Transport tuning strategies in MOF film synthesis – a perspective
Muhammad Yazid Bin
Zulkifli
 ab,
Rijia
Lin
a,
Milton
Chai
a,
Vicki
Chen
a and
Jingwei
Hou
ab,
Rijia
Lin
a,
Milton
Chai
a,
Vicki
Chen
a and
Jingwei
Hou
 *a
*a
aSchool of Chemical Engineering, University of Queensland, St Lucia, QLD 4072, Australia. E-mail: jingwei.hou@uq.edu.au
bSchool of Chemical Engineering, The University of New South Wales, Sydney, NSW 2052, Australia
First published on 7th June 2022
Abstract
Metal–organic frameworks (MOFs), sometimes also known as coordination polymers, are a very versatile group of materials consisting of metal nodes and organic linkers forming a tunable porous structure that can exist in different structural phases. The capability to synthesise MOF films allows for application in many different fields such as separation, energy, and catalysis. This perspective aims to explore the transport mechanism and tuning strategies in different types of MOF thin films tailored for different applications. Intracrystalline transport, which has been widely studied, has been shown to successfully aid in the selective transport of different molecules by adjusting the MOF's pore structure and environment. However, in the context of thin film, it is also important to consider interframework (for mixed matrix membrane (MMM) thin film) or intercrystalline (for polycrystalline thin film) based transport as it also directly impacts the overall transport of the thin film. Here we highlight the current intracrystalline and intercrystalline/interframework transport tuning strategies, as well as chart a course for future transport tuning strategies tailored for current and future applications.
Introduction
Metal–organic frameworks (MOFs) are a group of versatile porous materials that can be tuned to show specific transport behaviours. These frameworks are formed by coordinating metal ions/clusters and organic linkers, forming a highly accessible framework with varying pore volume and size, functionalisation, rigidity, and many other tuning variables. They are also known as porous coordination frameworks, a term that was initially used during one of the first studies of this kind of framework material by Tomic in 1965.1,2 Interest in the tunable properties of this material has resurfaced since the term MOF (a much more used term to describe this framework in more recent studies) was first used in 1995 by Yaghi et al., and is continually being studied to be further optimized for many different applications.3–5MOFs have also been shown to exist in many different crystalline phases, shapes and polymorphs – even with similar metal nodes and organic linkers.6–9 They can also be tuned to have different overall crystal sizes and shapes, towards their designated applications.10–13 Recently, MOFs have been shown to be capable of even existing in different structural phases such as amorphous, liquid and glassy phases,14–18 allowing the tunable capability of MOFs to be transferred between their microscopic structures and macroscopic form factors. Due to MOFs' high tunability, the host–guest interaction properties can be adjusted such that the separation and transport become more selective/permeable, thus beating the very basic Knudsen diffusion behaviour, which is typically one of the first aims for many MOF based thin film separations.
Since the first reported MOF, the different varieties of materials that fall under this porous structure family have found application in many different fields such as but not limited to separation, catalysis, sensing and biomedicine.19,20 One important aspect of MOF research is their thin film forms, which have managed to extend the usage of MOF materials as practical devices, particularly for a multitude of molecular-based separations. One of the more extensively studied transport pathways in MOF media is within the gas separation membrane field, due to its highly tunable pores that can selectively separate many different types of gases, from as small as helium to larger gas molecules such as light hydrocarbons.19,20 MOFs have also been applied for liquid-based separation, such as in the separation of oil emulsions, aromatics, fuels and aqueous organic mixtures.21–24 Transport of different substrates for catalysis in the thin film form has also gained traction in aiding a more efficient and useful catalysis–reactant interaction, which has found application in many fields such as biosensors and substrate size-selective catalysis. For example, Chen et al. have demonstrated the capability of Tb-mesoMOF to selectively facilitate the transport of two different substrates through its pore via size exclusion, which only allows the smaller sized substrate to react with the encapsulated myoglobin.25 In this perspective, we will look at recent developments in tuning strategies to regulate the transport behaviours in MOF thin films. This perspective will also shed light on the possible new future research opportunities in this important area.
MOF film transport behaviour tuning considerations
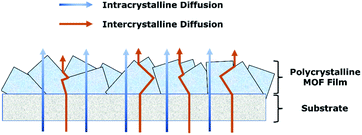
In monitoring the transport of different molecules through the porous MOF films, two main transport pathways, namely intracrystalline and intercrystalline transport, are both equally important. Intracrystalline transport is the transport mechanism through the intrinsic MOF porous frameworks. Tuning strategies for intracrystalline transport have been extensively studied, as it is initially much more straightforward to vary the MOFs' pore environment and properties by matching different compatible (or incompatible) organic ligands and metals together. The fundamental understanding of the interaction between different organic ligands and metal nodes in the earlier stage of MOF research – such as reticular chemistry and supermolecular building blocks – has fuelled newer research and guided the synthesis of more specific and efficient MOFs for a variety of applications including gas separation, catalysis and energy storage.26–29 For example, more recent intracrystalline diffusion improvement strategies are achieved via hybridisation of ligands/metal nodes or controlled defect introduction into MOFs, both cases allowing for improved selective transport in the form of MOF membranes.30,31 To fully utilise these various unique intracrystalline transport capabilities of MOFs on a more industrial scale for many different applications, a MOF film may be required, which leads to the importance of the second medium of transport, the intercrystalline transport.Intercrystalline or interframework transport is the transport between the selective MOF and its additional surrounding materials/substrates, be it a supporting polymeric framework as can be seen in mixed matrix membranes (MMMs), or other MOFs as can be seen in polycrystalline films. The intra and intercrystalline transport behaviours are equally important in governing the overall transport of the target molecules through the film. For example, the strategy of highly controlled oriented MOF growth and phase conversion of a crystalline MOF into the glassy phase has been shown to form good MOF membranes with minimised non-selective grain boundaries leading towards higher transport selectivity.32,33
Intracrystalline tuning strategies
Many different tuning approaches have been explored for intracrystalline transport in MOFs. This is to ensure that a suitable pore environment favourable for the transport of a target molecule is achieved to ensure more selective permeance or separation. The tuning can help change the properties of the pores such as the pore size and flexibility/rigidity, as well as the pore environment interactions with the target molecule. These tunings have been performed via many different strategies, which can be grouped into ligand modification, metal node variation, pore size modification/assisted separation, the introduction of guest molecules, MOF composites, and a mixture of these different strategies.Tuning by ligand control
Ligand modification is one of the most common ways to fine-tune the MOF pore environment for desired transport performance. As MOFs are very porous and rely on the interaction of the targeted molecules with the frameworks to facilitate diffusion through the structure, it is a common strategy to introduce functional groups within the pores, such as amino, carboxyl, nitro, and alkane/alkene groups. One of the most studied MOFs that uses this strategy is UiO-66, in which different functional groups such as UiO-66-NH2, UiO-66-COOH, and UiO-66-NO2 have been synthesised and used for various separation processes without a significant change in their crystal structure.34,35 These functionalisation effects on different separations have also been studied on other MOFs with easily functionalised organic linkers (e.g. terephthalic acid), such as in MIL-53, MIL-101, and IRMOF.36,37These functionalised ligands are usually used for targeted separation/transport processes. For example, polar groups such as NH2 and carboxyl groups are used for polar based separation due to their biased interactions between polar molecules. Amine functionalisation is one of the more widely studied functionalisation routes in many MOF structures, typically for CO2-targeted separations.38,39 This is due to the favourable interaction of CO2 with amine groups, allowing for enhanced CO2 selectivity. Polar –OH and –NO2 groups have also been used to help selectively adsorb/separate CO2 from other gas molecules via active diffusion.40 Functionalisation can also impact the resulting pore size of the MOFs, despite the similar crystal structure, leading to a better transport tuning based on molecular size differences. This can be seen for the UiO-66 series in the separation of gaseous I2 in Fig. 1.36 As a result, it is not as straightforward to select a specific functionalisation rule in all MOFs for specific transport as different kinds of MOFs may have different pore environment changes even with the same functional group. For example, in the case of I2 adsorptive transport, the adsorptive capacity of the MIL-53 series decreases in the order of Cl ≈ NH2 ≈ H > CH3 > Br, while in the UiO-66 series, the adsorptive capacity decreases in a different order of H > NH2 > CH3 > Cl > Br, which may be due to differing pore environments (different changes in pore size/volume, different resulting polarity strengths, etc.).36 Other than that, these polar groups have also been used to help separate very similar molecules with slight polar differences. For example, in the case of ethane/ethylene separation, which has almost similar chemical and physical properties, researchers have shown that polar groups such as sulfonate, perfluoro and amino groups help in the separation of these light hydrocarbons.41–43 Different functional groups are also used to introduce hydrophobicity in the structure. For example, small alkane chains have been used to introduce hydrophobicity into MOFs, allowing for much better hydrostability.44
 | ||
| Fig. 1 I2 adsorption capacity of the UiO-66 series with different functional groups based on its respective pore volume. Reproduced/adapted from ref. 36 with permission.36 | ||
This strategy of introducing functional groups can be implemented via two main methods. One of them involves immediately using the functionalised ligand during MOF formation or synthesis. The second method involves ligand exchange where a non-functionalised pore MOF is synthesised before functionalised ligands are introduced post-synthesis. A recent example can be seen from the modification of ZIF-7, where amino groups were introduced to form ZIF-7-NH2, leading to an evident increase in CO2/CH4 selectivity.45 However, some modifications are hard to perform in this way, due to the interaction of the additional functional groups with the metal nodes, forming a completely different MOF that does not have the free desired functional group in its open pore environment. This can be widely seen in carboxyl-based functionalisation, leading to a limited example of carboxyl functionalized MOFs as compared to other functional groups such as NH2. However, NH2 based functionalisation can be easily modified to accommodate different types of functional groups, typically done via post-synthetic modification. For example, Zhou et al. have managed to introduce the carboxyl group into MIL-101-NH2via post-synthetic modification, resulting in an enhanced selective CO2 adsorption, and thus improved CO2/N2 adsorption selectivity.46
Functionalisation of organic linkers has also been shown to directly impact the resultant MOF pore size and structure. Depending on the type of MOF, the change of pore size has different tendencies even with the same type of functional group. For example, with the addition of an amino group into the ligand, UiO-66-NH2 shows a smaller pore size as compared to the non-functionalised UiO-66.47 ZIF-7 however shows an increase in pore size as more NH2 groups are incorporated in up to 70% functionalised ligand proportion.45 A 100% converted amino functionalised ZIF-7 was not able to be achieved as the particle loses its crystallinity beyond 70% functionalisation.45 The effect of pore size on separation will be further explored later in this section.
A mixed linker approach can also be used to tune the MOF for specific diffusion. An example is in the previously mentioned ZIF-7-NH2, whereby only 70% NH2 benzimidazole linker can be used in the structure before the structure loses its crystallinity.45 Another interesting mixed linker MOF is ZIF-62. With both benzimidazole and imidazole as linkers, the MOF was endowed with an ultrahigh glass-forming ability.48 The glassy state of the metal–organic framework is a subset of the solid amorphous state of MOFs which has a clear transition temperature for transformation into a liquid-like state (Tg).49,50 This definition of the terminology of MOF glasses distinguishes it from other known amorphous states of MOFs and other types of amorphous solids. The main method to synthesise MOF glasses is via the quenching of a MOF from its liquid state. However, MOF glasses can also be synthesised via mechanical vitrification of crystalline MOFs or direct synthesis from the MOF's precursors.49,50 This capability allows crystalline ZIF-62 to form glass by melt-quenching, which improves its molecular sieving capability for CO2 against N2 and CH4 and has been shown to successfully and selectively improve the permeation of CO2 to the other larger gases.32,48 This meltable ZIF-62 MOF has also shown tunable selectivity in hydrocarbon separation.51 The meltable properties of ZIF-62 also allow for better control of inter-film formation leading to a much better intercrystalline transport, which will be discussed in the following section. A further example of the mixed linker strategy is the addition of another completely different connecting ligand that can aid in the transport of specific species. For example, in some coordination polymers (e.g. [Zn(HPO4)(H2PO4)2](imH2)2), phosphoric acid is added to the coordination polymer structure, which results in a chain of phosphoric acid within the framework structure.52 This chain of phosphoric acid, which is now part of the coordination polymer structure, allows for the continuous flow of protons through its structure, leading to enhanced proton transport.53
Tuning by metal nodes
MOFs can also be tuned by changing their metal node. This has been seen in many different MOFs such as ZIF-4, ZIF-62, ZIF-7, and MOF-74. Some of these MOFs may also be denoted using different names such as ZIF-7, which has zinc metal nodes but is denoted as ZIF-9 when substituted with cobalt metal nodes.9 In many cases, the substitution of metal nodes is usually for catalytic purposes, in which the metal nodes assist in a specific catalytic reaction.54 There have also been cases where the metal nodes are used to tune the diffusion of different matter through their structure. These MOFs usually contain open metal sites due to the removal of coordinated solvent molecules within the structure during synthesis.20 These open metal sites can act as Lewis acidic sites which allows for reversible binding of gas molecules through hemi-coordination or coulombic attractions.20 Some of the studied MOFs for separation based on their open metal sites include HKUST-1, M3(BTC)2, and most extensively M-MOF-74.20,55The effects of different open metal sites on gas adsorption have been studied extensively. For example, in terms of CO2 heat adsorption at the open metal sites in HKUST-1, the adsorption heats follow the sequence of Ni > Ru > Cu > Mo or Cr.20 He et al. also found that for M-MOF-74 where M denotes different types of metal centres, Mg and Co metal centres work best for CH4 based separation from other light hydrocarbons.57 Fe, Co or Mg centres perform better with C2 and C3 based hydrocarbon separations.57 Geier et al. also reported that Fe-MOF-74 has the strongest interaction with ethylene for ethylene-based separation, whilst Mn-MOF-74 has the strongest interaction with propylene for propylene-based separations.58 The open metal site in MOF-74 has also been shown to selectively adsorb benzene over cyclohexane via π-complexation as can be seen in Fig. 2.56 Although this benzene–cyclohexane separation was reported only as a powder adsorbent, MOF-74 has been repeatedly shown to be able to transfer its selective adsorbent capability towards thin film membrane separation, leading towards the possibility of utilising this in the form of a membrane in future research.59–62 Luo et al. reported the effects of different geometries of open metal sites on gas separation. They compared UTSA-74 with Zn-MOF-74 (two MOFs which have similar metals and ligands) with Zn2+ sites in different geometries, showing how the geometries caused a significant difference towards CO2 selectivity over acetylene during separation.63
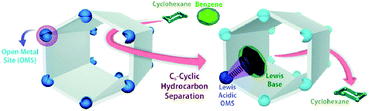 | ||
| Fig. 2 Role of metal site in MOF-74 towards the separation of benzene from cyclohexane. Reproduced/adapted from ref. 56 with permission from The Royal Society of Chemistry.56 | ||
Tuning for pore rigidity/flexibility
The flexibility of MOFs allows for the adsorption of different species of gases or other guest molecules at variable pressures to induce an aperture size change.64,65 This phenomenon is commonly referred to as the breathing effect or gate opening effect of MOFs, which has been seen in different MOFs such as MIL-53, ZIF-7 and ZIF-8.66–68 It is usually reversible, and the large pore or narrow pore phases of the “breathing MOFs” are determined by the osmotic thermodynamic potential.66,69 These pore changes are typically reliant on the flexible MOF's pore volume and guest chemical affinity and could affect the gas molecular transport through the MOF pores depending on the existing phases.69 It is also important to note that since the flexibility or pore opening capability in these MOFs is influenced by the chemical affinity within the pore environment, the pore opening capability varies with different transporting molecules. Due to the differing transport behaviour at different pressures, the gate opening mechanism is usually applied for pressure-based gas separation by flexible MOFs. Gases of different sizes can pass through a flexible MOF at different pressures, by overcoming the typical hydrogen bonds that hold the MOF's initial resting pore aperture structure.70 This is due to the interaction of the adsorbate with the linker within the MOF, allowing for the linker to change in conformation inside the MOF structure to allow for the molecules to pass at different pressures (‘breathing’ or ‘gate opening’).71 ZIF-7, despite having a very small pore size of ∼3 Å, was able to selectively separate ethylene from ethane, and CO2 from CH4via this mechanism.67,71 We have also demonstrated the capability of controlling this breathing effect via the crystal–glass composite structure.72 We have shown that the capability of permanently retaining the large pore phase of breathable MIL-53 is possible in a crystal–glass composite structure with glassy ZIF-62.72 This enables more CO2 adsorption in MIL-53 at a lower pressure as compared to its narrow pore structure which usually occurs at lower pressure.72The capacity to control pore stiffness also allows for selective gas transit. The presence of guest molecules coordinated within the structure can affect the rigidity of the pore in the framework. The coordinated water molecules present in UTSA-280 lead to a more rigid pore structure, resulting in great molecular sieving separation performance for ethylene over ethane.73 The absence of coordinated water molecules in the structure causes the structure to lose its rigidity, and thus lose its excellent gas separation capabilities. For ZIF-7, the DMF in the structure can be stripped if over-activated, resulting in a phase change from phase 1 to phase 2, which is a distorted version of phase 1.9 However, the impact of this more distorted version has yet to be fully studied in terms of gas transport within thin films.
A control of rigidity in MOFs has also been studied by Hou et al. to find the optimised component ratio to obtain the best transport selectivity across a Co-based ZIF-67/Zn-based ZIF-8 hybrid membrane as can be seen in Fig. 3.74 It was shown that by combining a rigid part (Co-containing ZIF-67) into a more flexible part (Zn-containing ZIF-8), the intrinsic flexible structure that could limit further enhancement of selectivity for propylene/propane with very similar sizes could be overcome, allowing for a selectivity of propylene/propane of ∼200 in the resulting mixed metal membrane.74 The control of rigidity combined with crystallisation kinetics was also shown to be able to form a much better inter-grain boundary structure, which will be discussed in later sections.
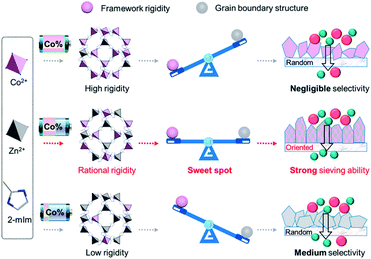 | ||
| Fig. 3 Balance between framework rigidity and flexibility with grain boundary structure towards sieving capability in MOF film, realised through metal node substitution. Reprinted (adapted) with permission from Q. Hou, S. Zhou, Y. Wei, J. Caro and H. Wang, J. Am. Chem. Soc., 2020, 142, 9582–9586. Copyright {2020} American Chemical Society.74 | ||
Tuning by guest molecules, secondary components and composites
Another more common method is to add specific molecules within the pores that do not impact the framework. These small molecules usually improve the transport or diffusion of specific molecules through different MOF structures. An example of this phenomenon is using an ionic liquid. For example, ionic liquids (e.g. 1-ethyl-3-methylimidazolium thiocyanate ([Emim][SCN])) were introduced into MOFs to improve the ion conductivity.75,76 There have also been instances where an ionic liquid was introduced into ZIF-8 to improve the transport of CO2.77On the other hand, the molecular transport through a MOF thin film can also be tuned by growing a secondary MOF layer. For example, a ZIF-8 on ZIF-7 composite membrane has been observed to enhance the selectivity of H2/CO2 separation.78 Furthermore, ZIF-67 has also been composited with ZIF-8 to selectively separate propylene from propane and managed to obtain a selectivity of ∼200, one of the highest selectivities for propylene/propane to date for MOF based membranes.79,80 Another example of MOF on MOF is the hybrid of UiO-66-NH2 and ZIF-8, leading to one of the highest performing monovalent ion-selective membranes for alkali metal to date.81 The ultrahigh selectivity is attributed to the effects of spatial hindrance and nucleophilic entrapment as the ions move across the hetero-structured MOF on MOF film, which is closely inspired by a biological ion channel responsible for regulating ion transport across cell membranes.81,82
A combination of these different strategies has also been found to be employed to enhance the transport of the target molecules through the selected MOF. For example, by combining the gate opening mechanism and the NH2 functionalisation, the ZIF-7-NH2 membrane resulted in a selective CO2/CH4 separation beyond the Robeson upper bound.45 While these intracrystalline strategies have certainly been developed to provide tunable transport performance needed for many different applications, a good intercrystalline tuning is also required for optimised usage in the form of thin films. This is to ensure that the full capabilities of these intracrystalline tunings are fully accessed and functioning during use. The issues and strategies for transport for intercrystalline diffusion are explored in the following section.
Intercrystalline/interframework tuning strategies
Intercrystalline/interframework transport is the transport of molecules between the MOF crystals, or between the selective MOF and its additional polymeric framework. This transport tuning is very important to ensure that the intracrystalline properties can be properly accessed and utilized during molecular transport through the MOF films. Current MOF thin film production mainly falls into two main categories: polycrystalline films or mixed matrix membranes (MMMs).Tuning strategies for mixed matrix films
Polymeric mixed matrix membranes provide one of the more common and easier methods to synthesize MOF based thin films. The selective MOFs are usually prepared in advance and dispersed in selective polymers and dried to form a selective thin film. However, one of the main issues usually found in these types of membranes is the poor interfacial compatibility between the MOF and the polymer matrix.83,84 It can result in the agglomeration of the MOF particles, and/or formation of void spaces between the MOF and its surrounding polymer. The presence of agglomeration can cause the MOF to be less evenly distributed throughout the matrix layer, while the presence of voids can limit and lower the interaction of the MOF with the adsorbent, thus lowering the capability of the MOF to actively govern the transport of different molecules through the film.A few different methods have been studied to solve these stated problems. One common method is MOF functionalisation, either throughout the MOFs or at least at the surface, with functional groups that interact well with the polymeric structure. This functionalisation can either promote hydrogen bonding between the MOF and the polymer matrix or has favourable hydrophobic/hydrophilic interaction, which has been shown to help increase the interfacial compatibility between the two components. For example, the use of amino groups has been widely found to improve the interfacial compatibility of MOFs with various polymeric substrates. The favourable interactions between NH2 groups and imide groups in polymers have been shown to help reduce the interfacial issues faced by many different MOFs.83 Hydrophobicity and hydrophilicity of the MOF filler and the polymer matrix have also been widely studied as a strategy to reduce the voids due to interfacial issues within the matrix.83
Other than that, in situ crystallisation of MOFs during the formation of polymers has also been seen. MOFs and polymers are formed in situ to allow for the covalent bonding of specific linkers with the polymer, thus eliminating the possible agglomeration or void formation.85–87 It was also found that the MOF particle size plays an important role in the prevention of agglomeration and void formation within the polymeric framework matrix.84 The agglomeration of MOFs within the polymer matrix could provide an alternative non-selective pathway as can be seen in Fig. 4, which is not desirable in selective transport. A recently developed method that combines the usage of small MOF nanoparticles with selective functionalisation has been shown to help mitigate agglomeration, allowing for higher MOF particle loading. Knebel et al. have introduced two different N-heterocyclic carbenes (NHCs) onto the surface of ZIF-67 nanoparticles, allowing for dispersion in previously non-dispersible solvents such as mesitylene.88 This functionalisation has allowed the ZIF-67 to be more liquid processible, allowing for better stability and dispersion when introduced into mixed matrix membranes. This leads to a much higher mixed matrix membrane loading not possible with non-functionalised ZIF-67, resulting in a more selective and permeable membrane for propylene/propane separation.88 Another approach to improve the interfacial compatibility between the MOF and its surrounding polymer matrix is changing the MOF phase to a more flowable phase within the structure to help remove void formation. This can be seen in our work in which we melted ZIF-62 particles within the polymer matrix itself.89 After melting and quenching the ZIF-62 particles while being confined, the initial void present within the structure was drastically reduced through the formation of the liquid intermediate phase, as probed by FIB-SEM analysis. In all these cases, the improvement of interactions has greatly improved the MOF to ensure a better film separation.
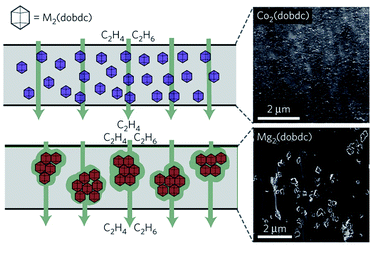 | ||
| Fig. 4 Diffusion pathway across the M2(dobdc) membrane with respect to MOF particle size. Reprinted/adapted with permission from Springer Nature Customer Service Centre GmbH: [Springer Nature], J. E. Bachman, Z. P. Smith, T. Li, T. Xu and J. R. Long, Enhanced ethylene separation and plasticization resistance in polymer membranes incorporating metal–organic framework nanocrystals, Nature Materials, 2016, 15, 845–849, copyright (2016).84 | ||
Tuning strategies for polycrystalline films
Polycrystalline films have been extensively investigated in recent decades, allowing MOFs to be used in a wide range of applications. Following this, a multitude of different MOF film synthesis methods has been studied and reviewed. There are three main intercrystalline/bulk tuning strategies that are crucial for regulating the transport of different molecular species through the MOF film: tuning of film thickness, substrate to film interaction, and MOF–MOF interaction.One of the more basic tuning strategies in MOF films is the tuning of film thickness itself. Early research on MOF film synthesis has seen examples of films with a thickness of around 20–30 μm for the case of ZIF-8, in sharp contrast to the more current thin films for ZIF-8 that have reached a thickness as small as 85 nm, which is a reduction of around 95%. The motivation for thinner MOF films is to reduce the diffusion time through the MOF, thus allowing for a much more efficient permeation.90–93 However, a thinner film can also pose a disadvantage over selective transport if the substrate to film interaction and MOF–MOF interaction in the crystalline layer is not designed properly. This will result in transport defects, which become more pronounced as the film becomes thinner.
One of the more pressing issues in polycrystalline MOF film formation is the compatibility between the MOF film and its substrate. Substrates are usually required to maintain the mechanical integrity of the MOF membrane when an ultrathin MOF film is targeted for high separation efficiency. By functionalising the substrate, we can help tune the MOF growth such that a better polycrystalline film is formed. This is commonly required as a necessity as the MOF heterogeneous nucleation on substrates, when compared with homogeneous nucleation, is usually unfavourable. For example, electrostatic repulsion can occur between alumina and some MOF precursors, leading to the inhibition of the MOF nucleation on its surface.94 Different kinds of functional groups such as tannic acid (TA)–Fe, polydopamine/polyethyleneimine (PDA/PEI), and TiO2–(3-aminopropyl)triethoxysilane have been developed to promote MOF growth.95–97
On a more related aspect, the selection of functional groups on the substrate plays an important role in the transport properties of the synthesized films. We investigated the ZIF-8 deposition process on differently functionalised substrates, i.e. TiO2 inorganic nanoparticle coated and PDA/PEI functionalised substrates.97 Functionalising the substrates with PDA/PEI results in stronger interaction with ZIF-8 during growth, leading to a slightly contracted MOF lattice structure.97 However, the usage of TiO2 on the substrate allows for less contraction and better preservation of the original inherent ZIF-8 crystal structure both at the interface and the bulk of the thin film (Fig. 5). This leads to a better-defined CO2 transport over bulky gases such as N2 and CH4, and thus a better selectivity.97 This contraction behaviour of MOFs at the interface may directly impact the initially engineered intercrystalline tuning design, leading to varied transport selectivity. Although transport will mainly be governed by the bulk of the film for a thick film, the current goal in this area is towards a thinner film for improved permeance. Therefore, the substrate to MOF interaction effect becomes increasingly important and should be given more attention in future research.
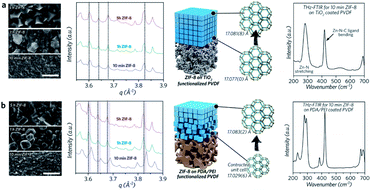 | ||
| Fig. 5 Effect of functionalisation towards the contraction of MOF film structure. Reprinted (adapted) with permission from J. Hou, P. D. Sutrisna, T. Wang, S. Gao, Q. Li, C. Zhou, S. Sun, H.-C. Yang, F. Wei, M. T. Ruggiero, J. A. Zeitler, A. K. Cheetham, K. Liang and V. Chen, ACS Appl. Mater. Interfaces, 2019, 11, 5570–5577. Copyright {2019} American Chemical Society.97 | ||
Another important property that governs transport in MOF films is through the intergrain boundaries. While many researchers have reported the non-selective behaviour of intergrain boundaries, this can also help to improve permeance through the film. The intergrain boundary or intergrain voids would be beneficial for applications which require high film permeance rather than selectivity, or processes that involve the transport of bulk materials that are larger than the MOF inherent pores, such as for membranes and films with immobilised catalysts or biocatalysts.98,99 In this case, the strategy for creating a highly packed yet highly permeable film via intergrain boundary diffusion is highly desired. Thin films with high intergrain boundary pathways can be easily formed via a simple dip-coating method involving immersion into a thick MOF solution to form a thick MOF coated film. These MOF particles will be densely packed while having a huge amount of intergrain voids for diffusion. However, intergrain boundaries are not as beneficial for molecular-based separation and ionic/charge conduction.
Intergrain boundaries in MOF films have been shown to provide a nonselective pathway in polycrystalline films, leading to poor selectivity for molecular transport through MOF films as can be seen in Fig. 6. For example, in comparison with the polycrystalline ZIF-8 membrane, the single crystal ZIF-8 membrane shows a significantly higher selectivity with good comparable permeance over other polycrystalline ZIF-8 membranes for different gas separation pairs.100 For molecular transport, a thicker film can be formed to mitigate the non-selective transport through the intergrain boundary by increasing the tortuosity in the film and allowing for more pore diffusion, but at a price of a much lower overall permeance/diffusivity.101 Intergrain boundaries have also been shown to lead towards higher surface roughness and discontinuity in electrical flow, both contributing towards poor conductivity and ionic transport through the polycrystalline film.102,103 Intergrain boundaries also provide conductivity resistance to the thin film, leading to a declined conductivity.104
Multiple strategies have been developed to overcome the non-selective transport through the intergrain boundary in polycrystalline thin films. Slow epitaxial/heteroepitaxial growth is shown to be able to minimise grain boundary effects, allowing for the intrinsic pore properties to be fully utilised, leading to higher CO2/N2 and propylene/propane selectivity.79,105 Oriented growth has also shown promise to mitigate the non-selective transport issue through intergrain boundaries. For example, UiO-66-NH2 grown by well-controlled in-plane orientation results in a much better-intergrown film with reduced grain boundary defects and enhanced transport selectivity performance.106 Wei et al. have also grown a well oriented ZIF-8 membrane with its selective face being the main window for transport.33 This well oriented ZIF-8 has been shown to help form a more tightly intergrown ZIF-8 film, coupled with selective transport through its 〈1 0 0〉 window, leading to an increase of ∼3 times in terms of selectivity for ethylene/ethane separation.33 Additionally, it is reported that the intergrain boundary can be tuned via manipulating the crystallisation kinetics of frameworks by bimetallic approaches, such as mixing Zn and Co as metal nodes in ZIFs.74,79,105 Co-based ZIFs are usually much more rigid, thus providing a much better pore environment for molecular sieving based separation. However, Co-based ZIFs usually have much faster crystallisation kinetics, leading to a very poor grain boundary structure.74 Zn-based ZIFs however usually have much slower crystallisation kinetics, and thus can form polycrystalline films with a better intergrain boundary. But due to their flexibility, they can have a less selective transport through their inherent framework pores.74 Combining these two metals allows for better polycrystalline growth kinetics, leading to a much better selective polycrystalline film transport. This is due to the presence of rigidity from the Co metal node while maintaining reasonable crystallisation kinetics, allowing for a minimised non-selective intergrain boundary.74
MOF glass, a new MOF phase recently discovered, has also shown promise in the thin film application. Due to its more continuous layer and ease of processing, MOF glass has been hailed as one of the solutions to the intergrain boundary issues in thin MOF film.32 The liquid phase of MOF glass allows for better interaction between the MOF elements, and the issue of grain boundary is also eliminated upon quenching.32 This glassy film has been widely studied for its conductive properties, exhibiting a good performance in proton transport properties.53 Glassy MOF and coordination polymer films have also been studied for gas separation processes fairly recently, showing good selective transport through the film.32,107 However, the study of glassy film transport is still very limited and opens to a much wider exploration for many different separation applications in the future.
Other than attempting to eliminate the intergrain boundaries, the intergrain boundaries can also be used to add functionality that can aid in the transport of the desired molecules. For example, in an amino-functionalized UiO-66 membrane, the grain surface allows for a much faster transport pathway for protons as compared to through their pores.108 The flow of aqueous solution through the ZIF-8 membrane was shown to be dominated by its intergrain boundary layer due to ZIF-8's hydrophobicity.101 This is apparent when the boundaries are grown carefully such that the defects in the intergrain boundaries are covered by hydrophobic 2-methylimidazole ligands, which further limits the transport of water through the film.109 Loading the intergrain boundary layer with a selective carrier is also another strategy to improve selective transport through the intergrain boundary layer. Yang et al. impregnated layered MOF membranes with AgBF4/ionic liquid to make the intergrain from non-selective pathways to a highly selective pathway for olefin–paraffin separation.110
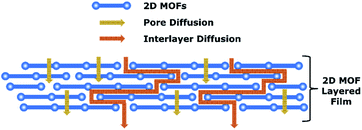
Another type of polycrystalline film is a film that was formed using MOF particles that have been synthesised using their typical solvothermal method and then deposited onto a substrate. However, with 3D MOFs, the packing density is very limited, which can lead to very poor selective intracrystalline separation due to the presence of voids within the MOF that greatly impacts the selectivity. This method is more viable for 2D MOFs due to its high packing density, which can be achieved via filtration coating, spin coating or self-assembly driven by the entropy terms. The tuning strategy of such 2D films is that it differs based on the two possible transport mechanisms – either through the MOF's pores or via interlayer spacing between the 2D MOF sheets. Transport that requires the film to go through the 2D MOF pores, as for the ZnbIm nanosheet, requires the stacking to be less laminar and much more disoriented.111 This helps prevent the pore blockage that may occur during stacking. This is starkly different to other 2D materials that prefer laminar stacking as it depends on the interlayer spacing to ensure selective transport. For example, graphene oxide membranes have been modified and functionalised to obtain the desired interlayer spacing suitable for the separation of organic matter and ionic species.112–114 Functionalised graphene oxide was shown to have a smaller operational interlayer spacing, leading to a lower transport of small ions.112 In this case of separation via an interlayer spacing based mechanism, it is more rational to have a more laminar stacking as the separation is based on the interlayer spacing rather than through the pores of the MOF. As 2D MOFs can be easily tuned to have specific pore and surface properties, it is important to ensure that the tuning is designed to suit the target separation mechanism. A combination of these strategies for 2D MOF film can also be implemented in future research to ensure improved transport. Future 2D MOF films can be tuned such that the pores of the 2D MOFs can selectively allow the transport of one species over another while having a well-tuned laminar interlayer spacing such as to also have selective transport via this pathway without blocking the pores.
Future applications and improvements in MOF thin film
Prospective tuning strategies
Although intracrystalline transport strategies have been extensively studied in the past, there remain possible new intracrystalline transport strategies that should be further explored. The creation of molecular pumps has provided a new area for intracrystalline transport tuning exploration in MOF thin films. A molecular pump is generally a mechanism that helps transport molecules against their concentration gradient. Currently, relatively little research has been conducted on this process in MOF thin films for selective transport. One example can be seen from the use of a porphyrin-based MOF membrane, in which the MOF can help pump cations through the membrane against the concentration gradient when subjected to light.115 This research however has only shown the pumping capability of the MOF and the selective capability of the ion pump has not yet been extensively explored. Thus, this strategy opens a new avenue for future MOF transport tuning design by including the possibility of ionic/molecular pumping in MOFs to allow for selective transport against the concentration gradient.Single crystal MOFs have been shown to have good transport selectivity and diffusion because the separation is largely governed by the intracrystalline diffusion in the single crystals.100,116,117 In this case, the intracrystalline design dominates the separation process without having to worry too much about intercrystalline diffusion. However, single crystals are usually very small – on the microscale – and thus are not viable to be made into a proper film. With better control over the synthesis conditions and substrate treatment, a macroscopic single-crystal MOF film could be formed on the surface of substrates, similar to the single-crystal metal halide perovskites formed between two clamping glass slides, to generate a large continuous monolith for film formation.118 Nevertheless, growing a sufficiently large crystal, to form a large enough good film, is usually very challenging as a slight lattice mismatch and defects in the structure can lead to the formation of more thermodynamically preferable polycrystalline film. Another approach is to control the substrate pore opening such that single crystals can easily sit on the substrate opening and ensure that transport through the substrate is fully governed by the single crystals. The performance of single crystals has been measured by sitting the crystal onto a very small hole and has shown good selectivity as shown in Fig. 7. This technology may be scaled up by covering multiple pore holes on the substrate with many single crystals. However, the current technology is still not advanced enough to upscale the formation of these multiple holes covered by multiple single crystals in a financially viable manner, leaving this subject open for further research. Future research may concentrate on functionalisation at the substrate pore opening, which could aid in regulating single crystal formation at the substrate pore opening.
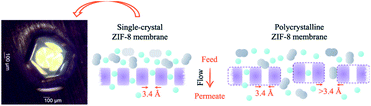 | ||
| Fig. 7 Transport pathways through single crystal and polycrystalline ZIF-8 as membrane. Reprinted from C. Chen, A. Ozcan, A. O. Yazaydin and B. P. Ladewig, Gas permeation through single-crystal ZIF-8 membranes, Journal of Membrane Science, 2019, 575, 209–216, copyright (2019), with permission from Elsevier.100 | ||
Another interesting material that requires a more in-depth transport tuning analysis is the recently discovered MOF glassy phase. Recent studies on MOF glass composites and functionalisation are focused on particle preparations. For example, halogenated and amino functionalised ZIF-62 glass particles showed interesting properties for gas separation, and possibly other separation processes.17,119 The intracrystalline transport of MOF glass can also be tuned by post-synthetic modification strategies as experienced by the widely studied MOF crystals as discussed. MOF glasses are promising candidates to be further explored in thin films and membranes mainly for separation processes. There have been very few MOF glass membranes/thin films that have been reported, and this opens a lot of exploration possibilities in MOF glass membrane separation.
A mixed-phase strategy, like a mixed matrix membrane, can also be explored with this glassy MOF phase. Selected MOF crystals can be surrounded by a better contacting phase such as the MOF glassy phase, thus eliminating or reducing the intergrain boundary issues faced by a typical polymeric mixed matrix membrane. We recently developed a crystal–glass MOF composite between ZIF-62 glass and MIL-53 crystals.72 The findings have shown good contact between MIL-53 crystals and ZIF-62 glass allowing for better hydrostability and phase control of MIL-53, leading to possible good molecular separation applications.72 While this strategy has been performed on a particle system, it is yet to be extensively explored in a thin film form, thus opening this area to a wide array of exploration opportunities. Similar to the polymeric mixed matrix membrane, the addition of selective and permeable MOF crystals into the MOF glass matrix could further help improve selectivity and permeance within the crystal–glass composite membrane. MOF glass has also been shown to have good contact with functional materials such as lead halide perovskites, leading to phenomenal stability and optoelectronic improvements.120 Good contact and porosity of MOF glass also allow for the possibility of a functional catalytic MOF glass thin film composite by applying a MOF with catalytic capability.121 Further tuning the transport selectivity and properties in MOF glass will also open its usage in selective catalysis processes, allowing for only specific species to go through and interact with the catalysts, thus further improving the selectivity and stability of the catalyst. The melting temperatures for the MOF/coordination polymer glasses have also recently been found to be able to reach reasonably low temperatures, down to 80 °C, allowing them to composite with other functional materials with low thermal stability, to further improve the transport properties, or to endow the material with additional properties.52
Another MOF configuration with possible further transport tuning is the 2D MOF layered thin films. As explained in the previous sections, a strategy using a hybrid of both transport mechanisms through the MOF pores and interlayer diffusion should be further explored for layered 2D MOF based thin films. This strategy may be more efficient as these two distinct transport mechanisms can work synergistically to promote the selectivity and permeability in the layered film as can be seen in Fig. 8. In terms of tuning the 2D MOF pores, strategies explained in the intracrystalline transport tuning sections such as pore functionalisation could be employed to preferentially transport one species of the molecule over another. This functionalisation technique can also be used to tune the interlayer spacing between the 2D MOFs, allowing for selective transport via interlayer diffusion without blocking the pores of the 2D MOFs. Tuning of a 2D material's interlayer spacing by functionalising the material has been proven to be successful in other materials such as graphene oxide. The interlayer spacing between graphene oxide sheets has been reduced when subjected to N-(trimethoxysilylpropyl)ethylenediamine triacetic acid (EDTA) functionalisation but could be increased when functionalised with t-butyl hydrazinecarboxylate or triethanolamine.122–124 Due to the ease of MOF functionalization, this technology can be easily transferrable to the synthesis of future selective two-dimensional MOFs for selective transport. Guest molecules can also be introduced to control the transport via interlayer diffusion. The guest molecules can not only help to adjust the interlayer spacing but also facilitate transport through the interlayer diffusion. The presence of water and ions has been shown to swell or shrink the interlayer spacing of various stacked 2D materials (such as graphene oxide).122,125,126 Thus the same strategy could be used to induce swelling or shrinking in stacked 2D MOF thin films. A selective guest molecule such as an ionic liquid can also be used based on this strategy to tune the interlayer spacing of 2D MOFs and provide an additional selective diffusion.
A MOF-on-MOF strategy for MOF thin film can also provide a possible tuning mechanism for selective transport. MOF-on-MOF growth can facilitate partial ligand/metal exchange coupled with the capability to close up non-selective intergrain boundaries. This can be seen in the ZIF-67–ZIF-8 MOF-on-MOF membrane that allows for the mitigation of non-selective intergrain boundary, coupled with possible optimal pore rigidity structure by a mixed metal framework that has allowed the membrane to achieve a very high propylene/propane selectivity of ∼200.74,79 This transport tuning strategy can be further applied for other molecular pairs with very similar chemical and physical properties, such as ethylene/ethane, whereby two MOFs with smaller pore size, but different rigidity and crystallisation kinetics could be combined in a MOF-on-MOF structure to help achieve similar transport selectivity performance. Another MOF-on-MOF strategy involves the formation of MOF film with different hierarchical pore sizes, where spatial hindrance could aid in the selective transport of molecules through the MOF thin film membrane.81 However, the growth of MOF-on-MOF films has been hindered by the crystallisation growth limitation between the different MOFs, where the lattice between the two different MOFs must closely match for the secondary MOF to successfully grow on the initial MOF layer.127,128 If there are clear differences in the lattice structure, a connecting molecule such as a surfactant or random copolymer glue is usually required for a successful secondary MOF film growth.81 A promising alternative is to introduce a glassy MOF phase as a secondary layer on the MOF-on-MOF film, where the issue of lattice mismatch is no longer relevant. The possibility of flux melting MOF glass provides the possibility of a hierarchical MOF glass pore structure by controlling the diffusion of the flux melted MOF into the bulk MOF glass phase during melting. The usage of a non-flux-meltable MOF will also be able to create a good hierarchical pore structure, as meltable MOF glasses have been shown to have good contact with other MOFs due to their flowability during the formation of a liquid state.72 These MOF-on-MOF strategies open a huge exploration possibility for selective transport in MOF thin film future research.
Prospective MOF thin film applications
One prospective future application for tunable film-based separation/transport lies in the field of quantum-based separation. H2 and its isotopes such as D2 and T2 have been applied in many different areas such as nuclear magnetic spectroscopy and nuclear fusion.129–133 However, current methods for obtaining the isotopes of hydrogen gas including the chemical exchange Girdler-sulfide method, cryogenic distillation and electrolysis are highly energy-intensive due to the very close physical properties of the isotopes (boiling point: 20.3 K for H2 and 23.6 K for D2).131,132 This property leads to a selectivity of only 3 at 20 K via cryogenic distillation.132 MOFs have been presented for quantum-based separation via two main methods, kinetic and chemical affinity-based quantum sieving.133 MOFs have shown that via quantum sieving based adsorption, they can reach a selectivity of more than 90 for D2/H2 separation, and over 200 for T2/H2 separation.131 For kinetic based separation, a smaller quantum effective pore size (QEPS) is much better, with QEPS lower than 3 Å having D2/H2 selectivity of more than 50. Meanwhile, for chemical affinity-based quantum sieving, larger pores with functionalisation have also shown good selectivity. For example, in the case of MOF-74, the presence of lone pair electrons in the pore allows for D2/H2 separation to occur despite the larger pores.129 Another MOF, FJI-Y11, has also shown good D2/H2 selectivity despite its considerably large pores (5.9 Å) due to the interaction between hydrogen with oxygen groups within the pores.134 However, all these studies have only been performed on a particle–adsorbent process basis. The capability of a MOF to be tuned to have the desired pore size and pore environment can certainly be extended towards membrane and thin film processes for quantum sieving based separation.It is important to address the intercrystalline challenges stated in the previous section for fully utilising the capability of pore engineering performed on MOFs. One possible solution is fusing MOF glass films for these hard to separate quantum molecules. By applying MOF glasses it is possible to remove the intergrain boundaries while having excellent contact with the substrate.32,107 One of the more widely studied MOF glasses, ZIF-62, has a pore size of ∼3.2 Å, which falls within the sweet spot for quantum-based separation.32 The pore size of ZIF-62, coupled with the absence of intergrain boundaries in ZIF-62 membranes, should make ZIF-62 membranes promising candidates for quantum sieving based separation in the future.
Scaling up of MOF-based thin films
As the technology for MOF-based thin films progresses, it is important to explore the larger-scale production of MOF-based thin films. This is to secure the transition from lab-scale to industrial-scale processes or end-user applications using MOF-based thin films. However, the formation of a large continuous area of MOF films remains a challenge as the formation of defects within the large area film could significantly reduce the selective transport capability of the resulting MOF film, especially for sensitive separation processes such as gas separation. As the technology continuously demands thinner selective films, it is important to develop scale-up methods/strategies that are able to consistently form continuous defect-free MOF films.Spraying is a successful method for forming larger MMM MOF membranes. A simple electrospraying method has been developed by Elsaidi et al. to introduce HKUST-1 based MMM film onto a substrate over a 116 cm2 area, with the possibility of further scale-up.135 This approach can also be combined with the method of forming a highly stable dispersed MOF solution developed by Knebel et al. to ensure increased MOF loading within the larger MMM film.88 The method involves functionalisation of the MOF outer surface with N-heterocyclic carbene ligands, which results in a stable MOF solution for up to a year. This allows for better storage life of the MOF precursor, allowing for easier scale-up into the industrial manufacturing processes.88 Quan et al. reported a one-step reactive extrusion compounding of MOF loaded polymer pellets.136 This method can be combined with in situ MOF formation during polymerisation of the MMM polymer matrix, as explained in the previous section on tuning strategies for mixed matrix membrane thin film, within the extruder.85–87 This can result in the formation of MOF–polymer pellets with high interfacial interaction between the MOF crystals and polymer matrix, leading to better selective transport. The resulting MOF polymer pellet can then be used to create excellent MOF MMMs using existing polymer membrane synthesis methods for industrial-scale manufacturing.
Other than MMMs, spray coating has also successfully been applied for larger-scale production of polycrystalline MOF thin films. Spray coating has been shown to successfully synthesise a large area of different MOFs including ZIF-67, ZIF-8, and HKUST-1, and is predicted to be able to produce even up to 1000 m2 area of the polycrystalline thin film membrane.137,138 Ma et al. demonstrated the capability of a semi-solid dip-coating method coupled with less than 15 minutes of thermal conversion to form a continuous ZIF-8 membrane over a large area of alumina substrate.139 Brown et al. also demonstrated the microfluidic-based contra-diffusion growth method to extend the large scale formation of ZIF-8 polycrystalline thin film beyond a flat sheet to a hollow fibre-based substrate.140 These methods have been proven to be excellent methods to produce MOF thin films over a large area and can be extended to form other functional selective MOFs to allow for the scale-up of other MOF thin films for different targeted applications.
Conclusion
MOFs are a family of porous materials with ultra-high tuning capabilities and are still being extensively researched today. The transport tuning in MOFs has always been a very important field of research due to the modifiable porous nature of MOFs. This results in MOFs finding application in many different processes that require specific transport of molecules through their pores, usually by selective transport. The capability of MOFs to be tuned is still actively studied, but the tuning of MOFs is starting to gravitate towards governing harder to separate molecules. For example, more investigation is starting to focus on the selective transport of molecules with smaller differences in their chemical and physical properties, such as in ethane/ethylene separation and multiple other monovalent ionic separations. Some complex separations such as quantum-based separation have also started to emerge, demonstrating the wide range of MOF's ability to selectively allow specific molecules to pass through when properly tuned. While a lot of focus has been placed on the intracrystalline transport of MOFs, more exploration should also be done on the larger context of film research, which is to focus on the intercrystalline tuning of MOF films. This is because the next phase of research for multiple different applications will require the use of a much larger area of active MOF separation to be more industrially attractive. Thus, to fully utilise the actively researched intercrystalline tuning capabilities of MOFs, an equal amount of research energy should be focused here too. Continuous research towards the tuning of these MOF materials will without a doubt help the advancement of the separation process in many different fields, such as but not limited to, gas separation, ionic separation, process intensification, fuel cell technologies, and catalysis.Conflicts of interest
There are no conflicts to declare.Acknowledgements
M. Y. B. Z. acknowledges the financial support from the Australian Government Research Training Program (RTP) Scholarship. J. H. acknowledges the financial support from the Australian Research Council (FT210100589) and the University of Queensland (2021002771). V. C. acknowledges funding by the Australian Government through the Australian Research Council through its Discovery Program (DP180103874). This research was supported by an AINSE Ltd Early Career Researcher Grant (ECRG).References
- M. Safaei, M. M. Foroughi, N. Ebrahimpoor, S. Jahani, A. Omidi and M. Khatami, TrAC, Trends Anal. Chem., 2019, 118, 401–425 CrossRef CAS.
- E. A. Tomic, J. Appl. Polym. Sci., 1965, 9, 3745–3752 CrossRef CAS.
- X. Zhang, Z. Chen, X. Liu, S. L. Hanna, X. Wang, R. Taheri-Ledari, A. Maleki, P. Li and O. K. Farha, Chem. Soc. Rev., 2020, 49, 7406–7427 RSC.
- O. M. Yaghi and H. Li, J. Am. Chem. Soc., 1995, 117, 10401–10402 CrossRef CAS.
- O. M. Yaghi, G. Li and H. Li, Nature, 1995, 378, 703–706 CrossRef CAS.
- R. N. Widmer, G. I. Lampronti, S. Chibani, C. W. Wilson, S. Anzellini, S. Farsang, A. K. Kleppe, N. P. M. Casati, S. G. MacLeod, S. A. T. Redfern, F.-X. Coudert and T. D. Bennett, J. Am. Chem. Soc., 2019, 141, 9330–9337 CrossRef PubMed.
- N. Zhu, M. J. Lennox, T. Düren and W. Schmitt, Chem. Commun., 2014, 50, 4207–4210 RSC.
- J. Lyu, X. Gong, S.-J. Lee, K. Gnanasekaran, X. Zhang, M. C. Wasson, X. Wang, P. Bai, X. Guo, N. C. Gianneschi and O. K. Farha, J. Am. Chem. Soc., 2020, 142, 4609–4615 CrossRef CAS PubMed.
- P. Zhao, G. I. Lampronti, G. O. Lloyd, M. T. Wharmby, S. Facq, A. K. Cheetham and S. A. T. Redfern, Chem. Mater., 2014, 26, 1767–1769 CrossRef CAS PubMed.
- Y. Chen, X. Huang, S. Zhang, S. Li, S. Cao, X. Pei, J. Zhou, X. Feng and B. Wang, J. Am. Chem. Soc., 2016, 138, 10810–10813 CrossRef CAS PubMed.
- X.-M. Liu, L.-H. Xie and Y. Wu, Inorg. Chem. Front., 2020, 7, 2840–2866 RSC.
- B. Valizadeh, T. N. Nguyen and K. C. Stylianou, Polyhedron, 2018, 145, 1–15 CrossRef CAS.
- Z. Wang, L. Liu, Z. Li, N. Goyal, T. Du, J. He and G. K. Li, Energy Fuels, 2022, 36, 2927–2944 CrossRef CAS.
- J. Fonseca, T. Gong, L. Jiao and H.-L. Jiang, J. Mater. Chem. A, 2021, 9, 10562–10611 RSC.
- J. Hou, A. F. Sapnik and T. D. Bennett, Chem. Sci., 2020, 11, 310–323 RSC.
- T. D. Bennett, J.-C. Tan, Y. Yue, E. Baxter, C. Ducati, N. J. Terrill, H. H.-M. Yeung, Z. Zhou, W. Chen, S. Henke, A. K. Cheetham and G. N. Greaves, Nat. Commun., 2015, 6, 8079 CrossRef CAS PubMed.
- J. Hou, M. L. Ríos Gómez, A. Krajnc, A. McCaul, S. Li, A. M. Bumstead, A. F. Sapnik, Z. Deng, R. Lin, P. A. Chater, D. S. Keeble, D. A. Keen, D. Appadoo, B. Chan, V. Chen, G. Mali and T. D. Bennett, J. Am. Chem. Soc., 2020, 142, 3880–3890 CrossRef CAS PubMed.
- J.-S. M. Lee, K. Otake and S. Kitagawa, Coord. Chem. Rev., 2020, 421, 213447 CrossRef CAS.
- Q. Qian, P. A. Asinger, M. J. Lee, G. Han, K. Mizrahi Rodriguez, S. Lin, F. M. Benedetti, A. X. Wu, W. S. Chi and Z. P. Smith, Chem. Rev., 2020, 120, 8161–8266 CrossRef CAS PubMed.
- R.-B. Lin, S. Xiang, W. Zhou and B. Chen, Chem, 2020, 6, 337–363 CAS.
- B.-M. Jun, Y. A. J. Al-Hamadani, A. Son, C. M. Park, M. Jang, A. Jang, N. C. Kim and Y. Yoon, Sep. Purif. Technol., 2020, 247, 116947 CrossRef CAS.
- J. Li, H. Wang, X. Yuan, J. Zhang and J. W. Chew, Coord. Chem. Rev., 2020, 404, 213116 CrossRef CAS.
- A. Nalaparaju and J. Jiang, Adv. Sci., 2021, 8, 2003143 CrossRef CAS PubMed.
- X. Li, Y. Liu, J. Wang, J. Gascon, J. Li and B. Van der Bruggen, Chem. Soc. Rev., 2017, 46, 7124–7144 RSC.
- Y. Chen, V. Lykourinou, T. Hoang, L.-J. Ming and S. Ma, Inorg. Chem., 2012, 51, 9156–9158 CrossRef CAS PubMed.
- O. M. Yaghi, ACS Cent. Sci., 2019, 5, 1295–1300 CrossRef CAS PubMed.
- O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705–714 CrossRef CAS PubMed.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- V. Guillerm, D. Kim, J. F. Eubank, R. Luebke, X. Liu, K. Adil, M. S. Lah and M. Eddaoudi, Chem. Soc. Rev., 2014, 43, 6141–6172 RSC.
- C. Zhu, Y. Peng and W. Yang, GreenChE, 2021, 2, 17–26 Search PubMed.
- X. Wang, Q. Lyu, T. Tong, K. Sun, L.-C. Lin, C. Y. Tang, F. Yang, M. D. Guiver, X. Quan and Y. Dong, Nat. Commun., 2022, 13, 266 CrossRef CAS PubMed.
- Y. Wang, H. Jin, Q. Ma, K. Mo, H. Mao, A. Feldhoff, X. Cao, Y. Li, F. Pan and Z. Jiang, Angew. Chem., Int. Ed., 2020, 59, 4365–4369 CrossRef CAS PubMed.
- R. Wei, X. Li, Z. Zhou, C. Chen, Y. Yuan, Z. Li, X. Li, X. Dong, D. Lu, Y. Han and Z. Lai, Sci. Adv., 2022, 8, eabm6741 CrossRef CAS PubMed.
- P. Zhao, N. Liu, C. Jin, H. Chen, Z. Zhang, L. Zhao, P. Cheng and Y. Chen, Inorg. Chem., 2019, 58, 8787–8792 CrossRef CAS PubMed.
- S. Biswas and P. Van Der Voort, Eur. J. Inorg. Chem., 2013, 2013, 2154–2160 CrossRef CAS.
- F. Salles and J. Zajac, Nanomaterials, 2021, 11, 2245 CrossRef CAS PubMed.
- Z. Ji, H. Wang, S. Canossa, S. Wuttke and O. M. Yaghi, Adv. Funct. Mater., 2020, 30, 2000238 CrossRef CAS.
- D. Bahamon, W. Anlu, S. Builes, M. Khaleel and L. F. Vega, Front. Chem., 2021, 8, 574622 CrossRef PubMed.
- R. W. Flaig, T. M. Osborn Popp, A. M. Fracaroli, E. A. Kapustin, M. J. Kalmutzki, R. M. Altamimi, F. Fathieh, J. A. Reimer and O. M. Yaghi, J. Am. Chem. Soc., 2017, 139, 12125–12128 CrossRef CAS PubMed.
- Z. H. Rada, H. R. Abid, J. Shang, H. Sun, Y. He, P. Webley, S. Liu and S. Wang, Ind. Eng. Chem. Res., 2016, 55, 7924–7932 CrossRef CAS.
- J. Pires, J. Fernandes, K. Dedecker, J. R. B. Gomes, G. Pérez-Sánchez, F. Nouar, C. Serre and M. L. Pinto, ACS Appl. Mater. Interfaces, 2019, 11, 27410–27421 CrossRef CAS PubMed.
- X.-W. Gu, J.-X. Wang, E. Wu, H. Wu, W. Zhou, G. Qian, B. Chen and B. Li, J. Am. Chem. Soc., 2022, 144, 2614–2623 CrossRef CAS PubMed.
- X. Wang, L. Li, Y. Wang, J.-R. Li and J. Li, CrystEngComm, 2017, 19, 1729–1737 RSC.
- H. Zhang, J. James, M. Zhao, Y. Yao, Y. Zhang, B. Zhang and Y. S. Lin, J. Membr. Sci., 2017, 532, 1–8 CrossRef CAS.
- L. Xiang, L. Sheng, C. Wang, L. Zhang, Y. Pan and Y. Li, Adv. Mater., 2017, 29, 1606999 CrossRef PubMed.
- F. Zhou, J. Zhou, X. Gao, C. Kong and L. Chen, RSC Adv., 2017, 7, 3713–3719 RSC.
- C. L. Luu, T. T. V. Nguyen, T. Nguyen and T. C. Hoang, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2015, 6, 025004 Search PubMed.
- A. Qiao, T. D. Bennett, H. Tao, A. Krajnc, G. Mali, C. M. Doherty, A. W. Thornton, J. C. Mauro, G. N. Greaves and Y. Yue, Sci. Adv., 2018, 4, eaao6827 CrossRef PubMed.
- N. Ma and S. Horike, Chem. Rev., 2022, 122, 4163–4203 CrossRef CAS PubMed.
- T. D. Bennett and S. Horike, Nat. Rev. Mater., 2018, 3, 431–440 CrossRef.
- L. Frentzel-Beyme, M. Kloß, P. Kolodzeiski, R. Pallach and S. Henke, J. Am. Chem. Soc., 2019, 141, 12362–12371 CrossRef CAS PubMed.
- S. Horike, S. S. Nagarkar, T. Ogawa and S. Kitagawa, Angew. Chem., Int. Ed., 2020, 59, 6652–6664 CrossRef CAS PubMed.
- M. Inukai, Y. Nishiyama, K. Honjo, C. Das, S. Kitagawa and S. Horike, Chem. Commun., 2019, 55, 8528–8531 RSC.
- Z. H. Syed, F. Sha, X. Zhang, D. M. Kaphan, M. Delferro and O. K. Farha, ACS Catal., 2020, 10, 11556–11566 CrossRef CAS.
- S. Liu, Q. Dong, Y. Zhou, S. Wang and J. Duan, Dalton Trans., 2020, 49, 17093–17105 RSC.
- S. Mukherjee, B. Manna, A. V. Desai, Y. Yin, R. Krishna, R. Babarao and S. K. Ghosh, Chem. Commun., 2016, 52, 8215–8218 RSC.
- Y. He, R. Krishna and B. Chen, Energy Environ. Sci., 2012, 5, 9107–9120 RSC.
- S. J. Geier, J. A. Mason, E. D. Bloch, W. L. Queen, M. R. Hudson, C. M. Brown and J. R. Long, Chem. Sci., 2013, 4, 2054–2061 RSC.
- J. H. Choe, H. Kim and C. S. Hong, Mater. Chem. Front., 2021, 5, 5172–5185 RSC.
- C.-H. Yeh, A. H. Khan, T. Miyazaki and J.-C. Jiang, Phys. Chem. Chem. Phys., 2021, 23, 12270–12279 RSC.
- D.-J. Lee, Q. Li, H. Kim and K. Lee, Microporous Mesoporous Mater., 2012, 163, 169–177 CrossRef CAS.
- N. Wang, A. Mundstock, Y. Liu, A. Huang and J. Caro, Chem. Eng. Sci., 2015, 124, 27–36 CrossRef CAS.
- F. Luo, C. Yan, L. Dang, R. Krishna, W. Zhou, H. Wu, X. Dong, Y. Han, T.-L. Hu, M. O'Keeffe, L. Wang, M. Luo, R.-B. Lin and B. Chen, J. Am. Chem. Soc., 2016, 138, 5678–5684 CrossRef CAS PubMed.
- Y. Du, B. Wooler, M. Nines, P. Kortunov, C. S. Paur, J. Zengel, S. C. Weston and P. I. Ravikovitch, J. Am. Chem. Soc., 2015, 137, 13603–13611 CrossRef CAS PubMed.
- S. A. Moggach, T. D. Bennett and A. K. Cheetham, Angew. Chem., Int. Ed., 2009, 48, 7087–7089 CrossRef CAS PubMed.
- F. Formalik, A. V. Neimark, J. Rogacka, L. Firlej and B. Kuchta, J. Colloid Interface Sci., 2020, 578, 77–88 CrossRef CAS PubMed.
- A. Arami-Niya, G. Birkett, Z. Zhu and T. E. Rufford, J. Mater. Chem. A, 2017, 5, 21389–21399 RSC.
- D. Fairen-Jimenez, S. A. Moggach, M. T. Wharmby, P. A. Wright, S. Parsons and T. Düren, J. Am. Chem. Soc., 2011, 133, 8900–8902 CrossRef CAS PubMed.
- F.-X. Coudert, M. Jeffroy, A. H. Fuchs, A. Boutin and C. Mellot-Draznieks, J. Am. Chem. Soc., 2008, 130, 14294–14302 CrossRef CAS PubMed.
- A. J. Fletcher, K. M. Thomas and M. J. Rosseinsky, J. Solid State Chem., 2005, 178, 2491–2510 CrossRef CAS.
- C. Gücüyener, J. van den Bergh, J. Gascon and F. Kapteijn, J. Am. Chem. Soc., 2010, 132, 17704–17706 CrossRef PubMed.
- J. Hou, C. W. Ashling, S. M. Collins, A. Krajnc, C. Zhou, L. Longley, D. N. Johnstone, P. A. Chater, S. Li, M.-V. Coulet, P. L. Llewellyn, F.-X. Coudert, D. A. Keen, P. A. Midgley, G. Mali, V. Chen and T. D. Bennett, Nat. Commun., 2019, 10, 2580 CrossRef PubMed.
- R.-B. Lin, L. Li, H.-L. Zhou, H. Wu, C. He, S. Li, R. Krishna, J. Li, W. Zhou and B. Chen, Nat. Mater., 2018, 17, 1128–1133 CrossRef CAS PubMed.
- Q. Hou, S. Zhou, Y. Wei, J. Caro and H. Wang, J. Am. Chem. Soc., 2020, 142, 9582–9586 CAS.
- X. Yu, N. S. Grundish, J. B. Goodenough and A. Manthiram, ACS Appl. Mater. Interfaces, 2021, 13, 24662–24669 CrossRef CAS PubMed.
- Q. Xu, X. Zhang, S. Zeng, L. Bai and S. Zhang, ACS Sustainable Chem. Eng., 2019, 7, 7892–7899 CrossRef CAS.
- Z. Zhang, X. Jia, Y. Sun, X. Guo, H. Huang and C. Zhong, GreenChE, 2021, 2, 104–110 Search PubMed.
- J. Sánchez-Laínez, A. Veiga, B. Zornoza, S. R. G. Balestra, S. Hamad, A. R. Ruiz-Salvador, S. Calero, C. Téllez and J. Coronas, J. Mater. Chem. A, 2017, 5, 25601–25608 RSC.
- H. T. Kwon, H.-K. Jeong, A. S. Lee, H. S. An and J. S. Lee, J. Am. Chem. Soc., 2015, 137, 12304–12311 CrossRef CAS PubMed.
- S. H. Yuan, A. P. Isfahani, T. Yamamoto, A. Muchtar, C. Y. Wu, G. Huang, Y. C. You, E. Sivaniah, B. K. Chang and B. Ghalei, Small Methods, 2020, 4, 2000021 CrossRef CAS.
- M. Abdollahzadeh, M. Chai, E. Hosseini, M. Zakertabrizi, M. Mohammad, H. Ahmadi, J. Hou, S. Lim, A. Habibnejad Korayem, V. Chen, M. Asadnia and A. Razmjou, Adv. Mater., 2022, 34, 2107878 CrossRef CAS PubMed.
- A. Razmjou, M. Asadnia, E. Hosseini, A. Habibnejad Korayem and V. Chen, Nat. Commun., 2019, 10, 5793 CrossRef CAS PubMed.
- V. Muthukumaraswamy Rangaraj, M. A. Wahab, K. S. K. Reddy, G. Kakosimos, O. Abdalla, E. P. Favvas, D. Reinalda, F. Geuzebroek, A. Abdala and G. N. Karanikolos, Front. Chem., 2020, 8, 534 CrossRef PubMed.
- J. E. Bachman, Z. P. Smith, T. Li, T. Xu and J. R. Long, Nat. Mater., 2016, 15, 845–849 CrossRef CAS PubMed.
- R. Lin, L. Ge, L. Hou, E. Strounina, V. Rudolph and Z. Zhu, ACS Appl. Mater. Interfaces, 2014, 6, 5609–5618 CrossRef CAS PubMed.
- Q. Qian, A. X. Wu, W. S. Chi, P. A. Asinger, S. Lin, A. Hypsher and Z. P. Smith, ACS Appl. Mater. Interfaces, 2019, 11, 31257–31269 CrossRef CAS PubMed.
- Q. Qian, W. S. Chi, G. Han and Z. P. Smith, Ind. Eng. Chem. Res., 2020, 59, 5432–5438 CrossRef CAS.
- A. Knebel, A. Bavykina, S. J. Datta, L. Sundermann, L. Garzon-Tovar, Y. Lebedev, S. Durini, R. Ahmad, S. M. Kozlov, G. Shterk, M. Karunakaran, I. D. Carja, D. Simic, I. Weilert, M. Klüppel, U. Giese, L. Cavallo, M. Rueping, M. Eddaoudi, J. Caro and J. Gascon, Nat. Mater., 2020, 19, 1346–1353 CrossRef CAS PubMed.
- R. Lin, J. Hou, M. Li, Z. Wang, L. Ge, S. Li, S. Smart, Z. Zhu, T. D. Bennett and V. Chen, Chem. Commun., 2020, 56, 3609–3612 RSC.
- H. Bux, C. Chmelik, R. Krishna and J. Caro, J. Membr. Sci., 2011, 369, 284–289 CrossRef CAS.
- H. Bux, F. Liang, Y. Li, J. Cravillon, M. Wiebcke and J. Caro, J. Am. Chem. Soc., 2009, 131, 16000–16001 CrossRef CAS PubMed.
- M. C. McCarthy, V. Varela-Guerrero, G. V. Barnett and H.-K. Jeong, Langmuir, 2010, 26, 14636–14641 CrossRef CAS PubMed.
- Y. Xiao, W. Zhang, Y. Jiao, Y. Xu and H. Lin, J. Membr. Sci., 2021, 624, 119101 CrossRef CAS.
- D. Zacher, O. Shekhah, C. Wöll and R. A. Fischer, Chem. Soc. Rev., 2009, 38, 1418–1429 RSC.
- M. Mohammad, M. Lisiecki, K. Liang, A. Razmjou and V. Chen, Appl. Mater. Today, 2020, 21, 100884 CrossRef.
- J. Hou, P. D. Sutrisna, Y. Zhang and V. Chen, Angew. Chem., Int. Ed., 2016, 55, 3947–3951 CrossRef CAS PubMed.
- J. Hou, P. D. Sutrisna, T. Wang, S. Gao, Q. Li, C. Zhou, S. Sun, H.-C. Yang, F. Wei, M. T. Ruggiero, J. A. Zeitler, A. K. Cheetham, K. Liang and V. Chen, ACS Appl. Mater. Interfaces, 2019, 11, 5570–5577 CrossRef CAS PubMed.
- M. Chai, A. Razmjou and V. Chen, J. Membr. Sci., 2021, 623, 118986 CrossRef CAS.
- M. Chai, S. Razavi Bazaz, R. Daiyan, A. Razmjou, M. Ebrahimi Warkiani, R. Amal and V. Chen, Chem. Eng. J., 2021, 426, 130856 CrossRef CAS.
- C. Chen, A. Ozcan, A. O. Yazaydin and B. P. Ladewig, J. Membr. Sci., 2019, 575, 209–216 CrossRef CAS.
- J. S. Tuninetti, M. Rafti, A. Andrieu-Brunsen and O. Azzaroni, Microporous Mesoporous Mater., 2016, 220, 253–257 CrossRef CAS.
- V. Rubio-Giménez, M. Galbiati, J. Castells-Gil, N. Almora-Barrios, J. Navarro-Sánchez, G. Escorcia-Ariza, M. Mattera, T. Arnold, J. Rawle, S. Tatay, E. Coronado and C. Martí-Gastaldo, Adv. Mater., 2018, 30, 1704291 CrossRef PubMed.
- E. M. Johnson, S. Ilic and A. J. Morris, ACS Cent. Sci., 2021, 7, 445–453 CrossRef CAS PubMed.
- G. Jiang, C. Qu, F. Xu, E. Zhang, Q. Lu, X. Cai, S. Hausdorf, H. Wang and S. Kaskel, Adv. Funct. Mater., 2021, 31, 2104300 CrossRef CAS.
- J. Zhu, H. Li, J. Hou, J. Liu, Y. Zhang and B. Van der Bruggen, AIChE J., 2020, 66, e16935 CAS.
- Y. Sun, C. Song, X. Guo and Y. Liu, ACS Appl. Mater. Interfaces, 2020, 12, 4494–4500 CrossRef CAS PubMed.
- J. Li, J. Wang, Q. Li, M. Zhang, J. Li, C. Sun, S. Yuan, X. Feng and B. Wang, Angew. Chem., Int. Ed., 2021, 60, 21304–21309 CrossRef CAS PubMed.
- K. Zhang, G.-H. Wen, S.-S. Bao, L.-Q. Wu and J.-G. Jia, ACS Appl. Energy Mater., 2020, 3, 8198–8204 CrossRef CAS.
- S. Eslava, L. Zhang, S. Esconjauregui, J. Yang, K. Vanstreels, M. R. Baklanov and E. Saiz, Chem. Mater., 2013, 25, 27–33 CrossRef CAS.
- K. Yang, Y. Ban and W. Yang, J. Membr. Sci., 2021, 639, 119771 CrossRef CAS.
- Y. Peng, Y. Li, Y. Ban, H. Jin, W. Jiao, X. Liu and W. Yang, Science, 2014, 346, 1356 CrossRef CAS PubMed.
- H. Huang, Y. Ying and X. Peng, J. Mater. Chem. A, 2014, 2, 13772–13782 RSC.
- T. Liu, L. Tian, N. Graham, B. Yang, W. Yu and K. Sun, Environ. Sci. Technol., 2019, 53, 11949–11959 CrossRef CAS PubMed.
- S. Valizadeh, L. Naji and M. Karimi, Sep. Purif. Technol., 2021, 263, 118392 CrossRef CAS.
- Y. Jiang, W. Ma, Y. Qiao, Y. Xue, J. Lu, J. Gao, N. Liu, F. Wu, P. Yu, L. Jiang and L. Mao, Angew. Chem., Int. Ed., 2020, 59, 12795–12799 CrossRef CAS PubMed.
- B. Joarder, J.-B. Lin, Z. Romero and G. K. H. Shimizu, J. Am. Chem. Soc., 2017, 139, 7176–7179 CrossRef CAS PubMed.
- A. Sorrenti, L. Jones, S. Sevim, X. Cao, A. J. deMello, C. Martí-Gastaldo and J. Puigmartí-Luis, J. Am. Chem. Soc., 2020, 142, 9372–9381 CrossRef CAS PubMed.
- X. Cheng, S. Yang, B. Cao, X. Tao and Z. Chen, Adv. Funct. Mater., 2020, 30, 1905021 CrossRef CAS.
- A. M. Bumstead, I. Pakamorė, K. D. Richards, M. F. Thorne, S. S. Boyadjieva, C. Castillo-Blas, L. N. McHugh, A. F. Sapnik, D. S. Keeble, D. A. Keen, R. C. Evans, R. S. Forgan and T. D. Bennett, Chem. Mater., 2022, 34, 2187–2196 CrossRef CAS PubMed.
- J. Hou, P. Chen, A. Shukla, A. Krajnc, T. Wang, X. Li, R. Doasa, L. H. G. Tizei, B. Chan, D. N. Johnstone, R. Lin, T. U. Schülli, I. Martens, D. Appadoo, M. S. Ari, Z. Wang, T. Wei, S. C. Lo, M. Lu, S. Li, E. B. Namdas, G. Mali, A. K. Cheetham, S. M. Collins, V. Chen, L. Wang and T. D. Bennett, Science, 2021, 374, 621–625 CrossRef CAS PubMed.
- V. Pascanu, G. González Miera, A. K. Inge and B. Martín-Matute, J. Am. Chem. Soc., 2019, 141, 7223–7234 CrossRef CAS PubMed.
- B. Lee, K. Li, H. S. Yoon, J. Yoon, Y. Mok, Y. Lee, H. H. Lee and Y. H. Kim, Sci. Rep., 2016, 6, 28052 CrossRef CAS PubMed.
- J. Bhagavathsingh, R. Pugulanthi, P. Devi, A. Angamuthu, D. K. Janapala, N. Moses, V. Sivasankaran, S. P. Anthony and R. Venkatasan, MRS Commun., 2022, 12, 37–44 CrossRef CAS.
- B. Song, C. Sizemore, L. Li, X. Huang, Z. Lin, K. Moon and C.-P. Wong, J. Mater. Chem. A, 2015, 3, 21789–21796 RSC.
- S. Liang, L. Mu, L. Chen, J. Jiang, Y. Yang and H. Fang, Chem.–Asian J., 2020, 15, 2346–2349 CrossRef CAS PubMed.
- Y. Yang, L. Mu, L. Chen, G. Shi and H. Fang, Phys. Chem. Chem. Phys., 2019, 21, 7623–7629 RSC.
- K. Ikigaki, K. Okada and M. Takahashi, ACS Appl. Nano Mater., 2021, 4, 3467–3475 CrossRef CAS.
- K. Ikigaki, K. Okada, Y. Tokudome, T. Toyao, P. Falcaro, C. J. Doonan and M. Takahashi, Angew. Chem., Int. Ed., 2019, 58, 6886–6890 CrossRef CAS PubMed.
- S. A. FitzGerald, C. J. Pierce, J. L. C. Rowsell, E. D. Bloch and J. A. Mason, J. Am. Chem. Soc., 2013, 135, 9458–9464 CrossRef CAS PubMed.
- G. Han, Y. Gong, H. Huang, D. Cao, X. Chen, D. Liu and C. Zhong, ACS Appl. Mater. Interfaces, 2018, 10, 32128–32132 CrossRef CAS PubMed.
- D. Liu, W. Wang, J. Mi, C. Zhong, Q. Yang and D. Wu, Ind. Eng. Chem. Res., 2012, 51, 434–442 CrossRef CAS.
- B. Paschke, D. Denysenko, B. Bredenkötter, G. Sastre, A. Wixforth and D. Volkmer, Chem.–Eur. J., 2019, 25, 10803–10807 CrossRef CAS PubMed.
- I. Savchenko, A. Mavrandonakis, T. Heine, H. Oh, J. Teufel and M. Hirscher, Microporous Mesoporous Mater., 2015, 216, 133–137 CrossRef CAS.
- Y. Si, X. He, J. Jiang, Z. Duan, W. Wang and D. Yuan, Nano Res., 2021, 14, 518–525 CrossRef CAS.
- S. K. Elsaidi, M. Ostwal, L. Zhu, A. Sekizkardes, M. H. Mohamed, M. Gipple, J. R. McCutcheon and D. Hopkinson, RSC Adv., 2021, 11, 25658–25663 RSC.
- Y. Quan, R. Shen, R. Ma, Z. Zhang and Q. Wang, ACS Sustainable Chem. Eng., 2022, 10, 7216–7222 CrossRef CAS.
- S. Hurrle, S. Friebe, J. Wohlgemuth, C. Wöll, J. Caro and L. Heinke, Chem.–Eur. J., 2017, 23, 2294–2298 CrossRef CAS PubMed.
- Z. Chen, R. Wang, T. Ma, J.-L. Wang, Y. Duan, Z.-Z. Dai, J. Xu, H.-J. Wang, J. Yuan, H.-L. Jiang, Y.-W. Yin, X.-G. Li, M.-R. Gao and S.-H. Yu, Angew. Chem., Int. Ed., 2021, 60, 14124–14130 CrossRef CAS PubMed.
- Q. Ma, K. Mo, S. Gao, Y. Xie, J. Wang, H. Jin, A. Feldhoff, S. Xu, J. Y. S. Lin and Y. Li, Angew. Chem., Int. Ed., 2020, 59, 21909–21914 CrossRef CAS PubMed.
- A. J. Brown, N. A. Brunelli, K. Eum, F. Rashidi, J. R. Johnson, W. J. Koros, C. W. Jones and S. Nair, Science, 2014, 345, 72–75 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |