres shortens with an increase in EuIII content. In either case, the radiative process accounted by the observed τ is the emission of light. Regarding τTb, the TbIII → EuIII ET is the main non-radiative deactivation pathway present in all systems. Thus, a higher number of EuIII ions in the MCA leads to a higher probability of ET, resulting in a faster depopulation of 5D4. In the case of τEu, besides the non-radiative contribution of the LMCT state, the higher number of EuIII ions leads to concentration quenching and increases the likelihood of EuIII occupying different crystallographic sites, which impacts the average τ.
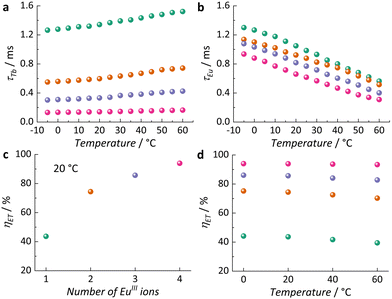
To better understand these photophysical properties and assess the MCAs' potential as τ-based luminescent thermometers, we collected variable temperature emission decay curves of TbIII 5D4 and EuIII 5D0, ranging from −5 °C to 60 °C (Fig. S8 and S9, ESI†). As illustrated in Fig. 3a and b, τTb increases with rising temperature, while τEu decreases under the same conditions. The trend for TbIII suggests that at higher temperatures excited vibrational levels coupled with the TbIII 5D4 have a higher probability of being occupied, as expected according to Boltzmann distribution, leading to a slower depopulation of this emitting state. Conversely, EuIII 5D0 becomes more easily deactivated at higher temperatures since it increases the probability of energy loss through non-radiative routes.
To gain a deeper understanding of these characteristics and assess the efficiency (ηET) of TbIII → EuIII ET in each system, structurally analogous MCAs were prepared by replacing the emitting EuIII ions with GdIII. As the first excited 4f level of GdIII (6P7/2, 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 cm−1) is too high in energy relative to both chp T1 and TbIII 5D4, it is an optically inactive ion in this system. Thus, we were able to compare the τ of the donor ion (TbIII) in the presence (τa) and the absence (τ0) of the acceptor ion (EuIII) (Fig. S10 ESI†), and calculate ηET using eqn (3).14
200 cm−1) is too high in energy relative to both chp T1 and TbIII 5D4, it is an optically inactive ion in this system. Thus, we were able to compare the τ of the donor ion (TbIII) in the presence (τa) and the absence (τ0) of the acceptor ion (EuIII) (Fig. S10 ESI†), and calculate ηET using eqn (3).14
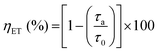 | (3) |
As depicted in Fig. 3c, at a constant temperature (20 °C), the increase of EuIII content in the MCA results in the enhancement of ηET, which corroborates TbIII → EuIII ET as the main pathway of non-radiative depopulation of TbIII 5D4. This leads to the population of EuIII 5D0 at the expense of TbIII 5D4. In general, the efficiency of ET processes is highly influenced by temperature, with significant effects typically observed at lower temperatures (<0 °C).15 In our study, as the temperature increases, the efficiency decreases (Fig. 3d and Table S2, ESI†). This effect is more pronounced in 1, where ηET decreases from 44.2% to 39.4%, and less evident in 4, where it changes from 94.0% to 93.4%. The varying behaviour is attributed to the more noticeable increase in τTb with temperature in 1 compared to 4.
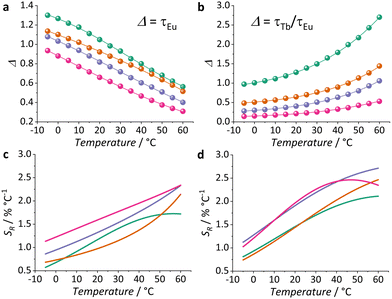
Based on these results, we explored the tuning of intra-cluster TbIII → EuIII ET of our series of MCAs to investigate their potential as τ-based thermometers. More specifically, we decided to take advantage of the two τ with different tendencies in our systems to investigate their suitability for ratiometric thermometry. We chose as Δ the ratio between τTb/τEu, and also τEu itself for comparison purposes, on the premise that the opposite trends of TbIII and EuIII, where τTb increases and τEu decreases according to temperature, would have a positive impact on sensitivity and favour a higher SR.
The temperature dependence of Δ is shown in Fig. 4a and b. A logistic function (eqn (S1), ESI†) was used for the mathematical fitting of Δ values. It is valid to point out that this function has no physical meaning and was used just for fitting purposes. To evaluate the thermometric performance, SR curves were obtained by applying the fitted function to eqn (1). Further details about thermometry data treatment, including thermal resolution (Fig. S11, ESI†), are available in the ESI.†
Using only τEu as a Δ, we observe an enhancement of SR according to the increase of EuIII ions in the MCA composition. While 1 shows a maximum SR (SRmax) value of 1.7% °C−1 at 60 °C, at this same temperature, 4 displays an SRmax of 2.3% °C−1. This behaviour is somehow expected once the LMCT state quenching depends on the number of EuIII present. Since this is the main non-radiative deactivation pathway of EuIII 5D0, it will govern the temperature dependence of τEu. On top of that, the increase of ηET seems to favor a higher SR. However, a beneficial impact on thermometry is observed only to a certain extent, as 3 and 4 exhibit the same trend and SRmax values.
When we consider the τTb/τEu ratiometric approach and compare it to τEu data (single approach), an interesting improvement of SRmax is observed. While 1 displays SRmax of 2.1% °C−1, 3 shows a value of 2.7% °C−1, both at 60 °C. This represents an enhancement higher than 22% for 1 and 15% for 3 in comparison to the single approach. Within the ratiometric approach and considering the trend observed for the other MCAs, 4 shows a slight reduction in performance, with SRmax of 2.5% °C−1 at 45 °C. In this case, in addition to the thermally-driven effect of LMCT on τEu, we also have to consider the behaviour of τTb itself. For all compositions, τTb exhibits a considerable increase according to temperature. The highest increase is observed for 3 (37%), while 4 (22%) has the lowest. Therefore, the degree of τTb temperature susceptibility greatly impacts the ratiometric approach outcome. While τTb/τEu improves the thermometric performance, one must consider how much advantage can be practically derived from each specific system being explored.
The values of SRmax obtained in this study are comparable to those observed for other τ-based thermometers (Table S4, ESI†), ranging from nanoparticles to molecular compounds. Notably, our MCAs exhibit significantly better performance than other reported molecular systems. To the best of our knowledge, this is the first time τ has been employed as a thermometric parameter for a LnIII-based MCA. This result highlights the unique advantages of MCAs as they strike a balance between nanoparticles and molecular systems, combining high nuclearity in a nanometric size with precise control over composition and coordination environment. We anticipate that the insights gained from this study will pave the way for the development of other dual-centre systems with potential for τ-based thermometry.
This work was supported by the Canadian Foundation for Innovation, and the Natural Sciences and Engineering Research Council of Canada.
Conflicts of interest
There are no conflicts to declare.Notes and references
- C. D. S. Brites, S. Balabhadra and L. D. Carlos, Adv. Opt. Mater., 2019, 7, 1801239 CrossRef.
- J. Zhou, B. Rosal, D. Jaque, S. Uchiyama, D. Jin, B. del Rosal, D. Jaque, S. Uchiyama and D. Jin, Nat. Methods, 2020, 17, 967–980 CrossRef CAS PubMed.
- A. Skripka, A. Benayas, R. Marin, P. Canton, E. Hemmer and F. Vetrone, Nanoscale, 2017, 9, 3079–3085 RSC.
- D. M. Lyubov, A. N. Carneiro Neto, A. Fayoumi, K. A. Lyssenko, V. M. Korshunov, I. V. Taydakov, F. Salles, Y. Guari, J. Larionova, L. D. Carlos, J. Long and A. A. Trifonov, J. Mater. Chem. C, 2022, 10, 7176–7188 RSC.
- A. Ćirić and M. D. Dramićanin, J. Lumin., 2022, 252, 119413 CrossRef.
- L. Li, X. Tang, Z. Wu, Y. Zheng, S. Jiang, X. Tang, G. Xiang and X. Zhou, J. Alloys Compd., 2019, 780, 266–275 CrossRef CAS.
- D. A. Gálico and M. Murugesu, Angew. Chem., Int. Ed., 2022, 61, e202204839 CrossRef PubMed.
- D. A. Gálico, C. M. S. Calado and M. Murugesu, Chem. Sci., 2023, 14, 5827–5841 RSC.
- D. A. Gálico, A. A. Kitos, J. S. Ovens, F. A. Sigoli and M. Murugesu, Angew. Chem., Int. Ed., 2021, 60, 6130–6136 CrossRef PubMed.
- D. A. Gálico, R. Ramdani and M. Murugesu, Nanoscale, 2022, 14, 9675–9680 RSC.
- K. Elzbieciak-Piecka, J. Drabik, D. Jaque and L. Marciniak, Phys. Chem. Chem. Phys., 2020, 22, 25949–25962 RSC.
- D. A. Gálico and M. Murugesu, ACS Appl. Mater. Interfaces, 2021, 13, 47052–47060 CrossRef PubMed.
- W. M. Yen, S. Shionoya and H. Yamamoto, Phosphor handbook, CRC Press, 2nd edn, 2007, pp. 844–885 Search PubMed.
- C. Piguet, J. C. G. Bünzli, G. Bernardinelli, G. Hopfgartner, A. F. Williams, J. C. G. Bünzli, G. Bernardinelli and G. Hopfgartner, J. Am. Chem. Soc., 1993, 115, 8197–8206 CrossRef CAS.
- D. Zhao, D. Yue, L. Zhang, K. Jiang and G. Qian, Inorg. Chem., 2018, 57, 12596–12602 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc03658h |
| This journal is © The Royal Society of Chemistry 2023 |