Microneedle-based technology for cell therapy: current status and future directions
Bo Zhi
Chen
ab,
Ze Qiang
Zhao
ab,
Mohammad-Ali
Shahbazi
*cd and
Xin Dong
Guo
 *ab
*ab
aState Key Laboratory of Organic–Inorganic Composites, Beijing University of Chemical Technology, Beijing 10029, China. E-mail: xdguo@buct.edu.cn
bBeijing Laboratory of Biomedical Materials, College of Materials Science and Engineering, Beijing University of Chemical Technology, Beijing, 100029, China
cDepartment of Biomedical Engineering, University Medical Center Groningen, University of Groningen, Antonius Deusinglaan 1, 9713 AV Groningen, The Netherlands. E-mail: m.a.shahbazi@umcg.nl
dDepartment of Pharmaceutical Nanotechnology, School of Pharmacy, Zanjan University of Medical Sciences, 45139-56184 Zanjan, Iran
First published on 11th May 2022
Abstract
With the growing technological innovations in medical treatments, cell-based therapies hold great potential as efficient tools against various previously incurable diseases by restoring or altering the function of certain sets of cells. Along this line, an essential factor to determine the success of cell therapy is the choice of cell delivery strategy. In recent years, a novel trend is blooming in the application of microneedle systems, which are based on the miniaturization of multiple needles within a patch to the micrometer dimensions, aimed at the delivery of therapeutic cells to the target site with high efficiency and in a minimally invasive manner. This review aims to demonstrate the advantages of exploiting microneedle-based technology as a new tool for cell therapy. The advancements of microneedle-based strategies for cell delivery are summarized in terms of two categories: cell-free and cell-loaded microneedle systems. The majority of research on microneedle-based cell therapy has shown promising results for tissue regeneration, cancer immunotherapy, skin immune monitoring and targeted cell delivery. Finally, current challenges and future perspectives toward the development and application of microneedles for cell therapy are also discussed.
1. Introduction
Cell therapy is a technique to transfer viable autologous or allogenic cells into a patient to elicit a therapeutic response, for example, by delivering a chimeric antigen receptor (CAR) T cell capable of attacking the malignant cells to treat blood cancer or transplanting hematopoietic stem cells to create bone marrow for the therapy of blood-related disorders.1,2 Since the first successful cell transplantation, which was reported by E. Donnall Thomas in 1957, the field of cell therapy has vigorously evolved and more than 8000 clinical trials for cell-based therapies have so far been conducted for a multitude of diseases (sources: ClinicalTrials.gov database, 2021).3,4 Multiple strategies including apply biomaterials, automated manufacture systems or synergistic techniques have been applied to produce cell-based therapies with high quality and mass production capacity in order to facilitate the translation of cell therapies to approved products.On the other hand, with cell therapies being one of the hot research fields in life sciences, it is critically important to develop a considerable delivery system capable of precisely delivering cells to the desired locations in the body while preserving their viability and functionality. Bolus injection and surgical graft are currently the typical modes of cell delivery in preclinical and clinical studies.5–7 Using a single needle injection technique, Chua et al. demonstrated that an intra-arterial injection of neural stem cells into the carotid artery with a preserved flow within the artery could improve stroke-associated damages in a preclinical model.8 Furthermore, various types of stem cells have also been employed to treat cardiac damage by intra-myocardial or intra-coronary injections, and most of the clinical results exhibited obvious therapeutic benefits in recovering the function of the damaged heart.9,10 Other cell injection approaches include the intra-nasal and intra-thecal injections of mesenchymal stem cells (MSCs) for neurologic disorders,11,12 local intra-muscular injection of bone marrow cells for tissue regeneration,13 and intra-venous injection of multipotent adult progenitor cells for traumatic brain injuries.14,15 However, despite several notable efforts that have been devoted to increasing the cell stability and viability during the injection process, the cell migration from the specific site of actions would lead to low engraftment efficiency and upsetting side effects, thus reducing the therapeutic efficacy and influencing the subsequent outcomes of the treatment. In this context, to enhance the localization of cells once delivered, biomaterial-based systems have been designed for cell encapsulation and further proliferation. For example, recent attempts for cell therapy by biomaterials include the entrapment of cardiomyocytes (CMs) in a gelatin-based 4D physiologically adaptable cardiac patch for myocardial infarction treatment16 and implantation of the autologous mesenchymal stem cell-loaded collagen hydrogel scaffold for articular cartilage defect repair.17 In this context, the main challenge in promoting biomaterial-incorporated cell therapies to widespread clinical adoption is delivering these therapeutic cells to the target tissue with high cell engraftment and patient compliance. Both the current bolus injection and surgical graft are associated with invasive and professional medical procedures, increasing the risk of infection and financial burden on patients.18 These challenges, in a sense, directed developments in cell therapy to alternative non-traumatic delivery strategies that are less destructive to tissues and do not cause pain. One highlight of recent efforts is the development of an engineered cell sheet, which has demonstrated successful outcomes in clinical trials for skin regeneration and corneal reconstruction.19 On the other hand, since the therapeutic cells are just cast onto the surface of the tissue without penetrating the deeper target regions, an enormous number of cells are required to achieve a therapeutic response due to the poor transplantation efficiency.
To overcome these limitations, researchers have started to re-explore microneedle (MN)-based systems for the controlled and targeted cell delivery in a minimally invasive and self-administrable manner. The MN system is based on the miniaturization of multiple needles within a patch to the micron range dimensions that can overcome the tissue barriers for the effective delivery of various kinds of pharmaceutical compounds without causing tissue damage and acute pain.20–22 Since the first silicon MN patch emerged as a new transdermal drug delivery enhancement system in 1998,23 to date, a large number of studies have reported MNs of different matrix materials (metal, ceramic, glass, silicon, polymer, etc.) and architecture designs (solid, hollow, coated, dissolvable, swollen, etc.) applied for drug delivery and biomarker detection.24–29 Given these motivations, a few visionary scientists have begun demonstrating the unique potential of MN-based systems for delivering therapeutic cells against various intractable diseases (Fig. 1). In this review, the recent advancements of the MN-based technology for cell therapy will be summarized, and the prospects and challenges of this field will also be discussed.
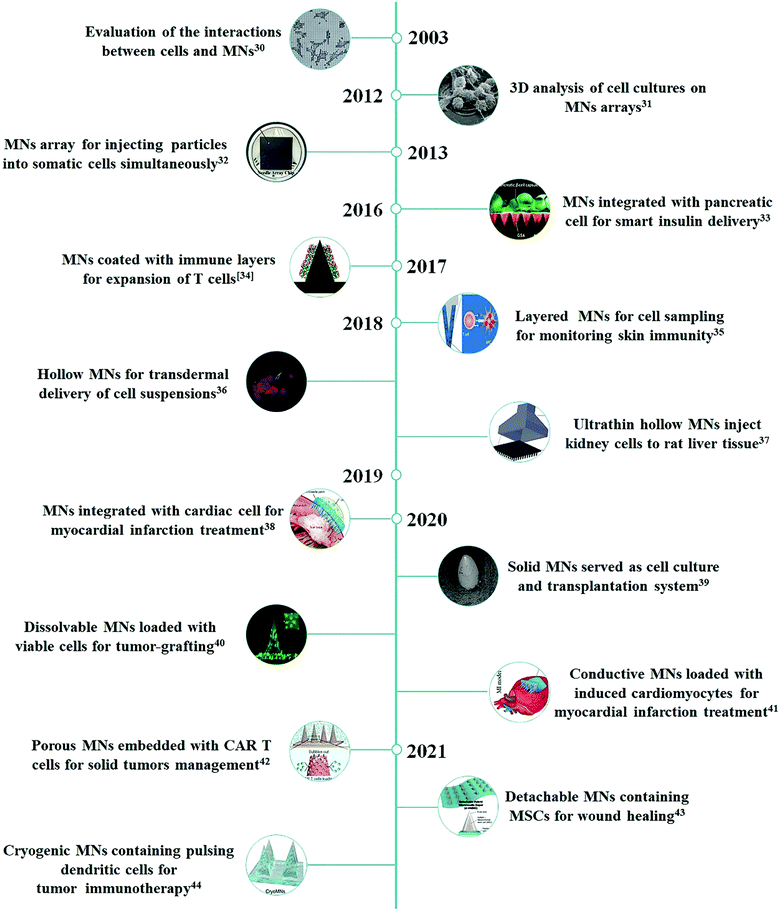 | ||
| Fig. 1 Chronological timeline of studies with MN-based technology for cell therapy.30–44 | ||
2. Cell–MN interactions
The interactions between cells and MNs are of fundamental relevance and contribute to the successful application of MN-based cell therapy. It has been proved that both the cell types and properties of the cultured platforms have a tremendous effect on cell adhesion, proliferation and transplantation.45,46 Understanding how the cultured cells interact with MN systems is crucial in extending the therapeutic impact of these cell therapies. As early as in the year 2003, Tan and his colleagues applied the silicone elastomer-based MN arrays as mechanically isolated sensors to manipulate and measure the mechanical interactions of cells lying on the bed of the MNs.30 Through controlling the cell adhesion on the MNs, the authors demonstrated that the size of focal adhesions has a positive correlation with the traction forces generated by cells at these adhesions area larger than 1 μm2. The cellular processes associated with the cell morphology, traction force, and focal adhesions affect the biological behavior of cells on the MNs. Furthermore, Friedmann et al. combined focused ion beam and scanning electron microscopy technologies to observe the interfaces between MN arrays and cell cultures in patch-on-chip systems and obtained the direct evidence of the influence of MN geometries on the biological behavior of cells.31 The obtained micrographs clearly show that the cells and MNs are closely connected, but no cell perforation occurred (Fig. 2). Moreover, cells cultivated on both the solid and hollow MN arrays exhibited a typical spread cell morphology with protuberances of the cell membrane as filopodia and microvilli appear, indicating the negligible effect of MNs on cell viability and functionality.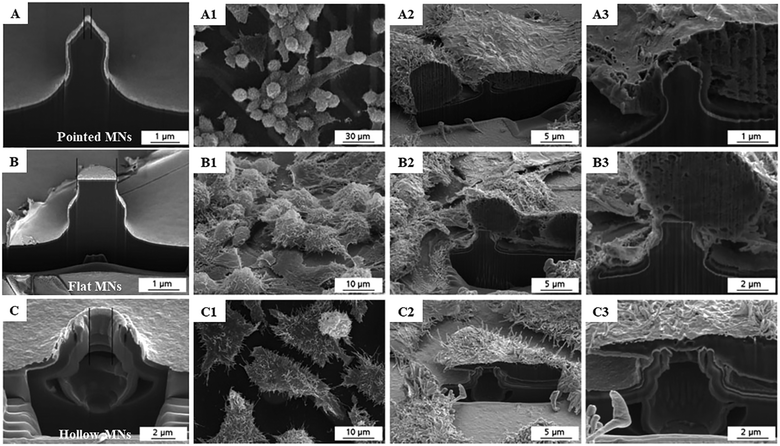 | ||
| Fig. 2 SEM micrographs of L-929 cells cultivated on three types of MNs: (A) pointed solid MNs, (B) flat MNs and (C) hollow MNs. (A1–C1) Cells on the MNs. (A2–C2) Cells were selected for cross-sectional preparation. (A3–C3) Section enlargement to show the MN–cell interface. (A1–A3) Micrographs depict a cell culture on the pointed needle. (B1–B3) Micrographs depict a cell culture on a flat MN. (C1–C3) Micrographs depict a cell culture on a hollow needle.31 | ||
On the other hand, besides the structures of MNs, the cell–MN interactions are also strongly dependent on the constituent materials of MNs. The determination and control of cell–material interactions are therefore critical to ensure the cellular function and therapeutic efficacy.47 A recent work by Chen et al. has shown the promising ability of polymer MN arrays for cell seeding and delivering.39 The authors found both the HaCaT and dermal papilla cells can be easily seeded on the poly-methyl methacrylate MNs with high viability and retention rates. Following 2–4 h of culture, the attached cells form a connective cellular sheet on the base of the MN arrays and migrate to the needle shafts gradually. The further in vitro cell delivery tests using the collagen hydrogel showed an 83.2% delivery efficiency of this cell-seeded MNs, demonstrating its potential application for cellular therapy. However, since the field of MN-based cell therapy has only been widely developed in the past few years, information about the interactions between cells and the matrix materials of MNs is very limited at the current stage. Future attempts in this field can thus consider to evaluate the cell behavior on the glass, silicon, metals, ceramics and especially polymeric biomaterial-based MNs. In addition, the effects of some physical properties (surface topography and materials stiffness) and chemical modifications (grafting of chemical groups, adhesion ligands and growth factors) of the MN materials on the cell behavior should also require to be systematically studied.
3. Cell delivery
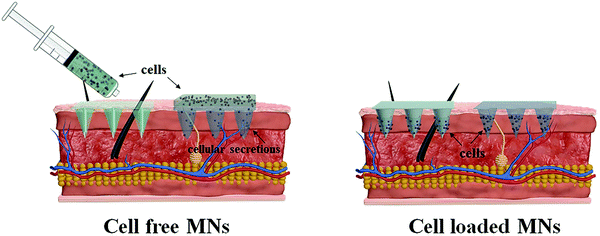
Taking lessons from the successful application of MN-based systems for precise drug delivery and efficient cell culture, attempts have been prompted to develop MN-based cell therapies that are capable of transferring therapeutic cells directly into the targeted sites in a minimally invasive manner, thereby maximizing therapeutic efficacy and improving medication compliance. The MN-based cell delivery studies reported in the literature so far can be broadly classified into two categories: cell free MNs and cell loaded MNs (Fig. 3). In the cell free MNs category, living cells are transported into the specific sites through the channels of the hollow MNs or the temporary pathway created by the swelling of MNs. Meanwhile, for the cell loaded MN systems, therapeutic cells are directly loaded into the MNs matrix and delivered along with the dissolution or degradation of the MNs after application. The key features of these two MN systems, in terms of the cell delivery strategy, MN type, materials for fabricating MNs, geometric parameters and applications, are summarized in Table 1, and the advantages and drawbacks of each MN type for cell delivery are also included.| Cell delivery strategy | Type | Approach | MNs design | Application | Advantages for cell delivery | Drawbacks for cell delivery | |
|---|---|---|---|---|---|---|---|
| Materials | Diameter (D)/height (H) | ||||||
| SiO2: silicon dioxide; Si3N4: silicon nitride; HA: hyaluronic acid; PVA: poly(vinyl alcohol); PBS: phosphate-buffered saline; DMSO: dimethyl sulfoxide; PVP: polyvinylpyrrolidone; GelMA: gelatin methacryloyl; PLGA: poly(lactic-co-glycolic) acid; CaCO3: calcium carbonate; and CAR T cells: chimeric antigen receptor T cells. | |||||||
| Cell free MNs | Hollow MNs | Infuse cell suspensions through the inner channel of MNs | Silicon36 | Array: 5 × 5 | • Delivery of melanocyte, keratinocyte and mixed epidermal cell suspensions for vitiligo treatment | • Great control over the amount and timing of cell delivery | • Shear force and local hypoxia may decrease cell viability |
| D: 75–150 μm | • Liquid formulation may simplify the use of the existing injectable cell formulations for delivery using MNs | • Probably associated with blockage and leakage | |||||
| H: 400–700 μm | • Require additional assistive devices that made the system complex and hard to handle | ||||||
| SiO2/Si3N437 | Wall thickness: 3–5 μm | • Inject Mardin–Darby canine kidney cells into rat liver tissue | |||||
| D: 30–50 μm | |||||||
| H: 100–500 μm | |||||||
| Reservoir-based swelling MNs | Cellular secretions from cell-reservoir diffuse into the tissue through the temporary pathway created by the swelling of MNs | Cross-linked HA33 | Array: 20 × 20 | • Transdermal delivery of insulin that is secreted by the pancreatic β-cells for treating diabetes | • Negligible risk of immune rejection | • Difficult to preserve the mechanical strength under high humidity conditions | |
| D: 400 μm | • Removing the limitation on dosing | ||||||
| H: 800 μm | • Intact removal from insertion sites with no waste left | • Difficult to maintain cell viability at the dry state | |||||
| PVA38 | Array: 20 × 20 | • Local delivery of regenerative factors that secreted by cardiac stromal cells into the injured myocardium to promote heart repair | |||||
| D: 300 μm | |||||||
| H: 600 μm | • Limited therapeutic efficacy due to the extended diffusion pathway | ||||||
| Cell loaded MNs | Dissolving MNs | Cells are directly encapsulated within the MNs and released into the tissue with the dissolution of the matrix materials | PBS/DMSO/sucrose44 | Array: 10 × 10 | • Dendritic cells are released with the melting of needles to elicit anti-tumor immune responses | • Allow simultaneous tissue insertion and cell delivery in one step | • Relative low dose of encapsulated cells |
| D: 350 μm | • Precise dose and rapid cell delivery | • Mechanical strength may be affected by moisture | |||||
| H: 900 μm | |||||||
| HA/maltose/PVP40 | Array: 10 × 10 | • Transdermal delivery of cancer cells for establishing tumor mouse models | |||||
| D: 300 μm | • Require strict shipping and storage conditions | ||||||
| H: 650 μm | • No sharp medical waste left | ||||||
| Biodegradable MNs | Cells are first enveloped in MNs shell or attached onto the porous MNs shafts and next delivered with the degradation of the MNs | GelMA/PLGA43 | Structure: core–shell | • Implant the mesenchymal stem cells-loaded MNs into the site of the wound for sustained delivery of the secreted therapeutic factors to facilitate wound regeneration | • Sufficient mechanical strength for reliable tissue insertion | • Cell inactivation during MNs manufacturing | |
| Aspect ratio: 1.5 | |||||||
| H: 750 μm | • Probably cause safety issue when the matrix materials are deposited within the application sites | ||||||
| CaCO3/PLGA48 | Array: 15 × 15 | • Local scattered seeding of CAR T cells in the tumor bed or in resection cavity for immunotherapy cancer treatment | • Easy application | ||||
| D: 250 μm | • Cell release characteristics are constrained by the degradation rate of the MNs materials | ||||||
| H: 1500 μm | |||||||
3.1 Cell free MN systems
Over the last few decades, knowledge and skills in the field of injection-based cell therapy are progressing fast enough that researchers have developed several related products for clinical use.49,50 Similar to the subcutaneous injection using a hypodermic needle, hollow MNs provide a defined conduit in each individual needle capable of localized delivering cell suspension into the targeted sites. Gualeni et al. first demonstrated the feasibility of hollow silicon MNs for precise, minimally invasive cell therapy application in the skin for vitiligo treatment.36 By using MNs with pore sizes ≥75 μm, both the individual and mixed cell types were effectively extruded through the MNs, and no noticeable cell loss or death was observed during the application process. All cell types displayed the normal adhesion to the culture platform and investigated maintained their distinctive morphologies following injection into the skin. Moreover, studies using immunofluorescence in excised human skin models showed that the delivered cells are aggregated within the insertion sites and expressed markers of melanocytic or keratinocyte differentiation, further indicating the safety and efficacy of hollow MNs for transdermal cell delivery. In addition to applications in the skin, hollow MNs have also been applied to inject functional cells directly into the other damaged tissues to enhance the therapeutic effect in a site-specific manner.37 Iliescu and co-workers developed a cell transplantation device consisting of SiO2/Si3N4-based ultrathin hollow MNs as the injection port, a commercial syringe as a cell container, and a 3D-printed chamber as the connector (Fig. 4A), which is able to achieve the multiple parallel equidistant delivery of Mardin–Darby canine kidney cells into rat liver tissue. However, on the other hand, it should also be noted that some part of the injected cells were pushed out rather than completely embedded within the tissues, which can be attributed to the micron-sized of the cell-flow holes as well as the pressure generated during the MN insertion and cell injection.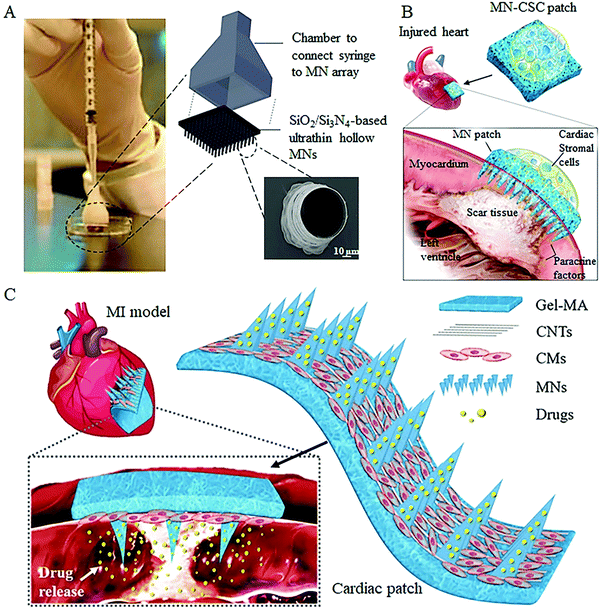 | ||
| Fig. 4 Representative cell free MN systems for cell therapy. (A) Hollow MN-based cell injection device.37 (B) Concept rendering of the cardiac cell-integrated swelling MN patch for the treatment of the injured heart.38 (C) Schematic showing the overall design of carbon nanotube (CNT) conductive hydrogel-forming MNs with drug encapsulation and cardiomyocyte (CM) alignment for treating myocardial infarction.41 | ||
To overcome this hurdle, the strategy to develop MN-based cell therapy without direct cell transferring or the pressure-driven process has been investigated in recent years. A research team led by Gu et al. has proposed a novel integrated MN patch that consists of swelling needle shafts and a cell-loaded hydrogel in the patch base.33,38 Following administration, the needle shafts can penetrate the tissue barrier to form the communication channels between the attached cells and the host tissue, through which the secretions of functional cells can be transported into the targeted tissues for disease treatments (Fig. 4B). The live/dead staining and quantitative analysis demonstrated that the viability and morphology of the integrated cells were not compromised when cocultured on MNs on days 1, 3, and 7. In subsequent tests with rats and pigs, the authors demonstrated the potential ability of this live cell-integrated MN system for therapeutic heart regeneration and improving diabetes treatment.
Furthermore, to increase the effectiveness of the cell-integrated MN system for treating myocardial infarction, Sun et al. encapsulated angiogenic and antiphlogistic drugs within the needle shafts and attached a carbon nanotube layer onto the hydrogel base to induce the directional growth of cardiomyocytes41 (Fig. 4C). In this improved system, the induced pluripotent stem cell-derived cardiomyocytes were used to ensure the orientation of differentiation and the encapsulated drugs were effectively delivered into the insertion site to facilitate the cardiac repair process. With the in vivo test in mouse myocardial infarction models, the induced cardiomyocyte-integrated MNs could be firmly attached to the beating heart and exhibited a benign therapeutic effect on heart disease. However, although these studies may aid in the development of next-generation precision cell therapies that better deliver therapeutic cells for tissue regeneration and disease treatments, further studies are required to optimize the application mode of the cell-integrated MNs patch since the cardiac MN patches are applied to the injured tissue through a highly invasive open-heart surgery at the current stage.
3.2 Cell loaded MN systems
Besides employing MNs as the transport channels of live cells or cell secretions, MN-based cell therapy can also be developed by embedding therapeutic cells within the needle shafts or directly using the cell suspension as the matrix materials of the whole MN patch. Compared to cell free MN systems, the cell loaded MNs systems allow the simultaneous tissue micro-perforation and cell delivery in one step, no additional auxiliary equipment is required and no sharp medical waste left.51,52 Moreover, the cell loaded MNs can more accurately control the number of delivered cells and effectively retain them at the targeted location for proliferation and therapy.53 For example, Lee et al. engineered a detachable hybrid microneedle depot (d-HMND) for localized stem cell delivery to support cell viability and accelerate wound healing.43 In this system, the therapeutic cells are first mixed with a cross-linked gelatin methacryloyl (GelMA) that can then be enveloped in the polylactic-co-glycolic acid (PLGA) shell to form hybrid MNs with a core–shell structure (Fig. 5A). GelMA containing nutrients (compressive modulus, 30 kPa) mimics a normal extracellular environment for improved cell adhesion and proliferation, while the much harder PLGA shell not only protects the cell–GelMA mixture but also provides mechanical strength for successful tissue penetration. Following the application of the d-HMND into the wound bed, the cell-loaded MNs are quickly detached from the substrate and transplanted into the site of the wound for the sustained delivery of the secreted therapeutic factors to facilitate wound regeneration. The author demonstrated that the d-HMND offers a high cell viability of above 90% for 24 h, and more importantly, the delivered cells did not lose their functionality as evidenced by their secretion of biomarkers even without nutrition supply from outside. With evaluations on the mouse skin wound model, the cell-loaded MNs indeed stimulated numerous critical parameters associated with wound healing and showed great benefits in regeneration therapy. Another exemplary study in this regard was reported by Li and colleagues, who loaded the CAR T cells in the pores of the PLGA MN shafts that can then be implanted into the solid tumor lesion or post-surgical resection cavity for improved anticancer efficacy.42 The porous structure of MNs enables cell adhesion during the implantation process; meanwhile, the array distribution of cell-loaded MNs ensures ample scattered cell delivery (Fig. 5B). In this system, a 15 × 15 MN array with a 144 mm2 area is capable of carrying around 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells and more than 50% of the loaded cells can be effectively delivered into the targeted site within 15 min. The analysis of the tumor size and the expression of tissue factors in tumor cells confirmed a superior therapeutic action of the cell-loaded MN array since the multipoint scattered delivering manner promotes cell interactions between the CAR T and tumor cells. With further evaluations on the orthotopic pancreatic tumor model, CAR T cell loaded MNs showed a more prominent tumor infiltration and immunotherapy outcome as compared with conventional deposition administration and intratumor injection.
000 cells and more than 50% of the loaded cells can be effectively delivered into the targeted site within 15 min. The analysis of the tumor size and the expression of tissue factors in tumor cells confirmed a superior therapeutic action of the cell-loaded MN array since the multipoint scattered delivering manner promotes cell interactions between the CAR T and tumor cells. With further evaluations on the orthotopic pancreatic tumor model, CAR T cell loaded MNs showed a more prominent tumor infiltration and immunotherapy outcome as compared with conventional deposition administration and intratumor injection.
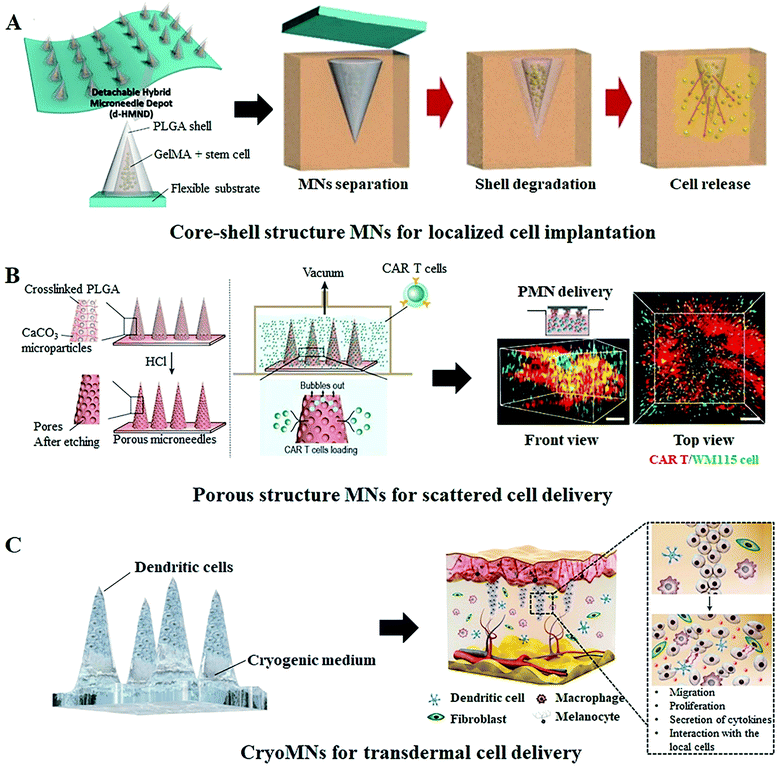 | ||
| Fig. 5 Representative cell loaded MN systems for cell therapy. (A) Schematic illustration of a detachable hybrid microneedle depot (d-HMND) for the localized delivery of mesenchymal stem cells (MSC). This d-HMND consists of an outer PLGA shell and an internal gelatin methacryloyl (GelMA)–MSC mixture (GMM).43 (B) Schematic of the CAR T cell-loaded porous MN fabrication process and its application in a 3D tumor model. The 3D reconstruction illustrates the homogeneous distribution of the delivered cells.42 (C) Schematic illustration of the transdermal delivery of cells using cryomicroneedles. The cryoMNs are made of the optimized cell medium and melt to release the pre-loaded cells for subsequent cell therapy after insertion into the skin.44 | ||
Instead of utilizing polymer MNs as the cell carrier, Chang et al. fabricated the cell-loaded MNs, termed “cryomicroneedles (CryoMNs)”, directly with the cell suspension using a stepwise cryogenic micromolding process.44 To enhance cell viability and delivery efficiency, phosphate-buffered saline (PBS) supplemented with dimethyl sulfoxide and sucrose was selected as the cryogenic medium and cells were selectively loaded into the tips of the needles (Fig. 5C). By inserting the CryoMNs into the skin, the pre-loaded cells were released with the melting of needles, and subsequently migrated and proliferated within the skin. In addition, the author found that, although the cell viability of ovalbumin-pulsed dendritic cells in CryoMNs was 71.4 ± 1.4%, these living cells could proliferate after being released from the MNs. Given these results, the authors further performed the proof-of-concept research in melanoma-bearing mice models to evaluate the therapeutic effect of ovalbumin-pulsed dendritic cell loaded CryoMNs. The results demonstrated that vaccination with CryoMNs elicited robust anti-tumor immune responses in the treated mice and provided stronger protection against tumors. This new CryoMN technology is also appropriate for other types of therapeutic cells and would even be compatible with patient-derived cells for personalized therapy.
On the other hand, besides delivering therapeutic cells for the treatment of diseases or disorders, MN systems have also been applied to the transdermal delivery of cancer cells for establishing tumor mouse models (Fig. 6).40 In this strategy, the B16–F10-murine-melanoma cells were mixed with hyaluronic/maltose/polyvinylpyrrolidone to form the cell-loaded dissolving MNs. After storage at 4 °C for 1 day, the prepared MNs could preserve 50.6 ± 12.0% of cell survivals and possess sufficient mechanical strength for reliable skin insertion. The in vivo tumor-grafting tests in mice demonstrated that even though 100 times lower numbers of cancer cells are delivered via MNs can create a bigger volume of tumor as compared to the transdermal injection method, which was attributed to the ability of polymer MNs to retain the delivered cells at the target sites and protect them from host immune clearance.54,55 The produced tumor showed massive tumor necrosis in the subcutaneous tissue or hypodermis with obvious tumor invasion up to the epidermis. Moreover, the tumor size can be effectively controlled by the numbers of the administered cells, and the further histopathological examination of the excised tumors demonstrated that the tumors induced by cancer cell-loaded MNs exhibited similar neoplastic cell features with that induced by the traditional hypodermic needle injection.
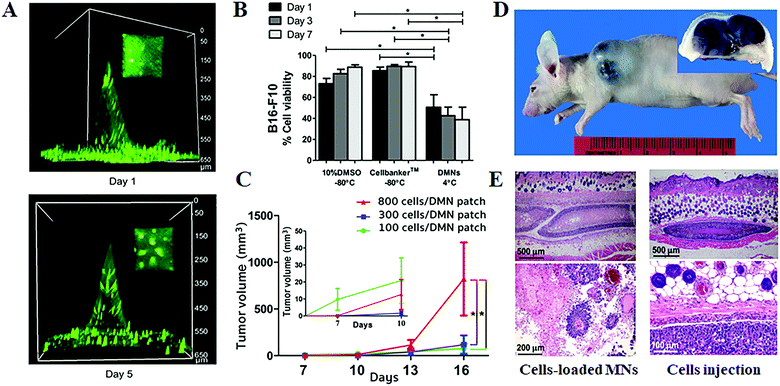 | ||
| Fig. 6 Transdermal delivery of cancer cells for establishing tumor models.40 (A) Three-dimensional fluorescence images of cancer cell-loaded dissolving MNs at days 1 and 5 post storage at 4 °C. (B) Cell viability after being kept under different conditions. (C) Tumor volumes at various times post the one-time administration of B16–F10-murine melanoma cell-loaded MNs. (D) Gross image (main image) and cross-section (inset) of the tumor induced by cancer cell-loaded MNs. (E) Histopathology of the excised melanoma from mice at day 16 post the one-time administration of cell-loaded MNs (left) and hypodermic needle injection of cell suspension (right). | ||
4. MN systems for cell sampling and immune cell stimulation
The MN systems could provide an easy-to-use and less-invasive way to overcome the tissue barrier to make it available to access compartments of the body to collect fluid or cell samples for medical monitoring.56–59 In recent years, researchers in Massachusetts Institute of Technology have developed a hydrogel-coated solid MN system for the longitudinal sampling of viable cells and interstitial fluid, enabling the parallel monitoring of skin-resident immunity (Fig. 7A).35 After skin insertion, the hydrogel layer swells upon contact with the interstitial fluid and withdraws the immune cells that reside in the insertion sites. When embedding the immunologic adjuvants and immune-boosting agents within the hydrogel layer, a single 1 cm2 MN patch (80 needles, 500 μm in length) can withdraw around 5000 live cells including ∼2500 leukocytes after a 24 h application, a more than two-fold increase from the blank MNs patch. Antigen-presenting cells (APCs) migrate into the MNs in response to the localized inflammation induced by the MN application, are activated by the adjuvants and take up the embedded antigens. Cytokines and chemokines produced by these activated APCs in turn promote the recruitment of T cells into the hydrogel layer. More importantly, with applying such an optimized MN system into the mice immunized with an immunogenic protein, the authors found that the skin-resident memory T cells withdrawn by MNs are not migrated back to the insertion sites to perturb the immune status of mice, indicating the potential of this cell sampling MN system for reliable immune monitoring.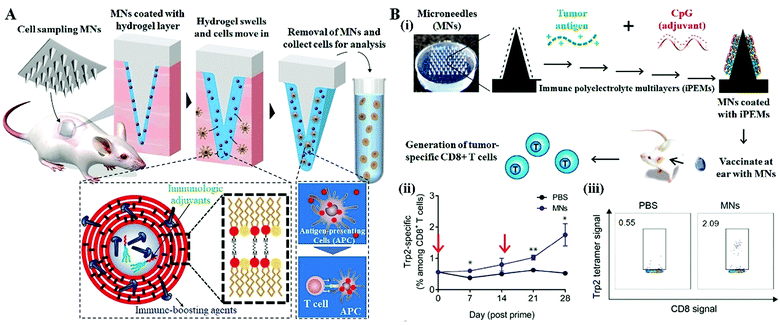 | ||
| Fig. 7 MN systems for immune cell monitoring and immunostimulation. (A) Schematic of the cell and fluid sampling MNs for skin immune monitoring.35 (B) Expansion of tumor-specific T cells using solid MNs coated with immune-polyelectrolyte multilayers (iPEMs).34 (i) Schematic of iPEM-coated MNs for promoting immune cell generation. (ii) Development of tumor-specific CD8+ T cell responses over 28 days. Arrows indicated prime and booster MN applications. (iii) Representative flow cytometry scatter plots demonstrating the development of a clear population of CD8+ T cells specific for the Trp2 epitope on day 28 post prime. | ||
Recently, several studies have begun demonstrating the enhanced cell therapy efficacy by using MN treatment as the stimuli.60,61 Zeng reported the in vivo expansion of tumor-specific T cells using solid MN coated with immune polyelectrolyte multilayers (iPEMs), showing more potent memory recall responses occurred during the MN application (Fig. 7B).34 The iPEMs are mainly composed of the conserved human melanoma antigen, tyrosinase-related protein 2 (Trp2), and CpG oligonucleotide-based toll like receptor (TRL) agonists that serve as adjuvants. Following the MN application in mice, the iPEMs cargo released from MNs in situ and incubated with primary immune cells promotes the uptake and activation of antigen-presenting cells, triggering functional responses in tumor-specific T cells during coculture.
On the other hand, microneedles themselves also offer a fascinating and intriguing cell stimulation feature. Following the MN insertion, the cells located around the needle holes likely sense the penetrations as new induced wound stimuli and therefore are in an active state that leads to a polarized electro-magnetic field in the inter-cellular electrolyte, promoting the release of growth factors.62 This results in proliferation and migration of fibroblasts, and thus further improving the therapeutic effect of the MN treatment.
5. Conclusion and future prospects
With cell therapy moving towards clinical trials and further commercialization, it is clear that selecting suitable cell delivery systems is becoming increasingly important since they may affect the retention, viability, and functionality of the therapeutic cells and therefore influence the subsequent treatment efficacy and future process development. An ideal cell delivery system should not only be able to precisely and reproducibly deliver cells to the target site in a less invasive manner but also ensure that the delivered cells work without causing side effects. Extensive research over the past decades provided ample proof of the potential of MN-based technology for medical therapy and diagnosis in a convenient and minimally invasive manner.63–66 The studies summarized in this review show the feasibility of the MN-based technology for cell delivery or cell sampling due to its innate advantages of pain-free mechanical disruption of the tissue barriers, better tissue compatibility, selective lesions targeting, and enhanced therapeutic efficacy with a reduction in dose and side effects. Compared to conventional cell injections or clinical implantation, the MN-based cell therapy can control the delivery of the therapeutic cells to the targeted sites with minimal tissue damage and is more suitable for self-administration by patients.However, since the cells are fragile and preservation of their functionality is indispensable for therapeutic efficacy, the development of MN-based cell therapy that can deliver therapeutic cells into the targeted tissue at the effective level for the desired duration of time remains a bottleneck problem. Extensive efforts are required to identify the best mode of cell delivery for specific disease treatment (cell free or cell loaded MN system), increase both the cell loading capacity and viability during the MN application process to achieve the effective therapeutic concentration, and retain the delivered cells at the desired location for the desired duration of time. Although various optimized biomaterials and cytokines have been utilized to support cell viability and functionality, the number of cells that can be loaded in the MNs is severely restricted by the structure of the MN system.67,68 This might be solved by the development of cell loaded MNs with high volume (e.g. funnel structure and larger dimensions) and high delivery efficiency (e.g. fully embeddable and higher density), or by applying the cell free MN system combined with assistive equipment (e.g. cell reservoir and external actuator). But how to realize the uniform distribution of cells within MNs and the accurate controlling of the number of cells that is delivered by every insertion of MNs require to be considered. On the other hand, the mechanical properties of the MN system also play a prominent role in both cell proliferation and delivery. So far, as we know, the cell viability and regenerative capacity are highly influenced by the surrounding microenvironment; thus, the cell-contacted materials should be similar to the extracellular matrix with an appropriate stiffness and biocompatibility.69,70 Moreover, the cell-based MNs should also possess sufficient mechanical strength for successful tissue penetration without needle fracture. For example, hydrogel MNs only in the solid-state are strong enough for tissue insertion, but the live cells just survived in the hydrated state.43 Although the core–shell structure or cell-coated MNs showed some practical potential, these strategies are generally associated with the complex fabrication process and harsh application requirements. In addition, the seeding or encapsulation of cells will weaken the mechanical strength of MNs and the variability of tissue characteristics among individuals also has a negative impact on the successful MN application. We envisage that a matched applicator would be helpful in achieving reproducible insertion ability.
Another challenge to the implementation of MN-based cell therapy is setting up a reliable manufacturing and storage system to culture and transport cells in the optimum environment for their survival and therapy. The substantial benefit of cell therapy requires that not only the cells be delivered into the correct target tissue, but also establish the therapeutic function within the tissue over time.71 More efforts should thus be focused on understanding how cells interact with the MN matrix and with the surrounding cells to guide the developing biomimetic MN systems that are capable of enhancing the cell function and extending therapeutic utility. On the other hand, the manufacturing processes so far applied for the fabrication of MN-based cell therapies largely resemble those employed in conventional MN systems. Unlike drug-loaded MNs, however, cells are biological materials that are notoriously difficult to grow and transport, requiring complex fabrication processes and specific storage conditions to generate consistent products without compromising their native activities and functions.72,73 Automation of the manufacturing processes, for example, integrating with sensors, robotics, or image acquisition and processing software, would be promising reproducible protocols for the development of MN-based cell therapy in the future.
Furthermore, there was definitely a concern about whether the application of MN-based cell therapy would cause unintended consequences.74,75 In some cases, the treatment outcome will not be as effective as expected and may prolong or worsen symptoms and the inappropriate migration of the delivered cells could also lead to tumor formation. Although animal research studies have been conducted for years, moving toward clinical trials remains a daunting challenge as it requires more comprehensive data on the physicochemical properties and biological evaluations of the MN systems. Future research studies should be devoted to determining the potential application site reactions, the biocompatibility of the MN materials, interactions between MN-based cell therapy and the microenvironments, and the immune rejection of the delivered cells and the therapeutic cell functionalities. Examination of the benefit-risk ratio to participants after the application of the MN-based cell therapy is crucial to facilitate clinical applications and future product translation (Fig. 8).
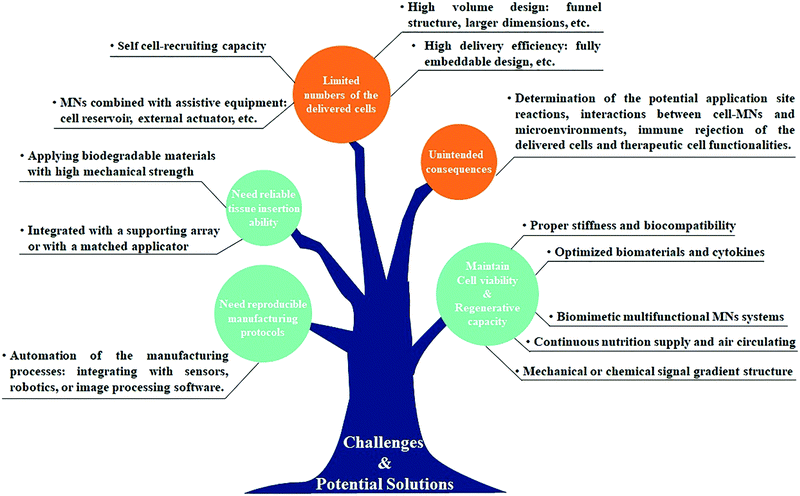 | ||
| Fig. 8 A summary of current challenges and potential solutions in the MN-based technology for cell therapy. | ||
The achievements of the MN-based technology over the past decades have bolstered efforts in recent years to develop MN-based cell therapy for a multitude of disease treatments. Interest in this area is dramatically increased and t many are hopeful of achieving clinical trials in the coming years. Future perspectives may involve the development of biomimetic or multifunctional MN systems capable of self-cell-recruiting, continuous nutrition supply and air circulating, avoiding the immune reaction, and endowing augmented therapeutic efficacy. We believe that the advances in materials science, along with an improved understanding of therapeutic cell functionalities, will allow the refinements of the current MN-based technology for cell therapy to make a strong contribution toward the currently unmet patient need.
Author contributions
B. Z. C.: conceptualization, investigation, and writing – original draft preparation. Z. Q. Z.: data curation. M. A. S.: writing – review and editing. X. D. G.: writing – review and editing and supervision. All the authors gave approval to the final version of the manuscript.Conflicts of interest
The authors declare that they have no conflicts of interest.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (51873015 and 52161145410), the Joint Project of BRC-BC (Biomedical Translational Engineering Research Center of BUCT-CJFH) (RZ2020-01) and the long-term subsidy mechanism from the Ministry of Finance and the Ministry of Education of PRC.References
- Z. Chen, Q. Hu and Z. Gu, Acc. Chem. Res., 2018, 51, 668–677 CrossRef CAS PubMed.
- A. L. Facklam, L. R. Volpatti and D. G. Anderson, Adv. Mater., 2020, 32, e1902005 CrossRef PubMed.
- https://clinicaltrials.gov/ct2/results?cond=cell+therapy&term=&cntry=&state=&city=&dist=, 2021.
- E. Curtis, J. R. Martin, B. Gabel, N. Sidhu, T. K. Rzesiewicz, R. Mandeville, S. Van Gorp, M. Leerink, T. Tadokoro, S. Marsala, C. Jamieson, M. Marsala and J. D. Ciacci, Cell Stem Cell, 2018, 22, 941–950.e946 CrossRef CAS PubMed.
- R. Dong, X. Zhao, B. Guo and P. X. Ma, ACS Appl. Mater. Interfaces, 2016, 8, 17138–17150 CrossRef CAS PubMed.
- D. J. Mooney and H. Vandenburgh, Cell Stem Cell, 2008, 2, 205–213 CrossRef CAS PubMed.
- C. C. Sheng, L. Zhou and J. Hao, BioMed Res. Int., 2013, 547902 Search PubMed.
- J. Y. Chua, A. V. Pendharkar, N. Wang, R. Choi, R. H. Andres, X. Gaeta, J. Zhang, M. E. Moseley and R. Guzman, J. Cereb. Blood Flow Metab., 2011, 31, 1263–1271 CrossRef PubMed.
- N. G. Campbell and K. Suzuki, J. Cardiovasc. Transl., 2012, 5, 713–726 CrossRef PubMed.
- S.-J. Park, R. Y. Kim, B.-W. Park, S. Lee, S. W. Choi, J.-H. Park, J. J. Choi, S.-W. Kim, J. Jang, D.-W. Cho, H.-M. Chung, S.-H. Moon, K. Ban and H.-J. Park, Nat. Commun., 2019, 10, 3123 CrossRef PubMed.
- L. Danielyan, S. Beer-Hammer, A. Stolzing, R. Schäfer, G. Siegel, C. Fabian, P. Kahle, T. Biedermann, A. Lourhmati, M. Buadze, A. Novakovic, B. Proksch, C. H. Gleiter, W. H. Frey and M. Schwab, Cell Transplant., 2014, 23, S123–S139 Search PubMed.
- J. Vaquero, M. Zurita, M. A. Rico, C. Aguayo, C. Bonilla, E. Marin, N. Tapiador, M. Sevilla, D. Vazquez, J. Carballido, C. Fernandez, G. Rodriguez-Boto and M. Ovejero, Cytotherapy, 2018, 20, 806–819 CrossRef PubMed.
- X. Che, J. Guo, X. Li, L. Wang and S. Wei, Mol. Med. Rep., 2016, 13, 681–688 CrossRef CAS PubMed.
- C. S. Cox, Jr., J. Juranek and S. Bedi, Transfusion, 2019, 59, 858–868 CrossRef PubMed.
- M. T. Harting, F. Jimenez, H. Xue, U. M. Fischer, J. Baumgartner, P. K. Dash and C. S. Cox, J. Neurosurg., 2009, 110, 1189–1197 CAS.
- H. Cui, C. Liu, T. Esworthy, Y. Huang, Z.-X. Yu, X. Zhou, H. San, S.-J. Lee, S. Y. Hann, M. Boehm, M. Mohiuddin, J. P. Fisher and L. G. Zhang, Sci. Adv., 2020, 6, eabb5067 CrossRef CAS PubMed.
- C. E. Kilmer, C. M. Battistoni, A. Cox, G. J. Breur, A. Panitch and J. C. Liu, ACS Biomater. Sci. Eng., 2020, 6, 3464–3476 CrossRef CAS PubMed.
- Q. Pagneux, R. Ye, L. Chengnan, A. Barras, N. Hennuyer, B. Staels, D. Caina, J. Osses, A. Abderrahmani, V. Plaisance, V. Pawlowski, R. Boukherroub, S. Melinte and S. Szunerits, Nanoscale Horiz., 2020, 5, 663–670 RSC.
- Y. Kobayashi, C. E.-J. Cordonier, Y. Noda, F. Nagase, J. Enomoto, T. Kageyama, H. Honma, S. Maruo and J. Fukuda, Sci. Rep., 2019, 9, 10415 CrossRef PubMed.
- Y. C. Kim, J. H. Park and M. R. Prausnitz, Adv. Drug Delivery Rev., 2012, 64, 1547–1568 CrossRef CAS PubMed.
- B. Z. Chen, L. Q. Zhang, Y. Y. Xia, X. P. Zhang and X. D. Guo, Sci. Adv., 2020, 6, eaba7260 CrossRef CAS PubMed.
- X. Jin, D. D. Zhu, B. Z. Chen, M. Ashfaq and X. D. Guo, Adv. Drug Delivery Rev., 2018, 127, 119–137 CrossRef CAS PubMed.
- S. Henry, D. V. McAllister, M. G. Allen and M. R. Prausnitz, J. Pharm. Sci., 1998, 87, 922–925 CrossRef CAS PubMed.
- B. Demir, L. Rosselle, A. Voronova, Q. Pagneux, A. Quenon, V. Gmyr, D. Jary, N. Hennuyer, B. Staels, T. Hubert, A. Abderrahmani, V. Plaisance, V. Pawlowski, R. Boukherroub, S. Vignoud and S. Szunerits, Nanoscale Horiz., 2022, 7, 174–184 RSC.
- M. R. Prausnitz, Adv. Drug Delivery Rev., 2004, 56, 581–587 CrossRef CAS PubMed.
- R. F. Donnelly, T. R. Singh, M. J. Garland, K. Migalska, R. Majithiya, C. M. McCrudden, P. L. Kole, T. M. Mahmood, H. O. McCarthy and A. D. Woolfson, Adv. Funct. Mater., 2012, 22, 4879–4890 CrossRef CAS PubMed.
- B. L. Zhang, X. P. Zhang, B. Z. Chen, W. M. Fei, Y. Cui and X. D. Guo, Microchem. J., 2021, 162, 105830 CrossRef CAS.
- H. Lee, C. Song, S. Baik, D. Kim, T. Hyeon and D. H. Kim, Adv. Drug Delivery Rev., 2018, 127, 35–45 CrossRef CAS PubMed.
- J. N. Zhang, B. Z. Chen, M. Ashfaq, X. P. Zhang and X. D. Guo, J. Ind. Eng. Chem., 2018, 65, 363–369 CrossRef CAS.
- J. L. Tan, J. Tien, D. M. Pirone, D. S. Gray, K. Bhadriraju and C. S. Chen, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 1484 CrossRef CAS PubMed.
- A. Friedmann, A. Cismak, C. Tautorat, P. J. Koester, W. Baumann, J. Held, J. Gaspar, P. Ruther, O. Paul and A. Heilmann, Scanning, 2012, 34, 221–229 CrossRef CAS PubMed.
- G. H. Teichert, S. Burnett and B. D. Jensen, J. Micromech. Microeng., 2013, 23, 095003 CrossRef CAS.
- Y. Q. Ye, J. C. Yu, C. Wang, N. Y. Nguyen, G. M. Walker, J. B. Buse and Z. Gu, Adv. Mater., 2016, 28, 3115–3121 CrossRef CAS PubMed.
- Q. Zeng, J. M. Gammon, L. H. Tostanoski, Y. C. Chiu and C. M. Jewell, ACS Biomater. Sci. Eng., 2017, 3, 195–205 CrossRef CAS PubMed.
- A. Mandal, A. V. Boopathy, L. K.-W. Lam, K. D. Moynihan, M. E. Welch, N. R. Bennett, M. E. Turvey, N. Thai, J. H. Van, J. C. Love, P. T. Hammond and D. J. Irvine, Sci. Transl. Med., 2018, 10, eaar2227 CrossRef PubMed.
- B. Gualeni, S. A. Coulman, D. Shah, P. F. Eng, H. Ashraf, P. Vescovo, G. J. Blayney, L. D. Piveteau, O. J. Guy and J. C. Birchall, Br. J. Dermatol., 2018, 178, 731–739 CrossRef CAS PubMed.
- F. S. Iliescu, J. C.-M. Teo, D. Vrtacnik, H. Taylor and C. Iliescu, Microsyst. Technol., 2018, 24, 2905–2912 CrossRef CAS.
- J. Tang, J. Wang, K. Huang, Y. Ye, T. Su, L. Qiao, M. T. Hensley, T. G. Caranasos, J. Zhang, Z. Gu and K. Cheng, Sci. Adv., 2018, 4, eaat9365 CrossRef CAS PubMed.
- Y.-H. Chen, D.-C. Lin, E. Chern and Y.-Y. Huang, Biomed. Microdevices, 2020, 22, 63 CrossRef CAS PubMed.
- P. Pukfukdee, W. Banlunara, T. Rutwaree, B. Limcharoen, P. Sawutdeechaikul, T. Pattarakankul, T. Sansureerungsikul, P. Toprangkobsin, A. Leelahavanichkul, R. Panchaprateep, P. Asawanonda, T. Palaga and S. Wanichwecharungruang, ACS Appl. Bio Mater., 2020, 3, 4581–4589 CrossRef CAS PubMed.
- L. Sun, X. Zhu, X. Zhang, G. Chen, F. Bian, J. Wang, Q. Zhou, D. Wang and Y. Zhao, Chem. Eng. J., 2021, 414, 128723 CrossRef CAS.
- H. Li, Z. Wang, E. Archibong, Q. Wu, G. Chen, Q. Hu, T. Ci, Z. Chen, J. Wang, D. Wen, H. Du, J. Jiang, J. Sun, X. Zhang, G. Dotti and Z. Gu, Natl. Sci. Rev., 2021, 9, nwab172 CrossRef PubMed.
- K. Lee, Y. Xue, J. Lee, H.-J. Kim, Y. Liu, P. Tebon, E. Sarikhani, W. Sun, S. Zhang, R. Haghniaz, B. Çelebi-Saltik, X. Zhou, S. Ostrovidov, S. Ahadian, N. Ashammakhi, M. R. Dokmeci and A. Khademhosseini, Adv. Funct. Mater., 2020, 30, 2000086 CrossRef CAS PubMed.
- H. Chang, S. W.-T. Chew, M. Zheng, D. C.-S. Lio, C. Wiraja, Y. Mei, X. Ning, M. Cui, A. Than, P. Shi, D. Wang, K. Pu, P. Chen, H. Liu and C. Xu, Nat. Biomed. Eng., 2021, 5, 1008–1018 CrossRef CAS PubMed.
- E. R. Ruskowitz and C. A. DeForest, Nat. Rev. Mater., 2018, 3, 17087 CrossRef CAS.
- B. M. Gumbiner, Cell, 1996, 84, 345–357 CrossRef CAS PubMed.
- L.-C. Cheng, X. Jiang, J. Wang, C. Chen and R.-S. Liu, Nanoscale, 2013, 5, 3547–3569 RSC.
- H. Li, Z. Wang, E. A. Ogunnaike, Q. Wu, G. Chen, Q. Hu, T. Ci, Z. Chen, J. Wang, D. Wen, H. Du, J. Jiang, J. Sun, X. Zhang, G. Dotti and Z. Gu, Natl. Sci. Rev., 2022, 9, nwab172 Search PubMed.
- M. L. Weiss, M. S. Rao, R. Deans and P. Czermak, Stem Cells Int., 2016, 1750697 Search PubMed.
- L. T. Wang, K. J. Liu, H. K. Sytwu, M. L. Yen and B. L. Yen, Stem Cells Transl. Med., 2021, 10, 1288–1303 CrossRef PubMed.
- B. Z. Chen, Y. Yang, B. B. Wang, M. Ashfaq and X. D. Guo, Int. J. Pharm., 2019, 556, 338–348 CrossRef CAS PubMed.
- B. Z. Chen, J. L. Liu, Q. Y. Li, Z. N. Wang, X. P. Zhang, C. B. Shen, Y. Cui and X. D. Guo, ACS Appl. Bio Mater., 2019, 2, 5616–5625 CrossRef CAS PubMed.
- J. A. Burdick, R. L. Mauck and S. Gerecht, Cell Stem Cell, 2016, 18, 13–15 CrossRef CAS PubMed.
- A. K. Grosskopf, G. A. Roth, A. A.-A. Smith, E. C. Gale, H. L. Hernandez and E. A. Appel, Bioeng. Transl. Med., 2020, 5, e10147 CAS.
- M. C. He, B. Z. Chen, M. Ashfaq and X. D. Guo, Drug Delivery Transl. Res., 2018, 8, 1034–1042 CrossRef CAS PubMed.
- P. P. Samant, M. M. Niedzwiecki, N. Raviele, V. Tran, J. Mena-Lapaix, D. I. Walker, E. I. Felner, D. P. Jones, G. W. Miller and M. R. Prausnitz, Sci. Transl. Med., 2020, 12, eaaw0285 CrossRef CAS PubMed.
- H. Lee, T. K. Choi, Y. B. Lee, H. R. Cho, R. Ghaffari, L. Wang, H. J. Choi, T. D. Chung, N. Lu, T. Hyeon, S. H. Choi and D.-H. Kim, Nat. Nanotechnol., 2016, 11, 566–572 CrossRef CAS PubMed.
- T. Liu, G. Luo and M. Xing, Adv. Ther., 2020, 3, 1900140 CrossRef CAS.
- L. Ventrelli, L. M. Strambini and G. Barillaro, Adv. Healthcare Mater., 2015, 4, 2606–2640 CrossRef CAS PubMed.
- G. S. Guvanasen, L. Guo, R. J. Aguilar, A. L. Cheek, C. S. Shafor, S. Rajaraman, T. R. Nichols and S. P. DeWeerth, IEEE Trans. Neural Syst. Rehabil. Eng., 2017, 25, 1440–1452 Search PubMed.
- S. Bhatnagar, K. Dave and V. V.-K. Venuganti, J. Controlled Release, 2017, 260, 164–182 CrossRef CAS PubMed.
- H. Liebl and L. C. Kloth, J. Am. Coll. Clin. Wound Spec., 2012, 4, 2–6 CrossRef PubMed.
- Y. Yang, L. Xu, D. Jiang, B. Z. Chen, R. Luo, Z. Liu, X. Qu, C. Wang, Y. Shan, Y. Cui, H. Zheng, Z. Wang, Z. L. Wang, X. D. Guo and Z. Li, Adv. Funct. Mater., 2021, 31, 2104092 CrossRef CAS.
- B. Z. Chen, M. Ashfaq, D. D. Zhu, X. P. Zhang and X. D. Guo, Macromol. Rapid Commun., 2018, 39, 1800075 CrossRef PubMed.
- H. Amani, M.-A. Shahbazi, C. D'Amico, F. Fontana, S. Abbaszadeh and H. A. Santos, J. Controlled Release, 2021, 330, 185–217 CrossRef CAS PubMed.
- V. Alimardani, S. S. Abolmaali, A. M. Tamaddon and M. Ashfaq, Drug Delivery Transl. Res., 2021, 11, 788–816 CrossRef PubMed.
- Q. Lei, J. Guo, F. Kong, J. Cao, L. Wang, W. Zhu and C. J. Brinker, J. Am. Chem. Soc., 2021, 143, 6305–6322 CrossRef CAS PubMed.
- A. Blocki, S. Beyer, J. Y. Dewavrin, A. Goralczyk, Y. Wang, P. Peh, M. Ng, S. S. Moonshi, S. Vuddagiri, M. Raghunath, E. C. Martinez and K. K. Bhakoo, Biomaterials, 2015, 53, 12–24 CrossRef CAS PubMed.
- Y. S. Pek, A. C. Wan and J. Y. Ying, Biomaterials, 2010, 31, 385–391 CrossRef CAS PubMed.
- O. Chaudhuri, L. Gu, D. Klumpers, M. Darnell, S. A. Bencherif, J. C. Weaver, N. Huebsch, H. P. Lee, E. Lippens, G. N. Duda and D. J. Mooney, Nat. Mater., 2016, 15, 326–334 CrossRef CAS PubMed.
- C. Raffin, L. T. Vo and J. A. Bluestone, Nat. Rev. Immunol., 2020, 20, 158–172 CrossRef CAS PubMed.
- C. F. Bellani, J. Ajeian, L. Duffy, M. Miotto, L. Groenewegen and C. J. Connon, Front. Nutr., 2020, 7, 575146 CrossRef PubMed.
- E. A. Guzzi, G. Bovone and M. W. Tibbitt, Small, 2019, 15, 1905421 CrossRef CAS PubMed.
- N. M. Toyserkani, M. G. Jørgensen, S. Tabatabaeifar, C. H. Jensen, S. P. Sheikh and J. A. Sørensen, Stem Cells Transl. Med., 2017, 6, 1786–1794 CrossRef PubMed.
- P. D. Lulla, I. Tzannou, S. Vasileiou, G. Carrum, C. A. Ramos, R. Kamble, T. Wang, M. Wu, M. Bilgi, A. P. Gee, S. Mukhi, B. Chung, L. Wang, A. Watanabe, M. Kuvalekar, M. Jeong, Y. Li, S. Ketkar, M. French-Kim, B. Grilley, M. K. Brenner, H. E. Heslop, J. F. Vera and A. M. Leen, Sci. Transl. Med., 2020, 12, eaaz3339 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |