Finely modulated asymmetric nonfullerene acceptors enabling simultaneously improved voltage and current for efficient organic solar cells†
Huanhuan
Gao
ab,
Xiangjian
Wan
 b,
Ziyi
Xuan
c,
Wei
Ma
*c,
Jingming
Xin
c,
Chenxi
Li
b and
Yongsheng
Chen
b,
Ziyi
Xuan
c,
Wei
Ma
*c,
Jingming
Xin
c,
Chenxi
Li
b and
Yongsheng
Chen
 *b
*b
aXi'an Key Laboratory of High Performance Oil and Gas Field Materials, School of Materials Science and Engineering, Xian Shiyou University, Shaanxi, Xi’an 710065, China
bKey Laboratory of Functional Polymer Materials, College of Chemistry, Renewable Energy Conversion and Storage Center (RECAST), Nankai University, Tianjin 300071, China. E-mail: yschen99@nankai.edu.cn
cState Key Laboratory for Mechanical Behavior of Materials, Xi’an Jiaotong University, Xi’an 710049, China. E-mail: msewma@mail.xjtu.edu.cn
First published on 19th October 2021
Abstract
It is a great challenge to simultaneously improve the short-circuit current density (Jsc) and open-circuit voltage (Voc) of organic solar cells (OSCs) owing to the trade-off effect between the two photovoltaic parameters. Delicate chemical structure modulation of the active layer materials is always one of the effective strategies to address this issue. In this work, following a simple and efficient strategy through fine-tuning the molecular configuration in combination with the side chain modulation to address the issue, three non-fullerene acceptors (NFAs), 5T-2C8-IN, 5T-2C8-Cl and 5T-2C8-2Cl with a five-thiophene (5T) fused asymmetric molecular backbone and octyl side chains at the terminal position of the molecular backbone, have been designed and synthesized. Among them, the 5T-2C8-2Cl based photovoltaic device showed a power conversion efficiency (PCE) of 13.02% with a simultaneously improved Voc of 0.802 V and a Jsc of 24.97 mA cm−2 compared with the device of the control acceptor 6T-2C8-2Cl with a PCE of 12.43%, a Voc of 0.785 V and a Jsc of 24.40 mA cm−2.
1. Introduction
Organic solar cells (OSCs) have witnessed a great progress in the past decade.1–4 The state-of-the-art OSCs have achieved impressive efficiencies of over 18% thanks to the invention of new active layer materials especially non fullerene acceptors (NFAs) and device engineering.5–10 NFAs with broad absorptions up to the near-infrared range have proved to be an effective strategy to harvest more solar irradiation and obtain enhanced Jsc values. However, the decrease of the bandgaps of acceptor materials inevitably leads to a concomitant reduction in the Voc value.11 Thus, the trade-off issue between the Voc and Jsc limits the overall PCE values of OSC devices. Obtaining balanced and best values for Voc and Jsc has become a great challenge for active material design and device optimization. Finely tuning the absorption ranges and energy level alignments of the active layers have been regarded as the effective way to address the above trade-off issue of Voc and Jsc.12–14 In fact, many efforts have been devoted to designing active layer materials, especially the donor–acceptor–donor (A–D–A) type NFAs from multiple perspectives to encounter this issue. Generally, A–D–A NFAs have a ladder-type electron-rich conjugation skeleton with two electron-deficient end groups.15–17 In order to precisely regulate NFAs’ properties, fine-tuning the chemical structures via modification of the central core,18–23 the side chains24–27 or end groups28–30 has attracted extensive attention and led to great success in the past decade.31–35In our previous work, we have developed a strategy to improve Voc and Jsc synergistically by a simple and effective side chain modulation on the six thiophene (6T) fused symmetric backbone of an A–D–A type acceptor.12 Recently, asymmetric NFAs have been proposed as a simple but effective strategy for precise regulation of molecular structures.36–39 It has been demonstrated that asymmetric structures could induce a larger dipole moment and an enhanced intramolecular structure charge transfer, thus leading to enhanced intermolecular charge transfer (ICT) and stronger intermolecular binding energy compared with the symmetric ones.40,41 In this work, based on our previous work of side chain modulation at the terminal position of the molecular backbone and the advantages of asymmetric acceptors, we have designed and synthesized three asymmetric NFAs, 5T-2C8-IN, 5T-2C8-Cl and 5T-2C8-2Cl with 5T fused asymmetric skeletons and octyl side chains at the terminal position of the molecular backbone. Among them, 5T-2C8-2Cl showed redshifted absorption with absorption edge up to 960 nm. Compared with the control molecule 6T-2C8-2Cl with a 6T fused symmetric molecular backbone,125T-2C8-2Cl demonstrated an up-shifted LUMO energy level but a slightly red-shifted absorption edge. The OSC based on PM6:5T-2C8-2Cl achieved a higher PCE of 13.02% with an enhanced Voc of 0.802 V and an improved Jsc of 24.97 mA cm−2 compared with the PM6:6T-2C8-2Cl based device with a PCE of 12.43%, a Voc of 0.785 V and a Jsc of 24.40 mA cm−2. These results demonstrate that careful molecular modulation from the perspective of an asymmetric molecular backbone as well as the side chain modulation is an effective strategy to address the challenge of the trade-off issue between Voc and Jsc.
2. Results and discussion
2.1. Synthesis and characterization
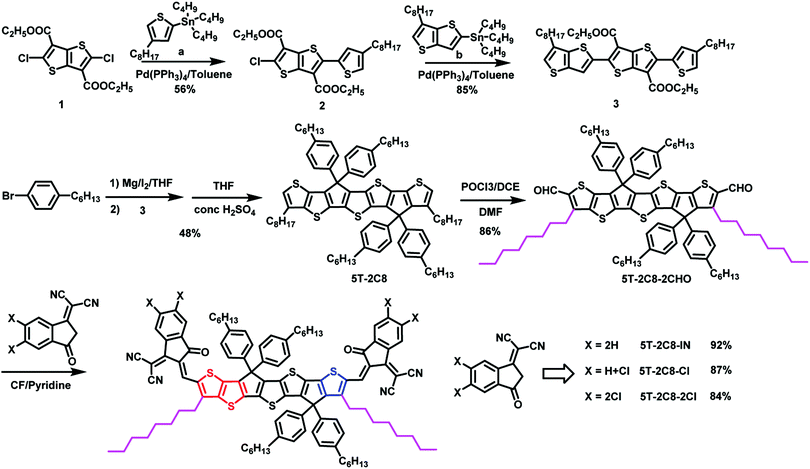
The synthetic route of the three asymmetric acceptors 5T-2C8-IN, 5T-2C8-Cl and 5T-2C8-2Cl is depicted in Scheme 1. The control symmetric acceptor 6T-2C8-2Cl was prepared following our reported method.12 The detailed synthesis procedures and characterization data of the three asymmetric acceptors are summarized in the ESI.† As shown in Scheme 1, the intermediate 3 was obtained with two step Stille coupling reactions with a and b. Then, the intermediate 5T-2C8 was synthesized via the intramolecular cyclization reaction from compound 3. Subsequently, the intermediate 5T-2C8 was reacted with the Vilsmeier–Haack reagent to afford dialdehyde 5T-2C8-2CHO. Finally, the target asymmetric acceptors were obtained through Knoevenagel condensation of 5T-2C8-2CHO with different end groups. The intermediates and the target molecules are well characterized by 1H NMR, 13C NMR, and HR-MS, the details of which are presented in the ESI.†2.2. Photophysical and electrochemical properties
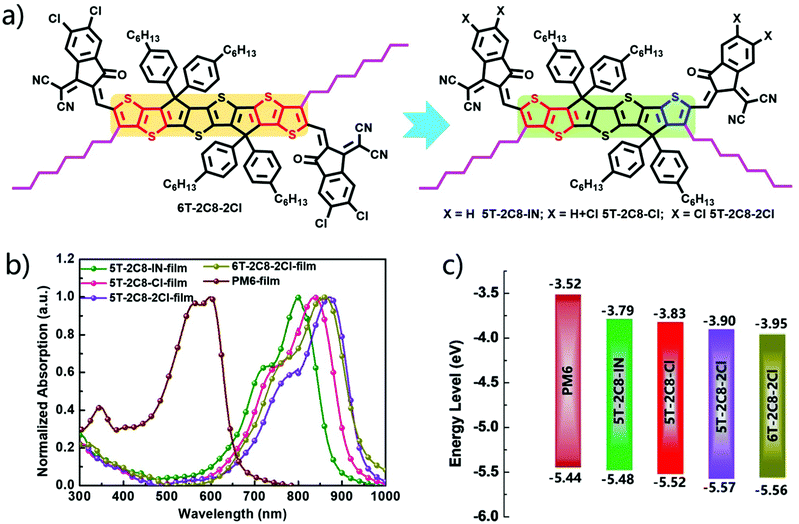
The ultraviolet-visible absorption spectra of 5T-2C8-IN, 5T-2C8-Cl, 5T-2C8-2Cl and 6T-2C8-2Cl both in diluted solution and thin films were studied to investigate the influence of the backbone structure and the number of chlorine atoms on the optical properties. As shown in Fig. S1 (ESI†), in diluted chloroform solution, the maximum absorption peaks of 5T-2C8-IN, 5T-2C8-Cl and 5T-2C8-2Cl were located at 770 (ε = 2.44 × 105 M−1 cm−1), 785 (ε = 2.61 × 105 M−1 cm−1), and 796 (ε = 2.64 × 105 M−1 cm−1), respectively. In contrast, 6T-2C8-2Cl showed redshifted absorption with maximum absorption peaks at 809 nm (ε = 2.41 × 105 M−1 cm−1) owing to its enlarged conjugation length. In the solid film state, these three 5T acceptors all showed clear red-shifted absorptions compared with their absorptions in solution, demonstrating strong intermolecular interactions (Fig. 1). Surprisingly, 5T-2C8-2Cl demonstrated a red-shifted absorption peak (871 nm) in comparison with the control molecule 6T-2C8-2Cl (860 nm). Besides, a density functional theoretical (DFT) calculation based on the B3LYP/6-31G(d) level by Gaussian 16 (Fig. S3, ESI†) has been performed to further investigate the performance variation. To simplify the calculation process, the long alkyl chains were replaced with methyl groups. It can be seen from the calculation results that the four acceptors all exhibit planar conjugations for their backbones, which is beneficial to achieve effective intermolecular π–π stacking and good charge transport channels. The configuration change of asymmetric 5T-2C8-2Cl shows a “C” shaped skeleton, which plays an important role in facilitating the intermolecular π–π stacking.42,43 In contrast, 6T-2C8-2Cl exhibits a “Z” shaped configuration as depicted in Fig. S3 (ESI†).44,45The energy levels of the three asymmetric NFAs together with 6T-2C8-2Cl for comparison were measured by cyclic voltammetry (Fig. S3b, ESI†) in acetonitrile on solid films. As shown in Fig. 1c, the HOMOs and LUMOs were estimated to be −5.48/−3.79, −5.52/−3.83, −3.57/−3.90 and −5.56/−3.95 eV for 5T-2C8-IN, 5T-2C8-Cl, 5T-2C8-2Cl and 6T-2C8-2Cl, respectively. These results indicate that the introduction of chlorine at the end groups indeed downshifts the energy levels, especially the LUMOs. Furthermore, the asymmetric acceptor 5T-2C8-2Cl demonstrated a relatively higher LUMO energy level compared to that of the control symmetric 6T-2C8-2Cl, which is expected to offer an improved Voc value for 5T-2C8-2Cl based OSCs (Table 1).
| Acceptors | λ CFmax (nm) | λ filmmax (nm) | ε (×105 M−1 cm−1) | λ filmedge (nm) | HOMO (eV) | LUMO (eV) | E CVg (eV) | E optg (eV) |
|---|---|---|---|---|---|---|---|---|
| 5T-2C8-IN | 770 | 801 | 2.44 | 895 | −5.48 | −3.79 | 1.69 | 1.39 |
| 5T-2C8-Cl | 785 | 838 | 2.61 | 939 | −5.52 | −3.83 | 1.69 | 1.32 |
| 5T-2C8-2Cl | 796 | 871 | 2.64 | 960 | −5.57 | −3.90 | 1.67 | 1.29 |
| 6T-2C8-2Cl | 809 | 860 | 2.41 | 959 | −5.56 | −3.95 | 1.61 | 1.29 |
2.3. Photovoltaic performances
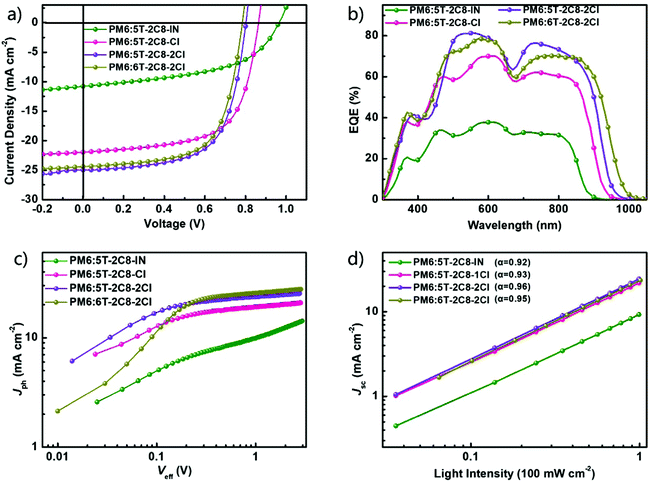
To evaluate the photovoltaic properties of 5T-2C8-IN, 5T-2C8-Cl and 5T-2C8-2Cl, OSCs with an inverted device structure of ITO/ZnO/PFN-Br/PM6:NFA/MoOx/Ag were fabricated, in which the wide band gap polymer PM6 was selected as the donor material owing to the matched energy levels and complementary absorptions with these acceptors. The device optimization process under different conditions is summarized in Tables S1–S9 (ESI†), including the donor/acceptor weight ratio (m%), the amount of additive (1,8-diiodine octane, DIO, vol%), thermal annealing (TA) conditions, etc. After systematic optimization, chlorobenzene was selected as the solvent, the optimized amount of DIO is 0.6% and the best TA temperature is 140 °C for the devices based on these four acceptors. The optimized photovoltaic parameters are summarized in Table 2 and the corresponding current density–voltage (J–V) curves are shown in Fig. 2a. As shown in Table 2, 5T-2C8-IN, 5T-2C8-Cl and 5T-2C8-2Cl based devices showed the best PCEs of 5.04%, 11.53% and 13.02%, respectively. The Voc of the three optimized devices decreased with the increasing number of chlorine atoms on the end groups owing to the down shifted LUMOs, while the Jsc was gradually enhanced due to the red-shifted and broadened absorption range. It is worth noting that the 5T-2C8-2Cl based device demonstrated an impressively higher PCE of 13.02% with the simultaneously improved Voc of 0.802 V and a Jsc of 24.97 mA cm−2 compared with the acceptor 6T-2C8-2Cl based device with a PCE of 12.43%, a Voc of 0.785 V and a Jsc of 24.40 mA cm−2. In addition, the 5T-2C8-2C based device with a conventional structure has also been fabricated and a comparable PCE of 12.20% has been achieved (Table S11, ESI†).| Devices | V oc (V) | FF | J sc (mA cm−2) | J EQEsc (mA cm−2) | PCEa (%) |
|---|---|---|---|---|---|
| a The PCE value was calculated from 20 devices. b The data were cited from ref. 50. | |||||
| PM6:5T-2C8-IN | 0.965 (0.963 ± 0.03) | 0.51 (0.50 ± 0.01) | 10.24 (10.12 ± 0.21) | 9.95 | 5.04 (4.82 ± 0.30) |
| PM6:5T-2C8-Cl | 0.864 (0.861 ± 0.03) | 0.64 (0.64 ± 0.01) | 20.86 (20.62 ± 0.26) | 19.91 | 11.53 (11.22 ± 0.23) |
| PM6:5T-2C8-2Cl | 0.802 (0.800 ± 0.03) | 0.65 (0.64 ± 0.02) | 24.97 (24.80 ± 0.20) | 23.77 | 13.02 (12.87 ± 0.23) |
| PM6:6T-2C8-2Cl | 0.785 (0.782 ± 0.04) | 0.65 (0.64 ± 0.01) | 24.40 (24.22 ± 0.17) | 23.80 | 12.43 (12.25 ± 0.18)b |
The external quantum efficiency (EQE) curves of the optimized devices are shown in Fig. 3b. For the three asymmetric 5T based devices, the EQE response was significantly enhanced after chlorination of the end groups and extended to around 960 nm for the 5T-2C8-2Cl based device. Compared with the 5T-2C8-2Cl device, the 6T-2C8-2Cl based device showed a relatively lower EQE response but a slightly broader EQE range, which is consistent with the blend film absorptions (Fig. S2, ESI†). The integrated Jsc values of the four optimized devices are in good agreement with the corresponding Jsc values measured from J–V curves (error of less than 5%).
2.4. Carrier dynamics study
For further evaluating the exciton dissociation and charge collection properties of these four NFA based solar cells, the photocurrent density (Jph) versus applied voltage (Veff) was measured. Herein, Jph = JL − JD, where JL and JD are the current densities under light illumination and in the dark, respectively, and Veff is calculated as Veff = Vo − Va, in which Va is the applied voltage and Vo is the voltage when Jph = 0.46,47 As shown in Fig. 3c, these four devices all reach saturation (Jsat) when Veff reaches ∼1.5 V, illustrating that at a higher voltage, the charge recombination is minimal for these devices. Then the value of Jph/Jsat representing the charge dissociation and the collection probability (P(E, T)) was studied to investigate the charge carrier dynamics. The devices based on 5T-2C8-IN, 5T-2C8-Cl, 5T-2C8-2Cl and 6T-2C8-2Cl gave Jph/Jsat values of 69%, 90%, 92% and 91% under short-circuit density conditions and 44%, 74%, 78% and 76%, under maximal power output conditions, respectively. The 5T-2C8-2Cl based device showed the highest Jph/Jsat values under short-circuit current density and maximal power output conditions, demonstrating the best exciton dissociation and efficient charge collection efficiency in these devices.48The light-intensity dependence (P) of Jsc was measured to further study the charge recombination behaviour of the corresponding devices (Fig. 2d). The light intensity (P) and Jsc abided the power-law equation of Jsc ∝ Pα, where the exponent α represents the bimolecular recommendation extent. The device with an α value ∼1 indicates the weaker degree of bimolecular recombination.47 The α values were 0.92, 0.93, 0.96 and 0.95 for 5T-2C8-IN, 5T-2C8-Cl, 5T-2C8-2Cl and 6T-2C8-2Cl based devices, respectively. These results demonstrate that the 5T-2C8-2Cl based device exhibited least bimolecular recombination among the devices based on these four acceptors.
The charge mobilities were measured using the space charge limit current (SCLC) method with the electron and hole only devices (Fig. S4, ESI†). The electron and hole mobilities of PM6:5T-2C8-2Cl and PM6:6T-2C8-2Cl based devices were calculated to be 6.05 × 10−5/3.99 × 10−4 and 2.85 × 10−5/5.08 × 10−5 cm−2 V−1 s−1, respectively, which are higher than those of PM6:5T-2C8-IN (9.13 × 10−6/3.38 × 10−5 cm−2 V−1 s−1) and PM6:5T-2C8-Cl (2.77 × 10−5/2.11 × 10−4 cm−2 V−1 s−1). The mobility values as well as the exciton dissociation and bimolecular recombination results indicate that the asymmetric molecular backbone and side chain modulation generate favourable effects on the device working process and is a promising strategy for the active layer material design to achieve high-efficiency OSCs.
2.5. Morphology characterization
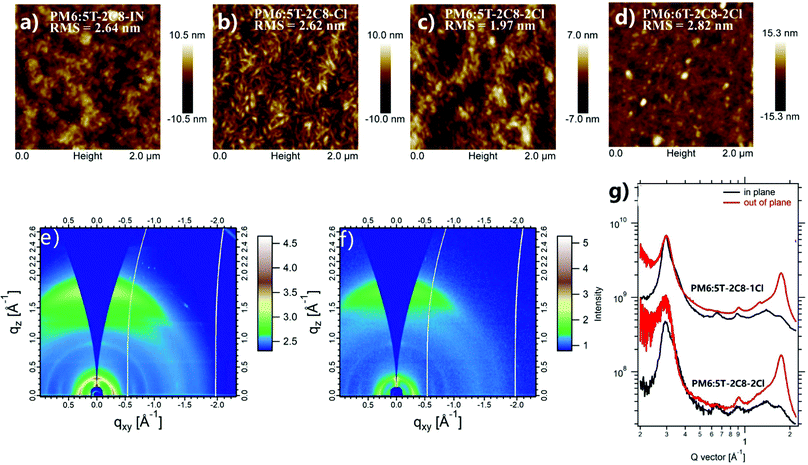
Atomic force microscopy (AFM) was used to investigate the blend films of the three asymmetric acceptors with PM6. As shown in Fig. 3a–d and the corresponding phase images in Fig. S5 (ESI†), PM6:5T-2C8-IN and PM6:5T-2C8-Cl blend films show relatively larger aggregates with root-mean-square (RMS) values of 2.64 and 2.62 nm, respectively, which is similar to that of the PM6:6T-2C8-2Cl (2.82 nm) blend film.49 Whereas, a nano-fibrillar network was observed in the 5T-2C8-2Cl blend film with a RMS surface roughness of 1.97 nm. Furthermore, the PM6:5T-2C8-2Cl based blend film exhibits a clear nanoscale network with a small root-mean-square (RMS) surface roughness of 1.97 nm, which indicates a well matched compatibility between PM6 and 5T-2C8-2Cl. Furthermore, grazing incidence wide angle X-ray scattering (GIWAXS) was used to investigate the molecular packing properties of 5T-2C8-Cl and 5T-2C8-2Cl based blend films. As shown in Fig. 3f, the 5T-2C8-Cl and 5T-2C8-2Cl based blend films adopt a face-on orientation with an intense π–π diffraction peak in the out-of-plane (OOP) direction and a lamellar diffraction peak in the in-plane (IP) direction. The 5T-2C8-2Cl blend film shows a clear π–π stacking diffraction peak (010) at 1.75 Å−1, with a π–π d-spacing distance of 3.59 Å in the OOP direction, which is more compact than that of the 5T-2C8-Cl blend film with π–π d-spacing distance of 3.61 Å (OOP diffraction peak (010) at 1.74 Å−1). In contrast, the PM6:6T-2C8-2Cl blend film exhibits an OOP diffraction peak at 1.82 Å−1 with a π–π d-spacing distance of 3.45 Å.12,50 Despite the slightly larger π–π distance in the PM6:5T-2C8-2Cl blend film compared with that of PM6:6T-2C8-2Cl blend film, the crystal coherence length (CCL) for the PM6:5T-2C8-2Cl blend film estimated from Scherrer's equation51 (CCL = 2πk/fwhm) by Gaussian fitting with the full-width at half-maximum (fwhm) is 24.1 Å, which is larger than that of the 6T-2C8-2Cl based blend film (CCL of 18.8 Å).50 This demonstrates the enhanced crystallinity in the blend film of PM6:5T-2C8-2Cl, facilitating the charge transport in the photovoltaic device. Compared with the “Z” type molecular skeleton of the symmetric acceptor 6T-2C8-2Cl, the “C” type configuration of the asymmetric acceptor 5T-2C8-2Cl should be the intrinsic factor that determines the packing difference of the two acceptor based blend films. The results also demonstrate that careful modulation of the molecular skeleton should be one of the effective ways for the morphology control of the active layer.3. Conclusions
In summary, following the strategy of fine-tuning the molecular configuration in combination with the side chain modulation to simultaneously improve the Voc and Jsc, three acceptors with asymmetric molecular skeletons and octyl side chains at the terminal position of the molecular backbones, 5T-2C8-IN, 5T-2C8-Cl and 5T-2C8-2Cl, have been designed and synthesized. Among them, the 5T-2C8-2Cl based solar cell demonstrated the best PCE of 13.02% with a simultaneously improved Jsc of 24.97 mA cm−2 and a Voc of 0.794 V, compared with the control symmetric acceptor 6T-2C8-2Cl based device with a PCE of 12.43%, a Jsc of 24.40 mA cm−2 and a Voc of 0.785 V. The results indicate that the Jsc and Voc can be simultaneously enhanced through careful molecular structural regulation. Considering the large variety of organic materials and current promising results in the OSC field, it is believed that high efficiency active layer materials can be rationally designed to encounter the issue of Jsc and Voc trade-off and obtain higher device performance.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Natural Science Foundation Research Project of Shaanxi Province (No. 2021JQ-595), the Scientific Research Program Funded by Shaanxi Provincial Education Department (No. 20JK0841), the NSFC (21935007, 21704082) and the Key Scientific and Technological Innovation Team Project of Shaanxi Province (2020TD-002). X-ray data were acquired at beamlines 7.3.3 at the Advanced Light Source, which is supported by the Director, Office of Science, Office of Basic Energy Sciences, the U.S. Department of Energy under Contract No. DE-AC02-05CH11231. The authors thank Chenhui Zhu at beamline 7.3.3 for assistance with data acquisition.Notes and references
- X. Wan, M. Zhang and Y. Chen, Chem. Soc. Rev., 2020, 49, 2828–2842 RSC.
- C. Yan, S. Barlow, Z. Wang, H. Yan, A. K. Y. Jen, S. R. Marder and X. Zhan, Nat. Rev. Mater., 2018, 3, 18003 CrossRef.
- G. Zhang, J. Zhao, P. C. Y. Chow, K. Jiang, J. Zhang, Z. Zhu, J. Zhang, F. Huang and H. Yan, Chem. Rev., 2018, 118, 3447–3507 CrossRef PubMed.
- L. Meng, Y. Zhang, X. Wan, C. Li, X. Zhang, Y. Wang, X. Ke, Z. Xiao, L. Ding, R. Xia, H.-L. Yip, Y. Cao and Y. Chens, Science, 2018, 361, 1094–1098 CrossRef.
- C. Li, J. Zhou, J. Song, J. Xu, H. Zhang, X. Zhang, J. Guo, L. Zhu, D. Wei, G. Han, J. Min, Y. Zhang, Z. Xie, Y. Yi, H. Yan, F. Gao, F. Liu and Y. Sun, Nat. Energy, 2021, 6, 605–613 CrossRef.
- Y. Lin, A. Magomedov, Y. Firdaus, D. Kaltsas, A. El-Labban, H. Faber, D. R. Naphade, E. Yengel, X. Zheng, E. Yarali, N. Chaturvedi, K. Loganathan, D. Gkeka, S. H. AlShammari, O. M. Bakr, F. Laquai, L. Tsetseris, V. Getautis and T. D. Anthopoulos, ChemSusChem, 2021, 14, 3569–3578 CrossRef CAS PubMed.
- G. Liu, R. Xia, Q. Huang, K. Zhang, Z. Hu, T. Jia, X. Liu, H. L. Yip and F. Huang, Adv. Funct. Mater., 2021, 2103283 CrossRef CAS.
- Y. Xu, Y. Cui, H. Yao, T. Zhang, J. Zhang, L. Ma, J. Wang, Z. Wei and J. Hou, Adv. Mater., 2021, 2101090 CrossRef CAS.
- P. Bi, S. Zhang, Z. Chen, Y. Xu, Y. Cui, T. Zhang, J. Ren, J. Qin, L. Hong, X. Hao and J. Hou, Joule, 2021, 5, 2408–2419 CrossRef CAS.
- J. Wang, Z. Zheng, Y. Zu, Y. Wang, X. Liu, S. Zhang, M. Zhang and J. Hou, Adv. Mater., 2021, 2102787 CrossRef CAS.
- B. Fan, X. Du, F. Liu, W. Zhong, L. Ying, R. Xie, X. Tang, K. An, J. Xin, N. Li, W. Ma, C. J. Brabec, F. Huang and Y. Cao, Nat. Energy, 2018, 3, 1051–1058 CrossRef CAS.
- H. H. Gao, Y. Sun, Y. Cai, X. Wan, L. Meng, X. Ke, S. Li, Y. Zhang, R. Xia, N. Zheng, Z. Xie, C. Li, M. Zhang, H. L. Yip, Y. Cao and Y. Chen, Adv. Energy Mater., 2019, 9, 1901024 CrossRef.
- Y. Qin, Y. Chen, Y. Cui, S. Zhang, H. Yao, J. Huang, W. Li, Z. Zheng and J. Hou, Adv. Mater., 2017, 29, 1606340 CrossRef.
- W. Zhao, S. Li, H. Yao, S. Zhang, Y. Zhang, B. Yang and J. Hou, J. Am. Chem. Soc., 2017, 139, 7148–7151 CrossRef CAS PubMed.
- G. Li, W.-H. Chang and Y. Yang, Nat. Rev. Mater., 2017, 2, 17043 CrossRef CAS.
- P. Cheng, G. Li, X. Zhan and Y. Yang, Nat. Photonics, 2018, 12, 131–142 CrossRef CAS.
- J. Hou, O. Inganas, R. H. Friend and F. Gao, Nat. Mater., 2018, 17, 119–128 CrossRef CAS.
- Y. Lin, J. Wang, Z. G. Zhang, H. Bai, Y. Li, D. Zhu and X. Zhan, Adv. Mater., 2015, 27, 1170–1174 CrossRef CAS.
- J. Yuan, Y. Zhang, L. Zhou, G. Zhang, H.-L. Yip, T.-K. Lau, X. Lu, C. Zhu, H. Peng, P. A. Johnson, M. Leclerc, Y. Cao, J. Ulanski, Y. Li and Y. Zou, Joule, 2019, 3, 1140–1151 CrossRef CAS.
- S. Li, L. Zhan, N. Yao, X. Xia, Z. Chen, W. Yang, C. He, L. Zuo, M. Shi, H. Zhu, X. Lu, F. Zhang and H. Chen, Nat. Commun., 2021, 12, 4627 CrossRef CAS.
- Z. Bi, K. Chen, L. Gou, Y. Guo, X. Zhou, H. B. Naveed, J. Wang, Q. Zhu, J. Yuan, C. Zhao, K. Zhou, S. Chandrabose, Z. Tang, Y. Yi, J. M. Hodgkiss, L. Zhang and W. Ma, J. Mater. Chem. A, 2021, 9, 16733–16742 RSC.
- M. Chang, Y. Zhang, B.-S. Lu, D. Sui, F. Wang, J. Wang, Y. Yang and B. Kan, Chem. Eng. J., 2022, 427, 131473 CrossRef CAS.
- T. Shan, K. Ding, L. Yu, X. Wang, Y. Zhang, X. Zheng, C. C. Chen, Q. Peng and H. Zhong, Adv. Funct. Mater., 2021, 31, 2100750 CrossRef CAS.
- K. Jiang, Q. Wei, J. Y. L. Lai, Z. Peng, H. K. Kim, J. Yuan, L. Ye, H. Ade, Y. Zou and H. Yan, Joule, 2019, 3, 3020–3033 CrossRef CAS.
- X. Zhang, C. Li, L. Qin, H. Chen, J. Yu, Y. Wei, X. Liu, J. Zhang, Z. Wei, F. Gao, Q. Peng and H. Huang, Angew. Chem., Int. Ed., 2021, 60, 17720–17725 CrossRef CAS.
- J. Subbiah, C. J. Lee, V. D. Mitchell and D. J. Jones, ACS Appl. Mater. Interfaces, 2020, 13, 1086–1093 CrossRef PubMed.
- J. Subbiah, B. Purushothaman, M. Chen, T. Qin, M. Gao, D. Vak, F. H. Scholes, X. Chen, S. E. Watkins, G. J. Wilson, A. B. Holmes, W. W. Wong and D. J. Jones, Adv. Mater., 2015, 27, 702–705 CrossRef CAS PubMed.
- S. Li, L. Ye, W. Zhao, S. Zhang, S. Mukherjee, H. Ade and J. Hou, Adv. Mater., 2016, 28, 9423–9429 CrossRef CAS PubMed.
- H. Lu, H. Jin, H. Huang, W. Liu, Z. Tang, J. Zhang and Z. Bo, Adv. Funct. Mater., 2021, 2103445 CrossRef CAS.
- H. Yu, S. Luo, R. Sun, I. Angunawela, Z. Qi, Z. Peng, W. Zhou, H. Han, R. Wei, M. Pan, A. M. H. Cheung, D. Zhao, J. Zhang, H. Ade, J. Min and H. Yan, Adv. Funct. Mater., 2021, 31, 2100791 CrossRef CAS.
- C. Tang, X. Ma, J. Y. Wang, X. Zhang, R. Liao, Y. Ma, P. Wang, P. Wang, T. Wang, F. Zhang and Q. Zheng, Angew. Chem., Int. Ed., 2021, 60, 2–12 CrossRef.
- B. Kan, H. Feng, X. Wan, F. Liu, X. Ke, Y. Wang, Y. Wang, H. Zhang, C. Li, J. Hou and Y. Chen, J. Am. Chem. Soc., 2017, 139, 4929–4934 CrossRef CAS.
- N. Qiu, H. Zhang, X. Wan, C. Li, X. Ke, H. Feng, B. Kan, H. Zhang, Q. Zhang, Y. Lu and Y. Chen, Adv. Mater., 2017, 29, 1604964 CrossRef.
- H. Feng, Y. Q. Q. Yi, X. Ke, J. Yan, Y. Zhang, X. Wan, C. Li, N. Zheng, Z. Xie and Y. Chen, Adv. Energy Mater., 2019, 9, 1803541 CrossRef.
- Z. Wang, R. Wang, Y. Mi, K. Lu, Y. Liu, C. Yang, J. Zhang, X. Liu, Y. Wang, Z. Shuai and Z. Wei, Chem. Mater., 2021, 33, 4578–4585 CrossRef CAS.
- C. Li, H. Fu, T. Xia and Y. Sun, Adv. Energy Mater., 2019, 9, 1900999 CrossRef.
- Y. Chen, R. Ma, T. Liu, Y. Xiao, H. K. Kim, J. Zhang, C. Ma, H. Sun, F. Bai, X. Guo, K. S. Wong, X. Lu and H. Yan, Adv. Energy Mater., 2021, 11, 2003777 CrossRef CAS.
- D. Hu, Q. Yang, Y. Zheng, H. Tang, S. Chung, R. Singh, J. Lv, J. Fu, Z. Kan, B. Qin, Q. Chen, Z. Liao, H. Chen, Z. Xiao, K. Sun and S. Lu, Adv. Sci., 2021, 8, 2004262 CrossRef CAS.
- W. Gao, H. Fu, Y. Li, F. Lin, R. Sun, Z. Wu, X. Wu, C. Zhong, J. Min, J. Luo, H. Y. Woo, Z. Zhu and A. K. Y. Jen, Adv. Energy Mater., 2020, 11, 2003177 CrossRef.
- Q. Zhu, D. Liu, Z. Lu, C. Gu, K. Zhang, X. Bao, Q. Li and R. Yang, J. Mater. Chem. A, 2019, 7, 4823–4828 RSC.
- S. Li, L. Zhan, Y. Jin, G. Zhou, T. K. Lau, R. Qin, M. Shi, C. Z. Li, H. Zhu, X. Lu, F. Zhang and H. Chen, Adv. Mater., 2020, 32, 2001160 CrossRef CAS.
- N. Zarrabi, O. J. Sandberg, S. Zeiske, W. Li, D. B. Riley, P. Meredith and A. Armin, Nat. Commun., 2020, 11, 5567 CrossRef CAS.
- G. Zhang, X. K. Chen, J. Xiao, P. C. Y. Chow, M. Ren, G. Kupgan, X. Jiao, C. C. S. Chan, X. Du, R. Xia, Z. Chen, J. Yuan, Y. Zhang, S. Zhang, Y. Liu, Y. Zou, H. Yan, K. S. Wong, V. Coropceanu, N. Li, C. J. Brabec, J. L. Bredas, H. L. Yip and Y. Cao, Nat. Commun., 2020, 11, 3943 CrossRef CAS.
- Z. Zhang, J. Yu, X. Yin, Z. Hu, Y. Jiang, J. Sun, J. Zhou, F. Zhang, T. P. Russell, F. Liu and W. Tang, Adv. Funct. Mater., 2018, 28, 1705095 CrossRef.
- D. Liu, L. Yang, Y. Wu, X. Wang, Y. Zeng, G. Han, H. Yao, S. Li, S. Zhang, Y. Zhang, Y. Yi, C. He, W. Ma and J. Hou, Chem. Mater., 2018, 30, 619 CrossRef CAS.
- L. Yang, X. Song, J. Yu, H. Wang, Z. Zhang, R. Geng, J. Cao, D. Baran and W. Tang, J. Mater. Chem. A, 2019, 7, 22279–22286 RSC.
- P. W. M. Blom, V. D. Mihailetchi, L. J. A. Koster and D. E. Markov, Adv. Mater., 2007, 19, 1551–1566 CrossRef CAS.
- C. M. Proctor, M. Kuik and T.-Q. Nguyen, Prog. Polym. Sci., 2013, 38, 1941–1960 CrossRef CAS.
- Z.-G. Zhang, B. Qi, Z. Jin, D. Chi, Z. Qi, Y. Li and J. Wang, Energy Environ. Sci., 2014, 7, 1966–1973 RSC.
- Y. Sun, H.-H. Gao, S. Wu, L. Meng, X. Wan, M. Li, Z. Ma, Z. Guo, S. Li, H. Zhang, C. Li and Y. Chen, Sci. China: Chem., 2021, 64, 608–615 CrossRef CAS.
- D.-M. Smilgies, J. Appl. Crystallogr., 2013, 46, 286 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1tc03793e |
| This journal is © The Royal Society of Chemistry 2022 |