Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Lead-free perovskite-inspired semiconductors for indoor light-harvesting – the present and the future
G. Krishnamurthy
Grandhi
a,
Lethy
Krishnan Jagadamma
 b,
Vipinraj
Sugathan
a,
Basheer
Al-Anesi
b,
Vipinraj
Sugathan
a,
Basheer
Al-Anesi
 a,
Debjit
Manna
a and
Paola
Vivo
a,
Debjit
Manna
a and
Paola
Vivo
 *a
*a
aHybrid Solar Cells, Faculty of Engineering and Natural Sciences, Tampere University, P.O. Box 541, FI-33014, Tampere, Finland. E-mail: paola.vivo@tuni.fi
bEnergy Harvesting Research Group, SUPA, School of Physics and Astronomy, University of St Andrews, North Haugh, St Andrews, KY16 9SS, UK
First published on 3rd July 2023
Abstract
Are lead-free perovskite-inspired materials (PIMs) the wise choice for efficient yet sustainable indoor light harvesting? This feature article outlines how wide-bandgap PIMs can provide a positive answer to this compelling question. The wide band gaps can hinder sunlight absorption, in turn limiting the solar cell performance. However, PIMs based on group VA of the periodic table can theoretically lead to an outstanding indoor power conversion efficiency up to 60% when their band gap is ∼2 eV. Yet, the research on PIM-based indoor photovoltaics (IPVs) is still in an early stage with highest indoor device efficiencies up to 10%. This article reviews the recent advancements on PIMs for IPVs and identifies the main limiting factors of device performance, thus suggesting effective strategies to address them. We emphasize the poor operational stability of the IPV devices of PIMs being the key bottleneck for the vast adoption of this technology. We believe that this report can provide a solid scaffolding for further researching this fascinating class of materials, ultimately supporting our vision that, upon extensive advancement of the stability and efficiency, PIMs with wide bandgap will become a contender for the next-generation absorbers for sustainable indoor light harvesting.
The urge to self-power the wireless Internet of Things (IoT) ecosystem in a cost-effective and sustainable way has recently led to a growing demand for indoor photovoltaics (IPVs).1 Among the various emerging IPV technologies, e.g., hydrogenated amorphous silicon (a-Si:H), dye-sensitized solar cells (DSSCs) and organic photovoltaics (OPVs), lead halide perovskites (LHPs) currently hold the record of the highest indoor power conversion efficiency (PCE(i)) exceeding 40% at 1000 lux illuminance.2 This has been ensured by the defect-tolerance nature of LHPs and their tunable absorption spectra to match those of the typical indoor light spectra. Peng et al. calculated the photovoltaic efficiency limits under 1000 lux white light-emitting diode (WLED) illumination as a function of the band gap (Fig. 1a) using the indoor spectroscopically limited maximum efficiency (i-SLME) method.3 According to this study, a maximum theoretical PCE(i) of ≈60% can be achieved for absorbers with band gaps around 1.9 eV. IPV devices employing LHPs demonstrate very high open-circuit voltage (VOC) values >1 V,4,5 which minimizes the number of cells to be connected in series to obtain the desired power for IoT applications. Nevertheless, the potential lead (Pb) release from LHP-based IPVs embedded within IoT devices represents a substantial toxicity risk and causes concerns for their recycling.6,7 Therefore, it is important to develop IPV materials that do not pose any threat to the environment or health during and after the lifecycle of the photovoltaic cells. Low-toxicity tin (Sn)-based perovskites led to PCE(i)s approaching 15%.8,9 However, Sn-based perovskites possess a few drawbacks, including the air-oxidation of Sn2+ to Sn4+.10,11 Efforts are continuously made to suppress the Sn2+ oxidation. Yang et al. demonstrated a PCE(i) of 12.81% with a VOC of 0.65 V by incorporating catechin with a hydroxyl functional group (as a doping agent) into Sn-based IPV devices to prevent Sn2+ oxidation and improve film morphology.12 The highest performing (PCE(i) of 17.57% under 1062 lux) Sn perovskite device was achieved via interlayer passivation with KSCN-based materials, where K+ and SCN− ions passivated both bulk and interface defects.13 The IPV device retained 80% of the original PCE(i) after 1200 h of storage in darkness in an inert gas atmosphere. But the stability of Sn-perovskites in air is still not sufficient for any practical application.12 Furthermore, their typical band gap values (∼1.3 V) are much lower than those desired for IPVs (∼2 eV).14 Perovskite-inspired materials (PIMs) comprising air-stable Group VA cations, such as antimony (III) (Sb3+) and bismuth (III) (Bi3+), have recently emerged as promising wide band gap absorbers with low toxicity. The Bi- and Sb-halides and chalcohalides display similar electronic structures as LHPs.15 Both Sb3+ and Bi3+ possess the valence electronic configuration of 6s2, like Pb2+. In addition to low toxicity,16 a high dielectric constant of these ions due to their highly polarizable nature may suppress the defect formation probability in the band gap regime.15,17
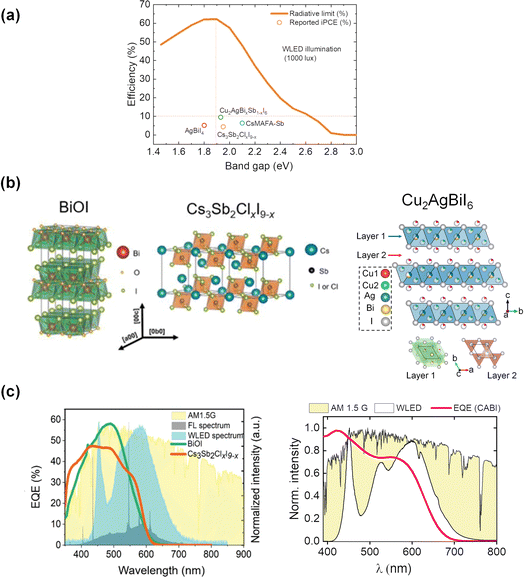 | ||
| Fig. 1 (a) Radiative-limit (RL) curve (taken from3) under indoor lighting conditions as a function of the band gap. The PCE(i) values of PIMs under WLED illumination at 1000 lux are also represented in the same figure. (b) Crystal structures of BiOI and Cs3Sb2(ClxI9−x), adapted from,3 and CABI (reproduced from24). (c) Comparison between the EQE of BiOI and Cs3Sb2ClxI9−x devices with the WLED, FL, and AM 1.5G spectra (left), reproduced from3 and the normalized EQE spectrum of a CABI device along with the AM 1.5G and WLED emission (color temperature: 4000 K) spectra (right). | ||
These materials typically crystallize in two-dimensional or layered structures (see the crystal structures of BiOI, Cs3Sb2ClxI9−x, and Cu2AgBiI6 (CABI) in Fig. 1b) and own band gaps in the 1.9–2.0 eV range.3,18 Their adoption as absorbers in single-junction solar cells led to modest efficiencies up to 4%19 mostly due to their wide band gaps, non-ideal for outdoor sunlight absorption. However, these large band gaps could be beneficial for applications in tandem solar cells and IPVs. This research is in its early infancy with only a handful of examples of PIM-based IPVs (Table 1) and with still no PIM-based tandem cells reported.18
| Active layer | Bandgap/type | Device structure | Light source | PCE(i) (%) | FF | J sc (μA cm−2) | V OC (V) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cs3Sb2[ClxI9−x] | 1.95 eV/near-direct | FTO/TiO2/m-TiO2(Cs3Sb2ClxI9−x)/Poly-TPD/Au | WLED | 4.4 | 0.40 | 76 | 0.47 | 3 |
| FL | 4.9 | 0.42 | 82 | 0.49 | ||||
| BiOI | 1.93 eV/direct | ITO/NiOx/BiOI/ZnO/Cr/Ag | WLED | 4.0 | 0.38 | 56 | 0.60 | 3 |
| FL | 4.4 | 0.40 | 62 | 0.60 | ||||
| AgBiI4 | 1.8 eV/direct | FTO/c-TiO2/AgBiI4/spiro-OMeTAD/Au | WLED | 5.17 | 0.56 | 5.7 | 0.55 | 21 |
| ATBiI4 (AT = 2-aminothiazolium) | 1.78 eV | FTO/c-TiO2/ATBiI4/PTAA/Ag | WLED | 0.52 | 0.50 | 4.35 | 0.71 | 23 |
| Cs2.4MA0.5FA0.1Sb2I8.5Cl0.5 | 2.1 eV/direct | FTO/c-TiO2/Cs2.4MA0.5FA0.1Sb2I8.5Cl0.5/P3HT/Au | WLED | 6.37 | 0.61 | 106.7 | 060 | 20 |
| Cu2AgBiI6 + HI | 1.94 eV/direct | FTO/c-TiO2/Cu2AgBiI6 + HI/Spiro-OMeTAD/Au | WLED | 4.7 | 0.65 | 80 | 0.60 | 24 |
| Cu2AgBiI6 | 1.91 eV/direct | FTO/c-TiO2/m-TiO2/Cu2AgBiI6/Spiro-OMeTAD/Au | WLED | 5.52 | 0.67 | 72 | 0.53 | 25 |
| Sb-alloyed Cu2AgBiI6 | 1.95 eV/direct | FTO/c-TiO2/m-TiO2/Cu2AgBiI6-Sb/Spiro-OMeTAD/Au | WLED | 9.53 | 0.67 | 128 | 0.51 | 26 |
Current research and outlook on PIM-based IPVs
As shown in Fig. 1a, the wide band gap values (∼2 eV) of PIMs ensure an enhanced photovoltaic performance under indoor vs. outdoor lighting conditions. The suitability of the wide band gap PIMs for IPVs can be corroborated by the great match between the external quantum efficiency (EQE) spectra of these materials and the WLED and fluorescence lamp (FL) spectra with respect to the AM 1.5G (1-Sun) spectrum (see Fig. 1c). The PCEs of the photovoltaic devices based on BiOI and Cs3Sb2ClxI9−x were enhanced from ∼1% under 1-Sun to 4–5% under indoor light sources, as demonstrated by Peng et al. in their first IPV study on PIMs in 2021.3 Though both the materials have bandgap values slightly larger than 1.9 eV, the corresponding IPV devices yielded moderate VOC values of 0.49 V (Cs3Sb2ClxI9−x) and 0.6 V (BiOI), which accounts for voltage losses of more than 1.3 V. Recently, we developed a triple-cation Sb-based PIM with an elemental composition of Cs2.4MA0.5FA0.1Sb2I8.5Cl0.5, CsMAFA-Sb (band gap ∼2 eV). Our design is the first example of hybrid organic–inorganic cation mixing involving Cs, methylammonium (MA), and formamidinium (FA) cations. The corresponding photovoltaic devices delivered a PCE(i) of 6.4% and an improved VOC of 0.6 V at 1000 lux illumination.20 In this case, the cation mixing reduced the defect density in the Sb-PIM layer of the device.20 Turkevych et al. explored the IPV potential of silver iodobismuthates deposited by co-evaporation.21 AgBiI4-based devices demonstrated a PCE(i) of 5.17% and a corresponding VOC of 0.55 V under 1000 lux WLED illumination. Replacing the Ag+ in the above composition with a 2-aminothiazolium (AT) cation, Arivazhagan et al.22 reported ATBiI4 photoactive thin films with a bandgap of 1.78 eV, leading to a corresponding modest PCE(i) of 0.54%. However, these devices displayed relatively high VOC values of 0.71 V under 1000 lux illumination.The authors attributed this enhanced performance to the reduced trap density and high built-in potential, which suppressed the interfacial charge accumulation.
The quaternary CABI with a band gap of 1.94 eV, first reported by Sansom et al.,18 has been recently investigated by us in IPVs. First, we achieved a PCE(i) of 4.7% and VOC of 0.6 V for CABI-based IPV devices in a planar architecture through microstructure engineering of the CABI absorber layer by hydroiodic acid (HI) additive.24 More recently, mesoscopic CABI IPV cells delivered a PCE(i) of 5.52% and 5.07% under WLED illuminations of 1000 and 100 lux, respectively.25 The thickness of the mesoporous titania (mp-TiO2) layer plays a critical role in maximizing the PCE(i) of the devices through fill factor (FF) and short-circuit current density (JSC) enhancements. Lately, we reported a PCE(i) of ∼10% (9.53%) under 1000 lux, i.e., almost double the previous value of 5.52%, for CABI devices upon Bi–Sb co-alloying (CABI-Sb).26 The efficiency increase was mainly due to the enhancement of JSC. Our CABI-Sb study demonstrates a significant advancement in the performance of PIM-based IPVs, promising a large room for further improvement in PCE(i) through VOC optimization. The CABI-Sb device displayed impressive PCE(i) values of 6.65% and 4.3% even at the low-light WLED intensities of 200 lux and 50 lux, respectively.
Suppressed carrier self-trapping is necessary
Strong electron–phonon coupling in Sb- and Bi-based PIMs leads to the excited-state trapping of charge carriers, thereby reducing their mobility and fundamentally limiting their optoelectronic device performance.27 Since the charge carrier diffusion lengths in these materials are typically low (≤100 nm)27,28 and fall within the absorber layer thickness in the photovoltaic devices, the self-trapping induced carrier mobility drop leads to a further reduction in the drift and diffusion lengths, in turn causing JSC and FF losses. The spectroscopic studies predict low carrier mobilities (∼1 cm2 V−1 s−1) in Bi- and Sb-based PIMs at room temperature arising from self-trapping mediated charge-carrier localization.29–31 Rondiya et al. summarized the JSC and PCE values of various Bi-based PIM photovoltaic devices, which are all well below their theoretical limits, partially due to the self-trapping nature of the charge carriers.32 The carrier-trapping process also leads to VOC losses and high dark current generation in PIM-based devices.The self-trapping can be tuned by modifying the local symmetry of the polyhedra involving carrier-trapping.33 Recently, we hypothesized the reduction of self-trapping in CABI by the partial alloying of some of the bismuth (III)-sites with antimony (III) ions, which leads to the local symmetry enhancement at the octahedral lattice sites of CABI.26 However, to unequivocally prove the increase in the number of free carriers in CABI-Sb through the lowering of the number of STEs, advanced studies like optical-pump tera-hertz-probe (OPTP) spectroscopy, should be performed30,34 The suppressed carrier self-trapping in CABI through Sb alloying enabled a significant enhancement of the JSC value of the corresponding IPV device. Similarly, mixing of the A-site of Cs3Sb2I9 with methylammonium and formamidinium ions resulted in a nearly undetectable luminescence signal,31 unlike the weak self-trapped exciton emission observed for the pristine Cs3Sb2I9.35 The cation mixing remarkably contributed to increase the PCE(i) of Sb-based IPVs from 4.9% to 6.4%.3,31 These observations suggest that compositional engineering holds a great promise to alter the symmetry of the lattice points involved in the self-trapped exciton (STE) process and diminish carrier trapping-related non-radiative losses in PIM-based photovoltaic cells. In addition, the compositional engineering was proposed to increase the mobility of charge carriers by reducing their effective masses in Cs2AgBiX6 (X = Br, Cl).36
Open circuit voltage (VOC) losses should be minimized
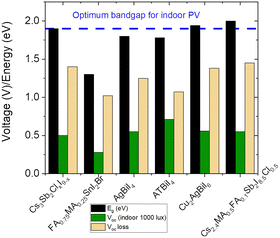
The theoretical optimum bandgap for IPVs 1.9–2.0 eV, and the highest predicted VOC is 1.4–1.5 eV.37,38 However, there are only a few reports on halide perovskite photovoltaics with the experimentally observed VOC of >1 V under indoor lighting conditions (1000 lux).39,40 Both reports are based on conventional LHP compositions (i.e., CsPbI2Br and CH3NH3PbI2−xBrClx).A systematic comparison between the optical bandgap (∼1.8–2.0 eV) of the PIMs and their reported VOC values (0.5–0.6 V) under indoor illumination reveals that the PIM compositions suffer a high VOC loss of ∼1.3–1.4 V (Fig. 2), which is huge compared to what has been reported for the LHP devices under 1-Sun illumination (less than 0.5 V). A voltage loss of ∼0.2 V is expected and systematically observed while comparing the VOC of LHP devices under indoor and 1-Sun illuminations due to the differences in light intensity. To maximise the IPV efficiency of these PIMs, it is essential to reduce the difference between the bandgap and the observed VOC.
Recently, Bahadani41 has quantified the different energy losses in IPVs considering GaAs and GaInP as test cells and a warm WLED as the illumination source. For all the cases, the highest energy loss was due to the thermalisation losses (30–40%) and to the junction loss (20–30%). However, these cells demonstrated relatively low non-radiative recombination losses (∼4 to 8%).
For the photovoltaic devices in general (and specifically for perovskite solar cells), three different voltage loss pathways (due to Shockley–Queisser limit, radiative losses, and Shockley–Read–Hall (SRH)-type losses) have been previously reported.42–46 Recently, Dong et al.47 have quantified these losses for perovskite solar cells (Cs/FA/MA-LHP) as 273 mV, 3.12 mV, and 250.07 mV (for 1-Sun illumination), respectively. In the case of halide perovskites, the typical trap density of the order of ∼1015 cm−3 is comparable to or slightly lower than the typical photogenerated carrier density of ∼1015–1016 cm−3 under 1-sun illumination.48 Still, these perovskite solar cells show remarkable efficiencies under low-intensity indoor/solar illumination (trap-dominated regime) because of the defect-tolerant nature of the LHPs or the entirely different charge trapping/de-trapping mechanism in these materials compared to other PV materials.49
In the case of Pb-free PIMs, the reported trap density is even higher than that of the halide Pb-based perovskites. Ran et al.50 and Ivaturi et al.23 reported trap densities of 1018–1017cm−3 for PIM-based films, which are at least one-to-two orders of magnitude higher than those of the Pb-based halide perovskites. The devices with lower trap density showed a higher VOC of 0.8 V under 1-sun illumination. To achieve a high VOC (>1 V) for PIM-based IPVs, one good starting point would be to obtain high VOC under 1-Sun and reduce the voltage losses under indoor lighting conditions. Thus, to maximise the VOC of PIM-based indoor photovoltaics, there should be more investigation to explore the nature and energy of the traps (shallow, or deep), their density distribution as a function of composition, the formation energy required, and the feasible trap reduction methodologies. Fig. 3, adapted from reference51 shows how reducing the trap density can still retain a high VOC even at low illumination intensities.
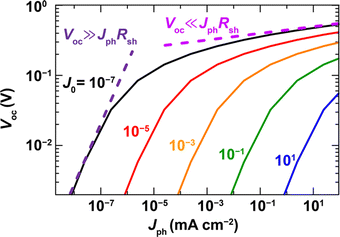 | ||
| Fig. 3 The simulated graph showing how reducing the leakage current can sustain high photovoltage even under low intensity illumination (adapted from105). | ||
However, it is interesting to note that the VOC losses for the best-performing CABI-Sb (PCE(i) = 9.5%) and CsMAFA-Sb (PCE(i) = 6.4%) PIM-based IPV devices are 0.13 V and 0.20 V, respectively when the illumination source changes from 1-Sun to 1000 lux WLED.26,31 Modest VOC losses of ≈0.2 V have been reported even for high-performing IPVs.52,53 This suggests that VOC losses due to lowering the light intensity are not very high in the IPV devices of these two materials. However, the low indoor VOC values of just ∼0.6 V in these devices are due to already low VOC values achieved under 1-Sun, particularly in the case of CABI.24–26,28 Hoye and co-workers examined the performance limiting factors of Cs2Ag(SbxBi1−x)Br6 solar cells.54 They demonstrated that sub-band gap states are accountable for the VOC losses. Similarly, sub-band gap states are detected in CABI;18 however, their influence on CABI devices is not yet studied. On the other hand, density functional theory calculations have suggested that Sb-alloying in CABI increases the defect or vacancy formation energy.26 While the co-alloying has nearly doubled the JSC of the CABI IPV devices, it only resulted in very small enhancement in their VOC values. The low VOC values of the devices despite the suppressed intrinsic defect formation and carrier-self trapping could be explained by the non-radiative recombination of the charge carriers at the surface (interfacial recombination) or the grain boundaries (within the bulk) of the CABI layer. Such undesired recombination process may be promoted by the typical sub optimal morphology of CABI,18,24 which has been so far only partially addressed through additive and compositional engineering strategies.24,26 Therefore, morphological engineering and surface passivation strategies should be employed, inspired by works on both LHPs55 and other PIMs,56–58 to mitigate the VOC losses arising from SRH and interface recombination and push their PCE(i)s towards their theoretical limits of 50–60% (see Fig. 1a). The low VOC values also call for exploring different hole transport and electron transport layers (HTLs and ETLs) than those typically used in LHP-based devices (e.g., Spiro-OMeTAD and P3HT as HTLs; titanium and tin oxides as ETLs), as a good energy level matching is also crucial to achieving high VOC.
Device operational stability needs immediate attention
Lead-free PIM films are usually stable when stored in air owing to the stable oxidation states of Bi3+ and Sb3+. For instance, the X-ray diffraction (XRD) patterns of CABI and Sb-PIM collected before and after months of air storage show no changes (see Fig. 4a), indicating their high phase stability. In another study, CABI and AgBiI4 stored in the air displayed no signs of degradation after exposure to solar light for one week.18 However, long-term stability of corresponding IPV cells is compulsory for their commercial applications. The stability of the photovoltaic cells is expected to be longer under indoor illumination than 1-Sun due to mild operation conditions, such as low-light intensity and no drastic variations in the humidity and light intensity. Commonly, the reported stability of LHP IPV cells has been assessed in terms of shelf-lifetime trends. For instance, Cheng et al. demonstrated the long-term stability of triple-anion methylammonium LHP cells with nearly 95% of their original PCE value retained after 2000 h of storage under indoor light.40 Chen et al. reported the longest known shelf-life stability for LHP IPV cells based on a vacuum-deposited LHP layer, which shows a negligible loss in efficiency under continuous exposure to indoor light of intensities between 200 and 1000 lux for 16 months.59 On the contrary, such long-term stability studies have not been yet reported for PIM-based IPV cells. In addition, to mimic the real-world behavior of the IPV cells, their operational stability should be carefully examined, similarly to the case of LHP solar cells.60 Among the available standard protocols to assess the operational stability of halide perovskite photovoltaics, maximum power point tracking (MPPT), which monitors the device performance under constant electric load and continuous illumination, sheds light on the intrinsic stability of the devices.61 As a case study, we report the data related to IPVs employing CABI or triple-cation Sb-based PIM (CsMAFA-Sb). The performance of the unencapsulated devices in air (RH ≈ 50%) is monitored at the maximum power point (MPP) under continuous WLED (1000 lux) illumination (see Fig. 4b and c). The CABI IPV cell retains nearly 50% of its original PCE after 120 h of continuous operation. On the other hand, the CsMAFA-Sb device loses 40% of its original PCE already after 7 hours of air exposure. The operational stability of both CABI and CsMAFA-Sb IPVs is poor,62 which leaves them still far from any practical utilization. This is in contrast with the respectable shelf-lifetime stability of CABI and CsMAFA-Sb films in the air (Fig. 4a).20,24 These observations exemplify that absorber material stability does not guarantee the corresponding device stability. In addition, the CABI device degrades more rapidly under 1-Sun (Fig. 4d) compared to the WLED illumination. This hints at the accelerated degradation of the device under high-light intensity illumination.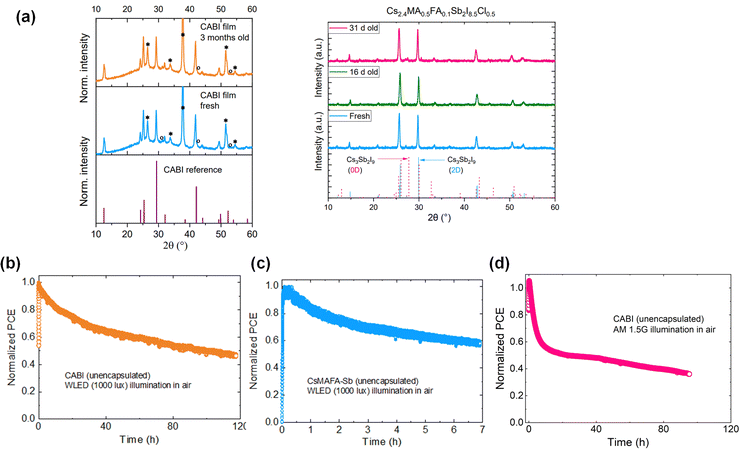 | ||
| Fig. 4 (a) XRD patterns of fresh and aged CABI films stored in air (reproduced from24) and fresh and aged CsMAFA-Sb films stored in dry air (RH of 15%) (reproduced from31). MPP tracking of unencapsulated devices (in their n–i–p planar device architecture) of (b) CABI and (c) CsMAFA-Sb under WLED (1000 lux) illumination, and (d) CABI under 1-Sun illumination in air (RH ∼50%). | ||
The degradation mechanisms of the photovoltaic devices of LHPs under 1-Sun illumination are widely studied and quite well-established.60 One of the common degradation mechanisms in the LHP devices, as far as the absorber layer is concerned, is the ion migration under electric bias and illumination.60,63 Recently, Kulkarni et al. demonstrated that vacancy or defect-induced ion migration is a major degradation pathway of the Ag-Bi-I PIM-based solar cells under ambient storage.64 This could be a plausible degradation pathway in many wide-bandgap PIMs as they often comprise a large number of defects.65 However, further research should be performed to understand the underlying causes of the degradation in PIM-based photovoltaic cells under 1-Sun and indoor illuminations. In addition, effective strategies should be applied to extend the device lifetime of PIMs. Li et al. summarized the literature on various efficient strategies, which involve charged defect passivation, compositional tuning, surface passivation by organic and inorganic layers, and so on, to improve the operational stability of the LHP solar cells.60 Although the optoelectronic behaviour of PIMs differs from LHPs, a good starting point may be to explore some of the strategies mentioned by Li et al., which may also improve the VOC values and other device parameters.
Prospect IPV absorbers
To address the above-mentioned instability and toxicity concerns of LHPs, the development of novel, and inherently more stable, PIMs has been the focus of recent research. Yet, more efforts are required to expand the library of potential non-toxic IPV absorbers. The following PIM candidates for IPVs are proposed based on (i) suitability of band gaps (1.9–2.0 eV) for indoor light-harvesting, (ii) reasonable PCEs under 1-Sun, (iii) optoelectronic properties suitable for photovoltaic applications, (iv) high defect-tolerance, and (v) air-stability.Three-dimensional (3D) materials
Cesium germanium iodide (CsGeI3), with a band gap of ∼1.89 eV, has been explored for solar cell applications, yielding a PCE of 4.94%. This is one of the most efficient photovoltaic absorbers among the Pb-free perovskite structures.66 Ba2AgIO6, a low-temperature processable oxide analogue of halide perovskites, is another stable and encouraging alternative with a band gap (1.9 eV) close to the ideal value required for IPV absorbers.673D MASbSI2 (band gap = 2.03 eV) was recently found suitable for photovoltaic applications (PCE = 3.08%).68
Two-dimensional (2D) materials
PEA2(Ge/Sn)I4 possesses a band gaps around 2.1–2.2 eV.69,70 These are the candidates with the highest PCEs under 1-Sun when considering wide band gap materials ideal for IPV applications. For example, (PEA)2GeI4 exhibits luminescence at room temperature with a moderate lifetime, showing promise for photovoltaic applications. Another popular family of compounds, A3(Sb/Bi)2X9, with bandgaps in the 1.9–2.1 eV range is also not much explored for IPV applications and can be a great prospect considering their favaourable optoelectronic properties.71–74 Among these, MA3Sb2I9−xClx (band gap = 2.2 eV) yielded a PCE of 3.34% under 1-Sun,75 which makes it a potential IPV absorber. Sb–Bi co-alloying in these materials may favourably modify their optoelectronic properties, as in the case of CABI. Other 2D candidates for consideration can be MA2PdCl4 (band gap = 2.2 eV) and CsBi3I10 (band gap = 1.8 eV).76Double perovskites and vacancy-ordered double perovskites, A2Ag(Sb/Bi)X6 (A = Cs and MA; X = Br and Cl), having bandgap tunability over a wide range (1.6–2.6 eV), offer great potential as candidates that remain unexplored for IPV applications.77–82 The double perovskite Cs2AgBiBr6, with a PCE of 2.8% and bandgap around 2.2 eV, is the most explored PIM when it comes to solar cells.83 Additionally, the high air-stability and the earth abundant nature of the ivolved elements make Cs2AgBiBr6 ideal candidates for IPV applications. Cs2TiBr6 (band gap = 1.8 eV), a vacancy-double perovskite with a reported PCE (1-Sun) of ≈ 3.3%, is also a potential IPV absorber.84,85 Cs2PdX6, with tunable band gaps over the 1.6–2.22 eV range by halide compositional engineering, are suitable for different indoor light environments.86,87
Although the IPV performance of a silver iodobismuthate with the composition of AgBiI4 has been already examined,21 another member of the same family, Ag3BiI6 that yielded PCE values up to 4.2–5.4% (and notable JSC and FF) under 1-Sun due to improved charge transport88 may ensure >10% IPV efficiencies. Antimony sulfoiodide (SbSI) with a band gap of ≈ 2.2 eV, high defect-tolerance (dielectric constant in the order of 104), and high stability offers a great promise for IPV applications.89 The IPV performance of BiOI, a deep-trap tolerant,90 can be further improved if the morphology-related limitations are addressed.91
Furthermore, we believe that the compositional engineering with the introduction of organic cations such as methylammonium (MA) and formadinimium (FA), by increasing the overall dielectric constant of the material, may improve the defect tolerance by reducing the capture cross-section of the charged defects.31,92 In addition to the materials listed so far, air-stable, wide band gap chalcogenide perovskites with tuneable band gaps of 1.73–2.87 eV93 can be worth investigating for their IPV performance. Preliminary DFT calculations suggest that BaZrS3 (band gap ∼ 1.8 eV) is defect-tolerant, similarly to LHPs.94 Low-temperature processing of high-quality chalcogenide perovskite films must be established to realize their full commercialisation potential.
Recently there has been much interest in the application of machine learning (ML) in hybrid perovskite solar cells for screening novel compositions, optimising the fabrication processes, and developing more stable compositions.95,96 While designing novel compositions of perovskite-inspired Pb-free materials, the available materials parameters of the corresponding Pb-containing perovskites can provide the desirable input ML parameters required for the optimisation.97 This would reduce the initial stages of experimentation for the implementation of ML. Even though there are no reports yet demonstrating the use of ML in optimising IPVs, the same design principles developed for the LHP solar cells can be extended to the perovskite IPVs as well, upon appropriate modifications in the input data set. Since there are numerous available publications on the bandgap tuning of halide perovskites, their electrical properties, crystal structure, thin film fabrication etc., the ‘data preparation’ stage of the ML workflow is relatively straightforward.98 The application of ML in indoor PV has the potential to boost the efficiency towards the theoretical limit of 50–60% by rapid material design, discovery, and PV property evaluation.
By combining the unique strengths of high throughput synthesis and ML, recently Sun et al.99 have discovered 75 PIM compositions in just 2 months, which are suitable for energy harvesting applications. A few dozens of Pb-free PIMs were also discovered in an accelerated matter, which allowed avoiding the slow incremental trial and error method. The most impressive aspect was the development of four compounds and 18 alloy compositions of Pb-free perovskite-inspired compositions in the thin film form, which otherwise existed only in the crystal bulk form, in turn enabling their adoption in many thin film architecture optoelectronic devices.
In addition to the material screening for the photovoltaic applications,1,100 the suitability of artificial intelligence in predicting the stability, and the reap, rest, and recovery (3R) of halide perovskites has been also reported and may applied also to PIM-based IPVs to boost their credibility for future practical applications.101
In conclusion, PIMs have had a promising start for IPVs. Their low VOC values of ∼0.5–0.6 V remain the major drawback restricting them from reaching PCE(i) values beyond 10% and towards their theoretical limits. The wide band gap nature and typical sub optimal morphology of PIMs typically lead to dominant non-radiative recombination both in their bulk and at their surface or interfaces. Our studies on CsMAFA-Sb and CABI-Sb indicate that cation co-alloying is a promising strategy to improve the defect tolerance of PIMs. We believe that the combination of compositional engineering and microstructure enhancement is the key to significantly boosting the efficiency of lead-free perovskite-inspired IPVs. In all the PIM-based IPV studies, the champion devices under 1-Sun are typically examined under indoor lighting. However, it has been realized in LHPs that the best solar cell performance does not necessarily guarantee the highest indoor device performance.102,103 Therefore, parameters such as absorber layer thickness, type of charge transport layers, and device architecture should be separately optimized for the PIM-based devices under indoor illumination.
Expanding the library of IPV absorbers by combined computational and experimental methods helps in two ways. Firstly, it helps identifying highly promising candidates. Secondly, it provides a general understanding of the behavior of PIMs under low-light intensities, which will help in modifying the existing IPV materials and rationalize the design of new absorbers. Materials with high defect-tolerance and long diffusion lengths can be discovered through experimental characterizations and ML calculations. In addition to the band gap requirements, the next-generation IPV absorbers should preferably comprise low-toxic and earth-abundant elements. Absorbers with low exciton binding energies, high thermodynamic stabilities, and direct band gap absorption will be promising. Moreover, the absorbers should be solution-processed at low temperatures for cost-effectiveness, large-scale fabrication, and deposition on flexible substrates. The morphology of the PIM-based thin films is far inferior to that of LHPs in terms of both grain size and smoothness. Despite the constant efforts to improve the microstructural properties of PIMs through solution- and vacuum-based methods engaging numerous modifications (e.g., solvent engineering, additives, doping, temperature tuning, and post-annealing treatments), the thin films are often non-continuous (incomplete coverage) and contain significantly large pin holes, which create shunt pathways that cause undesired non-radiative recombination during the IPV device operation. Studies focusing on the understanding of the film crystallization kinetics of the PIMs should be carried out to identify the factors affecting the crystal growth and determine the nucleation mechanisms, which, in turn, will enable high-quality PIM-based thin films. In addition to electronic structure examination, deep crystal structural analysis is also necessary. For instance, the deep trap formation in BiOI is restricted due to its long Bi–I bonds. Furthermore, the band gap requirement may not be strictly applicable in all the cases. For example, despite the low band gap of 1.4 eV of a quaternary chalcogenide halide, Sn2SbS2I3, its strong absorption in the 400–650 nm range,104 where the emission intensities of WLEDs are the highest, may still allow their IPV devices perform efficiently. The PIMs show poor device stability in stark contrast to their high material stability in the air. In addition to the simple monitoring of the shelf-life stability of the devices, MPPT and other standard operational stability characterizations (e.g., accelerated indoor testing involving day-night cycles) should be widely adopted to demonstrate the potential of PIM-based devices towards commercialization. Therefore, regardless of their performance, an enhanced stability will be even more crucial for the future of PIM-based IPVs. Although the PIMs typically consist of low-toxicity elements, we strongly encourage the research community to investigate the practicality of the IPV devices from a life-cycle perspective. The cradle-to-grave life-cycle assessment of IPVs will provide insights on the energy payback time and the impact of the devices on the environment, which are important parameters in the context of the global PV market. Overall, enhancing the long-term operational stability and efficiency of the PIM-based photovoltaic devices under indoor illumination will lead us closer to the goal of cost-effective, low-toxic, and high-performance IPVs for indoor-located, self-powered IoT devices.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Miss. Noora Lamminen for the support with the maximum power point data. G. K. G. thanks Tampere Institute for Advanced Study for the postdoctoral funding. P. V. and V. S. acknowledge the financial support of Jane and Aatos Erkko foundation (SOL-TECH project) and Academy of Finland (Decision No. 347772). B.A-A. thanks Vilho, Yrjö and Kalle Väisälä Fund of the Finnish Academy of Science and Letters for the financial support. This work is part of the Academy of Finland Flagship Programme, Photonics Research and Innovation (PREIN), Decision No. 320165.References
- I. Mathews, S. N. Kantareddy, T. Buonassisi and I. M. Peters, Joule, 2019, 3, 1415–1426 CrossRef CAS.
- C. Dong, X. Li, C. Ma, W. Yang, J. Cao, F. Igbari, Z. Wang and L. Liao, Adv. Funct. Mater., 2021, 31, 2011242 CrossRef CAS.
- Y. Peng, T. N. Huq, J. Mei, L. Portilla, R. A. Jagt, L. G. Occhipinti, J. L. Macmanus-driscoll, R. L. Z. Hoye and V. Pecunia, Adv. Energy Mater., 2020, 11, 2002761 CrossRef.
- Z. Guo, A. K. Jena, I. Takei, G. M. Kim, M. A. Kamarudin, Y. Sanehira, A. Ishii, Y. Numata, S. Hayase and T. Miyasaka, J. Am. Chem. Soc., 2020, 142, 9725–9734 CrossRef CAS PubMed.
- Z. Guo, S. Zhao, N. Shibayama, A. Kumar Jena, I. Takei and T. Miyasaka, Adv. Funct. Mater., 2022, 32, 2207554 CrossRef CAS.
- H. Needleman, Annu. Rev. Med., 2004, 55, 209–222 CrossRef CAS PubMed.
- J. Li, H.-L. Cao, W.-B. Jiao, Q. Wang, M. Wei, I. Cantone, J. Lü and A. Abate, Nat. Commun., 2020, 11, 1–5 CrossRef CAS PubMed.
- W. F. Yang, J. J. Cao, C. Dong, M. Li, Q. S. Tian, Z. K. Wang and L. S. Liao, Appl. Phys. Lett., 2021, 118, 023501 CrossRef CAS.
- W. F. Yang, J. J. Cao, J. Chen, K. L. Wang, C. Dong, Z. K. Wang and L. S. Liao, Solar RRL, 2021, 5, 1–7 Search PubMed.
- W. Ke, C. C. Stoumpos and M. G. Kanatzidis, Adv. Mater., 2021, 31, 1803230 CrossRef PubMed.
- J. Cao and F. Yan, Energy Environ. Sci., 2021, 14, 1286 RSC.
- W. F. Yang, J. J. Cao, C. Dong, M. Li, Q. S. Tian, Z. K. Wang and L. S. Liao, Appl. Phys. Lett., 2021, 118, 023501 CrossRef CAS.
- J.-J. Cao, Y.-H. Lou, W.-F. Yang, K.-L. Wang, Z.-H. Su, J. Chen, C.-H. Chen, C. Dong, X.-Y. Gao and Z.-K. Wang, Chem. Eng. J., 2022, 433, 133832 CrossRef CAS.
- K. Ruhle and M. Kasemann, Photovoltaic Specialists Conference (PVSC) IEEE 39th, 2013, 2651–2654.
- R. E. Brandt, V. Stevanović, D. S. Ginley and T. Buonassisi, MRS Commun., 2015, 5, 265–275 CrossRef CAS.
- R. Mohan, Nat. Chem., 2010, 2, 336 CrossRef CAS PubMed.
- L. C. Lee, T. N. Huq, J. L. MacManus-Driscoll and R. L. Z. Hoye, APL Mater., 2018, 6, 84502 CrossRef.
- H. C. Sansom, G. Longo, A. D. Wright, L. R. V. Buizza, S. Mahesh, B. Wenger, M. Zanella, M. Abdi-Jalebi, M. J. Pitcher and M. S. Dyer, J. Am. Chem. Soc., 2021, 143, 3983–3992 CrossRef CAS PubMed.
- I. Turkevych, S. Kazaoui, E. Ito, T. Urano, K. Yamada, H. Tomiyasu, H. Yamagishi, M. Kondo and S. Aramaki, ChemSusChem, 2017, 10, 3754–3759 CrossRef CAS PubMed.
- N. Lamminen, G. K. Grandhi, F. Fasulo, A. Hiltunen, H. Pasanen, M. Liu, B. Al-Anesi, A. Efimov, H. Ali-Löytty, K. Lahtonen, P. Mäkinen, A. Matuhina, A. B. Muñoz-García, M. Pavone and P. Vivo, Adv. Energy Mater., 2022, 13, 2203175 CrossRef.
- I. Turkevych, S. Kazaoui, N. Shirakawa and N. Fukuda, Jpn. J. Appl. Phys., 2021, 60, 2–6 CrossRef.
- V. Arivazhagan, F. Gun, R. K. K. Reddy, T. Li, M. Adelt, N. Robertson, Y. Chen and A. Ivaturi, Sustainable Energy Fuels, 2022, 6, 3179–3186 RSC.
- V. Arivazhagan, F. Gun, R. K. K. Reddy, T. Li, M. Adelt, N. Robertson, Y. Chen and A. Ivaturi, Sustainable Energy Fuels, 2022, 6, 3179–3186 RSC.
- G. K. Grandhi, B. Al-Anesi, H. Pasanen, H. Ali-Löytty, K. Lahtonen, S. Granroth, N. Christian, A. Matuhina, M. Liu, A. Berdin, V. Pecunia and P. Vivo, Small, 2022, 18, 2203768 CrossRef CAS PubMed.
- G. K. Grandhi, S. Toikkonen, B. Al-Anesi, V. Pecunia and P. Vivo, Sustainable Energy Fuels, 2022, 7, 66–73 RSC.
- B. Al-Anesi, G. K. Grandhi, A. Pecoraro, V. Sugathan, N. S. M. Viswanath, H. Ali-Löytty, M. Liu, T.-P. Ruoko, K. Lahtonen, D. Manna, S. Toikkonen, A. B. Muñoz-García, M. Pavone and P. Vivo, ChemRxiv, 2023, preprint DOI:10.26434/chemrxiv-2023-nb5jj.
- B. Wu, W. Ning, Q. Xu, M. Manjappa, M. Feng, S. Ye, J. Fu, S. Lie, T. Yin and F. Wang, Sci. Adv., 2021, 7, eabd3160 CrossRef CAS PubMed.
- N. Pai, M. Chatti, S. O. Fürer, A. D. Scully, S. R. Raga, N. Rai, B. Tan, A. S. R. Chesman, Z. Xu, K. J. Rietwyk, S. S. Reddy, Y. Hora, G. A. Sepalage, N. Glück, M. Lira-Cantú, U. Bach and A. N. Simonov, Adv. Energy Mater., 2022, 2201482 CrossRef CAS.
- A. D. Wright, L. R. V. Buizza, K. J. Savill, G. Longo, H. J. Snaith, M. B. Johnston and L. M. Herz, J. Phys. Chem. Lett., 2021, 12, 3352–3360 CrossRef CAS PubMed.
- L. R. V. Buizza, A. D. Wright, G. Longo, H. C. Sansom, C. Q. Xia, M. J. Rosseinsky, M. B. Johnston, H. J. Snaith and L. M. Herz, ACS Energy Lett., 2021, 6, 1729–1739 CrossRef CAS PubMed.
- N. Lamminen, G. K. Grandhi, F. Fasulo, A. Hiltunen, H. Pasanen, M. Liu, B. Al-Anesi, A. Efimov, H. Ali-Löytty and K. Lahtonen, Adv. Energy Mater., 2023, 13, 2203175 CrossRef CAS.
- S. R. Rondiya, R. A. Jagt, J. L. MacManus-Driscoll, A. Walsh and R. L. Z. Hoye, Appl. Phys. Lett., 2021, 119, 220501 CrossRef CAS.
- B. Luo, D. Liang, S. Sun, Y. Xiao, X. Lian, X. Li, M.-D. Li, X.-C. Huang and J. Z. Zhang, J. Phys. Chem. Lett., 2019, 11, 199–205 CrossRef PubMed.
- L. R. V. Buizza and L. M. Herz, Adv. Mater., 2021, 33, 2007057 CrossRef CAS PubMed.
- A. Hiltunen, N. Lamminen, H. Salonen, M. Liu and P. Vivo, Sustainable Energy Fuels, 2022, 6, 217–222 RSC.
- J. Leveillee, G. Volonakis and F. Giustino, J. Phys. Chem. Lett., 2021, 12, 4474–4482 CrossRef CAS PubMed.
- M. Freunek, M. Freunek and L. M. Reindl, IEEE J. Photovolt., 2012, 3, 59–64 Search PubMed.
- J. K. W. Ho, H. Yin and S. K. So, J. Mater. Chem. A, 2020, 8, 1717–1723 RSC.
- S. Jiang, Y. Bai, Z. Xu, F. Wang, L. Xia, Y. Yang, C. Li and Z. Tan, Small Methods, 2022, 6, 1–9 Search PubMed.
- R. Cheng, C. Chung, H. Zhang, F. Liu, W. Wang, Z. Zhou, S. Wang, A. B. Djurišić and S. Feng, Adv. Energy Mater., 2019, 9, 1901980 CrossRef CAS.
- B. H. Hamadani, Appl. Phys. Lett., 2020, 117, 043904 CrossRef CAS PubMed.
- M. Azzouzi, T. Kirchartz and J. Nelson, Trends Chem., 2019, 1, 49–62 CrossRef CAS.
- C. Dong, J. Chen, C. H. Chen, Y. R. Shi, W. F. Yang, K. L. Wang, Z. K. Wang and L. S. Liao, Nano Energy, 2022, 94, 106866 CrossRef CAS.
- J. Liu, S. Chen, D. Qian, B. Gautam, G. Yang, J. Zhao, J. Bergqvist, F. Zhang, W. Ma, H. Ade, O. Inganäs, K. Gundogdu, F. Gao and H. Yan, Nat. Energy, 2016, 1, 1–48 Search PubMed.
- T. Kirchartz, P. Kaienburg and D. Baran, J. Phys. Chem. C, 2018, 122, 5829–5843 CrossRef CAS.
- L. Krückemeier, U. Rau, M. Stolterfoht and T. Kirchartz, Adv. Energy Mater., 2020, 10, 1902573 CrossRef.
- C. Dong, J. Chen, C. H. Chen, Y. R. Shi, W. F. Yang, K. L. Wang, Z. K. Wang and L. S. Liao, Nano Energy, 2022, 94, 106866 CrossRef CAS.
- J. M. Richter, M. Abdi-Jalebi, A. Sadhanala, M. Tabachnyk, J. P. H. Rivett, L. M. Pazos-Outón, K. C. Gödel, M. Price, F. Deschler and R. H. Friend, Nat. Commun., 2016, 7 Search PubMed.
- S. D. Stranks, ACS Energy Lett., 2017, 2, 1515–1525 CrossRef CAS.
- C. Ran, Z. Wu, J. Xi, F. Yuan, H. Dong, T. Lei, X. He and X. Hou, J. Phys. Chem. Lett., 2017, 8, 394–400 CrossRef CAS PubMed.
- S. Hwang and T. Yasuda, Polym. J., 2023, 55, 297 CrossRef CAS.
- H. Yin, J. K. W. Ho, S. H. Cheung, R. J. Yan, K. L. Chiu, X. Hao and S. K. So, J. Mater. Chem A, 2018, 6, 8579–8585 RSC.
- Y. Cui, Y. Wang, J. Bergqvist, H. Yao, Y. Xu, B. Gao, C. Yang, S. Zhang, O. Inganäs and F. Gao, Nat. Energy, 2019, 4, 768–775 CrossRef CAS.
- Z. Li, Y.-T. Huang, L. Mohan, S. J. Zelewski, R. H. Friend, J. Briscoe and R. L. Z. Hoye, Solar RRL, 2022, 6, 2200749 CrossRef CAS.
- Z. Guo, A. K. Jena, G. M. Kim and T. Miyasaka, Energy Environ. Sci., 2022, 15, 3171–3222 RSC.
- Q. Zhang, C. Wu, X. Qi, F. Lv, Z. Zhang, Y. Liu, S. Wang, B. Qu, Z. Chen and L. Xiao, ACS Appl. Energy Mater., 2019, 2, 3651–3656 CrossRef CAS.
- Y. Guo, J. Zhou, F. Zhao, Y. Wu, J. Tao, S. Zuo, J. Jiang, Z. Hu and J. Chu, Nano Energy, 2021, 88, 106281 CrossRef CAS.
- J. Li, J. Duan, J. Du, X. Yang, Y. Wang, P. Yang, Y. Duan and Q. Tang, ACS Appl. Mater. Interfaces, 2020, 12, 47408–47415 CrossRef CAS PubMed.
- C.-Y. Chen, W.-H. Lee, S.-Y. Hsiao, W.-L. Tsai, L. Yang, H.-L. Lin, H.-J. Chou and H.-W. Lin, J. Mater. Chem. A, 2019, 7, 3612–3617 RSC.
- N. Li, X. Niu, Q. Chen and H. Zhou, Chem. Soc. Rev., 2020, 49, 8235–8286 RSC.
- M. V. Khenkin, E. A. Katz, A. Abate, G. Bardizza, J. J. Berry, C. Brabec, F. Brunetti, V. Bulović, Q. Burlingame and A. Di Carlo, Nat. Energy, 2020, 5, 35–49 CrossRef.
- G. Li, Z. Su, L. Canil, D. Hughes, M. H. Aldamasy, J. Dagar, S. Trofimov, L. Wang, W. Zuo and J. J. Jerónimo-Rendon, Science, 1979, 2023(379), 399–403 Search PubMed.
- Y. Yuan and J. Huang, Acc. Chem. Res., 2016, 49, 286–293 CrossRef CAS PubMed.
- A. Kulkarni, F. Ünlü, N. Pant, J. Kaur, C. Bohr, A. K. Jena, S. Öz, M. Yanagida, Y. Shirai and M. Ikegami, Solar RRL, 2021, 5, 2100077 CrossRef CAS.
- A. M. Ganose, D. O. Scanlon, A. Walsh and R. L. Z. Hoye, Nat. Commun., 2022, 13, 1–4 Search PubMed.
- L.-J. Chen, RSC. Adv., 2018, 8, 18396–18399 RSC.
- G. Volonakis, N. Sakai, H. J. Snaith and F. Giustino, J. Phys. Chem. Lett., 2019, 10, 1722–1728 CrossRef CAS PubMed.
- R. Nie, A. Mehta, B. Park, H.-W. Kwon, J. Im and S. Il Seok, J. Am. Chem. Soc., 2018, 140, 872–875 CrossRef CAS PubMed.
- P. Cheng, T. Wu, J. Zhang, Y. Li, J. Liu, L. Jiang, X. Mao, R.-F. Lu, W.-Q. Deng and K. Han, J. Phys. Chem. Lett., 2017, 8, 4402–4406 CrossRef CAS PubMed.
- Z. Xu, D. B. Mitzi, C. D. Dimitrakopoulos and K. R. Maxcy, Inorg. Chem., 2003, 42, 2031–2039 CrossRef CAS PubMed.
- C. Zuo and L. Ding, Angew. Chem., 2017, 129, 6628–6632 CrossRef.
- C. W. M. Timmermans, S. O. Cholakh and G. Blasse, J. Solid State Chem., 1983, 46, 222–233 CrossRef CAS.
- A. J. Lehner, D. H. Fabini, H. A. Evans, C.-A. Hébert, S. R. Smock, J. Hu, H. Wang, J. W. Zwanziger, M. L. Chabinyc and R. Seshadri, Chem. Mater., 2015, 27, 7137–7148 CrossRef CAS.
- S. Sun, N. T. P. Hartono, Z. D. Ren, F. Oviedo, A. M. Buscemi, M. Layurova, D. X. Chen, T. Ogunfunmi, J. Thapa and S. Ramasamy, Joule, 2019, 3, 1437–1451 CrossRef CAS.
- Y. Yang, C. Liu, M. Cai, Y. Liao, Y. Ding, S. Ma, X. Liu, M. Guli, S. Dai and M. K. Nazeeruddin, ACS Appl. Mater. Interfaces, 2020, 12, 17062–17069 CrossRef CAS PubMed.
- G.-X. Liang, X.-Y. Chen, Z.-H. Chen, H.-B. Lan, Z.-H. Zheng, P. Fan, X.-Q. Tian, J.-Y. Duan, Y.-D. Wei and Z.-H. Su, J. Phys. Chem. C, 2019, 123, 27423–27428 CrossRef CAS.
- A. H. Slavney, T. Hu, A. M. Lindenberg and H. I. Karunadasa, J. Am. Chem. Soc., 2016, 138, 2138–2141 CrossRef CAS PubMed.
- F. Wei, Z. Deng, S. Sun, F. Zhang, D. M. Evans, G. Kieslich, S. Tominaka, M. A. Carpenter, J. Zhang and P. D. Bristowe, Chem. Mater., 2017, 29, 1089–1094 CrossRef CAS.
- E. T. McClure, M. R. Ball, W. Windl and P. M. Woodward, Chem. Mater., 2016, 28, 1348–1354 CrossRef CAS.
- F. Wei, Z. Deng, S. Sun, N. T. P. Hartono, H. L. Seng, T. Buonassisi, P. D. Bristowe and A. K. Cheetham, Chem. Commun., 2019, 55, 3721–3724 RSC.
- J. Kangsabanik, V. Sugathan, A. Yadav, A. Yella and A. Alam, Phys. Rev. Mater., 2018, 2, 55401 CrossRef CAS.
- V. Sugathan, J. Kangsabanik, A. Alam and A. Yella, Energy Fuels, 2022, 36, 11100–11107 CrossRef CAS.
- X. Yang, Y. Chen, P. Liu, H. Xiang, W. Wang, R. Ran, W. Zhou and Z. Shao, Adv. Funct. Mater., 2020, 30, 2001557 CrossRef CAS.
- W. Zhang, K. Tao, C. Ji, Z. Sun, S. Han, J. Zhang, Z. Wu and J. Luo, Inorg. Chem., 2018, 57, 4239–4243 CrossRef CAS PubMed.
- M. Chen, M.-G. Ju, A. D. Carl, Y. Zong, R. L. Grimm, J. Gu, X. C. Zeng, Y. Zhou and N. P. Padture, Joule, 2018, 2, 558–570 CrossRef CAS.
- N. Sakai, A. A. Haghighirad, M. R. Filip, P. K. Nayak, S. Nayak, A. Ramadan, Z. Wang, F. Giustino and H. J. Snaith, J. Am. Chem. Soc., 2017, 139, 6030–6033 CrossRef CAS PubMed.
- T. J. Huang, Z. X. Thiang, X. Yin, C. Tang, G. Qi and H. Gong, Chem. – Eur. J., 2016, 22, 2146–2152 CrossRef CAS PubMed.
- N. Pai, J. Lu, T. R. Gengenbach, A. Seeber, A. S. R. Chesman, L. Jiang, D. C. Senevirathna, P. C. Andrews, U. Bach and Y. Cheng, Adv. Energy Mater., 2019, 9, 1803396 CrossRef.
- R. Nie, H. Yun, M. Paik, A. Mehta, B. Park, Y. C. Choi and S. Il Seok, Adv. Energy Mater., 2018, 8, 1701901 CrossRef.
- T. N. Huq, L. C. Lee, L. Eyre, W. Li, R. A. Jagt, C. Kim, S. Fearn, V. Pecunia, F. Deschler and J. L. MacManus-Driscoll, Adv. Funct. Mater., 2020, 30, 1909983 CrossRef CAS.
- R. L. Z. Hoye, L. C. Lee, R. C. Kurchin, T. N. Huq, K. H. L. Zhang, M. Sponseller, L. Nienhaus, R. E. Brandt, J. Jean and J. A. Polizzotti, Adv. Mater., 2017, 29, 1702176 CrossRef PubMed.
- R. C. Kurchin, P. Gorai, T. Buonassisi and V. Stevanovic, Chem. Mater., 2018, 30, 5583–5592 CrossRef CAS.
- S. Perera, H. Hui, C. Zhao, H. Xue, F. Sun, C. Deng, N. Gross, C. Milleville, X. Xu and D. F. Watson, Nano Energy, 2016, 22, 129–135 CrossRef CAS.
- X. Wu, W. Gao, J. Chai, C. Ming, M. Chen, H. Zeng, P. Zhang, S. Zhang and Y.-Y. Sun, Sci. China Mater., 2021, 64, 2976–2986 CrossRef CAS.
- Y. Liu, X. Tan, J. Liang, H. Han, P. Xiang and W. Yan, Adv. Funct. Mater., 2023, 2214271 CrossRef CAS.
- Q. Tao, P. Xu, M. Li and W. Lu, NPJ Comput. Mater., 2021, 7, 1–18 CrossRef.
- A. Mannodi-Kanakkithodi and M. K. Y. Chan, Energy Environ. Sci., 2022, 1930–1949 RSC.
- Q. Tao, P. Xu, M. Li and W. Lu, NPJ Comput. Mater., 2021, 7, 1–18 CrossRef.
- S. Sun, N. T. P. Hartono, Z. D. Ren, F. Oviedo, A. M. Buscemi, M. Layurova, D. X. Chen, T. Ogunfunmi, J. Thapa, S. Ramasamy, C. Settens, B. L. DeCost, A. G. Kusne, Z. Liu, S. I. P. Tian, I. M. Peters, J. P. Correa-Baena and T. Buonassisi, Joule, 2019, 3, 1437–1451 CrossRef CAS.
- X. Cai, F. Liu, A. Yu, J. Qin, M. Hatamvand, I. Ahmed, J. Luo, Y. Zhang, H. Zhang and Y. Zhan, Light: Sci. Appl., 2022, 11 Search PubMed.
- J. M. Howard, E. M. Tennyson, B. R. A. Neves and M. S. Leite, Joule, 2019, 3, 325–337 CrossRef CAS.
- H. K. H. Lee, J. Barbé, S. M. P. Meroni, T. Du, C. Lin, A. Pockett, J. Troughton, S. M. Jain, F. De Rossi and J. Baker, Solar RRL, 2019, 3, 1800207 CrossRef.
- M. H. Ann, J. Kim, M. Kim, G. Alosaimi, D. Kim, N. Y. Ha, J. Seidel, N. Park, J. S. Yun and J. H. Kim, Nano Energy, 2020, 68, 104321 CrossRef CAS.
- R. Nie, K. S. Lee, M. Hu, M. J. Paik and S. Il Seok, Matter, 2020, 3, 1701–1713 CrossRef.
- S. Hwang and T. Yasuda, Polym. J., 2022, 1–20 Search PubMed.
| This journal is © The Royal Society of Chemistry 2023 |