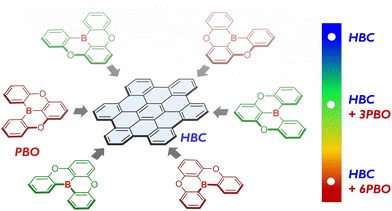
Distorted B/O-containing nanographenes with tunable optical properties†
Jiaxiang
Guo
a,
Tianyu
Zhang
a,
Zeyi
Li
a,
Kaiqi
Ye
 a,
Yue
Wang
a,
Yue
Wang
 a and
Chuandong
Dou
a and
Chuandong
Dou
 *ab
*ab
aState Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun 130012, P. R. China. E-mail: chuandong@jlu.edu.cn
bJiangsu Engineering Laboratory of Novel Functional Polymeric Materials, Soochow University, Suzhou, 215123, P. R. China
First published on 6th February 2023
Abstract
We report the synthesis of two B/O-containing nanographenes, which feature the fusion of three or six planar B/O-heterocycles onto one hexa-peri-hexabenzocoronene π-framework. Incorporation of the B/O-heterocycles not only leads to distorted geometries, but also modulates the electronic structures and results in gradually red-shifted absorptions and fluorescence.
Polycyclic aromatic hydrocarbons (PAHs) and synthetic nanographenes (NGs) have received great attention owing to their intriguing topological structures and widespread applications in electronics, spintronics and bioimaging.1 Incorporating heterocycles is an efficient strategy not only to construct diverse structures but also to modulate the electronic nature and properties.2 Indeed, a variety of heterocyclic NGs containing B, N, O, P or S atoms have been dramatically developed, which display interesting optical and electronic properties.3 Moreover, nonplanar or contorted PAHs possess unusual conformations, molecular stacking modes and functions, such as disturbed π–π stacking, which is of importance for supramolecular assembly or semiconductors employing intermolecular electronic interactions.4 Therefore, the construction of distorted heterocyclic NGs has emerged as an attractive research topic from both the synthetic chemistry and materials science point of view.
Hexa-peri-hexabenzocoronene (HBC), owning a planar configuration with a diameter of over 1 nm, is one of the most representative NG molecules.5 Introducing non-hexagonal rings or defective edges into the HBC scaffold has afforded various structure-extended NGs with amazing nonplanar topologies.6 For instance, chiral NGs and even graphene nanoribbons containing single/multiple helical regions have been developed as a novel series of circularly polarized luminescence emitters.6b,d Severely contorted or negatively curved NGs based on HBC have also been synthesized, which display attractive electronic structures and molecular assembly behaviours.6e However, HBC-based heterocyclic NGs are rarely explored, though incorporation of heterocycles onto HBC may modulate its molecular conformation and alter its physical properties. Typically, hexa-peri-hexabenzoborazinocoronene bearing an internal B3N3 ring, prepared using solution-phase and on-surface synthetic chemistry, exhibits a larger energy gap in comparison to all-carbon HBC.7
Working in a research area of heterocyclic analogs of polycyclic hydrocarbons, we recently reported rapid π-extension of conjugated organoboranes as an effective methodology for creating nanoscale B-doped PAHs.8 One example is a new kind of pristine B-doped molecular ribbon, which not only has contorted geometries with up to 2.2 nm in length but also displays unexpected photochromism properties and charge-transporting properties. In this study, we disclose hybridization of HBC and B/O-heterocycles to design distorted heterocyclic NGs (Fig. 1). Two B/O-containing NGs (HBC-3BO and HBC-6BO) that feature the fusion of three or six planar B/O-heterocycles (PBO) onto one HBC π-framework were synthesized. These B/O-heterocycles produce distorted configurations owing to the formation of multiple helical regions, and moreover, modulate their electronic structures and thus optical properties, as revealed by detailed theoretical and experimental studies.
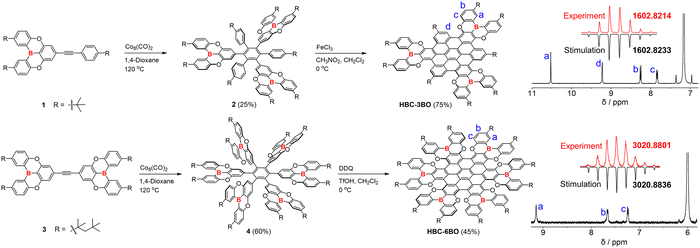
Scheme 1 shows the synthesis of these two B/O-containing NGs, in which the B/O-heterocycle was first reported by Hatakeyama et al. and was recently employed to develop B-containing organic diradicaloids.9,10 A cyclotrimerization reaction of alkyne derivative 1 with Co2(CO)8 as the catalyst afforded key precursor 2 in 25% yield. Intramolecular cyclodehydrogenation (i.e., the Scholl reaction) of 2 with an excess of FeCl3 produced HBC-3BO in 75% yield as an orange-yellow solid. For HBC-6BO, the branched alkyl chains were used to enhance its solubility. The cyclotrimerization reaction was similarly performed on 3 to form 4. In the cyclodehydrogenation reaction of 4, 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) and trifluoromethanesulfonic acid (TfOH) were used to efficiently generate HBC-6BO as a pink solid in 45% yield, whereas FeCl3 led to a mixture of fully and partially fused products.11 These two B/O-containing NGs have good stability and can be purified by silica gel column chromatography under ambient conditions.
The structures of HBC-3BO and HBC-6BO were confirmed by NMR spectroscopy and high-resolution mass spectrometry (HRMS). The HRMS spectra exhibit intense signals at m/z = 1602.8214 for HBC-3BO and 3020.8801 for HBC-6BO along with clear isotopic distributions, which agree well with the calculated molecular mass and simulated patterns of their molecular formula of C114H105B3O6 and C210H240B6O12, respectively (Scheme 1). The well-resolved 1H NMR spectra of HBC-3BO and HBC-6BO were recorded in C6D6 and C2D2Cl4 at room temperature, respectively. It is notable that HBC-3BO and HBC-6BO exhibit only four and three different signals in the aromatic region, respectively, which are clearly assigned to the protons. These simple proton signals also demonstrate their highly symmetric structures in solution.
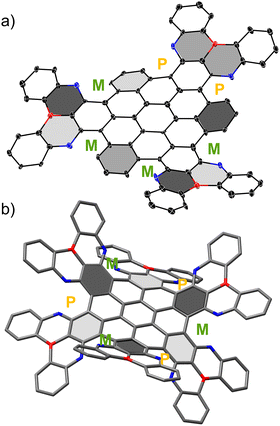
Single crystals of HBC-3BO suitable for X-ray structural analysis were obtained by slow diffusion of CH3OH into its CHCl3 solution. Twenty-five hexagons are fused together to form a large skeleton that features the fusion of three PBO moieties onto HBC with the formation of six cove regions. Thus, a distorted geometry with the (P, P, M, M, M, and M) conformation is obtained due to the steric repulsion in the cove regions (Fig. 2).
Torsional angles of these helical regions range from 6.5(17)° to 23.4(15)°, indicative of the moderately contorted topology of the framework and facile conversion from one conformation to others (Fig. S1, ESI†). Despite the distorted configuration, HBC-3BO shows a slipped face-to-face packing mode, accompanied by a large π–π overlap and a small π–π stacking distance of 3.35 Å (Fig. S3, ESI†). For HBC-6BO, we did not obtain its single crystals due to the flexible branched alkyl chains. We thus optimized its model compound HBC-6BO′ for structural analysis. According to the density functional theory (DFT) calculations (B3LYP/6-311G(d) level), there are fourteen possible stereoisomers caused by B/O-containing [5]-helicenes, and notably, the waggling (P, M, P, M, P, and M) conformation has the lowest total energy (Fig. S8, ESI†). Six PBO moieties adopt an “up-down” arrangement with an enhanced torsional angle of 38.3°.12 In addition, the B/O-containing [5]-helicene, as the structural fragment of HBC-6BO′, has a theoretically estimated activation energy (ΔG+) of 20.8 kcal mol−1 for conformational conversion, which is slightly smaller than that (24.1 kcal mol−1) of all-carbon [5]-helicene (Fig. S9, ESI†). These relatively small ΔG+ values suggest that the conformations of HBC-6BO may show facile interconversion in solution, which is consistent with the 1H NMR result.
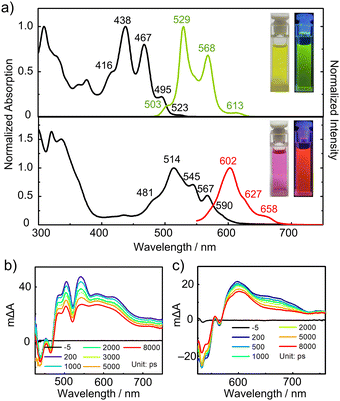
The optical properties of these B/O-containing NGs are greatly influenced by the B/O-heterocycles. As shown in Fig. 3, HBC-3BO and HBC-6BO in toluene display yellow and purple colors, respectively, and well-resolved vibronic absorption spectra. While HBC-3BO exhibits a main adsorption band with maxima (λabs) at 438/467 nm along with shoulder peaks at 416/495/523 nm, HBC-6BO shows a absorption maxima at 514/545 nm and shoulder peaks at 481/567/590 nm. According to the onset adsorption, the optical band gap (Egopt) values are calculated to be 2.44 eV for HBC-3BO and 2.10 eV for HBC-6BO. Strong green and red fluorescence is observed for their toluene solutions, respectively. In the fluorescence spectra, HBC-3BO has maximum emission peaks (λem) at 529/568 nm and weak tails at 503/613 nm, whereas HBC-6BO exhibits one main emission band at 602 nm along with shoulder peaks at 627/658 nm. The fluorescence quantum yields and lifetimes are determined to be 18% and 4.6/21.4 ns for HBC-3BO and 20% and 5.7/10.2 ns for HBC-6BO (Fig. S6, ESI†). In comparison to HBC containing six t-butyl groups (tBu-HBC), HBC-3BO and HBC-6BO display significantly red-shifted absorption bands by 78 and 154 nm, respectively (Table S1, ESI†).13 Moreover, from tBu-HBC to HBC-3BO and HBC-6BO, the main emission bands are bathochromically shifted by 36 and 109 nm, respectively, along with increased fluorescence quantum yields. The remarkably red-shifted absorption and emissions for B/O-containing NGs can be attributed to the great electronic effects of B/O-heterocycles (vide infra).
The excited-state dynamics were investigated by performing transient absorption (TA) spectroscopy measurements on HBC-3BO and HBC-6BO in toluene. As shown in the TA spectra (Fig. 3b and c), broad excited-state absorption (ESA) bands are observed at 480–740 nm for HBC-3BO and 560–740 nm for HBC-6BO. The ground-state bleach (GSB) signals appear at 425–480 nm for HBC-3BO and at 520–550 nm for HBC-6BO, which match well with their low-energy absorption peaks of the steady-state absorption spectra, respectively. While the stimulated emission (SE) signal is observed at 528 nm as a negative and overwhelming bump for HBC-3BO, such a signal of HBC-6BO cannot be detected probably because of the complete overwhelming by its ESA band.14 The excited-state lifetimes (τ) are determined to be 17.6 ps and 21.8 ns for HBC-3BO and 61.0 ps and 24.8 ns for HBC-6BO, respectively, based on the fitting of the decay profiles by a two-exponential function (Fig. S7, ESI†). These short and relatively long lifetimes can be ascribed to internal conversion and radiative relaxation processes, respectively.15
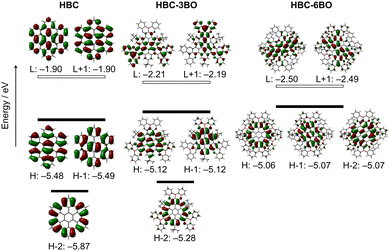
To elucidate the effects of the B/O-heterocycles on the electronic structure of HBC, we performed density functional theory (DFT) calculations at the B3LYP/6-311G(d) level of theory on the model compounds HBC, HBC-3BO′ and HBC-6BO’. Their Kohn–Sham molecular orbitals from HOMO−2 to LUMO+1 are shown in Fig. 4. While the LUMO and LUMO+1 as well as the HOMO and HOMO−1 in HBC are well delocalized on the π-skeleton and almost identical in energy, the HOMO−2 with a lower energy is localized on its peripheral region. From HBC to HBC-3BO′, the distributions of the HOMO, HOMO−1 and HOMO−2 are slightly modulated only with elevated energies by 0.3–0.6 eV. In contrast, the LUMO and LUMO+1 are lowered by 0.3 eV in energy along with their distributions extending to the B/O-heterocycles, in which the vacant p orbital of the B atom contributes significantly. From HBC-3BO′ to HBC-6BO′, the LUMO and LUMO+1 are further decreased by 0.3 eV in energy accompanied by the perturbed distributions, and the HOMO, HOMO−1 and HOMO−2 have almost identical energy due to the obviously increased energy by 0.22 eV for the HOMO−2. As a result, from HBC to HBC-3BO′ and HBC-6BO′, the energy gap is gradually decreased. These changes indicate that incorporation of the B/O-heterocycles onto HBC significantly impacts its electronic structure, especially the electronic energy levels and distributions of molecular orbitals, which can be attributed to the synergetic effects of the electron-accepting B atoms and electron-donating O atoms in the B/O-heterocycles.
To gain insight into the absorption properties of these B/O-containing NGs, we conducted time-dependent DFT (TD-DFT) calculations on HBC-3BO′ and HBC-6BO′. The absorption band at 438/467 nm of HBC-3BO is assignable to the allowed electronic transitions of S0 → S3, S0 → S4, S0 → S5 and S0 → S6 (Fig. S10, ESI†). For HBC-6BO, the absorbance observed around 514/545 nm is assigned to the transitions of S0 → S5 and S0 → S6 (Fig. S11, ESI†). The theoretical spectra including energies, wavelengths and oscillator strengths agree well with the experimental results. It is noteworthy that their LUMO and LUMO+1 are all involved in these electronic transitions. It is thus suggested that the B atom in the B/O-heterocycle plays an important role in producing the red-shifted absorption of B/O-containing NGs.
In summary, we synthesized two B/O-containing nanographenes, HBC-3BO and HBC-6BO, which feature the fusion of three or six planar B/O-heterocycles onto one HBC π-framework. Incorporation of the B/O-heterocycles leads to multiple helical regions, thus producing their obviously distorted geometries. Moreover, these B/O-heterocycles greatly impact on the electronic structure of HBC and thus give rise to the gradually red-shifted absorptions and fluorescence of B/O-containing NGs, for instance, the maximum emission peak is 493 nm for tBu-HBC, 529 nm for HBC-3BO and 602 nm for HBC-6BO. This study thus proves that hybridization of B/O-heterocycles and π-systems is a promising strategy toward the construction of distorted heterocyclic NGs together with modulations of the electronic structures and properties, which may be applied to the production of sophisticated π materials.
Support has been provided by the Jilin Scientific and Technological Development Program (No. 20220101054JC) and the National Natural Science Foundation of China (No. 22175074). We thank the crystallography reviewer for his kind help on the single-crystal data.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) A. Narita, X.-Y. Wang, X. Feng and K. Müllen, Chem. Soc. Rev., 2015, 44, 6616–6643 RSC; (b) M. Grzybowski, B. Sadowski, H. Butenschön and D. T. Gryko, Angew. Chem., Int. Ed., 2020, 59, 2998–3027 CrossRef CAS PubMed; (c) Y. Segawa, H. Ito and K. Itami, Nat. Rev. Mater., 2016, 1, 15002 CrossRef CAS; (d) J. Liu and X. Feng, Angew. Chem., Int. Ed., 2020, 59, 23386–23401 CrossRef CAS PubMed.
- (a) M. Hirai, N. Tanaka, M. Sakai and S. Yamaguchi, Chem. Rev., 2019, 119, 8291–8331 CrossRef CAS PubMed; (b) A. Borissov, Y. K. Maurya, L. Moshniaha, W.-S. Wong, M. Żyła-Karwowska and M. Stępień, Chem. Rev., 2022, 122, 565–788 CrossRef CAS PubMed; (c) W. Jiang, Y. Li and Z. Wang, Acc. Chem. Res., 2014, 47, 3135–3147 CrossRef CAS PubMed.
- (a) S. R. Peurifoy, T. J. Sisto, F. Ng, M. L. Steigerwald, R. Chen and C. Nuckolls, Chem. Rec., 2019, 19, 1050–1061 CrossRef CAS PubMed; (b) X.-Y. Wang, X. Yao, A. Narita and K. Müllen, Acc. Chem. Res., 2019, 52, 2491–2505 CrossRef CAS PubMed; (c) E. von Grotthuss, A. John, T. Kaese and M. Wagner, Asian J. Org. Chem., 2018, 7, 37–53 CrossRef; (d) X. Yang, S. M. Elbert, F. Rominger and M. Mastalerz, J. Am. Chem. Soc., 2022, 144, 9883–9892 CrossRef CAS PubMed; (e) S. Seifert, K. Shoyama, D. Schmidt and F. Würthner, Angew. Chem., Int. Ed., 2016, 55, 6390–6395 CrossRef CAS PubMed.
- (a) I. R. Márquez, S. Castro-Fernández, A. Millán and A. G. Campaña, Chem. Commun., 2018, 54, 6705–6718 RSC; (b) Chaolumen, I. A. Stepek, K. E. Yamada, H. Ito and K. Itami, Angew. Chem., Int. Ed., 2021, 60, 23508–23532 CrossRef CAS PubMed; (c) R. K. Dubey, M. Melle-Franco and A. Mateo-Alonso, J. Am. Chem. Soc., 2021, 143, 6593–6600 CrossRef CAS PubMed; (d) J. Liu, B.-W. Li, Y.-Z. Tan, A. Giannakopoulos, C. Sanchez-Sanchez, D. Beljonne, P. Ruffieux, R. Fasel, X. Feng and K. Müllen, J. Am. Chem. Soc., 2015, 137, 6097–6103 CrossRef CAS PubMed; (e) Y. Tanaka, N. Fukui and H. Shinokubo, Nat. Commun., 2020, 11, 3873 CrossRef CAS PubMed; (f) Y. Chen, C. Lin, Z. Luo, Z. Yin, H. Shi, Y. Zhu and J. Wang, Angew. Chem., Int. Ed., 2021, 60, 7796–7801 CrossRef CAS PubMed; (g) W. Zeng, H. Phan, T. S. Herng, T. Y. Gopalakrishna, N. Aratani, Z. Zeng, H. Yamada, J. Ding and J. Wu, Chem, 2017, 2, 81–92 CrossRef CAS; (h) S. H. Pun and Q. Miao, Acc. Chem. Res., 2018, 51, 1630–1642 CrossRef CAS PubMed.
- J. Wu, W. Pisula and K. Müllen, Chem. Rev., 2007, 107, 718–747 CrossRef CAS PubMed.
- (a) Y. Zhang, S. H. Pun and Q. Miao, Chem. Rev., 2022, 122, 14554–14593 CrossRef CAS PubMed; (b) C. Cruz, S. Castro-Fernández, E. Maçôas, A. Millán and A. Campaña, Synlett, 2019, 997–1002 CAS; (c) W.-S. Wong and M. Stępień, Trends Chem., 2022, 4, 573–576 CrossRef CAS; (d) Y. Zhu, X. Guo, Y. Li and J. Wang, J. Am. Chem. Soc., 2019, 141, 5511–5517 CrossRef CAS PubMed; (e) S. H. Pun, C. K. Chan, J. Luo, Z. Liu and Q. Miao, Angew. Chem., Int. Ed., 2018, 57, 1581–1586 CrossRef CAS PubMed.
- (a) M. Krieg, F. Reicherter, P. Haiss, M. Ströbele, K. Eichele, M.-J. Treanor, R. Schaub and H. F. Bettinger, Angew. Chem., Int. Ed., 2015, 54, 8284–8286 CrossRef CAS PubMed; (b) J. Dosso, J. Tasseroul, F. Fasano, D. Marinelli, N. Biot, A. Fermi and D. Bonifazi, Angew. Chem., Int. Ed., 2017, 56, 4483–4487 CrossRef CAS PubMed.
- (a) W. Sun, J. Guo, Z. Fan, L. Yuan, K. Ye, C. Dou and Y. Wang, Angew. Chem., Int. Ed., 2022, 61, e2022092 Search PubMed; (b) L. Yuan, J. Guo, Y. Yang, K. Ye, C. Dou and Y. Wang, CCS Chem., 2022 DOI:10.31635/ccschem.022.202101738.
- H. Hirai, K. Nakajima, S. Nakatsuka, K. Shiren, J. Ni, S. Nomura, T. Ikuta and T. Hatakeyama, Angew. Chem., Int. Ed., 2015, 54, 13581–13585 CrossRef CAS PubMed.
- (a) J. Guo, Y. Yang, C. Dou and Y. Wang, J. Am. Chem. Soc., 2021, 143, 18272–18279 CrossRef CAS PubMed; (b) X. Tian, J. Guo, W. Sun, L. Yuan, C. Dou and Y. Wang, Chem. – Eur. J., 2022, 28, e202200045 CAS.
- (a) X. Yang, F. Rominger and M. Mastalerz, Angew. Chem., Int. Ed., 2019, 58, 17577–17582 CrossRef CAS PubMed; (b) T. Dumslaff, Y. Gu, G. M. Paternò, Z. Qiu, A. Maghsoumi, M. Tommasini, X. Feng, F. Scotognella, A. Narita and K. Müllen, Chem. Sci., 2020, 11, 12816–12821 RSC.
- (a) M. Żyła-Karwowska, H. Zhylitskaya, J. Cybińska, T. Lis, P. J. Chmielewski and M. Stępień, Angew. Chem., Int. Ed., 2016, 55, 14658–14662 CrossRef PubMed; (b) M. Navakouski, H. Zhylitskaya, P. J. Chmielewski, T. Lis, J. Cybińska and M. Stępień, Angew. Chem., Int. Ed., 2019, 58, 4929–4933 CrossRef CAS PubMed.
- J. M. Fernández-García, P. J. Evans, S. Medina Rivero, I. Fernández, D. García-Fresnadillo, J. Perles, J. Casado and N. Martín, J. Am. Chem. Soc., 2018, 140, 17188–17196 CrossRef PubMed.
- T. Dumslaff, Y. Gu, G. M. Paternò, Z. Qiu, A. Maghsoumi, M. Tommasini, X. Feng, F. Scotognella, A. Narita and K. Müllen, Chem. Sci., 2020, 11, 12816–12821 RSC.
- G. M. Paternò, Q. Chen, X.-Y. Wang, J. Liu, S. G. Motti, A. Petrozza, X. Feng, G. Lanzani, K. Müllen, A. Narita and F. Scotognella, Angew. Chem., Int. Ed., 2017, 56, 6753–6757 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, thermal and photophysical properties, single-crystal analysis, and theoretical calculations. CCDC 2221600. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2cc06376j |
| This journal is © The Royal Society of Chemistry 2023 |