Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A NIR fluorescent probe for the detection of renal damage based on overrepresentation of alanine aminopeptidase enzyme†
Marcia
Domínguez
ab,
Kathleen
Meyer
c,
Félix
Sancenón
abde,
Juan F.
Blandez
*abd,
Manuel
Serrano
*cf and
Ramón
Martínez-Máñez
 *abde
*abde
aInstituto Interuniversitario de Investigación de Reconocimiento Molecular y Desarrollo Tecnológico (IDM), Universitat Politècnica de València, Universitat de València, Spain
bCIBER de Bioingeniería, Biomateriales y Nanomedicina, Instituto de Salud Carlos III, Spain
cInstitute for Research in Biomedicine (IRB Barcelona), Barcelona Institute of Science and Technology (BIST), Barcelona, 08028, Spain. E-mail: manuel.serrano@irbbarcelona.org
dUnidad Mixta de Investigación en Nanomedicina y Sensores, Universitat Politècnica de València, Instituto de Investigación Sanitaria La Fe, Spain. E-mail: juablaba@upvnet.upv.es; rmaez@qim.upv.es
eUnidad Mixta UPV-CIPF de Investigación en Mecanismos de Enfermedades y Nanomedicina, Universitat Politècnica de València, Centro de Investigación Príncipe Felipe, Spain
fCatalan Institution for Research and Advanced Studies (ICREA), Barcelona, 08010, Spain
First published on 31st January 2023
Abstract
Kidney damage generates changes at the phenotypic and genotypic levels that allow its monitoring using different biomarkers in blood, urine or serum. Among these biomarkers, kidney failure causes the urine overrepresentation of the alanine aminopeptidase (APN) enzyme. Here, we describe the design of a molecular probe (NB-ALA) based on the Nile Blue fluorophore (NB), which can detect the APN enzyme in urine by simple fluorometric measurements.
The kidneys’ primary function is to filter waste products from the blood and remove excess water. In addition, the kidneys are responsible for preserving the balance of salts and minerals (such as calcium, phosphorus, sodium, and potassium), controlling blood pressure, regulating the production of erythropoietin to prevent anemia, and maintaining the body homeostasis. However, the kidneys are very sensitive organs that can suffer from a large number of pathologies that lead to renal fibrosis in the majority of cases. Early-stage detection of these diseases is of great importance because they usually progress over time, increasing the damage and difficulty of recovery treatments.1,2
One of the most common clinical complications in hospitalized and critically ill patients is acute kidney injury.3 There are numerous factors that may predispose to the development of this disease, such as advanced age, chronic infections, diabetes, hypertension, immune disorders, underlying renal and hepatic problems, prostatic hypertrophy and bladder obstruction.4
Acute kidney injury is usually diagnosed by the accumulation of end products of nitrogen metabolism (urea and creatinine) or by decreased urine output, or by both factors.5 However, serum creatinine cannot be used as a sensitive early biomarker, since it requires a decrease in the glomerular filtration rate of at least 50% and there is a moderately long time lag between this reduction and its translation into an increase in serum levels.6,7 Moreover, these levels depend on multiple variables such as age, gender, diet, muscle metabolism, medication, or hydration. In the same way, serum urea levels may increase under certain conditions such as corticosteroid treatment, gastrointestinal bleeding, or a high-protein diet, limiting its use as a renal dysfunction biomarker.8,9 Besides changes in creatinine or urea serum levels, kidney damage also induces urinary overrepresentation of the enzyme alanine aminopeptidase (APN). Aminopeptidases degrade the N-terminal residue of oligopeptides, producing smaller peptides and free amino acids. APN is an exopeptidase located in the renal microvillus membranes of the brush border/proximal renal epithelial cells and other plasmatic membranes within the intestines. Upon damage of the renal epithelium, enzymes such as APN are released and secreted into the urine.10–12 As a result, their presence in urine can be employed as a kidney degradation biomarker even when urinary macroglobulin remains in normal ranges, allowing their use as an early biomarker of renal damage.13,14 APN has also been used as a cancer biomarker and several fluorogenic molecular probes had been developed for its monitoring.15,16 However, APN detection in urine samples as a potential early biomarker of kidney damage is a much less explored field (Table S1, ESI†).10,11
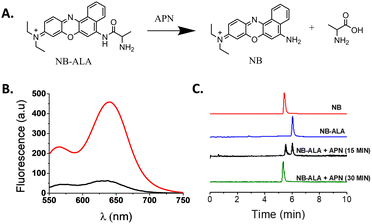
Based on the above and following our interest in designing fluorogenic probes for the detection of biomarkers,17,18 we report herein the design and preparation of compound NB-ALA, which is a fluorescent APN probe based on Nile Blue (NB). NB is a low-cost commercial fluorophore that meets the requirements established by the Food and Drug Administration (FDA) for use in humans and whose emission band is centered in the near-infrared area (NIR).19NB-ALA can sensitively and selectively detect the enzyme APN in aqueous solutions and in doped urine. Besides, APN detection by NB-ALA is also validated in a murine model of acute kidney injury by direct urine fluorescence measurements (Fig. 1).
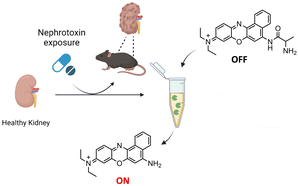 | ||
| Fig. 1 Schematic representation of the application of the NB-ALA probe for the detection of kidney damage through urine samples. | ||
NB-ALA was synthetized using a two-step protocol (Fig. S1, ESI†). In the first step, Fmoc-Ala-OH was covalently linked through the formation of an amide bond with the NB fluorophore. Then, in the second step, the Fmoc protecting group was removed with piperidine, yielding NB-ALA with a 31% global yield. NB-ALA and its intermediate were characterized by 1H-NMR, 13C-NMR and mass spectrometry (see ESI†). NB-ALA was designed in such a way that, after APN hydrolysis, NB was released (Fig. 2A). H2O–DMSO 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v solution of NB-ALA shows a weak emission band at 630 nm upon excitation at 530 nm (ΦNB-ALA = 0.00028). However, in the presence of APN enzyme, a ca. 10-fold enhancement in emission band at 630 nm was observed (Fig. 2B, ΦNB = 0.01 and Table S2, ESI†). This emission enhancement is ascribed to the APN-induced hydrolysis of the NB-ALA probe, which yields free NB (Fig. 2A).
1 v/v solution of NB-ALA shows a weak emission band at 630 nm upon excitation at 530 nm (ΦNB-ALA = 0.00028). However, in the presence of APN enzyme, a ca. 10-fold enhancement in emission band at 630 nm was observed (Fig. 2B, ΦNB = 0.01 and Table S2, ESI†). This emission enhancement is ascribed to the APN-induced hydrolysis of the NB-ALA probe, which yields free NB (Fig. 2A).
HPLC-MS studies, carried out with NB-ALA probe alone and in the presence of APN enzyme, confirmed the proposed hydrolysis reaction. In this respect, the chromatogram of the probe alone showed a single peak at 6.02 min whereas after 15 min in the presence of enzyme the intensity of this signal is reduced with the subsequent appearance of a new peak at 5.47 min, which is ascribed to NB formed upon NB-ALA hydrolysis (Fig. 2C and Fig. S4, ESI†). Besides, after 30 min of enzyme addition the 6.02 min peak of NB-ALA completely disappeared confirming the complete hydrolysis of the probe.
In an additional study, different amounts of APN were added to a solution of NB-ALA (H2O–DMSO 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
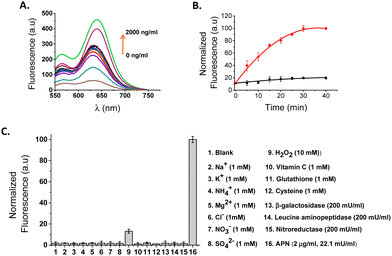
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 20 μM) and the emission spectra (λexc = 530 nm) were recorded after incubation of the samples at 37 °C for 30 min. The activity of the APN enzyme was calculated as 22.1 mU mL−1, using Ala-7-amido-4-methylcoumarin (Ala-AMC) as a reference substrate (Fig. S5 and eqn (S1), ESI†). As shown in Fig. 3A, a marked emission enhancement was observed proportional to the amount of enzyme added and due to the APN enzyme induced hydrolysis of NB-ALA and the subsequent release of free NB. From these data, a limit of detection for APN as low as 1 ng mL−1 was calculated using the eqn (S2) (Fig. S6, ESI†).
1 v/v, 20 μM) and the emission spectra (λexc = 530 nm) were recorded after incubation of the samples at 37 °C for 30 min. The activity of the APN enzyme was calculated as 22.1 mU mL−1, using Ala-7-amido-4-methylcoumarin (Ala-AMC) as a reference substrate (Fig. S5 and eqn (S1), ESI†). As shown in Fig. 3A, a marked emission enhancement was observed proportional to the amount of enzyme added and due to the APN enzyme induced hydrolysis of NB-ALA and the subsequent release of free NB. From these data, a limit of detection for APN as low as 1 ng mL−1 was calculated using the eqn (S2) (Fig. S6, ESI†).
Additionally, a kinetic study of NB-ALA hydrolysis in the presence or absence of the APN enzyme (2 μg mL−1, 22.1 mU mL−1) was carried out (Fig. 3B). The fluorescence emission of the NB-ALA solution (H2O–DMSO 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 20 μM) remained stable in the absence of APN enzyme. In sharp contrast, upon the addition of the APN enzyme (2 μg mL−1, 22.1 mU mL−1), a progressive fluorescence enhancement at 630 nm due to NB was observed reaching the maximum in ca. 30 min (Table S3, ESI†). Finally, the emission response of NB-ALA in the presence of potentially interfering species such as anions, cations, small biomolecules (vitamin C, glutathione, cysteine) and enzymes (β-galactosidase, leucine aminopeptidase, nitroreductase, APN) was also tested (Fig. 3C). This study demonstrated that only in the presence of APN was a marked emission enhancement at 630 nm observed.
1 v/v, 20 μM) remained stable in the absence of APN enzyme. In sharp contrast, upon the addition of the APN enzyme (2 μg mL−1, 22.1 mU mL−1), a progressive fluorescence enhancement at 630 nm due to NB was observed reaching the maximum in ca. 30 min (Table S3, ESI†). Finally, the emission response of NB-ALA in the presence of potentially interfering species such as anions, cations, small biomolecules (vitamin C, glutathione, cysteine) and enzymes (β-galactosidase, leucine aminopeptidase, nitroreductase, APN) was also tested (Fig. 3C). This study demonstrated that only in the presence of APN was a marked emission enhancement at 630 nm observed.
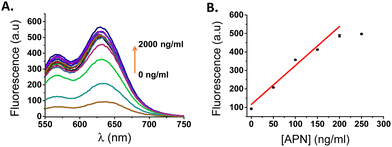
Considering the promising results obtained with NB-ALA in aqueous solutions, we checked the ability of the probe to detect APN in a more clinically relevant environment. For this purpose, a human urine sample was collected from a healthy volunteer. The concentration of APN enzyme was negligible in this urine as confirmed using the aminopeptidase N/ANPEP ELISA kit (see ESI,† Table S4). In order to validate the ability of the probe for APN enzyme detection in urine, NB-ALA was added to human urine samples that were doped with different amounts of APN (probe concentration 20 μM). The fluorescence signal was recorded after incubation at 37 °C for 30 min (Fig. 4A). A progressive emission enhancement was obtained directly related to the amount of APN (5-fold at 2 μg mL−1 of enzyme, 22.1 mU mL−1). From the obtained calibration curve (Fig. 4B) a limit of detection of 5.4 ng mL−1 for APN in doped human urine was calculated using eqn (S2) (see ESI†).
Subsequently, the urine sample was spiked with different amounts of APN (50, 100 and 200 ng mL−1), NB-ALA was added, and the mixture was incubated at 37 °C for 30 min. Finally, the emission at 630 nm was measured and the APN concentration was calculated using the calibration curve shown in Fig. 4B. The obtained results are shown in Table 1. The probe NB-ALA successfully detected APN in human urine spiked with the enzyme with recoveries ranging from 98.5 to 99.0%.
| APN spiked (ng mL−1) | APN detected (ng mL−1) | % of APN found by NB-ALA |
|---|---|---|
| 50 | 49.5 | 99.0 |
| 100 | 98.5 | 98.5 |
| 200 | 197.8 | 98.9 |
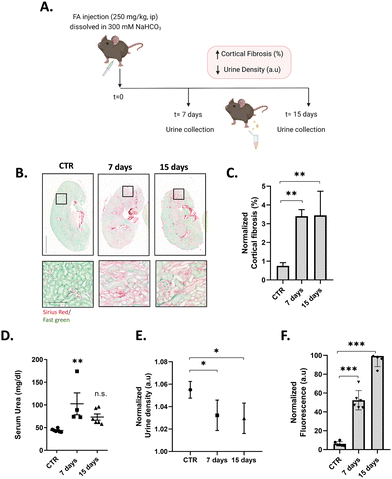
To test the ability of NB-ALA to detect endogenous APN enzyme in a pre-clinical setting, urine samples from mice with acute kidney damage and fibrosis were evaluated. To generate acute kidney damage, C57BL/6 J mice were one time injected intraperitoneally (i.p.) with a high concentration (250 mg kg−1) of folic acid (FA), which is a well-known experimental model of kidney damage.20 The pathological features underlying FA-induced acute kidney injury are direct and indirect tubular damage and oxidative stress, which triggers tubular epithelial cell (TEC) necrosis, senescence, and cytokine release.21 In this model, serum urea and serum creatinine levels recover with time (or rather compensates while some renal damage remains) (Fig. 5D)22 and the only pathological reading that can be related to renal damage is urine density, which was monitored with time.
Urine samples of FA-treated mice were collected at 0 (control, CTR), 7 and 15 days after FA injection (Fig. 5A). To analyse the animal model, the mice were euthanized, the kidneys were harvested, and tissue sections were embedded in paraffin and stained with Sirius red/Fast green in order to visualize and quantify fibrosis (Fig. 5B, C and Fig. S7, ESI†). CTR animals did not present signs of cortical fibrosis, whereas mice after 7 and 15 days of FA treatment developed fibrosis. In order to assess whether fibrosis resulted in a decline of renal function, urine was collected, and their density measured (Fig. 5E), showing a significative reduction in the values for sample of animals after 7 and 15 days of FA treatment, corroborating renal failure.
To validate the effectiveness of NB-ALA in determining kidney failure by APN detection, urine samples collected at 0, 7 and 15 days were treated with NB-ALA (final concentration of 20 μM) and incubated at 37 °C for 30 min before measuring the fluorescence emission signal at 630 nm (λexc = 530 nm). Fluorescence measurements were performed without any previous treatment of urine (Fig. 5F). Interestingly, significant differences in fluorescence signal were found in urine samples 7 days after FA treatment (6-fold). Furthermore, urine samples from 15 days FA post-treatment showed an 11-fold enhancement. These experiments demonstrate that the NB-ALA can be used for the detection of renal damage in a mouse model of kidney injury.
In summary, we show herein the synthesis and characterization of NB-ALA, a NIR fluorescent probe for APN detection. NB-ALA is weakly emissive, however, in the presence of APN enzyme, NB-ALA is hydrolysed releasing the highly emissive NB fluorophore. NB-ALA was functional in water and in APN-doped human urine samples. Additionally, the NB-ALA probe was validated in a murine renal fibrosis model induced by FA. An emission signal was only observed in urine from fibrotic kidneys of FA-treated mice. This study demonstrates the potential applications of the NB-ALA probe for the sensitive and selective detection of APN to non-invasively determine the burden of renal damage. NB-ALA is a promising probe that could be employed for a range of applications including the monitoring of treatments with nephrotoxic drugs that induce acute kidney damage or to determine regeneration after renal damage.
The authors thank the Spanish Government (PID2021-126304OB-C41) and the Generalitat Valenciana (PROMETEO CIPROM/2021/007) for support and acknowledge the financial support from the FEDER fund of the European Union (IDIFEDER/2021/044). This work was also supported by CIBER-Consorcio Centro de Investigación Biomédica en Red-(CB06/01/2012), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación. M. D.-R. is grateful for his predoctoral fellowship Grisolias to the Genelalitat Valenciana (GRISOLIAP/2019/144). J. F. B. is thankful for his postdoctoral fellowship Sara Borrell from ISCIII (CD19/00038).
Conflicts of interest
There are no conflicts to declare.Notes and references
- J. G. Abuelo, N. Engl. J. Med., 2007, 357, 797–805 CrossRef CAS PubMed.
- L. He, Q. Wei and J. Liu, et al. , Kidney Int., 2017, 92(5), 1071–1083 CrossRef PubMed.
- J. A. Kellum, P. Romagnani, G. Ashuntantang, C. Ronco, A. Zarbock and H.-J. Anders, Nat. Rev. Dis. Primers, 2021, 7, 52 CrossRef PubMed.
- S. M. Bagshaw and R. Bellomo, Curr. Opin. Crit. Care, 2007, 13(6), 638–644 CrossRef PubMed.
- J. Y. C. Soo, J. Jansen, R. Masereeuw and M. H. Little, Nat. Rev. Nephrol., 2018, 14, 378–393 CrossRef CAS PubMed.
- X. Wang, et al. , J. Am. Soc. Nephrol., 2006, 17, 2900–2909 CrossRef CAS PubMed.
- P. K. Bhatraju, et al. , JAMA Netw. Open, 2020, 3, 1–12 Search PubMed.
- M. A. Perazella and S. G. Coca, Nat. Rev. Nephrol., 2013, 9, 484–490 CrossRef CAS PubMed.
- O. Dong-Jin, Renal Failure, 2020, 42(1), 154–165 CrossRef PubMed.
- P. Cheng, Q. Miao, J. Huang, J. Li and K. Pu, Anal. Chem., 2020, 92, 6166–6172 CrossRef CAS PubMed.
- X. He, Y. Xu, W. Shi and H. Ma, Anal. Chem., 2017, 89(5), 3217–3221 CrossRef CAS PubMed.
- L. Z. Chen, W. Sun, W. H. Li, J. Li, L. P. Du, W. F. Xu, H. Fang and M. Y. Li, Anal. Methods, 2012, 4, 2661–2663 RSC.
- L. Z. Chen, W. Sun, J. Li, Z. Z. Liu, Z. Ma, W. Zhang, L. P. Du, W. F. Xu, H. Fang and M. Y. Li, Org. Biomol. Chem., 2013, 11, 378–382 RSC.
- J. Li, L. Z. Chen, W. X. Wu, W. Zhang, Z. Ma, Y. N. Cheng, L. P. Du and M. Y. Li, Anal. Chem., 2014, 86, 2747–2751 CrossRef CAS PubMed.
- H. Li, Q. Yao, W. Sun, K. Shao, Y. Lu, J. Chung, D. Kim, J. Fan, S. Long, J. Du, Y. Li, J. Wang, J. Yoon and X. Peng, J. Am. Chem. Soc., 2020, 142(13), 6381–6389 CrossRef CAS PubMed.
- X. Zhou, H. Li, Ch Shi, F. Xu, Z. Zhang, Q. Yao, H. Ma, W. Sun, K. Shao, J. Du, S. Long, J. Fan, J. Wang and X. Peng, Biomaterials, 2020, 253, 120089 CrossRef CAS PubMed.
- M. Xiao, W. Sun, J. Fan, J. Cao, Y. Li, K. Shao, M. Li, X. Li, Y. Kang, W. Zhang, S. Long, J. Du and X. Peng, Adv. Funct. Mater., 2018, 1805128, 1–9 Search PubMed.
- W. Sun, S. Guo, C. Hu, J. Fan and X. Peng, Chem. Rev., 2016, 116, 7768–7817 CrossRef CAS PubMed.
- J. Mérian, J. Gravier, F. Navarro and I. Texier, Molecules, 2012, 17, 5564–5591 CrossRef PubMed.
- Z. Hu, H. Zhang, S. Yang, X. Wu, D. He, K. Cao and W. Zhang, Oxid. Med. Cell. Longevity, 2019, 8, 1–9 Search PubMed.
- O. E. Aparicio-Trejo, P. Rojas-Morales, S. H. Avila-Rojas, J. C. León-Contreras, R. Hernández-Pando, A. P. Jiménez-Uribe, R. Prieto-Carrasco, L. G. Sánchez-Lozada, J. Pedraza-Chaverri and E. Tapia, Int. J. Mol. Sci., 2020, 21, 6512 CrossRef CAS PubMed.
- J. A. Kellum, Crit. Care Clin., 2015, 31, 621–632 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cc05408f |
| This journal is © The Royal Society of Chemistry 2023 |