Open Access Article
Open Access ArticleChemometers: an integrative tool for chemical assessment in multimedia environments
Elisa
Rojo-Nieto
 *a and
Annika
Jahnke
*a and
Annika
Jahnke
 *ab
*ab
aHelmholtz Centre for Environmental Research – UFZ, Department of Ecological Chemistry, Permoserstr. 15, 04318 Leipzig, Germany. E-mail: elisa.rojo-nieto@ufz.de; annika.jahnke@ufz.de
bInstitute for Environmental Research, RWTH Aachen University, 52074 Aachen, Germany
First published on 24th February 2023
Abstract
We propose novel chemometers – passive equilibrium samplers of, e.g., silicone – as an integrative tool for the assessment of hydrophobic organic compounds in multimedia environments. The traditional way of assessing levels of organic pollutants across different environmental compartments is to compare the chemical concentration normalized to the major sorptive phase in two or more media. These sorptive phases for hydrophobic organic compounds differ between compartments, e.g., lipids in biota and organic carbon in sediments. Hence, comparability across media can suffer due to differences in sorptive capacities, but also extraction protocols and bioavailability. Chemometers overcome these drawbacks; they are a common, universal and well-defined polymer reference phase for sampling of a large range of nonpolar organic pollutants in different matrices like biota, sediment and water. When bringing the chemometer into direct contact with the sample, the chemicals partition between the sample and the polymer until thermodynamic equilibrium partitioning is established. At equilibrium, the chemical concentrations in the chemometers can be determined and directly compared between media, e.g., between organisms of different trophic levels or inhabiting different areas, between organs within an organism or between biotic and abiotic compartments, amongst others. Chemometers hence allow expressing the data on a common basis, as the equilibrium partitioning concentrations in the polymer, circumventing normalizations. The approach is based on chemical activity rather than total concentrations, and as such, gives a measure of the “effective concentration” of a compound or a mixture. Furthermore, chemical activity is the main driver for partitioning, biouptake and toxicity. As an additional benefit, the extracts of the chemometers only require limited cleanup efforts, avoiding introduction of a bias between chemicals of different persistence, and can be submitted to both chemical analysis and/or bioanalytical profiling.
Introduction
Thermodynamic gradients are a major driver of numerous processes in and between environmental media, including the partitioning and fate of organic pollutants. They help to identify the direction of diffusion of chemicals and to quantify the potential for spontaneous physicochemical processes, such as sorption and partitioning1 as well as bioaccumulation. Thermodynamic gradients can be characterized as gradients in, e.g., chemical activity, but also in fugacity or freely dissolved concentrations. These metrics all have in common that they allow for understanding and predicting environmental processes. In this article, we use the chemical activity concept.To determine the chemical activity of an environmental contaminant in a medium, equilibrium sampling devices can be used, a special format of which are polymeric passive equilibrium samplers, referred to here as “chemometers”. Chemometers consist of polymers, such as, e.g., silicone rubber for neutral, nonpolar hydrophobic organic compounds (HOCs), in diverse formats, tailor-made for their specific purpose. The HOCs studied with silicone chemometers usually span a wide range of hydrophobicities, with typical log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW ranges from 2.5 to 9.0, where KOW is the compound-specific octanol/water partition coefficient.
KOW ranges from 2.5 to 9.0, where KOW is the compound-specific octanol/water partition coefficient.
For passive equilibrium sampling, the chemometer is brought in contact with the medium to be studied. The chemicals then partition between the sample and the chemometer until a thermodynamic equilibrium between the two phases is reached. At equilibrium, chemicals still move between the two phases, but the concentrations remain constant. The same applies for the chemical activity which can be described as follows (eqn (1)):
| a = γsilicone × Csilicone | (1) |
Mayer et al.2 introduced an analogy between equilibrium sampling devices, such as chemometers, and thermometers: like thermometers, which are deployed in direct contact with the sample until they reach thermal equilibrium with their surroundings, chemometers are brought into thermodynamic equilibrium with the medium they are immersed in. Thermometers then allow us to read the temperature, whereas chemometers give an indication of the chemical activity of a compound in the medium of interest. As has been stated above, chemical activity is the main driver of the free concentrations of pollutants (Cfree) available for processes such as biouptake and partitioning as well as toxicity. Chemometers applied to single or multiple environmental media allow for comparison between (i) organisms of different trophic levels or inhabiting different areas, (ii) organs within an organism or, (iii) biotic and abiotic compartments, amongst others. Their deployment in different media circumvents normalization of the chemical concentration to the relevant sorptive phases common in traditional approaches. Hence, chemometers imply a lower risk of introducing bias.
Instead of single chemicals, chemometers take up complex mixtures of pollutants with widely varying physicochemical properties from a broad range of media. Furthermore, they allow for the assessment of the chemical activity gradient between media, approached by the ratio of concentrations of a chemical in the chemometers equilibrated with different media, samples or compartments. Silicone has proven useful for this purpose because it enriches HOCs in a similar way as biota lipids, and even complex sample types have been shown not to alter the sorptive capacity of the silicone.3
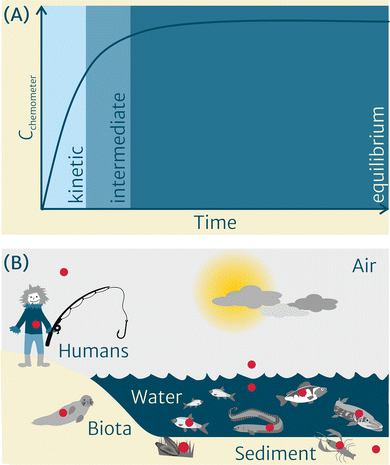
Once in direct contact with the sample, at first the uptake curve of chemicals into the chemometer is steep and linear, as a result of the strong gradient in chemical activity between both phases (Fig. 1(A)). It then flattens, showing a curvilinear intermediate phase, until it reaches a plateau when equilibrium is approached. Following the sampling, the chemometer is retrieved from the sample, its surface is thoroughly cleaned, and the chemicals from the chemometer are extracted using a suitable solvent or mixture(s) of solvents. Depending on the medium that was sampled and the analytical requirements, a non-destructive4 or destructive cleanup5 can be used to remove matrix constituents and enhance the analytical result. However, in general, chemometer extracts require less cleanup compared to traditional exhaustive solvent extraction methods due to lower amounts of co-extracted matrix, such as lipids, which is one of the benefits of this approach. Following cleanup, the extracts of chemometers equilibrated with multimedia environments (Fig. 1(B)) can be submitted to chemical profiling and/or bioanalytical characterization.
Several prerequisites apply for successfully using chemometers, as summarized by Mayer et al.:2 (i) the chemometer must be applied under negligible depletion conditions, i.e., not more than 5% of the chemical mass present/available in the sample must be transferred into the sampler in order not to alter the equilibrium concentration (i.e., not to perturb chemical activity); (ii) the response time of the chemometer must be sufficiently short to correctly mirror fluctuations in environmental levels, which can be ensured by a suitable design, including maximizing its surface area-to-volume ratio; and (iii) the sorptive properties of the chemometer must not be altered by immersion in complex matrices.3
The same working principle, but in reversed mode, is known as passive dosing, where a polymer is pre-loaded with a mixture of HOCs (resembling environmental or artificial mixtures) and used for dosing those compounds into air or aqueous media. Thanks to passive dosing, stable exposure concentrations can be maintained over time, even for HOCs, as required amongst others for bioaccumulation assessment and (eco)toxicological studies.6–11
In recent years, we have teamed up in a group of scientists to jointly work towards widely establishing the chemometer concept in the multimedia environment (Fig. 1(B)) within the project CHEMO-RISK.
CHEMO-RISK covered four major applications (Fig. 2): (i) thermodynamics-based bioaccumulation assessment, by deploying chemometers in abiotic and biotic media of different trophic levels in a Swedish background lake combined with chemical profiling; (ii) internal exposure and effects, by applying chemometers in diverse tissues of marine mammals from the North and Baltic Seas and submitting them to chemical and bioanalytical profiling, subsequently linked in an iceberg modeling approach; (iii) developing chemometers for non-invasive human exposure assessment, applied, amongst others, on the study participants’ skin; and (iv) in a final step, applying chemometers to investigate patterns of known and unknown chemicals across samples and media, in order to identify, e.g., bioaccumulative chemicals from comparison of peak areas, without the need of knowing their identity a priori. The latter approach may provide useful for prioritization of unknown peaks that are interesting to identify, e.g., if a peak shows explicit biomagnification in a trophic network or can be linked to adverse effects, e.g., via effect-directed analysis.
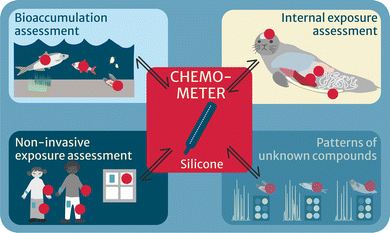 | ||
| Fig. 2 Four applications of chemometers (red circles) in the project CHEMO-RISK, of which “Patterns of unknown compounds” is being developed. | ||
According to the underlying concept, the chemometer constitutes of a common polymeric reference phase – in our case silicone – which is equilibrated with diverse sample types and media, allowing for direct comparison of the concentrations of a chemical in the silicone equilibrated with the multimedia environment. Since the enrichment of potentially hazardous environmental chemicals in lipids of biota, including humans, often is a major concern, we may then translate the concentration in the silicone, Csilicone, to the concentration in model lipids, Clipid, using lipid/silicone partition coefficients, Klipid/silicone (ref. 12–19) according to eqn (2):
| Clipid = Csilicone × Klipid/silicone. | (2) |
In general, equilibrium partitioning concentrations in silicone at equilibrium with environmental media converted to a lipid basis are considered more comprehensible for scientists, regulators, and the general public.20 In this regard, the chemometer approach with the derived equilibrium partitioning concentrations in lipids has similarity to the equilibrium lipid partitioning concentrations introduced by Webster et al.21 The major advancement is that we combine the unique features of chemometers in quantifying freely dissolved concentrations and chemical activity and make use of directly comparing chemical concentrations in the silicone, whereas Webster et al. worked with data from all kinds of studies, mostly based on traditional exhaustive solvent (i.e., total) extractions. As such, the pioneering approach by Webster et al. considered total chemical loads, not taking into account that strong sorption to organic phases may reduce bioavailability in many multimedia systems.
Current environmental risk assessment usually compares the total concentrations of a single chemical in the main surrounding compartment relevant for the exposure of the organism to the highest concentration that did not show adverse effects in standard test species (e.g., the no observed effect level/concentration, NOEL/NOEC). This approach entails a number of major shortcomings, such as that (a) exposure and effect are evaluated separately in standard settings, hence ignoring site-specific factors; (b) the bioavailability of the chemical is not taken into account; (c) application of different methods hampers comparability across studies; (d) evaluation of single chemicals precludes taking into account mixture effects; and (e) ecosystem health assessments are not directly transferrable to human health.
Chemometers represent a major advancement in this context because they (a) are used in site-specific assessments to integrate exposure and effect assessment by allowing to submit extracts from identical chemometers to both chemical profiling and bioanalytical characterization; (b) represent the fraction of the total concentration of the chemical that is bioavailable; (c) provide a standardized approach applicable to diverse environmental media for enhanced comparability; (d) cover a broad chemical universe; and (e) can be applied for environmental biota and humans, allowing for direct comparability of the outcomes.22,23
State-of-the-art in multimedia passive sampling
In recent years, a range of peer-reviewed scientific publications regarding the use of chemometers in one or more environmental compartments has been published, and Table 1 compiles a related literature overview. The criteria for inclusion, in addition to applying polymeric passive samplers in environmental compartments to determine concentrations and/or characterize effects, included a restriction to studies that focused on nonpolar HOCs (amenable to gas chromatographic (GC) separation coupled to mass spectrometric (MS) detection). Table 1 summarizes a synopsis of these studies, aiming to provide a general impression of the application of chemometers in environmental studies of HOCs until now, but it does not claim to be an exhaustive review of all the studies published up to date. In the table it can be observed that the main polymers used in this regard are silicone and polyethylene (PE), together with polyurethane foam (PUF). What also becomes obvious is an increase of studies in recent years, which partly is a result of the advantages that this approach brings to environmental assessments.| Ref. | Chemicals of concern | Polymer(s) | Environmental compartment | Evaluation | |||||
|---|---|---|---|---|---|---|---|---|---|
| Atmosphere | Water | Sediment /SPM | Env. biota | Humans | Chemical analysis | Bioassays | |||
| Alkon et al. (2022)27 | Pesticides, PYRs | Silicone (wristbands) | × | × | |||||
| Allan et al. (2013)28 | PAHs, PCBs, OCPs and CHCs | Silicone | × | × | |||||
| Allan et al. (2021)29 | PAHs, PCBs, OCPs and CHCs | Silicone | × | × | |||||
| Arcury et al. (2021)30 | Pesticides | Silicone (wristbands) | × | × | |||||
| Baumer et al. (2020)31 | PCBs, OCPs and CHCs | Silicone | × | × | |||||
| Baumer et al. (2021a)32 | PAHs, PCBs, OCPs, PBDEs and CHCs | Silicone | × | × | |||||
| Baumer et al. (2021b)33 | PAHs, PCBs, pesticides, PBDEs, CHCs, OFRs and musks | Silicone | × | × | × | ||||
| Beckingham et al. (2013)34 | PCBs, OCPs and CHCs | POM | × | × | |||||
| Bergmann et al. (2017)35 | PCBs, pesticides, PAHs, and PCPPs | Silicone (wristbands) | × | × | |||||
| Booij et al. (2016)36 | Review | Silicone | × | ||||||
| Chen et al. (2020)37 | PCBs | Silicone | × | × | |||||
| Chen et al. (2022)38 | PCBs | Silicone | × | × | |||||
| Claessens et al. (2015)39 | PAHs, PCBs, pesticides and farmaceuticals | Silicone | × | × | × | ||||
| Cornelissen et al. (2008)40 | PCDD/Fs and PCBs | POM | × | × | |||||
| Dixon et al. (2018)41 | PAHs | Silicone (wristbands) | × | × | |||||
| Figueiredo et al. (2017)42 | PCBs | Silicone and PE | × | × | × | ||||
| Fuchte et al. (2020)43 | PAHs | Silicone | × | × | |||||
| Global atmospheric passive sampling (GAPS) network44 | POPs, e.g., PCBs, OCPs | PUF | × | ||||||
| Harley et al. (2019)45 | Pesticides | Silicone (wristbands) | × | × | |||||
| Hendryx et al. (2020)46 | PAHs | Silicone (wristbands) | × | × | |||||
| Jahnke et al. (2009)47 | PCBs | Silicone | × | × | |||||
| Jahnke et al. (2011)48 | PCBs | Silicone | × | × | |||||
| Jahnke et al. (2012)49 | PCBs | Silicone | × | × | |||||
| Jahnke et al. (2014a)50 | HCB and PCBs | Silicone | × | × | × | ||||
| Jahnke et al. (2014b)51 | HCB and PCBs | Silicone | × | × | |||||
| Jahnke et al. (2018)52 | — | Silicone | × | × | |||||
| Jin et al. (2015)53 | PCBs, PBDEs and PCDDs | Silicone | × | × | |||||
| Jonker et al. (2018)54 | PAHs and PCBs | PE, silicone and polyacrylate | × | × | |||||
| Khairy et al. (2015)55 | PCBs | PE | × | × | × | ||||
| Khairy and Lohmann (2017)56 | PBDEs | PE | × | × | × | × | |||
| Kile et al. (2016)57 | PBDEs | Silicone (wristbands) | × | × | |||||
| Lang et al. (2018)58 | PCBs | Silicone | × | × | |||||
| Lao et al. (2016)59 | PAHs, PCBs, PBDEs, OCPs and musks | PMMA | × | × | × | ||||
| Li et al. (2013)60 | — | Silicone | × | × | |||||
| Li et al. (2014)14 | PBDEs | Silicone | × | × | |||||
| Lohmann et al. (2012)61 | PCBs and OCPs | PE | × | × | |||||
| Lohmann et al. (2013)62 | PBDEs | PE | × | × | × | ||||
| Mäenpää et al. (2011)22 | PCBs | Silicone | × | × | |||||
| Mäenpää et al. (2015b)63 | PCBs | Silicone | × | × | × | ||||
| McDonough et al. (2016)64 | PBDEs and NHFRs | PE | × | × | × | ||||
| Meierdierks et al. (2021)65 | PAHs | PE | × | × | |||||
| Meierdierks et al. (2022)66 | PAHs | PE | × | × | × | ||||
| Meire et al. (2016)67 | OCPs | PE | × | × | × | ||||
| Mustajärvi et al. (2017)68 | — | Silicone | × | ||||||
| Muz et al. (2020)69 | PAHs, PCBs, PBDEs, CHCs, PYRs, OCPs, UV filters and musks | Silicone | × | × | × | ||||
| Nguyen et al. (2020)70 | Flame retardants | Silicone (incl. wristbands) | × | × | |||||
| Niu et al. (2020)71 | PAHs, PCBs, PBDEs, CHCs, PYRs, OCPs, UV filters and musks | Silicone | × | × | |||||
| Niu et al. (2021)26 | PAHs, PCBs, PBDEs, CHCs, PYRs, OCPs, UV filters and musks | Silicone | × | × | × | ||||
| Pintado-Herrera et al. (2020)72 | PAHs, PCBs, pesticides, OPFRs, UV filters and musks | Silicone | × | × | |||||
| Reche et al. (2020)73 | PAHs | Silicone (wristbands) | × | × | |||||
| Reddam et al. (2020)74 | OPEs | Silicone (wristbands) | × | × | |||||
| Reichenberg et al. (2008)23 | PAHs | Silicone | × | × | |||||
| Reiter et al. (2022)75 | — | Silicone | × | × | |||||
| Rohlman et al. (2019)76 | PAHs | Silicone (wristbands) | × | × | |||||
| Rojo-Nieto and Perales (2015)77 | PAHs | Silicone | × | × | |||||
| Rojo-Nieto et al. (2019)78 | PAHs, PCBs and OCPs | Silicone | × | × | |||||
| Ruge et al. (2015)79 | PAHs and PBDEs | PE | × | × | × | ||||
| Rusina et al. (2017)80 | PCBs, OCPs and CHCs | Silicone | × | × | |||||
| Sacks and Lohmann (2012)81 | PBDEs | PE | × | × | × | ||||
| Samon et al. (2022)82 | Review | Silicone (wristbands) | × | × | |||||
| Schäfer et al. (2015)83 | PCBs | Silicone | × | × | |||||
| Schuster et al. (2021)84 | POPs, e.g., PCBs, OCPs | PUF | × | ||||||
| Smedes et al. (2013)85 | PCBs and PAHs | Silicone | × | × | |||||
| Smedes et al. (2020)86 | PCBs, PBDEs, OCPs and CHCs | Silicone | × | × | × | ||||
| Strandberg et al. (2022)87 | PAHs | PUF | × | ||||||
| Smith and Jeong (2021)6 | — | Silicone | × | ||||||
| Tuduri et al. (2005)88 | PCBs | PUF | × | ||||||
| Travis et al. (2020)89 | PCBs, flame retardants and pesticides | Silicone (wristbands) | × | × | |||||
| Vrana et al. (2018)90 | PAHs, PCBs and CHCs | Silicone and PE | × | × | |||||
| Vrana et al. (2019)91 | PAHs, PCBs, PBDEs, OCPs and CHCs | Silicone | × | × | |||||
| Wang et al. (2020)92 | Flame retardants (PBDEs and NBFRs) | Silicone (wristbands) | × | × | |||||
| Wernicke et al.(2022a)24 | PAHS, PCBs and OCPs | Silicone | × | × | |||||
| Wernicke et al. (2022b)25 | PAHS, PCBs and OCPs | Silicone | × | × | × | ||||
| Wise et al. (2022)93 | PBDEs, NBFRs and OPEs | Silicone (wristbands) | × | × | |||||
| Xie et al. (2021)94 | OPEs | Silicone (wristbands) | × | × | |||||
| Young et al. (2021)95 | PCBs, PBDEs, BFRs, OPEs, pesticides and PAHs | Silicone (wristbands) | × | ||||||
Soils and sediments were the first compartments to be equilibrated with chemometers,22,23 using ex situ setups, which can be performed in a non-depletive mode due to the high sorptive capacity of such samples for HOCs. Furthermore, equilibration times of several weeks are facilitated by adding agents such as sodium azide to stabilize the samples. Suspended particulate matter (SPM) from aquatic environments has been studied likewise.24–26
More recently, biota have been sampled with chemometers,38,78,96 and nowadays, methods are established even for lean tissues, based on frequent mixing of the samples to avoid local depletion, that make it feasible to reach equilibrium within a reasonable time frame, i.e., before the tissue starts to decay. The methods were originally developed for fish muscle tissue and whole fish,78,80 but have recently been adapted to include different organs and tissues of marine mammals75 and humans.33 Regarding the study of human exposure, as a result of ethical limitations, non-invasive formats such as silicone wristbands or similar devices have usually been applied.82 Those devices comprise the disadvantage that they measure not only the release of chemicals from the human body via the skin, but also, at least partially, the environmental exposure through air. An exception are few studies such as Baumer et al.33 using in tissue sampling in body donors post-mortem.
Contrarily, in water it is still challenging to achieve equilibrium partitioning of HOCs, especially for the more hydrophobic compounds. According to the literature,83 the uptake of certain HOCs (up to a log KOW of 5.5) from water into chemometers may take as long as 4 months until equilibrium is reached even under agitated conditions. However, for compounds with a log KOW >5.5 it remains challenging to reach equilibrium within a reasonable time frame, and for some compounds it will not even be reached after 6 months. Hence, chemometers have mainly been used in kinetic mode in water, sampling in the linear uptake phase (Fig. 1(A)). The results are then usually extrapolated to concentrations in equilibrium conditions based on the dissipation of performance reference compounds (PRCs) that had been loaded into the polymer prior to their exposure to water.97
The same approach is commonly used for atmospheric sampling.97 In air, the most commonly used polymer is PUF, together with PE (more precisely low-density PE, LDPE).44,55,56,62,64,65,67,79,84,87,88 Some studies44,84 have aimed to deploy chemometers for sampling the air over several seasons and years, allowing to establish temporal trends, and thus to evaluate, e.g., the effectiveness of control measures of POPs on a global scale.
Summarizing, chemometers have recently been used successfully in diverse environmental compartments (Table 1), but there remains an enormous potential for development and extension. In the next sections we will focus on novel approaches and ongoing advancements regarding the use of chemometers in multimedia environments and the next steps to be addressed.
Major advancements
Within our project CHEMO-RISK, we have achieved major improvements towards establishing chemometers as powerful and solid tools for environmental and human health assessment in three application areas: thermodynamics-based bioaccumulation, internal exposure and effect assessment and non-invasive human exposure assessment (Fig. 2).Thermodynamics-based bioaccumulation
Bioaccumulation of environmental chemicals in aquatic biota is of concern for the involved species and related ecosystem health, but also for human exposure in case of consumption. In this, “bioaccumulation” includes both uptake from the organism's abiotic environment (bioconcentration) and from its diet (i.e., biomagnification across trophic levels). Opposed to bioconcentration, which is expected to yield equal chemical activity, biomagnification is supposed to be accompanied by an increase in chemical activity from one trophic level to the next one,98 but this assumption has to date not been experimentally confirmed.To characterize bioaccumulation, diverse metrics have been proposed derived from the ratio of total concentrations of a pollutant in the organism, normalized to its lipid fraction in the case of HOCs, over its concentrations in the main exposure medium normalized to the major sorptive phase, e.g., organic carbon in sediment. Examples include: bioconcentration factors (BCF = Cbiota/Cwater) for uptake from the abiotic environment, (b) biomagnification factors (BMF = Cpredator/Cprey) for uptake via food, (c) biota/sediment accumulation factors (BSAF = Cbiota/Csediment), (d) trophic magnification factors (TMF) and (e) fugacity or activity ratios.
To date, activity ratios have mainly been calculated from traditional monitoring data, i.e., from sorption phase-normalized concentrations.99 Drawbacks of this normalization approach include differences in sorptive capacities, e.g., for “lipids” (in many cases operationally defined as extractable organic matter) between storage lipids and membrane lipids, opposed to other sorptive phases such as proteins of relevance in lean tissues78,100–102 or for “organic carbon” relative to black carbon in different fractions as a function of site-specific conditions, hence limiting comparability. The overall goal regarding activity ratios is to express the data on a common basis to enable direct comparison, circumventing normalizations, which becomes feasible when directly comparing Cchemometer across samples.
Novel chemometers have been developed in the CHEMO-RISK project to extend their applicability domain, previously restricted to samples rich in transporter agents, such as lipids or organic carbon, to enable equilibration with biota of different lipid contents78,103 and sediment for application in a well-defined model ecosystem, the Swedish Lake Ången. The concentrations of the sampled pollutants in the chemometer can then be directly compared across the different compartments and trophic levels to provide insights into the thermodynamic factors affecting bioaccumulation. However, it has to be kept in mind that a larger amount of sample is necessary in this application compared to exhaustive extraction, to ensure working in non-depletive mode.
The results from this project have proven that chemometers are a promising tool to evaluate chemical pollutants in biota, allowing to determine metrics such as the TMF directly using the concentrations determined in the chemometers, hence avoiding the need of normalization to the major sorptive phases.103 The results further corroborate the general assumption that biomagnification is accompanied by an increase in chemical activity. Taking into account eqn (1), if biomagnification is accompanied by an increase in the concentrations in the chemometers, it implies an increase of chemical activity in the higher trophic level opposed to the lower trophic level, which was observed in our study.103 Within this work it has thus been experimentally confirmed that biomagnification across the food web implies an increase in the concentrations of the chemometers equilibrated with species from higher trophic levels, equivalent to an increase in chemical activity.
Furthermore, the use of chemometers in different biotic and abiotic compartments has extended their application to determine activity ratios between biota and their surrounding abiotic compartments (e.g., sediments), using eqn (3):
| abiota/asediment = Csilicone⇌biota/Csilicone⇌sed | (3) |
Chen et al.37,38 recently developed another interesting approach to study the thermodynamics of biomagnification through the application of chemometers in food before and after passing the gastrointestinal tract (feces) of different zoo-based predator species.37,38 Regarding bioaccumulation studies, Allan et al.,105 Adolfsson-Erici106et al. and O’Connell et al.107 have explored the feasibility of in vivo sampling following implantation of chemometers for studying HOCs through the insertion of a silicone sampler into trout. Other researchers such as Narváez Valderrama et al.108 follow this research line.
Considering the sampling in air as another abiotic compartment and the potential comparison with biota samples, in the CHEMO-RISK project we studied human exposure in the indoor environment by means of thin silicone films attached to the kitchen windows of the study participants for non-invasive human exposure assessment (see Fig. 2). No PRCs were used in this case to avoid elevated exposure. The results are still under evaluation, but the setup might represent an interesting approach to compare concentrations in chemometers of the identical polymer deployed in different human-related compartments, further addressing the characterization of the human exposome.
Internal exposure and effect assessment
Internal exposure to a chemical at the target site is known to be best suited to explain observed effects.109 However, in many cases only external exposure data are accessible, which implies a limited degree of correlation between exposure and effects. For total concentrations in either exposure medium (water or sediment) or biota tissues, normalization-related challenges apply: tissues have different compositions including diverse fractions of storage lipids vs. membrane lipids, and in lean tissues other sorptive phases such as proteins gain importance.100Chemometers open up for characterizing chemical activity in target tissues following in tissue equilibration of the polymer, avoiding the need for normalization. The simultaneous uptake of very diverse compounds into the polymer moreover allows for investigation of chemical exposure and mixture toxicity across different organs: chemometers have the inherent advantage of transferring mixtures of nonpolar HOCs into the extract either submitted to analytical determination or dosed into, e.g., bioassays without changing the chemical composition of the mixture.2,52,75 As an additional benefit, chemometers transfer less disturbing matrix constituents such as lipids into the extracts; hence non-destructive cleanup procedures are often sufficient, limiting alterations of the mixture composition.
In our project, chemometers have been developed to study mixtures of chemicals from in tissue sampling of different organs, i.e., blubber, liver, kidney and brain tissues of stranded marine mammals.32,75 The related hypothesis was that HOCs mainly accumulate in storage lipids and that the chemical activity hence would be the highest in blubber tissues. However, the results of the bioanalytical profiling (oxidative stress response and activation of xenobiotic metabolism) showed only minor differences for the oxidative stress response (AREc32), while blubber extracts did not activate the aryl hydrocarbon receptor (AhR-CALUX) up to concentrations where cytotoxicity occurred.32,75 Only the peroxisome proliferator-activated receptor gamma (PPARγ-bla) indicated an elevated chemical activity in chemometers equilibrated with liver, followed by kidney, brain, and blubber tissues.75
Subsequent chemical profiling of a wide range of legacy and emerging pollutants in the extracts was performed but did not show clear tissue-specific patterns.110 The reason may be that those chemicals that are known to activate PPARγ-bla were not specifically targeted in the chemical analysis,69 which is strong support to complementarily work with chemical screening and bioanalytical profiling to capture the whole exposome of an organism. In combination with chemical profiling, the observed effects can then be related to the measured chemicals in an approach called “iceberg modeling”111 to determine to which extent the known chemicals contribute to the observed effects. Our recent study indicated that for highly contaminated individuals and assays such as AhR-CALUX in which legacy HOCs elicit strong effects, a large fraction of both the specific effect and the observed cytotoxicity can be explained by the measured chemicals.110 Contrarily, the fraction explained in the other assays was well below 1% as far as specific effects are concerned, whereas regarding cytotoxicity, the explained fractions were between 0.1 and 10%.
Non-invasive human exposure assessment
The fate and effects of environmental chemicals in humans is of major concern, but very challenging to assess due to ethical issues and the related limited availability of study material. Regarding chemometers, in the last decade, silicone wristbands have been proposed as a non-invasive chemical assessment tool and have been used in multiple studies to characterize personal exposure in a variety of populations such as in Samon et al.82 However, with this approach it is challenging to reach equilibrium with the chemicals sequestered from the human skin within a reasonable time frame due to the thickness of the wristband and limited contact with the skin. Furthermore, as is exposed not only to the human skin but also to the surrounding air, clothes, etc., the origin of the sampled chemicals is poorly defined and may be heavily impacted, e.g., by particles deposited on the wristbands or personal care products used by the study participant. This may be the reason why many studies apply the wristbands with a perspective on passive air sampling. In addition, being in direct contact with the skin, some of the procedures that are commonly used to extrapolate concentrations in chemometers applied in the kinetic uptake phase to equilibrium partitioning concentrations (such as, e.g., the use of PRCs) are not suitable in this setup.In order to overcome those drawbacks, in our project new chemometers have been designed as non-invasive alternatives for human exposure assessment112 with a perspective to subsequent comparison to patterns and levels in human blood. We have assessed different sampler formats, including thin silicone skin patches that offer a larger contact area between the chemometer and the human skin, applied to the upper thigh of the study participants112 and compared the obtained results to the performance of the commonly used silicone wristbands. In a pre-study, we assessed the uptake kinetics for 1d, 3d, or 5d of exposure. Furthermore, different combinations of isolation layers, consisting of medical gauze and a weaved activated carbon fabric, have been tested to isolate uptake from either the skin or the surrounding air. In addition, silicone films attached to the study participants’ kitchen windows have been tested to investigate the external exposure of the study participants from indoor air.
Comparison of the results for the different isolation layers indicated that the activated carbon fabric was not suitable in our setting because not only did it isolate the silicone skin patches from airborne chemicals, it also depleted the silicone of the sampled compounds.112 The gauze, however, proved suitable to prevent deposition of contaminants onto the silicone chemometer. Comparison of the sampled contaminant patterns and burdens showed that the wristbands had enhanced sensitivity as they sampled substantially higher levels in the silicone, despite their thickness and limited, poorly controlled contact to the skin, due to airborne deposition being the major uptake pathway. However, the extracts of the wristbands contained much more disturbing matrix so that certain time windows of the chromatograms were hardly quantifiable if at all. Overall, as in the case of the silicone wristbands, drawbacks of the silicone skin patches such as limited uptake rates, etc. imply that these chemometers do not reach steady state with the human body either. Some preliminary results113 showed that, even if certain benefits of the skin patches were observed relative to the wristbands, including cleaner chromatograms, improvements in the design are still necessary to be able to successfully apply them in large-scale monitoring or cohort studies.
Another interesting approach regarding the study of the human exposome using chemometers was described in Baumer et al.,33 where post-mortem samples of different tissues from body donors were equilibrated with chemometers and submitted to both chemical analysis and bioassays. This research opens up a new pathway for investigating the human lifelong exposure to mixtures of chemicals. Further chemometer approaches to study the human exposome have been explored for human biomonitoring, such as silicone breast implants, which equilibrate in the body over time and provide a measure of the overall body burden.107,114
Next steps
To fully establish chemometers as powerful and solid tools in environmental and human health assessment, further steps to be taken include the following four aspects: (i) fostering the development and use of identical polymers for all environmental and human compartments or translation into concentrations in the same polymer; (ii) achieving equilibrium partitioning for chemometers in water; (iii) overcoming challenges in studies of the human exposome; and (iv) identifying patterns of known, suspected and unknown chemicals across multimedia compartments.Identical polymers or conversion into the same polymeric phase
For each polymeric chemometer, irrespective of whether it is silicone, PE or any other polymer, there is a large variety of materials available. These distinct polymers have slightly different sorptive characteristics for chemicals. Unless the identical polymer is used for chemometers applied in every environmental compartment, allowing for direct and unbiased comparison, the equilibrium partitioning concentration in the polymers require translation into the same material to be comparable.For this conversion, polymer/polymer partition coefficients are needed, which are only partly available in the literature for a subset of the polymers and a range of nonpolar HOCs.115,116 As a consequence, efforts have to be taken in order to (i) establish a solid and wide set of highly precise polymer/polymer partition coefficients that allow for those translations; (ii) further develop the chemometers applied in certain environmental compartments to be applicable also in the other compartments. Although some steps have already been taken in that direction, such us using silicone sheets (normally used for biota and water) for sediments or using coated jars (normally used for soil/sediments/SPM) for biota,37,38 in most of the cases different chemometers are still used for sampling of nonpolar HOCs in the different environmental compartments within a study which then have to be translated for direct comparability.
Achieving equilibrium partitioning in water
As mentioned above, achieving equilibrium partitioning between a chemometer and water is very challenging within a reasonable time frame for many of the nonpolar, highly hydrophobic HOCs. Due to the very high polymer/water partition coefficients for HOCs, equilibrating the samplers with negligible depletion of the sample requires huge volumes of water, rendering ex situ equilibration in the laboratory, e.g., under agitation, impossible. Thus, the sampling with chemometers has to be done on the field.The usual procedure is to measure the concentrations in the kinetic uptake phase (Fig. 1(A)) and extrapolate them to concentrations at equilibrium (i) either by using field dissipation data of a set of PRCs covering the range of physicochemical properties of the analytes for calculating the sampling rates in the field deployment or (ii) using sampling rates derived under laboratory conditions, which are challenging to extrapolate to field conditions. According to the guideline by Smedes and Booij,117 for using PCRs: the retained PRC fraction f can be calculated for each PRC by the ratio of the amount left in the field sampler Nt upon retrieval from the field and the measured original amount as derived from the laboratory controls (average) N0 according to eqn (4):
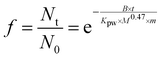 | (4) |
Then, the sampling rate Rs for each compound can be calculated as follows in eqn (5):
| Rs = B/M0.47. | (5) |
Some progress has been made in this direction, aiming to reduce the water boundary layer to increase uptake rates into the chemometer, as well as towards minimizing the thickness of the chemometers to maximize the surface area in contact with the sample relative to the sampler volume. Examples include (i) a device referred to as dynamic passive sampler,90,91 (ii) the pre-equilibration of the chemometers with another medium, e.g. sediment, from the study site, prior to exposure to water in order to start from a chemical activity close to equilibrium to allow faster attainment of equilibrium partitioning with water,91 or (iii) the use of silicone-coated jars under a continuous movement of water which appeared to be promising for moderately hydrophobic contaminants.103 Despite those advancements, equilibrium partitioning between chemometers and water remains to date unsolved in a practical way.
Overcoming challenges in human studies
To date, most of the studies regarding humans have focused on non-invasive human exposure assessment through external devices, such as silicone wristbands. As stated above, those approaches do not reach equilibrium with the human body via the skin, but rather (also) sample other compartments such as the surrounding air. In order to comprehensively study the human exposome, further approaches need to be explored, such as the ones proposed by Abel et al. (in prep.),112 applying different formats of external chemometers, or by Baumer et al.33 equilibrating the chemometers post-mortem with different human tissues, but these approaches are still of limited scope and/or restricted to post-mortem investigations. In order to broaden the scope of human biomonitoring with chemometers despite very restrictive ethics, one interesting approach may be to use samples that are taken during regular surgeries collected as by-products. These leftovers could provide a valuable set of samples, in particular if complemented by detailed medical information about the study participants.Chemical patterns across multimedia compartments
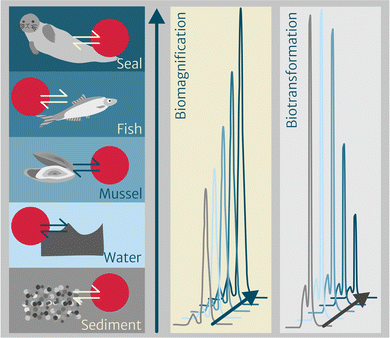
One of the major advantages of applying chemometers to study the environmental fate and bioaccumulation of pollutants is the direct comparability between chemometers equilibrated with different multimedia environmental compartments. As such, chemometers help overcoming substantial bias related to the multitude of methods and approaches applied to date which all reduce comparability. The use of activity ratios (section “Thermodynamics-based bioaccumulation” and eqn (3)) is one possibility to compare between compartments when targeted analysis yields quantifiable concentrations of a chemical in each chemometer. This approach has been applied successfully in Rojo-Nieto et al. (in prep.)103 for sediments and aquatic biota from different trophic levels.Beyond that targeted application, chemometer extracts are also ideally suited for suspect screening and nontargeted analysis. By exploring the extracts in a nontargeted way, peak patterns across different sample types can be studied without knowing the identity of the underlying compounds. In our project, we have explored chemometer-based pattern analysis for chemical profiling to identify increasing or decreasing peaks in biota from one location covering diverse trophic levels,118 in which increasing peak areas up the food web indicate biomagnification and decreasing peak areas may point at chemicals that are biotransformed (Fig. 3). Until now, suspect screening and nontarget analysis have been applied to chemometer extracts exposed to abiotic compartments, mainly to water for less hydrophobic compounds,119–121 but not yet to biota.
Another approach has focused on chemical patterns between different organs and tissues of marine mammals122 (i.e., blubber, liver, kidney and brain) to characterize the chemical mixture and potentially identify yet unrecognized chemicals that strongly biomagnify or are biotransformed as a result of enzymatic action, for example. The underlying hypothesis is that identical chemometers equilibrated with different tissues within one organism or across individuals from the same environment allow for an initial screening based on comparing chromatograms, allowing for peaks with interesting patterns to be selected for potential identification. In this context, chemicals known to strongly biomagnify (e.g., hexachlorinated PCBs) or to be subject to biotransformation (e.g., certain PAHs) can help to establish the related workflows. If successful within different biota and/or tissues, future applications may address the additional challenge of comparing abiotic to biotic compartments.
Abandoned research lines
In our efforts to design versatile, robust and reliable multimedia chemometers, the following approaches could not be successfully established and were hence abandoned: (i) silicone-coated glass wool for equilibration in the water phase; (ii) silicone nets produced by electrospinning for equilibration in the water phase; (iii) chemometers made of different polymers for the water phase, as a chemical proxy for the exposome of diverse tissues of aquatic biota; (iv) identical cleanup methods for chemical analysis and bioanalytical profiling. The ideas behind were: (i) and (ii) to maximize the surface area in contact with the water while allowing high water flow to reduce the water boundary layer and increase uptake kinetics; (iii) to mimic different tissues using diverse polymers that enrich less hydrophobic compounds than the HOCs targeted with silicone; (iv) to use the identical clean-up for all chemometer-based studies which proved impossible in our work so far due to reduced instrument lifetime of samples submitted to the cleanup used for biotesting on the one hand and blank issues of the cleanup used for chemical analysis in bioanalytical assessment on the other hand.110Besides those abandoned research lines, some challenges have been addressed and partially overcome: (i) the impact of co-dosed lipids in cell-based bioassays and (ii) background contamination of silicone mono- and oligomers in the chemometer extracts. Those obstacles have been overcome as follows: (i) the effect of the co-dosed lipids has been quantified and modeled in the bioassays,123 and environmental concentrations can now be corrected for co-extracted lipids; (ii) the co-extraction of silicone mono- and oligomers has been evaluated and discussed in Muz et al.4 and it can partially be solved applying the non-destructive cleanup protocol. In addition, using different solvents for extracting the chemicals from the chemometers could minimize the co-extraction of any polymeric matrix.
Conclusions
Chemometers have proven to be promising tools for environmental and human health assessment of a broad chemical universe of nonpolar HOCs in multimedia environments. As such, they give highly valuable additional information to traditional exhaustive extraction methods, making chemometers in certain cases the superior approach. The unique concept allows for direct comparison of chemical profiles and levels across abiotic and biotic environmental media, across organisms or between organs, over time and space (for temporal and geographical trends) and is expected to generate novel insights into the environmental fate and effects of multiple pollutants. Furthermore, pattern analysis may help to find relevant candidates for the identification of important, yet unknown pollutants. The approach has also proven promising for human health assessment and is being tested within suspect and nontargeted screening. Finally, whereas the focus of CHEMO-RISK was on method development, validation and proof-of-concept of multimedia passive sampling with chemometers, the follow-up project EXPOSO-METER will apply the established chemometers to give a comprehensive overview of the exposome of humans, terrestrial mammals and marine mammals (Fig. 4).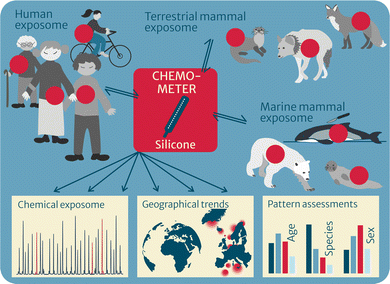 | ||
| Fig. 4 Chemometers to characterize the exposome in top predators such as terrestrial and marine mammals as well as humans. | ||
Author contributions
E. R. N. contributed to conceptualization, methodology, investigation, validation, writing – original draft and supervision. A. J. was responsible for conceptualization, validation, resources, writing – original draft, visualization, supervision, funding acquisition and project administration (https://www.elsevier.com/authors/policies-and-guidelines/credit-author-statement).Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work presented here represents the core outcome of the CHEMO-RISK project (http://www.ufz.de/chemo-risk) supported by the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement no. 715173). Additional work that has been included is part of the Helmholtz Association-funded project EXPOSO-METER (http://www.ufz.de/exposo-meter) supported within the funding program of first-time appointments of excellent women scientists (grant agreement no. W2/W3-126). The manuscript could be improved substantially based on insightful comments by Theo Wernicke, Eva Reiter and Sebastian Abel. We gratefully acknowledge Philipp Mayer for initiating this research line and for his guidance in our first and subsequent steps in this research field. We thank each member of the “Chemometers Group”, two anonymous reviewers and numerous other colleagues for the good discussions and valuable comments.References
- F. Reichenberg and P. Mayer, Environ. Toxicol. Chem., 2006, 25, 1239–1245 CrossRef CAS PubMed.
- P. Mayer, J. Tolls, J. L. M. Hermens and D. Mackay, Environ. Sci. Technol., 2003, 37, 184–191 CrossRef PubMed.
- A. Jahnke and P. Mayer, J. Chromatogr. A, 2010, 1217, 4765–4770 CrossRef CAS PubMed.
- M. Muz, E. Rojo-Nieto and A. Jahnke, Environ. Toxicol. Chem., 2021, 40, 2693–2704 CrossRef CAS PubMed.
- F. Smedes, J. Sobotka, T. P. Rusina, P. Fialová, P. Carlsson, R. Kopp and B. Vrana, Environ. Sci. Technol., 2020, 54, 7942–7951 CrossRef CAS PubMed.
- K. E. C. Smith and Y. Jeong, Environ. Sci. Technol., 2021, 55, 9538–9547 CrossRef CAS PubMed.
- K. E. C. Smith, G. J. Oostingh and P. Mayer, Chem. Res. Toxicol., 2010, 23, 55–65 Search PubMed.
- P. Mayer, J. Wernsing, J. Tolls, P. G. J. de Maagd and D. T. H. M. Sijm, Environ. Sci. Technol., 1999, 33, 2284–2290 CrossRef CAS.
- K. E. C. Smith, N. Dom, R. Blust and P. Mayer, Aquat. Toxicol., 2010, 98, 15–24 CrossRef CAS PubMed.
- P. Mayer and M. Holmstrup, Environ. Sci. Technol., 2008, 42, 7516–7521 CrossRef CAS PubMed.
- E. Rojo-Nieto, K. E. C. Smith, J. A. Perales and P. Mayer, Aquat. Toxicol., 2012, 120–121, 27–34 CrossRef CAS PubMed.
- A. Jahnke, M. S. McLachlan and P. Mayer, Chemosphere, 2008, 73, 1575–1581 CrossRef CAS PubMed.
- F. Smedes, Chemosphere, 2019, 223, 748–757 CrossRef CAS PubMed.
- H. Li, B. Zhang, Y. Wei, F. Wang, M. J. Lydy and J. You, Environ. Sci. Technol., 2014, 48, 6957–6964 CrossRef CAS PubMed.
- K. Mäenpää, M. T. Leppänen, K. Figueiredo, F. Tigistu-Sahle and R. Käkelä, Arch. Environ. Contam. Toxicol., 2015, 68, 193–203 CrossRef PubMed.
- B. I. Escher, C. E. Cowan-Ellsberry, S. Dyer, M. R. Embry, S. Erhardt, M. Halder, J. H. Kwon, K. Johanning, M. T. T. Oosterwijk, S. Rutishauser, H. Segner and J. Nichols, Chem. Res. Toxicol., 2011, 24, 1134–1143 Search PubMed.
- S. Endo, B. Mewburn and B. I. Escher, Chemosphere, 2013, 90, 505–511 CrossRef CAS PubMed.
- Y. Pei, H. Li and J. You, Sci. Total Environ., 2017, 598, 385–392 CrossRef CAS PubMed.
- L. Jin, C. Gaus, L. van Mourik and B. I. Escher, Environ. Sci. Technol., 2013, 47, 7982–7988 CrossRef CAS PubMed.
- F. Smedes, Chemosphere, 2019, 223, 748–757 CrossRef CAS PubMed.
- E. Webster, D. Mackay and K. Qiang, J. Great Lakes Res., 1999, 25, 318–329 CrossRef CAS.
- K. Mäenpää, M. T. Leppänen, F. Reichenberg, K. Figueiredo and P. Mayer, Environ. Sci. Technol., 2011, 45, 1041–1047 CrossRef PubMed.
- F. Reichenberg, F. Smedes, J. A. Jönsson and P. Mayer, Chem. Cent. J., 2008, 2, 1–10 CrossRef PubMed.
- T. Wernicke, S. Abel, B. I. Escher, J. Koschorreck, H. Rüdel and A. Jahnke, Ecotoxicol. Environ. Saf., 2022, 232, 113285 CrossRef CAS PubMed.
- T. Wernicke, E. Rojo-Nieto, A. Paschke, C. Nogueira Tavares, M. Brauns and A. Jahnke, Environ. Sci. Eur., 2022, 34 DOI:10.1186/s12302-022-00644-w.
- L. Niu, J. Ahlheim, C. Glaser, R. Gunold, L. Henneberger, M. König, M. Krauss, M. Schwientek, C. Zarfl and B. I. Escher, Environ. Sci. Technol., 2021, 55, 5106–5116 CrossRef CAS PubMed.
- A. Alkon, R. B. Gunier, K. Hazard, R. Castorina, P. D. Hoffman, R. P. Scott, K. A. Anderson and A. Bradman, J. Pediatr. Health Care, 2022, 36, 34–45 CrossRef PubMed.
- I. J. Allan, K. Bæk, T. O. Haugen, K. L. Hawley, A. S. Høgfeldt and A. D. Lillicrap, Environ. Sci. Technol., 2013, 47, 11660–11667 CrossRef CAS PubMed.
- I. J. Allan, B. Vrana, J. de Weert, A. Kringstad, A. Ruus, G. Christensen, P. Terentjev and N. W. Green, Sci. Rep., 2021, 11, 1–12 CrossRef PubMed.
- T. A. Arcury, H. Chen, S. A. Quandt, J. W. Talton, K. A. Anderson, R. P. Scott, A. Jensen and P. J. Laurienti, Sci. Total Environ., 2021, 763, 144233 CrossRef CAS PubMed.
- A. Baumer, B. I. Escher, J. Landmann and N. Ulrich, Anal. Bioanal. Chem., 2020, 412, 7295–7305 CrossRef CAS PubMed.
- A. Baumer, S. Jäsch, N. Ulrich, I. Bechmann, J. Landmann and B. I. Escher, Environ. Sci. Technol., 2021, 55, 9097–9108 CrossRef CAS PubMed.
- A. Baumer, S. Jäsch, N. Ulrich, I. Bechmann, J. Landmann, A. Stöver and B. I. Escher, Environ. Int., 2021, 157, 106867 CrossRef CAS PubMed.
- B. Beckingham and U. Ghosh, Chemosphere, 2013, 91, 1401–1407 CrossRef CAS PubMed.
- A. J. Bergmann, P. E. North, L. Vasquez, H. Bello, M. del C. G. Ruiz and K. A. Anderson, J. Exposure Sci. Environ. Epidemiol., 2017, 27, 560–568 CrossRef CAS PubMed.
- K. Booij, C. D. Robinson, R. M. Burgess, P. Mayer, C. A. Roberts, L. Ahrens, I. J. Allan, J. Brant, L. Jones, U. R. Kraus, M. M. Larsen, P. Lepom, J. Petersen, D. Pröfrock, P. Roose, S. Schäfer, F. Smedes, C. Tixier, K. Vorkamp and P. Whitehouse, Environ. Sci. Technol., 2016, 50, 3–17 CrossRef CAS PubMed.
- Y. Chen, Y. D. Lei, J. Wensvoort and F. Wania, Environ. Sci. Technol., 2020, 54, 6842–6849 CrossRef CAS PubMed.
- Y. Chen, Y. D. Lei, J. Wensvoort, S. Gourlie and F. Wania, Environ. Sci. Technol., 2022, 56, 9497–9504 CrossRef CAS PubMed.
- M. Claessens, E. Monteyne, K. Wille, L. Vanhaecke, P. Roose and C. R. Janssen, Mar. Pollut. Bull., 2015, 93, 9–19 CrossRef CAS PubMed.
- G. Cornelissen, K. Wiberg, D. A. G. Broman, H. P. H. Arp, Y. Persson, K. Sundqvist and P. Jonsson, Environ. Sci. Technol., 2008, 42, 8733–8739 CrossRef CAS PubMed.
- H. M. Dixon, R. P. Scott, D. Holmes, L. Calero, L. D. Kincl, K. M. Waters, D. E. Camann, A. M. Calafat, J. B. Herbstman and K. A. Anderson, Anal. Bioanal. Chem., 2018, 410, 3059–3071 CrossRef CAS PubMed.
- K. Figueiredo, K. Mäenpää, M. Lyytikäinen, J. Taskinen and M. T. Leppänen, Sci. Total Environ., 2017, 601–602, 340–345 CrossRef CAS PubMed.
- H. E. Fuchte, A. Schäffer, K. Booij and K. E. C. Smith, Environ. Sci. Technol., 2020, 54, 15759–15767 CrossRef CAS PubMed.
- T. Harner, et al., Global Atmosphere Passive Sampling (GAPS) Network, https://gml.noaa.gov/obop/mlo/programs/coop/gaps/gaps.html.
- K. G. Harley, K. L. Parra, J. Camacho, A. Bradman, J. E. S. Nolan, C. Lessard, K. A. Anderson, C. M. Poutasse, R. P. Scott, G. Lazaro, E. Cardoso, D. Gallardo and R. B. Gunier, Sci. Total Environ., 2019, 652, 1022–1029 CrossRef PubMed.
- M. Hendryx, S. Wang, K. A. Romanak, A. Salamova and M. Venier, Environ. Pollut., 2020, 257, 113501 CrossRef CAS PubMed.
- A. Jahnke, P. Mayer, D. Broman and M. S. McLachlan, Chemosphere, 2009, 77, 764–770 CrossRef CAS PubMed.
- A. Jahnke, P. Mayer, M. Adolfsson-Erici and M. S. Mclachlan, Environ. Toxicol. Chem., 2011, 30, 1515–1521 CrossRef CAS PubMed.
- A. Jahnke, P. Mayer and M. S. McLachlan, Environ. Sci. Technol., 2012, 46, 10114–10122 CrossRef CAS PubMed.
- A. Jahnke, P. Mayer, M. S. McLachlan, H. Wickström, D. Gilbert and M. Macleod, Environ. Sci.: Processes Impacts, 2014, 16, 464–472 RSC.
- A. Jahnke, M. MacLeod, H. Wickström and P. Mayer, Environ. Sci. Technol., 2014, 48, 11352–11359 CrossRef CAS PubMed.
- A. Jahnke, A. Sobek, M. Bergmann, J. Bräunig, M. Landmann, S. Schäfer and B. I. Escher, Environ. Sci.: Processes Impacts, 2018, 20, 1667–1679 RSC.
- L. Jin, B. I. Escher, C. J. Limpus and C. Gaus, Chemosphere, 2015, 138, 292–299 CrossRef CAS PubMed.
- M. T. O. Jonker, S. A. Van Der Heijden, D. Adelman, J. N. Apell, R. M. Burgess, Y. Choi, L. A. Fernandez, G. M. Flavetta, U. Ghosh, P. M. Gschwend, S. E. Hale, M. Jalalizadeh, M. Khairy, M. A. Lampi, W. Lao, R. Lohmann, M. J. Lydy, K. A. Maruya, S. A. Nutile, A. M. P. Oen, M. I. Rakowska, D. Reible, T. P. Rusina, F. Smedes and Y. Wu, Environ. Sci. Technol., 2018, 52, 3574–3582 CrossRef CAS PubMed.
- M. Khairy, D. Muir, C. Teixeira and R. Lohmann, Environ. Sci. Technol., 2015, 49, 13787–13797 CrossRef CAS PubMed.
- M. A. Khairy and R. Lohmann, Environ. Sci. Technol., 2017, 51, 9062–9071 CrossRef CAS PubMed.
- M. L. Kile, R. P. Scott, S. G. O’Connell, S. Lipscomb, M. MacDonald, M. McClelland and K. A. Anderson, Environ. Res., 2016, 147, 365–372 CrossRef CAS PubMed.
- S. C. Lang, P. Mayer, A. Hursthouse, D. Kötke, I. Hand, D. Schulz-Bull and G. Witt, Chemosphere, 2018, 191, 886–894 CrossRef CAS PubMed.
- W. Lao, Y. Hong, D. Tsukada, K. A. Maruya and J. Gan, Environ. Sci. Technol., 2016, 50, 13470–13476 CrossRef CAS PubMed.
- J. Y. Li, J. Y. M. Tang, L. Jin and B. I. Escher, Environ. Toxicol. Chem., 2013, 32, 2888–2896 CrossRef CAS PubMed.
- R. Lohmann, J. Klanova, P. Kukucka, S. Yonis and K. Bollinger, Environ. Sci. Technol., 2012, 46, 10471–10479 CrossRef CAS PubMed.
- R. Lohmann, J. Klanova, P. Kukucka, S. Yonis and K. Bollinger, Environ. Sci. Technol., 2013, 47, 13967–13975 CrossRef CAS PubMed.
- K. Mäenpää, M. T. Leppänen, K. Figueiredo, P. Mayer, D. Gilbert, A. Jahnke, C. Gil-Allué, J. Akkanen, I. Nybom and S. Herve, Environ. Toxicol. Chem., 2015, 34, 2463–2474 CrossRef PubMed.
- C. A. McDonough, G. Puggioni, P. A. Helm, D. Muir and R. Lohmann, Environ. Sci. Technol., 2016, 50, 9133–9141 CrossRef CAS PubMed.
- J. Meierdierks, C. Zarfl, B. Beckingham and P. Grathwohl, Environ. Sci.: Atmos., 2021, 1, 253–266 CAS.
- J. Meierdierks, C. Zarfl, B. Beckingham and P. Grathwohl, ACS Environ. Au, 2022, 2(6), 536–548 CrossRef CAS.
- R. O. Meire, M. Khairy, A. C. Targino, P. M. A. Galvão, J. P. M. Torres, O. Malm and R. Lohmann, Chemosphere, 2016, 144, 2175–2182 CrossRef CAS PubMed.
- L. Mustajärvi, A. K. Eriksson-Wiklund, E. Gorokhova, A. Jahnke and A. Sobek, Environ. Sci.: Processes Impacts, 2017, 19, 1404–1413 RSC.
- M. Muz, B. I. Escher and A. Jahnke, Environ. Sci. Technol., 2020, 54, 15861–15871 CrossRef CAS PubMed.
- L. v Nguyen, S. Gravel, F. Labrèche, B. Bakhiyi, M. A. Verner, J. Zayed, L. M. Jantunen, V. H. Arrandale and M. L. Diamond, Environ. Sci. Technol., 2020, 54, 15277–15286 CrossRef CAS PubMed.
- L. Niu, E. Carmona, M. König, M. Krauss, M. Muz, C. Xu, D. Zou and B. I. Escher, Environ. Sci. Technol., 2020, 54, 13197–13206 CrossRef CAS PubMed.
- M. G. Pintado-Herrera, I. J. Allan, E. González-Mazo and P. A. Lara-Martín, Environ. Sci. Technol., 2020, 54, 6693–6702 CrossRef CAS PubMed.
- C. Reche, M. Viana, B. L. van Drooge, F. J. Fernández, M. Escribano, G. Castaño-Vinyals, M. Nieuwenhuijsen, P. E. Adami and S. Bermon, Sci. Total Environ., 2020, 717, 137161 CrossRef CAS PubMed.
- A. Reddam, G. Tait, N. Herkert, S. C. Hammel, H. M. Stapleton and D. C. Volz, Environ. Int., 2020, 136, 105499 CrossRef CAS PubMed.
- E. B. Reiter, B. I. Escher, U. Siebert and A. Jahnke, Environ. Int., 2022, 165, 107337 CrossRef CAS PubMed.
- D. Rohlman, H. M. Dixon, L. Kincl, A. Larkin, R. Evoy, M. Barton, A. Phillips, E. Peterson, C. Scaffidi, J. B. Herbstman, K. M. Waters and K. A. Anderson, BMC Public Health, 2019, 19 DOI:10.1186/s12889-019-7217-z.
- E. Rojo-Nieto and J. A. Perales, Environ. Sci.: Processes Impacts, 2015, 17, 1331–1339 RSC.
- E. Rojo-Nieto, M. Muz, J. Koschorreck, H. Rüdel and A. Jahnke, Chemosphere, 2019, 220, 501–504 CrossRef CAS PubMed.
- Z. Ruge, D. Muir, P. Helm and R. Lohmann, Environ. Sci. Technol., 2015, 49, 13777–13786 CrossRef CAS PubMed.
- T. P. Rusina, P. Carlsson, B. Vrana and F. Smedes, Environ. Sci. Technol., 2017, 51, 11250–11257 CrossRef CAS PubMed.
- V. P. Sacks and R. Lohmann, Environ. Pollut., 2012, 162, 287–293 CrossRef CAS PubMed.
- S. M. Samon, S. C. Hammel, H. M. Stapleton and K. A. Anderson, Environ. Int., 2022, 169 Search PubMed.
- S. Schäfer, C. Antoni, C. Möhlenkamp, E. Claus, G. Reifferscheid, P. Heininger and P. Mayer, Chemosphere, 2015, 138, 856–862 CrossRef PubMed.
- J. K. Schuster, T. Harner, A. Eng, C. Rauert, K. Su, K. C. Hornbuckle and C. W. Johnson, Environ. Sci. Technol., 2021, 55, 9479–9488 CrossRef CAS PubMed.
- F. Smedes, L. A. van Vliet and K. Booij, Environ. Sci. Technol., 2013, 47, 510–517 CrossRef CAS PubMed.
- F. Smedes, T. P. Rusina, P. Fialova, R. Kopp and B. Vrana, Environ. Sci. Technol., 2020, 54, 7942–7951 CrossRef CAS PubMed.
- B. Strandberg, C. Österman, H. Koca Akdeva, J. Moldanová and S. Langer, Polycyclic Aromat. Compd., 2022, 42, 448–459 CrossRef CAS.
- L. Tuduri, T. Harner and H. Hung, Environ. Pollut., 2006, 144, 377–383 CrossRef CAS PubMed.
- S. C. Travis, D. S. Aga, E. I. Queirolo, J. R. Olson, M. Daleiro and K. Kordas, Sci. Total Environ., 2020, 740, 140136 CrossRef CAS PubMed.
- B. Vrana, F. Smedes, I. Allan, T. Rusina, K. Okonski, K. Hilscherová, J. Novák, P. Tarábek and J. Slobodník, Sci. Total Environ., 2018, 636, 1597–1607 CrossRef CAS PubMed.
- B. Vrana, T. Rusina, K. Okonski, R. Prokeš, P. Carlsson, R. Kopp and F. Smedes, Sci. Total Environ., 2019, 664, 424–435 CrossRef CAS PubMed.
- Y. Wang, A. Peris, M. R. Rifat, S. I. Ahmed, N. Aich, L. V. Nguyen, J. Urík, E. Eljarrat, B. Vrana, L. M. Jantunen and M. L. Diamond, Sci. Total Environ., 2020, 720, 137480 CrossRef CAS PubMed.
- C. F. Wise, S. C. Hammel, N. J. Herkert, M. Ospina, A. M. Calafat, M. Breen and H. M. Stapleton, Environ. Sci. Technol., 2022, 56, 1149–1161 CrossRef CAS PubMed.
- Q. Xie, Q. Guan, L. Li, X. Pan, C. L. Ho, X. Liu, S. Hou and D. Chen, Environ. Pollut., 2021, 282, 117011 CrossRef CAS PubMed.
- A. S. Young, N. Herkert, H. M. Stapleton, J. G. Cedeño Laurent, E. R. Jones, P. MacNaughton, B. A. Coull, T. James-Todd, R. Hauser, M. L. Luna, Y. S. Chung and J. G. Allen, Environ. Int., 2021, 156, 106727 CrossRef CAS PubMed.
- T. P. Rusina, P. Carlsson, B. Vrana and F. Smedes, Environ. Sci. Technol., 2017, 51, 11250–11257 CrossRef CAS PubMed.
- K. Booij, F. Smedes and E. M. van Weerlee, Chemosphere, 2002, 46, 1157–1161 CrossRef CAS PubMed.
- F. A. P. C. Gobas, X. Zhang and R. Wells, Environ. Sci. Technol., 1993, 27, 2855–2863 CrossRef CAS.
- L. P. Burkhard, J. A. Arnot, M. R. Embry, K. J. Farley, R. A. Hoke, M. Kitano, H. A. Leslie, G. R. Lotufo, T. F. Parkerton, K. G. Sappington, G. T. Tomy and K. B. Woodburn, Integr. Environ. Assess. Manage., 2012, 8, 17–31 CrossRef CAS PubMed.
- A. M. H. DeBruyn and F. A. P. C. Gobas, Environ. Toxicol. Chem., 2007, 26, 1803–1808 CrossRef CAS PubMed.
- A. Jahnke, J. Holmbäck, R. A. Andersson, A. Kierkegaard, P. Mayer and M. MacLeod, Environ. Sci. Technol. Lett., 2015, 2, 193–197 CrossRef CAS.
- K. Mäenpää, M. T. Leppänen, K. Figueiredo, F. Tigistu-Sahle and R. Käkelä, Arch. Environ. Contam. Toxicol., 2015, 68, 193–203 CrossRef PubMed.
- E. Rojo-Nieto, et al., From TMFs to multimedia activity ratios: chemometers as a versatile tool to study bioaccumulation of HOCs in aquatic ecosystems (in prep.).
- I. J. Allan, B. Vrana and A. Ruus, Environ. Sci. Technol., 2022, 56, 7945–7953 CrossRef CAS PubMed.
- I. J. Allan, K. Bæk, T. O. Haugen, K. L. Hawley, A. S. Høgfeldt and A. D. Lillicrap, Environ. Sci. Technol., 2013, 47, 11660–11667 CrossRef CAS PubMed.
- M. Adolfsson-Erici, G. Åkerman and M. S. McLachlan, Chemosphere, 2012, 88, 62–68 CrossRef CAS PubMed.
- S. G. O’Connell, N. I. Kerkvliet, S. Carozza, D. Rohlman, J. Pennington and K. A. Anderson, Environ. Int., 2015, 85, 182–188 CrossRef PubMed.
- J. F. Narvaez Valderrama, et al., 13th IPSW 2022, in Abstract book – Conference proceedings.
- B. I. Escher and J. L. M. Hermens, Environ. Sci. Technol., 2004, 38, 455A–462A CrossRef CAS PubMed.
- E. B. Reiter, et al., Chemical Profiling and Bioanalytical Measurement of Passive Sampling Extracts of Different Organs from Marine Mammals (under review).
- P. A. Neale, S. Ait-Aissa, W. Brack, N. Creusot, M. S. Denison, B. Deutschmann, K. Hilscherová, H. Hollert, M. Krauss, J. Novák, T. Schulze, T. B. Seiler, H. Serra, Y. Shao and B. I. Escher, Environ. Sci. Technol., 2015, 49, 14614–14624 CrossRef CAS PubMed.
- S. Abel, et al., Comparing Non-Invasive Passive Sampling Approaches to Monitor Human Exposure to Environmental Contaminants (in prep.).
- S. Abel, et al., SETAC Europe 32 Annual Meeting. Abstract book – Conference proceedings (223–224), 2022.
- I. J. Allan, K. Bæk, A. Kringstad, H. E. Roald and K. V. Thomas, Environ. Int., 2013, 59, 462–468 CrossRef CAS PubMed.
- D. Gilbert, G. Witt, F. Smedes and P. Mayer, Anal. Chem., 2016, 88, 5818–5826 CrossRef CAS PubMed.
- K. Booij, F. Smedes and I. J. Allan, Guidelines for determining polymer-water and polymer-polymer partition coefficients of organic compounds, ICES Techniques in Marine Environmental Sciences, 2017, vol. 61., 2017 Search PubMed.
- F. Smedes and K. Booij, ICES Techniques in Marine Environmental Sciences, 2012, vol. 52, p. 20 Search PubMed.
- M. Muz, et al., Investigating patterns of known and unknown chemicals across samples and media using chemometers (in prep.).
- L. L. Hohrenk-Danzouma, M. Vosough, V. I. Merkus, F. Drees and T. C. Schmidt, Environ. Sci. Technol., 2022, 56, 5466–5477 CrossRef CAS PubMed.
- X. Gao, P. Huang, Q. Huang, K. Rao, Z. Lu, Y. Xu, G. W. Gabrielsen, I. Hallanger, M. Ma and Z. Wang, Environ. Pollut., 2019, 253, 1–10 CrossRef CAS PubMed.
- R. Guibal, S. Lissalde, A. Charriau, G. Poulier, N. Mazzella and G. Guibaud, J. Chromatogr. A, 2015, 1387, 75–85 CrossRef CAS PubMed.
- V. J. Schacht, et al., Chemometers for exploring patterns of known and unknown chemicals in organs of marine mammals (in prep.).
- E. B. Reiter, A. Jahnke, M. König, U. Siebert and B. I. Escher, Environ. Sci. Technol., 2020, 54, 4240–4247 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |