Horizontal expansion of biicosahedral M13-based nanoclusters: resolving decades-long questions†
Chuanjun
Zhou‡
ab,
Peiyao
Pan‡
ab,
Xiao
Wei
ab,
Zidong
Lin
c,
Cheng
Chen
c,
Xi
Kang
 *ab and
Manzhou
Zhu
*ab and
Manzhou
Zhu
 *ab
*ab
aDepartment of Chemistry and Centre for Atomic Engineering of Advanced Materials, Anhui Province Key Laboratory of Chemistry for Inorganic/Organic Hybrid Functionalized Materials, Anhui University, Hefei 230601, P. R. China. E-mail: kangxi_chem@ahu.edu.cn; zmz@ahu.edu.cn
bKey Laboratory of Structure and Functional Regulation of Hybrid Materials, Ministry of Education, Anhui University, Hefei 230601, P. R. China
cInstitutes of Physical Science and Information Technology, Anhui University, Hefei, Anhui 230601, P. R. China
First published on 23rd September 2022
Abstract
For metal nanoclusters with the “cluster of clusters” intramolecular evolution pattern, most efforts have been made towards the vertical superposition of icosahedral nanobuilding blocks (e.g., from mono-icosahedral Au13 to bi-icosahedral Au25 and tri-icosahedral Au37), while the horizontal expansion of these rod-shaped multi-icosahedral aggregates was largely neglected. We herein report the horizontal expansion of the biicosahedral M25 cluster framework, yielding an [Au19Ag12(S-Adm)6(DPPM)6Cl7]2+ nanocluster that contains an Au13Ag12 kernel and six Au1(DPPM)1(S-Adm)1 peripheral wings. The structural determination of [Au19Ag12(S-Adm)6(DPPM)6Cl7]2+ resolved a decades-long question towards rod-shaped multi-icosahedral aggregates: how to load bidentate phosphine and bulky thiol ligands onto the nanocluster framework? The structural comparison between [Au19Ag12(S-Adm)6(DPPM)6Cl7]2+ and previously reported [Au13Ag12(PPh3)10Cl8]2+ or [Au13Ag12(SR)5(PPh3)10Cl2]2+ rationalized the unique packing of Au1(DPPM)1(S-Adm)1 motif structures on the surface of the former nanocluster. Overall, this work presents the horizontal expansion of rod-shaped multi-icosahedral nanoclusters, which provides new insights into the preparation of novel icosahedron-based aggregates with both vertically and horizontally growing extensions.
New conceptsThe “cluster of clusters” evolution was one of the most fascinating features of intramolecular assembled nanoclusters. Hundreds of clusters have been constructed by systematically aggregating icosahedral M13 nanobuilding blocks, and these rod-shaped aggregates were extensively studied owing to the strong electron coupling between substituent icosahedral units. However, most efforts were made towards the vertical superposition of icosahedral nanobuilding blocks (e.g., from mono-icosahedral Au13 to bi-icosahedral Au25 and tri-icosahedral Au37), while the horizontal expansion of these rod-shaped multi-icosahedral aggregates was largely neglected. In this work, based on a newly developed M31 nanocluster template, the horizontal expansion of the biicosahedral M25 dimer has been accomplished. The atomic-level structural determination of the Au19Ag12(S-Adm)6(DPPM)6Cl7 cluster resolved two decades-long questions for rod-shaped multi-icosahedral aggregates, including (i) how to load bidentate phosphine ligands onto the nanocluster shoulder and (ii) how to arrange bulky thiol ligands onto the nanocluster waist. This work significantly broadens the research towards the “cluster of clusters” evolution pattern of metal nanoclusters. |
1. Introduction
Metal nanoclusters have served as an emerging class of modular nanomaterials owing to their atomically precise structures and intriguing physical–chemical properties.1–8 Besides, due to their programmably structure-dependent performances, metal nanoclusters or cluster-based hybrids have been customized for widespread applications such as catalysis, chemical sensors, drug delivery, biological imaging, and so on.9–18 Typically, the structure of nanoclusters is composed of an internal metallic kernel and a peripheral ligand shell.19–30 The structural determination of the metallic kernels of clusters at the atomic level has considerably inspired nanoscientists on a long-lasting question in nanoscience: what are the evolution modes from small-sized metallic complexes to nanoclusters or from nanoclusters to large-sized nanoparticles?1,31 Indeed, such a question has puzzled nanoscientists for decades because of two objective deficiencies of metal nanoparticles: the nonuniform molecule size and the uncertain surface environment.With the continuous accumulation of the determined structures of metal nanoclusters, several intracluster evolution patterns have been observed experimentally, including cluster of clusters, kernel fusion, interpenetration, shell-by-shell, layer-by-layer, tetrahedron-based vertex-sharing, etc.31 Among these patterns, the “cluster of clusters”, which is first proposed in the 1980s, represents one of the most fascinating features of intramolecular assembled nanoclusters.32–35 The icosahedral M13 nanobuilding blocks can be aggregated by a vertex-sharing mode (e.g., rod-shaped Au25, Pt2Ag23, Au37, Pt3Ag44, and Ag61,36–40 and pan-shaped Au34Cu3, Pt3Ag33, and Au60),41–43 a face-sharing mode (e.g., Au38 and Au27Cd1),44,45 or an interpenetrating mode (e.g., Au8Ag55, Au8Ag57, and Au156).46,47
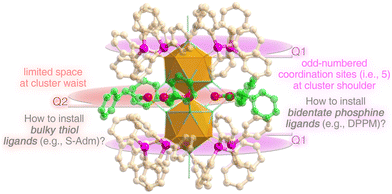
Among the cluster of clusters, rod-shaped aggregates of M13 (M = Au/Ag/Cu/Pt/Pd/Ni) nanobuilding blocks have been extensively studied because of the strong electron coupling between substituent icosahedral units.31–35,40 However, most efforts were made towards the vertical superposition of icosahedral units (e.g., from mono-icosahedral Au13 to bi-icosahedral Au25 and tri-icosahedral Au37), while the horizontal expansion of these rod-shaped multi-icosahedral aggregates is largely neglected.31 Besides, several questions remain unsolved regarding these nanoclusters. For example, for rod-shaped Au25(PPh3)10(SR)5Cl2 with five monodentate PPh3 at each shoulder and five thiol ligands at the waist,36 the outcome remains mysterious by substituting the monodentate PPh3 with bidentate phosphine ligands or regulating the SR as bulky thiol ligands in terms of two objective contradictions: (i) the odd-numbered coordination sites at the nanocluster shoulder (i.e., 5) versus the even-numbered coordination ability (i.e., 2n) of the bidentate phosphines, and (ii) the limited space at the nanocluster waist versus the large steric hindrance of bulky thiols. The problem solving towards such rod-shaped cluster models calls for more efforts.
Herein, the horizontal expansion of the rod-shaped biicosahedral M25 nanocluster was accomplished, yielding an [Au19Ag12(S-Adm)6(DPPM)6Cl7]2+ nanocluster (Au19Ag12 for short; S-Adm = 1-adamantanethiol; DPPM = bis(diphenylphosphino)methane) that consists of a biicosahedral Au13Ag12 kernel, seven chlorine ligands, and six Au1(DPPM)1(S-Adm)1 peripheral wings. Compared with the rod-shaped [Au13Ag12(SR)5(PPh3)10Cl2]2+ or [Au13Ag12(PPh3)10Cl8]2+ (Au13Ag12-S or Au13Ag12-Cl for short), the horizontal extension of unique Au1(DPPM)1(S-Adm)1 surface units in Au19Ag12 was rationalized by the substitution of monodentate PPh3 with bidentate DPPM as well as the introduction of bulky S-Adm ligands. Control experiments were carried out to validate the decisive roles of both bidentate DPPM and bulky S-Adm in constructing the Au19Ag12 nanocluster. In this context, Au19Ag12 has served as a cluster model for resolving the abovementioned longstanding questions in rod-shaped nanoclusters with the “cluster of clusters” intramolecular evolution pattern.
2. Results and discussion
The rod-shaped Au13Ag12-S nanocluster follows a biicosahedral configuration via sharing a vertex Au atom between two Au7Ag6 nanobuilding blocks.48 The Au atoms at the nanocluster shoulder are capped by monodentate PPh3 ligands, and the Ag atoms at the nanocluster waist are stabilized by SC2H4Ph ligands. Two longstanding questions remain unsolved for this nanocluster template (Scheme 1).(i) Altering monodentate PPh3 into bidentate phosphine ligands at the nanocluster shoulder (Scheme 1, Q1): five PPh3 ligands are anchored onto each cluster shoulder via Au–P interactions, which puts forward a contradiction between the odd-numbered coordination sites (i.e., 5) and the even-numbered coordination ability (i.e., 2n) of the bidentate phosphines (e.g., DPPM).
(ii) Arranging bulky thiol ligands into the limited space at the nanocluster waist (Scheme 1, Q2): five –SC2H4Ph ligands are arranged at the waist of Au13Ag12-Svia Ag–S–Ag interactions. The waist space of the nanocluster is limited by considering the steric hindrance of the M25 metallic kernel from the inside out as well as the hindrance of the phosphine ligands from both sides to the middle. Accordingly, the thiol ligands in reported biicosahedral M25 nanoclusters are almost accompanied by alkyl chains or benzene rings with relatively small steric resistances. However, it remains challenging to install bulky thiol ligands (e.g., –S-Adm) into this limited space.
Collectively, it is unclear how the nanocluster framework undergoes self-adaption in the presence of bidentate phosphine ligands or bulky thiol ligands, and a comprehensive understanding of these longstanding questions calls for a new nanocluster template established from the biicosahedral M25 nanostructure.
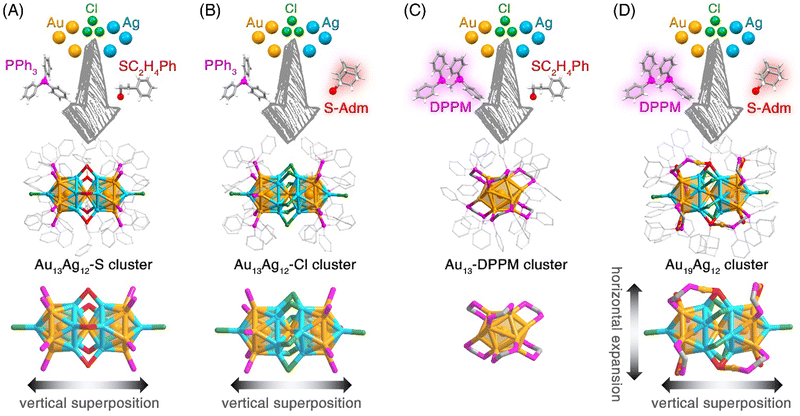
Several efforts have been made to install bidentate DPPM or bulky S-Adm ligands onto the M25 nanocluster surface (Scheme 2). The rod-shaped Au13Ag12-S nanocluster was synthesized by directly reducing the Au-Ag-Cl-PPh3-SC2H4Ph complexes with NaBH4 (Scheme 2A). A set of control experiments were performed (Scheme 2B–D).
(i) The single thiol ligand control (Scheme 2B): the SC2H4Ph was substituted by the bulky S-Adm ligand, while the other experimental conditions remained unchanged. The Au13Ag12-Cl nanocluster was obtained without surface thiol ligands.49 The Au13Ag12-Cl nanocluster followed a similar configuration to Au13Ag12-S with the only difference of waist stabilizers. For Au13Ag12-Cl, six chlorine ligands stabilized the biicosahedral configuration via Ag–Cl–Ag interactions, playing the same role as SC2H4Ph ligands in Au13Ag12-S. In other words, compared with SC2H4Ph, the bulky thiol ligand was challenging to be installed at the nanocluster waist, which was probably due to the large steric hindrance of S-Adm relative to the limited space.
(ii) The single phosphine ligand control (Scheme 2C): the substitution of PPh3 with DPPM yielded a homogold [Au13(DPPM)6]3+ (Au13-DPPM for short) nanocluster. Accordingly, the biicosahedral configuration of M25 was broken to give rise to a mono-icosahedral nanocluster. The twelve surface Au atoms of the Au13-DPPM nanocluster were capped by six DPPM ligands with an Au–DPPM–Au bonding mode. The obtained Au13-DPPM nanocluster was the same as the previously reported one that was prepared via a thiol addition-induced synthetic procedure.50 Of note, the Ag component was absent in the final Au13-DPPM. Such an absence might result from the instability of the possible AgxAu13−x(DPPM)6 products that only the homogold Au13-DPPM nanocluster survived.
(iii) The dual thiol and phosphine ligand control (Scheme 2D): the PPh3 and SC2H4Ph were substituted by bidentate DPPM and bulky S-Adm ligands simultaneously, which produced an Au19Ag12 nanocluster. The Au19Ag12 contained an M25 biicosahedron that was co-stabilized by Cl, DPPM, and S-Adm ligands. The rod-shaped M25 kernel was maintained by comparing the overall structure of Au19Ag12 to that of Au13Ag12-S or Au13Ag12-Cl, while the surface DPPM–Au–SAdm motif structures followed a horizontal expansion mode. Collectively, based on the horizontally expanded biicosahedral Au19Ag12 nanocluster, both bidentate phosphine and bulky thiol ligands were installed onto the surface of rod-shaped nanoclusters.
The ESI-MS results of the Au19Ag12 nanocluster showed two groups of mass signals around 2853 and 4297 Da, corresponding to [Au31−xAgx(S-Adm)6(DPPM)6Cl7]3+ and [Au31−xAgx(S-Adm)6(DPPM)6Cl7]2+ (x = 10–14), respectively (Fig. S1, ESI†). The excellent agreement of the experimental and simulated isotope patterns illustrated that the measured formulas of the largest peaks within these two groups were [Au19Ag12(S-Adm)6(DPPM)6Cl7]3+ and [Au19Ag12(S-Adm)6(DPPM)6Cl7]2+ (Fig. S1, insets, ESI†). In addition, ESI-MS results demonstrated that several metal positions of the Au19Ag12 nanocluster framework were jointly occupied by Au/Ag, which was reminiscent of the composition of Au13Ag12-S.48 By combining the ESI-MS results and the crystal data, such Au31−xAgx(S-Adm)6(DPPM)6Cl7 nanoclusters are called Au19Ag12 in this work (see Tables S5–S7, ESI,† for more details of the naming of the three alloy nanoclusters in this work). Besides, previous studies of M13-based cluster aggregates demonstrated that each icosahedral unit tended to hold eight free valence electrons within the nanocluster framework.31 Accordingly, for the [Au19Ag12(S-Adm)6(DPPM)6Cl7]2+ mass signal, the nominal electron count of the Au19Ag12 nanocluster was determined as 16 (i.e., 31(Au + Ag) − 6(S-Adm) − 7(Cl) − 2(charge) = 16e),51 tallying with the two icosahedral units in the cluster framework. The presence of [Au31−xAgx(S-Adm)6(DPPM)6Cl7]3+ signals originated from the loss of an electron from the nanocluster molecule, which has also been observed previously in Ag44(SR)30 and Pt1Ag31(SR)16(DPPM)3Cl3 nanoclusters.52,53
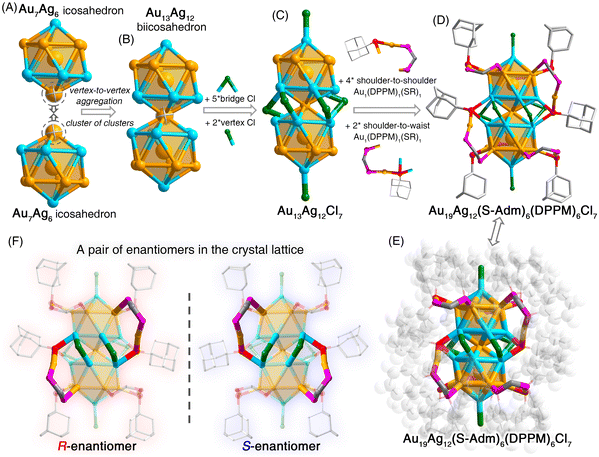
The structural anatomy of the Au19Ag12 nanocluster is shown in Fig. 1 and Fig. S2 (ESI†). First, two Au7Ag6 icosahedral nanobuilding blocks were assembled via sharing the vertex Au atom, giving rise to an Au13Ag12 biicosahedral kernel (Fig. 1A and B). Then, seven chlorine ligands, including five bridge Cl ligands at the waist and two Cl ligands at the vertex, were bonded onto the Au13Ag12 biicosahedron to yield an Au13Ag12Cl7 structure (Fig. 1C). Finally, the Au13Ag12Cl7 structure was enwrapped by six Au1(DPPM)1(S-Adm)1 surface motif units to generate the overall structure of Au19Ag12 (Fig. 1D and E). Among these DPPM–Au–SAdm surface motifs, four were anchored onto the nanocluster with a shoulder-to-shoulder pattern, while the other two followed a shoulder to waist pattern. As shown in Fig. 1D and E, all bidentate DPPM ligands dually bound with a shoulder Au atom and a motif Au atom, while the bulky S-Adm ligands connected a shoulder Au atom and a motif Au atom (for motifs with a shoulder-to-shoulder pattern) or two waist Ag atoms and a motif Au atom (for motifs with a shoulder-to-waist pattern).
Two nanocluster enantiomers of the Au19Ag12 nanocluster were observed in the crystal lattice (Fig. 1F and Fig. S3, ESI†). Fig. 1F shows the mirrored structures of R-nanocluster and S-nanocluster enantiomers. Furthermore, the crystallographic packing of these enantiomers followed a “lamellar eutectic” pattern, and the interlayer distance was determined as 28.897 Å from the (100) plane (Fig. S3, ESI†). Such a crystallographic packing pattern has been observed in several other crystal lattices consisting of nanocluster enantiomers.54,55
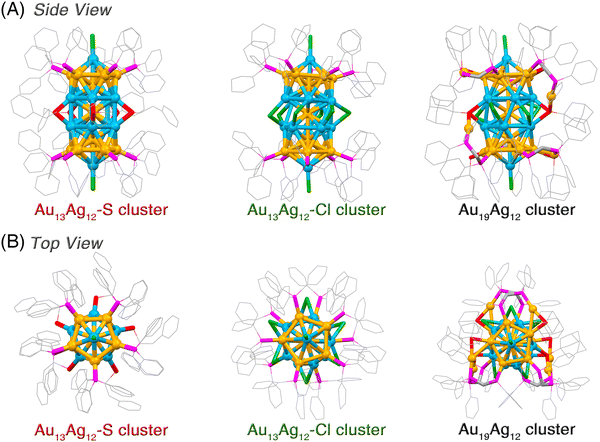
Structurally, both Au13Ag12-S and Au13Ag12-Cl nanoclusters follow a “cluster of clusters” evolution mode and are assembled through the vertical superposition of icosahedral nanobuilding blocks. By comparison, the Au19Ag12 nanocluster is constructed by horizontally expanding the rod-like biicosahedral M25 cluster framework with several denotative Au1(DPPM)1(S-Adm)1 motif structures (Fig. 2). A combination of Au13Ag12-S, Au13Ag12-Cl, and Au19Ag12 nanoclusters forms a platform for investigating the ligand effect in vertically and horizontally extending the M25 framework (Fig. 2). It is suggested that the biicosahedral M25 framework is robust enough to load five thiol ligands or six chlorine ligands into the limited space at the nanocluster waist, yielding the Au13Ag12-S or Au13Ag12-Cl, respectively. Besides, although the bulky S-Adm ligands cannot be included into the limited space of the M25 framework, the further introduction of DPPM ligands triggers the self-adaption of the M25 framework. As a result, six couples of DPPM and S-Adm ligands are anchored onto the framework surface by tying six Au atoms. For the assembly between two M13 icosahedra, because of the pulling effect of surface Au1(DPPM)1(S-Adm)1 motif structures, the distortion degree of icosahedral Au7Ag6 nanobuilding blocks in Au19Ag12 is higher than that in Au13Ag12-S or Au13Ag12-Cl.
The comparison of the corresponding bond lengths is depicted in Fig. S4 (ESI†). Specifically, the bond length distributions of these M13-assembled nanoclusters (i.e., Au13Ag12-S, Au13Ag12-Cl, and Au19Ag12) are similar in terms of the Au(kernel)–M(icosahedral surface), M(icosahedral surface)–M(icosahedral surface), Ag(icosahedral surface)–Cl(vertex), and Au(icosahedral surface)–P(shoulder) bonds (M = Au/Ag; Fig. S4A–D, ESI†), which results from the robust assembly between two icosahedral subunits. The Ag(icosahedral surface)–Cl(waist) bond lengths of Au19Ag12 are much longer than those of Au13Ag12-Cl, and such an elongation originates from the more twisted assembly between two icosahedral Au7Ag6 subunits in Au19Ag12 (Fig. S4E, ESI†). The most significant difference in bond length lies in the Ag(icosahedral surface)–S(waist) interactions between Au19Ag12 and Au13Ag12-S nanoclusters, where the average Ag(icosahedral surface)–S(waist) bond length in Au19Ag12 is 2.515 Å, much longer than that of Au13Ag12-S (2.432 Å; Fig. S4F, ESI†). Such a difference is rational, given that the S-Adm ligand in Au19Ag12 is bulkier than SC2H4Ph in Au13Ag12-S, resulting in extended metal–thiol interactions in Au19Ag12 so that the bulky S-Adm ligands can be installed into the limited space at the cluster waist.
Because of their different molecular configurations and intercluster interactions, these four M13-based nanoclusters exhibit disparate crystal packing modes (Fig. S5, ESI†). The ligand-directed intercluster packings of these M13-based nanoclusters were then compared. Specifically, both Au13Ag12-S and Au13Ag12-Cl nanoclusters contain C–H⋯π and H⋯Cl interactions, while there are only C–H⋯π interactions in the crystal structure of Au13-DPPM. For the Au19Ag12 nanocluster, several types of intercluster interactions are detected, including C–H⋯π, H⋯Cl, and π⋯π interactions (Fig. S5, ESI†).
The optical absorptions of these icosahedron-based nanoclusters were then compared. The CH2Cl2 solution of the Au19Ag12 nanocluster showed three distinct absorptions at 455, 515, and 710 nm and two shoulder bands at 415 and 535 nm (Fig. S6, ESI†). By comparison, the UV-vis spectrum of the Au13Ag12-S nanocluster displayed three absorptions at 425, 480, and 650 nm, while those of the Au13Ag12-Cl nanocluster were centered at 420, 510, and 645 nm. The mono-icosahedral Au13-DPPM nanocluster exhibited an intense band at 440 nm and a shoulder band around 800 nm (Fig. S7, ESI†). Such differences in optical absorptions of these icosahedron-based nanoclusters originated from their distinct electronic structures. Yuan et al. have unveiled the strong electron coupling between substituent icosahedral units based on the mono-icosahedral Ag13 and tetra-icosahedral Ag61 nanoclusters, which resulted in the red-shift of their main absorptions.40 In this work, the red-shifted absorption was also observed by comparing the intense optical bands of bi-icosahedral Au19Ag12, Au13Ag12-S, and Au13Ag12-Cl nanoclusters (710, 650, and 645 nm, respectively) with the main absorption of the mono-icosahedral Au13-DPPM nanocluster (440 nm), which might also arise from the electron coupling between the substituent icosahedral subunits. Of note, for the Au13-DPPM nanocluster, its 440 nm absorption was regarded as the main absorption of the icosahedral kernel since the M13-based absorptions (or electronic transitions) were always located in nearby areas.56–58 In addition, owing to the complete protection of the Au13Ag12 biicosahedral kernel by bridging Cl and surface Au1(DPPM)1(SR)1 structures, the Au19Ag12 nanocluster was highly stable and could maintain its composition and configuration within 20 days, derived from its unchanged optical absorptions over time (Fig. S8, ESI†).
The photoluminescent properties of these four nanoclusters were then compared. As shown in Fig. S9, ESI† the mono-icosahedral Au13-DPPM was non-emissive, while the other three biicosahedral nanoclusters were emissive. Specifically, the PL intensity of these emissive nanoclusters followed a sequence of Au13Ag12-S > Au13Ag12-Cl > Au19Ag12. As for the emissive wavelength, the Au19Ag12, Au13Ag12-S, and Au13Ag12-Cl nanoclusters emitted at 825, 735, and 765 nm, respectively. In this context, the emission wavelength of Au19Ag12 occurred in the NIR-I region and exhibited a significant red-shift relative to those of Au13Ag12-S and Au13Ag12-Cl (Fig. S9, ESI†).
3. Conclusions
In summary, the horizontal expansion of the rod-like biicosahedral M25 cluster framework was accomplished, yielding an [Au19Ag12(S-Adm)6(DPPM)6Cl7]2+ nanocluster. The structural determination resolved two decades-long questions towards rod-shaped multi-icosahedral nanoclusters, including (i) how to install bidentate phosphine ligands onto the nanocluster shoulder and (ii) how to load bulky thiol ligands into the limited space of the nanocluster waist? A combination of the new [Au19Ag12(S-Adm)6(DPPM)6Cl7]2+ nanocluster in this work and previously reported [Au13Ag12(PPh3)10Cl8]2+ or [Au13Ag12(SR)5(PPh3)10Cl2]2+ constituted a platform for mapping out the ligand effect in vertically/horizontally expanding icosahedral M13-based nanoclusters. Overall, this work presents the horizontal expansion of rod-shaped nanoclusters, which broadens the research towards the “cluster of clusters” and hopefully inspires more work on constructing M13-based aggregates from vertically and horizontally growing extensions.Data availability
All the data supporting this article have been included in the main text and the ESI.†Author contributions
C. Z. and P. P. carried out the experiments and analyzed the data. X. W., Z. L. and C. C. assisted the analysis. X. K. and M. Z. designed the project, analyzed the data, and wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support provided by the National Natural Science Foundation of China (21631001, 21871001, and 22101001), the Ministry of Education, and the University Synergy Innovation Program of Anhui Province (GXXT-2020-053).References
- R. Jin, C. Zeng, M. Zhou and Y. Chen, Chem. Rev., 2016, 116, 10346–10413 CrossRef CAS PubMed.
- I. Chakraborty and T. Pradeep, Chem. Rev., 2017, 117, 8208–8271 CrossRef CAS PubMed.
- X. Kang, Y. Li, M. Zhu and R. Jin, Chem. Soc. Rev., 2020, 49, 6443–6514 RSC.
- S. Sharma, K. Chakrahari, J. Saillard and C. W. Liu, Acc. Chem. Res., 2018, 51, 2475–2483 CrossRef CAS.
- T. Kawawaki, Y. Kataoka, M. Hirata, Y. Iwamatsu, S. Hossain and Y. Negishi, Nanoscale Horiz., 2021, 6, 409–448 RSC.
- Y. Jin, C. Zhang, X. Dong, S. Zang and T. C. W. Mak, Chem. Soc. Rev., 2021, 50, 2297–2319 RSC.
- J. Yan, B. K. Teo and N. Zheng, Acc. Chem. Res., 2018, 51, 3084–3093 CrossRef CAS PubMed.
- X. Kang and M. Zhu, Chem. Soc. Rev., 2019, 48, 2422–2457 RSC.
- A. W. Cook and T. W. Hayton, Acc. Chem. Res., 2018, 51, 2456–2464 CrossRef CAS PubMed.
- N. Xia and Z. Wu, Chem. Sci., 2021, 12, 2368–2380 RSC.
- S. Kenzler and A. Schnepf, Chem. Sci., 2021, 12, 3116–3129 RSC.
- G. Salassa and T. Bürgi, Nanoscale Horiz., 2018, 3, 457–463 RSC.
- M. Agrachev, M. Ruzzi, A. Venzo and F. Maran, Acc. Chem. Res., 2019, 52, 44–52 CrossRef CAS PubMed.
- K. Kwak and D. Lee, Acc. Chem. Res., 2019, 52, 12–22 CrossRef CAS.
- B. Nieto-Ortega and T. Bürgi, Acc. Chem. Res., 2018, 51, 2811–2819 CrossRef CAS.
- M. Hesari, H. Ma and Z. Ding, Chem. Sci., 2021, 12, 14540–14545 RSC.
- X.-Q. Liang, Y.-Z. Li, Z. Wang, S.-S. Zhang, Y.-C. Liu, Z.-Z. Cao, L. Feng, Z.-Y. Gao, Q.-W. Xue, C.-H. Tung and D. Sun, Nat. Commun., 2021, 12, 4966 CrossRef CAS.
- E. Ubasart, I. M. Marin, J. M. Asensio, G. Mencia, Á. López-Vinasco, C. García-Simón, I. Rosal, R. Poteau, B. Chaudret and X. Ribas, Nanoscale Horiz., 2022, 7, 607–615 RSC.
- S. Li, X.-Y. Dong, K.-S. Qi, S.-Q. Zang and T. C. W. Mak, J. Am. Chem. Soc., 2021, 143, 20574–20578 CrossRef CAS.
- R. W. Y. Man, H. Yi, S. Malola, S. Takano, T. Tsukuda, H. Häkkinen, M. Nambo and C. M. Crudden, J. Am. Chem. Soc., 2022, 144, 2056–2061 CrossRef CAS PubMed.
- T. Kawawaki, Y. Kataoka, M. Hirata, Y. Akinaga, R. Takahata, K. Wakamatsu, Y. Fujiki, M. Kataoka, S. Kikkawa, A. S. Alotabi, S. Hossain, D. J. Osborn, T. Teranishi, G. G. Andersson, G. F. Metha, S. Yamazoe and Y. Negishi, Angew. Chem., Int. Ed., 2021, 60, 21340–21350 CrossRef CAS PubMed.
- Z.-J. Guan, R.-L. He, S.-F. Yuan, J.-J. Li, F. Hu, C.-Y. Liu and Q.-M. Wang, Angew. Chem., Int. Ed., 2022, 61, e202116965 CAS.
- H. Yi, S. M. Han, S. Song, M. Kim, E. Sim and D. Lee, Angew. Chem., Int. Ed., 2021, 60, 22293–22300 CrossRef CAS PubMed.
- G. Deng, B. K. Teo and N. Zheng, J. Am. Chem. Soc., 2021, 143, 10214–10220 CrossRef CAS PubMed.
- G.-T. Xu, X.-Y. Chang, K.-H. Low, L.-L. Wu, Q. Wan, H.-X. Shu, W.-P. To, J.-S. Huang and C.-M. Che, Angew. Chem., Int. Ed., 2022, 61, e202200748 CAS.
- G. Li, X. Sui, X. Cai, W. Hu, X. Liu, M. Chen and Y. Zhu, Angew. Chem., Int. Ed., 2021, 60, 10573–10576 CrossRef CAS.
- S. Lee, M. S. Bootharaju, G. Deng, S. Malola, H. Häkkinen, N. Zheng and T. Hyeon, J. Am. Chem. Soc., 2021, 143, 12100–12107 CrossRef CAS.
- X. Kang, X. Wei, S. Jin, Q. Yuan, X. Luan, Y. Pei, S. Wang, M. Zhu and R. Jin, Proc. Natl. Acad. Sci. U.S.A., 2019, 116, 18834–18840 CrossRef CAS.
- M. Qu, H. Li, L.-H. Xie, S.-T. Yan, J.-R. Li, J.-H. Wang, C.-Y. Wei, Y.-W. Wu and X.-M. Zhang, J. Am. Chem. Soc., 2017, 139, 12346–12349 CrossRef CAS PubMed.
- N. A. Sakthivel and A. Dass, Acc. Chem. Res., 2018, 51, 1774–1783 CrossRef CAS.
- X. Kang and M. Zhu, Coord. Chem. Rev., 2019, 394, 1–38 CrossRef CAS.
- B. K. Teo and H. Zhang, Coord. Chem. Rev., 1995, 143, 611–636 CrossRef CAS.
- B. K. Teo and H. Zhang, Inorg. Chim. Acta, 1988, 144, 173–176 CrossRef CAS.
- B. K. Teo and H. Zhang, Polyhedron, 1990, 9, 1985–1999 CrossRef CAS.
- B. K. Teo and H. Zhang, J. Cluster Sci., 1990, 2, 223–228 CrossRef.
- Y. Shichibu, Y. Negishi, T. Watanabe, N. K. Chaki, H. Kawaguchi and T. Tsukuda, J. Phys. Chem. C, 2007, 111, 7845–7847 CrossRef CAS.
- M. S. Bootharaju, S. M. Kozlov, Z. Cao, M. Harb, N. Maity, A. Shkurenko, M. R. Parida, M. N. Hedhili, M. Eddaoudi, O. F. Mohammed, O. M. Bakr, L. Cavallo and J. Basset, J. Am. Chem. Soc., 2017, 139, 1053–1056 CrossRef CAS PubMed.
- R. Jin, C. Liu, S. Zhao, A. Das, H. Xing, C. Gayathri, Y. Xing, N. L. Rosi, R. R. Gil and R. Jin, ACS Nano, 2015, 9, 8530–8536 CrossRef CAS PubMed.
- T.-H. Chiu, J.-H. Liao, F. Gam, I. Chantrenne, S. Kahlal, J.-Y. Saillard and C. W. Liu, J. Am. Chem. Soc., 2019, 141, 12957–12961 CrossRef CAS PubMed.
- S. Yuan, C. Xu, W. Liu, J. Zhang, J. Li and Q. Wang, J. Am. Chem. Soc., 2021, 143, 12261–12267 CrossRef CAS.
- S. Yang, J. Chai, T. Chen, B. Rao, Y. Pan, H. Yu and M. Zhu, Inorg. Chem., 2017, 56, 1771–1774 CrossRef CAS.
- S. Yang, J. Chai, Y. Lv, T. Chen, S. Wang, H. Yu and M. Zhu, Chem. Commun., 2018, 54, 12077–12080 RSC.
- Y. Song, F. Fu, J. Zhang, J. Chai, X. Kang, P. Li, S. Li, H. Zhou and M. Zhu, Angew. Chem., Int. Ed., 2015, 54, 8430–8434 CrossRef CAS PubMed.
- H. Qian, W. T. Eckenhoff, Y. Zhu, T. Pintauer and R. Jin, J. Am. Chem. Soc., 2010, 132, 8280–8281 CrossRef CAS.
- L. Xu, Q. Li, T. Li, J. Chai, S. Yang and M. Zhu, Inorg. Chem. Front., 2021, 8, 4820–4827 RSC.
- S. Jin, M. Zhou, X. Kang, X. Li, W. Du, X. Wei, S. Chen, S. Wang and M. Zhu, Angew. Chem., Int. Ed., 2020, 59, 3891–3895 CrossRef CAS PubMed.
- F. Hu, Z. Guan, G. Yang, J. Wang, J. Li, S. Yuan, G. Liang and Q. Wang, J. Am. Chem. Soc., 2021, 143, 17059–17067 CrossRef CAS PubMed.
- S. Wang, X. Meng, A. Das, T. Li, Y. Song, T. Cao, X. Zhu, M. Zhu and R. Jin, Angew. Chem., Int. Ed., 2014, 53, 2376–2380 CrossRef CAS PubMed.
- B. K. Teo and H. Zhang, Angew. Chem., Int. Ed. Engl., 1992, 104, 445–447 CrossRef.
- S. Jin, W. Du, S. Wang, X. Kang, M. Chen, D. Hu, S. Chen, X. Zou, G. Sun and M. Zhu, Inorg. Chem., 2017, 56, 11151–11159 CrossRef CAS PubMed.
- M. Walter, J. Akola, O. Lopez-Acevedo, P. D. Jadzinsky, G. Calero, C. J. Ackerson, R. L. Whetten, H. Grönbeck and H. Häkkinen, Proc. Natl. Acad. Sci. U.S.A., 2008, 105, 9157–9162 CrossRef CAS.
- A. Desireddy, B. E. Conn, J. Guo, B. Yoon, R. N. Barnett, B. M. Monahan, K. Kirschbaum, W. P. Griffith, R. L. Whetten, U. Landman and T. P. Bigioni, Nature, 2013, 501, 399–402 CrossRef CAS.
- X. Kang, S. Jin, L. Xiong, X. Wei, M. Zhou, C. Qin, Y. Pei, S. Wang and M. Zhu, Chem. Sci., 2020, 11, 1691–1697 RSC.
- X. Kang, F. Xu, X. Wei, S. Wang and M. Zhu, Sci. Adv., 2019, 5, eaax7863 CrossRef CAS PubMed.
- C. Xu, Q. Yuan, X. Wei, H. Li, H. Shen, X. Kang and M. Zhu, Chem. Sci., 2022, 13, 1382–1389 RSC.
- M. Zhu, C. M. Aikens, F. J. Hollander, G. C. Schatz and R. Jin, J. Am. Chem. Soc., 2008, 130, 5883–5885 CrossRef CAS.
- K. L. D. M. Weerawardene, P. Pandeya, M. Zhou, Y. Chen, R. Jin and C. M. Aikens, J. Am. Chem. Soc., 2009, 141, 18715–18726 CrossRef.
- J. Kong, W. Zhang, Y. Wu and M. Zhou, Aggregate, 2022 DOI:10.1002/agt2.207.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2183917, 2163571, 2163572 and 2163804. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2nh00321j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |