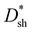Highly stable photomultiplication-type organic photodetectors with single polymers containing intramolecular traps as the active layer†
Zijin
Zhao‡
a,
Baiqiao
Liu‡
ab,
Chunyu
Xu
a,
Longtao
Li
a,
Ming
Liu
 a,
Kaixuan
Yang
a,
Kaixuan
Yang
 a,
Sang Young
Jeong
d,
Han Young
Woo
a,
Sang Young
Jeong
d,
Han Young
Woo
 d,
Guangcai
Yuan
*c,
Weiwei
Li
d,
Guangcai
Yuan
*c,
Weiwei
Li
 *b and
Fujun
Zhang
*b and
Fujun
Zhang
 *a
*a
aKey Laboratory of Luminescence and Optical Information, Ministry of Education, Beijing Jiaotong University, Beijing, 100044, People's Republic of China. E-mail: fjzhang@bjtu.edu.cn
bBeijing Advanced Innovation Center for Soft Matter Science and Engineering & State Key Laboratory of Organic–Inorganic Composites, Beijing University of Chemical Technology, Beijing, 100029, People's Republic of China. E-mail: liweiwei@iccas.ac.cn
cInstitute for Display and Sensor Devices, BOE Technology Group Co. Ltd., No. 9 Dize Road, BDA, Beijing 100176, People's Republic of China. E-mail: yuanguangcai@boe.com.cn
dOrganic Optoelectronic Materials Laboratory, Department of Chemistry, College of Science, Korea University, 02841, Seoul, Republic of Korea
First published on 4th May 2022
Abstract
Photomultiplication-type organic photodetectors (PM-OPDs) have attracted enormous interest owing to their high sensitivity toward weak light and, especially, due to their excellent parasitic stability when using a single polymer as the active layer. Herein, three different polymers, DCP1-3, were synthesized with different amounts of PC61BM pendants as intramolecular traps, and they were successfully applied to high-stability PM-OPDs. Photogenerated electrons will be trapped by suspended PC61BM in the polymers to induce interfacial band-bending for hole tunneling injection, and the injected holes can be efficiently transported along the channels of the polymer under bias. The thickness of the ultrathin PFN–Br interfacial layer was optimized to decrease the dark current density. An EQE of 19100% at 365 nm was obtained for DCP3-based PM-OPDs using PFN–Br as the interfacial layer under a bias of 20 V. The optimized PM-OPDs exhibit excellent stability, with no photocurrent decay after 70 days of storage in a nitrogen-filled glove box, which is attributed to the locking of donor and acceptor segments through covalent links in the polymer. The optimized PM-OPDs can be employed to measure the heart rate (HR) of humans under different pulsatile conditions, indicating the promising application prospects of PM-OPDs with a single polymer as the active layer.
Introduction
Photomultiplication-type organic photodetectors (PM-OPDs) have drawn increasing attention due to their external quantum efficiency (EQE) values of much higher than 100% and their small dark current density (JD) values.1–4 The small JD values of PM-OPDs originate from the single-charge-carrier transport channels in the active layers with donor:acceptor weight ratios of ∼ 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1
1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, in which the presence of much less donor or acceptor will form large amounts of isolated charge traps in the bulk heterojunction active layers.5–8 The presence of more trapped charge near the electrode will generate a Coulomb force, inducing interfacial band-bending for opposite-charge tunneling injection from the external circuit and leading to the large photocurrent density (JL) values of PM-OPDs.9–12 Great achievements in the field of PM-OPDs have been obtained via controlling molecular arrangements, charge mobility, and photogenerated exciton distributions, and via interfacial engineering, introducing third components, and employing double-layer strategies.7,9,13–15 Yang and co-workers employed the liquid–crystal material BTR as a regulator to enhance the performance of PM-OPDs; thanks to the optimized molecular arrangement improving hole mobility in the active layer, the EQE value of PM-OPDs could be increased 2.5-fold upon incorporating an appropriate amount of BTR into the active layer.15 The EQE of optimal PM-OPDs can be improved to 19300% at 660 nm under a bias of −20 V. Wu et al. utilized ITO/PEIE with a work function of 3.6 eV as an electrode to increase the interfacial hole injection barrier, achieving a JD value of 2.53 × 10−4 mA cm−2 and responsivity (R) of 42.0 A W−1 for PM-OPDs with P3HT
100, in which the presence of much less donor or acceptor will form large amounts of isolated charge traps in the bulk heterojunction active layers.5–8 The presence of more trapped charge near the electrode will generate a Coulomb force, inducing interfacial band-bending for opposite-charge tunneling injection from the external circuit and leading to the large photocurrent density (JL) values of PM-OPDs.9–12 Great achievements in the field of PM-OPDs have been obtained via controlling molecular arrangements, charge mobility, and photogenerated exciton distributions, and via interfacial engineering, introducing third components, and employing double-layer strategies.7,9,13–15 Yang and co-workers employed the liquid–crystal material BTR as a regulator to enhance the performance of PM-OPDs; thanks to the optimized molecular arrangement improving hole mobility in the active layer, the EQE value of PM-OPDs could be increased 2.5-fold upon incorporating an appropriate amount of BTR into the active layer.15 The EQE of optimal PM-OPDs can be improved to 19300% at 660 nm under a bias of −20 V. Wu et al. utilized ITO/PEIE with a work function of 3.6 eV as an electrode to increase the interfacial hole injection barrier, achieving a JD value of 2.53 × 10−4 mA cm−2 and responsivity (R) of 42.0 A W−1 for PM-OPDs with P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM (100
PC61BM (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, wt/wt) as the active layer under a bias of −20 V.14 Zhao et al. reported narrowband double-layered PM-OPDs with one response peak at 660 nm, a full-width at half-maximum (FWHM) of less than 35 nm, and an optimal EQE of 1120% under a bias of −20 V, in which the double-layered PM-OPDs were constructed with P3HT
1, wt/wt) as the active layer under a bias of −20 V.14 Zhao et al. reported narrowband double-layered PM-OPDs with one response peak at 660 nm, a full-width at half-maximum (FWHM) of less than 35 nm, and an optimal EQE of 1120% under a bias of −20 V, in which the double-layered PM-OPDs were constructed with P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P-TPD (9
P-TPD (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, wt/wt) as the optical field adjusting (OFA) layer and P3HT
2, wt/wt) as the optical field adjusting (OFA) layer and P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (50
PC71BM (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, wt/wt) as the photomultiplication (PM) layer via transfer-printing technology.16 The phase separation of two-component donor:acceptor films is usually unstable due to inevitable molecular aggregation, and this is harmful to the long-term stability of PM-OPDs.17–19 Stability is one key prerequisite for the real-world application of PM-OPDs. However, it is highly challenging to maintain long-term stability in relation to phase separation in bulk heterojunction (BHJ) active layers.
1, wt/wt) as the photomultiplication (PM) layer via transfer-printing technology.16 The phase separation of two-component donor:acceptor films is usually unstable due to inevitable molecular aggregation, and this is harmful to the long-term stability of PM-OPDs.17–19 Stability is one key prerequisite for the real-world application of PM-OPDs. However, it is highly challenging to maintain long-term stability in relation to phase separation in bulk heterojunction (BHJ) active layers.
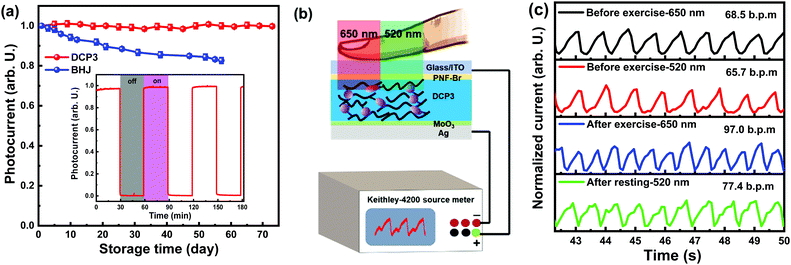
In this work, we proposed a smart strategy to overcome the long-term stability issues connected with PM-OPDs via developing polymers with donor and acceptor segments locked through covalent links, in which suspended PC61BM in the polymers was used as an intramolecular electron trap to induce interfacial band-bending for efficient hole tunneling injection. Three fullerene-containing polymers, named DCP1-3, were synthesized according to the synthetic procedure shown in Scheme S1 (ESI†), and the chemical structures of these polymers are depicted in Fig. 1a. The molecular structures of the monomers and polymers were confirmed through 1H and 13C nuclear magnetic resonance spectra and mass spectrometry measurements, as shown in Fig. S1–S4 (ESI†). The polymers contain PBDB-T units as a conjugated backbone and donor segments. Functionalized units with PC61BM pendants are connected to the PBDB-T conjugated backbone through covalent links, and the attached PC61BM pendants are used as intramolecular electron traps. The amounts of PC61BM pendants in the polymers DCP1-3 are estimated to be 1 wt%, 2 wt%, and 5 wt%, respectively. The charge mobility of the polymers was measured based on the space charge limited current (SCLC) method,20–22 as exhibited in Fig. S5 (ESI†). The hole mobility (μh) and electron mobility (μe) values of DCP1-3 are 3.8 × 10−5 and 1.2 × 10−9 cm2 V−1 s−1; 6.3 × 10−5 and 4.3 × 10−9 cm2 V−1 s−1; and 9.8 × 10−5 and 4.3 × 10−9 cm2 V−1 s−1, respectively. The rather low μe values of the polymers are mainly due to the lack of successive electron transport channels in the polymers with low amounts of PC61BM pendants. The absorption spectra and photoluminescence (PL) spectra of the polymers DCP1-3 and PBDB-T were recorded and are shown in Fig. 1b. The absorption spectra of the polymers are almost identical with PBDB-T because the polymers are mainly constructed from PBDB-T units with only small amounts of PC61BM segments. It is interesting that the PL emission of the polymers is markedly quenched as the acceptor segment content increases, indicating that photogenerated excitons can be sufficiently dissociated into free charge carriers assisted by the suspended PC61BM segments in the polymers. The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) levels of the polymers were measured via the cyclic voltammetry (CV) method, as shown in Fig. S6 (ESI†). The energy levels of the used materials are illustrated in Fig. 1c; slight variations in energy levels are induced upon the incorporation of different amounts of acceptor segments. Fig. 1d displays the structure of a PM-OPD with an inverted architecture of ITO/PFN–Br/active layer/MoO3/Ag, in which the thickness of PFN–Br was adjusted to suppress JD and further optimize the PM-OPD performance. The optimized PM-OPDs show an EQE of 19100% at 365 nm under a bias of 20 V and they also exhibit excellent stability, with less than 2% photocurrent decay after 70 days of storage.
 | ||
| Fig. 1 (a) The chemical structures of the polymers. (b) Absorption and PL spectra of PBDB-T and polymer films. (c) Energy levels of the polymers, ITO/PFN–Br,23 and the MoO3/Ag electrode. (d) The device architecture of a PM-OPD with a single polymer as the active layer. | ||
Results and discussion
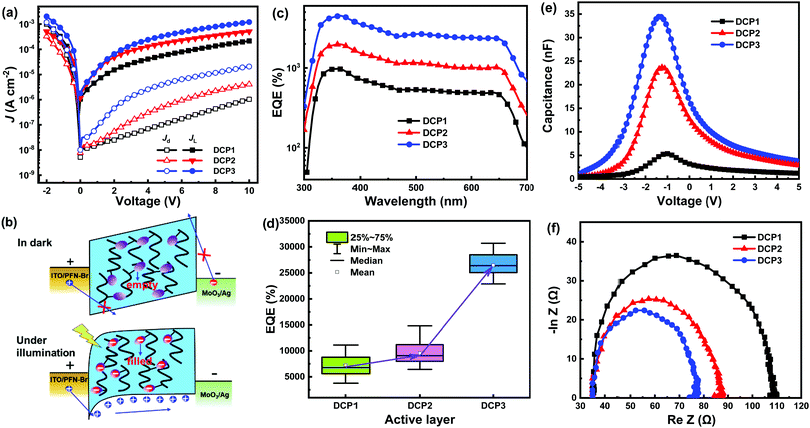
The current density versus voltage (J–V) curves of PM-OPDs with DCP1-3 as the active layer were measured under dark conditions and under white light illumination with an intensity of ∼1 mW cm−2, as shown in Fig. 2a. The JD and JL values of PM-OPDs are enhanced with an increase in forward bias (the ITO electrode is positively biased), which is mainly attributed to enhanced hole injection and transport in response to a large electric field. The JD and JL values of PM-OPDs with DCP3 as the active layer are larger than those of PM-OPDs with DCP1 or DCP2 as the active layer under the same bias; this can be easily explained based on the relatively high μh of DCP3 (9.8 × 10−5 cm2 V−1 s−1). Meanwhile, the electron current passing through the PM-OPDs should be negligible due to the low μe of ∼10−9 cm2 V−1 s−1 (compared with the μh value of ∼10−5 cm2 V−1 s−1) and the electron injection barrier of ∼1.4 eV between MoO3/Ag and the LUMO of the polymer. Under forward bias, holes will be injected from PFN–Br/ITO into the HOMO of the polymer and transported along the channels formed by the polymers. The JL values of all PM-OPDs are two orders of magnitude larger than the corresponding JD values under the same forward bias, which should arise due to enhanced hole tunneling injection from PFN–Br/ITO assisted by trapped electrons in PC61BM pendants under light illumination. Schematic diagrams are provided to exhibit the charge dynamics in PM-OPDs under dark and light conditions, as shown in Fig. 2b. Under dark conditions, it is difficult for holes to be injected into the active layer due to the large triangular barrier separating PFN–Br/ITO electrons from the polymer HOMO, resulting in the low JD values of the PM-OPDs.24–26 Under illuminated conditions, photogenerated excitons can be dissociated into free charge carriers in the polymer containing PC61BM pendants. Photogenerated holes will be transported along the channels of the polymer and collected by the MoO3/Ag electrode. Photogenerated electrons will be trapped by PC61BM pendants due to its low content in the polymer. Trapped electrons near PFN–Br/ITO will cause interfacial band-bending, narrowing the hole injection barrier from a triangular shape to a wedge shape. Holes will be readily injected into the active layer via tunneling through the wedge barrier, leading to large JL values in PM-OPDs with a single polymer as the active layer. The photoinduced current density (JPi) is obtained based on the difference between JL and JD, representing the photo-response abilities of a PM-OPD.27 The JL value of a PBDB-T-based device is slightly larger than JD, indicating the poor photo-response abilities of the PBDB-T-based device, as depicted in Fig. S7a (ESI†). The maximum EQE of the PBDB-T-based device is lower than 50% under an applied bias of 10 V without photocurrent multiplication, as shown in Fig. S7b (ESI†), indicating that the PC61BM pendants play a vital role in PM-OPDs with DCP1-3 as the active layer.The EQE spectra of all PM-OPDs were obtained under a bias of 10 V, as displayed in Fig. 2c. It is apparent that all single-polymer-based PM-OPDs exhibit a broad response range from 300 to 700 nm, corresponding well with the photon harvesting range of the polymers. It is notable that DCP3-based PM-OPDs have relatively large EQEs compared to DCP1- and DCP2-based PM-OPDs under the same bias, which can be explained based on the relatively high exciton dissociation efficiency and μh value shown by the DCP3-based active layer. Photogenerated excitons can be sufficiently dissociated into free charges in DCP3 with a relatively high PC61BM content, as confirmed based on the markedly quenched PL emission of DCP3 exhibited in Fig. 1b.
More photogenerated electrons will be trapped in DCP3 due to its relatively high PC61BM content, which can induce sufficient interfacial band-bending for hole tunneling injection. The injected holes will be sufficiently transported along the channels formed by DCP3 due to its large μh value of 9.8 × 10−5 cm2 V−1 s−1 compared with those of DCP1 and DCP2. The EQE spectra of single-polymer-based PM-OPDs were measured under different biases, and these are shown in Fig. S8 (ESI†). The EQE values of all PM-OPDs rapidly increase as the bias increases, which is mainly attributed to improved hole tunneling injection and hole transport under a large bias.28Fig. 2d exhibits the distributions of the EQE values of all PM-OPDs under a bias of 20 V; the statistical results for each kind of single-polymer-based PM-OPD were obtained based on 15 devices prepared from different batches. The median values of the EQE distributions for DCP1-3-based PM-OPDs are 6700%, 8900%, and 26300%, respectively, under a bias of 20 V.
To investigate the charge dynamics in detail in single-polymer-based PM-OPDs, capacitance versus voltage (C–V) measurements were performed under one sun simulated light illumination at a frequency of 10 kHz, as exhibited in Fig. 2e. It is apparent that the capacitance of the PM-OPDs increases as the amount of PC61BM pendants increases in the polymers, indicating the improved electron accumulation properties of polymer films with more PC61BM. These improved electron accumulation properties are conducive to inducing interfacial band-bending, allowing hole tunneling injection from the external circuit. The hole-transport properties of all PM-OPDs can also be investigated based on electrochemical impedance spectroscopy (EIS) measurements. Nyquist plots of all PM-OPDs were obtained in the frequency range of 10 KHz to 8 MHz under a bias of 5 V and one sun simulated light illumination, as shown in Fig. 2f. The charge transport resistance (Rct) can be estimated based on the diameter of the semicircle. It is apparent that Rct gradually decreases for PM-OPDs with different active layers in the order DCP1 > DCP2 > DCP3, indicating that the hole transport properties can be enhanced when using DCP3 film. The enhanced hole transport properties of DCP3 films can be attributed to the improved molecular arrangement upon incorporating more PC61BM pendants.
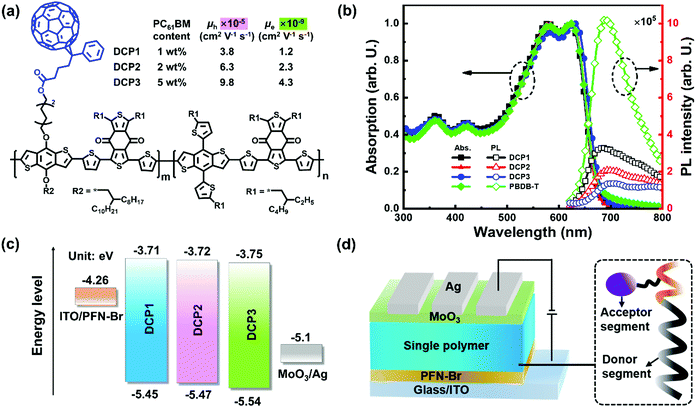
Grazing incidence wide-angle X-ray scattering (GIWAXS) analysis was carried out on polymers containing different amounts of PC61BM to investigate the molecular arrangements in the DCP1-3 films. The two-dimensional (2D) GIWAXS patterns of DCP1-3 films are exhibited in Fig. 3a–c, respectively. The in-plane (IP) and out-of-plane (OOP) line profiles of the GIWAXS patterns of the polymers are shown in Fig. 3d. Distinct (100) lamellar peaks (q ≈ 0.29 Å−1) and (200) lamellar peaks (q ≈ 0.65 Å−1) in the IP direction and (010) π–π stacking peaks (q ≈ 1.67 Å−1) in the OOP direction can be simultaneously observed from the profiles of the polymer films, indicating that the polymers prefer to adopt a face-on molecular orientation, allowing efficient hole transport along the normal direction to the substrate.29,30 Meanwhile, the gradual sharpening of the IP (100) lamellar peak and OOP (010) π–π stacking peak can be clearly observed with an increase in the PC61BM content from the profiles of the DCP1-3 films. To evaluate the degree of molecular crystallinity, the crystal coherence length (CCL) values of the IP (100) lamellar peak and OOP (010) π–π stacking peak were calculated according to the Scherrer equation: CCL = 2πk/Δq,31–33 where k denotes the Scherrer constant (≈ 0.9) and Δq denotes the FWHM of the diffraction peak. The CCL values of the IP (100) lamellar peak and OOP (010) π–π stacking peak are 6.5 nm/1.8 nm for DCP1, 7.2 nm/3.2 nm for DCP2, and 8.8 nm/3.9 nm for DCP3, respectively. The gradual increase in CCL values suggests the presence of improved molecular packing upon incorporating more PC61BM pendants, leading to the gradual enhancement of the μh value with PC61BM content for the polymers DCP1-3.
 | ||
| Fig. 3 (a)–(c) 2D GIWAXS patterns of DCP1-3 film samples, respectively. (d) Scattering profiles of the corresponding film samples with respect to the OOP and IP directions. | ||
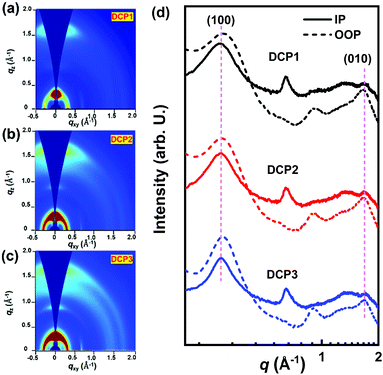
Specific detectivity (D*) is a critical figure of merit for photodetectors, denoting the capacity of a photodetector to sense a faint light signal.34–36 Supposing that shot noise mainly contributes to the total noise, D* can be evaluated from the shot-noise-limited specific detectivity 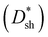 , which can be calculated from the following equation:37–39
, which can be calculated from the following equation:37–39
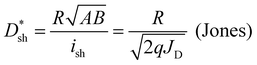 | (1) |
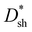 values of PM-OPDs can be obtained according to eqn (1), as shown in Fig. 4a. It is apparent that a
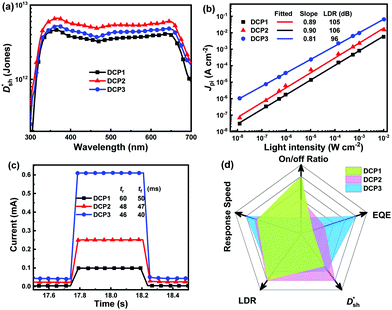
values of PM-OPDs can be obtained according to eqn (1), as shown in Fig. 4a. It is apparent that a 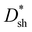 value of over 9 × 1011 Jones can be obtained in the region of 310–700 nm for all PM-OPDs under a bias of 10 V. The maximum
value of over 9 × 1011 Jones can be obtained in the region of 310–700 nm for all PM-OPDs under a bias of 10 V. The maximum 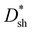 values of PM-OPDs with DCP1-3 as the active layer are 4.6 × 1012, 6.6 × 1012, and 5.2 × 1012 Jones, respectively, under a bias of 10 V. The
values of PM-OPDs with DCP1-3 as the active layer are 4.6 × 1012, 6.6 × 1012, and 5.2 × 1012 Jones, respectively, under a bias of 10 V. The 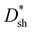 value of the DCP3-based PM-OPDs is lower than that of the DCP2-based PM-OPDs under a bias of 10 V owing to the relatively large JD value of DCP3-based PM-OPDs, indicating that suppressing JD is beneficial for enhancing the detectivity of PM-OPDs.40,41 The linear dynamic range (LDR) describes the range over which the output signal of a photodetector changes linearly with the input signal, and this can be calculated through the equation:42,43
value of the DCP3-based PM-OPDs is lower than that of the DCP2-based PM-OPDs under a bias of 10 V owing to the relatively large JD value of DCP3-based PM-OPDs, indicating that suppressing JD is beneficial for enhancing the detectivity of PM-OPDs.40,41 The linear dynamic range (LDR) describes the range over which the output signal of a photodetector changes linearly with the input signal, and this can be calculated through the equation:42,43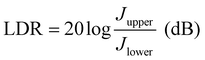 | (2) |
Response speed is also one of the important parameters for evaluating photodetectors.44,45 To evaluate the response speeds of all PM-OPDs, the transient photocurrent was recorded under a bias of 10 V and 532 nm light illumination with an intensity of 10 mW cm−2, and the excited light was modulated through an electronic shutter with a period of 1 s, as shown in Fig. 4c. The rise time (tr) and fall time (tf) decrease from 60 to 48 to 46 ms and from 50 to 47 to 40 ms, respectively, for the PM-OPDs with DCP1, DCP2, and DCP3, respectively, as the active layer. It is well known that more electron traps formed from PC61BM pendants can rapidly capture more photogenerated electrons, leading to sufficient interfacial band-bending as soon as possible.46 The decreased tr value of PM-OPDs with DCP3 as the active layer should be attributed to enhanced hole tunneling injection and better hole transport in the DCP3 film. The tf value of the PM-OPDs corresponds to the release or recombination time of trapped electrons in PC61BM, which strongly depends on the number of injected holes per unit time from the external circuit. Once the excited light is turned off, the injected holes will rapidly recombine with trapped electrons in PC61BM, resulting in the termination of interfacial band-bending and limited hole tunneling injection. The shorter tf value of PM-OPDs with DCP3 as the active layer can be well explained based on the relatively large transient photocurrent. Curves plotting the on/off current ratios of PM-OPDs were obtained according to the J–V curves of PM-OPDs in the dark and under light illumination, as exhibited in Fig. S10 (ESI†). To intuitively evaluate the overall performances of the PM-OPDs, the EQE, 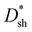 , LDR, response speed, and on/off ratio values of all PM-OPDs under a bias of 10 V were gathered in the form of a radar map, as illustrated in Fig. 4d. Although DCP3-based PM-OPDs exhibit the optimal EQE value and response speed, the corresponding
, LDR, response speed, and on/off ratio values of all PM-OPDs under a bias of 10 V were gathered in the form of a radar map, as illustrated in Fig. 4d. Although DCP3-based PM-OPDs exhibit the optimal EQE value and response speed, the corresponding 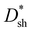 , LDR, and on–off ratio are lower than those of the other kinds of PM-OPDs due to the relatively large JD value of PM-OPDs with DCP3 as the active layer. To suppress the JD value of PM-OPDs with DCP3 as the active layer, the thickness of the interfacial PFN–Br layer was finely optimized via adjusting the concentration of PFN–Br solution (CPFN–Br) while maintaining the other preparation conditions. The thickness of the PFN–Br layer should increase along with an increase in CPFN–Br.
, LDR, and on–off ratio are lower than those of the other kinds of PM-OPDs due to the relatively large JD value of PM-OPDs with DCP3 as the active layer. To suppress the JD value of PM-OPDs with DCP3 as the active layer, the thickness of the interfacial PFN–Br layer was finely optimized via adjusting the concentration of PFN–Br solution (CPFN–Br) while maintaining the other preparation conditions. The thickness of the PFN–Br layer should increase along with an increase in CPFN–Br.
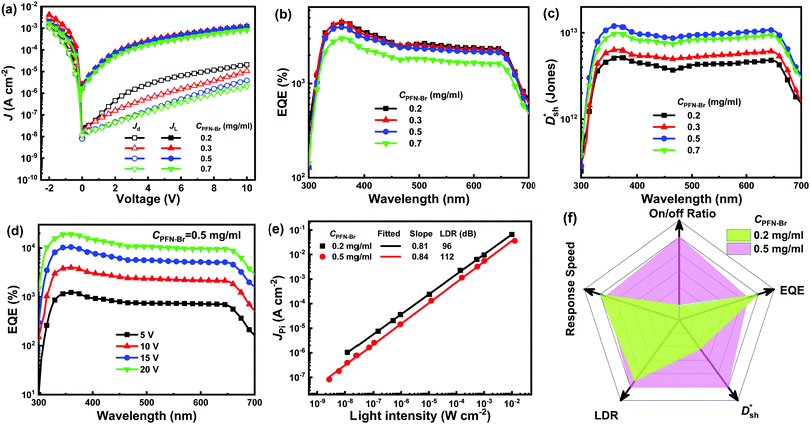
The J–V curves of DCP3-based PM-OPDs with different PFN–Br layer thicknesses were obtained and are shown in Fig. 5a. Obviously, the JD value of PM-OPDs is gradually reduced upon increasing CPFN–Br. The hole injection barrier will be enlarged upon increasing the thickness of the PFN–Br interlayer via using a higher-concentration solution. It will be more difficult for holes to be injected into the active layer with increased PFN–Br layer thickness, resulting in the suppression of JD. EIS measurements of DCP3-based PM-OPDs with different CPFN–Br values under dark conditions were performed, and the results are shown in Fig. S11 (ESI†). Rct clearly increases as CPFN–Br is increased from 0.2 to 0.7 mg ml−1, which should result from an enhanced hole injection barrier as a result of an increase in CPFN–Br. The JL value of the PM-OPDs can be kept almost constant as CPFN–Br increases from 0.2 to 0.5 mg ml−1 but it slightly decreases as CPFN–Br increases to 0.7 mg ml−1. The on/off ratio of PM-OPDs can be enhanced from 58 to 397 as CPFN–Br increases from 0.2 to 0.7 mg ml−1, resulting from a gradual decrease in JD, as shown in Fig. S12 (ESI†). The EQE and 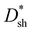 spectra of DCP3-based PM-OPDs with different levels of CPFN–Br were investigated under a bias of 10 V, and the results are displayed in Fig. 5b and c, respectively. The EQE values of DCP3-based PM-OPDs slightly decrease as CPFN–Br increases from 0.2 to 0.7 mg ml−1 due to the increased hole injection barrier. The maximum EQE values of DCP3-based PM-OPDs are 4510%, 4500%, 3970%, and 3040% under a bias of 10 V when CPFN–Br is 0.2, 0.3, 0.5, and 0.7 mg ml−1, respectively. The
spectra of DCP3-based PM-OPDs with different levels of CPFN–Br were investigated under a bias of 10 V, and the results are displayed in Fig. 5b and c, respectively. The EQE values of DCP3-based PM-OPDs slightly decrease as CPFN–Br increases from 0.2 to 0.7 mg ml−1 due to the increased hole injection barrier. The maximum EQE values of DCP3-based PM-OPDs are 4510%, 4500%, 3970%, and 3040% under a bias of 10 V when CPFN–Br is 0.2, 0.3, 0.5, and 0.7 mg ml−1, respectively. The 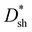 values of PM-OPDs are enhanced as CPFN–Br increases from 0.2 to 0.5 mg ml−1 thanks to the suppression of JD, and then there is a slight decrease when CPFN–Br increases to 0.7 mg ml−1 due to the weakened hole tunneling injection. The maximum
values of PM-OPDs are enhanced as CPFN–Br increases from 0.2 to 0.5 mg ml−1 thanks to the suppression of JD, and then there is a slight decrease when CPFN–Br increases to 0.7 mg ml−1 due to the weakened hole tunneling injection. The maximum 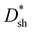 value of 1.2 × 1013 Jones at 365 nm under a bias of 10 V can be obtained for PM-OPDs with a PFN–Br layer prepared from 0.5 mg ml−1 solution. The use of the optimal PFN–Br layer will lead to acceptable EQE values and rather low JD values in PM-OPDs.
value of 1.2 × 1013 Jones at 365 nm under a bias of 10 V can be obtained for PM-OPDs with a PFN–Br layer prepared from 0.5 mg ml−1 solution. The use of the optimal PFN–Br layer will lead to acceptable EQE values and rather low JD values in PM-OPDs.
The EQE spectra of DCP3-based PM-OPDs with a PFN–Br layer prepared at a solution concentration of 0.5 mg ml−1 were investigated under different bias levels, and they are shown in Fig. 5d. It is apparent that the EQE values of the optimal PM-OPDs are dramatically enhanced upon increasing the bias due to enhanced hole tunneling injection from the PFN–Br/ITO electrode and accelerated hole transport in the active layer under a large electric field. An EQE of 19100% at 365 nm is achieved for the optimal PM-OPDs under a bias of 20 V. The LDR of optimal PM-OPDs was investigated via measuring J–V curves at different light intensities, and JPi as a function of the light intensity under a bias of 10 V is shown in Fig. 5e. The JPi values of the optimal PM-OPDs linearly vary from 2.6 × 10−2 to 1.6 × 10−7 A cm−2 as the light intensity decreases from 12.6 mW cm−2 to 2.7 nW cm−2, giving a LDR of 112 dB. The LDR of the optimal PM-OPDs is larger than that of 96 dB for control PM-OPDs prepared with a CPFN–Br value of 0.2 mg ml−1 due to the suppression of JD. It is apparent that the slope of a curve of JPiversus light-intensity for the optimum PM-OPDs is larger than that of the control PM-OPDs, resulting from the suppressed recombination of trapped electrons and injected holes. The tr and tf values of the optimal PM-OPDs are 46 and 49 ms, respectively, which are slightly increased in comparison with those of the control PM-OPDs, resulting from the slightly weakened hole tunneling injection, as shown in Fig. S13 (ESI†). To intuitively compare the parameters of the optimal PM-OPDs and the control PM-OPDs, radar map analysis of the key parameters of EQE, 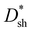 , LDR, response speed, and on/off ratio is displayed in Fig. 5f. Apparently, the overall performance of PM-OPDs can be well improved and balanced via optimizing the thickness of the PFN–Br layer, primarily resulting from the large suppression of JD and the maintenance of JL.
, LDR, response speed, and on/off ratio is displayed in Fig. 5f. Apparently, the overall performance of PM-OPDs can be well improved and balanced via optimizing the thickness of the PFN–Br layer, primarily resulting from the large suppression of JD and the maintenance of JL.
The optimal PM-OPDs without encapsulation were stored in a nitrogen-filled glove box to investigate their long-term stability. The photocurrent of the optimal PM-OPDs was recorded under a bias of 10 V every 4 days, as shown in Fig. 6a. It is apparent that the optimal PM-OPDs exhibit less than 2% photocurrent decay after 70 days of storage, indicating the superior long-term stability of the PM-OPDs due to the polymer locking the donor and acceptor segments via covalent links.47,48 The photocurrent of PBDB-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM (95
PC61BM (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, w/w) BHJ-based PM-OPDs undergoes a relatively fast decay process, as shown in Fig. 6a. BHJ-based PM-OPDs show approximately 20% photocurrent decay after 57 days of storage, resulting from molecular aggregation in the blended donor:acceptor active layer. The photocurrent versus illumination time curve of optimal PM-OPDs after 70 days of storage is shown in the inset of Fig. 6a, indicating the excellent stability of single-polymer-based PM-OPDs.
5, w/w) BHJ-based PM-OPDs undergoes a relatively fast decay process, as shown in Fig. 6a. BHJ-based PM-OPDs show approximately 20% photocurrent decay after 57 days of storage, resulting from molecular aggregation in the blended donor:acceptor active layer. The photocurrent versus illumination time curve of optimal PM-OPDs after 70 days of storage is shown in the inset of Fig. 6a, indicating the excellent stability of single-polymer-based PM-OPDs.
Photoplethysmography (PPG) testing was performed to demonstrate the potential application of the optimal PM-OPDs. An optimal PM-OPD was contented to a Keithley 4200 source meter to realize the application of the PPG sensor, as illustrated in Fig. 6b. When light passes through the finger, the transmitted light can be detected by the PM-OPD. The PPG signal can be obtained via recording the variations in photocurrent resulting from periodic variations in the transmitted light intensity due to the systolic and diastolic phases of the cardiac cycle.49–52 The heart rate (HR) of a human can be evaluated based on the period of the PPG signal, as shown in Fig. 6c. A detailed description of the use of PM-OPDs for monitoring human heart rates is given in the ESI.† The current from the PM-OPD was monitored under a bias of 5 V in air to measure the absorption of red (650 nm) or green (520 nm) light by the blood. Clear and repeated PPG signals can be obtained using red or green light due to the high sensitivity of the optimized PM-OPDs to 650 and 520 nm light. The pulsatile signal can be distinctly extracted from the output current, and the heart rates (HRs) of one author were evaluated to be 68.5 and 65.7 beats per minute (bpm) before exercise based on red and green light, respectively. The measured HR values for the author using both red and green light are in the normal range for humans. The slight fluctuations of the HR value of the author under resting conditions should be attributed to fluctuations in the pulsatile signal. The HR value was enhanced to 97.0 bpm for the author after exercise. Then, the HR value dropped to 77.4 bpm for the author after having a rest. The PM-OPDs can be used to measure the HRs of humans under different pulsatile conditions due to the high sensitivity of PM-OPDs to slight changes in light intensity.
Conclusions
In summary, three polymers, DCP1-3, with different amounts of PC61BM pendants as intramolecular traps were carefully synthesized, and they were successfully applied in PM-OPDs with the configuration of ITO/PFN–Br/active layer/MoO3/Ag. The photocurrent multiplication is attributed to hole tunneling injection from the PFN–Br/ITO electrode induced by interfacial trapped electrons in PC61BM pendants under forward bias. The DCP3-based PM-OPDs show the highest EQE values due to the relatively high hole-transport abilities and exciton dissociation efficiency of DCP3 film. The overall performance of DCP3-based PM-OPDs can be further enhanced via adjusting the thickness of the PFN–Br layer to suppress JD. A maximum EQE of 19100% is realized under a bias of 20 V when using the optimal PM-OPDs with a PFN–Br layer prepared from solution with a concentration of 0.5 mg ml−1. Meanwhile, the optimized PM-OPDs show enhanced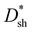 , LDR, and on/off ratio values of 1.2 × 1013 Jones, 104 dB, and 390, respectively, under a bias of 10 V compared to values of 5.2 × 1012 Jones, 95 dB, and 58, respectively, for control PM-OPDs with a PFN–Br layer prepared from solution with a CPFN–Br value of 0.2 mg ml−1. PM-OPDs with a single polymer as the active layer show excellent stability after 70 days of storage without any encapsulation, indicating their great application potential. Meanwhile, the HR of an author under different pulsatile conditions can be successfully recorded, indicating the strong detection abilities of the PM-OPDs toward weak light without a current amplified circuit.
, LDR, and on/off ratio values of 1.2 × 1013 Jones, 104 dB, and 390, respectively, under a bias of 10 V compared to values of 5.2 × 1012 Jones, 95 dB, and 58, respectively, for control PM-OPDs with a PFN–Br layer prepared from solution with a CPFN–Br value of 0.2 mg ml−1. PM-OPDs with a single polymer as the active layer show excellent stability after 70 days of storage without any encapsulation, indicating their great application potential. Meanwhile, the HR of an author under different pulsatile conditions can be successfully recorded, indicating the strong detection abilities of the PM-OPDs toward weak light without a current amplified circuit.
Author contributions
Z. Zhao, G. Yuan, and F. Zhang conceived the idea and wrote this paper. Z. Zhao fabricated all the PM-OPD samples, conducted measurements, and performed data analysis. B. Liu and W. Li designed and synthesized the polymers and performed the characterization. C. Xu, M. Liu, and K. Yang participated in data analysis and manuscript preparation. Z. Zhao and L. Li conducted the heart-rate measurements. S. Jeong and H. Woo conducted the GIWAXS measurements. F. Zhang directed the project.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are thankful for financial support from the Fundamental Research Funds for the Central Universities (Grant No. 2021YJS178), National Natural Science Foundation of China (Grant No. 61975006, 62175011 and 92163128), and the Beijing Natural Science Foundation (Grant No. 4192049 and JQ21006).References
- Z. Zhao, C. Xu, L. Niu, X. Zhang and F. Zhang, Laser Photonics Rev., 2020, 14, 2000262 CrossRef CAS
.
- J. Kublitski, A. Fischer, S. Xing, L. Baisinger, E. Bittrich, D. Spoltore, J. Benduhn, K. Vandewal and K. Leo, Nat. Commun., 2021, 12, 4259 CrossRef CAS PubMed
.
- M. Liu, J. Wang, Z. Zhao, K. Yang, P. Durand, F. Ceugniet, G. Ulrich, L. Niu, Y. Ma, N. Leclerc, X. Ma, L. Shen and F. Zhang, J. Phys. Chem. Lett., 2021, 12, 2937 CrossRef CAS PubMed
.
- J. Kim, M. Kang, S. Lee, C. So and D. S. Chung, Adv. Mater., 2021, 33, 2104689 CrossRef CAS PubMed
.
- J. Kim, C. So, M. Kang, K. M. Sim, B. Lim and D. S. Chung, Mater. Horiz., 2021, 8, 276 RSC
.
- Z. Lan, Y. Lei, W. Kin, E. Chan, S. Chen, D. Luo and F. Zhu, Sci. Adv., 2020, 6, eaaw8065 CrossRef CAS PubMed
.
- J. Wang, S. C. Chen, Z. Yin and Q. Zheng, J. Mater. Chem. C, 2020, 8, 14049 RSC
.
- D. K. Neethipathi, H. S. Ryu, M. S. Jang, S. Yoon, K. M. Sim, H. Y. Woo and D. S. Chung, ACS Appl. Mater. Interfaces, 2019, 11, 21211 CrossRef CAS PubMed
.
- Z. Zhao, J. Wang, C. Xu, K. Yang, F. Zhao, K. Wang, X. Zhang and F. Zhang, J. Phys. Chem. Lett., 2020, 11, 366 CrossRef CAS PubMed
.
- H. R. Sim, M. Kang, S. H. Yu, G. H. Nam, B. Lim and D. S. Chung, Adv. Opt. Mater., 2020, 9, 2001836 CrossRef
.
- M. S. Jang, S. Yoon, K. M. Sim, J. Cho and D. S. Chung, J. Phys. Chem. Lett., 2018, 9, 8 CrossRef CAS PubMed
.
- C. Wang, J. Lai, Q. Chen, F. Zhang and L. Chen, Nano Lett., 2021, 21, 8474 CrossRef CAS PubMed
.
- S. Yoon, G. S. Lee, K. M. Sim, M. J. Kim, Y. H. Kim and D. S. Chung, Adv. Funct. Mater., 2021, 31, 2006448 CrossRef CAS
.
- Y. Wu, K. Fukuda, T. Yokota and T. Someya, Adv. Mater., 2019, 31, 1903687 CrossRef CAS PubMed
.
- K. Yang, Z. Zhao, M. Liu, Z. Zhou, K. Wang, X. Ma, J. Wang, Z. He and F. Zhang, Chem. Eng. J., 2022, 427, 131802 CrossRef CAS
.
- Z. Zhao, M. Liu, K. Yang, C. Xu, Y. Guan, X. Ma, J. Wang and F. Zhang, Adv. Funct. Mater., 2021, 31, 2106009 CrossRef CAS
.
- N. Li, J. D. Perea, T. Kassar, M. Richter, T. Heumueller, G. J. Matt, Y. Hou, N. S. Guldal, H. Chen, S. Chen, S. Langner, M. Berlinghof, T. Unruh and C. J. Brabec, Nat. Commun., 2017, 8, 14541 CrossRef CAS PubMed
.
- C. Li, X. Wu, X. Sui, H. Wu, C. Wang, G. Feng, Y. Wu, F. Liu, X. Liu, Z. Tang and W. Li, Angew. Chem., Int. Ed., 2019, 58, 15532 CrossRef CAS PubMed
.
- M. Jorgensen, K. Norrman, S. A. Gevorgyan, T. Tromholt, B. Andreasen and F. C. Krebs, Adv. Mater., 2012, 24, 580 CrossRef PubMed
.
- C. Xu, K. Jin, Z. Xiao, Z. Zhao, X. Ma, X. Wang, J. Li, W. Xu, S. Zhang, L. Ding and F. Zhang, Adv. Funct. Mater., 2021, 31, 2107934 CrossRef CAS
.
- W. Xu, X. Ma, J. H. Son, S. Y. Jeong, L. Niu, C. Xu, S. Zhang, Z. Zhou, J. Gao, H. Y. Woo, J. Zhang, J. Wang and F. Zhang, Small, 2022, 18, 2104215 CrossRef CAS PubMed
.
- S. Zhang, X. Ma, C. Xu, W. Xu, S. Y. Jeong, H. Y. Woo, Z. Zhou, X. Zhang and F. Zhang, Macromol. Rapid Commun., 2022 DOI:10.1002/marc.202200345
.
- L. Chen, C. Xie and Y. Chen, Adv. Funct. Mater., 2014, 24, 3986 CrossRef CAS
.
- S. G. Han, H. Lee, W. Choi, D. Lee, S. Kim, Y. Sung, S. Kim and K. Cho, Adv. Funct. Mater., 2021, 31, 2102087 CrossRef CAS
.
- Z. Zhao, C. Li, L. Shen, X. Zhang and F. Zhang, Nanoscale, 2020, 12, 1091 RSC
.
- D. Guo, L. Yang, J. Zhao, J. Li, G. He, D. Yang, L. Wang, A. Vadim and D. Ma, Mater. Horiz., 2021, 8, 2293 RSC
.
- Z. Zhao, B. Liu, C. Xu, M. Liu, K. Yang, X. Zhang, Y. Xu, J. Zhang, W. Li and F. Zhang, J. Mater. Chem. C, 2021, 9, 5349 RSC
.
- K. Yang, J. Wang, Z. Zhao, Z. Zhou, M. Liu, J. Zhang, Z. He and F. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 21565 CrossRef CAS PubMed
.
- L. Lv, W. Dang, X. Wu, H. Chen, T. Wang, L. Qin, Z. Wei, K. Zhang, G. Shen and H. Huang, Macromolecules, 2020, 53, 10636 CrossRef CAS
.
- Y. Wei, J. Yu, L. Qin, H. Chen, X. Wu, Z. Wei, X. Zhang, Z. Xiao, L. Ding, F. Gao and H. Huang, Energy Environ. Sci., 2021, 14, 2314 RSC
.
- Y. Wu, J. Guo, W. Wang, Z. Chen, Z. Chen, R. Sun, Q. Wu, T. Wang, X. Hao, H. Zhu and J. Min, Joule, 2021, 5, 1800 CrossRef CAS
.
- J. Kim, C. W. Joo, S. Z. Hassan, S. H. Yu, M. Kang, J.
E. Pi, S. Y. Kang, Y. S. Park and D. S. Chung, Mater. Horiz., 2021, 8, 3141 RSC
.
- M. Liu, J. Wang, K. Yang, Z. Zhao, Z. Zhou, Y. Ma, L. Shen, X. Ma and F. Zhang, J. Mater. Chem. C, 2021, 9, 6357 RSC
.
- Y. Yao, Q. Ou, K. Wang, H. Peng, F. Fang, Y. Shi, Y. Wang, D. I. Asperilla, Z. Shuai and P. Samori, Nat. Commun., 2021, 12, 3667 CrossRef CAS PubMed
.
- C. Fuentes-Hernandez, W. Chou, T. Khan, L. Diniz, J. Lukens, F. Larrain, V. Rodriguez-Toro and B. Kippelen, Science, 2020, 370, 698 CrossRef CAS PubMed
.
- X. Wang, L. Lv, L. Li, Y. Chen, K. Zhang, H. Chen, H. Dong, J. Huang, G. Shen, Z. Yang and H. Huang, Adv. Funct. Mater., 2016, 26, 6306 CrossRef CAS
.
- Y. Chen, Y. Zheng, Y. Jiang, H. Fan and X. Zhu, J. Am. Chem. Soc., 2021, 143, 4281 CrossRef CAS PubMed
.
- Y. Wei, H. Chen, T. Liu, S. Wang, Y. Jiang, Y. Song, J. Zhang, X. Zhang, G. Lu, F. Huang, Z. Wei and H. Huang, Adv. Funct. Mater., 2021, 31, 2106326 CrossRef CAS
.
- T. Yokota, K. Fukuda and T. Someya, Adv. Mater., 2021, 33, 2004416 CrossRef CAS PubMed
.
- B. Park, J. Jung, D. H. Lim, H. Lee, S. Park, M. Kyeong, S. J. Ko, S. H. Eom, S. H. Lee, C. Lee and S. C. Yoon, Adv. Funct. Mater., 2021, 31, 2108026 Search PubMed
.
- W. Jang, B. G. Kim, S. Seo, A. Shawky, M. S. Kim, K. Kim, B. Mikladal, E. I. Kauppinen, S. Maruyama, I. Jeon and D. H. Wang, Nano Today, 2021, 37, 101081 CrossRef CAS
.
- J. Liu, J. Jiang, S. Wang, T. Li, X. Jing, Y. Liu, Y. Wang, H. Wen, M. Yao, X. Zhan and L. Shen, Small, 2021, 17, 2101316 CrossRef CAS PubMed
.
- C. Li, H. Wang, F. Wang, T. Li, M. Xu, H. Wang, Z. Wang, X. Zhan, W. Hu and L. Shen, Light: Sci. Appl., 2020, 9, 31 CrossRef CAS PubMed
.
- A. Pospischil, M. Humer, M. M. Furchi, D. Bachmann, R. Guider, T. Fromherz and T. Mueller, Nat. Photonics, 2013, 7, 892 CrossRef CAS
.
- J. Vanderspikken, W. Maes and K. Vandewal, Adv. Funct. Mater., 2021, 31, 2104060 CrossRef CAS
.
- Z. Zhao, B. Liu, C. Xie, Y. Ma, J. Wang, M. Liu, K. Yang, Y. Xu, J. Zhang, W. Li, L. Shen and F. Zhang, Sci. China: Chem., 2021, 64, 1302 CrossRef CAS
.
- Y. He, T. Heumüller, W. Lai, G. Feng, A. Classen, X. Du, C. Liu, W. Li, N. Li and C. J. Brabec, Adv. Energy Mater., 2019, 9, 1900409 CrossRef
.
- S. Liang, X. Jiang, C. Xiao, C. Li, Q. Chen and W. Li, Acc. Chem. Res., 2021, 54, 2227 CrossRef CAS PubMed
.
- Y. Khan, A. E. Ostfeld, C. M. Lochner, A. Pierre and A. C. Arias, Adv. Mater., 2016, 28, 4373 CrossRef CAS PubMed
.
- S. Park, K. Fukuda, M. Wang, C. Lee, T. Yokota, H. Jin, H. Jinno, H. Kimura, P. Zalar, N. Matsuhisa, S. Umezu, G. C. Bazan and T. Someya, Adv. Mater., 2018, 30, 1802359 CrossRef PubMed
.
- J. Liu, Y. Wang, H. Wen, Q. Bao, L. Shen and L. Ding, Sol. RRL, 2020, 4, 2000139 CrossRef
.
- G. Simone, D. Tordera, E. Delvitto, B. Peeters, A. J. J. M. Breemen, S. C. J. Meskers, R. A. J. Janssen and G. H. Gelinck, Adv. Opt. Mater., 2020, 8, 1901989 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2tc01297a |
| ‡ Zijin Zhao and Baiqiao Liu contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |