Open Access Article
Open Access ArticleAdvances in antimicrobial hydrogels for dental tissue engineering: regenerative strategies for endodontics and periodontics
Deniz
Atila
* and
Vignesh
Kumaravel
 *
*
International Centre for Research on Innovative Biobased Materials (ICRI-BioM) – International Research Agenda, Lodz University of Technology, Żeromskiego 116, 90-924, Lodz, Poland. E-mail: deniz.atila@p.lodz.pl; vignesh.kumaravel@p.lodz.pl
First published on 28th August 2023
Abstract
Dental tissue infections have been affecting millions of patients globally leading to pain, severe tissue damage, or even tooth loss. Commercial sterilizers may not be adequate to prevent frequent dental infections. Antimicrobial hydrogels have been introduced as an effective therapeutic strategy for endodontics and periodontics since they have the capability of imitating the native extracellular matrix of soft tissues. Hydrogel networks are considered excellent drug delivery platforms due to their high-water retention capacity. In this regard, drugs or nanoparticles can be incorporated into the hydrogels to endow antimicrobial properties as well as to improve their regenerative potential, once biocompatibility criteria are met avoiding high dosages. Herein, novel antimicrobial hydrogel formulations were discussed for the first time in the scope of endodontics and periodontics. Such hydrogels seem outstanding candidates especially when designed not only as simple volume fillers but also as smart biomaterials with condition-specific adaptability within the dynamic microenvironment of the defect site. Multifunctional hydrogels play a pivotal role against infections, inflammation, oxidative stress, etc. along the way of dental regeneration. Modern techniques (e.g., 3D and 4D-printing) hold promise to develop the next generation of antimicrobial hydrogels together with their limitations such as infeasibility of implantation.
1. Introduction
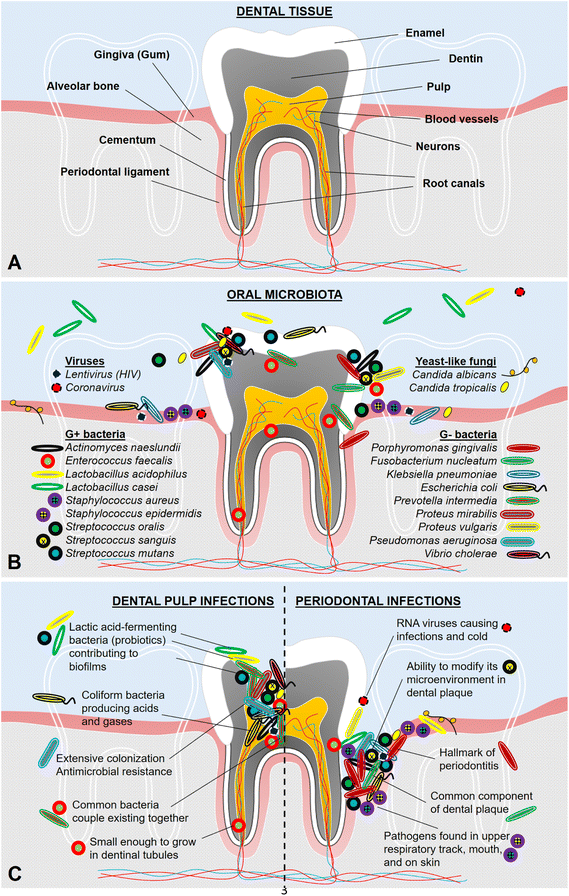
Issues regarding oral health dampen daily life quality and the well-being of individuals remarkably which has been faced by as common as 1 in 2 people worldwide.1 According to the estimations, the global burden of dental diseases amounted to $298 and $144 billion annually associated with direct and indirect costs, respectively.2 Dental infections prominently underlie oral diseases including tooth decay (i.e., dental caries), endodontic disorders (i.e., diseases inside teeth), and periodontal illnesses (i.e., diseases in the surroundings of teeth). Dental tissue parts are illustrated in Fig. 1A. Pathogens specifically select surfaces to adhere according to the type and density of protein adsorbed onto the surface. Dental surfaces in contact with saliva and plasma are highly prone to adsorb a vast amount of protein constituting attractive chemical characteristics to oral microbiota.3 Hundreds of different microbial species inhabit the oral cavity including both hard tissue parts such as the surface of teeth as well as soft oral tissues, namely gums, cheek walls, and tongue surfaces.4,5 Common types of pathogens found in oral microbiota are represented in Fig. 1B. These pathogens may be able to reach dental pulp depending on the presence and extent of dental caries.6 The spatial difference between dental pulp and periodontal infections is displayed in Fig. 1C.Treatment strategies developed for endodontic and periodontal infections have been a critical issue since subsequent tooth loss may occur when these tissues are damaged by severe infections and inflammation generated. In addition, long-term consequences of oral diseases and tooth loss tend to further influence other mechanisms in the entire body regarding systemic health (e.g., weight loss, diabetes, cardiovascular diseases, dementia, Alzheimer's disease, and spatial cognitive impairment).5,7–11 Therefore, a huge amount of effort must be put to develop biomaterials by sorting out oral health problems faced in dentistry. The selection of biomaterials is being done considering the end product's potential physical, chemical, and biological characteristics.12 Otherwise, safety and durability concerns of these biomaterials may cause troubles such as toxicity or allergenicity as well as too fast or too slow biodegradation.
Tissue engineering is a multidisciplinary field that aims to restore and/or replace malfunctioning tissues or organs using scaffolds. To achieve this goal, tissue-engineered constructs should mimic native tissue features and indigenous tissue environment of the targeted body part. In endodontics and periodontics, basic requirements to succeed in scaffold design can be listed as similar viscoelastic properties to dental pulp or gum tissues, high accessibility to each corner of small defects with safe implantation, and suitable rate of biodegradation in consistent with new tissue formation.
(i) Viscoelasticity: Among the various biomaterials used for scaffolding in tissue engineering applications, hydrogels (i.e., hydrophilic three-dimensional (3D) networks of natural or synthetic polymers with enormous water retention capability) have been considered favorable scaffolds both for endodontics and periodontics.13 Hydrogels can mimic the native extracellular matrix (ECM) of soft tissues owing to their viscoelastic properties; which encapsulate cells and contain a huge amount of water providing an active transport of biological molecules/cellular wastes and a delivery of bioactive agents loaded into hydrogels.14,15
(ii) Accessibility & applicability: Injectability of hydrogel is another concern to treat small 3D defects encountered in endodontics and periodontics. Such injectable formulations reaching voids/gaps out fully are superior to scaffolds with pre-determined shapes, which often cannot fit into the irregular tiny volume of the pulp cavity, root canals, and periodontal pockets.16 The pre-shaped 3D scaffolds have the risk of harming the fragile soft tissue remaining at the defect site during implantation.17 Hence, injectable hydrogels with optimal viscoelastic properties are considered one of the ideal materials having the lowest degree of invasiveness and the highest degree of sterility due to their injectability.18
(iii) Biodegradability: Average tissue regeneration time was roughly estimated as 2–3 weeks for dental pulp19,20 and 2–6 weeks for periodontal tissue,21,22 (although it may alter depending on material type used in treatment and patient-specific situation). In order not to hinder the regeneration, the implanted hydrogel should be degrading gradually and replacing with the newly formed tissue while the natural tissue reconstruction proceeds. Tailoring biodegradability of hydrogels is possible by methods like crosslinking.23 In the case of infections, the defect environment becomes acidic so that hydrogel durability should be adjusted in the acidic pH.24 Alternatively, pH-responsive hydrogels can be designed to stimulate degradation and thus, drug release at lower pH in the presence of pathogens after implantation.25
To further improve the hydrogel characteristics, antimicrobial properties should be included within hydrogel formulations since infections can lead to a huge delay in the healing process at the defect site.26 Especially for dental tissue engineering applications, residual pathogens left after disinfection of the diseased region may cause a repeated infection scenario by contaminating the surrounding tissue as well as the implanted biomaterial.27 Thus, antimicrobial properties introduced to these types of biomaterials can play a critical role in promoting tissue regeneration.
The timeline of hydrogel utilization in the focus of endodontics and periodontics is presented in Fig. 2, starting from the birth of the term “hydrogel”28 and followed by the first covalently crosslinked hydrogel.29 Among all biomaterials popular recently, hydrogels were introduced as the first materials developed for biomedical applications.30 Then, antimicrobial drug delivery from hydrogels was reported in the biomedical field.31 Initial studies were carried out to extend the hydrogel utilization as root canal filling materials in endodontics32 without any antimicrobial action. As pioneer studies regarding controlled release from hydrogels in particular to dental applications, the use of hydrogels for fluoride ion delivery was introduced for the prevention of demineralization of dental tissue.33 Soon after, antimicrobial drug delivery was reported by using tetracycline in periodontics;34 however, hydrogels were not included in this study. In the field of endodontics, antimicrobial root canal materials including types of sealers, pastes, and cement were tested to evaluate their efficacy.35 Intrinsically antimicrobial hydrogels were developed for periodontal reconstruction36 and for blocking the microchannels of dentin,37 even though no information concerning their injectability was reported. Just before entering the next decade, superporous hydrogels started to be studied as drug delivery platforms.38 Additionally, the past three generations of hydrogels39 were presented simultaneously in the historical timeline. Within the 2000s, antimicrobial eugenol from clove oil was incorporated into hydrogels towards periodontics, which was called oil-in-hydrogel dispersions.40 Injectable thermosensitive hydrogels with antimicrobial properties were designed against periodontal pathogens.41 In the following decade, smart hydrogels have been termed around 2010, which referred to in situ-forming hydrogels crosslinked covalently under physiological conditions possessing minimal or non-toxic features.39 In this era, self-assembling peptide hydrogels were used to encapsulate dental pulp stem cells and growth factors for endodontics.42 In light of the newly emerging techniques, 3D-printed hydrogels were fabricated for endodontics43 and periodontics,44 respectively. Meanwhile, four-dimensional (4D)-printed hydrogels have been recently developed,45 although hydrogels with antimicrobial property have not been studied in endodontics or periodontics yet. Such materials can transform themselves in terms of morphology, physical or chemical structure, and functionality upon exposure to a predetermined stimulus in a certain environment, e.g., a specific defect site in the body. In periodontics, antimicrobial hydrogels responsive to a highly specific stimulus (i.e., an enzyme production by periodontitis-causing bacteria) were developed.23 Also, hydrogels targeting the same bacteria were prepared by an alternative approach, in which oxygen carriers were incorporated into the hydrogels to inhibit the growth of these anaerobic pathogens.46 Furthermore, metal–organic frameworks (MOFs) were combined with hydrogels for antimicrobial drug delivery in periodontics lately.47 As a new avenue in hydrogel science, hydrogels composed of branched DNA have been produced by the enzymatic ligation method and used for drug delivery applications.48 These hydrogels were able to respond to enzymes and substrates in their microenvironment. In this way, DNA hydrogels could convert the substrates into proteins, which is a brand-new way of producing proteins. To our knowledge, however, no examples of antimicrobial DNA hydrogels could be encountered neither in endodontics nor periodontics yet. Coming closer to the present, 3D-printed antimicrobial hydrogels have emerged in periodontics.49
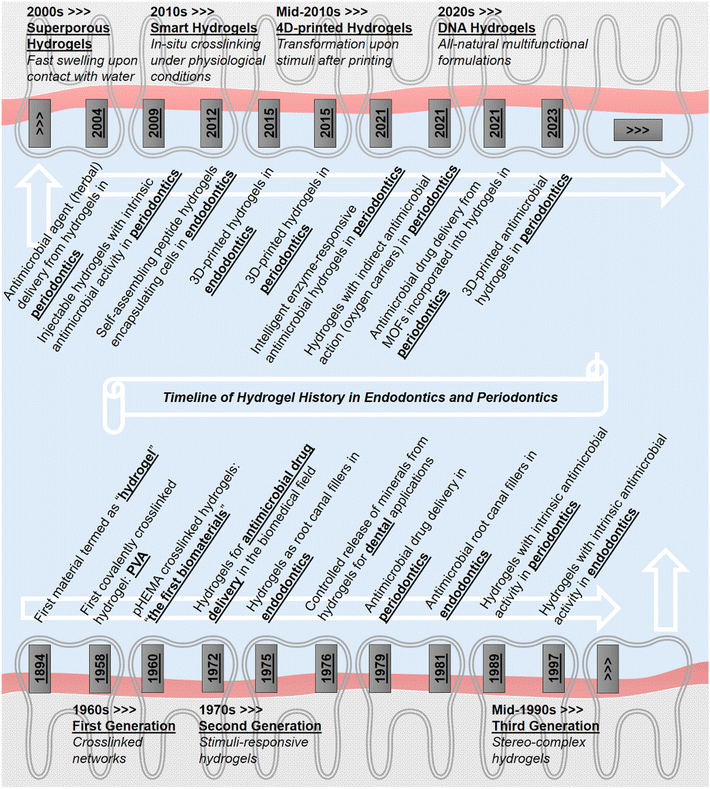 | ||
| Fig. 2 Schematic of chronological history of antimicrobial hydrogels in the scope of endodontics and periodontics. | ||
During the last few years, antimicrobial hydrogels have only been reviewed from a broad spectrum of applications.50,51 The engineering of antimicrobial hydrogels exclusively for endodontics and periodontics has not been reported elsewhere. Herein, the modern strategies to design hydrogels specifically for endodontics and periodontics bearing antimicrobial properties either intrinsically or in a drug-/particle-releasing manner have been reviewed for the first time. The antimicrobial efficacy of the novel hydrogels concerning specific pathogens is compared together with their biocompatibility level for the host cells. Inherently antimicrobial hydrogels composed of peptides and/or polymers as well as hydrogels combined with the addition of antimicrobial drugs/particles are classified. The key findings concerning the state-of-the-art of antimicrobial hydrogels are discussed. Finally, challenges and prospects are highlighted including dextrous biomaterials (e.g., DNA) and advanced fabrication techniques (e.g., 4D printing). During the study selection process, the first criteria set was whether the antimicrobial hydrogels were formulated to be placed into the targeted treatment area or not. Studies related to the application of hydrogels in endodontics or periodontics by using dental pulp stem cells, periodontal ligament stem cells, etc. were included. Development of hydrogels according to the unique features of dental defect sites (e.g., to induce odontogenic differentiation, to inhibit local pathogens, to deliver molecules in a local enzyme-responsive manner, to facilitate angiogenesis by releasing specific secretomes produced by dental tissue cells, etc.) were emphasized. PubMed, Scopus, Web of Science, and Google Scholar were used to search the key findings of antimicrobial hydrogels for endodontics and periodontics from 2018 to the present. Comparisons among other hydrogel formulations in the biomedical field were included where they are necessary.
2. Antimicrobial hydrogels developed for endodontics
Within endodontic concerns, the first and the most important task is to protect the pulp (i.e., the innermost part of the tooth) by protecting the enamel (i.e., the outermost cover of the tooth). Prevention of pulp infections depends on an effective restraining of the decomposition of the enamel. Such decay of the enamel occurs due to the demineralization of the inorganic material-based tissue. This is mainly caused by frequent exposure to acidic foods and lack of regular oral hygiene as well as eventual microbial accumulation at a massive level (i.e., dental plaque formation). Once such plaques are formed, they produce acidic by-products that synergistically contribute to the decaying process.6 At later stages of the enamel demineralization without treatment, defects (i.e., dental caries) may grow towards the dentin layer underneath, which finally connects the oral cavity to the pulp. Eventually, microbial invasion of the soft tissue containing nerves and blood vessels takes place with pain and inflammation52 so that the need for endodontic treatments emerges.Common endodontic therapies involve root canal treatment, in which the infected pulp is removed entirely, and the pulp cavity is sterilized53 by traditional disinfectants such as saline, calcium hydroxide (Ca(OH)2), sodium hypochlorite (NaOCl), EDTA/citric acid, and antibiotic pastes.54–56 However, pathogens may remain inside dentinal tubules due to the narrow and curved topology of the tubules, where the attainability and dispersion level of antimicrobial agents is diminished.57 The use of antimicrobial exposure at higher dosages is not preferable since it may cause toxic effects on tissue-forming cells within the host tissue.55 Moreover, NaOCl caused the degradation of dentin matrix protein 1 and dentin sialophosphoprotein which are involved in the odontoblastic differentiation process of dental pulp stem cells.58 Most recently, to avoid the loss of the entire pulp, a new generation of biomaterials with antimicrobial agents has been involved in endodontics for long-term antimicrobial action.59,60 Besides antimicrobial action, biocompatibility, biodegradability, and porosity of the scaffolds should be considered for the successful revitalization of the pulp.61 Furthermore, utilizing cells, biologically active molecules, and morphologically suitable scaffolds can be an option to promote pulp tissue regeneration more and to restore the functions of the pulp.56,62–64 Antimicrobial hydrogels recently developed for endodontics and their multifunctional properties (if there are any) were listed in Table 1.
| Materials | Gelling method | Biodegradability | Antimicrobial part | Biocompatibility | Multifunctionality | Ref. |
|---|---|---|---|---|---|---|
| RGD-functionalized multicomponent peptide hydrogels: Biogelx™-S or Biogelx™-RGD | Self-assembly | — | Intrinsic | In vitro tests | Angiogenesis | 52 |
| Peptides | Dental pulp stem/stromal cells | |||||
| Highest groups: no difference among the groups | ||||||
| Period: 3 days | ||||||
| Ultrashort (<8 amino acids) (naphthalene-2-ly)-acetyl-diphenylalanine-dilysine-OH peptide hydrogels | Self-assembly | — | Intrinsic | In vitro tests | Angiogenesis | 65 |
| Peptides with positively charged lysine residues | Dental pulp stem/stromal cells | |||||
| Highest groups: no difference among the groups | ||||||
| Period: 14 days | ||||||
| Methacrylated hyaluronic acid hydrogels incorporated with platelet lysate | Photo-crosslinking | — | Intrinsic | In vitro tests | Increased cellular metabolism Biomineralization capacity | 66 |
| Platelet lysate | Human dental pulp cells | |||||
| Highest group: hydrogels with platelet lysate | ||||||
| Period: 21 days | ||||||
| Cellularized fibrin-alone hydrogels and cellularized fibrin hydrogels supplemented with chitosan | Fibrinogen polymerization under thrombin control | — | Intrinsic | In vitro tests | Regeneration | 26 |
| Chitosan | Dental pulp-mesenchymal stem/stromal cells | |||||
| Highest groups: no difference between the groups | ||||||
| Period: 7 days | ||||||
| Fibrin and fibrin-chitosan hydrogels | Fibrinogen polymerization under thrombin control | — | Intrinsic | In vivo tests | Immunomodulation | 67 |
| Chitosan | Pulpotomized Sprague-Dawley rat incisors | Regeneration | ||||
| Highest group: no difference between the groups | ||||||
| Period: 1 day | ||||||
| Chitosan-alone and chitosan loaded with secretomes of stem cells from human exfoliated deciduous teeth (SHED) hydrogels | Crosslinking by glycerol phosphate | — | Intrinsic | In vitro tests | Cell homing | 68 |
| Chitosan | Human apical papilla stem cells | Angiogenesis | ||||
| Highest group: hydrogels loaded with SHED secretomes | ||||||
| Period: 3 days | ||||||
| Chitosan associated with gelatin and microparticulate dentin by using genipin | Crosslinking by genipin and freeze-drying | In vitro tests | Intrinsic | In vitro tests | Odontogenic differentiation | 69 |
| Fastest group: chitosan hydrogels (>50%) | Chitosan | Human cells from the apical papilla | ||||
| Period: 32 days | Highest group: chitosan/gelatin/genipin hydrogels | |||||
| Medium: lysozyme in SBF | Period: 8 days | |||||
| Fibrin hydrogels and self-assembling peptide (RADA-16) hydrogels with or without chlorite-oxidized oxyamylose (COAM) | Fibrinogen polymerization under thrombin control and self-assembling | — | Intrinsic | In vitro tests | Regeneration | 70 |
| Chlorite-oxidized oxyamylose | Human dental pulp stem cells | |||||
| Highest group: fibrin hydrogels hydrogels with COAM | ||||||
| Period: 7 days | ||||||
| Photo-cross-linkable chlorhexidine-laden GelMA hydrogels | Photo-crosslinking | In vitro tests | Drugs | In vitro tests | — | 71 |
| Fastest group: GelMA hydrogels without chlorhexidine (∼100%) | Chlorhexidine | Stem cells from human exfoliated deciduous teeth | ||||
| Period: 28 days | Highest group: hydrogels loaded with 0.12% chlorhexidine | |||||
| Medium: collagenase type I | Period: 14 days | |||||
| GelMA hydrogels containing clindamycin- or metronidazole-laden electrospun PLGA fibers | Photo-crosslinking | In vitro tests | Drugs | In vitro tests | — | 72 |
| Fastest groups: all hydrogels incorporated with one or both drugs (100%) | Clindamycin and metronidazole | Stem cells from human exfoliated deciduous teeth | ||||
| Period: 14 days | Highest groups: no difference among the groups | |||||
| Medium: collagenase type I | Period: 21 days | |||||
| GelMA hydrogels engineered with ciprofloxacin-eluting short nanofibers or β-cyclodextrin-inclusion complex of ciprofloxacin | Photo-crosslinking | In vitro tests | Drugs | In vitro tests | — | 73 |
| Fastest groups: 2.5% GelMA hydrogels with free drug or the drug-containing forms (100%) | Ciprofloxacin | Human dental pulp stem cells | ||||
| Period: 7 days | Highest groups: 2.5% GelMA hydrogels, 2.5% GelMA with drug-loaded fibers, and 10% GelMA with drug-inclusion complex fibers | |||||
| Medium: collagenase A | Period: 7 days | |||||
| Methylcellulose hydrogels loaded with low concentrations of double antibiotic pastes (DAP) | Sol–gel transition | — | Drugs | In vitro tests | — | 74 |
| DAP (ciprofloxacin and metronidazole) | Human dental pulp stem cells | |||||
| Highest groups: DAP-free and 1 mg mL−1 DAP-containing hydrogels | ||||||
| Period: 3 days | ||||||
| Fibrin or chitosan-fibrin hydrogels with triple antibiotic paste (TAP), modified TAP, or double antibiotic paste (DAP) | Fibrinogen polymerization under thrombin control | In vitro tests | Drugs + intrinsic | In vitro tests | — | 75 |
| Fastest groups: no difference among the groups (100%) | Antibiotic pastes + chitosan | Human dental pulp stem cells | ||||
| Period: 21 days | Highest groups: fibrin-alone, fibrin with modified TAP, and fibrin with DAP hydrogels | |||||
| Medium: PBS | Period: 14 days | |||||
| Methylcellulose hydrogels containing diclofenac, triple antibiotic paste, or double antibiotic paste | Stirring | — | Drugs | — | — | 76 |
| Diclofenac and triple or double antibiotic pastes | ||||||
| Fibrin hydrogels loaded with free clindamycin, incorporated with PLA nanoparticles, or clindamycin-loaded PLA nanoparticles | Surfactant-free nanoprecipitation | — | Drug-loaded nanoparticles | In vitro tests | — | 27 |
| Clindamycin | Dental pulp mesenchymal stem cells | |||||
| Highest groups: no difference among the groups | ||||||
| Period: 2 days | ||||||
| GelMA hydrogels incorporated with minocycline- or clindamycin-loaded electrospun PLGA fibers | Photo-crosslinking | In vitro tests | Drug-loaded microparticles | In vitro tests | Angiogenesis | 77 |
| Fastest group: hydrogels with 2.5% minocycline-loaded electrospun fibers (100%) | Minocycline or clindamycin | Stem cells from human exfoliated deciduous teeth | ||||
| Period: 14 days | Highest groups: all hydrogels with drug-loaded fibers | |||||
| Medium: collagenase A | Period: 28 days | |||||
| In vivo tests | In vivo tests | |||||
| Fastest group: no difference | Subcutaneous implantation into Fischer 344 male rats | |||||
| Period: 7 days | Highest groups: hydrogels with 2.5% clindamycin-loaded fibers | |||||
| Period: 7 days | ||||||
| Silver nanoparticle-carrying thermoreversible hydrogels composed of poloxamers P188 and P407 | Thermoreversible self-assembling | — | Nanoparticles | In vitro tests | — | 78 |
| Silver nanoparticles | Human periodontal ligament fibroblasts | |||||
| Highest groups: hydrogels with 4 μg mL−1 of AgNO3 or 4–8 μg mL−1 of nanoparticles | ||||||
| Period: 3 days | ||||||
| Chitosan hydrogels incorporated with silver-doped bioactive glass particles | Sol–gel transition | — | Ion-doped microparticles + intrinsic | In vitro tests | Bacterial growth-responsive immunomodulation | 79 |
| Silver ions + chitosan | Human dental pulp cells | Odontogenic differentiation | ||||
| Highest groups: only one hydrogel group was tested, and it was biocompatible | ||||||
| Period: 7 days | ||||||
| Pluronic F127–alginate hydrogel releasing nitric oxide and fluoride ions from Pluronic micelles | Crosslinking via a calcium chloride | — | Chemical compound-loaded nanoparticles | In vitro tests | Prevention of enamel demineralization | 6 |
| Nitric oxide | Human gingival fibroblasts and human osteoblasts | |||||
| Highest groups: no difference among the groups | ||||||
| Period: 1 day |
2.1. Hydrogels containing intrinsically antimicrobial peptides for endodontics
Hydrogels with inherent antimicrobial properties are extensively preferred to treat dental infections since they provide homogeneous and well-dispersed antimicrobial action throughout the hydrogel volume. A schematic illustration of various hydrogels with intrinsic antimicrobial properties is shown in Fig. 3A. The consistent antimicrobial action of the polymeric network can be favorable to disinfect the pathogens by direct contact, which otherwise may stay inside the small voids, cracks, and gaps in the dental tissue. Such hydrogels are favorable for maintaining a long-term antimicrobial action against microbial colonization that would be able to re-emerge after implantation.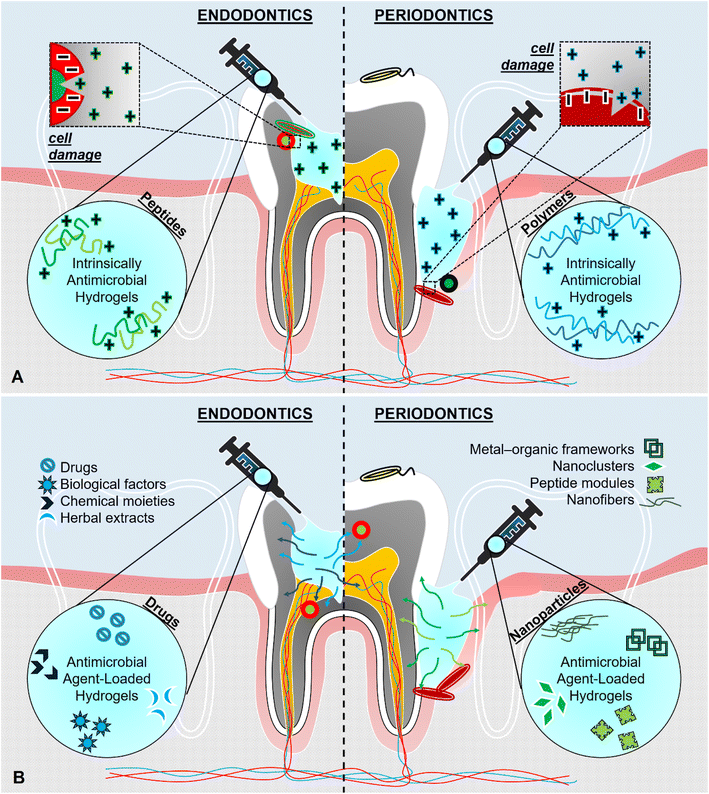 | ||
| Fig. 3 Schematic of (A) hydrogels with intrinsic antimicrobial features, and (B) hydrogels incorporated with antimicrobial agents. | ||
Antimicrobial peptides, also called host defense peptides, are products of the immune system in the body, which can also be designed artificially with specific amino acid sequences.80 In addition to regulatory functions upon inflammation and modulation of the immune response, cationic antimicrobial peptides attach to the bacterial cell membrane and cause a deformation, followed by leakage of cytoplasm. In consequence, severe disruption on the bacterial cell wall damages bacteria fatally.81 The efficacy of the peptides on antimicrobial activity could vary concerning the peptide-to-lipid ratio of the cell membrane of different strains of microorganisms. Conformational changes in microbial membranes made by peptides differ according to the peptide structure (i.e., α-helical and the β-sheet); thus, their mechanism of action through the membranes. These peptides also favor the presence of commensal (non-pathogenic) microbes over harmful ones.82 Hydrogels composed of antimicrobial peptides have been receiving remarkable attention due to their biocompatibility, biodegradability, and self-assembly, besides the strong and selective antimicrobial action.
In a recent study, the antimicrobial action of multicomponent self-assembling peptide hydrogels (Biogelx™), which are commercially available, were examined against several oral pathogens, namely Staphylococcus aureus, Enterococcus faecalis, and Fusobacterium nucleatum.52 These commercial hydrogels were functionalized with the cell-adhesive motif Arginine-Glycine-Asparagine (RGD) to enhance cell spreading and expansion since cells can recognize these RGD motifs and bind to them via the sites of integrin on the cell membrane.83 RGD enhanced the adhesion of dental pulp cells in many reports previously.84,85 In the mentioned study, dental pulp stem/stromal cells could release secretomes within the volume of the hydrogels, which is critical to promote angiogenesis.52 During in vitro antimicrobial activity studies, these peptide hydrogels with or without RGD sequences were effective against Staphylococcus aureus and Enterococcus faecalis biofilms. On the other hand, the antimicrobial action of the peptides was detected against Fusobacterium nucleatum, only in the absence of RGD. This study demonstrated that the peptide modifications (i.e., RGD incorporation) for tissue regeneration might contribute to the antimicrobial performance of the biomaterials. In a parallel study, peptide modifications to improve the ability to hinder microbial growth have been investigated, in which ultrashort peptides (i.e., [naphthalene-2-ly]-acetyl-diphenylalanine-dilysine-OH) consisting of less than 8 amino acids, could self-assemble into hydrogels.65 Those peptides were artificially combined with antimicrobial motifs (e.g., positively charged lysine residues), to prevent infection and enhance healing. Experiments were conducted to analyze the characteristics of the peptides (either in the solution or hydrogel forms) that had an impact on the antimicrobial action. These peptides prevented the biofilm formation of Staphylococcus aureus and Enterococcus faecalis when they were utilized in the solution form. The hydrogels of the same composition exerted antimicrobial action for Enterococcus faecalis and Fusobacterium nucleatum; however, they failed for Staphylococcus aureus. The physical state of the peptides affected the antimicrobial action against the same bacterium species, which implied that even the gelation degree of hydrogels could alter the antimicrobial efficacy. This was attributed to the multi-featured mechanism of action of these peptides, involving not only the interaction capacity with an individual bacterium but also the ability to infiltrate into biofilms. It was also noted that peptide solutions might penetrate the biofilms more efficiently. On the other hand, peptide hydrogels would enhance cell-based tissue engineering techniques since body cells are well-supported within the 3D environment of hydrogels.
Through the path of combinational strategies, several materials can be employed in a single hydrogel formulation to assess the synergistic effects of host defense peptides and other antimicrobial agents, as well as to facilitate the multifunctionality of the hydrogels. Recently, such antimicrobial peptides (IDR-1002) were added to PVA/chitosan nanofibers together with the antimicrobial drug – ciprofloxacin for dental pulp regeneration and revascularization.86 However, they were not incorporated into the hydrogel counterparts after carrying out the biodegradability tests for the fibers and the hydrogels. A specific fiber group exhibited an optimal time for degradation (21 days) for dental pulp reconstruction. Hence, no further experiments were conducted for the hydrogel groups. Nevertheless, this study demonstrated that host defense peptides could serve well as a part of scaffolds, besides their neat use. In the future, they may be incorporated into other types of hydrogel formulations within the concept of novel antimicrobial designs for endodontics.
Protein sources like platelet lysate could also exert antimicrobial action like peptides. In a current study, platelet lysate (i.e., a natural mixture of proteins taking a key role in the wound healing process) was incorporated in hyaluronic acid hydrogels primarily to enhance dental pulp regeneration by regulating cell migration.66 The platelet lysate-containing hydrogels increased the cellular metabolism and biomineralization capacity of dental pulp stem cells. However, antimicrobial tests were not conducted in this study, although the antimicrobial action of platelet lysates was reported in the literature.87 It was previously demonstrated that the platelet lysates prevented the adhesion, proliferation, and biofilm formation of Staphylococcus aureus. The selective antimicrobial action of the platelet lysates for specific types of bacteria was reported.88 However, the antimicrobial mechanism of action of the platelet lysates has not been completely determined yet.
2.2. Hydrogels containing intrinsically antimicrobial polymers for endodontics
Antimicrobial polymers have been widely utilized to develop novel hydrogels. For instance, ε-poly(L-lysine)89 and poly(ethylene imine)90 were converted into antimicrobial hydrogel formulations for biomedical applications, despite not being designed specifically for endodontics. Chitosan is well-pronounced among the other antimicrobial polymers and extensively incorporated into hydrogels due to its inherent antibacterial properties against a myriad of Gram-positive and Gram-negative bacteria as well as fungi, yeast, and algae.91 Indeed, chitosan, one of the most abundant natural polymers, is a biocompatible linear polysaccharide derived from chitin and consists of acetylated and deacetylated units (i.e., N-acetyl-D-glucosamine and β-(1→4)-linked D-glucosamine) with positively charged groups.91,92 The antimicrobial mechanism of action of chitosan has been prominently associated with its cationic domains, which naturally interact with the negatively charged microbial cell membranes.Recently, cellularized fibrin hydrogels were prepared through fibrinogen polymerization under thrombin control and they were supplemented with chitosan for dental pulp regeneration.26 The antibacterial effect of the fibrin/chitosan hydrogels on Enterococcus faecalis (i.e., bacterium persistent in root canals even after endodontic disinfection procedures) was significantly higher as compared to the fibrin-alone networks and control groups. The hydrogels cellularized with dental pulp-mesenchymal stem/stromal cells provided a suitable environment for the cells to deposit a 3D-collagenous network around them. Therefore, the capability of the fibrin/chitosan hydrogels to construct a native ECM would facilitate healing, which can be considered one of the most essential processes during tissue regeneration.
Similarly, fibrin and fibrin/chitosan hydrogels were fabricated for dental pulp therapy, in which the immune response to the hydrogels in the pulpotomized rat incisors was assessed.67 Antimicrobial action and cell viability of such hydrogels were previously reported for endodontics.26 Regarding the inflammatory response, M1/M2 polarization of macrophages was analyzed, and pro-regenerative macrophage phenotypes were promoted specifically in the chitosan-enriched fibrin hydrogel group. The upregulation level of interleukin-6 gene expression, which is normally upregulated after infection or trauma, was not altered by the addition of chitosan into the hydrogels. These investigations demonstrated that chitosan could be used for multifunctional purposes including antimicrobial action, immunomodulation, and others. The approach of merging antimicrobial action and immunomodulation in a single hydrogel design may lead to an effective regeneration since the immune system of the body harms the defect site enormously to eliminate infections.
In another study, multifunctional hydrogels composed of high molecular weight chitosan with low acetylation levels were designed through a novel approach. Chitosan hydrogels loaded with secretomes of stem cells from human exfoliated deciduous teeth were developed.68 One of the main aims of the study was to achieve cell homing, which is defined by the orientation of resident stem cells by chemoattractant molecules signaling for migration, proliferation, and differentiation of cells as well as angiogenesis.93 The cell homing-based strategy was assured by adding high concentrations of tropic factors (i.e., secretomes) into the hydrogels and by the sustained release of these factors. The bacteriostatic effect was observed up to 24 h in the chitosan groups while Enterococcus faecalis growth was suppressed better up to 48 h in the presence of chitosan compared to the groups without chitosan.
The antimicrobial action of chitosan could also be improved by combining it with other natural ingredients. In a recent report, chitosan scaffolds associated with gelatin and microparticulate dentin of 0.3–53 μm were fabricated by crosslinking with genipin (noting that genipin can itself enhance odontogenic differentiation contributing to the multifunctionality of the scaffolds).69 Even though these scaffolds were not injectable hydrogels, they possessed a degree of swelling. The potential capability of these hydrogels to fit into root canals was displayed. Enterococcus faecalis growth was reduced in the chitosan/gelatin/dentin scaffolds between 24 and 48 h. Bacterial adhesion was observed in all groups including chitosan and chitosan/gelatin whereas a relatively less level of microbial attachment was detected on the chitosan/gelatin/dentin scaffolds. Therefore, the antimicrobial performance of chitosan may further increase when it is placed into the pulp cavity in contact with collagen-containing surroundings and dentinal walls.
Alternative to chitosan, chlorite-oxidized oxyamylose polysaccharide was synthesized by a two-step oxidation of amylose. The polymer was blended with fibrin and self-assembling peptide (RADA-16) to produce two types of hydrogels.70 Although it was known that this polyanionic polysaccharide derivative (i.e., chlorite-oxidized oxyamylose) possessed inherent antibacterial and antiviral activities (besides immunomodulatory properties), the antimicrobial performances of these hydrogels were not evaluated in this report. Nonetheless, fibrin hydrogels with chlorite-oxidized oxyamylose were suggested as a good candidate for endodontic regeneration according to the results of the in vitro studies conducted using dental pulp stem cells.
2.3. Hydrogels incorporated with antimicrobial drugs for endodontics
Polymers without any antimicrobial action against pathogens have also been considered candidate materials for endodontics owing to other properties of the polymers to generate functionality other than antimicrobial action. In that case, external antimicrobial agents were incorporated into hydrogels in various forms. These ingredients may involve synthetic drugs, herbal extracts, and biologically or chemically active molecules as well as inorganic or organic micro-/nanoparticles with different configurations (which are discussed in the next section 2.4). The schematic illustration of hydrogels incorporated with various antimicrobial agents is shown in Fig. 3B.One of the most common techniques in the removal of microbial infection in dentistry is the application of medicaments (e.g., Ca(OH)2) which destroy the pathogens by direct contact.74 Nevertheless, their performance might be inadequate against specific species which can endure high pH or high temperatures such as Enterococcus faecalis.78 One-step advanced version of the medicaments involved in dentistry is called double or triple antibiotic pastes composed of antibiotics.94 However, the drawbacks of these pastes can be listed as cytotoxicity for the host tissue, systemic allergic reactions, antagonism, and bacterial resistance.71,74 To this extent, local drug delivery systems have been put forward for the replacement of the traditional methods mentioned above to provide a prolonged release of antimicrobial agents at the site of concern effectively in a host cell-friendly way.95 Furthermore, researchers have been looking for alternative solutions rather than drugs such as the use of plant extracts to avoid the disadvantages of synthetics such as ulcerative lesions, burning sensation, fluorosis, and enamel erosion.96
In a recent drug delivery study, photocrosslinkable chlorhexidine-loaded methacrylated gelatin (GelMA) hydrogels were prepared with a broad spectrum of antimicrobial action against endodontic pathogens.71 GelMA hydrogels with different concentrations of the drug (0.12, 0.5, 1, 2, and 5%) were compared to each other and with the control (2% of chlorhexidine without GelMA). It is worth noting that it is crucial to focus on the elimination of the highly persistent bacterium Enterococcus faecalis which might inhabit the tiniest spaces such as dentinal tubules due to its smaller size and stay there with an eminent endurance to long-term starvation even after sterilization procedures applied.97 The agar diffusion assay showed that GelMA loaded with 2 and 5% of chlorhexidine exhibited statistically higher antimicrobial activity against Enterococcus faecalis and reached the level of control in 24 h. GelMA groups with the same drug concentrations were significantly effective to inhibit the growth of Actinomyces naeslundii compared to the lower drug concentrations. Nonetheless, the efficacy of the control group was higher than that of all hydrogel groups. During anti-biofilm formation experiments, the hydrogels exhibited a more prominent antimicrobial action in an increasing fashion proportional to the increasing drug concentration than the control did. GelMA with 1, 2, and 5% of chlorhexidine displayed a total inhibition of biofilm formation of the microbial culture obtained from the supragingival plaque of healthy adults.
In another controlled release study, GelMA hydrogels containing clindamycin- or metronidazole-laden electrospun poly DL-lactide-co-glycolide (PLGA) fibers were formulated.72 In the first step, clindamycin or metronidazole was added to the PLGA solution and electrospun into fibrous mats. After that, the electrospun fibers were reduced in size via the cryo-milling process. Lastly, GelMA hydrogels were mixed with clindamycin- or metronidazole-loaded PLGA fibers. Clindamycin-containing hydrogel systems were effective on all bacteria species tested (i.e., Actinomyces naeslundii, Fusobacterium nucleatum, and Enterococcus faecalis). Metronidazole-containing groups were not able to inhibit the Gram-positive Enterococcus faecalis. This could be associated with Gram-negative bacteria that are prone to be affected by metronidazole;98 although they are usually more resistant to antibiotics.99 The reduced resistance of Gram-positive bacteria to antibiotics has been attributed to the thick (20–80 nm) peptidoglycan layer as an outer cover on their cell membrane, which makes them absorb the chemicals around them easily. The choice of drugs should be considered since specific drugs could exhibit unexpected efficacy against certain types of pathogens.
In another GelMA-containing study, GelMA hydrogels were engineered with ciprofloxacin-eluting short nanofibers or ciprofloxacin/β-cyclodextrin-inclusion complex-eluting short nanofibers.73 Polydioxanone/ciprofloxacin fibers were electrospun with or without the β-cyclodextrin-inclusion complex of ciprofloxacin and cut by cryo-cutting. The results demonstrated the solubility of the antimicrobial agents was tailored using the inclusion complex together with the tunable degradation profile of GelMA hydrogels. Hence, the non-cytotoxic dosages of the drug could be applied. The preclusion of the biofilm deposited by Enterococcus faecalis was promoted mostly in the group of hybrid hydrogels containing ciprofloxacin and its complex. Additionally, the short nanofibers spread throughout the hydrogel volume (3D) were superior to the electrospun fibers in a mat (2D) form for releasing antimicrobial agents influentially owing to the higher surface area of the cut and dispersed fibers inside the hydrogels. The use of 3D hydrogels providing a wet surrounding environment for the fibers embedded within the hydrogels became advantageous for drug release. These formulations were also suggested for periodontal applications in the report, besides pulpal treatments.
As another approach, double antibiotic pastes were recently incorporated into hydrogels to evaluate their antimicrobial action against Enterococcus faecalis and Prevotella intermedia (i.e., a well-known bacteria couple existing together with the ability to deposit biofilm in a well-organized manner), while also testing their cytotoxicity on dental pulp stem cells.74 Methylcellulose hydrogels loaded with several concentrations (i.e., 1, 5, and 10 mg mL−1) of ciprofloxacin/metronidazole pastes displayed antimicrobial activity against Enterococcus faecalis-only and both Enterococcus faecalis- and Prevotella intermedia-infected dentin samples. Even though all mentioned paste concentrations, as well as the control group (only Ca(OH)2), exhibited antimicrobial action in a proportionally increasing manner in response to the concentration increase, only the lowest concentration (1 mg mL−1) was non-toxic. The cells could proliferate and differentiate at this lowest paste concentration, resulting in nodule formations by biomineralization. The improper concentrations of the antibiotic pastes were determined as 5, and 10 mg mL−1 regarding cell viability and osteo/odontogenic differentiation. Taken together, it must be noted that even if the antimicrobial action is aimed as a core of designing antimicrobial hydrogels, the biocompatibility of these hydrogels should also be checked not to damage tissue-forming cells in contact with the hydrogels.
In another similar study, fibrin or chitosan/fibrin hydrogels loaded with different combinations of antibiotic pastes; (i) triple antibiotic paste-original = metronidazole, ciprofloxacin, and minocycline, (ii) triple-modified = metronidazole, ciprofloxacin, and clindamycin, and lastly (iii) double-original = metronidazole and ciprofloxacin, were tested against Enterococcus faecalis.75 This study revealed that the fibrin hydrogels could exert an antimicrobial action once the pastes were incorporated into the hydrogels. The chitosan/fibrin hydrogels alone (without the pastes) could also decrease microbial colony formation due to the intrinsic antimicrobial property of the chitosan component. Double antibiotic paste-loaded chitosan/fibrin hydrogel groups were determined as the best to inhibit bacterial growth because of the impact of the paste additives and chitosan together. This formulation was able to ensure cell viability, spreading, and biomineralization more effectively when compared to the performances of all other combinations of different hydrogels and antibiotic pastes. Also the fibrin groups without chitosan displayed more biocompatible features, which could be linked to the presence of cell-adhesive motifs within fibrin and its many other cell-friendly functions.100 On the other hand, triple antibiotic pastes were found more detrimental for cells compared to double antibiotic counterparts, due to the higher load of drugs, as expected. This study showed that commercial antibiotic pastes could be combined with other green materials and their cytotoxicity could be tailored in this way.
Most recently, methylcellulose-based hydrogels were loaded with an antimicrobial drug diclofenac to overcome root canal infections.76 The antimicrobial efficacy of the diclofenac-loaded hydrogels was compared to the hydrogel groups which were loaded with triple antibiotic pastes, double antibiotic pastes, or Ca(OH)2. Antimicrobial activity tests were conducted by using 3-week-old polymicrobial root canal biofilms grown on human radicular dentine. The hydrogels loaded with 5% diclofenac exhibited significantly higher antimicrobial effects than the other groups during confocal scanning laser microscopy analyses. As a result, these alternative hydrogels can be considered better formulations compared to conventional medicaments currently used.
2.4. Hydrogels incorporated with antimicrobial micro-/nanoparticles for endodontics
Basic drug delivery approaches can be improved/renewed by the utilization of more complex release systems involving functional and porous micro-/nanoparticles. By loading drugs into particles before loading them into hydrogels, side effects of drugs could be avoided once the release was sustained at non-toxic doses for a longer time. The inefficiency of some antimicrobials has been able to be sorted out mostly by adding more effective components into hydrogels. Such particles consisting of noble metals (e.g., gold, silver, copper, platinum, etc.) have been combined with the hydrogels owing to their strong antimicrobial activity.101 On the other hand, MOFs or porous coordination polymers acquire immense potential in biomedical applications including drug delivery since they are porous 3D materials consisting of metal nodes connected by organic linkers.102 Nevertheless, MOFs have not been widely utilized within antimicrobial hydrogel formulations for endodontics, despite their versatility. In other instances, the fabrication of nanoparticles from intrinsically antimicrobial polymers such as quaternary ammonium poly(ethylene imine) was reported among recent investigations on dental applications,103 even though their use in hydrogels to serve an antimicrobial activity has been reported yet neither endodontics nor periodontics.As a recent improvement in endodontics, fibrin hydrogels were incorporated with clindamycin-loaded poly (D, L) lactic acid (PLA) nanoparticles prepared by a surfactant-free nanoprecipitation method.27 PLA nanoparticles with an average diameter of ∼155 nm did not change in size after drug loading, as well as their homogeneous distribution within the hydrogels was proven. It was important to ensure the drug release from the nanoparticles in touch with dentinal walls upon injection of the hydrogels. Since the cytotoxicity concept is a major concern for both metallic and polymeric nanoparticles, the biocompatibility of the hydrogels with the drug-loaded nanoparticles was tested by culturing dental pulp mesenchymal stem cells. The cells displayed 75% viability after 48 h, which is considered non-toxic. The drug release was greatly delayed in the nanocomposite/hydrogel formulations compared to the hydrogels with free clindamycin without the nanoparticles. The antimicrobial efficacy of both types of hydrogels with the drug was confirmed against Enterococcus faecalis. Nonetheless, the prolonged drug release was more favorable for the long-term antimicrobial action after implantation.
In another study, antimicrobial drugs were loaded into electrospun fibers to fabricate antimicrobial-eluting microparticles. Then, GelMA hydrogels were incorporated with minocycline- or clindamycin-loaded PLGA fibers after the cryo-milling step.77 Their antimicrobial properties against pathogens associated with root canal infections (Actinomyces naeslundii, Fusobacterium nucleatum, and Enterococcus faecalis) were tested. Actinomyces naeslundii was affected by both hydrogel groups while Enterococcus faecalis was resistant to clindamycin-containing hydrogels. Antibiofilm efficacy was confirmed for the hydrogels-containing drugs compared to the controls. Meanwhile, minocycline-releasing hydrogels were less effective to promote the formation of capillary-like networks of endothelial cells in vitro compared to clindamycin-releasing counterparts. A well-dispersed vascularization with functioning blood vessels was observed in the hydrogel groups with clindamycin during in vivo studies.
Toward metallic particle applications, silver nanoparticle-carrying thermo-reversible hydrogels composed of poloxamers P188 and P407 were prepared for root canal therapy.78 These poloxamers, composed of polyethylene oxide and polypropylene oxide units, had surfactant characteristics and could self-assemble into micelles. Hence, they were convenient to fabricate thermo-reversible hydrogels, by which gelation took place at temperatures close to body temperature whereas they became liquid at room temperature. Therefore, the injectability of these systems was enhanced at room temperature while they could be converted into hydrogel scaffolds at body temperature delivering antimicrobial nanoparticles in a controlled manner. Antimicrobial performances of the nanocomposite hydrogels with two concentrations of nanoparticles (16 and 32 μg mL−1) were evaluated. The inhibition of the Enterococcus faecalis biofilm formation for 9 d was more effective than the controls (i.e., blank poloxamer hydrogels and Ca(OH)2). The cytotoxicity of the hydrogels was investigated by using primary human periodontal ligament fibroblasts, where the hydrogels with nanoparticle concentrations ranging between 4 and 32 μg mL−1 were found non-toxic up to 72 h.
Similarly, silver doping was chosen as a strategy in a parallel work, in which bioactive glass particles (<35 μm-sized) were doped with silver and incorporated into chitosan hydrogels undergoing sol–gel transition for vital pulp therapy.79 The viability of primary human dental pulp cells was not negatively affected in vitro, indicating the biocompatibility of these hydrogel systems. Odontogenic differentiation of the cells treated with the hydrogels was evident by an increase in ALP enzyme activity, and upregulation of odontogenic biomarkers compared to the groups of the inflamed cells. The composite hydrogels displayed immunomodulatory characteristics upon exposure to Escherichia coli lipopolysaccharides, which could be considered a bacterial growth-responsive anti-inflammatory feature. During antimicrobial activity tests, the hydrogel groups were able to inhibit the growth of Streptococcus mutans and Lactobacillus casei strains and no microbial colony was detectable after 24 h. Taken together, these antimicrobial hydrogels hold promise for effective multifunctional use to enhance dental regeneration.
Towards mimicking the natural defense mechanisms of the body, nitric oxide was combined with Pluronic F127–alginate hydrogels lately.6 Host cells produce antimicrobial nitric oxide molecules in the gas state to kill pathogens by infiltrating through biofilms and exerting oxidative and nitrosative stresses.104 With this knowledge, the hydrogels were loaded with nitric oxide-containing micelles in this study. Also, fluoride ions were added to the micelles to prevent enamel decay. The hydrogels exerted antimicrobial action against Streptococcus mutans with a 97.59% success on the reduction of pathogenic viability. The previously formed biofilms dampened 48.8% after 24 h by the nitric oxide release. On the other hand, the fluoride release from the hydrogels obstructed the demineralization of the model hydroxyapatite discs. Lastly, in vitro, tests confirmed the cytocompatibility of the hydrogels. Thus, mimicking the endogenous ways of protection found in the body can be an intelligent choice to develop new biomaterials to treat infections faced in dentistry.
Antimicrobial hydrogels hold great promise in regenerative endodontics compared to traditional approaches such as disinfection-only and capping of the dental pulp. Especially, the formulations targeting to inhibit the pulp infection-associated pathogens (e.g., Enterococcus faecalis) or utilizing the dentin microparticulates to restore dentin-pulp complex have been providing quite specific solutions for endodontics. Other than these, the antimicrobial hydrogels developed against infections can be considered possible alternatives for periodontics too.
3. Antimicrobial hydrogels developed for periodontics
Detrimental infections can also be located in the peripheral tissues surrounding teeth, defined as periodontitis. This disease may lead to severe harm both in hard (cementum and alveolar bone) or/and soft (periodontal ligament and gingiva) periodontal tissues supporting teeth.105 Together with a deteriorating immune response, it eventually culminates in a periodontal pocket formation, gingival recession, bleeding, teeth mobility/migration, and malfunctioning in mastication, besides aesthetic concerns.46,106 Once the periodontal pocket deepens dramatically, not only aerobes but also anaerobic microbes can populate inside the pocket such as Porphyromonas gingivalis which is considered one of the hallmarks of periodontal infections.107 In this regard, periodontal treatments frequently become obligatory for such severe periodontitis cases.Periodontal treatments to facilitate the attachment and restoration of tissue around teeth have been classified as nonsurgical and surgical procedures which are mechanical debridement of the tooth and open flap debridement, respectively.108 Surgical protocols can be applied in the absence or presence of membranes and bone grafts (i.e., autografts, allografts, and alloplasts). Such approaches with the membranes are termed guided bone regeneration and guided tissue regeneration. Various biological molecules can be utilized to mediate regeneration such as enamel matrix proteins, platelet-rich plasma, and platelet-rich fibrin.95,109 Tissue engineering of multifunctional biomaterials has been introduced to the field of periodontics with novel designs of nanoparticles, and drug delivery platforms for controlled and prolonged antimicrobial action.110–112 Antimicrobial hydrogels recently developed for periodontics and their multifunctional properties (if there are any) are listed in Table 2.
| Materials | Gelling method | Biodegradability | Antimicrobial part | Biocompatibility | Multifunctionality | Ref. |
|---|---|---|---|---|---|---|
| Self-assembling peptide (P11-4 and P11-28/29) hydrogels loaded with tetracycline, ciprofloxacin, and doxycycline | Self-assembly | — | Intrinsic + drugs | In vitro tests | Osteogenic differentiation | 113 |
| Self-assembling peptides + tetracycline, ciprofloxacin, and doxycycline | Human dental follicle stem cells | |||||
| Highest group: P11-4 peptide hydrogels loaded with the antibiotics | ||||||
| Period: 14 days | ||||||
| Hydrogels composed of hydroxypropyl methylcellulose/hyaluronic acid, glycerol, and bomidin peptide | Thermo-sensitive gelation | — | Intrinsic | In vitro tests | — | 114 |
| Antimicrobial peptide bomidin | Human gingival fibroblasts | |||||
| Highest groups: hydroxypropyl methylcellulose/hyaluronic acid/glycerol hydrogels with or without bomidin | ||||||
| Period: 5 days | ||||||
| Poly(ethylene glycol) diacrylate/short antimicrobial peptide hydrogels loaded with stromal cell-derived factor-1 | Thermo-sensitive gelation and crosslinking by the crosslinker dithiothreitol | In vitro tests | Intrinsic | In vitro tests | Osteogenesis | 23 |
| Fastest group: hydrogels prepared by gelation only (100%) | Short antimicrobial peptides | Periodontal ligament stem cells | Osteogenic differentiation | |||
| Period: 14 days | Highest groups: all hydrogels containing stromal cell-derived factor-1 | |||||
| Medium: PBS, 1 nM gingipain R1 protein in PBS, and 10 nM gingipain R1 protein in PBS | Period: 5 days | |||||
| In vivo tests | ||||||
| Male Wistar rats with periodontitis | ||||||
| Highest group: crosslinked hydrogels containing stromal cell-derived factor-1 | ||||||
| Period: 28 days | ||||||
| Chitosan hydrogels containing Nal-P-113 peptides and polydopamine nanoparticles | Thermo-sensitive gelation | In vitro tests | Intrinsic | In vitro tests | Antioxidant activity | 115 |
| Fastest group: there was only one group-hydrogels with the peptide and the nanoparticles (∼80%) | Antimicrobial peptide Nal-P-113 + chitosan | Human gingival epithelial cells | Anti-inflammatory activity | |||
| Period: 16 days | Highest groups: no difference among the groups | |||||
| Medium: PBS | Period: 1 day | |||||
| In vivo tests | ||||||
| Male Sprague-Dawley rats with experimental periodontitis | ||||||
| Highest group: hydrogels with the peptide and the nanoparticles | ||||||
| Period: 3 days | ||||||
| Hydrogels of poloxamer 407, alginate sodium, and cellulose derivatives with the mixture of Scutellariae baicalensis radix extract and chitosan | Thermo-sensitive gelation in situ based on poloxamer 407 | — | Intrinsic | — | Anti-inflammatory activity | 116 |
| Scutellariae baicalensis radix extract and chitosan | Antioxidant activity | |||||
| Ferrous ion-chelating activity | ||||||
| Anti-hyaluronidase activity | ||||||
| Thymol-loaded dodecenylsuccinic anhydride-modified chitosan hydrogels | Crosslinking by acetic acid | — | Intrinsic + drugs | In vitro tests | Antioxidant activity | 117 |
| Chitosan + thymol | 3T3 mouse fibroblasts | |||||
| Highest groups: unmodified chitosan hydrogels | ||||||
| Period: 1 day | ||||||
| In vivo tests | ||||||
| Periodontitis rat model | ||||||
| Highest groups: unmodified thymol-loaded chitosan hydrogels | ||||||
| Period: 7 days | ||||||
| In situ poloxamer 407 and chitosan gel containing levofloxacin and metronidazole | Thermo-responsive gelation in situ based on poloxamer 407 | — | Intrinsic + drugs | — | — | 118 |
| Chitosan + levofloxacin and metronidazole | ||||||
| Chitosan/β-glycerophosphate hydrogels loaded with BMP-7 and ornidazole | Thermo-sensitive gelation | In vitro tests | Intrinsic + drugs | In vivo tests | Regeneration | 119 |
| Fastest group: no difference among the groups (∼80%) | Chitosan + ornidazole | Class III furcation defects in male beagles | ||||
| Period: 28 days | Highest groups: hydrogels loaded with BMP-7-alone or BMP-7 and ornidazole | |||||
| Medium: lysozyme in DMEM | Period: 8 weeks | |||||
| Poly(vinyl alcohol) hydrogels crosslinked by chitosan microcapsules loaded with metronidazole | Dynamic covalent bonding and ionic interaction | In vitro tests | Intrinsic + drugs | In vitro tests | — | 120 |
| Fastest group: hydrogels crosslinked by 2% of the microcapsules loaded with metronidazole (>40%) | Chitosan + metronidazole | Human gingival fibroblasts | ||||
| Period: 28 days | Highest group: hydrogels with 25 μg mL−1 of drug | |||||
| Medium: PBS | Period: 1 day | |||||
| In vivo tests | ||||||
| Male Wistar rat periodontitis model | ||||||
| Highest group: only one group was tested, and it was biocompatible | ||||||
| Period: 7 days | ||||||
| Flurbiprofen-loaded chitosan hydrogel carrying triclosan-loaded poly-ε-caprolactone nanoparticles | Solvent displacement/crosslinking by hydrochloric acid | — | Intrinsic + drugs | In vivo tests | Anti-inflammatory activity | 121 |
| Chitosan + triclosan and flurbiprofen | Sprague-Dawley male rats with induced experimental periodontitis | |||||
| Highest group: nanogel group (there was only one) | ||||||
| Period: 7 days | ||||||
| Hydrogels composed of GelMA modified with quaternary ammonium groups and unmodified GelMA | Photo-crosslinking | — | Intrinsic | In vitro tests | Regeneration | 122 |
| Quaternary ammonium groups | Immortalized human gingival fibroblasts | Anti-inflammatory activity | ||||
| Highest group: unmodified GelMA/modified GelMA 25/75 hydrogels | ||||||
| Period: 2 days | ||||||
| Surfactin and herbmedotcin-loaded hydrogels composed of cellulose nanofibers and κ-carrageenan oligosaccharide nanoparticles | Crosslinking by epichlorohydrin and heating-freezing methods | — | Drugs | In vitro tests | Anti-inflammatory activity | 123 |
| Surfactin and Herbmedotcin | Human gingival fibroblast cells | Antioxidant activity | ||||
| Highest groups: hydrogels without surfactin and herbmedotcin | ||||||
| Period: 1 day | ||||||
| Poly(acrylic acid) hydrogels containing metronidazole | One-step gamma-ray irradiation crosslinking | — | Drugs | In vitro tests | — | 124 |
| Metronidazole | Swiss mouse embryo-NIH/3T3 fibroblasts | |||||
| Highest groups: no difference among the groups | ||||||
| Period: 1 day | ||||||
| Doxycycline and/or lipoxin A4-loaded thermo-reversible poly(isocyanopeptide) hydrogels | Thermo-reversible gelation | In vivo tests | Drugs | In vivo tests | Anti-inflammatory activity | 21 |
| No difference among the groups (residual dressings were left.) | Doxycycline | Beagle dogs with naturally occurring periodontitis | ||||
| Period: 2 weeks | Highest groups: no difference among the groups | |||||
| Period: 6 weeks | ||||||
| Curdlan/polydopamine composite loaded with acetate chlorhexidine | Physical gelation | — | Drugs + photothermal | In vitro tests | — | 125 |
| Near-infrared-responsive delivery of acetate chlorhexidine | Human periodontal ligament cells | |||||
| Highest group: hydrogels without curdlan | ||||||
| Period: 5 days | ||||||
| GelMA hydrogels with mesoporous silica-coated gold nanobipyramids-loaded with minocycline | Photo-crosslinking | — | Drugs + photothermal | In vitro tests | — | 126 |
| Near-infrared-responsive delivery of minocycline | L929 fibroblast cells | |||||
| Test 1 Different concentrations of the particles | ||||||
| Highest groups: no difference among the groups | ||||||
| Period: 1 day | ||||||
| Test 2 Exposure to irradiation | ||||||
| Highest groups: 04–08 W cm−2 | ||||||
| Period: 5 min | ||||||
| Oxidized dextran and phenylboronic acid-functionalized poly(ethylene imine) hydrogels loaded with doxycycline and metformin | In situ gel formation of Schiff base | In vivo tests | Drugs | In vitro tests | Anti-inflammatory activity | 127 |
| Fastest group: no difference among the groups (>90%) | ROS-responsive delivery of doxycycline | L929 fibroblast cells | Pro-osteogenic activity | |||
| Period: 14 days | Highest groups: no difference among the groups | |||||
| Medium: subcutaneous region of Kunming mice | Period: 3 days | |||||
| In vivo tests | ||||||
| Male Kunming mice | ||||||
| Highest groups: no difference among the groups | ||||||
| Period: 14 days | ||||||
| Metronidazole-loaded methacrylated-poly-γ-glutamic acid hydrogels containing chlorhexidine-loaded methacrylated-poly-γ-glutamic acid nanoparticles | Blue-light photo-polymerization | — | Drugs | In vitro tests | — | 128 |
| pH-Sensitive delivery of chlorhexidine and metronidazole | MG63 cells | |||||
| Highest groups: only one hydrogel group was tested and it was biocompatible | ||||||
| Period: 7 days | ||||||
| Hydrogels composed of green tea extracts | Thermo-responsive gelation based on poloxamer 407 and carbopol 934 | — | Herbal antimicrobials | — | — | 129 |
| Green tea extracts | ||||||
| Gold nanoparticle-modified PVA hydrogels loaded with epigallocatechin gallate | Sonication | — | Herbal antimicrobials + nanoparticles + photothermal delivery | In vitro tests | Bone regeneration | 130 |
| Near-infrared-responsive delivery of epigallocatechin gallate + gold nanoparticles | Bone marrow mesenchymal stem cells and human umbilical vein endothelial cells | Angiogenesis | ||||
| Highest groups: no difference among the groups | ||||||
| Period: 5 days | ||||||
| In vivo tests | ||||||
| Periodontitis rat model | ||||||
| Highest groups: no difference among the groups | ||||||
| Period: 4 weeks | ||||||
| Poly(vinyl alcohol)/chitosan composite hydrogels incorporated with silver nanoparticles | Freeze–thawing and electrostatic interactions | — | Nanoparticles + intrinsic | In vitro tests | — | 131 |
| Silver nanoparticles + chitosan | Primary human dermal fibroblasts | |||||
| Highest groups: hydrogels without nanoparticles | ||||||
| Period: 2 days | ||||||
| Carbopol 940® (polymer of acrylic acid) hydrogel containing minocycline and zinc oxide nanoparticle-loaded serum albumin microspheres | Dispersion in hydrogels | — | Nanoparticle-loaded microparticles + drugs | In vitro tests | — | 22 |
| Zinc oxide nanoparticles + minocycline | Gingival cells | |||||
| Highest groups: nanoparticles without hydrogels were tested. All were biocompatible | ||||||
| Period: 1 day | ||||||
| In vivo tests | ||||||
| Periodontitis rat model | ||||||
| Highest group: hydrogels loaded with drug/particles | ||||||
| Period: 2 weeks | ||||||
| Nanosilver-incorporated halloysite nanotubes/GelMA hybrid hydrogels | Photo-crosslinking | — | Nanoparticles | In vitro tests | Osteo-immunomodulation | 132 |
| Nanosilver | Human periodontal ligament stem cells | Bone regeneration | ||||
| Highest groups: no difference among the groups | ||||||
| Period: 7 days | ||||||
| In vivo tests | ||||||
| Female Sprague-Dawley rats | ||||||
| Highest group: hydrogels with nanotubes and nanoparticles | ||||||
| Period: 2 months | ||||||
| Methacrylic polyphosphoester and methacrylic gelatin hydrogels doped with dexamethasone-loaded ZIF nanocomposites | Photo-crosslinking | — | MOFs + drugs | In vitro tests | Anti-inflammatory activity | 133 |
| Zinc ions + dexamethasone | Human gingival fibroblasts and rat bone mesenchymal stem cells | Immunomodulation | ||||
| Highest groups: hydrogels with 0–30 μg mL−1 of nanoparticles | Regeneration | |||||
| Period: 2 days | Osteogenic differentiation | |||||
| In vivo tests | ||||||
| Periodontitis rat model | ||||||
| Highest group: only one hydrogel group was tested | ||||||
| Period: 4 weeks | ||||||
| Hydroxyethyl cellulose hydrogels containing iodine loaded into crosslinked cyclodextrin MOFs | Magnetic stirring at RT | — | MOFs + drugs | In vivo tests | Anti-inflammatory activity | 47 |
| Iodine loaded into crosslinked cyclodextrin MOFs | Experimental periodontitis rat model | Inhibition of bone resorption | ||||
| Highest group: only one hydrogel group was tested and it was biocompatible | ||||||
| Period: 4 weeks | ||||||
| GelMA hydrogels incorporated with ZIF-8 | Photo-crosslinking | In vitro tests | MOFs | In vitro tests | Bone regeneration | 134 |
| Fastest group: only one group of hydrogels was tested (>50%) | Zinc ions | Rat bone mesenchymal stem cells | Biomineralization | |||
| Period: 7 days | Highest groups: no difference among the groups | Anti-inflammatoryactivity | ||||
| Medium: collagenase-2 | Period: 7 days | |||||
| In vivo tests | ||||||
| Male Wistar rats | ||||||
| Highest group: hydrogels with ZIF-8 | ||||||
| Period: 4 weeks | ||||||
| Ionic hydrogels composed of gallic acid-modified chitosan and poly(N-hydroxyethyl acrylamide) combined with copper nanodots | Crosslinking | — | Nanoparticles | In vitro tests | Anti-inflammatory activity | 135 |
| Copper nanodots | RAW 264.7 cells | Antioxidant activity | ||||
| Highest group: neat hydrogels | ||||||
| Period: 1 day | ||||||
| In vivo tests | ||||||
| Female Sprague Dawley rat model with periodontitis | ||||||
| Highest group: no difference among the groups | ||||||
| Period: 7 days |
3.1. Hydrogels containing intrinsically antimicrobial peptides for periodontics
In the scope of periodontics, inherently antimicrobial peptides and polymers (mostly polysaccharides and polyzwitterions)136 as exemplified in the endodontics section above, can also be regarded as good options for the selection of an effective hydrogel material against oral pathogens. Recent improvements related to such antimicrobial hydrogels for periodontics were elaborated on below.Human saliva contains proteins including antimicrobial peptides as a defense mechanism against Porphyromonas gingivalis and a regulatory factor regarding the inflammatory process.137 Hence, natural host defense peptides could be undoubtedly counted as one of the most suitable raw materials to develop biocompatible and antimicrobial hydrogels for periodontics.
Recently, the intrinsic antimicrobial activity of self-assembling peptide hydrogels (β-sheet forming P11-4 and P11-28/29 hydrogels, consisting of 11 amino acids) was analyzed recently.113 Besides their inherent antimicrobial characteristics, these peptides were also tested as drug delivery platforms for tetracycline, ciprofloxacin, and doxycycline. The stock solutions of the drugs were prepared initially. Then, they were added to the peptide (P11-4 or P11-28/29)-containing buffer solutions by adjusting the amount of the drug according to the final concentration targeted. P11-28/29 hydrogels showed a significantly higher antibacterial effect on the periodontal pathogen Porphyromonas gingivalis compared to P11-4 hydrogels. Since the antimicrobial effect of these peptides was governed by their charge functionality (e.g., charge density, accessibility, and amphiphilicity), the low antimicrobial performance of P11-4 was associated with its overall negative charge caused by glutamic acid residues. On the other hand, the superiority of P11-28/29 was arisen from the highly positively charged P11-28 part, containing a vast amount of ornithine (i.e., an amino acid similar to lysine in structure). Nonetheless, both P11-4 and P11-28/29 hydrogels could not inhibit the growth of Streptococcus sanguinis, which was related to the differences in membrane compositions of these Gram-negative and positive bacteria. Yet, the mechanism has not been well-described. The drug release profiles were considered favorable up to 120 h and the drug incorporation did not alter the fibril formation involved in the self-assembly process. This study can be regarded as a good example of combining intrinsically antimicrobial peptides with drugs to increase their capacity to act as antimicrobial hydrogels or to introduce multifunctionality into biomaterials.
As injectable hydrogel systems, hydroxypropyl methylcellulose, hyaluronic acid, and glycerol were used to form thermo-sensitive hydrogels as intraperiodontal pocket materials to treat periodontitis.114 Then, a BMAP-27-derived recombinant antimicrobial peptide Bomidin was embedded into the hydrogels. The injectability of the hydrogels and in situ gelation (thermo-sensitive) features around body temperature were confirmed as well as their cytocompatibility by using human gingival fibroblast cells in vitro. The antimicrobial examinations (colony counting and zone of inhibition assays) demonstrated that the hydrogels containing Bomidin exhibited significantly stronger antimicrobial performances against Porphyromonas gingivalis, Staphylococcus aureus, and Escherichia coli compared to the hydrogel groups without Bomidin.
Among smart, stimuli-responsive hydrogels developed for periodontics, polyethylene glycol diacrylate/short antimicrobial peptide hydrogels loaded with stromal cell-derived factor-1 (namely chemokine CXCL12 proteins, capable of facilitating bone regeneration by recruiting endogenous stem cells) were designed.23 In this formulation, the specifically designed functional peptide module (i.e., anchor peptide + short antimicrobial peptide + anchor peptide) was blended with hydrogel solutions. The blend was stimulated for gelation at 37 °C (1st stimulus, thermo-responsive property). The cleavage of the anchor peptides was stimulated in response to gingipain (i.e., an enzyme secreted by Porphyromonas gingivalis), resulting in the release of the short antimicrobial peptides from the hydrogels (2nd stimulus, gingipain-responsive property). The hydrogels released the short antimicrobial peptides in a continuous and controlled way in the enzyme solution approximately within 10 d, which could not be observed in the other groups without the intelligent peptide module and led to an initial burst release. The growth of Porphyromonas gingivalis was strongly inhibited in the groups with the functional peptide modules. These hydrogels enhanced the migration and differentiation of periodontal ligament stem cells in vitro, in addition to the regulation of the inflammation process and osteogenesis in a rat periodontitis model triggered by Porphyromonas gingivalis infection in vivo. Such kinds of hydrogel designs can be formulated to treat certain types of diseases in a highly specific manner.
As multi-component hydrogels with multifunctionality, injectable and thermo-sensitive chitosan hydrogels were combined with antimicrobial peptides (Nal-P-113) and polydopamine nanoparticles for periodontitis therapy.115 The peptides and nanoparticles released from the hydrogels within 13 d. The antibacterial activity of Nal-P-113-containing hydrogel groups showed around 99% efficacy against Streptococcus gordonii, Fusobacterium nucleatum, and Porphyromonas gingivalis. Although the antimicrobial effect of chitosan is well-known, the impact of the peptides was mentioned prominently and presented in the results obtained during the antimicrobial activity measurements. The nanoparticle release resulted in 80% antioxidant activity. Further studies demonstrated that a synergistic therapeutic effect of the use of the antimicrobial chitosan/peptides and the antioxidant nanoparticles was observed in vivo regarding the suppression of alveolar bone loss and the inhibition of the local inflammatory response in the rat periodontitis model.
3.2. Hydrogels containing intrinsically antimicrobial polymers for periodontics
In periodontics, antimicrobial hydrogels have been prepared by using inherently antimicrobial polymers or adding antimicrobial moieties to the polymeric chains.122 In the literature, antimicrobial characteristics of some polymers were evaluated against microbes causing periodontitis and dental caries;138 however, they were not investigated in the hydrogel formulation. Instead of that, these polymers were added to the bacterial solutions to test their bactericidal activity. Herein, antimicrobial polymers-based hydrogel formulation for periodontics studies were exclusively discussed.In an herbal treatment study, thermo-sensitive hydrogels of poloxamer 407, alginate sodium, and cellulose derivatives (with a mixture of Scutellariae baicalensis radix extract and chitosan) were produced.116 The binary mixture of the plant extract and chitosan having a ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 was added in two amounts to the placebo samples to reach the final concentrations of 2 and 4%. Two placebo samples (containing deionized water, sodium alginate, hydroxypropyl cellulose, methylcellulose, poly(ethylene glycol)—PEG400, and poloxamer 407 in different formulations) were used to form hydrogels. The final hydrogels were prepared by mixing a specific amount of the binary mixture with the placebo samples. In addition to anti-inflammatory and antioxidant features, this plant extract had active units (i.e., baicalin) exerting antimicrobial action against periodontal pathogens. The synergistic effects of the plant extract and chitosan on ferrous ion-chelating and hyaluronidase inhibition were shown. Their synergistic effects on preventing bacteria growth for four types of Gram-positive bacteria (Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus mutants, and Actinomyces naeslundii), three types of Gram-negative bacteria (Escherichia coli, Proteus mirabilis, and Prevotella intermedia), two types of yeast-like fungi (Candida albicans and Candida tropicalis), and a Gram-positive lactic acid bacterium (Lactobacillus acidophilus) were revealed. This study represented the potential of herbal extracts and antimicrobial polymers utilized together to display synergistic effects on the antimicrobial performance of hydrogels.
500 was added in two amounts to the placebo samples to reach the final concentrations of 2 and 4%. Two placebo samples (containing deionized water, sodium alginate, hydroxypropyl cellulose, methylcellulose, poly(ethylene glycol)—PEG400, and poloxamer 407 in different formulations) were used to form hydrogels. The final hydrogels were prepared by mixing a specific amount of the binary mixture with the placebo samples. In addition to anti-inflammatory and antioxidant features, this plant extract had active units (i.e., baicalin) exerting antimicrobial action against periodontal pathogens. The synergistic effects of the plant extract and chitosan on ferrous ion-chelating and hyaluronidase inhibition were shown. Their synergistic effects on preventing bacteria growth for four types of Gram-positive bacteria (Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus mutants, and Actinomyces naeslundii), three types of Gram-negative bacteria (Escherichia coli, Proteus mirabilis, and Prevotella intermedia), two types of yeast-like fungi (Candida albicans and Candida tropicalis), and a Gram-positive lactic acid bacterium (Lactobacillus acidophilus) were revealed. This study represented the potential of herbal extracts and antimicrobial polymers utilized together to display synergistic effects on the antimicrobial performance of hydrogels.
In another report regarding the combination of two active components in a hydrogel, chitosan was used as a delivery platform for the antimicrobial agent thymol. The antimicrobial and antioxidant performance of thymol-loaded dodecenylsuccinic anhydride-modified chitosan hydrogels was evaluated in vitro and in vivo.117 During in vitro studies, complete inhibition of Staphylococcus aureus growth and a reduction in Pseudomonas aeruginosa growth were confirmed for 2 d. In addition, the free radical scavenger activity was observed for 5 d. On the other hand, during in vivo examinations, the periodontal damage decreased after a week in thymol-containing hydrogel groups compared to other groups in a periodontitis rat model. Using a similar strategy, the antimicrobial activity of in situ poloxamer 407/chitosan hydrogels loaded with two types of drugs, levofloxacin (against aerobes) and metronidazole (against anaerobes), were investigated.118 The injectable hydrogels with 1.5% w/v of chitosan were mucoadhesive and thermoresponsive, which could become a gel near body temperature. The drug-loaded hydrogels were more effective against Staphylococcus aureus, Escherichia coli, Vibrio cholera, Klebsiella pneumonia, and Proteus vulgaris up to 24 mm of the zone of inhibition. Yet, the hydrogel itself also led to a smaller zone of inhibition (2–4 mm) for only 4 strains of bacteria (Staphylococcus aureus, Escherichia coli, Vibrio cholera, and Klebsiella pneumonia) among all. This result was attributed to the intrinsically antimicrobial chitosan component of the hydrogels. Although there was not any possible reason for the unsuccessful antimicrobial action of chitosan on Proteus vulgaris mentioned in the report, in another previous study, similar findings were stated regarding the Proteus vulgaris growth on chitosan/alginate microspheres.139 The zone of inhibition for Staphylococcus aureus, Enterococcus faecalis, and Pseudomonas aeruginosa was recorded as higher than that of Proteus vulgaris. This implied that Proteus vulgaris might be more resistant to antimicrobials than its counterparts inhabiting the oral cavity.
Currently, the regenerative properties of hydrogels regarding periodontal class III furcation defects were evaluated. This report included the preparation of chitosan/β-glycerophosphate hydrogels loaded with bone morphogenetic protein-7 (BMP-7) and an antibiotic (ornidazole) and their applications in male beagles in vivo.119 Throughout the design of the hydrogels, β-glycerophosphate was incorporated to modulate the gelation properties whereas BMP-7 was utilized to stimulate cementogenesis, which is critical for the regeneration of the lost tissue in periodontal defects. Ornidazole governed the antimicrobial action together with chitosan so that chitosan/ornidazole-containing groups led to the formation of remarkably wider inhibitory halos around the hydrogels against Porphyromonas gingivalis, compared to those around the neat chitosan hydrogels. In another recent and multi-strategical study, PVA hydrogels were crosslinked by chitosan microcapsules loaded with metronidazole for periodontitis therapy, in which chitosan units were evenly decorated throughout the hydrogel volume since it was used as the crosslinker.120 These hydrogels showed sustained bacteriostatic and sterilizing performances against Porphyromonas gingivalis and Fusobacterium nucleatum for 14 d as same as the effect observed in the positive control (i.e., Periocline). All other hydrogel combinations including chitosan-alone, and metronidazole-alone groups contaminated before two weeks.
In a similar investigation, the antimicrobial features of flurbiprofen (anti-inflammatory)-loaded chitosan hydrogels with triclosan (antimicrobial)-loaded poly-ε-caprolactone (PCL) nanoparticles were examined.121 Indeed, the hydrogels were described as nanogels since they had 100–330 nm size in diameter as blank controls. Nanogels became larger (150–400 nm) once the drugs were loaded into them. The nanogels exhibited pH-dependent swelling and degradation behavior in the simulated saliva fluid. The drug-loaded formulations showed approximately 2 times higher antibacterial performance against Escherichia coli and Staphylococcus aureus than the groups that the drug mixture was applied directly (without using nanogels). This was ascribed to the enhancement of drug dissolution due to the decrement in crystallinity of the drugs within nanogel structures, which eventually led to enhanced drug solubility. The surface area for the drug release was amplified by the utilization of PCL nanoparticles inside the nanogels. The increment in the drug solubility and release assured the effective release of the antimicrobial drug from the hydrogel systems.
In another study, polymers with no antimicrobial activity were modified by chemical moieties to generate this action. GelMA polymers were combined with GelMA modified with quaternary ammonium groups (–NR3+) to prepare antimicrobial hydrogels.122 Although GelMA with a high degree of substitution of methacrylamide groups was appropriate to improve the degree of crosslinking, the number of free amino groups of this GelMA was not enough for the functionalization with quaternary ammonium groups in this case. The free functional groups were maintained more by using GelMA with a low degree of substitution of methacrylamide groups for further modification. By using these two types of GelMA, the crosslinking efficiency and antimicrobial property of the hydrogels could be optimized. Different hydrogel groups developed by changing the GelMA ratio (unmodified/modified: 50/50 or 25/75) were compared. These hydrogels exhibited a limited level of bacteriostatic effect against Porphyromonas gingivalis. However, the bacterial virulence was greatly diminished. This result might imply that the antimicrobial groups incorporated into GelMA might inhibit the bacterial enzymes such as gingipain, yet might not kill them directly. During in vitro studies conducted by using immortalized human gingival fibroblast cells, the regenerative and anti-inflammatory capacities of these hydrogels were observed higher than the control group (unmodified GelMA). GelMA loaded with chlorhexidine (the other control group) was reported as cytotoxic. These GelMA formulations were proposed as good alternatives to drug-containing treatments due to the harmful effects of drugs including potential carcinogenicity.
3.3. Hydrogels incorporated with antimicrobial drugs for periodontics
Antimicrobial agents utilized for endodontics mentioned in section 2.3 can also be considered good candidates to endow antimicrobial activity to hydrogels used in periodontics. Herein, the most recent hydrogel systems combined with antimicrobial additives, which were particularly designed for periodontal treatments, were discussed in detail. Antimicrobial drugs have been included in hydrogels to reduce their toxicity upon direct application by sustaining their release from the 3D hydrogels at the local defect site of periodontal pockets.Surfactin and Herbmedotcin®-loaded hydrogels composed of cellulose nanofibers and κ-carrageenan oligosaccharide nanoparticles were produced for periodontitis treatment.123 The composite hydrogels showed strong activity in terms of both zone of inhibition and reduction in biofilm formation against directly or indirectly periodontitis-related strains of bacteria; Streptococcus mutans, Porphyromonas gingivalis, Fusobacterium nucleatum, and Pseudomonas aeruginosa. The antimicrobial action was mainly governed by dual release of the antimicrobial agents; (i) Herbmedotcin® (i.e., a patented commercial antibacterial agent composed of super quantum dots and natural organic materials) with positive charges generating its antimicrobial action and (ii) surfactin mainly synthesized by Bacillus subtilis and defined as an antiviral, antibacterial, antifungal, and antimycoplasma biosurfactant. Carrageenan (i.e., a polysaccharide extracted from red seaweed) is also known for its antiviral features as well as anti-inflammatory property. These formulations were tested for multifunctionality, where it was verified that the hydrogels were antimicrobial, antioxidant, and anti-inflammatory.
Recently, poly(acrylic acid) hydrogels containing a periodontal drug (i.e., metronidazole) were fabricated by a one-step gamma-ray irradiation-induced crosslinking procedure.124 These bio-adhesive polymeric networks were suitable for drug loading and the gradual delivery of it. With the help of the controlled release of the drug, the antibacterial activity of the hydrogels was observed against Escherichia coli, Staphylococcus aureus, and Streptococcus mutans, which were highly correlated with the formation of dental caries and periodontitis. The antibacterial action of the hydrogel films increased in proportion to the increasing drug content. Additionally, the hydrogels were more effective against Streptococcus mutants compared to the other strains. The cytocompatibility of the hydrogels was also confirmed in vitro by using Swiss mouse embryo-NIH/3T3 fibroblasts. These hydrogels were suggested as good candidates for the treatment of periodontitis.
In another study, the antimicrobial property of doxycycline (antibiotic) and/or lipoxin A4 (anti-inflammatory)-loaded thermo-reversible polyisocyanopeptide (PIC) hydrogels was examined.21 Since high polymer concentrations may cause cytotoxic effects at the local site of application, PIC was selected due to its capability to form hydrogels in low polymer concentrations in a thermo-reversible manner, which eventually resulted in the syringeability of the solutions. In vivo studies experimented with dogs suffering from naturally occurring periodontitis showed that the bacterial growth of subgingival microflora decreased significantly in the groups containing lipoxin A4 and doxycycline/lipoxin A4. Interestingly, the anti-inflammatory drug (lipoxin A4) released from PIC hydrogels could also be effective concerning antimicrobial action without using the antimicrobial drug. This finding implied a strong correlation between immunomodulation and antimicrobial action. These lipoxin A4-loaded hydrogel groups exhibited anti-inflammatory effects by reducing interleukin-8 levels. The gingival attachment was enhanced around 0.6 mm more in these groups than the control treated with mechanical debridement (i.e., a conventional periodontal treatment including the removal of dental plaque by scaling, followed by root planning).
Alternatively, photothermal methods were also introduced into antibacterial drug delivery applications. In a recent report, curdlan (i.e., a water-insoluble polysaccharide synthesized by bacteria) with a linear (1→3)-β-glucan structure was modified by polydopamine in terms of swelling, gelling, and morphological properties to obtain a stable network in the hydrogel form.125 Then, curdlan/polydopamine composite hydrogels were loaded with acetate chlorhexidine. The antimicrobial action was synergistically created by both (i) the acetate chlorhexidine delivery triggered photothermally upon near-infrared light irradiation in a controlled manner and (ii) the thermal effect itself facilitated the bacterial membrane rupture and eventually caused cell death. It resulted in a 99.9% bacteriostatic rate for Staphylococcus aureus and Escherichia coli.
Similarly, GelMA hydrogels with gold nanobipyramids coated by mesoporous silica were developed for periodontal infections.126 The sustained release of minocycline could be involved in the system for the long-term owing to the ordered pores and favorable surface area of silica for high drug loads. Moreover, gold nanobipyramids served as photothermal agents with near-infrared tunability due to the strong surface plasmon resonance and photothermal conversion performance. The minocycline delivery was improved under the near-infrared light (808 nm) exposure by this system. The photothermal effect against the bacterial growth mentioned above for the curdlan/polydopamine composite hydrogels125 was observed in this study too. As a result, the hydrogels showed remarkable antimicrobial activity against Porphyromonas gingivalis. Such near-infrared light-responsive systems were less toxic to biological tissues due to the relatively small absorption and scattering coefficients of the near-infrared light compared to the UV light.
In a most recent report, another bacteria-derived polysaccharide was selected to treat chronic periodontitis with diabetes mellitus disease, in which reactive oxygen species (ROS) were produced extensively.127 ROS-responsive oxidized dextran and phenylboronic acid-functionalized poly(ethylene imine) hydrogels loaded with doxycycline (antibiotic) and metformin (oral anti-hyperglycemic agent) were fabricated for local drug release. A pronounced antibacterial effect on Staphylococcus aureus, Escherichia coli, and Porphyromonas gingivalis was reported. Furthermore, the synergistic therapeutic effect of the dual delivery of doxycycline and metformin was observed in a rat model having chronic periodontitis with diabetes mellitus in vivo.
In another multi-strategical research, metronidazole-loaded methacrylated-poly-γ-glutamic acid hydrogels containing chlorhexidine-loaded methacrylated-poly-γ-glutamic acid nanoparticles were developed as pH-responsive drug delivery platforms for periodontitis therapies.128 Metronidazole was a well-known antibiotic used for the treatment of periodontal infections while chlorhexidine was an antiseptic used as a gold standard to test newly developed drugs and drug delivery systems. The dual release of metronidazole from the hydrogels and chlorhexidine from the nanoparticles within the hydrogels exerted antimicrobial action against Escherichia coli on agar plates. The release of the drugs occurred within 12 h for metronidazole and 7 d for chlorhexidine in a strongly pH-dependent manner for the latter. The noticeable delay observed in the chlorhexidine release was associated with the location of the drug since it was loaded into nanoparticles and then encapsulated within the hydrogels. Such prolonged delivery can be considered beneficial not to load high doses of medicines with side effects for the host tissue, but to load less amount of them releasing in a controlled manner at the local defect site. Thus, it can sustain the delivery of the effective local dose of drugs for a long time.
3.4. Hydrogels incorporated with antimicrobial herbal extracts for periodontics
Synthetic drugs have been occasionally replaced by natural herbal extracts to eliminate the drawbacks of synthetic drugs. Interestingly, similar examples were not extensively produced for endodontics. Herbal antimicrobials can be also combined with micro-/nanoparticles to suppress oral infections in periodontics. One way or another, the reduction of drug exposure (by decreasing drug concentration, controlling their delivery, replacing them with green molecules, etc.) is the key concern to prevent the development of antimicrobial resistance led by constant antimicrobial drug intake.In a recent report regarding phytotherapeutics, thermo-reversible hydrogels containing green tea extracts were prepared due to their antimicrobial and antioxidant potential.129 The hydrogels released phytochemicals (i.e., epigallocatechin gallate) in an effective manner. After the antimicrobial activity of the green tea extract was confirmed against a variety of oral pathogens (Streptococcus mutants, Porphyromonas gingivalis, Prevotella intermedia, Fusobacterium nucleatum, and Aggregatibacter actinomycetemcomitans), the antimicrobial performance of the hydrogels was examined. The killing efficiency of the green tea extracts increased over time for all the tested bacteria while the level of efficacy varied depending on the pathogen type. Meanwhile, the hydrogels containing green tea extracts resulted in a significantly larger zone of inhibition against Porphyromonas gingivalis than that of the control (i.e., chlorhexidine gel – the gold standard antiplaque agent used in periodontitis treatments). Such herbal replacements with antimicrobial drugs may have a promising future in dental infection treatments regarding the effective release of antimicrobial phytochemical moieties (e.g., green tea which is rich in flavanols and gallic acid derivatives stated in the study above) and, should be explored more.
In another nanocomposite hydrogel formulation, gold nanoparticle-modified PVA hydrogels loaded with epigallocatechin gallate were prepared.130 The herbal component epigallocatechin gallate (i.e., a polyphenol found in green tea) was chosen since it possesses many features including antibacterial, antioxidant, and osteoprotective characteristics. The hydrogels displayed a photothermal ability in response to the exposure to near-infrared light which facilitated the epigallocatechin gallate release. During molecular biology assays, the composites showed significant inhibitory effects against the biofilm formation of Staphylococcus aureus and Escherichia coli. These hydrogels facilitated bone regeneration and angiogenesis in vitro. Bone tissue regeneration was also promoted during in vivo studies as well as the antimicrobial action of the hydrogels was confirmed.
3.5. Hydrogels incorporated with antimicrobial micro-/nanoparticles for periodontics
As described in the endodontics section, it is a versatile strategy to mix hydrogels with antimicrobial constituents other than neat antimicrobial drugs. Nowadays, advancements regarding hydrogels incorporated with antimicrobial micro-/nanoparticles (e.g., spheres, tubes, fibers, metallic particles, etc.) have involved more variety in periodontics, compared to endodontics. For instance, MOFs were incorporated into hydrogels for the treatment of periodontal infections.Silver nanoparticles were recently incorporated into hydrogels for periodontics. PVA/chitosan composite hydrogels containing silver nanoparticles (spherical, ∼9 nm in diameter) were produced by double crosslinking, namely freeze-thawing as well as electrostatic interactions.131 Their mechanical properties could be altered by changing (i) the fractions of PVA and oxalic acid (i.e., the crosslinker) to chitosan and (ii) the number of freeze–thaw cycles. Stiffer hydrogels could be produced by increasing these parameters mentioned. In this way, the nanoparticle release was lowered remarkably. Such control over metallic nanoparticle release is critical for biocompatibility. Concentrations of silver nanoparticles were changing within the interval of 0 to 8.3 wt%. The hydrogels exhibited antimicrobial activity against Staphylococcus aureus, Klebsiella Pneumoniae, and Porphyromonas gingivalis when the silver nanoparticle concentration was even low as 0.33 wt%. However, cytotoxicity increased for primary human dermal fibroblast adult cells at higher nanoparticle concentrations. The most convenient concentrations of silver nanoparticles were determined as 0.7 and 1.4% with effective antimicrobial action and adequate cytocompatibility for periodontal treatments. Nevertheless, there were no evaluations reported regarding the injectability of these stiff hydrogels or the ease of their application during implantation.
Recently, an antimicrobial drug (minocycline) and metal oxide nanoparticles (zinc oxide – ZnO) were combined in a single hydrogel formulation to facilitate the disinfection performance of the biomaterial for periodontitis treatment. Carbopol 940® (polymer of acrylic acid) hydrogels were prepared, which contained serum albumin microspheres loaded with minocycline and ZnO nanoparticles.22 The results demonstrated that the combination of minocycline and ZnO nanoparticles exhibited the expected synergistic effect on amplifying the antimicrobial action against Streptococcus oralis, Porphyromonas gingivalis, Streptococcus sanguis, and Prevotella intemedia. In another metallic nanoparticle-containing study, silver nanoparticles-incorporated halloysite nanotubes/GelMA hybrid hydrogels were engineered to investigate the osteoimmunomodulatory and antimicrobial properties in vitro and in vivo.132 Nanosilver was chosen due to its superior biocompatibility compared to silver and its possible anti-inflammatory features. In this specific design, halloysite nanotubes (i.e., naturally occurring aluminosilicate nanotubes) were utilized to conjugate silver nanoparticles to the GelMA hydrogel network by strong electrostatic interactions so that antibacterial activity would be sustained. The use of halloysite nanotubes led to the formation of a specific nanotopography for osteoimmunomodulation. During the inhibition zone tests, the hydrogels containing silver nanoparticles and halloysite nanotubes displayed a broad-spectrum antibacterial activity against Escherichia coli and Staphylococcus aureus.
Recently, several studies regarding the use of MOFs have been introduced in periodontics. Methacrylic polyphosphoester and GelMA hydrogels doped with dexamethasone-loaded MOFs were fabricated as an injectable formulation.133 It is well-known that porous zeolitic imidazolate frameworks-8 – ZIF-8 (i.e., a type of zinc-based MOFs), possess remarkable antibacterial properties owing to the gradual metal ion release from the MOFs in an acid-responsive manner. It makes these MOFs favorable to trigger the antimicrobial release in the acidic microenvironment of dental plaques. To this extent, zinc-based MOFs were designed specifically to be loaded with dexamethasone to provide anti-inflammatory and antimicrobial features for periodontitis treatment. After the addition of MOFs into the hydrogels, the antibacterial efficacy was enhanced against two common dental plaque bacteria strains (i.e., Streptococcus mutants and Porphyromonas gingivalis) compared to the control. The zone of inhibition in the hydrogel groups with the neat MOFs was statistically higher than that of the hydrogels with dexamethasone-loaded MOFs since the release of zinc ions from the MOFs decreased slightly in the presence of dexamethasone. Together with the anti-inflammatory effect of dexamethasone, these nanocomposite hydrogels dampened the periodontal inflammation in rats with periodontitis while diminishing bone loss in vivo.
In a parallel MOF study, MOFs crosslinked with cyclodextrin were loaded with iodine and then they were suspended in hydroxyethyl cellulose hydrogels to treat periodontal pocket formations in periodontitis.47 Since iodine is a broad-spectrum antimicrobial agent, it could effectively act against the growth of bacteria, viruses, fungi, and chlamydia without any bacterial resistance. The superior porosity and surface area of MOFs could facilitate a sustained release pattern for iodine at the local defect region. The results revealed that the hydrogels released iodine in a prolonged manner in response to artificial saliva stimulation. That means that the sustained iodine release would be achieved in a simulated oral environment. Nonetheless, there were no antimicrobial studies conducted in this report, despite the expected antimicrobial action of the iodine delivery. Instead of the antimicrobial tests in vitro, the hydrogels were evaluated using an experimental periodontitis rat model. The hydrogel groups exhibited a similar efficacy to the control group – minocycline hydrochloride ointment (i.e., a common antibiotic used in periodontitis) regarding the reduction in the depth of periodontal pocket in vivo. In other instances of MOF applications, GelMA hydrogels were incorporated with ZIF-8 for the treatment of periodontitis.134 The injectability of the hydrogels was confirmed and they exhibited antimicrobial action against Porphyromonas gingivalis through the sustained Zn2+ ion release from ZIF-8 embedded into the hydrogels. In vitro studies demonstrated that these hydrogels were non-toxic and promoted osteogenic differentiation of bone mesenchymal stem cells as well as biomineralization on ECM. The hydrogels enhanced bone regeneration in rat models with alveolar bone defects while microbial growth and inflammation were alleviated in rat models with periodontal inflammation.
As another example of antimicrobial nanoparticles, copper nanodots were combined with dual network hydrogel systems of gallic acid-modified chitosan and poly(N-hydroxyethyl acrylamide).135 In this formulation, adhesive properties of the hydrogels (thus, penetration through gingival tissue) were improved by adding choline glycolic acid ionic liquid, inspired by mussels which possess polyphenols attaching to tissues by hydrogel bonds in nature. For generating antimicrobial and antioxidant characteristics, copper nanodots were utilized having triple enzyme-like activities; (i) peroxidase-like activity promoted antimicrobial action under acidic conditions, while (ii) superoxide dismutase- and (iii) catalase-like activities render the hydrogels free radical scavengers under neutral conditions. The antimicrobial activity of the hydrogels was proven against Staphylococcus aureus, Escherichia coli, and Streptococcus mutans by the plate counting method, whereas anti-inflammatory properties were confirmed in vitro and in vivo.
According to the recent studies reported here, antimicrobial hydrogels were mostly studied in the field of periodontics, compared to endodontics. One of the reasons for this phenomenon could be anatomical features of periodontal region. Since it is open and connected throughout the mouth, periopathogens can transfer by saliva, and tongue contact. However, dental pulp is more isolated compared to periodontal tissue, which leads to a limitation of the spreading of infections from one tooth to another.
In periodontics, a more diverse array of materials and formulations were utilized compared to endodontics. Some antimicrobial hydrogels in periodontics were designed in a highly specific manner (e.g., gingipain-responsive hydrogels as described above), which cannot work for endodontics. It might be speculated that the state-of-the-art antimicrobial hydrogels would be a step further in the future applications of antimicrobial hydrogels. Undoubtedly, these advancements would also shed light on the way of novel applications for endodontics.
4. Challenges and future prospects
Combining hydrogels with antimicrobial properties brings advantages to treat dental pulp and periodontal defects since infections are among major causes of dental health issues. While researchers have been working on the development of the antimicrobial hydrogel design, various strategies are proposed to overcome the challenges in this field. Undoubtedly, biocompatibility is considered one of the most essential concerns for successful hydrogel/scaffold production as described above. To fabricate more tissue-friendly hydrogel composites, the incorporation of non-toxic additives into hydrogels and/or selection of biocompatible materials are one of the best approaches (such as the utilization of peptides or polymers with intrinsic antimicrobial features). Antimicrobial action can be prolonged by using these inherently antimicrobial materials. Nevertheless, they might lack pronounced antimicrobial efficacy for a broad spectrum of pathogens inhabiting the dental environment unless these hydrogels are combined with another antimicrobial ingredient. The incorporation of antimicrobial agents (such as drugs or metallic nanoparticles) into hydrogel systems exhibit higher performance against microbial growth than bio-derived materials, although they might be cytotoxic at high doses utilized to ensure broad spectrum action. As a challenging factor, long-term antimicrobial activity with biosafety is an issue for these hydrogel systems. Nowadays, it has been tried to be maintained by putting both together; (i) intrinsically antimicrobial hydrogels upon their contact with the pathogens and/or (ii) the controlled release of antimicrobial moieties from the hydrogels. To endow long-term antimicrobial action, the release kinetics of antimicrobials can be fine-tuned by playing with biodegradation or chemical features of the hydrogels. Also, antimicrobial herbal extracts can be introduced to hydrogels to eliminate antimicrobial resistance caused by long-term exposure to synthetic drugs. Besides their chemical properties, the physical features of hydrogels are also important to achieve easy application and physical compatibility with the tissue itself, as described above. Advanced 3D printing techniques are promising candidates to generate tissue-mimicking morphology and other properties; however, the limitation for such formulations might be difficulties in implantation. Due to the unique anatomy of these dental tissues, injectable and/or stimuli-responsive hydrogels taking the shape of the defect after implantation are superior to others as non-invasive applications. Other matters postponing tissue regeneration such as inflammation are listed among the limitations that can affect the performance of the hydrogels. Since tissue regeneration is a complex process, multifunctional hydrogels are advantageous to promote the healing by not only eliminating infections at the defect site but also modulating biological processes and overall host tissue response. The schematic representation of challenges and prospects is shown in Fig. 4.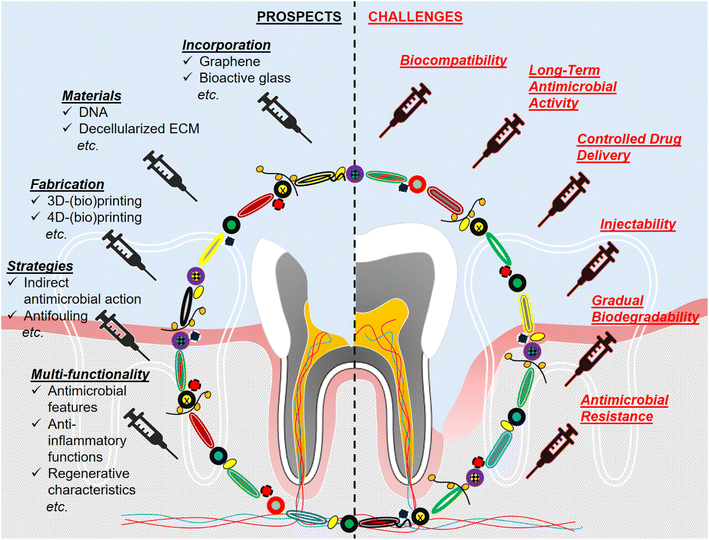 | ||
| Fig. 4 Graphical illustration of prospects and challenges regarding the antimicrobial hydrogel design in endodontics and periodontics. | ||
For translations of antimicrobial hydrogels into clinics, the above-mentioned limitations should be addressed. Up to date, several trials on patients have been reported regarding the use of antimicrobial hydrogels in endodontics and periodontics. In a series of case studies, methylcellulose hydrogels loaded with double or triple antibiotic pastes were tested to patients’ necrotic permanent immature teeth for regenerative endodontics.140 The symptoms of the disease totally disappeared after the application of the hydrogels with triple antibiotic pastes and control medicaments (i.e., the standard calcium hydroxide treatment) when the patients were examined after 12–24 months, although the pulp vitality could not be achieved. In a clinical trial to treat periodontitis, pluronic F-127 hydrogels with curcumin were applied to patients’ periodontal pockets locally.141 After 6 months, the growth of periopathogens (Porphyromonas gingivalis, Prevotella intermedia, Fusobacterium nucleatum, and Fusobacterium capnocytophaga) were significantly reduced by the hydrogel treatment. In another application for chronic periodontitis, hydrogels composed of carbopol (934) polymer and flax seed (i.e., Linum usitatissimum) oil (antimicrobial agent) was tested on 20 patients.142 Also, flurbiprofen (anti-inflammatory agent) was combined with the carbopol as a second hydrogel group and again tested on 20 patients, besides the control group (20 patients). As a result, flax seed hydrogels enhanced the periodontal healing most among the groups after 3 months. Even though a few other trials were reported,143 it seems that there is still a gap in the biomedical field to provide antimicrobial hydrogels for endodontics and periodontics.
4.1. Biocompatibility and tissue regeneration
Cytotoxicity is a problem faced when antimicrobial materials are aimed to be involved in hydrogels that will be in contact with body parts. Alternative antimicrobial agents with less cytotoxicity and more efficient antimicrobial performance against a wide range of pathogens would be greatly preferable in this aspect. For instance, carbonaceous materials (e.g., graphene) are among the convenient additives for hydrogels to provide antimicrobial, antiviral, and antifungal features.144,145 Such graphene derivatives were investigated in dentistry to induce bone repair, implant coating, etc.146–148 However, to our knowledge, there has not been any research reported on neither endodontics nor periodontics about their utilization within hydrogels so far, which particularly aim to mimic the soft part of native tissue for soft tissue regeneration, including antimicrobial activity measurements. Furthermore, graphene-based implants had a risk of mechanical failure after implantation due to potential structural defects formed during the manufacturing process for hard tissue regeneration.149 Yet, it would not pose difficulties for its applications of soft tissue equivalents. Graphene-containing hydrogels can be designed for dental pulp regeneration since a high mechanical strength is not a requirement in this field. In addition, even though further research is still needed, owing to their intrinsic electroconductive characteristics, carbon-based materials might enhance neuroregeneration besides their antimicrobial action.150 It would support the entire tissue repair during dental pulp regeneration since neuroregeneration is a slow process and often hard to achieve. Nonetheless, it is worth noting that the critical properties of graphene-derived materials (e.g., biocompatibility, biodegradability, antimicrobial activity, and drug loading capability) could remarkably change depending on their size, shape, modifications, structural defects, purity, and dosage.148 Optimization studies are required before clinical use.As another aspect of developing biomaterials in the form of hydrogels for endodontics and periodontics, bioactivity for the effective promotion of tissue regeneration stands as a concern. In this regard, bioactive glasses, (i.e., a type of synthetic surface-active bio-ceramics with the original composition of 45 wt% SiO2, 24.5 wt% CaO, 24.5 wt% Na2O, 6.0 wt% P2O5), or other compositions, have been taking outstanding attention due to their highly bioactive behavior as a scaffold material. When bioactive glasses were placed into the defect site, they exhibited resorbable characteristics. Hence, their dissolution products and ionic exchanges could facilitate bone bonding and modulate protein adsorption, leading to cell adhesion, differentiation, and biomineralization as well as angiogenesis.16,151,152 Apart from bone tissue engineering, such integration of scaffolds with the surrounding tissue is essential in dental applications too (e.g., to trigger dentin mineralization at the walls of dental pulp during the natural restoration process or bone formation around the periodontal pocket). Furthermore, the regenerative potential of bioactive glasses was demonstrated not only for hard but also for soft tissues with limited renewal capacity.153 Bioactive glasses were incorporated into hydrogels to investigate their osteoconductivity inside hydrogels and also to test the changes in the elasticity of hydrogels.151 As evidenced earlier, antimicrobial features could be added to bioactive glass particles by doping metal ions (e.g., silver, copper, and iron) into the molecular structure.154 However, these bioactive glass formulations combined with hydrogels have not been studied extensively within the focus of endodontics and periodontics showing an antimicrobial action against a broad spectrum of pathogens. Nevertheless, there are a few recent reports that confirm the antimicrobial and anti-inflammatory activity of silver-doped bioactive glasses in hydrogels for dental pulp regeneration.79,155 More of those hydrogel studies would be appreciated in the field of dental tissue engineering in the future, owing to the great potential of bioactive glasses.
Natural-origin materials have been mostly bringing superior characteristics to artificial tissue constructs regarding biodegradation, biocompatibility, and enhancement of biological processes compared to synthetic counterparts. Decellularized ECM hydrogels are one of the favorable substances to stimulate new tissue formation at the damaged region since ECM is a naturally secreted network of macromolecular structures that support cells and distribute throughout the intra-spaces within tissues, providing micro-skeletons essential for cell/tissue growth.156 The scaffolds composed of decellularized natural tooth bud ECM were the only biomaterial capable of facilitating tooth regeneration most (i.e., closest to the real size of a natural tooth) with adult dental cells.157 As another ECM utilization, the powder-like demineralized bone matrix has been widely applied in periodontal and orthopedic regeneration; yet they were not in the category of hydrogels.158 Several studies reported ECM hydrogels for endodontics159–161 and periodontics162 without any antimicrobial properties. In the biomedical field, decellularized ECM hydrogels with the incorporation of antimicrobial activity (i) by integrating it with thiolated chitosan for skin regeneration,163 (ii) by combining with chitosan for bone regeneration,164 and by loading with drugs for bone regeneration165 were developed recently. Such antimicrobial hydrogels can be good candidates for potential dental tissue engineering applications. In the dental field, natural ECM hydrogels containing bioactive glass ceramic with silver ions were designed as antimicrobial hydrogels for endodontics,166 even though it was not reported very recently. These hydrogels were prepared by using a porcine urinary bladder matrix as a raw material. The bladder matrices were decellularized to produce the ECM-based hydrogels, which was followed by mixing the pre-gel of ECM with a silver-doped bioactive glass powder. The hydrogel composites showed a long-lasting antimicrobial activity against Escherichia coli and Enterococcus faecalis bacteria, commonly faced during dental pulp infections. Therefore, further research on ECM hydrogels (e.g., different tissues as an ECM source and in vivo studies) deserves specific attention particularly for endodontics and periodontics at this stage of the state-of-the-art.
4.2. Anti-inflammatory response
As one of several key phenomena diminishing tissue regeneration regarding endodontics and periodontics, the inflammatory response of the body must be taken into account while developing strategies in dental tissue engineering. Infections trigger the inflammation process in the body, which in fact, is a defense mechanism. However, under severe inflammatory conditions caused by chronic infections, harsh external stimuli such as a dramatic change in pH or temperature due to the intensely provoked host response become uncontrollable for the host cells. In the presence of repetitive microbial colonization, inflammation might substantially end up in necrosis of the tissue.155,167,168 Hence, anti-inflammatory characteristics would be better to be incorporated into novel hydrogels to overcome the detrimental impacts of infections from different aspects, instead of spotting only antimicrobial features. Several examples of such multifunctional hydrogels developed for dental treatments were reported recently.21,23,121,133 Nevertheless, it can still be considered as a new area that should be explored more to assure a synergistic impact of antimicrobial and anti-inflammatory actions on infected and thus, inflamed tissue neogenesis towards future directions of endodontics and periodontics.4.3. Indirect antimicrobial action
Many strategies have been focusing on the removal of the infection by killing the pathogens in a regular approach to an antimicrobial hydrogel design. Indeed, inhibition of microbial adhesion to surfaces, namely antifouling, is the blockage of the initial stage of potential pathogen accumulation. It could serve well since it stops the process of microbial growth before a well-established infection, which is harder to remove once formed. In this regard, a few hydrogels with antifouling features have been introduced to treat or coat implant surfaces and dental devices due to their ability to diminish biofilm formation and foreign body reaction against the implant.169,170 Recently, injectable antifouling hydrogels composed of ABA triblock copolymer (A block: catechol functionalized polyethylene glycol and B block: poly[2-(methacryloyloxy)-ethyl]trimethylammonium iodide) were fabricated through self-assembly for biomedical applications and displayed self-healing property.171 Such kind of multifunctionality could be validated as promising with the antifouling, antimicrobial, and self-healing performance of hydrogel upon injection into pulp cavity for endodontics. Through the path of periodontics, a current study was reported which included a novel antifouling hydrogel system consisting of self-strengthening features. The hydrogel was initially easily injectable and comfortable for the surrounding soft tissue upon injection, followed by a gradual strengthening by crosslinking.172 Antifouling ability was assured by the poly(sulfobetaine methacrylate-co-glycidyl methacrylate) part of the hydrogels for 8 d. Considering the potential advancements, future investigations related to endodontics and periodontics can serve as complementary modalities assisting the active antimicrobial performance of the hydrogels. Nevertheless, hydrogels possessing only antifouling features may not be rated as highly effective for enclosed defects.Instead of depending on the direct effect of antimicrobials incorporated into hydrogels, novel strategies regarding indirectly generating an antimicrobial influence against microbial growth without utilizing any antimicrobial additives have been introduced to the area of hydrogel applications for dental tissue regeneration. Most recently, hyaluronic acid and xanthan hydrogels containing an extracellular oxygen carrier M101 (i.e., hemoglobin extracted from the blood of a marine worm Arenicola marina which can carry 40 times higher amount of oxygen than human hemoglobin does) were prepared.46 In this study, local oxygen delivery was aimed to prevent hypoxia in the periodontal pocket so that the pocket microenvironment would be bactericidal for anaerobic Porphyromonas gingivalis. Hence, the bacteria would not impair healing by accumulating subgingival biofilms. Besides, the potential anti-inflammatory features of M101 were reported.173 On the other hand, oxygen-generating biomaterials are advantageous in many aspects such as providing sufficient oxygen for the host tissue cells migrating through the scaffolds with the lack of vasculature.174 Taken together, further research on similar approaches holds promise not only to overcome infections in a non-toxic and effective manner but also to promote host cell viability in the future of endodontics and periodontics.
4.4. Novel biomaterials and multifunctionality
To generate multiple functions by using a single natural raw material has been a tough goal to achieve till the last few years. Fortunately, deoxyribonucleic acid (DNA) hydrogels possessing biologically active properties in various ways were introduced to the biomedical area recently. Throughout the novel hydrogel science, a block copolymer and polyanion DNA have been used as a raw material with immense unique and superior features such as self-assembly governed by Watson–Crick base-paring rules, nano-sized controllability, responsiveness upon pH, enzyme, ions, and biomolecules after a particular design, intrinsic biocompatibility, controlled phase transformation, tunable mechanical properties, and biodegradability as well as advanced drug delivery by specific molecular recognition with target region and well-integration with other bioactive agents for synergistic therapeutic impact.175–177 Despite all superior properties, DNA hydrogels have been rarely prepared in dental tissue engineering so far. Very recently, DNA hydrogels encapsulating cytokines with anti-inflammatory action were fabricated for the treatment of diabetic alveolar injury in the periodontal region.178 These injectable hydrogels sustained a long-term release of cytokine interleukin-10 for immunomodulation while possessing biodegradability and osteogenic properties, making them promising materials to be utilized as therapeutics in periodontal diseases. Such injectable DNA hydrogels are still in need of being further explored and evaluated through antimicrobial performance in endodontics and periodontics.4.5. Advanced additive manufacturing techniques
3D-printing (without cells) or 3D-bioprinting (with cells) of hydrogels is highly prominent to have control over the internal and external morphology of the structure with the utilization of newly emerging bioinks. Such precise control over the design would be expected to be able to facilitate homogeneous vascularization, regeneration, and dispersed ECM deposition throughout the scaffold.100,179 Recently, 3D-printed hydrogels (alginate/gelatin) enhanced dental pulp stem cell adhesion, proliferation, and differentiation compared to traditional counterparts for dental tissue engineering.180 Nevertheless, non-injectable features were the challenges for those systems, rendering them invasive approaches with a need for open surgery for the implantation. Among antimicrobial 3D-printed hydrogels developed for biomedical applications, methacrylated o-acetyl-galactoglucomannan (i.e., a photo-cross-linkable polysaccharide) hydrogels with nanocomposite lignin nanoparticles surface-embedded with silver nanoparticles were fabricated by digital light processing.101 The hydrogels showed antimicrobial performances against Escherichia coli and Staphylococcus aureus, together with the limitation of the non-syringeable property again. As another example of bioinks, methacrylated whey protein isolates (derived from milk) have been recently introduced into the biomedical field.181 These photo-cross-linkable bioinks could be 3D-printed into hydrogels, microspheres, and other patterned shapes. Although the antimicrobial properties of whey protein were reported, these 3D-printed materials were not examined regarding their antimicrobial performance. In the category of synthetic polymers, hydrogel inks were produced by 3D-printable triblock copolypeptides recently.182 By designing the blocks, the properties of the final product could be governed involving antimicrobial characteristics for biomedical applications. Taken together, there are even fewer studies that can be encountered at the intersection of the 3D-printed hydrogels and antimicrobial functions in endodontics and periodontics. Most recently, composite antimicrobial hydrogels consisting of carbon nanotube, chitosan, and sodium alginate were developed for the treatment of periodontitis.49 Their cytocompatibility was confirmed using human periodontal ligament cells. Also, these hydrogels exerted an antimicrobial action against Porphyromonas gingivalis.Limitations regarding the predetermined shape of 3D-printed hydrogels and their non-injectable properties have been attempted to be resolved by more sophisticated techniques. 4D printing has been recently introduced into the medical field with an extra dimension “time”, in which specifically designed 3D-printed material would be able to respond to external stimuli through alternations in its morphology, function, etc. by the time.183 Shape memory polymers and hydrogels were stated as one of the most favorable smart materials for 4D printing applications because they can autonomously be converted into a desired configuration/state upon exposure to changes in environmental factors such as moisture or ion concentration. Then, they can turn back to their original 3D-printed form once the stimulus is removed.184,185 Future applications of such hydrogels designed by 4D-printing approaches can be regarded as quite promising for soft and hard tissue regeneration in endodontics and periodontics owing to their potential self-adaptability into irregular cavities and curved defects along the narrow volume of oral space after implantation.186 The current focus of 3D & 4D printing has been mainly concentrated on prosthodontics in the field of dentistry, instead of endodontics and periodontics.185,187 Moreover, 4D-printed hydrogels have been receiving attention for disease-specific and condition-dependent drug delivery formulations involving the precise release of multiple bioactive agents and control over the release time and the targeted local release region in an intelligent manner.186–188 Such kind of drug delivery platforms or stimuli-responsive biomaterials sensitive to intrinsic (pH, enzyme, ROS, etc.) and extrinsic (temperature, light, ultrasound, mechanical force, electric or magnetic fields, etc.) signals189 would surely stand for ground-breaking era regarding the impactful antimicrobial action of dental hydrogels. Antimicrobial bioinks such as chitosan could also be incorporated into these formulations to trigger inherent antimicrobial action, besides the multifunctionality of hydrogels.187 Any improvement in antimicrobial 4D hydrogel design may prevail over a requirement in endodontics and periodontics.
5. Summary
The oral cavity is a moist environment continuously exposed to food contact and open air. None of the other tissues in the body have such features regarding the tendency to get contaminated by pathogens. Thus, dental infections stand for a global challenge due to daily pain, discomfort, and many other complications directly related to their persistent presence in the mouth, which ruin the life quality of patients. This situation directs dental therapies towards the elimination of infections in the first place to achieve the ultimate goal – dental regeneration. Common sterilization techniques are often unable to remove all pathogens in the open defect site in oral space while being toxic from mild to moderate for the host tissue. Moreover, small-volume defects mostly faced in endodontics and periodontics are hard to treat due to constraints regarding physical access and isolation of the defect region after the therapeutic operation. To this extent, innovative strategies are required, in which antimicrobial hydrogels with injectable characteristics are superior among all tissue-engineered constructs including scaffolds with a predetermined shape.• Throughout the findings, it is worth noting that intrinsically antimicrobial hydrogels are preferable for a prolonged antimicrobial action since the hydrogel itself acquires the desired chemical features. Thus, it is supposed to be antimicrobial until it completely degrades within the damaged tissue site. Since such an antimicrobial property is inherent, it cannot be improved more unless desired modifications or incorporations into the hydrogel material are carried out.
• Antimicrobial drug delivery can be aimed at achieving a higher efficiency compared to intrinsically antimicrobial hydrogels. In this case, the amount of drug loaded as well as the release mechanism and duration can be fine-tuned by playing with constituent components of the hydrogels. For instance, hydrogel composition, stiffness, and degradation features could be optimized, besides drug encapsulation efficiency by micro-/nanoparticles.
• Drug delivery platforms can be regarded as advantageous to introduce several drugs for multiple purposes to enhance tissue regeneration in various ways, instead of just targeting the inhibition of the microbial infection. Nevertheless, antimicrobial resistance and biocompatibility always remain critical concerns that should be addressed once the drug-releasing systems are involved in any therapy.
• Hydrogels composed of DNA or decellularized ECM can be superior alternatives to ensure biocompatibility and other functions at the same time in the path of novel approaches. Multifunctional hydrogel formulations possess an incredible potential to cure the infection-associated tissue loss encountered in endodontics and periodontics. In most cases, antimicrobial action alone is not enough to overcome the problems in the way of regeneration starting from the severe infection state and turning to the natural (healthy) state back. Such complete regeneration is the topmost goal of assisted healing in dental tissue engineering.
• Anti-inflammatory and antifouling features will be welcomed as complementary properties and effective enhancers of the performance of antimicrobial hydrogels. These processes (e.g., infection and inflammation) are interpenetrating with one another prominently in the body so that they should not be deliberated separately. Indeed, innovative biomaterials such as graphene and bioactive glasses are deemed to be auspicious candidates in this content. For instance, bioactive glasses can be utilized to facilitate bone regeneration and biomineralization of tissue.
• Regarding novel fabrication methodologies, the research on 3D- or 4D-(bio)printing using bioinks has been accumulating currently. However, a few reports concerning antimicrobial hydrogels have been placed in endodontics and periodontics so far. Thus, antimicrobial bioinks possess great potential to fill the gap in the development of 3D- or 4D-printed hydrogels with intrinsic antimicrobial action, besides numerous advanced properties (e.g., precise morphological features with stimuli-responsiveness).
Author contributions
Deniz Atila: conceptualization, writing – original draft, visualization, writing – review and editing; Vignesh Kumaravel: conceptualization, supervision, writing – review and editing, validation, resources, project administration.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was conducted as a part of the International Research Agendas PLUS programme of the Foundation for Polish Science, co-financed by the European Union under the European Regional Development Found (MAB PLUS/2019/11).References
- A. Righolt, M. Jevdjevic, W. Marcenes and S. Listl, Global-, regional-, and country-level economic impacts of dental diseases in 2015, J. Dent. Res., 2018, 97(5), 501–507 CrossRef CAS PubMed.
- S. Listl, J. Galloway, P. Mossey and W. Marcenes, Global economic impact of dental diseases, J. Dent. Res., 2015, 94(10), 1355–1361 CrossRef CAS PubMed.
- J. G. S. Souza, M. M. Bertolini, R. C. Costa, B. E. Nagay, A. Dongari-Bagtzoglou and V. A. R. Barão, Targeting implant-associated infections: titanium surface loaded with antimicrobial, iScience, 2021, 24(1), 102008 CrossRef CAS PubMed.
- L. Gao, T. Xu, G. Huang, S. Jiang, Y. Gu and F. Chen, Oral microbiomes: more and more importance in oral cavity and whole body, Protein Cell, 2018, 9(5), 488–500 CrossRef CAS PubMed.
- M. Tsukasaki, RANKL and osteoimmunology in periodontitis, J. Bone Miner. Metab., 2021, 39(1), 82–90 CrossRef CAS PubMed.
- L. M. Estes Bright, M. R. Garren, M. Ashcraft, A. Kumar, H. Husain, E. J. Brisbois and H. Handa, Dual Action Nitric Oxide and Fluoride Ion-Releasing Hydrogels for Combating Dental Caries, ACS Appl. Mater. Interfaces, 2022, 14(19), 21916–21930 CrossRef CAS PubMed.
- P. P. Coll, A. Lindsay, J. Meng, A. Gopalakrishna, S. Raghavendra, P. Bysani and D. O'Brien, The prevention of infections in older adults: oral health, J. Am. Geriatr. Soc., 2020, 68(2), 411–416 CrossRef PubMed.
- W. Thomson and Y. Barak, Tooth loss and dementia: a critical examination, J. Dent. Res., 2021, 100(3), 226–231 CrossRef CAS PubMed.
- B. Luo, Q. Pang and Q. Jiang, Tooth loss causes spatial cognitive impairment in rats through decreased cerebral blood flow and increased glutamate, Arch. Oral Biol., 2019, 102, 225–230 CrossRef CAS PubMed.
- D. Koletsi, A. Iliadi, G. N. Tzanetakis, M. Vavuranakis and T. Eliades, Cardiovascular disease and chronic endodontic infection. Is there an association? A systematic review and meta-analysis, Int. J. Environ. Res. Public Health, 2021, 18(17), 9111 CrossRef PubMed.
- A. F. Fouad, Diabetes mellitus as a modulating factor of endodontic infections, J. Dent. Educ., 2003, 67(4), 459–467 CrossRef PubMed.
- R. A. Bapat, A. Parolia, T. Chaubal, S. Dharamadhikari, A. M. Abdulla, N. Sakkir, S. Arora, P. Bapat, A. M. Sindi and P. Kesharwani, Recent update on potential cytotoxicity, biocompatibility and preventive measures of biomaterials used in dentistry, Biomater. Sci., 2021, 9(9), 3244–3283 RSC.
- M. Huang, Y. Huang, L. Hongyu, Z. Tang, Y. Chen, Z. Huang, S. Xu, J. Du and B. Jia, Hydrogels for Treatment of Oral and Maxillofacial Diseases: Current Research, Challenge, and Future Directions, Biomater. Sci., 2022, 10(22), 6413–6446 RSC.
- M. K. Yazdi, V. Vatanpour, A. Taghizadeh, M. Taghizadeh, M. R. Ganjali, M. T. Munir, S. Habibzadeh, M. R. Saeb and M. Ghaedi, Hydrogel membranes: A review, Mater. Sci. Eng., C, 2020, 114, 111023 CrossRef CAS PubMed.
- Y. Chen, D. Diaz-Dussan, D. Wu, W. Wang, Y.-Y. Peng, A. B. Asha, D. G. Hall, K. Ishihara and R. Narain, Bioinspired self-healing hydrogel based on benzoxaborole-catechol dynamic covalent chemistry for 3D cell encapsulation, ACS Macro Lett., 2018, 7(8), 904–908 CrossRef CAS PubMed.
- E. Zeimaran, S. Pourshahrestani, A. Fathi, N. A. bin Abd Razak, N. A. Kadri, A. Sheikhi and F. Baino, Advances in bioactive glass-containing injectable hydrogel biomaterials for tissue regeneration, Acta Biomater., 2021, 136, 1–36 CrossRef CAS PubMed.
- Z. Siddiqui, B. Sarkar, K.-K. Kim, N. Kadincesme, R. Paul, A. Kumar, Y. Kobayashi, A. Roy, M. Choudhury and J. Yang, Angiogenic hydrogels for dental pulp revascularization, Acta Biomater., 2021, 126, 109–118 CrossRef CAS PubMed.
- X. M. Keutgen, K. J. Ornell, A. Vogle, O. Lakiza, J. Williams, P. Miller, K. S. Mistretta, N. Setia, R. R. Weichselbaum and J. M. Coburn, Sunitinib-Loaded Chondroitin Sulfate Hydrogels as a Novel Drug-Delivery Mechanism for the Treatment of Pancreatic Neuroendocrine Tumors, Ann. Surg. Oncol., 2021, 28(13), 8532–8543 CrossRef PubMed.
- D. Pankajakshan, S. L. Voytik-Harbin, J. E. Nör and M. C. Bottino, Injectable highly tunable oligomeric collagen matrices for dental tissue regeneration, ACS Appl. Bio Mater., 2020, 3(2), 859–868 CrossRef CAS PubMed.
- Z. C. T. Zaw, N. Kawashima, T. Kaneko and T. Okiji, Angiogenesis during coronal pulp regeneration using rat dental pulp cells: Neovascularization in rat molars in vivo and proangiogenic dental pulp cell-endothelial cell interactions in vitro, J. Dent. Sci., 2022, 17(3), 1160–1168 CrossRef PubMed.
- B. Wang, H. E. Booij-Vrieling, E. M. Bronkhorst, J. Shao, P. H. Kouwer, J. A. Jansen, X. F. Walboomers and F. Yang, Antimicrobial and anti-inflammatory thermo-reversible hydrogel for periodontal delivery, Acta Biomater., 2020, 116, 259–267 CrossRef CAS PubMed.
- J. Mou, Z. Liu, J. Liu, J. Lu, W. Zhu and D. Pei, Hydrogel containing minocycline and zinc oxide-loaded serum albumin nanopartical for periodontitis application: preparation, characterization and evaluation, Drug Delivery, 2019, 26(1), 179–187 CrossRef CAS PubMed.
- S. Liu, Y.-N. Wang, B. Ma, J. Shao, H. Liu and S. Ge, Gingipain-responsive thermosensitive hydrogel loaded with SDF-1 facilitates in situ periodontal tissue regeneration, ACS Appl. Mater. Interfaces, 2021, 13(31), 36880–36893 CrossRef CAS PubMed.
- Q. Dong, D. Zu, L. Kong, S. Chen, J. Yao, J. Lin, L. Lu, B. Wu and B. Fang, Construction of antibacterial nano-silver embedded bioactive hydrogel to repair infectious skin defects, Biomater. Res., 2022, 26(1), 36 CrossRef CAS PubMed.
- Z. Lu, J. Zhang, Z. Yu, Q. Liu, K. Liu, M. Li and D. Wang, Hydrogel degradation triggered by pH for the smart release of antibiotics to combat bacterial infection, New J. Chem., 2017, 41(2), 432–436 RSC.
- M. Ducret, A. Montembault, J. Josse, M. Pasdeloup, A. Celle, R. Benchrih, F. Mallein-Gerin, B. Alliot-Licht, L. David and J.-C. Farges, Design and characterization of a chitosan-enriched fibrin hydrogel for human dental pulp regeneration, Dent. Mater., 2019, 35(4), 523–533 CrossRef CAS PubMed.
- M. Bekhouche, M. Bolon, F. Charriaud, M. Lamrayah, D. Da Costa, C. Primard, A. Costantini, M. Pasdeloup, S. Gobert and F. Mallein-Gerin, Development of an antibacterial nanocomposite hydrogel for human dental pulp engineering, J. Mater. Chem. B, 2020, 8(36), 8422–8432 RSC.
- J. Bemmelen, Der Hydrogel und das kristallinische Hydrat des Kupferoxydes, Z. Anorg. Chem., 1894, 5, 466 CrossRef.
- A. Danno, Gel formation of aqueous solution of polyvinyl alcohol irradiated by gamma rays from cobalt-60, J. Phys. Soc. Jpn., 1958, 13(7), 722–727 CrossRef CAS.
- O. Wichterle and D. Lim, Hydrophilic gels for biological use, Nature, 1960, 185(4706), 117–118 CrossRef.
- R. M. Nalbandian, R. L. Henry and H. S. Wilks, Artifical skin. II. Pluronic F–127 silver nitrate or silver lactate gel in the treatment of thermal burns, J. Biomed. Mater. Res., 1972, 6(6), 583–590 CrossRef CAS PubMed.
- D. W. Rising, M. Goldman and S. M. Brayton, Histologic appraisal of three experimental root canal filling materials, J. Endod., 1975, 1(5), 172–177 CrossRef CAS PubMed.
- D. Cowsar, O. Tarwater and A. Tanquary, Controlled release of fluoride from hydrogels for dental applications, ACS Publications, 1976 Search PubMed.
- J. Goodson, A. Haffajee and S. Socransky, Periodontal therapy by local delivery of tetracycline, J. Clin. Periodontol., 1979, 6(2), 83–92 CrossRef CAS PubMed.
- D. Ørstavik, Antibacterial properties of root canal sealers, cements and pastes, Int. Endod. J., 1981, 14(2), 125–133 CrossRef PubMed.
- R. Muzzarelli, G. Biagini, A. Pugnaloni, O. Filippini, V. Baldassarre, C. Castaldini and C. Rizzoli, Reconstruction of parodontal tissue with chitosan, Biomaterials, 1989, 10(9), 598–603 CrossRef CAS PubMed.
- X. Qu, A. Wrzyszczynski, K. Pielichowski, J. Pielichowski, E. Adamczak, S. Morge, L. Lindén and J. Rabek, Polymerization of chitosan-acrylic salt for use in dentistry, J. Macromol. Sci., Part A: Pure Appl.Chem., 1997, 34(5), 881–899 CrossRef.
- H. Omidian, K. Park and J. G. Rocca, Recent developments in superporous hydrogels, J. Pharm. Pharmacol., 2007, 59(3), 317–327 CrossRef CAS PubMed.
- S. J. Buwalda, K. W. Boere, P. J. Dijkstra, J. Feijen, T. Vermonden and W. E. Hennink, Hydrogels in a historical perspective: From simple networks to smart materials, J. Controlled Release, 2014, 190, 254–273 CrossRef CAS PubMed.
- B. K. Jadhav, K. R. Khandelwal, A. R. Ketkar and S. S. Pisal, Formulation and evaluation of mucoadhesive tablets containing eugenol for the treatment of periodontal diseases, Drug Dev. Ind. Pharm., 2004, 30(2), 195–203 CrossRef CAS PubMed.
- Q. X. Ji, X. G. Chen, Q. S. Zhao, C. S. Liu, X. J. Cheng and L. C. Wang, Injectable thermosensitive hydrogel based on chitosan and quaternized chitosan and the biomedical properties, J. Mater. Sci.: Mater. Med., 2009, 20, 1603–1610 CrossRef CAS PubMed.
- K. M. Galler, J. D. Hartgerink, A. C. Cavender, G. Schmalz and R. N. D'Souza, A customized self-assembling peptide hydrogel for dental pulp tissue engineering, Tissue Eng., Part A, 2012, 18(1–2), 176–184 CrossRef CAS PubMed.
- J. Y. Park, J.-H. Shim, S.-A. Choi, J. Jang, M. Kim, S. H. Lee and D.-W. Cho, 3D printing technology to control BMP-2 and VEGF delivery spatially and temporally to promote large-volume bone regeneration, J. Mater. Chem. B, 2015, 3(27), 5415–5425 RSC.
- Y. Ma, Y. Ji, G. Huang, K. Ling, X. Zhang and F. Xu, Bioprinting 3D cell-laden hydrogel microarray for screening human periodontal ligament stem cell response to extracellular matrix, Biofabrication, 2015, 7(4), 044105 CrossRef PubMed.
- Y. Dong, S. Wang, Y. Ke, L. Ding, X. Zeng, S. Magdassi and Y. Long, 4D printed hydrogels: fabrication, materials, and applications, Adv. Mater. Technol., 2020, 5(6), 2000034 CrossRef CAS.
- H. Özçelik, F. Batool, M. Corre, A. Garlaschelli, G. Conzatti, C. Stutz, C. Petit, E. Delpy, F. Zal and E. Leize-Zal, Characterization of a hyaluronic acid-based hydrogel containing an extracellular oxygen carrier (M101) for periodontitis treatment: An in vitro study, Int. J. Pharm., 2021, 605, 120810 CrossRef PubMed.
- S. Lu, X. Ren, T. Guo, Z. Cao, H. Sun, C. Wang, F. Wang, Z. Shu, J. Hao and S. Gui, Controlled release of iodine from cross-linked cyclodextrin metal-organic frameworks for prolonged periodontal pocket therapy, Carbohydr. Polym., 2021, 267, 118187 CrossRef CAS PubMed.
- D. Wang, Y. Hu, P. Liu and D. Luo, Bioresponsive DNA hydrogels: beyond the conventional stimuli responsiveness, Acc. Chem. Res., 2017, 50(4), 733–739 CrossRef CAS PubMed.
- L. Suo, H. Wu, P. Wang, Z. Xue, J. Gao and J. Shen, The improvement of periodontal tissue regeneration using a 3D–printed carbon nanotube/chitosan/sodium alginate composite scaffold, J. Biomed. Mater. Res., Part B, 2023, 111(1), 73–84 CrossRef CAS PubMed.
- L. Tallet, V. Gribova, L. Ploux, N. E. Vrana and P. Lavalle, New smart antimicrobial hydrogels, nanomaterials, and coatings: Earlier action, more specific, better dosing?, Adv. Healthcare Mater., 2021, 10(1), 2001199 CrossRef CAS PubMed.
- R. Kundu and P. Payal, Antimicrobial Hydrogels: Promising Soft Biomaterials, ChemistrySelect, 2020, 5(46), 14800–14810 CrossRef CAS.
- M. E. Afami, I. El Karim, I. About, A. D. Krasnodembskaya, G. Laverty and F. T. Lundy, Multicomponent peptide hydrogels as an innovative platform for cell-based tissue engineering in the dental pulp, Pharmaceutics, 2021, 13(10), 1575 CrossRef CAS PubMed.
- R. Swimberghe, A. De Clercq, R. De Moor and M. Meire, Efficacy of sonically, ultrasonically and laser–activated irrigation in removing a biofilm–mimicking hydrogel from an isthmus model, Int. Endod. J., 2019, 52(4), 515–523 CrossRef CAS PubMed.
- N. Shetty, T. Mathew, A. Shetty, M. N. Hegde and S. Attavar, Ozonated water as an irrigant in disinfecting root canal systems-a systematic review, Evid.-Based Dent., 2022, 1–5 CAS.
- M. Khoshkhounejad, M. S. Afshar, F. Jabalameli, M. Emaneini and M. Sharifian, Cytotoxicity evaluation of minimum antibacterial values of different medicaments used in endodontic regenerative procedures, Eur. J. Dent., 2019, 13(04), 514–520 CrossRef PubMed.
- A. M. Acevedo-Jake, A. Griffith, N. Kadincesme, K. Dabek, D. Hindi, K. K. Kim, Y. Kobayashi, E. Shimizu and V. Kumar, Cells and material-based strategies for regenerative endodontics, Bioact. Mater., 2021, 14, 234–249 Search PubMed.
- D. Dasgupta, S. Peddi, D. K. Saini and A. Ghosh, Mobile nanobots for prevention of root canal treatment failure, Adv. Healthcare Mater., 2022, 11(14), 2200232 CrossRef CAS PubMed.
- L. Casagrande, F. Demarco, Z. Zhang, F. Araujo, S. Shi and J. Nör, Dentin-derived BMP-2 and odontoblast differentiation, J. Dent. Res., 2010, 89(6), 603–608 CrossRef CAS PubMed.
- F. T. Lundy, C. R. Irwin, D. F. McLean, G. J. Linden and I. A. El Karim, Natural antimicrobials in the dental pulp, J. Endod., 2020, 46(9), S2–S9 CrossRef PubMed.
- M. Samiei, M. Fathi, J. Barar, N. Fathi, N. Amiryaghoubi and Y. Omidi, Bioactive hydrogel-based scaffolds for the regeneration of dental pulp tissue, J. Drug Delivery Sci. Technol., 2021, 64, 102600 CrossRef CAS.
- I. Medina-Fernandez and A. D. Celiz, Acellular biomaterial strategies for endodontic regeneration, Biomater. Sci., 2019, 7(2), 506–519 RSC.
- D. Atila, C.-Y. Chen, C.-P. Lin, Y.-L. Lee, V. Hasirci, A. Tezcaner and F.-H. Lin, In vitro evaluation of injectable Tideglusib-loaded hyaluronic acid hydrogels incorporated with Rg1-loaded chitosan microspheres for vital pulp regeneration, Carbohydr. Polym., 2022, 278, 118976 CrossRef CAS PubMed.
- E. Ahmadian, A. Eftekhari, S. M. Dizaj, S. Sharifi, M. Mokhtarpour, A. N. Nasibova, R. Khalilov and M. Samiei, The effect of hyaluronic acid hydrogels on dental pulp stem cells behavior, Int. J. Biol. Macromol., 2019, 140, 245–254 CrossRef CAS PubMed.
- D. Atila, V. Hasirci and A. Tezcaner, Coaxial electrospinning of composite mats comprised of core/shell poly (methyl methacrylate)/silk fibroin fibers for tissue engineering applications, J. Mech. Behav. Biomed. Mater., 2022, 128, 105105 CrossRef CAS PubMed.
- M. E. Afami, I. El Karim, I. About, S. M. Coulter, G. Laverty and F. T. Lundy, Ultrashort Peptide hydrogels display antimicrobial activity and enhance angiogenic growth factor release by dental pulp stem/stromal cells, Materials, 2021, 14(9), 2237 CrossRef CAS PubMed.
- L. D. Almeida, P. S. Babo, C. R. Silva, M. T. Rodrigues, J. Hebling, R. L. Reis and M. E. Gomes, Hyaluronic acid hydrogels incorporating platelet lysate enhance human pulp cell proliferation and differentiation, J. Mater. Sci.: Mater. Med., 2018, 29(6), 1–11 CrossRef CAS PubMed.
- E. Renard, J. Amiaud, L. Delbos, C. Charrier, A. Montembault, M. Ducret, J. Farges, L. David, B. Alliot-Licht and A. Gaudin, Dental pulp inflammatory/immune response to a chitosan-enriched fibrin hydrogel in the pulpotomised rat incisor, Eur. Cells Mater., 2020, 40, 74–87 CrossRef CAS PubMed.
- G. L. Carvalho, G. Sarra, G. T. Schröter, L. S. R. G. Silva, S. K. K. Ariga, F. Gonçalves, H. V. Caballero-Flores and M. S. Moreira, Pro–angiogenic potential of a functionalized hydrogel scaffold as a secretome delivery platform: An innovative strategy for cell homing–based dental pulp tissue engineering, J. Tissue Eng. Regener. Med., 2022, 16(5), 472–483 CrossRef CAS PubMed.
- H. Caballero-Flores, C. K. Nabeshima, G. Sarra, M. S. Moreira, V. E. Arana-Chavez, M. M. Marques and M. E. de Lima Machado, Development and characterization of a new chitosan-based scaffold associated with gelatin, microparticulate dentin and genipin for endodontic regeneration, Dent. Mater., 2021, 37(7), e414–e425 CrossRef CAS PubMed.
- M. EzEldeen, B. Toprakhisar, D. Murgia, N. Smisdom, O. Deschaume, C. Bartic, H. Van Oosterwyck, R. V. S. Pereira, G. Opdenakker and I. Lambrichts, Chlorite oxidized oxyamylose differentially influences the microstructure of fibrin and self assembling peptide hydrogels as well as dental pulp stem cell behavior, Sci. Rep., 2021, 11(1), 1–12 CrossRef PubMed.
- J. S. Ribeiro, C. K. Sanz, E. A. Münchow, N. Kalra, N. Dubey, C. E. C. Suárez, J. C. Fenno, R. G. Lund and M. C. Bottino, Photocrosslinkable methacrylated gelatin hydrogel as a cell-friendly injectable delivery system for chlorhexidine in regenerative endodontics, Dent. Mater., 2022, 38(9), 1507–1517 CrossRef CAS PubMed.
- J. S. Ribeiro, E. A. Münchow, E. A. Bordini, N. S. Rodrigues, N. Dubey, H. Sasaki, J. C. Fenno, S. Schwendeman and M. C. Bottino, Engineering of Injectable Antibiotic-laden Fibrous Microparticles Gelatin Methacryloyl Hydrogel for Endodontic Infection Ablation, Int. J. Mol. Sci., 2022, 23(2), 971 CrossRef CAS PubMed.
- J. S. Ribeiro, A. Daghrery, N. Dubey, C. Li, L. Mei, J. C. Fenno, A. Schwendeman, Z. Aytac and M. C. Bottino, Hybrid antimicrobial hydrogel as injectable therapeutics for oral infection ablation, Biomacromolecules, 2020, 21(9), 3945–3956 CrossRef CAS PubMed.
- P. W. McIntyre, J. L. Wu, R. Kolte, R. Zhang, R. L. Gregory, A. Bruzzaniti and G. H. Yassen, The antimicrobial properties, cytotoxicity, and differentiation potential of double antibiotic intracanal medicaments loaded into hydrogel system, Clin. Oral Investig., 2019, 23(3), 1051–1059 CrossRef PubMed.
- H. Aksel, F. Mahjour, F. Bosaid, S. Calamak and A. A. Azim, Antimicrobial activity and biocompatibility of antibiotic-loaded chitosan hydrogels as a potential scaffold in regenerative endodontic treatment, J. Endod., 2020, 46(12), 1867–1875 CrossRef PubMed.
- M. Ruiz-Linares, J. F. Monroy-Rojas, C. Solana, P. Baca, B. Aguado, A. Soriano-Lerma, M. T. Arias-Moliz and C. M. Ferrer-Luque, Antimicrobial potential of new diclofenac hydrogels for disinfection in regenerative endodontics: An in vitro and ex vivo study, Int. Endod. J., 2023, 56(1), 103–117 CrossRef PubMed.
- N. Dubey, J. Ribeiro, Z. Zhang, J. Xu, J. Ferreira, Q. Liu, L. Mei, J. C. Fenno, A. Schwendeman and S. Schwendeman, Gelatin Methacryloyl Hydrogel as an Injectable Scaffold with Multi-therapeutic Effects to Promote Antimicrobial Disinfection and Angiogenesis for Regenerative Endodontics, J. Mater. Chem. B, 2023, 11(17), 3823–3835 RSC.
- T. Liu, A. Aman, M. Ainiwaer, L. Ding, F. Zhang, Q. Hu, Y. Song, Y. Ni and X. Tang, Evaluation of the anti-biofilm effect of poloxamer-based thermoreversible gel of silver nanoparticles as a potential medication for root canal therapy, Sci. Rep., 2021, 11(1), 1–16 CrossRef PubMed.
- N. Zhu, X. Chatzistavrou, L. Ge, M. Qin, P. Papagerakis and Y. Wang, Biological properties of modified bioactive glass on dental pulp cells, J. Dent., 2019, 83, 18–26 CrossRef CAS PubMed.
- M. Magana, M. Pushpanathan, A. L. Santos, L. Leanse, M. Fernandez, A. Ioannidis, M. A. Giulianotti, Y. Apidianakis, S. Bradfute and A. L. Ferguson, The value of antimicrobial peptides in the age of resistance, Lancet Infect. Dis., 2020, 20(9), e216–e230 CrossRef CAS PubMed.
- Q.-Y. Zhang, Z.-B. Yan, Y.-M. Meng, X.-Y. Hong, G. Shao, J.-J. Ma, X.-R. Cheng, J. Liu, J. Kang and C.-Y. Fu, Antimicrobial peptides: mechanism of action, activity and clinical potential, Mil. Med. Res., 2021, 8(1), 1–25 Search PubMed.
- K. F. Johnstone and M. C. Herzberg, Antimicrobial peptides: Defending the mucosal epithelial barrier, Front. Oral Health, 2022, 3, 958480 CrossRef PubMed.
- J. Zhao, Y. Zhou, J. Yan, J. Liu, L. Wang, X. Zhang, Y. Lou and K. Que, Effects of phase–transited lysozyme on adhesion, migration, and odontogenic differentiation of human dental pulp cells: An in vitro study, Int. Endod. J., 2022, 56(4), 475–485 CrossRef PubMed.
- G. V. Kulkarni, B. Chen, J. P. Malone, A. S. Narayanan and A. George, Promotion of selective cell attachment by the RGD sequence in dentine matrix protein 1, Arch. Oral Biol., 2000, 45(6), 475–484 CrossRef CAS PubMed.
- F. Asghari Sana, M. Çapkın Yurtsever, G. Kaynak Bayrak, E.Ö Tunçay, A. S. Kiremitçi and M. Gümüşderelioğlu, Spreading, proliferation and differentiation of human dental pulp stem cells on chitosan scaffolds immobilized with RGD or fibronectin, Cytotechnology, 2017, 69, 617–630 CrossRef CAS PubMed.
- M. G. da Costa Sousa, G. C. de Almeida, D. C. M. Mota, R. A. da Costa, S. C. Dias, S. N. Limberger, F. Ko, L. T. Lin, E. F. Haney and H. Etayash, Antibiofilm and immunomodulatory resorbable nanofibrous filing for dental pulp regenerative procedures, Bioact. Mater., 2022, 16, 173–186 CrossRef PubMed.
- R. Costa-Almeida, A. R. Franco, T. Pesqueira, M. B. Oliveira, P. S. Babo, I. B. Leonor, J. F. Mano, R. L. Reis and M. E. Gomes, The effects of platelet lysate patches on the activity of tendon-derived cells, Acta Biomater., 2018, 68, 29–40 CrossRef CAS PubMed.
- A. Shariati, A. Moradabadi, E. Ghaznavi-Rad, M. Dadmanesh, M. Komijani and F. Nojoomi, Investigation into antibacterial and wound healing properties of platelets lysate against Acinetobacter baumannii and Klebsiella pneumoniae burn wound infections, Ann. Clin. Microbiol. Antimicrob., 2021, 20(1), 1–9 CrossRef PubMed.
- X. Wang, J. Qi, W. Zhang, Y. Pu, R. Yang, P. Wang, S. Liu, X. Tan and B. Chi, 3D-printed antioxidant antibacterial carboxymethyl cellulose/ε-polylysine hydrogel promoted skin wound repair, Int. J. Biol. Macromol., 2021, 187, 91–104 CrossRef CAS PubMed.
- B. Bhattacharjee, L. Jolly, R. Mukherjee and J. Haldar, An easy-to-use antimicrobial hydrogel effectively kills bacteria, fungi, and influenza virus, Biomater. Sci., 2022, 10(8), 2014–2028 RSC.
- Z. Deng, T. Wang, X. Chen and Y. Liu, Applications of chitosan-based biomaterials: a focus on dependent antimicrobial properties, Mar. Life Sci. Technol., 2020, 2(4), 398–413 CrossRef.
- M. Rajabi, M. McConnell, J. Cabral and M. A. Ali, Chitosan hydrogels in 3D printing for biomedical applications, Carbohydr. Polym., 2021, 260, 117768 CrossRef CAS PubMed.
- J. L. Liesveld, N. Sharma and O. S. Aljitawi, Stem cell homing: From physiology to therapeutics, Stem Cells, 2020, 38(10), 1241–1253 CrossRef PubMed.
- J. L. Wu, P. W. McIntyre, J. M. Hong, G. H. Yassen and A. Bruzzaniti, Effects of radiopaque double antibiotic pastes on the proliferation, alkaline phosphatase activity and mineral deposition of dental pulp stem cells, Arch. Oral Biol., 2020, 117, 104764 CrossRef CAS PubMed.
- H. Rajeshwari, D. Dhamecha, S. Jagwani, M. Rao, K. Jadhav, S. Shaikh, L. Puzhankara and S. Jalalpure, Local drug delivery systems in the management of periodontitis: A scientific review, J. Controlled Release, 2019, 307, 393–409 CrossRef PubMed.
- B. Ashrafi, M. Rashidipour, A. Marzban, S. Soroush, M. Azadpour, S. Delfani and P. Ramak, Mentha piperita essential oils loaded in a chitosan nanogel with inhibitory effect on biofilm formation against S. mutans on the dental surface, Carbohydr. Polym., 2019, 212, 142–149 CrossRef CAS PubMed.
- S. Tang, H. Zhang, L. Mei, K. Dou, Y. Jiang, Z. Sun, S. Wang, M. S. Hasanin, J. Deng and Q. Zhou, Fucoidan-derived carbon dots against Enterococcus faecalis biofilm and infected dentinal tubules for the treatment of persistent endodontic infections, J. Nanobiotechnol., 2022, 20(1), 1–16 CrossRef PubMed.
- K. G. Bhat, P. Ingalagi, S. Patil, S. Patil and G. Pattar, Antimicrobial susceptibility pattern of oral Gram negative anaerobes from Indian subjects, Anaerobe, 2021, 70, 102367 CrossRef CAS PubMed.
- N. A. Mahat, N. S. M. Nor and S. A. Shamsudin, Effects of Positive Carbon Quantum Dots on Gram-negative Bacteria as an Antimicrobial Agent, J. Inorg. Organomet. Polym. Mater., 2022,(32), 2428–2440 CrossRef CAS.
- B. A. de Melo, Y. A. Jodat, E. M. Cruz, J. C. Benincasa, S. R. Shin and M. A. Porcionatto, Strategies to use fibrinogen as bioink for 3D bioprinting fibrin-based soft and hard tissues, Acta Biomater., 2020, 117, 60–76 CrossRef CAS PubMed.
- L. Wang, Q. Wang, A. Slita, O. Backman, Z. Gounani, E. Rosqvist, J. Peltonen, S. Willför, C. Xu and J. M. Rosenholm, Digital light processing (DLP) 3D-fabricated antimicrobial hydrogel with a sustainable resin of methacrylated woody polysaccharides and hybrid silver-lignin nanospheres, Green Chem., 2022, 24(5), 2129–2145 RSC.
- J. Y. Lim, L. Goh, K.-i. Otake, S. S. Goh, X. J. Loh and S. Kitagawa, Biomedically-relevant metal organic framework-hydrogel composites, Biomater. Sci., 2023, 11(8), 2661–2677 RSC.
- M. Chrószcz and I. Barszczewska-Rybarek, Nanoparticles of quaternary ammonium polyethylenimine derivatives for application in dental materials, Polymer, 2020, 12(11), 2551 Search PubMed.
- M. A. De Groote and F. C. Fang, NO inhibitions: antimicrobial properties of nitric oxide, Clin. Infect. Dis., 1995, 21(Supplement_2), S162–S165 CrossRef CAS PubMed.
- N. Dubey, J. A. Ferreira, A. Daghrery, Z. Aytac, J. Malda, S. B. Bhaduri and M. C. Bottino, Highly tunable bioactive fiber-reinforced hydrogel for guided bone regeneration, Acta Biomater., 2020, 113, 164–176 CrossRef CAS PubMed.
- Y. Almoshari, R. Ren, H. Zhang, Z. Jia, X. Wei, N. Chen, G. Li, S. Ryu, S. M. Lele and R. A. Reinhardt, GSK3 inhibitor-loaded osteotropic Pluronic hydrogel effectively mitigates periodontal tissue damage associated with experimental periodontitis, Biomaterials, 2020, 261, 120293 CrossRef CAS PubMed.
- S. Huang, T. He, F. Yue, X. Xu, L. Wang, P. Zhu, F. Teng, Z. Sun, X. Liu and G. Jing, Longitudinal multi-omics and microbiome meta-analysis identify an asymptomatic gingival state that links gingivitis, periodontitis, and aging, mBio, 2021, 12(2), e03281–e03220 CAS.
- Z. Akram, S. S. Shafqat, M. O. Niaz, A. Raza and M. Naseem, Clinical efficacy of photodynamic therapy and laser irradiation as an adjunct to open flap debridement in the treatment of chronic periodontitis: a systematic review and meta–analysis, Photodermatol., Photoimmunol. Photomed., 2020, 36(1), 3–13 CrossRef PubMed.
- X. Xu, Z. Gu, X. Chen, C. Shi, C. Liu, M. Liu, L. Wang, M. Sun, K. Zhang and Q. Liu, An injectable and thermosensitive hydrogel: Promoting periodontal regeneration by controlled-release of aspirin and erythropoietin, Acta Biomater., 2019, 86, 235–246 CrossRef CAS PubMed.
- H. N. Woo, Y. J. Cho, S. Tarafder and C. H. Lee, The recent advances in scaffolds for integrated periodontal regeneration, Bioact. Mater., 2021, 6(10), 3328–3342 CrossRef CAS PubMed.
- X. Bao, J. Zhao, J. Sun, M. Hu and X. Yang, Polydopamine nanoparticles as efficient scavengers for reactive oxygen species in periodontal disease, ACS Nano, 2018, 12(9), 8882–8892 CrossRef CAS PubMed.
- A. Radhi, D. Mohamad, F. S. A. Rahman, A. M. Abdullah and H. Hasan, Mechanism and factors influence of graphene-based nanomaterials antimicrobial activities and application in dentistry, J. Mater. Res. Technol., 2021, 11, 1290–1307 CrossRef CAS.
- F. Koch, K. Ekat, D. Kilian, T. Hettich, O. Germershaus, H. Lang, K. Peters and B. Kreikemeyer, A Versatile Biocompatible Antibiotic Delivery System Based on Self–Assembling Peptides with Antimicrobial and Regenerative Potential, Adv. Healthcare Mater., 2019, 8(13), 1900167 CrossRef PubMed.
- D. Wu, P. Wang, Q. Wu, C. H. Chu, C. Lei, W. Wu, S. Ma, J. Lv and C. Tang, Preparation and characterization of Bomidin-loaded thermosensitive hydrogel for periodontal application, J. Mater. Res., 2022, 37(18), 3021–3032 CrossRef CAS.
- S. Ma, X. Lu, X. Yu, Y. Du, S. Xu, M. Li, C. Peng, Z. Liu and J. Deng, An injectable multifunctional thermo-sensitive chitosan-based hydrogel for periodontitis therapy, Biomater. Adv., 2022, 142, 213158 CrossRef CAS PubMed.
- J. Chanaj-Kaczmarek, T. Osmałek, E. Szymańska, K. Winnicka, T. M. Karpiński, M. Dyba, M. Bekalarska-Dębek and J. Cielecka-Piontek, Development and Evaluation of Thermosensitive Hydrogels with Binary Mixture of Scutellariae baicalensis radix Extract and Chitosan for Periodontal Diseases Treatment, Int. J. Mol. Sci., 2021, 22(21), 11319 CrossRef CAS PubMed.
- M. I. Alvarez Echazu, M. E. Antona, O. Perna, C. E. Olivetti, G. S. Alvarez, E. V. Macri, C. J. Perez, M. Czerner, S. M. Friedman and M. F. Desimone, Dodecenylsuccinic anhydride modified chitosan hydrogels for the sustained delivery of hydrophobic drugs. The case of thymol buccal delivery, J. Appl. Polym. Sci., 2022, 139(1), 51432 CrossRef CAS.
- M. Bansal, N. Mittal, S. K. Yadav, G. Khan, P. Gupta, B. Mishra and G. Nath, Periodontal thermoresponsive, mucoadhesive dual antimicrobial loaded in situ gel for the treatment of periodontal disease: Preparation, in vitro characterization and antimicrobial study, J. Oral Biol. Craniofacial Res., 2018, 8(2), 126–133 CrossRef PubMed.
- S. Zang, R. Mu, F. Chen, X. Wei, L. Zhu, B. Han, H. Yu, B. Bi, B. Chen and Q. Wang, Injectable chitosan/β-glycerophosphate hydrogels with sustained release of BMP-7 and ornidazole in periodontal wound healing of class III furcation defects, Mater. Sci. Eng., C, 2019, 99, 919–928 CrossRef CAS PubMed.
- Z. Dong, Y. Sun, Y. Chen, Y. Liu, C. Tang and X. Qu, Injectable adhesive hydrogel through a microcapsule cross-link for periodontitis treatment, ACS Appl. Bio Mater., 2019, 2(12), 5985–5994 CrossRef CAS PubMed.
- N. Aminu, S.-Y. Chan, M.-F. Yam and S.-M. Toh, A dual-action chitosan-based nanogel system of triclosan and flurbiprofen for localised treatment of periodontitis, Int. J. Pharm., 2019, 570, 118659 CrossRef CAS PubMed.
- N. Vargas-Alfredo, M. Munar-Bestard, J. M. Ramis and M. Monjo, Synthesis and Modification of Gelatin Methacryloyl (GelMA) with Antibacterial Quaternary Groups and Its Potential for Periodontal Applications, Gels, 2022, 8(10), 630 CrossRef CAS PubMed.
- A. Johnson, F. Kong, S. Miao, H.-T. V. Lin, S. Thomas, Y.-C. Huang and Z.-L. Kong, Therapeutic effects of antibiotics loaded cellulose nanofiber and κ-carrageenan oligosaccharide composite hydrogels for periodontitis treatment, Sci. Rep., 2020, 10(1), 1–23 CrossRef PubMed.
- J.-O. Jeong, J.-S. Park, E. J. Kim, S.-I. Jeong, J. Y. Lee and Y.-M. Lim, Preparation of radiation cross-linked poly (Acrylic acid) hydrogel containing metronidazole with enhanced antibacterial activity, Int. J. Mol. Sci., 2019, 21(1), 187 CrossRef PubMed.
- X. Tong, X. Qi, R. Mao, W. Pan, M. Zhang, X. Wu, G. Chen, J. Shen, H. Deng and R. Hu, Construction of functional curdlan hydrogels with bio-inspired polydopamine for synergistic periodontal antibacterial therapeutics, Carbohydr. Polym., 2020, 245, 116585 CrossRef CAS PubMed.
- J. Lin, Z. He, F. Liu, J. Feng, C. Huang, X. Sun and H. Deng, Hybrid hydrogels for synergistic periodontal antibacterial treatment with sustained drug release and NIR-responsive photothermal effect, Int. J. Nanomed., 2020, 15, 5377 CrossRef CAS PubMed.
- X. Zhao, Y. Yang, J. Yu, R. Ding, D. Pei, Y. Zhang, G. He, Y. Cheng and A. Li, Injectable hydrogels with high drug loading through B–N coordination and ROS-triggered drug release for efficient treatment of chronic periodontitis in diabetic rats, Biomaterials, 2022, 282, 121387 CrossRef CAS PubMed.
- J. Bako, F. Toth, J. Gall, R. Kovacs, A. Csík, I. Varga, A. Sculean, R. Zelko and C. Hegedus, Combined release of antiseptic and antibiotic drugs from visible light polymerized biodegradable nanocomposite hydrogels for periodontitis treatment, Pharmaceutics, 2022, 14(5), 957 CrossRef CAS PubMed.
- R. Hr, S. Jagwani, P. A. Shenoy, K. Jadhav, S. Shaikh, S. P. Mutalik, P. Mullick, S. Mutalik, S. Jalalpure and M. S. Sikarwar, Thermoreversible gel of green tea extract: Formulation and evaluation for the management of periodontitis, J. Drug Delivery Sci. Technol., 2022, 76, 103765 CrossRef CAS.
- Z. Dong, Y. Lin, S. Xu, L. Chang, X. Zhao, X. Mei and X. Gao, NIR-triggered tea polyphenol-modified gold nanoparticles-loaded hydrogel treats periodontitis by inhibiting bacteria and inducing bone regeneration, Mater. Des., 2023, 225, 111487 CrossRef CAS.
- I. Popescu, M. Constantin, I. M. Pelin, D. M. Suflet, D. L. Ichim, O. M. Daraba and G. Fundueanu, Eco-Friendly Synthesized PVA/Chitosan/Oxalic Acid Nanocomposite Hydrogels Embedding Silver Nanoparticles as Antibacterial Materials, Gels, 2022, 8(5), 268 CrossRef CAS PubMed.
- Q. Ou, K. Huang, C. Fu, C. Huang, Y. Fang, Z. Gu, J. Wu and Y. Wang, Nanosilver-incorporated halloysite nanotubes/gelatin methacrylate hybrid hydrogel with osteoimmunomodulatory and antibacterial activity for bone regeneration, Chem. Eng. J., 2020, 382, 123019 CrossRef CAS.
- N. Li, L. Xie, Y. Wu, Y. Wu, Y. Liu, Y. Gao, J. Yang, X. Zhang and L. Jiang, Dexamethasone-loaded zeolitic imidazolate frameworks nanocomposite hydrogel with antibacterial and anti-inflammatory effects for periodontitis treatment, Mater. Today Bio, 2022, 16, 100360 CrossRef CAS PubMed.
- Y. Liu, T. Li, M. Sun, Z. Cheng, W. Jia, K. Jiao, S. Wang, K. Jiang, Y. Yang and Z. Dai, ZIF-8 modified multifunctional injectable photopolymerizable GelMA hydrogel for the treatment of periodontitis, Acta Biomater., 2022, 146, 37–48 CrossRef CAS PubMed.
- Y.-R. Gao, W.-X. Zhang, R. Xue, S. Yang and J.-H. Wang, Ionic gel incorporating copper nanodots with antibacterial and antioxidant dual functions for deep tissue penetration treatment of periodontitis in rats, Biomater. Sci., 2023, 11(10), 3547–3560 RSC.
- Q. Chen, Y. He, Q. Li, K. Yang, L. Sun, H. Xu and R. Wang, Intelligent design and medical applications of antimicrobial hydrogels, Colloid Interface Sci. Commun., 2023, 53, 100696 CrossRef CAS.
- B. E. Blancas-Luciano, J. Zamora-Chimal, P. G. da Silva-de Rosenzweig, M. Ramos-Mares and A. M. Fernández-Presas, Macrophages immunomodulation induced by Porphyromonas gingivalis and oral antimicrobial peptides, Odontology, 2023, 1–15 Search PubMed.
- S. Dima, Y.-Y. Lee, I. Watanabe, W.-J. Chang, Y.-H. Pan and N.-C. Teng, Antibacterial effect of the natural polymer ε-polylysine against oral pathogens associated with periodontitis and caries, Polymers, 2020, 12(6), 1218 CrossRef CAS PubMed.
- R. Thaya, B. Vaseeharan, J. Sivakamavalli, A. Iswarya, M. Govindarajan, N. S. Alharbi, S. Kadaikunnan, M. N. Al-Anbr, J. M. Khaled and G. Benelli, Synthesis of chitosan-alginate microspheres with high antimicrobial and antibiofilm activity against multi-drug resistant microbial pathogens, Microb. Pathog., 2018, 114, 17–24 CrossRef CAS PubMed.
- A. H. Sabrah, M. M. Hammad, F. K. Wahab, A. AlHadidi, N. A. Salim, A. F. Alelaimat and I. Khatib, A prospective case series in Regenerative endodontics: the effective use of diluted antibiotic hydrogels in endodontic regeneration procedures, Saudi Dent. J., 2023, 1–7 Search PubMed.
- M. Bhatia, S. S. Urolagin, K. B. Pentyala, S. B. Urolagin, K. Menaka and S. Bhoi, Novel therapeutic approach for the treatment of periodontitis by curcumin, J. Clin. Diagn. Res., 2014, 8(12), ZC65 Search PubMed.
- R. Pappu, J. Varghese, K. Koteshwara, V. Kamath, R. Lobo and K. Nimmy, Evaluation of biodegradable gel containing flax seed extract (Linum usitatissimum) as a targeted drug delivery for management of chronic periodontitis, J. Herb. Med., 2019, 15, 100254 CrossRef.
- ClinicalTrials.gov, National Library of Medicine/National Center for Biotechnology Information, 2023. https://clinicaltrials.gov/ct2/results?cond=oral&term=hydrogel&cntry=&state=&city=&dist= (accessed 08 July 2023).
- S. Duan, R. Wu, Y.-H. Xiong, H.-M. Ren, C. Lei, Y.-Q. Zhao, X.-Y. Zhang and F.-J. Xu, Multifunctional antimicrobial materials: From rational design to biomedical applications, Prog. Mater. Sci., 2022, 125, 100887 CrossRef CAS.
- Y. Kong, X. Li, L. Wang, Z. Zhang, X. Feng, J. Liu, C. Chen, L. Tong and J. Zhang, Rapid Synthesis of Graphdiyne Films on Hydrogel at the Superspreading Interface for Antibacteria, ACS Nano, 2022, 16(7), 11338–11345 CrossRef CAS PubMed.
- M. Z. I. Nizami, S. Takashiba and Y. Nishina, Graphene oxide: A new direction in dentistry, Appl. Mater. Today, 2020, 19, 100576 CrossRef.
- X. Qi, F. Jiang, M. Zhou, W. Zhang and X. Jiang, Graphene oxide as a promising material in dentistry and tissue regeneration: A review, Smart Mater. Med., 2021, 2, 280–291 CrossRef.
- M. Tahriri, M. Del Monico, A. Moghanian, M. T. Yaraki, R. Torres, A. Yadegari and L. Tayebi, Graphene and its derivatives: Opportunities and challenges in dentistry, Mater. Sci. Eng., C, 2019, 102, 171–185 CrossRef CAS PubMed.
- D. Rokaya, V. Srimaneepong, P. Thunyakitpisal, J. Qin, V. Rosa and J. Sapkota, Potential applications of graphene-based nanomaterials in biomedical, dental, and implant applications, in Advances in dental implantology using nanomaterials and allied technology applications, Springer, 2021, pp. 77–105 Search PubMed.
- B. C. Heng, Y. Bai, X. Li, X. Zhang and X. Deng, Extrapolating neurogenesis of mesenchymal stem/stromal cells on electroactive and electroconductive scaffolds to dental and oral-derived stem cells, Int. J. Oral Sci., 2022, 14(1), 1–9 CrossRef PubMed.
- S. P. Sevari, F. Shahnazi, C. Chen, J. C. Mitchell, S. Ansari and A. Moshaverinia, Bioactive glass–containing hydrogel delivery system for osteogenic differentiation of human dental pulp stem cells, J. Biomed. Mater. Res., Part A, 2020, 108(3), 557–564 CrossRef CAS PubMed.
- F. Zhao, Z. Yang, H. Xiong, Y. Yan, X. Chen and L. Shao, A bioactive glass functional hydrogel enhances bone augmentation via synergistic angiogenesis, self-swelling and osteogenesis, Bioact. Mater., 2023, 22, 201–210 CrossRef CAS PubMed.
- S. Majumdar, S. Gupta and S. Krishnamurthy, Multifarious applications of bioactive glasses in soft tissue engineering, Biomater. Sci., 2021, 9(24), 8111–8147 RSC.
- N. Gupta, D. Santhiya, S. Murugavel, A. Kumar, A. Aditya, M. Ganguli and S. Gupta, Effects of transition metal ion dopants (Ag, Cu and Fe) on the structural, mechanical and antibacterial properties of bioactive glass, Colloids Surf., A, 2018, 538, 393–403 CrossRef CAS.
- N. Zhu, X. Chatzistavrou, P. Papagerakis, L. Ge, M. Qin and Y. Wang, Silver-doped bioactive glass/chitosan hydrogel with potential application in dental pulp repair, ACS Biomater. Sci. Eng., 2019, 5(9), 4624–4633 CrossRef CAS PubMed.
- W. Zhang, A. Du, S. Liu, M. Lv and S. Chen, Research progress in decellularized extracellular matrix-derived hydrogels, Regener. Ther., 2021, 18, 88–96 CrossRef CAS PubMed.
- W. Zhang, B. Vazquez, D. Oreadi and P. Yelick, Decellularized tooth bud scaffolds for tooth regeneration, J. Dent. Res., 2017, 96(5), 516–523 CrossRef CAS PubMed.
- A. Ebrahimi Sadrabadi, P. Baei, S. Hosseini and M. Baghaban Eslaminejad, Decellularized extracellular matrix as a potent natural biomaterial for regenerative medicine, Cell Biol. Transl. Med., 2020, 13, 27–43 Search PubMed.
- H. Aksel, D. Sarkar, M. H. Lin, A. Buck and G. T.-J. Huang, Cell-derived extracellular matrix proteins in colloidal microgel as a self-assembly hydrogel for regenerative endodontics, J. Endod., 2022, 48(4), 527–534 CrossRef PubMed.
- J. Li, Z. Rao, Y. Zhao, Y. Xu, L. Chen, Z. Shen, Y. Bai, Z. Lin and Q. Huang, A decellularized matrix hydrogel derived from human dental pulp promotes dental pulp stem cell proliferation, migration, and induced multidirectional differentiation in vitro, J. Endod., 2020, 46(10), 1438–1447 CrossRef PubMed.
- H. Bakhtiar, M. Pezeshki-Modaress, Z. Kiaipour, M. Shafiee, M. R. Ellini, A. Mazidi, S. Rajabi, S. Z. Benisi, S. N. Ostad and K. Galler, Pulp ECM-derived macroporous scaffolds for stimulation of dental-pulp regeneration process, Dent. Mater., 2020, 36(1), 76–87 CrossRef CAS PubMed.
- R.-X. Wu, X.-T. He, J.-H. Zhu, Y. Yin, X. Li, X. Liu and F.-M. Chen, Modulating macrophage responses to promote tissue regeneration by changing the formulation of bone extracellular matrix from filler particles to gel bioscaffolds, Mater. Sci. Eng., C, 2019, 101, 330–340 CrossRef CAS PubMed.
- F. Yu, H. Zheng, X. Li, E.-N. Mohamed, E.-H. Hany, Y. Morsi, J. Li, J. Wu and X. Mo, A photocrosslinking antibacterial decellularized matrix hydrogel with nanofiber for cutaneous wound healing, Colloids Surf., B, 2022, 217, 112691 CrossRef CAS PubMed.
- S. Datta, A. P. Rameshbabu, K. Bankoti, M. Roy, C. Gupta, S. Jana, A. K. Das, R. Sen and S. Dhara, Decellularized bone matrix/oleoyl chitosan derived supramolecular injectable hydrogel promotes efficient bone integration, Mater. Sci. Eng., C, 2021, 119, 111604 CrossRef CAS PubMed.
- B. Fang, P. Qiu, C. Xia, D. Cai, C. Zhao, Y. Chen, H. Wang, S. Liu, H. Cheng and Z. Tang, Extracellular matrix scaffold crosslinked with vancomycin for multifunctional antibacterial bone infection therapy, Biomaterials, 2021, 268, 120603 CrossRef CAS PubMed.
- X. Chatzistavrou, J. C. Fenno, D. Faulk, S. Badylak, T. Kasuga, A. R. Boccaccini and P. Papagerakis, Fabrication and characterization of bioactive and antibacterial composites for dental applications, Acta Biomater., 2014, 10(8), 3723–3732 CrossRef CAS PubMed.
- K. Chachlioutaki, C. Karavasili, E. Adamoudi, A. Tsitsos, V. Economou, C. Beltes, N. Bouropoulos, O. L. Katsamenis, R. Doherty and A. Bakopoulou, Electrospun Nanofiber Films Suppress Inflammation In Vitro and Eradicate Endodontic Bacterial Infection in an E. faecalis-Infected Ex Vivo Human Tooth Culture Model, ACS Biomater. Sci. Eng., 2022, 8(5), 2096–2110 CrossRef CAS PubMed.
- E. Cross, S. M. Coulter, S. Pentlavalli and G. Laverty, Unravelling the antimicrobial activity of peptide hydrogel systems: current and future perspectives, Soft Matter, 2021, 17(35), 8001–8021 RSC.
- T. Huang, C. Tu, T. Zhou, Z. Yu, Y. Wang, Q. Yu, K. Yu, Z. Jiang, C. Gao and G. Yang, Antifouling poly (PEGMA) grafting modified titanium surface reduces osseointegration through resisting adhesion of bone marrow mesenchymal stem cells, Acta Biomater., 2022, 153, 585–595 CrossRef CAS PubMed.
- L. Peng, L. Chang, M. Si, J. Lin, Y. Wei, S. Wang, H. Liu, B. Han and L. Jiang, Hydrogel-coated dental device with adhesion-inhibiting and colony-suppressing properties, ACS Appl. Mater. Interfaces, 2020, 12(8), 9718–9725 CrossRef CAS PubMed.
- L. Li, B. Yan, J. Yang, W. Huang, L. Chen and H. Zeng, Injectable self-healing hydrogel with antimicrobial and antifouling properties, ACS Appl. Mater. Interfaces, 2017, 9(11), 9221–9225 CrossRef CAS PubMed.
- Y. Yang, J. Yuan, Y. Ni, Y. Gu, J. Zhou, W. Yuan, S. Xu, L. Che, S. Y. Zheng, W. Sun, D. Zhang and J. Yang, Spatiotemporal self-strengthening hydrogels for oral tissue regeneration, Composites, Part B, 2022, 243, 110119 CrossRef CAS.
- F. Batool, C. Stutz, C. Petit, N. Benkirane-Jessel, E. Delpy, F. Zal, E. Leize-Zal and O. Huck, A therapeutic oxygen carrier isolated from Arenicola marina decreased P. gingivalis induced inflammation and tissue destruction, Sci. Rep., 2020, 10(1), 1–14 CrossRef PubMed.
- V. K. Nikolopoulos, R. Augustine and G. Camci-Unal, Harnessing the potential of oxygen-generating materials and their utilization in organ-specific delivery of oxygen, Biomater. Sci., 2023, 11(5), 1567–1588 RSC.
- C. Yao, R. Zhang, J. Tang and D. Yang, Rolling circle amplification (RCA)-based DNA hydrogel, Nat. Protoc., 2021, 16(12), 5460–5483 CrossRef CAS PubMed.
- F. Li, J. Tang, J. Geng, D. Luo and D. Yang, Polymeric DNA hydrogel: Design, synthesis and applications, Prog. Polym. Sci., 2019, 98, 101163 CrossRef CAS.
- F. Mo, K. Jiang, D. Zhao, Y. Wang, J. Song and W. Tan, DNA hydrogel-based gene editing and drug delivery systems, Adv. Drug Delivery Rev., 2021, 168, 79–98 CrossRef CAS PubMed.
- W. Li, C. Wang, Z. Wang, L. Gou, Y. Zhou, G. Peng, M. Zhu, J. Zhang, R. Li and H. Ni, Physically Cross-Linked DNA Hydrogel-Based Sustained Cytokine Delivery for In Situ Diabetic Alveolar Bone Rebuilding, ACS Appl. Mater. Interfaces, 2022, 14(22), 25173–25182 CrossRef CAS PubMed.
- M. B. Sordi, A. Cruz, M. C. Fredel, R. Magini and P. T. Sharpe, Three-dimensional bioactive hydrogel-based scaffolds for bone regeneration in implant dentistry, Mater. Sci. Eng., C, 2021, 124, 112055 CrossRef CAS PubMed.
- H. Yu, X. Zhang, W. Song, T. Pan, H. Wang, T. Ning, Q. Wei, H. H. Xu, B. Wu and D. Ma, Effects of 3-dimensional bioprinting alginate/gelatin hydrogel scaffold extract on proliferation and differentiation of human dental pulp stem cells, J. Endod., 2019, 45(6), 706–715 CrossRef PubMed.
- Z. Hu, W. Cao, L. Shen, Z. Sun, K. Yu, Q. Zhu, T. Ren, L. Zhang, H. Zheng and C. Gao, Scalable milk-derived whey protein hydrogel as an implantable biomaterial, ACS Appl. Mater. Interfaces, 2022, 14(25), 28501–28513 CrossRef CAS PubMed.
- R. Murphy, S. Kordbacheh, D. Skoulas, S. Ng, K. Suthiwanich, A. M. Kasko, S.-A. Cryan, D. Fitzgerald-Hughes, A. Khademhosseini and A. Sheikhi, Three-dimensionally printable shear-thinning triblock copolypeptide hydrogels with antimicrobial potency, Biomater. Sci., 2021, 9(15), 5144–5149 RSC.
- N. G. Willemen, M. A. Morsink, D. Veerman, C. F. da Silva, J. C. Cardoso, E. B. Souto and P. Severino, From oral formulations to drug-eluting implants: using 3D and 4D printing to develop drug delivery systems and personalized medicine, Bio-Des. Manuf., 2021, 1–22 Search PubMed.
- M. Champeau, D. A. Heinze, T. N. Viana, E. R. de Souza, A. C. Chinellato and S. Titotto, 4D printing of hydrogels: a review, Adv. Funct. Mater., 2020, 30(31), 1910606 CrossRef CAS.
- D. Khorsandi, A. Fahimipour, P. Abasian, S. S. Saber, M. Seyedi, S. Ghanavati, A. Ahmad, A. A. De Stephanis, F. Taghavinezhaddilami and A. Leonova, 3D and 4D printing in dentistry and maxillofacial surgery: Printing techniques, materials, and applications, Acta Biomater., 2021, 122, 26–49 CrossRef CAS PubMed.
- M. Javaid, A. Haleem, R. P. Singh, S. Rab, R. Suman and L. Kumar, Significance of 4D printing for dentistry: Materials, process, and potentials, J. Oral Biol. Craniofacial Res., 2022, 12(3), 388–395 CrossRef PubMed.
- A. Sheikh, M. A. Abourehab and P. Kesharwani, The clinical significance of 4D printing, Drug Discovery Today, 2022, 103391 Search PubMed.
- S. Zu, Z. Zhang, Q. Liu, Z. Wang, Z. Song, Y. Guo, Y. Xin and S. Zhang, 4D printing of core–shell hydrogel capsules for smart controlled drug release, Bio-Des. Manuf., 2022, 5(2), 294–304 CrossRef CAS.
- B. Zhang, D. Lu and H. Duan, Recent advances in responsive antibacterial materials: design and application scenarios, Biomater. Sci., 2023, 11(2), 356–379 RSC.
| This journal is © The Royal Society of Chemistry 2023 |