Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recyclable optical bioplastics platform for solid state red light harvesting via triplet–triplet annihilation photon upconversion†
Pankaj
Bharmoria
 a,
Fredrik
Edhborg
a,
Fredrik
Edhborg
 a,
Hakan
Bildirir
a,
Hakan
Bildirir
 a,
Yoichi
Sasaki
a,
Yoichi
Sasaki
 b,
Shima
Ghasemi
a,
Anders
Mårtensson
a,
Nobuhiro
Yanai
b,
Shima
Ghasemi
a,
Anders
Mårtensson
a,
Nobuhiro
Yanai
 b,
Nobuo
Kimizuka
b,
Nobuo
Kimizuka
 b,
Bo
Albinsson
b,
Bo
Albinsson
 a,
Karl
Börjesson
a,
Karl
Börjesson
 c and
Kasper
Moth-Poulsen
c and
Kasper
Moth-Poulsen
 *ade
*ade
aDepartment of Chemistry and Chemical Engineering, Chalmers University of Technology, Kemivägen 4, Gothenburg 412 96, Sweden. E-mail: pankajb@chalmers.se; kasper.moth-poulsen@chalmers.se
bDepartment of Applied Chemistry, Graduate School of Engineering, Center for Molecular Systems (CMS), Kyushu University, 744 Moto-oka, Nishi-ku, Fukuoka 819-0395, Japan
cDepartment of Chemistry and Molecular Biology, Kemivägen 10, Gothenburg 412 96, Sweden
dThe Institute of Materials Science of Barcelona, ICMAB-CSIC, Bellaterra, Barcelona, 08193, Spain
eCatalan Institution for Research & Advanced Studies, ICREA, Pg. Lluís Companys 23, Barcelona, Spain
First published on 16th August 2022
Abstract
Sustainable photonics applications of solid-state triplet–triplet annihilation photon upconversion (TTA-UC) are limited by a small UC spectral window, low UC efficiency in air, and non-recyclability of polymeric materials used. In a step to overcome these issues, we have developed new recyclable TTA-UC bioplastics by encapsulating TTA-UC chromophores liquid inside the semicrystalline gelatin films showing broad-spectrum upconversion (red/far-red to blue) with high UC efficiency in air. For this, we synthesized a new anionic annihilator, sodium-TIPS-anthracene-2-sulfonate (TIPS-AnS), that combined with red/far-red sensitizers (PdTPBP/Os(m-peptpy)2(TFSI)2), a liquid surfactant Triton X-100 reduced (TXr) and protein gelatin (G) formed red/far-red to blue TTA-UC bioplastic films just by air drying of their aqueous solutions. The G-TXr-TIPS-AnS-PdTPBP film showed record red to blue (633 to 478 nm) TTA-UC quantum yield of 8.5% in air. The high UC quantum yield has been obtained due to the fluidity of dispersed TXr containing chromophores and oxygen blockage by gelatin fibers that allowed efficient diffusion of triplet excited chromophores. Further, the G-TXr-TIPS-AnS-Os(m-peptpy)2(TFSI)2 bioplastic film displayed far-red to blue (700–730 nm to 478 nm) TTA-UC, demonstrating broad-spectrum photon harvesting. Finally, we demonstrated the recycling of G-TXr-TIPS-AnS-PdTPBP bioplastics by developing a downstream approach that gives new directions for designing future recyclable photonics bioplastic materials.
Introduction
Photon upconversion (PUC) is a process of transforming low-energy photons into high-energy photons.1–3 Among the known PUC processes,1–8 triplet–triplet annihilation photon upconversion (TTA-UC) has an advantage over energy transfer upconversion (ETU), excited-state absorption (ESA), and photon avalanche (PA), due to the operation at flexible spectral ranges, and at low excitation intensities.9–11 TTA-UC occurs in an ensemble of chromophores, wherein a sensitizer after absorbing low energy generates triplet states, followed by triplet energy transfer (TET) to the annihilator via a Dexter energy transfer mechanism. The annihilator triplets then undergo TTA to form an emissive singlet state, which radiates the anti-Stokes delayed fluorescence (Fig. S1†).12,13 Due to the high UC quantum yields at low excitation intensities,14–16 TTA-UC has attracted a plethora of applications not limited to photocatalysis,17 biological imaging,18 3-D printing19 and photovoltaics.20,21In photovoltaics, TTA-UC can increase the efficiency of solar cells, by upconverting the otherwise transmitted sub-band gap photons to photons corresponding to the bandgap. But, practical integration with photovoltaics requires efficient solid-state TTA-UC materials with a broad UC spectral window (Red/NIR to vis).22,23 However, realizing efficient solid-state TTA-UC materials face several challenges like aggregation-induced emission quenching, back energy transfer, triplet quenching by molecular oxygen, and lack of sensitizer–annihilator pairs with suitable triplet energies.11,24,25 These challenges have been addressed partly in many proofs-of-concept solid-state green to blue TTA-UC systems.24–26 However, practical applications demand an extension of the photon harvesting window to red or far-red/near-infrared photons (far-red/NIR), which is the key goal of this work.12,20–23 Existing literature on solid-state red to blue molecular TTA-UC is limited to examples of chromophore doped synthetic polymeric films of polyurethanes,27,28 methyl acrylate,29 TTA-UC crystals (ΦUC = 5.6%),30 and chromophores loaded into liquid nanocapsules dispersed in cross-linked cellulose nanofiber films with ΦUC = 8.2% in deaerated conditions.31 Regarding solid-state near-infrared/far-red to visible (NIR/far-red to vis) molecular TTA-UC, different approaches to upconvert NIR/far-red light to red, yellow, green, blue, and violet light in PVA-doped chromophore nanoparticles,32–34 metal–organic frameworks35 and crystals,36 amorphous solid microparticles,37 and chromophores doped polystyrene film have been reported.38
However, low ΦUC in air and non-recyclable petroleum-derived plastics used in these systems remains key practical challenges for their sustainable applications. Many petroleum based-plastics are non-biodegradable which alarmingly leads to interference with the ecological and food cycles in the form of bulk plastic waste and/or microplastics.39,40 Moreover, it might be hard to recycle chromophores used in such plastics for photonics applications. Therefore, it is of great importance to develop techniques for plastic41 and dye recycling or explore the potential use of biopolymers42–45 as an alternative for solid-state TTA-UC. To address this issue, we previously developed a proof-of-concept photon upconversion bioplastic approach for green to blue TTA-UC with ΦUC = 7.8% in air.46 However, a limited UC spectral window remains an issue, due to the lack of ionic annihilators with suitable triplet energies to couple with red/far-red/NIR sensitizers, which is a key requisite for fabricating optically active TTA-UC bioplastics.
In this work we have addressed this challenge by synthesizing a new ionic annihilator; sodium TIPS-anthracene 2-sulfonate (TIPS-AnS). TIPS-AnS has a suitable triplet energy (T1 ∼ 1.41 eV47,48) to pair with a red sensitizer, Pd(II) meso-tetraphenyl tetrabenzoporphine (PdTPBP, T1 = 1.55 eV).49,50 Hence, the prepared gelatin-TX-100-reduced-TIPS-AnS-PdTPBP (G-TXr-TIPS-AnS-PdTPBP) bioplastic film showed an efficient red-to-blue TTA-UC (anti-stokes shift, ΔE = 0.62 eV), with a high UC quantum yield, ΦUC = 8.5% (50% theoretical maximum) and a reasonably low excitation intensity of Ith = 95 mW cm−2. This is a record ΦUC for solid-state red to blue TTA-UC in air. The UC spectral range was further expanded to harvest far-red photons (700–730 nm) by pairing TIPS-AnS with a far-red/NIR sensitizer, Os(m-peptpy)2(TFSI)2 (T1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.51
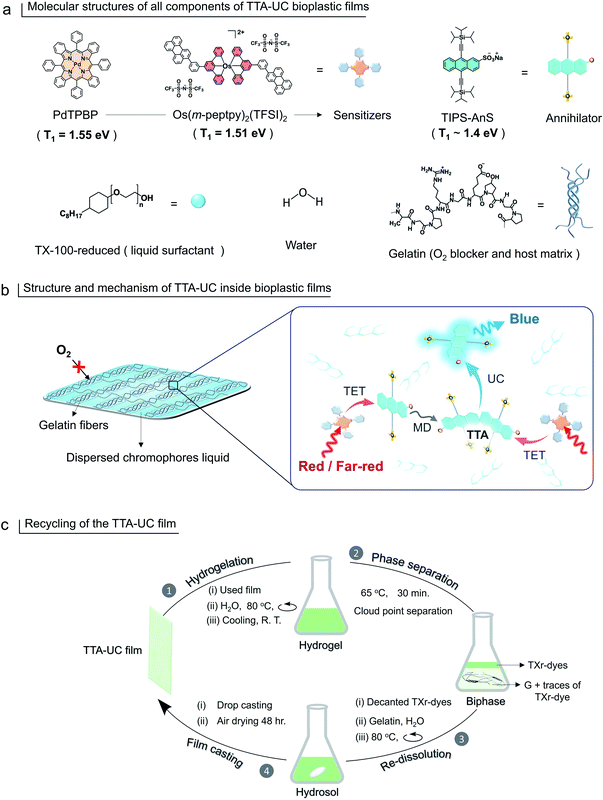
1.51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) eV51) to form G-TXr-Os(m-peptpy)2(TFSI)2-TIPS-AnS, far-red to blue TTA-UC bioplastic film. Hence, compared to our previous report46 we have formulated new sensitizer–annihilator pairs with suitable triplet energies that expand the UC spectral window to the far-red region. Further, we replaced the toxic TX-100 (currently being phased out in Europe due to legislation) with the globally acceptable TX-100-reduced (TXr)52 to dissolve chromophores in the film. Interestingly, this small change in chemical structure drastically changed the film nanostructure wherein TXr was found as a continuously dispersed liquid unlike the isolated droplets of TX-100 in the gelatin film reported by us previously.46 Finally, to avoid the post-utilization waste of developed bioplastics, a proof-of-concept downstream recycling approach of “cloud point extraction of TXr-chromophores” from the used G-TXr-TIPS-AnS-PdTPBP film has been developed. The extracted TXr-chromophores were reused to fabricate a new TTA-UC film. Hence, the highly efficient and recyclable TTA-UC bioplastics developed in this work convey a new direction to the solid-state TTA-UC field for sustainable broad-spectrum photon harvesting. The molecular structure of gelatin, TXr, PdTPBP, Os(m-peptpy)2(TFSI)2, and TIPS-AnS are shown in Fig. 1a, and a pictorial presentation of the mechanism of TTA-UC in the films, and recycling are shown in Fig. 1b and c.
eV51) to form G-TXr-Os(m-peptpy)2(TFSI)2-TIPS-AnS, far-red to blue TTA-UC bioplastic film. Hence, compared to our previous report46 we have formulated new sensitizer–annihilator pairs with suitable triplet energies that expand the UC spectral window to the far-red region. Further, we replaced the toxic TX-100 (currently being phased out in Europe due to legislation) with the globally acceptable TX-100-reduced (TXr)52 to dissolve chromophores in the film. Interestingly, this small change in chemical structure drastically changed the film nanostructure wherein TXr was found as a continuously dispersed liquid unlike the isolated droplets of TX-100 in the gelatin film reported by us previously.46 Finally, to avoid the post-utilization waste of developed bioplastics, a proof-of-concept downstream recycling approach of “cloud point extraction of TXr-chromophores” from the used G-TXr-TIPS-AnS-PdTPBP film has been developed. The extracted TXr-chromophores were reused to fabricate a new TTA-UC film. Hence, the highly efficient and recyclable TTA-UC bioplastics developed in this work convey a new direction to the solid-state TTA-UC field for sustainable broad-spectrum photon harvesting. The molecular structure of gelatin, TXr, PdTPBP, Os(m-peptpy)2(TFSI)2, and TIPS-AnS are shown in Fig. 1a, and a pictorial presentation of the mechanism of TTA-UC in the films, and recycling are shown in Fig. 1b and c.
Experimental section
Materials
All solvents and reagents were used as received. All solvents used in this work were purchased from Fischer Scientific. Palladium(II) meso-tetraphenyl porphine (PdTPBP) was purchased from Frontier Scientific. TX-100-reduced, sodium anthraquinone-2-sulfonate, (triisopropylsilyl)acetylene (97%), tin(II) chloride reagent grade (98%), gelatin type A porcine skin (80 to 120 g bloom) were purchased from Sigma Aldrich. The Os(m-peptpy)2(TFSI)2 was synthesized in our lab (Kimizuka Lab, Kyushu University, Japan). The synthesis and characterization of this sensitizer is published recently elsewhere.51 TIPS-anthracene-2-sulfonate was synthesized using the procedure given below.Synthesis and purification of sodium TIPS-anthracene 2-sulfonate
The synthetic procedure was adapted from the literature on the synthesis of non-sulfonated (triisopropylsilyl)acetylene-anthracene compounds.53,54 The two-step reaction involves the addition of lithium (triisopropylsilyl)acetylide to the carbonyls of sodium anthraquinone-2-sulfonate and the following elimination via SnCl2 to form the targeted anthracene derivative. In detail; 1.6 mL of (triisopropylsilyl)acetylene (TIPS) was dissolved in 20 mL anhydrous THF under a nitrogen atmosphere and cooled to −78 °C. 4.4 mL n-butyl lithium (1.6 M in hexane) was added to the solution dropwise, and the mixture was further stirred at −78 °C for 1 h. In the following, the prepared Li-TIPS salt solution was heated to room temperature for a few minutes (5–7 min) and transferred (via a cannula) to a flask containing the solution of dried (to get rid of the adsorbed water) 1 g sodium anthraquinone-2-sulfonate in 20 mL anhydrous THF at −78 °C under nitrogen atmosphere. The pale-yellow mixture was stirred at −78 °C for 30 min. and heated to room temperature for an overnight stirring (ca. 15 h). The final orange suspension was quenched with 5 mL of water, and the solid was filtrated using a Büchner funnel and flask. The filtrate was dissolved in diethyl ether and extracted with water. The collected organic phase was dried using sodium sulfate, filtrated, and the solvent evaporated to obtain a crude orange solid to be processed in the next step. The crude orange solid was dissolved in 5 mL THF and slowly added to the flask containing the 2 g SnCl2 in 10 mL THF. The mixture turned to a thick dark red solution, which was diluted using 10 mL THF and heated to 45 °C to be stirred overnight. The mixture was then quenched with 20 mL water and extracted using an excess of diethyl ether 3 times. The organic phase was dried by using sodium sulfate, and the solvent of the separated organic solution was removed under vacuum. The brown solid was washed with EtOH and purified via a silica gel column (ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol/99
methanol/99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The collected compound was then recrystallized in MeOH, yielding a 5% overall reaction. The yellow precipitates of the compound obtained after drying were checked for 1H-NMR, 13C-NMR, and mass analysis to ascertain the purity (Fig. S2–S4†).
1). The collected compound was then recrystallized in MeOH, yielding a 5% overall reaction. The yellow precipitates of the compound obtained after drying were checked for 1H-NMR, 13C-NMR, and mass analysis to ascertain the purity (Fig. S2–S4†).
1H (d-THF, 400 MHz) δ 9.25 (s, 1H), 8.68–8.60 (m, 2H), 8.52 (d, 1H), 8.20 (d, 1H), 7.63 (dd, 2H), 1.3–0.8 (m, 42H).
13C (d-THF & CDCl3 mix, 100 MHz) δ 33.22, 132.19, 131.94, 131.73, 130.68, 128.81, 126.66, 126.49, 126.46, 126.39, 124.46, 124.34, 119.35, 117.68; 105.02, 104.10, 102.45, 102.11; 17.90, 10.78.
m/z [M+H]+ calculated 641.2911, found 641.2917.
Sample preparation
Measurements
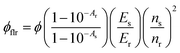 | (1) |
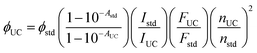 | (2) |
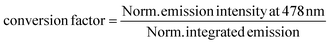 | (3) |
The obtained value was used to obtain to calculate integrated emission intensity without reabsorption using 0.016 as a conversion factor.
Results and discussion
Synthesis and characterization of sodium TIPS-anthracene-2-sulfonate
TIPS-AnS was synthesized in a two-step synthesis involving the addition of lithium (triisopropylsilyl)acetylide to the carbonyls of sodium anthraquinone-2-sulfonate and the following elimination via SnCl2 to form the targeted anthracene derivative (see methods for detailed synthesis procedure). The purity of TIPS-AnS was confirmed from 1H-NMR, 13C-NMR, and HR-MS analysis (Fig. S2–S4†). The photophysical characterization of TIPS-AnS was carried out in THF and TXr. The absorption spectrum of TIPS-AnS in THF showed vibronic peaks at 392, 415, and 440 nm, whereas the emission maximum was observed at 449 nm, followed by peaks at 476 nm and 506 nm (Fig. S7†). In TXr the TIPS-AnS showed absorption at 394, 416, and 441 nm, and emission at 452, 478, and 510 nm. (Fig. S8†). Compared to the non-sulfonated TIPS-anthracene in cyclohexane, the absorption and emission spectra of TIPS-AnS in THF are red-shifted by 1 nm and 7 nm respectively (Fig. S9†).47,48 The fluorescence lifetime (τf) and fluorescence quantum yield (Φf) of TIPS-AnS in TXr were found to be, τf = 7.1 ns (Fig. S10†) and Φf = 89%. The TIPS-anthracene has a suitable triplet energy (T1 ∼ 1.4 eV)47 for exergonic triplet sensitization by PdTPBP (T1 = 1.55 eV,49,50 Fig. S8†) and Os(m-peptpy)2(TFSI)2 (T1 = 1.65 eV51). Therefore, we paired TIPS-AnS with PdTPBP and Os(m-peptpy)2(TFSI)2 to fabricate a G-TXr bioplastics film to investigate solid-state red/far-red to blue TTA-UC.Preparation and structural and optical characterization of G-TXr-TIPS-AnS-PdTPBP films
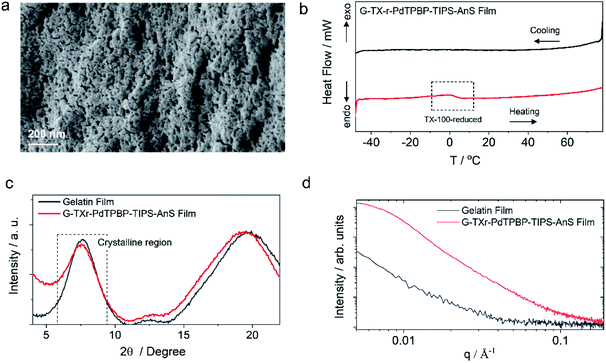
G-TXr-TIPS-AnS-PdTPBP films were prepared by drop-casting of their hot aqueous solutions on glass plates, followed by air drying for 48 h (see methods section and Fig. S5†). The semi-transparent films were obtained after drying due to the inter-chain cross-linking of gelatin upon dehydration, which resulted in an oxygen impermeable three-dimensional thick fiber network.56 Three different films were prepared at different TIPS-AnS concentrations (5, 2.5, and 1 mmol kg−1), and keeping the same concentration of TXr (10.5%), gelatin (89.4%), and PdTPBP (27 μmol kg−1). The transparency of G-TXr-TIPS-AnS-PdTPBP films increases with an increase in the concentration of anionic TIPS-AnS which is a characteristic feature of these biopolymer-surfactant-chromophores co-assembled molecular systems.46,57 Before, TIPS-AnS, we also tried non-ionic TIPS-anthracene, and tetratert-butylperylene, to pair with PdTPBP or Os(m-peptpy)2(TFSI)2 to fabricate bioplastics. However, these films are quite scattering and not suitable for correct TTA-UC characterization. Hence, justify the need for annihilators with ionicity or other designs suitable for co-assembly with the protein-surfactant system to fabricate such bioplastics. As per TIPS-AnS concentration, the prepared films were denoted as sample-I (TIPS-AnS = 5 mmol kg−1), sample-II (TIPS-AnS = 2.5 mmol kg−1), and sample-III (TIPS-AnS = 1 mmol kg−1) in the manuscript and ESI.† The variation in TIPS-AnS concentrations from sample-I to sample-III can be seen from their comparative absorption spectra (Fig. S11†).Sample-I was used for structural characterization. A cross-section SEM image of sample-I shows a porous structure with thick fiber networks (Fig. 2a). It is different from the G-TX-DPAS-PtOEP film published previously by us;46 wherein liquid droplets of TX-100 surrounded by gelatin fibers were observed. Hence, in these films (sample-I to III) TXr could be present as a dispersed liquid rather than isolated droplets. This observation was further supported by temperature-dependent differential scanning calorimetry (DSC) thermograms of sample-I wherein a glass transition (Fig. 2b) was observed near the melting temperature of TXr at around 3 °C (Fig. S12†). It is different from the sharp endothermic peak observed for isolated TX-100 droplets in the G-TX-DPAS-PtOEP film published previously.46 The semicrystalline structure of the G-TXr-TIPS-AnS-PdTPBP film was confirmed from the broad peak at 2θ = 7.6° in the wide-angle X-ray scattering (WAXS) pattern (Fig. 2c) corresponding to the crystalline triple helices of gelatin with an inter-helix distance of 1.2 nm.46,58 The higher scattering of X-ray at small-angle (SAXS) in G-TXr-TIPS-AnS-PdTPBP film compared to gelatin film confirmed densely ordered nanostructure of dispersed TXr-TIPS-AnS-PdTPBP phase in the gelatin (Fig. 2d).59 The time sweep dynamic mechanical analysis of the film confirmed good mechanical stability with a constant storage modulus of 2300 MPa. (Fig. S13†).46
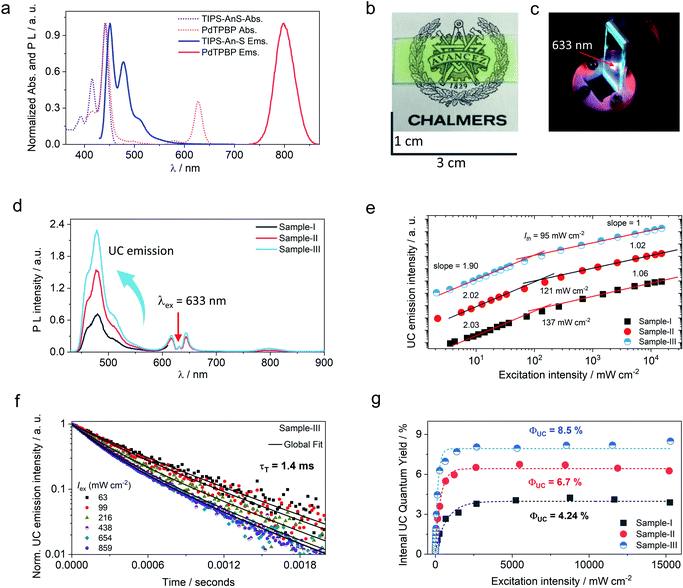
The molecular dispersion of chromophores (PdTPBP and TIPS-AnS) in the G-TXr film was confirmed by measuring their separate absorption and photoluminescence (PL) spectra (Fig. 3a). The absorption spectra of the G-TXr-TIPS-AnS film showed vibronic peaks at 393, 416, and 441 nm, whereas the emission maximum was observed at 451 nm, followed by peaks at 479 nm and 510 nm. Absorption spectra of the G-TXr-PdTPBP film showed a characteristic Soret band at 443 nm and Q bands at 581 and 628 nm, whereas the phosphorescence maximum was observed at 798 nm (1.55 eV).49,50 The similarity of these spectra with the absorption/emission peaks of TIPS-AnS and PdTPBP in the native TXr liquid (Fig. S8†) indicates their molecular dispersion in these films. However, TIPS-AnS showed slightly lower Φf = 79% and τf = 5.4 ns (Fig. S14,† violet line) in the G-TXr-TIPS-AnS film (TIPS-AnS = 200 μmol kg−1) compared to that in native TXr liquid (Φf = 89% and τf = 7.1 ns). The concentration-dependent emission decay profiles of TIPS-AnS in the presence of sensitizer in sample-I to sample-III showed a further decrease in τf to around 4.4 ns (Fig. S14†). These changes in τf can be assigned to the change in the chromophore's environment and structure of the film.60 We also observed the concentration-dependent secondary inner filter effect of TIPS-AnS in G-TXr-TIPS-AnS-PdTPBP films, from the decrease in its emission maximum at 451 nm (Fig. S15†).61 No change in emission decay profiles of TIPS-AnS in sample-I to sample-III with and without polarizer discards any fluorescence anisotropic effects.
TTA-UC measurements of G-TXr-TIPS-AnS-PdTPBP films
Upon continuous excitation with a 633 nm laser, the G-TXr-TIPS-AnS-PdTPBP film (Fig. 3b) showed bright up-converted blue fluorescence in the air at room temperature (Fig. 3c). The upconversion fluorescence spectra showed an emission maximum at 478 nm with an anti-Stokes shift, ΔEUC = 0.62 eV (Fig. 3d).62 The absence of normal emission maximum at 451 nm in up-conversion emission spectra confirmed the secondary inner filter effect. A significant quenching of PdTPBP phosphorescence at 798 nm confirmed triplet sensitization of TIPS-AnS by PdTPBP in the G-TXr-TIPS-AnS-PdTPBP films (Fig. 3d and S16†). The double logarithmic plots of excitation intensity dependency of up-conversion emission at 478 nm of G-TXr-TIPS-AnS-PdTPBP films with the change in slopes from 2 to 1 confirmed the sensitized TTA-UC mechanism (Fig. 3e).A long triplet excited-state lifetime of annihilator, τT = 1.4, 1.5, and 1.7 ms in sample-III, sample-II, and sample-I, respectively, confirmed the protection of annihilator triplets against quenching by molecular oxygen (Fig. 3f and S17†). The τT was determined from the global fitting of excitation intensity-dependent up-conversion emission decay kinetics according to a previously developed method using eqn (1).63–67
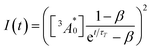 | (4) |
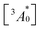 is the concentration of triplet excited annihilators at time zero, and β is a dimensionless parameter describing the relative initial rate of triplet deactivation by TTA and spontaneous triplet decay.68 An increase in β with excitation intensity in sample-III confirmed a rise in annihilator triplet deactivation by TTA rather than spontaneous decay (Fig. S18†). A reference TTA-UC experiment in the aerated TXr-TIPS-AnS-PdTPBP solution (Fig. S19,†λex = 633 nm laser, Iex = 15 W cm−2) showing no upconversion emission confirmed the role played by gelatin in the protection of chromophore triplets against oxygen quenching in the film.
is the concentration of triplet excited annihilators at time zero, and β is a dimensionless parameter describing the relative initial rate of triplet deactivation by TTA and spontaneous triplet decay.68 An increase in β with excitation intensity in sample-III confirmed a rise in annihilator triplet deactivation by TTA rather than spontaneous decay (Fig. S18†). A reference TTA-UC experiment in the aerated TXr-TIPS-AnS-PdTPBP solution (Fig. S19,†λex = 633 nm laser, Iex = 15 W cm−2) showing no upconversion emission confirmed the role played by gelatin in the protection of chromophore triplets against oxygen quenching in the film.
A maximum internal UC quantum yield, ΦUC = 8.5% (50% theoretical maximum) was observed in sample-III. The ΦUC was calculated by a relative method using cresyl violet perchlorate (CV)55 as a reference standard in G-TXr-TIPS-AnS-CV film (see methods section for detailed procedure). The internal ΦUC was calculated by reabsorption correction using normalized emission spectra of TIPS-AnS in TXr in the absence of a sensitizer (Fig. S6†). Without reabsorption correction, a maximum external ΦUC = 7.3% was observed in sample-III (Fig. S20†). The ΦUC = 8.5% is comparable to the record value for red to blue TTA-UC, ΦUC = 8.2% in deaerated nanocellulose film29 and is higher than ΦUC = 5.6% reported in deaerated PdTPBP/DPA crystals.28
The high ΦUC observed for sample-III in the air can also be accounted for high PdTPBP-to-TIPS-AnS triplet energy transfer (ΦTET = 94%). The ΦTET was calculated from phosphorescence lifetimes in the presence (τP) and absence (τPo) of TIPS-AnS in the G-TXr-PdTPBP film (Fig. S21†) using eqn (2).
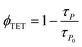 | (5) |
Interestingly, ΦUC decreased upon increasing the concentration of TIPS-AnS from sample-III to sample-I even after correction for secondary inner filter effects. It can be due to the nanoaggregation of the excited state of TIPS-AnS which could not be detected in UV-vis or emission spectra of sample-I to sample-III (Fig. S22 and S15†). To further ascertain the concertation effect of TIPS-AnS, we carried out TTA-UC experiments at even higher concentrations of TIPS-AnS (25 mmol kg−1) in the G-TXr-TIPS-AnS-PdTPBP film denoted as sample-X. Interestingly we did not observe any UC emission in the sample-X. The emission spectrum of TIPS-AnS in sample-X is slightly red-shifted compared to that in sample-I (Fig. S23†). Additionally, sample-X showed a low apparent Φf = 6.2%, compared to Φf = 79% in the film with 200 μmol kg−1 of TIPS-AnS (measured by an absolute method using an integration sphere). Together these results indicate that the excited state of TIPS-AnS is quenched at high concentrations, possibly due to the aggregation or structural changes in the film.
The protection against photodegradation of chromophores in the G-TXr-TIPS-AnS-PdTPBP film was confirmed from stable UC emission upon continuous excitation with a 633 nm laser for 5600 s (Fig. S24†). One mechanism of photodegradation is a reaction between the chromophore and singlet oxygen, where the singlet oxygen is generated by triplet sensitization from a chromophore. Stable UC emission in deaerated TXr-TIPS-AnS-PdTPBP solution upon continuous irradiation at high laser excitation intensity of 15.5 W cm−2 (Fig. S25†) confirmed that the sensitizer and annihilator are stable against photodegradation in absence of oxygen. However, the TXr-TIPS-AnS-PdTPBP films showed a minor decrease in the UC signal upon continuous excitation for 1800 s (Fig. S26†) and the UC stability time decreased with higher excitation intensity. This can be due to minor photodegradation of the chromophores caused by the reaction with residual O2 in the film. It was confirmed by a slight decrease in absorbance of chromophores after TTA-UC measurements (Fig. S27†).
Further, to understand the TTA-UC operation inside the trapped TXr liquid in the film, we measured UC properties in deaerated TXr-TIPS-AnS-PdTPBP solution with [PdTPBP] = 5 μM and [TIPS-AnS] = 1 mM, these are the same initial concentrations of chromophores used to prepare sample-I before casting. The deaerated TXr-TIPS-AnS-PdTPBP solution showed an increase in UC emission with an increase in excitation intensity (Fig. S28a†). The Ith = 415 mW cm−2 and ΦUC = 5.7% observed in the TXr-TIPS-AnS-PdTPBP solution is comparable to that observed for film sample-I (Fig. S28b and c†). The lower Ith observed in the film could be due to a five-fold increase in sensitizer concentration compared to degassed TXr liquid.
Generalization of the concept to far-red to blue TTA-UC
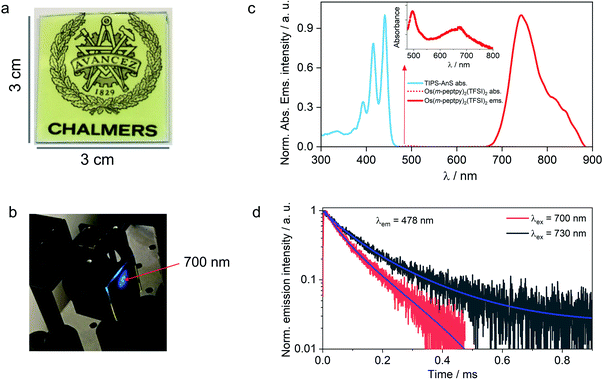
The versatility of the developed strategy is generalized for far-red to blue TTA-UC by paring an S-T sensitizer, Os(m-peptpy)2(TFSI)2 recently published by us (Sasaki et al.,51Fig. 1b) with TIPS-AnS in a G-TXr-TIPS-AnS-Os(m-peptpy)2(TFSI)2 film (Fig. 4a, see methods for detailed preparation procedure). The S-T sensitizers are metal–organic complex molecules that show direct absorption of ground-state singlet to excited triplet state in the far-red/NIR region. These sensitizers are useful to avoid the energy losses during the inter-system crossing in a sensitized TTA-UC process.51 The Os(m-peptpy)2(TFSI)2 shows a long phosphorescence lifetime, τp = 80 μs due to the perylene units at the meta position acting as a triplet reservoir, which minimizes the heavy atom effect of Osmium.51 The absorption spectra of Os(m-peptpy)2(TFSI)2 show vibronic peaks at 424, 450, 495, and 673 nm whereas emission spectra of Os(m-peptpy)2(TFSI)2 show triplet metal to ligand charge transfer (3MLCT) emission at 753 nm (1.65 eV) and phosphorescence emission from the perylene unit (3pPe) at 822 nm (1.51 eV, λex = 640 nm, Fig. S29†).51In the G-TXr-TIPS-AnS-Os(m-peptpy)2(TFSI)2 film, Os(m-peptpy)2(TFSI)2 shows absorption peaks at 496 and 674 nm with a tail ending in the far-red region (inset Fig. 4c), whereas 3MLCT emission was observed at 743 nm (1.67 eV) and 3pPe at 820 nm (1.51 eV) upon λex = 640 nm excitation. The triplet energy of Os(m-peptpy)2(TFSI)2 at 1.51 eV is suitable for exergonic triplet sensitization of TIPS-AnS (T1 ≈ 1.4 eV).47,48 The G-TXr-TIPS-AnS-Os(m-peptpy)2(TFSI)2 film showed blue UC emission, observed with naked eyes upon excitation with 700 nm, nanosecond pulsed laser, Iex = 0.9 mJ pulse−1 (Fig. 4b). The UC emission was further confirmed from the rise and decay in emission signals at λem = 478 nm, upon excitation with 700 nm and 730 nm far-red nanosecond pulsed laser excitation with an anti-stokes shift, ΔEUC = 0.752 eV (Fig. 4d).62 Hence, through the successful execution of this work, we have now expanded the photon harvesting spectral range of TTA-UC bioplastics from 500 to 730 nm.46
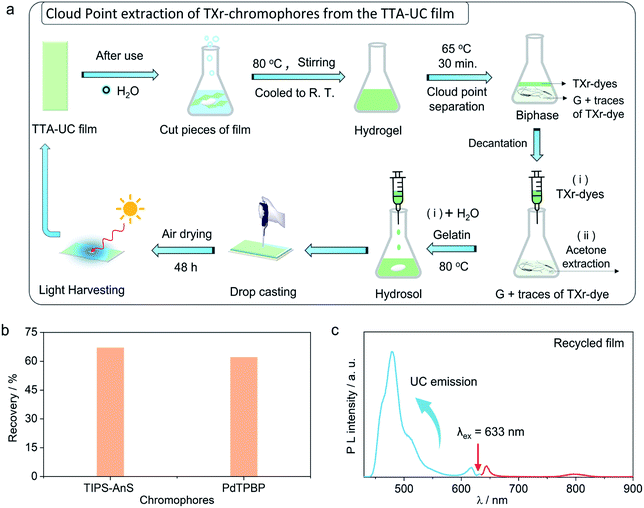
Recycling of the chromophores from the G-TXr-TIPS-AnS-PdTPBP bioplastics
The use of petroleum-based plastics in photonics is attractive due to their tunable molding, mechanical flexibility, and optical transparency.69 However, non-biodegradation of most of them, and non-recyclability of toxic chromophores fabricated inside them renders them unattractive from a sustainability perspective.37,38 To avoid the similar fate of our TTA-UC bioplastics we developed a new downstream recycling approach of “cloud point extraction of TXr-chromophores” from the waste G-TXr-TIPS-AnS-PdTPBP bioplastics (Fig. 5a). The cloud point of a nonionic surfactant is the temperature at which it begins to phase separate from the solid due to incomplete dissolution.70 The TXr has a cloud point of 65 °C in water71 and hence underwent phase-separation from the G-TXr-TIPS-AnS-PdTPBP hydrogel when kept at 65 °C for 30 min (Fig. 5a and S30†). The phase-separated TXr-chromophores top layer was extracted using a syringe. The remaining TXr-chromophores mixture in the gelatin phase was re-extracted using acetone as antisolvent (see methods for detailed extraction procedure). The re-extracted TXr-chromophores mixtures were dissolved in 1 mL of water separately to record their absorption spectra to calculate % chromophores extraction (Fig. S31†).Overall, 67% (55% + 12%) of [TIPS-AnS] and 62% (56% + 6%) of [PdTPBP] could be re-extracted using cloud point and acetone extraction (Fig. 5b). The re-extracted TXr-chromophores mixture was used to prepare G-TXr-TIPS-AnS-PdTPBP film by dissolving in fresh gelatin (Fig. 5a and S30†). The air-dried film showed blue upconversion emission upon excitation with a 633 nm laser in the air (Fig. 5c). The waste gelatin can be discarded for biodegradation after proper washing.
Conclusions
This work establishes TTA-UC bioplastics as recyclable solid-state upconversion materials for broad-spectrum solar light harvesting. The results demonstrate our idea of realizing sustainable and efficient solid-state TTA-UC materials by encapsulating TTA-UC chromophores liquid inside the biopolymer film for efficient channelization of triplet energy in the air. Moreover, it also established the necessity of an ionic annihilator to fabricate transparent TTA-UC bioplastic films made of gelatin with TXr as chromophore dispersing surfactant. The TTA-UC bioplastics are very easy to prepare in an energy-efficient ambient condition without any complicated procedures previously reported to prepare solid-state TTA-UC materials of petroleum-based polymers. Moreover, the simple recycling approach developed in this work to re-extract the expensive and toxic chromophores give them a significant advantage over existing solid-state TTA-UC materials of petroleum-based polymers on grounds of sustainability.In conclusion, we have developed new G-TXr-TIPS-AnS-PdTPBP and G-TXr-TIPS-AnS-Os(m-peptpy)2(TFSI)2 bioplastic films to upconvert red and far-red light to blue light. To realize this, we synthesized a new ionic annihilator, sodium-TIPS-anthracene-2-sulfonate (TIPS-AnS), with suitable triplet energy to pair with PdTPBP/Os(m-peptpy)2(TFSI)2 triplet sensitizers and consequent fabrication into photon upconverting bioplastics. Chromophores in the bioplastics diffuse mainly in the dispersed TXr liquid phase for efficient triplet energy transfer, followed by efficient triplet–triplet annihilation due to the oxygen protection provided by thick gelatin fiber networks. Consequently, the developed red to blue G-TXr-TIPS-AnS-PdTPBP bioplastics showed a record UC quantum yield, ΦUC = 8.5% in the solid-state in the air with an anti-Stokes shift of 0.62 eV. The developed strategy was generalized for far-red to blue TTA-UC in the G-TXr-TIPS-AnS-Os(m-peptpy)2(TFSI)2 film with an anti-Stokes shift of 0.75 eV upon 730 nm far-red laser excitation, thus expand the photon harvesting window of TTA-UC bioplastics from 500–730 nm.44 Finally, we developed a simple and effective recycling route of “cloud point extraction of chromophores” from G-TXr-TIPS-AnS-PdTPBP bioplastic film, demonstrating circular use of key functional components and illustrating how to avoid future post-utilization leaching of chromophores into the environment. Using this route, we could recycle 67% of TIPS-AnS and 62% of PdTPBP along with TXr. The recycled TXr-chromophores mixture was successfully re-used to fabricate new TTA-UC bioplastics thus establishing circularity of the developed bioplastics platforms. The developed strategies offers a new direction to design recyclable optical bioplastics materials in energy harvesting applications to strengthen the concept of circular bioeconomy.
Author contributions
P. B. and K. M. P. conceived this idea and obtained financial support for the work. P. B. led the experimental work assisted by F. E., H. B., Y. S., S. G., A. M. Kimizuka lab (Y. S., N. Y., and N. K.) provided the far-red/NIR sensitizer. F. E., K. B., B. A., and K. M. P. assisted P. B. in data analysis. P. B. and K. M. P. wrote the first draft of the manuscript. All authors participated in interpretation of the results and provided input on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Pankaj Bharmoria acknowledges Marie Skłodowska-Curie Actions – European Commission post-doctoral grant (NIRLAMS, Grant agreement ID: 844972) for research funding. Hakan Bildirir and Kasper Moth-Poulsen acknowledges funding from the Swedish Energy Agency, the Swedish Research Agency FORMAS, the Swedish Strategic Foundation, and the K & A Wallenberg foundation. Bo Albinsson acknowledges Swedish Energy Agency and the Swedish Research Council (VR). Nobuhiro Yanai acknowledges JSPS KAKENHI (grant numbers JP20H02713, JP20K21211, JP20H05676, JP18J21140).Notes and references
- C. A. Parker and C. G. Hatchard, Proc. R. Soc. London, Ser. A, 1962, 269, 574–584 Search PubMed.
- F. Auzel, Chem. Rev., 2004, 104, 39–174 CrossRef PubMed.
- S. Baluschev, T. Miteva, V. Yakutkin, G. Nelles, A. Yasuda and G. Wegner, Phys. Rev. Lett., 2006, 97, 1 CrossRef PubMed.
- S. Baluschev, V. Yakutkin, T. Miteva, Y. Avlasevich, S. Chernov, S. Aleshchenkov, G. Nelles, A. Cheprakov, A. Yasuda, K. Müllen and G. Wegner, Angew. Chem., Int. Ed., 2007, 46, 7693–7696 CrossRef CAS PubMed.
- L. Agazzi, K. Wörhoff and M. Pollnau, J. Phys. Chem. C, 2013, 117, 6759–6776 CrossRef CAS.
- J. Bergstrand, Q. Liu, B. Huang, X. Peng, C. Würth, U. Resch-Genger, Q. Zhan, J. Widengren, H. Ågren and H. Liu, Nanoscale, 2019, 11, 4959–4969 RSC.
- B. Golesorkhi, I. Taarit, H. Bolvin, H. Nozary, J.-R. Jiménez, C. Besnard, L. Guénée, A. Fürstenbergad and C. Piguet, Dalton Trans., 2021, 50, 7955–7968 RSC.
- M.-F. Joubert, Opt. Mater., 1999, 11, 181–203 CrossRef CAS.
- T. F. Schulze and T. W. Schmidt, Energy Environ. Sci., 2015, 8, 103–125 RSC.
- S. Wen, J. Zhou, P. J. Schuck, Y. D. Suh, T. W. Schmidt and D. Jin, Nat. Photonics, 2019, 13, 828–838 CrossRef CAS.
- V. Gray, K. Moth-Poulsen, B. Albinsson and M. Abrahamsson, Coord. Chem. Rev., 2018, 362, 54–71 CrossRef CAS.
- P. Bharmoria, H. Bildirir and K. Moth-Poulsen, Chem. Soc. Rev., 2020, 49, 6529–6554 RSC.
- R. R. Islangulov, J. Lott, C. Weder and F. N. Castellano, J. Am. Chem. Soc., 2007, 129, 12652–12653 CrossRef CAS PubMed.
- W. Sun, A. Ronchi, T. Zhao, J. Han, A. Monguzzi and P. Duan, J. Mater. Chem. C, 2021, 9, 14201–14208 RSC.
- Y. Wei, Y. Li, Z. Li, X. Xu, X. Cao, X. Zhou and C. Yang, Inorg. Chem., 2021, 60, 19001–19008 CrossRef CAS PubMed.
- N. Harada, Y. Sasaki, M. Hosoyamada, N. Kimizuka and N. Yanai, Angew. Chem., Int. Ed., 2021, 60, 142–147 CrossRef CAS PubMed.
- B. D. Ravetz, A. B. Pun, E. M. Churchill, D. N. Congreve, T. Rovis and L. M. Campos, Nature, 2019, 565, 343–346 CrossRef CAS.
- Q. Liu, M. Xu, T. Yang, B. Tian, X. Zhang and F. Li, ACS Appl. Mater. Interfaces, 2018, 10, 9883–9888 CrossRef CAS.
- S. N. Sanders, T. H. Schloemer, M. K. Gangishetty, D. Anderson, M. Seitz and D. N. Congreve, Nature, 2022, 604, 474–478 CrossRef CAS PubMed.
- E. M. Gholizadeh, S. K. K. Prasad, Z. L. Teh, T. Ishwara, S. Norman, A. J. Petty II, J. H. Cole, S. Cheong, R. D. Tilley, J. E. Anthony, S. Huang and T. W. Schmidt, Nat. Photonics, 2020, 14, 585–590 CrossRef CAS.
- B. Albinsson and A. Olesund, Nat. Photonics, 2020, 14, 528–530 CrossRef CAS.
- J. Pedrini and A. Monguzzi, J. Photonics Energy, 2017, 8, 022005 Search PubMed.
- B. S. Richards, D. Hudry, D. Busko, A. Turshatov and I. A. Howard, Chem. Rev., 2021, 121, 9165–9195 CrossRef CAS.
- B. Joarder, N. Yanai and N. Kimizuka, J. Phys. Chem. Lett., 2018, 9, 4613–4624 CrossRef CAS PubMed.
- J. Alves, J. Feng, L. Nienhaus and T. W. Schmidt, J. Mater. Chem. C, 2022, 10, 7783–7798 RSC.
- P. Duan, N. Yanai, Y. Kurashige and N. Kimizuka, Angew. Chem., Int. Ed., 2015, 54, 7544–7549 CrossRef CAS.
- T. N. Singh-Rachford and F. N. Castellano, J. Phys. Chem. Lett., 2010, 1, 195–200 CrossRef CAS.
- C. Li, C. Koenigsmann, F. Deng, A. Hagstrom, C. A. Schmuttenmaer and J.-H. Kim, ACS Photonics, 2016, 3, 784–790 CrossRef CAS.
- R. Vadrucci, A. Monguzzi, F. Saenz, B. D. Wilts, Y. C. Simon and C. Weder, Adv. Mater., 2017, 29, 1702992 CrossRef.
- L. Li, Y. Zeng, J. Chen, T. Yu, R. Hu, G. Yang and Y. Li, J. Phys. Chem. Lett., 2019, 10, 6239–6245 CrossRef CAS.
- A. J. Svagan, D. Busko, Y. Avlasevich, G. Glasser, S. Baluschev and K. Landfester, ACS Nano, 2014, 8, 8198–8207 CrossRef CAS PubMed.
- S. Amemori, Y. Sasaki, N. Yanai and N. Kimizuka, J. Am. Chem. Soc., 2016, 138, 8702–8705 CrossRef CAS PubMed.
- M. Kinoshita, Y. Sasaki, S. Amemori, N. Harada, Z. Hu, L. Zonghao, L. K. Ono, Y. Qi, N. Yanai and N. Kimizuka, ChemPhotoChem, 2020, 4, 5271–5278 CrossRef CAS.
- Y. Sasaki, S. Amemori, H. Kouno, N. Yanai and N. Kimizuka, J. Mater. Chem. C, 2017, 5, 5063–5067 RSC.
- B. Joarder, A. Mallick, Y. Sasaki, M. Kinoshita, R. Haruki, Y. Kawashima, N. Yanai and N. Kimizuka, ChemNanoMat, 2020, 6, 916–919 CrossRef CAS.
- R. Haruki, Y. Sasaki, K. Masutani, N. Yanai and N. Kimizuka, Chem. Commun., 2020, 56, 7017–7020 RSC.
- A. Abulikemu, Y. Sakagami, C. Heck, K. Kamada, H. Sotome, H. Miyasaka, D. Kuzuhara and H. Yamada, ACS Appl. Mater. Interfaces, 2019, 11, 20812–22081 CrossRef CAS.
- E. Radiunas, M. Dapkevićius, S. Raišys, S. Juršenas, A. Jozeliunaite, T. Javorskis, U. Šinkevičiūtė, E. Orentas and K. Kazlauskas, Phys. Chem. Chem. Phys., 2020, 22, 7392–7403 RSC.
- C. Ripkena, D. G. Kotsifaki and S. N. Chormaic, Sci. Total Environ., 2020, 760, 143927 CrossRef PubMed.
- M. Trainic, J. M. Flores, I. Pinkas, M. L. Pedrotti, F. Lombard, G. Bourdin, G. Gorsky, E. Boss, Y. Rudich, A. Vardi and I. Koren, Commun. Earth Environ., 2020, 1(64), 1–9 Search PubMed.
- A. H. Tullo, Chem. Eng. News, 2019, 97, 33 Search PubMed.
- L. Yuan, L. B. Kurnaz and C. Tang, Nat. Sustain., 2021, 4, 837–838 CrossRef.
- R. M. Cywar, N. A. Rorrer, C. B. Hoyt, G. T. Beckham and E. Y.-X. Chen, Nat. Rev. Mater., 2022, 7, 83–103 CrossRef CAS.
- R. Xiong, J. Luan, S. Kang, C. Ye, S. Singamaneni and V. V. Tsukruk, Chem. Soc. Rev., 2020, 49, 983–1031 RSC.
- B. E. Droguet, H.-L. B. Liang, P. R. M. Frka-Petesic, M. F. L. D. Volder, J. J. Baumberg and S. Vignolini, Nat. Mater., 2022, 21, 352–358 CrossRef CAS PubMed.
- P. Bharmoria, S. Hisamitsu, Y. Sasaki, T. S. Kang, M.-a. Morikawa, B. Joarder, K. Moth-Poulsen, H. Bildirir, A. Mårtensson, N. Yanai and N. Kimizuka, J. Mater. Chem. C, 2021, 9, 11655–11661 RSC.
- J. K. H. Pun, J. K. Gallaher, L. Frazer, S. K. K. Prasad, C. B. Dover, R. W. MacQueen and T. W. Schmidt, J. Photonics Energy, 2018, 8, 022006 Search PubMed.
- N. Nishimura, V. Gray, J. R. Allardice, Z. Zhang, A. Pershin, D. Beljonne and A. Rao, ACS Mater. Lett., 2019, 1, 660–664 CrossRef CAS.
- J. Zhou, Q. Liu, W. Feng, Y. Sun and F. Li, Chem. Rev., 2015, 115, 395–465 CrossRef CAS PubMed.
- L. Huang, T. Le, K. Huang and G. Han, Nat. Commun., 2021, 12, 1898 CrossRef PubMed.
- Y. Sasaki, N. Yanai and N. Kimizuka, Inorg. Chem., 2022, 61, 5982–5990 CrossRef CAS PubMed.
- J.-B. Farcet, J. Kindermann, M. Karbiener and T. R. Kreil, Eng. Reports., 2019, 1, e12078 CAS.
- D. P. Lydon, L. Porrès, A. Beeby, T. B. Mardera and P. J. Low, New J. Chem., 2005, 29, 972–976 RSC.
- D. J. Dibble, R. Kurakake, A. G. Wardrip, A. Bartlett, R. Lopez, J. A. Linares, M. Firstman, A. M. Schmidt, M. J. Umerani and A. A. Gorodetsky, Org. Lett., 2018, 20, 502–505 CrossRef CAS PubMed.
- K. Rurack and M. Spieles, Anal. Chem., 2011, 83, 1232–1242 CrossRef CAS PubMed.
- A. V. Tobolskv, Nature, 1967, 215, 509–510 Search PubMed.
- P. Bharmoria, S. Hisamitsu, H. Nagatomi, T. Ogawa, M.-a. Morikawa, N. Yanai and N. Kimizuka, J. Am. Chem. Soc., 2018, 34, 10848–10855 CrossRef PubMed.
- A. Bigi, S. Panzavolta and K. Rubini, Biomaterials, 2004, 25, 5675–5680 CrossRef CAS PubMed.
- R. Vadrucci, A. Monguzzi, F. Saenz, B. D. Wilts, Y. C. Simon and C. Weder, Adv. Mater., 2017, 29, 1702992 CrossRef.
- R. Tigoianu, A. Airinei and D. D. Ortansa, Rev. Chim., 2010, 61, 491–494 CAS.
- M. Kubista, R. Sjöback, S. Eriksson and B. Albinsson, Analyst, 1994, 119, 417–419 RSC.
- Y. Zhou, F. N. Castellano, T. W. Schmidt and K. Hanson, ACS Energy Lett., 2020, 5, 2322–2326 CrossRef CAS.
- F. Edhborg, H. Bildirir, P. Bharmoria, K. Moth-Poulsen and B. Albinsson, J. Phys. Chem. B, 2021, 125, 6255–6263 CrossRef CAS PubMed.
- A. Olesund, V. Gray, J. Martensson and B. Albinsson, J. Am. Chem. Soc., 2021, 143, 5745–5754 CrossRef CAS.
- S. M. Bachilo and R. B. Weisman, J. Phys. Chem. A, 2000, 104, 7711 CrossRef CAS.
- Y. Y. Cheng, B. Fückel, T. Khoury, R. G. C. R. Clady, M. J. Y. Tayebjee, N. J. Ekins-Daukes, M. J. Crossley and T. W. Schmidt, J. Phys. Chem. Lett., 2010, 1, 1795–1799 CrossRef CAS.
- F. Edhborg, A. Olesund and B. Albinsson, Photochem. Photobiol. Sci., 2022, 21, 1143–1158 CrossRef CAS PubMed.
- A. Haefele, J. Blumhoff, R. S. Khnayzer and F. N. Castellano, J. Phys. Chem. Lett., 2012, 3, 299–303 CrossRef CAS.
- H. Chen, H. Li and M. Li, Sci. Rep., 2012, 2, 1–6 Search PubMed.
- R. Aveyard, B. P. Binks, S. Clark and P. D. I. Fletcher, J. Chem. Technol. Biotechnol., 1990, 48, 161–171 CrossRef CAS.
- Triton™ X-100 reduced, https://www.sigmaaldrich.com/SE/en/product/SIGMA/X100RS.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ta04810h |
| This journal is © The Royal Society of Chemistry 2022 |