Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Two boron atoms versus one: high-performance deep-blue multi-resonance thermally activated delayed fluorescence emitters†‡
Chin-Yiu
Chan§
*a,
Subeesh
Madayanad Suresh§
b,
Yi-Ting
Lee§
a,
Youichi
Tsuchiya
 a,
Tomas
Matulaitis
b,
David
Hall
bc,
Alexandra M. Z.
Slawin
a,
Tomas
Matulaitis
b,
David
Hall
bc,
Alexandra M. Z.
Slawin
 b,
Stuart
Warriner
d,
David
Beljonne
b,
Stuart
Warriner
d,
David
Beljonne
 c,
Yoann
Olivier
c,
Yoann
Olivier
 e,
Chihaya
Adachi
e,
Chihaya
Adachi
 *af and
Eli
Zysman-Colman
*af and
Eli
Zysman-Colman
 *b
*b
aCenter for Organic Photonics and Electronics Research (OPERA), Kyushu University, Motooka, Nishi, Fukuoka 819-0395, Japan. E-mail: chinyiu.chan@opera.kyushu-u.ac.jp
bOrganic Semiconductor Centre, EaStCHEM School of Chemistry, University of St Andrews, St Andrews, KY16 9ST, UK. E-mail: eli.zysman-colman@st-andrews.ac.uk
cLaboratory for Chemistry of Novel Materials, University of Mons, 7000, Mons, Belgium
dSchool of Chemistry, University of Leeds, Woodhouse Lane, Leeds, UK
eLaboratory for Computational Modeling of Functional Materials, Namur Institute of Structured Matter, Université de Namur, Rue de Bruxelles, 61, B-5000 Namur, Belgium
fInternational Institute for Carbon Neutral Energy Research (I2CNER), Kyushu University, 744 Motooka, Nishi, Fukuoka 819-0395, Japan. E-mail: adachi@cstf.kyushu-u.ac.jp
First published on 21st July 2022
Abstract
Two new deep-blue narrowband multi-resonant emitters, 1B-DTACrs and 2B-DTACrs, one of which shows thermally activated delayed fluorescence (TADF), based on boron, nitrogen, and oxygen doped nanographenes are reported. Devices based on 2B-DTACrs showed an EQEmax of 14.8% and CIE coordinates of (0.150, 0.044), which are very close to the BT.2020 requirement for blue pixels.
Thermally activated delayed fluorescence (TADF) materials can achieve 100% internal quantum efficiency (IQE) by harvesting both singlet and triplet excitons. In 2012, Adachi and co-workers first reported organic light-emitting diodes (OLEDs) that showed IQEs of 100% based on organic donor–acceptor (D–A) type TADF emitters.1 Successively, a wide variety of D–A type TADF emitters have been developed, resulting in devices showing high external quantum efficiencies (EQEs) of over 20% for blue, green, and red emission colors.2–6 Nonetheless, D–A type TADF emitters usually possess broad emission bands that result in devices with poor color purity pixels. To achieve the BT.2020 standard for high-definition displays, narrowband electroluminescence (EL) in devices that show high EQE is highly desired.
Beyond the D–A type emitters, in 2016, Hatakeyama and co-workers first reported pure-blue boron-containing multi-resonance TADF (MR-TADF) emitters that feature narrowband emissions and high photoluminescence quantum yields (ΦPL, PLQYs).7 The narrowband emission behaviour of MR-TADF emitters is the most suitable for high-definition display with a wide colour gamut, i.e., BT.2020 standard. Since then, the development of MR-TADF emitters has attracted much attention.8–10 To date, there have been numerous reports on high-efficiency OLEDs based on MR-TADF emitters with blue, green, and red emissions.8–13 Nonetheless, there are a limited number of reports on high-efficiency MR-TADF emitters that can fulfil the requirement of the colour gamut for the blue pixel, i.e., Commission Internationale de lÉclairage coordinates (CIEx,y) = (0.131, 0.046).14 As a result, it is necessary to develop high-efficiency deep-blue MR-TADF emitters that satisfy the colour gamut requirement of the BT.2020 standard, i.e., CIEy ≤ 0.05.
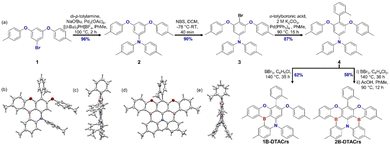
Here, we report two boron-based deep-blue emitters containing a mixed O/N donor atom system, one of which is MR-TADF. 1B-DTACrs and 2B-DTACrs were synthesized in five steps starting from 3,5-difluorobromobenzene. The key borylation reaction proceeded in 62% and 58% yield, respectively, for 1B-DTACrs and 2B-DTACrs from the common intermediate 4. Site selective borylation was achieved by controlling the reaction temperature and the amount of BBr3 in the reaction (Fig. 1). The identity and purity of both emitters were confirmed by a combination of 1H NMR (Fig. S13 and S17, ESI†), 13C NMR (Fig. S14 and S18, ESI†) spectroscopy, melting point determination, high-resolution mass spectrometry (Fig. S15 and S19, ESI†), single crystal X-ray diffraction (Fig. 1), and elemental analysis (Fig. S16 and S20, ESI†). 1B-DTACrs and 2B-DTACrs both display deep-blue narrowband emission at 440 and 447 nm and FWHMs of 30 and 26 nm, respectively, in the device. However, 1B-DTACrs is TADF-inactive while 2B-DTACrs is TADF-active. Devices based on 1B-DTACrs and 2B-DTACrs demonstrated maximum external quantum efficiencies (EQEmax) of 1.3 and 14.8%, respectively, together with corresponding CIE coordinates of (0.154, 0.049) and (0.150, 0.044), which are very close to the BT.2020 standard, i.e., (0.131, 0.046).
Crystals for 1B-DTACrs were obtained by slow diffusion of hexane into a saturated solution of the emitter in THF. Crystals of 2B-DTACrs were obtained directly from a temperature gradient sublimation method (Fig. 1). The ditolylamine (DTA) unit adopts a propeller structure. In the case of 1B-DTACrs, where there is only one boron in the compound, the MR-TADF core remains nearly planar. The o-tolyl unit is approximately perpendicular to the aromatic core in both emitters. Yasuda and co-workers reported BSBS-N1,15 a structurally analogous sulfur-containing compound to 2B-DTACrs, which was described as having a highly distorted structure, whereas 2B-DTACrs possesses a nearly planar geometry at the B,O-substituted pentacene core with an out-of-plane alignment of two aryl rings of the DTA unit.15 There are no significant intermolecular π–π interactions in the solid state for either compound, which may be a consequence of the orthogonally disposed o-tolyl unit.
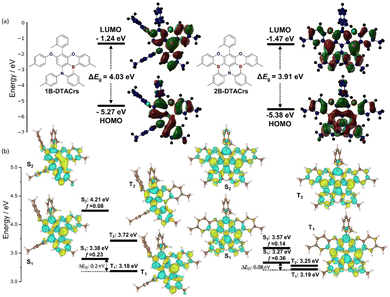
The ground- and excited-state properties of 1B-DTACrs and 2B-DTACrs were modelled using DFT and spin-component scaling second order approximate coupled-cluster (SCS-CC2) methods, respectively (Fig. 2). Both compounds possess difference density distributions between the ground and excited-state reminiscent of other MR-TADF emitters.10 Addition of the second boron atom in 2B-DTACrs resulted in a small stabilization in the HOMO and LUMO energy levels compared to those of 1B-DTACrs and a corresponding small decrease (0.12 eV) in the band gap (Fig. 2(a)). The S1 state is stabilized from 3.38 eV in 1B-DTACrs to 3.27 eV in 2B-DTACrs, while the T1 states are essentially isoenergetic. Thus, there is a much smaller ΔEST value of 0.08 eV for 2B-DTACrs compared to 0.20 eV of 1B-DTACrs. The oscillator strength (f) for the S0–S1 transition is higher at 0.36 for 2B-DTACrs compared to 0.23 for 1B-DTACrs likely for the apparent largest delocalization of the S1 excited state. Both the S2 and T2 states of 2B-DTACrs are also stabilized compared to those of 1B-DTACrs. The small energy difference between S1 and T2 (ΔEST2) is of particular interest as many reports have highlighted that reverse intersystem crossing (RISC) occurs via intermediate triplet states.16 The ΔEST2 drops from 0.34 eV in 1B-DTACrs to 0.02 eV in 2B-DTACrs. For each compound, spin-orbital coupling (SOC) is modest between S1 and T1, calculated to be 0.07 cm−1 and 0.04 cm−1 for 1B-DTACrs and 2B-DTACrs, respectively. This increases to 0.81 cm−1 and 0.98 cm−1 between S1 and T2 for 1B-DTACrs and 2B-DTACrs, respectively (Table S3, ESI†). These results suggest that RISC likely proceeds from T2 to S1, particularly for 2B-DTACr.17 Tables S1–S3 (ESI†) provide a full summary of the excited state energies, transitions and SOC values.
The electrochemical properties were measured using cyclic voltammetry (CV) and differential pulse voltammetry (DPV) in deaerated dichloromethane (DCM) with 0.1 M tetra-n-butylammonium hexafluorophosphate as the supporting electrolyte (Fig. S21 and Table S4, ESI†). The oxidation potentials, Eox, determined from the peak of the first oxidation wave of the DPV, are 1.07 V and 1.17 V versus a saturated calomel electrode (SCE), respectively, for 1B-DTACrs and 2B-DTACrs. The corresponding reduction potentials, Ered, are −2.10 V and −1.89 V, respectively, versus SCE for 1B-DTACrs and 2B-DTACrs. The corresponding HOMO and LUMO levels were calculated to be −5.42 eV and −2.24 eV for 1B-DTACrs.18 The HOMO and LUMO energy levels are stabilized to −5.51 eV and −2.46 eV, respectively, for 2B-DTACrs. The electrochemical gap is thus reduced from 3.17 eV for 1B-DTACrs to 3.05 eV for 2B-DTACrs, in line with the trend predicted by the gas-phase DFT calculations.
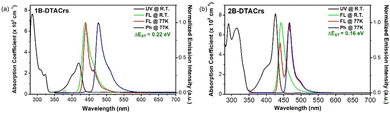
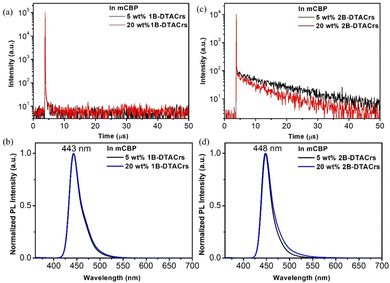
The photophysical properties of 1B-DTACrs and 2B-DTACrs were firstly examined in dilute toluene solutions (10−5 M). The photophysical data are shown in Fig. 3, and Table 1. Both emitters showed absorption bands below 350 nm, which are assigned as locally excited (LE) π–π* transitions, while the absorption bands beyond 400 nm are assigned as short-range charge transfer (SRCT) bands. Both compounds possess narrow high-energy bands assigned to transitions to the S1 state, with molar absorptivity coefficients, ε, of 3 and 8 × 105 M−1 cm−1 for 1B-DTACrs and 2B-DTACrs, respectively, in line with the larger computed f associated with the S0 → S1 transitions (vide supra). The higher calculated f value and measured ε in 2B-DTACrs originates from the extended conjugation in the compound.191B-DTACrs and 2B-DTACrs emit in the deep-blue in toluene at 438 and 443 nm, respectively. The PL spectra of 1B-DTACrs and 2B-DTACrs are narrow with full-width at half-maxima (FWHM) values of 27 and 21 nm, respectively. The narrowband emission coupled with the small Stokes-shifts reflect the rigid nature of these compounds and the small degree of reorganization in the excited state. The ΦPL in degassed toluene solutions of 1B-DTACrs is 99%, while 2B-DTACrs showed a rather lower ΦPL of 62%. When doped in a 3,3′-di(9H-carbazol-9-yl)-1,1′-biphenyl (mCBP) host at 5 wt% of 1B-DTACrs or 2B-DTACrs, the ΦPL values remain high at 83 and 74%, respectively. Both doped films display emissions (Fig. 4) solely originated from the corresponding emitter, which indicate an efficient energy transfer from mCBP to the emitter. The transient decay profiles of both doped films reveal that 1B-DTACrs showed no TADF behaviour. By contrast, 2B-DTACrs showed TADF behaviour with a delayed lifetime (τd) of 13.1 μs. The TADF behaviour of 2B-DTACrs was further confirmed by temperature-dependent transient emission decay measurement (Fig. S22, ESI†). The ΔEST values of the mCBP doped films were determined from the difference between the onsets of the fluorescence and phosphorescence spectra at 77 K (Fig. S23, ESI†). The fairly large ΔEST in 1B-DTACrs-doped film (0.21 eV) may account for the non-TADF behaviour while that of 2B-DTACrs is smaller at 0.16 eV. The rate constants of doped films of 1B-DTACrs and 2B-DTACrs were calculated, and the data are summarized in Table 1. The reverse intersystem crossing rate (kRISC) of 2B-DTACrs was found to be 1.3 × 105 s−1, which is amongst the fastest kRISC for deep-blue MR-TADF emitters with CIEy ≤ 0.10 (Table S5, ESI†).
| In toluene | 5 wt% in mCBP | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Material | λ abs (nm) | λ max (nm) | FWHM (nm) | Φ Air (%) | Φ Ar (%) | ΔEST (eV) | λ max (nm) | FWHM (nm) | ΔEST (eV) | Φ Ar (%) | Φ p (%) | Φ d (%) | τ p (ns) | τ d (μs) | k r (108 s−1) | k nr (107 s−1) | k ISC (108 s−1) | k RISC (105 s−1) |
| a PLQY in aerated toluene. b PLQY in argon-saturated toluene. c Prompt intensity. d Delayed intensity. | ||||||||||||||||||
| 1B-DTACrs | 420 | 438 | 27 | 68 | 99 | 0.22 | 443 | 28 | 0.21 | 83 | — | — | 10 | — | 0.8 | 1.7 | 0.2 | — |
| 2B-DTACrs | 425 | 443 | 21 | 20 | 64 | 0.16 | 448 | 24 | 0.16 | 74 | 35 | 39 | 2 | 13.1 | 1.7 | 6.1 | 3.3 | 1.3 |
We next fabricated vacuum-deposited OLEDs using 1B-DTACrs and 2B-DTACrs as the emitters. The following device configuration was used: indium-tin-oxide (ITO)-coated glass (100 nm)/HAT-CN (10 nm)/TAPC (30 nm)/mCP (10 nm)/mCP: 5 wt% of 1B-DTACrs or 2B-DTACrs (20 nm)/DPEPO (10 nm)/TmPyPB (30 nm)/Liq (2 nm)/Al (100 nm). 1,4,5,8,9,11-Hexaazatriphenyl-enehexacarbonitrile (HAT-CN) is the hole-injection layer, 1,1-bis[(di-4-tolylamino)phenyl]cyclohexane (TAPC) is the hole-transporting layer, 1,3-bis(N-carbazolyl)benzene (mCP) is used for electron-blocking and host layers, bis[2-(diphenylphosphino)phenyl]ether oxide (DPEPO) is the hole-blocking layer, 1,3,5-tris(3-pyridyl-3-phenyl)benzene (TmPyPB) is the electron-transporting layer, and 8-hydroxyquinolinolato-lithium (Liq) and Al are the electron injection and cathode layers, respectively. Fig. 5, Fig. S24–S27 (ESI†) and Table 2 depict all the device characteristics. Devices based on 1B-DTACrs (Device I) and 2B-DTACrs (Device II) produced deep-blue narrowband emission at 440 and 447 nm, respectively, which are consistent to their corresponding PL spectra. The EL spectra clearly confirm the complete exciton confinement in both devices. The FWHM of Device I is 30 nm, which is slightly wider than that of Device II (FWHM = 26 nm). The corresponding CIEx,y are (0.154, 0.049) and (0.150, 0.044), respectively. Although the color gamuts of both devices are very close to the BT.2020 standard for the blue pixel, i.e., (0.131, 0.046), the efficiencies of two devices varied significantly. Taking advantage of enhanced exciton harvesting ability of 2B-DTACrs, Device II demonstrated an excellent EQEmax of 14.8%, which is one of the highest performing deep-blue MR-TADF OLEDs with CIEy ≤ 0.05 (Table S5, ESI†);14 there is, however, considerable efficiency roll-off beyond 100 cd m−2. By contrast, Device I only showed a poor EQEmax of 1.3%, which is in part due to the fact that only singlet excitons can be utilized. Additionally, the poor carrier transporting properties in device I, in which a high turn-on voltage was obtained, may contribute to the lower EQE than the theoretical value (5%).
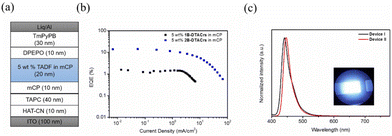 | ||
| Fig. 5 Device performance of TADF OLEDs. (a) Device structure; (b) EQE versus current density; (c) EL spectra of devices at 10 cd m−2 (inset: photo of Device II). | ||
In conclusion, we presented two deep-blue p,n-doped nanographene emitters (1B-DTACrs and 2B-DTACrs), in which 2B-DTACrs with two boron atoms is TADF-active while 1B-DTACrs with only one boron atom is TADF inactive. It is noteworthy that the emitter 2B-DTACrs does not show red-shifted emission. Devices based on 2B-DTACrs achieved a high EQEmax of 14.8% and CIEx,y of (0.150, 0.044), which are one of highest efficiency deep-blue MR-TADF devices reported to date. Importantly, the CIE coordinates effectively meet the colour gamut requirement for the BT.2020 standard for the blue pixel.
The authors acknowledge Ms N. Nakamura and Ms K. Kusuhara for their technical assistance with this research. This work was supported financially by the JSPS Core-to-Core Program (grant number: JPJSCCA20180005) and Kyulux Inc. S. M. S. acknowledges support from the Marie Skłodowska-Curie Individual Fellowship (grant agreement No 838885 NarrowbandSSL). We would like to thank the Leverhulme Trust (RPG-2016-047) for financial support. E. Z.-C. is a Royal Society Leverhulme Trust Senior Research fellow (SRF\R1\201089). We thank the EPRSC for funding (EP/R035164/1). Computational resources have been provided by the Consortium des Équipements de Calcul Intensif (CÉCI), funded by the Fonds de la Recherche Scientifiques de Belgique (F. R. S.-FNRS) under Grant No. 2.5020.11, as well as the Tier-1 supercomputer of the Fédération Wallonie-Bruxelles, infrastructure funded by the Walloon Region under the grant agreement n1117545. Y. O. acknowledges funding by the Fonds de la Recherche Scientifique-FNRS under Grant no. F.4534.21 (MIS-IMAGINE). D. B. is a FNRS Research Director.
Conflicts of interest
There are no conflicts to declare.Notes and references
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Nature, 2012, 492, 234–238 CrossRef CAS PubMed.
- M. Y. Wong and E. Zysman-Colman, Adv. Mater., 2017, 29, 1605444 CrossRef PubMed.
- D. H. Ahn, S. W. Kim, H. Lee, I. J. Ko, D. Karthik, J. Y. Lee and J. H. Kwon, Nat. Photonics, 2019, 13, 540–546 CrossRef CAS.
- H. Kaji, H. Suzuki, T. Fukushima, K. Shizu, K. Suzuki, S. Kubo, T. Komino, H. Oiwa, F. Suzuki, A. Wakamiya, Y. Murata and C. Adachi, Nat. Commun., 2015, 6, 8476 CrossRef CAS PubMed.
- T.-L. Wu, M.-J. Huang, C.-C. Lin, P.-Y. Huang, T.-Y. Chou, R.-W. Chen-Cheng, H.-W. Lin, R.-S. Liu and C.-H. Cheng, Nat. Photonics, 2018, 12, 235–240 CrossRef CAS.
- Y.-L. Zhang, Q. Ran, Q. Wang, Y. Liu, C. Hänisch, S. Reineke, J. Fan and L.-S. Liao, Adv. Mater., 2019, 31, 1902368 CrossRef CAS PubMed.
- T. Hatakeyama, K. Shiren, K. Nakajima, S. Nomura, S. Nakatsuka, K. Kinoshita, J. Ni, Y. Ono and T. Ikuta, Adv. Mater., 2016, 28, 2777–2781 CrossRef CAS PubMed.
- J.-M. Teng, Y.-F. Wang and C.-F. Chen, J. Mater. Chem. C, 2020, 8, 11340–11353 RSC.
- H. Lee, D. Karthik, R. Lampande, J. H. Ryu and J. H. Kwon, Front. Chem., 2020, 8, 373 CrossRef CAS PubMed.
- S. Madayanad Suresh, D. Hall, D. Beljonne, Y. Olivier and E. Zysman-Colman, Adv. Funct. Mater., 2020, 30, 1908677 CrossRef CAS.
- N. Ikeda, S. Oda, R. Matsumoto, M. Yoshioka, D. Fukushima, K. Yoshiura, N. Yasuda and T. Hatakeyama, Adv. Mater., 2020, 32, 2004072 CrossRef CAS PubMed.
- M. Yang, I. S. Park and T. Yasuda, J. Am. Chem. Soc., 2020, 142, 19468–19472 CrossRef CAS PubMed.
- Y. Zhang, D. Zhang, T. Huang, A. J. Gillett, Y. Liu, D. Hu, L. Cui, Z. Bin, G. Li, J. Wei and L. Duan, Angew. Chem., Int. Ed., 2021, 60, 20498–20503 CrossRef CAS PubMed.
- I. S. Park, M. Yang, H. Shibata, N. Amanokura and T. Yasuda, Adv. Mater., 2022, 34, 2107951 CrossRef CAS PubMed.
- M. Nagata, H. Min, E. Watanabe, H. Fukumoto, Y. Mizuhata, N. Tokitoh, T. Agou and T. Yasuda, Angew. Chem., Int. Ed., 2021, 60, 20280–20285 CrossRef CAS PubMed.
- I. Lyskov and C. M. Marian, J. Phys. Chem. C, 2017, 121, 21145–21153 CrossRef CAS.
- S. M. Pratik, V. Coropceanu and J.-L. Brédas, ACS Mater. Lett., 2022, 4, 440–447 CrossRef CAS.
- N. G. Connelly and W. E. Geiger, Chem. Rev., 1996, 96, 877–910 CrossRef CAS PubMed.
- A. Pershin, D. Hall, V. Lemaur, J.-C. Sancho-Garcia, L. Muccioli, E. Zysman-Colman, D. Beljonne and Y. Olivier, Nat. Commun., 2019, 10, 597 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2171192 and 2171191. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2cc03347j |
| ‡ The research data supporting this publication can be accessed at https://doi.org/10.17630/12747024-df19-4158-906b-1b0d26dbf5b3 |
| § These three authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |