Open Access Article
Open Access ArticlePhotoredox synthesis of 6- and 7-membered ring scaffolds via N-centered radicals†
William
Boiledieu
,
Maxime
De Abreu
 ,
Claire
Cuyamendous‡
,
Claire
Cuyamendous‡
 ,
Diana
Lamaa‡
,
Diana
Lamaa‡
 ,
Philippe
Belmont
,
Philippe
Belmont
 and
Etienne
Brachet
and
Etienne
Brachet
 *
*
Université Paris Cité, UMR 8038 CNRS, Faculté de Pharmacie, F-75006 Paris, France. E-mail: etienne.brachet@u-paris.fr
First published on 13th July 2022
Abstract
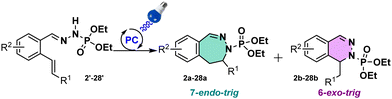
N-Containing heterocycles are important scaffolds due to their ubiquitous presence in bioactive compounds. Their synthesis has been considered as an important research field. In this work we report the access to 6- and 7-membered rings via a photoinduced strategy. To our knowledge, this work represents the first exemple of photo-induced 7-endo-trig cyclization with N-centered radicals.
Synthesis of N-containing heterocycles remains an important challenge in organic chemistry, given their ubiquitous presence in nature as well as in pharmaceuticals and agrochemicals.1 Consequently, researchers have been particularly interested in the synthesis of new N-heterocycles in order to develop bioactive compounds with unprecedented structures. To this aim, we intended to synthesize 6- and 7-membered ring scaffolds, 1,2-dihydrophthalazines and 2,3-dihydrobenzodiazepines respectively. Indeed, these structures are found in important pharmaceutical compounds such as AMPA receptor antagonists2 or antimicrobial agent.3
Thus, developing access to such derivatives in a sustainable and efficient way is a useful sought-after objective. To this aim, our research is focused on an attractive strategy, the visible light catalysis, which uses light as an exclusive energy source. Using such strategy, radicals can be generated under smooth conditions and engaged in the formation of new chemical bonds.4 In recent years, C–C bond formation has been intensively studied in a Diversity-Oriented Synthesis (DOS) fashion,5 but investigating C–N bond formation would empower medicinal chemistry projects aiming at designing new N-containing heterocycles. Thus, we envisioned to build unexplored N-containing 6- and 7-membered rings derivatives. Importantly, visible light induced 7-membered ring formation remains rare in the literature and totally unknown for the synthesis of 2,3-dihydrobenzodiazepines6 or for the 6-membered ring systems of 1,2-dihydrophthalazines. We propose their synthesis using alkenyl-substituted phosphonohydrazones through an Oxidative Deprotonation Electron Transfer (ODET) strategy.7 Thus, we studied the cyclization of a N-centered radical (NCR) onto a pendant alkene in order to perform the designed reactions (Scheme 1). Previously, we developed a methodology using N-tosylhydrazones as radical precursors,8 however the cyclization onto alkenes was in our hands inefficient in an intramolecular fashion. We supposed that the sulfonyl group of tosylhydrazones could be the limiting part for this reaction, in consequence, we turned our attention on phosphonohydrazones derivatives.
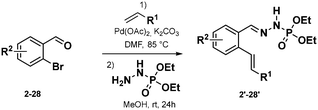
Phosphonohydrazones were obtained straightforwardly by a Heck-coupling (or other), followed by a condensation with a hydrazine unit, giving a direct access to our starting materials (Scheme 2, see ESI† for more detail).
With these products in hand, we evaluated the possibility to generate a NCR. Following the Baldwin's rules, we expected the formation of the 6-membered ring.9,10 However, to our great surprise, the 7-membered ring scaffold has been formed during the reaction in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio towards the 6-membered ring scaffold. We were surprised by this cyclization since with sulfonohydrazones we never observed such behaviour on alkynes. This observation confirmed our suspicions on the sulfonyl group being the limiting unit in our previous investigations.8 In addition, 7-endo-trig reactions are scarcely reported compared to 6-exo-trig reactions, and to the best of our knowledge unknown under visible light catalysis. This observation encouraged us to look deeper into this reactivity.
1 ratio towards the 6-membered ring scaffold. We were surprised by this cyclization since with sulfonohydrazones we never observed such behaviour on alkynes. This observation confirmed our suspicions on the sulfonyl group being the limiting unit in our previous investigations.8 In addition, 7-endo-trig reactions are scarcely reported compared to 6-exo-trig reactions, and to the best of our knowledge unknown under visible light catalysis. This observation encouraged us to look deeper into this reactivity.
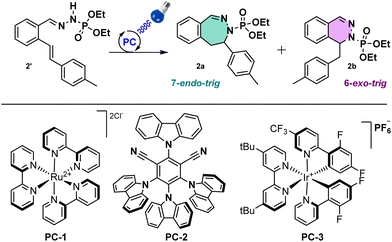
Starting with in-house optimized conditions (Table 1, entry 1),11 we were glad to obtain 40% of the C–N bond formation with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 6-exo- and 7-endo-trig cyclization products. We then proceeded to an extensive optimization of the reaction conditions. First, a modification of the photocatalyst (PC) from PC-1 to PC-2, PC-3 (Table 1, entries 2 and 3) or other PCs (see ESI†) gave lower yields. We then switched the base from tBuONa to KOH, pyridine (Table 1, entries 4 and 5), or others (see ESI†), increasing the yield of the desired product to 46% with KOH (3 equiv.). About the same results (49%, Table 1, entry 6) were obtained decreasing equivalents of KOH (1.5 equiv.). Modifying the solvent did not improve the outcome (see ESI† for full details), for instance no reaction was observed with MeCN (Table 1, entry 7), with an exception for a mixture of MeCN/H2O (9/1) with 1.5 equiv. of KOH that gave a yield of 65% (Table 1, entry 8). Interestingly, in this entry a 4
1 mixture of 6-exo- and 7-endo-trig cyclization products. We then proceeded to an extensive optimization of the reaction conditions. First, a modification of the photocatalyst (PC) from PC-1 to PC-2, PC-3 (Table 1, entries 2 and 3) or other PCs (see ESI†) gave lower yields. We then switched the base from tBuONa to KOH, pyridine (Table 1, entries 4 and 5), or others (see ESI†), increasing the yield of the desired product to 46% with KOH (3 equiv.). About the same results (49%, Table 1, entry 6) were obtained decreasing equivalents of KOH (1.5 equiv.). Modifying the solvent did not improve the outcome (see ESI† for full details), for instance no reaction was observed with MeCN (Table 1, entry 7), with an exception for a mixture of MeCN/H2O (9/1) with 1.5 equiv. of KOH that gave a yield of 65% (Table 1, entry 8). Interestingly, in this entry a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in favour of the 7-membered ring product 2a was observed. Finally, the reaction didn’t occur in the absence of light, catalyst or base, indicating that the reaction is photoinduced (Table 1, entries 9–11). After this optimization, the best ratio and the best yield for the 7-membered ring were obtained with the following conditions: phosphonohydrazone (1 equiv.), KOH (1.5 equiv.), Ru(bpy)3Cl2·6H2O as PC (2.5 mol%) and a mixture of MeCN and H2O (9/1) (0.5 mL/0.3 mmol), under 450 nm irradiation at 20 °C.
1 ratio in favour of the 7-membered ring product 2a was observed. Finally, the reaction didn’t occur in the absence of light, catalyst or base, indicating that the reaction is photoinduced (Table 1, entries 9–11). After this optimization, the best ratio and the best yield for the 7-membered ring were obtained with the following conditions: phosphonohydrazone (1 equiv.), KOH (1.5 equiv.), Ru(bpy)3Cl2·6H2O as PC (2.5 mol%) and a mixture of MeCN and H2O (9/1) (0.5 mL/0.3 mmol), under 450 nm irradiation at 20 °C.
| Entry | Photocatalyst (PC) | Base (equiv.) | Solvents | Yield (%) | Ratio 2a/2b |
|---|---|---|---|---|---|
a To an oven-dried sealable glass vial were added phosphonohydrazone (1 equiv.), potassium hydroxide (1.5 equiv.), Ru(bpy)3Cl2·6H2O as a photocatalyst (2.5 mol%) and a mixture of MeCN and H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (0.5 mL/0.3 mmol), then sealed with 20 mm crimp caps and stirred under 450 nm irradiation at 20 °C. After completion of the reaction, the mixture was purified by silica gel column chromatography.
b Without light. See the complete table in the ESI. 1) (0.5 mL/0.3 mmol), then sealed with 20 mm crimp caps and stirred under 450 nm irradiation at 20 °C. After completion of the reaction, the mixture was purified by silica gel column chromatography.
b Without light. See the complete table in the ESI.
|
|||||
| 1 | PC-1 | tBuONa (3) | MeOH | 40 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | PC-2 | tBuONa (3) | MeOH | 0 | — |
| 3 | PC-3 | tBuONa (3) | MeOH | 20 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | PC-1 | KOH (3) | MeOH | 46 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | PC-1 | Pyridine (3) | MeOH | 0 | — |
| 6 | PC-1 | KOH (1.5) | MeOH | 49 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | PC-1 | KOH (1.5) | MeCN | 0 | 0 |
| 8 | PC-1 | KOH (1.5) | MeCN/H2O (9/1) | 65 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9b | PC-1 | KOH (1.5) | MeCN/H2O (9/1) | 0 | — |
| 10 | — | KOH (1.5) | MeCN/H2O (9/1) | 0 | — |
| 11 | PC-1 | — | MeCN/H2O (9/1) | 0 | — |
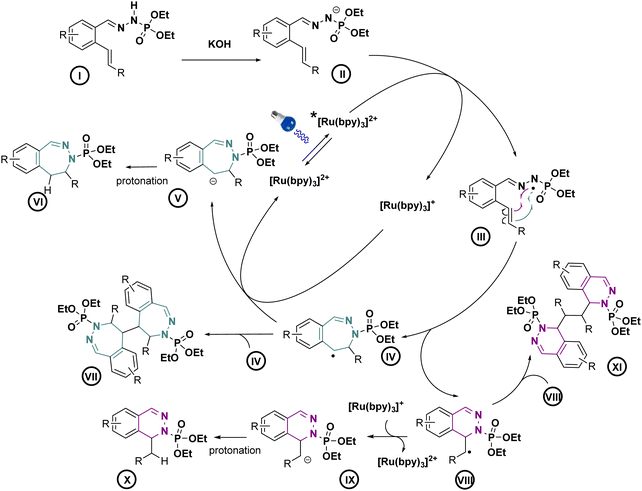
With these optimized conditions, we wanted then to investigate the substrate scope of the reaction. Gratifyingly, the reaction proceeded on a variety of substituted compounds to give the desired cyclized products with good yields (compounds 2–28). At first, several modifications have been made on the alkene chain (R1, Scheme 3). We were pleased to obtain the unsubstituted styrene product with a total selectivity for the 7-membered ring 3a with 50% yield. In addition, the 2-alkyl-1,1-diaryethylene starting material underwent efficiently the reaction affording the corresponding diastereoisomers 4a with 20 and 22% yields. A mixture of 6- and 7-membered rings was obtained starting from styrenes bearing electron-donating groups on different positions with moderate yields (compounds 5–7, 22–44%). However, except for the fluoro derivative (8), a total selectivity towards 6-membered ring products has been observed while using electron-deficient styrenes. Indeed, phenyls bearing ester, nitrile and nitro groups gave the corresponding products with moderate yields (24–44%, compounds 9–11) while pyridines gave 45–46% yield of the desired 6-membered rings (compounds 12–13). Moreover, aliphatic alkenes gave 6-membered rings with 30 and 48% yields (compounds 14–15), while no reaction occurred with a tetrasubstituted akene (16). Next, we have studied the variations around the aromatic cycle (R2). The reaction worked efficiently in the presence of electron-donating, electron-withdrawing groups as well as heterocycles (compounds 17–21). To our delight, we formed exclusively the corresponding 7-membered rings with moderate to good yields (34–60%). Finally, compounds bearing different substitutions, both on R1 and R2 positions, proceeded successfully affording a variety of 6- or/and 7-membered scaffolds (compounds 22–28, 21–73%) (see ESI†). Following this scope investigation, electron rich or neutral groups on R1 seem to promote the formation of the 7-membered ring, however, electron poor groups promote 6-membered ring products. These divergent results could demonstrate the major influence of electronic effects in the outcome of the reaction. Nonetheless, one example must be further discussed: the selectivity for the product 8 which favoured the formation of the 7-membered ring. We propose that this selectivity is due to the known mismatching electronic effects of fluorine on the electronic density on the alkene double bond due to its −I and +M effects, mesomeric effect being here predominant for the regioselectivity thus leading to the 7-membered ring as for 22a.12 In addition, counter ion, here the K+, is probably involved in stabilizing the attack during the transition state, as already described in the literature.13 To gain insight of the reaction mechanism, we have launched the reaction in presence of TEMPO, diphenyl disulfide and NCS in order to trap the formed radical. Unfortunately, no radical adducts could be identified, however, the cyclization reaction did not occur confirming that this transformation is certainly radical-induced. Based on our work and the literature,14 we thus propose that the first step is the deprotonation of the starting material I with the base giving the anion II.
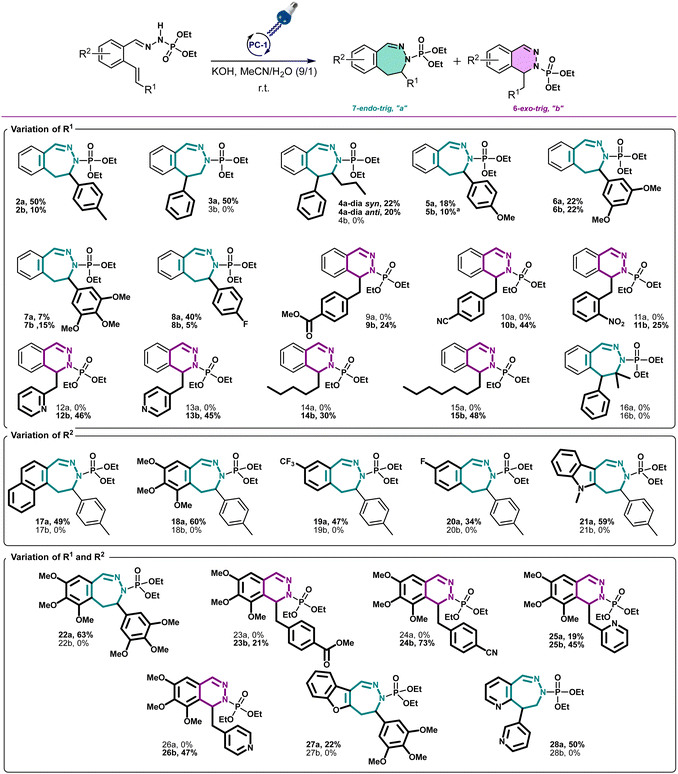 | ||
Scheme 3 Scope of the reaction of 6- and 7-membered ring formation. All yields were for isolated compounds. a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) The dimer of 5b has been isolated. The dimer of 5b has been isolated. | ||
This latter anion can be easily oxidized by the excited photocatalyst, as seen in cyclic voltammetry, our model substrate possesses an oxidation potential of 0.67 V vs. SCE. This radical can then follow two different pathways depending on the substitution of the double bound. Electron rich double bond substitutions preferentially induce 7-membered ring intermediate IV and electron poor ones promote the 6-membered ring intermediate VIII as seen in the Scheme 4.
Then, the effective reduction of the generated radical by the photocatalyst15 allows to produce, respectively, anions V and IX, leading after protonation to the final compounds VI and X. The dimer XI on the compound 5b has been isolated in a small quantity allowing us to suppose that the dimer VII could also be formed.
In conclusion, herein we report for the first time a 7-endo-trig cyclization induced under photoredox conditions,16 upon the generation of an NCR. The outcome of the cyclization process (6-exo versus 7-endo) seems to be electronically-driven based on the evaluation of the scope of this reaction.
The reactivity of the photo-induced NCR leads to a 6-exo- or/and a 7-endo-trig cyclization process paving the way for a large variety of exquisite and unknown structures, related to the benzodiazepine or dihydrophthalazine families, of upmost importance for further biological investigations.
Authors acknowledge the MESR for the financial support of W. B. and M. D. A., E. B. thanks the Université Paris Cité for financial support (Ipso-RM11J2IDXD7) and the ANR for financial support (Lightning, JCJC ANR-21-CE07-0017-01).
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) H. M. L. Davies and E. J. Sorensen, Chem. Soc. Rev., 2009, 38, 2981 RSC; (b) D. H. Brown Ripin and C. A. Lewis, in Practical Synthetic Organic Chemistry: Reactions, Principles, and Techniques, ed. S. Caron, Wiley-VCH, Weinheim, 2020, p. 377 Search PubMed.
- G. Szénási, M. Vegh, G. Szabo, S. Kertesz, G. Kapus, M. Albert, Z. Greff, I. Ling, J. Barkoczy, G. Simig, M. Spedding and L. G. Harsing, Neurochem. Int., 2008, 52, 166 CrossRef PubMed.
- E. W. Barrow, J. Dreier, S. Reinelt, P. C. Bourne and W. W. Barrow, Antimicrob. Agents Chemother., 2007, 51, 4447 CrossRef CAS PubMed.
- (a) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322 CrossRef CAS PubMed; (b) J.-R. Chen, X.-Q. Hu, L.-Q. Lu and W.-J. Xiao, Chem. Soc. Rev., 2016, 45, 2044 RSC; (c) L. Marzo, S. K. Pagire, O. Reiser and B. König, Angew. Chem., Int. Ed., 2018, 57, 10034 CrossRef CAS PubMed; (d) C. R. J. Stephenson, T. P. Yoon and D. W. C. MacMillan, in Visible Light Photocatalysis in Organic Chemistry, ed. C. R. J. Stephenson, T. P. Yoon and D. W. C. MacMillan, Wiley-VCH, Weinheim, 2018 CrossRef; (e) M. De Abreu, P. Belmont and E. Brachet, Eur. J. Org. Chem., 2020, 1327 CrossRef CAS.
- M. D. Burke and S. L. Schreiber., Angew. Chem., Int. Ed., 2004, 43, 46 CrossRef PubMed.
- (a) D. Xu, H. Li, G. Pan, P. Huang, J. Oberkofler, R. M. Reich and H. Guo, Org. Lett., 2020, 22, 4372 CrossRef CAS PubMed; (b) Y. Zhao, J. R. Chen and W. J. Xiao, Org. Lett., 2018, 20, 224 CrossRef CAS PubMed.
- (a) H. Jiang and A. Studer, CCS Chem., 2019, 1, 38 CAS; (b) X. Tao and Q. Zhang, Chem. Soc. Rev., 2016, 45, 3069 RSC; (c) P. Cassie, S. Fenner and J. A. Murphy, Chem. Rev., 2022, 122, 8181 CrossRef PubMed.
- E. Brachet, L. Marzo, M. Selkti, B. König and P. Belmont, Chem. Sci., 2016, 7, 5002 RSC.
- (a) J. E. Baldwin, J. Chem. Soc., Chem. Commun., 1976, 18, 734 RSC; (b) K. Gilmore and I. V. Alabugin, Chem. Rev., 2011, 111, 6513 CrossRef CAS PubMed.
- (a) C. Jacob, H. Baguia, A. Dubart, S. Oger, P. Thilmany, J. Beaudelot, C. Deldaele, S. Peruško, Y. Landrain, B. Michelet, S. Neale, E. Romero, C. Moucheron, V. Van Speybroeck, C. Theunissen and G. Evano, Nat. Commun., 2022, 13, 560 CrossRef CAS PubMed; (b) I. V. Alabugin and C. Hu, Chem. Catal., 2021, 5, 976 CrossRef; (c) J. W. Tucker and C. R. Stephenson, Org. Lett., 2011, 13, 5468 CrossRef CAS PubMed.
- M. De Abreu, M. Selkti, P. Belmont and E. Brachet, Adv. Synth. Catal., 2020, 362, 2216 CrossRef.
- M. B. Smith and J. March, Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, Wiley-Interscience, New York, 6th edn, 2007, ISBN 978-0-471-72091-1 Search PubMed.
- (a) X. Peng, B. M. K. Tong, H. Hirao and S. Chiba, Angew. Chem., Int. Ed., 2014, 53, 1959 CrossRef CAS PubMed; (b) J. P. Barham, T. N. J. Fouquet and Y. Norikane, Org. Biomol. Chem., 2020, 18, 2063 RSC; (c) M. J. P. Mandigma, M. Domański and J. P. Barham, Org. Biomol. Chem., 2020, 18, 7697 RSC.
- (a) X. Q. Hu, J. R. Chen, Q. Wei, F. L. Liu, Q. H. Deng, A. M. Beauchemin and W. J. Xiao, Angew. Chem., Int. Ed., 2014, 53, 12163 CrossRef CAS PubMed; (b) M. De Abreu, P. Belmont and E. Brachet, J. Org. Chem., 2021, 86(5), 3758 CrossRef CAS PubMed; (c) J.-R. Chen, X.-Q. Hu, L.-Q. Lu and W.-J. Xiao, Chem. Soc. Rev., 2016, 45, 2044 RSC.
- J. Grimshaw, Electrochemical Reactions and Mechanisms in Organic Chemistry, Elsevier, Amsterdam, 2000, vol. 8, p. 89 Search PubMed.
- T. Kleine, K. Bergander, R. Fröhlich, B. Wibbeling and E.-U. Würthwein, J. Org. Chem., 2011, 76, 1979 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cc02780a |
| ‡ Contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |