Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A practical synthesis of 1,3-disubstituted cubane derivatives†
Nahin
Kazi
 ab,
Marine C.
Aublette
ab,
Marine C.
Aublette
 ab,
Sarah L.
Allinson
ab,
Sarah L.
Allinson
 b and
Susannah C.
Coote
b and
Susannah C.
Coote
 *a
*a
aDepartment of Chemistry, Lancaster University, Bailrigg, LA1 4YB, UK. E-mail: s.coote@lancaster.ac.uk
bDivision of Biomedical and Life Sciences, Faculty of Health and Medicine, Lancaster University, Lancaster, UK
First published on 31st May 2023
Abstract
A robust multigram-scale synthesis of 1,3-disubstituted cubanes (previously only available on milligram-scale) is reported. The approach exploits a readily available enone intermediate previously used for the synthesis of 1,4-disubstituted cubanes, by introducing a novel Wharton transposition to access useful quantities of 1,3-disubstituted cubanes for diverse applications.
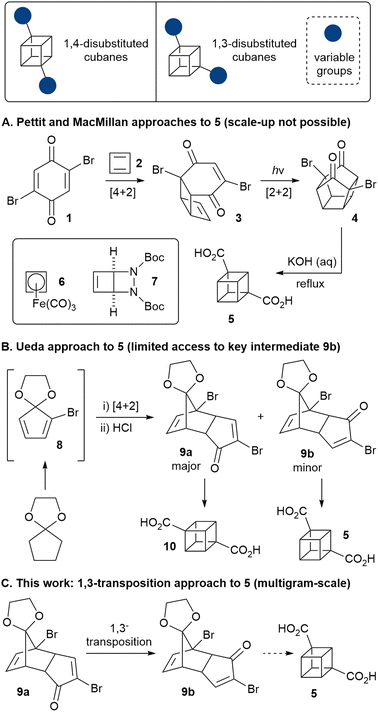
Ever since Eaton and Cole's landmark synthesis of the cubane system,1 chemists have been fascinated by this highly strained, non-natural cage motif.2 More recently, attention has turned to developing applications of cubanes, particularly within medicinal chemistry, where it has been suggested that cubanes may act as bioisosteres for the benzene ring.3–5 However, the vast majority of work on the applications of cubanes has been focused on 1,4-disubstituted cubanes (Fig. 1), as only these derivatives are available on multigram-scale. Other substitution patterns (such as 1,3-disubstituted cubanes), are accessible only in milligram quantities, and/or their synthesis requires long synthetic sequences often starting from the corresponding 1,4-disubstituted derivatives. The lack of access to multigram quantities of cubanes that bear different substitution patterns continues to preclude a full evaluation of the potential of cubanes in medicinal chemistry (as well as other areas), thus new synthetic approaches are required to bridge this gap.
 | ||
| Fig. 1 Disubstituted cubanes; previous synthetic approaches to 1,3-disubstituted cubanes (A/B); this work (C). | ||
Only two distinct direct routes to 1,3-disubstituted cubanes have been reported. The 3-step Pettit route (Scheme 1; A) features cyclobutadiene (2) as a key reagent, which undergoes Diels–Alder reaction with dibromoquinone 1.6 This is a particularly elegant and extremely concise route, but is not scalable, as it relies on the cyclobutadieneiron(tricarbonyl) complex (6), which is not commercial, is highly toxic and must be synthesised from expensive precursors.7
The Pettit approach was very recently revisited by MacMillan and co-workers,4 exploiting bicyclic diazetidine 78 as a new cyclobutadiene precursor. However, cyclobutadiene is very reactive and cycloadduct 3 is air- and moisture-sensitive, thus the route is feasible only on small scales (the sequence was demonstrated on 1 mmol scale), limiting its practicality. On the other hand, Ueda and co-workers showed that enone 9b could be converted to cubane 5 (Scheme 1; B),9 but this route is not viable for scale-up, due to the difficulty in accessing the key enone 9b in appreciable quantities – it was obtained in only 4.5% yield (after exhaustive chromatography) as a side product of the synthesis of the regioisomeric enone 9a (a precursor to 1,4-cubane dicarboxylate 10, and available on kilogram scale).
Herein, we demonstrate a robust approach to 5 that allows the straightforward multigram-scale synthesis of 1,3-cubane diester 11 as well as various novel 1,3-disubstituted cubane derivatives. A key component of our approach is the 1,3-transposition of enone 9a into enone 9b, thus enabling the preparation of both 1,4- and 1,3-disubstituted cubanes from a common, easily accessible intermediate.
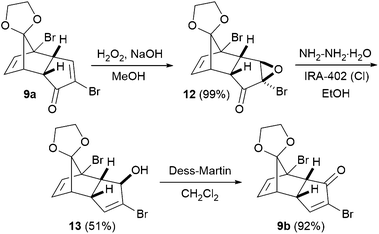
To start with, enone 9a was prepared from cyclopentanone according to Tsanaktsidis's pilot scale synthesis10 using standard laboratory glassware, followed by selective mono-deketalisation.11 The synthesis was routinely carried out on scales of several hundred grams (see the ESI† for details). 9a has previously been used in the synthesis of cubane 1,4-dicarboxylic acid,12 and to access the corresponding 1,3-disubstituted cubanes, we envisaged converting 9a into its isomeric enone 9bvia a Wharton transposition sequence.13 Thus, nucleophilic epoxidation of enone 9a gave epoxide 12 as a single diastereoisomer in essentially quantitative yield, with no purification required (Scheme 1). The subsequent Wharton reaction required some optimisation, since in addition to 13, the corresponding debrominated allylic alcohol was produced in varying amounts depending on the reaction conditions. Limiting the number of equivalents of hydrazine employed largely suppressed the debromination, but slowed the reaction. Thus, the optimized conditions involved gentle heating of a solution of 12 and hydrazine in ethanol in the presence of an basic resin for 72 hours, furnishing allylic alcohol 13 in 51% yield. Subsequent oxidation using Dess-Martin periodinane gave enone 9b in 92% yield, with the 3-step Wharton transposition sequence being carried out successfully on decagram scale.
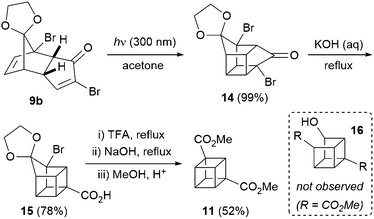
Next, enone 9b underwent facile [2+2] photocycloaddition upon irradiation at 300 nm, in acetone as both solvent and triplet sensitiser (Scheme 2). The reaction was performed on decagram scale and cycloadduct 14 was obtained in essentially quantitative yield after simple evaporation of the solvent. As previously noted by Ueda,9 attempts to effect a double Favorskii-type ring contraction (after deketalisation of 14) failed to yield diacid 5, hence two separate ring contraction reactions were carried out. Acid 15 was obtained in 78% yield upon heating in aqueous sodium hydroxide, and deprotection of the ketal in 15 was best performed by heating in trifluoroacetic acid. Attempts to perform this deprotection in sulfuric acid (as is common in the 1,4-cubane series10 and as reported by Ueda on small scales for the 1,3-cubane9) resulted in significant decomposition and an arduous aqueous extraction on multigram scales; pleasingly, both issues could be avoided simply by employing trifluoroacetic acid. The crude product was taken on directly into the second Favorskii-type ring contraction, which took place under more forcing conditions, and acid-mediated esterification of the resulting diacid in methanol gave 1,3-cubane diester 11 in 52% yield over the three steps – a significant improvement on the 25% yield obtained previously.9 Interestingly, Ueda and co-workers obtained a ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 11 and open-cage derivative 16 (which presumably derives from initial Haller–Bauer reaction14 instead of closure of the cubane), thus decreasing the overall yield of cubane 11. This undesired reactivity was also observed under our conditions through monitoring the ring contraction reaction by 1H NMR spectroscopy (with solvent suppression), but to our surprise, the cubane core was regenerated under the acidic esterification conditions employed, and no trace of 16 was detected. Thus, the choice of esterification conditions is crucial in optimising the yield of 11, with the milder esterification conditions chosen by the Ueda group (diazomethane) leading to significant amounts of the undesired open-cage product 16.
1 mixture of 11 and open-cage derivative 16 (which presumably derives from initial Haller–Bauer reaction14 instead of closure of the cubane), thus decreasing the overall yield of cubane 11. This undesired reactivity was also observed under our conditions through monitoring the ring contraction reaction by 1H NMR spectroscopy (with solvent suppression), but to our surprise, the cubane core was regenerated under the acidic esterification conditions employed, and no trace of 16 was detected. Thus, the choice of esterification conditions is crucial in optimising the yield of 11, with the milder esterification conditions chosen by the Ueda group (diazomethane) leading to significant amounts of the undesired open-cage product 16.
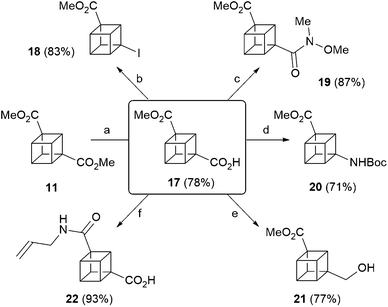
Finally, 1,3-cubane diester 11 underwent selective monosaponification to give carboxylic acid 17 (Scheme 3), which is a versatile intermediate en route to diverse 1,3-disubstituted cubanes. Thus, in addition to the cross-coupling methodology recently reported by the MacMillan group,417 also undergoes standard functional group interconversions, and five novel functionalised 1,3-disubstituted cubanes were prepared in short order, through iodination of the carboxylic acid moiety to give 18, formation of the corresponding Weinreb amide 19, Curtius rearrangement to give 20, and selective borane-mediated reduction to give alcohol 21. In addition, aminolysis of the ester moiety in 17 through heating in allylamine gave amide 22 in excellent yield (Scheme 3).
In summary, we have developed a multigram-scale synthesis of dimethyl 1,3-cubane dicarboxylate (11), which was previously available only on milligram scale, and demonstrated that 11 can be readily converted into a variety of different 1,3-disubstituted cubane derivatives via acid-ester intermediate 17. Key to the success of the approach was the development of a Wharton 1,3-transposition sequence of readily available enone 9a into enone 9b, such that both 1,4- and 1,3-disubstituted cubanes can now be easily prepared from a single intermediate. This work will expedite investigations into the applications of 1,3-disubstituted cubanes, which have so far been frustrated by the paucity of accessible 1,3-disubstituted cubanes. Now that appreciable quantities are available, applications are envisaged in particular within medicinal chemistry (e.g. as bioisosteres for meta-substituted benzene rings), but diverse applications can easily be envisaged in a broad range of other areas (for example, in materials chemistry, supramolecular chemistry and biochemistry).
This work was supported by funding from North West Cancer Research (LFSL2018-23, Studentship to MCA).
Conflicts of interest
There are no conflicts to declare.References
- P. E. Eaton and T. W. Cole, J. Am. Chem. Soc., 1964, 88, 962–964 CrossRef.
- K. F. Biegasiewicz, J. R. Griffiths, G. P. Savage, J. Tsanaktsidis and R. Priefer, Chem. Rev., 2015, 115, 6719–6745 CrossRef CAS PubMed.
- B. A. Chalmers, H. Xing, S. Houston, C. Clark, S. Ghassabian, A. Kuo, B. Cao, A. Reitsma, C.-E. P. Murray, J. E. Stok, G. M. Boyle, C. J. Pierce, S. W. Littler, D. A. Winkler, P. V. Bernhardt, C. Pasay, J. J. De Voss, J. McCarthy, P. G. Parsons, G. H. Walter, M. T. Smith, H. M. Cooper, S. K. Nilsson, J. Tsanaktsidis, G. P. Savage and C. M. Williams, Angew. Chem., Int. Ed., 2016, 55, 3580–3585 Search PubMed.
- M. P. Wiesenfeldt, J. A. Rossi-Ashton, I. B. Perry, J. Diesel, O. L. Garry, F. Bartels, S. C. Coote, X. Ma, C. S. Yeung, D. J. Bennett and D. W. C. MacMillan, Nature, 2023 DOI:10.1038/s41586-023-06021-8.
- For reviews of cubanes and other hydrocarbon structures as bioisosteres: (a) M. A. M. Subbaiah and N. A. Meanwell, J. Med. Chem., 2021, 64, 14046–14128 CrossRef CAS PubMed; (b) E. G. Tse, S. D. Houston, C. M. Williams, G. P. Savage, L. M. Rendina, I. Hallyburton, M. Anderson, R. Sharma, R. S. Obach and M. H. Todd, J. Med. Chem., 2020, 63, 11585–11601 CrossRef CAS PubMed; (c) T. A. Reekie, C. M. Williams, L. M. Rendina and M. Kassiou, J. Med. Chem., 2019, 62, 1078–1095 CrossRef CAS PubMed; (d) S. D. Houston, T. Fahrenhorst-Jones, H. Xing, B. A. Chalmers, M. L. Sykes, J. E. Stok, C. F. Soton, J. M. Burns, P. V. Bernhardt, J. J. De Voss, G. M. Boyle, M. T. Smith, J. Tsanaktsidis, G. P. Savage, V. M. Avery and C. M. Williams, Org. Biomol. Chem., 2019, 17, 6790–6798 RSC; (e) K. J. Flanagan, S. S. R. Bernhard, S. Plunkett and M. O. Senge, Chem. – Eur. J., 2019, 25, 6941–6954 CrossRef CAS PubMed; (f) P. K. Mykhailiuk, Org. Biomol. Chem., 2019, 17, 2839–2849 RSC; (g) J. Wlochal, R. D. M. Davies and J. Burton, Org. Lett., 2014, 16, 4094–4097 CrossRef CAS PubMed.
- J. C. Barborak, L. Watts and R. Pettit, J. Am. Chem. Soc., 1966, 88, 1328–1329 CrossRef CAS.
- (a) R. Pettit and J. Henery, Org. Synth., 1970, 50, 21 CrossRef CAS; (b) M. Rosenblum and C. Gatsonis, J. Am. Chem. Soc., 1967, 89, 5074–5075 CrossRef CAS.
- T. K. Britten, P. D. Kemmitt, N. R. Halcovitch and S. C. Coote, Org. Lett., 2019, 22, 9232–9235 CrossRef PubMed.
- T. Nigo, T. Hasegawa, Y. Kuwatani and I. Ueda, Bull. Chem. Soc. Jpn., 1993, 66, 2068–2072 CrossRef CAS.
- M. J. Falkiner, S. W. Littler, K. J. McRae, G. P. Savage and J. Tsanaktsidis, Org. Process Res. Dev., 2013, 17, 1503–1509 CrossRef CAS.
- N. B. Chapman, J. M. Key and K. J. Toyne, J. Org. Chem., 1970, 35, 3860–3867 CrossRef CAS.
- S. Lal, A. Bhattacharjee, A. Chowdhury, N. Kumbhakarna and I. N. N. Namboothiri, Chem. – Asian J., 2022, 17, e202200489 CrossRef CAS PubMed.
- (a) J. J. Li, in Name Reactions for Functional Group Transformations, ed. J. J. Li and E. J. Corey, John Wiley & Sons, Inc., 2007, ch. 2, pp 152–158 CrossRef; (b) P. S. Wharton and D. H. Bohlen, J. Org. Chem., 1961, 26, 3615–3616 CrossRef CAS.
- (a) M. Goverdhan and R. V. Venkateswaran, Tetrahedron, 2000, 56, 1399–1422 CrossRef; (b) J. P. Gilday and L. A. Paquette, Org. Prep. Proced. Int., 1990, 22, 167–201 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc02164e |
| This journal is © The Royal Society of Chemistry 2023 |