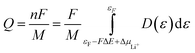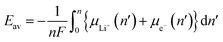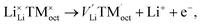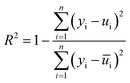Open Access Article
Open Access ArticleDesigning positive electrodes with high energy density for lithium-ion batteries
Masashi
Okubo
 ab,
Seongjae
Ko
a,
Debasmita
Dwibedi
a and
Atsuo
Yamada
ab,
Seongjae
Ko
a,
Debasmita
Dwibedi
a and
Atsuo
Yamada
 *ab
*ab
aDepartment of Chemical System Engineering, School of Engineering, The University of Tokyo, Hongo 7-3-1, Bunkyo-ku, Tokyo 113-8656, Japan. E-mail: yamada@chemsys.t.u-tokyo.ac.jp
bElemental Strategy Initiative for Catalysts & Batteries (ESICB), Kyoto University, Nishikyo-ku, Kyoto 615-8510, Japan
First published on 5th March 2021
Abstract
The development of efficient electrochemical energy storage devices is key to foster the global market for sustainable technologies, such as electric vehicles and smart grids. However, the energy density of state-of-the-art lithium-ion batteries is not yet sufficient for their rapid deployment due to the performance limitations of positive-electrode materials. The development of large-capacity or high-voltage positive-electrode materials has attracted significant research attention; however, their use in commercial lithium-ion batteries remains a challenge from the viewpoint of cycle life, safety, and cost. In this review, after summarizing the limitation issues associated with large-capacity/high-voltage positive electrodes and already attempted technical solutions, a machine-learning technique is applied to analyze the reported dataset to hierarchize various technical solutions by their effectiveness in improving performance. The proposed study highlights the importance of integrating systematic experimental data collection with modern data analysis techniques for rational development of large-capacity/high-voltage positive electrodes. The scope is extended to important technical issues with other cell components, such as electrolytes and additives, binders, conductive carbon, current collectors, and impurity control for total optimization.
1. Introduction
Lithium-ion batteries (LIBs) have been widely used in consumer electronics, which has resulted in the rapid growth of the LIB market in the 2000s. In the 2010s, the deployment of electric vehicles (EVs), which was accelerated by favorable governmental policies, reached a global stock of 5 million EVs in 2018, which further increased the LIB production to greater than 100 GW h per year.1 As the EV stock is predicted to reach 250 million in 2030, the automotive industry is eagerly seeking LIB technology that realizes long drive distances, low cost, and a robust cycle life for EVs, bearing in mind the second use of LIBs as stationary energy storage.The EV drive distance is linked to the energy density of the LIB. While the existing LIB cells possess an energy density of approximately 200–250 W h kg−1, the national targets of most leading countries are greater than 300 W h kg−1 for EV market growth.2 However, LIBs comprising conventional positive and negative electrodes have already approached their performance limits, and they can hardly reach these national targets due to the relatively moderate capacities/voltages of the electrodes.3 Battery engineers are attempting to exploit the large-capacity or high-voltage positive electrodes or large-capacity negative electrodes for increasing the energy density of LIBs. Indeed, the capacity of negative electrodes in the recent commercial LIBs has been successfully increased using silicon or silicon oxides as electrode additives.4
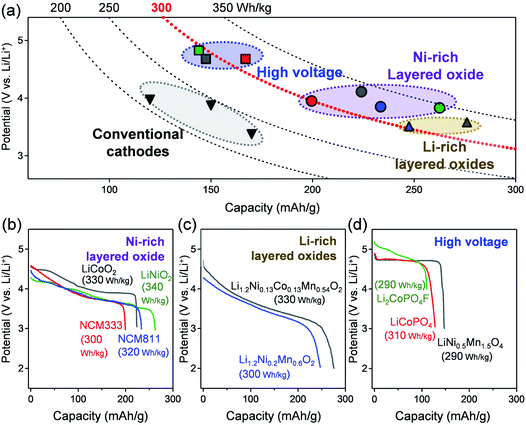
In contrast, despite decades of intensive research, the use of large-capacity or high-voltage positive electrodes remains challenging. Positive-electrode materials are typically classified into three categories, i.e. layered oxides (LiTMO2, TM: transition metal), spinel oxides (LiTM2O4), and polyanionic compounds (LixTMy(XOn)z, X: P, S, B, Si, etc.).5–10 For example, the layered oxide, LiCoO2, delivers a specific capacity of approximately 140–150 mA h g−1 at an average voltage of 3.9 V vs. Li/Li+ when cycled with an upper cut-off voltage of 4.2 V vs. Li/Li+.5 Meanwhile, LiMn2O4 is a conventional spinel oxide that delivers a specific capacity of approximately 120–130 mA h g−1 at an average voltage of 4.0 V vs. Li/Li+ (upper cut-off voltage of 4.2 V vs. Li/Li+).6 Finally, LiFePO4 is a typical polyanionic compound that can deliver a specific capacity of approximately 160–170 mA h g−1 at an average voltage of 3.4 V vs. Li/Li+.7,11 Although all these electrode materials have been used in commercial LIBs, their available specific capacity is far below the requirement of the EV industry (Fig. 1a), which has devoted considerable efforts in developing large-capacity/high-voltage positive electrodes.
Nickel-rich layered oxides are the most promising large-capacity positive electrode, as they deliver a specific capacity greater than 200 mA h g−1 (Fig. 1b).12–14 Lithium-rich layered oxides are another important family of layered oxides with a large specific capacity of >250 mA h g−1 (Fig. 1c).15–17 High-voltage positive-electrode materials, such as spinel oxides and polyanionic compounds, operating at an average voltage of >4.7 V vs. Li/Li+ have also been studied intensively (Fig. 1d).18–21 Theoretically, their effective adoption in LIBs will result in energy densities greater than 300 W h kg−1 (Fig. 1a).
However, even when we intuitively consider the intercalation chemistry of a positive-electrode reaction as nLi+ + ne− + A → LinA (where A is host), a large capacity corresponds to a large n, whereas a high voltage corresponds to a low lithium chemical potential (μLi) of the host. For both the cases, electrode reactions are associated with a large standard reaction Gibbs energy of the electrode material (|ΔrGo| = Σn|μLi|), which generally leads to the formation of unstable (de)lithiated phases and/or large change in the unit cell volume during (de)lithiation; this, in turn, rapidly degrades the reversible capacity upon cycling. Further, a large difference between the standard reaction Gibbs energies of positive and negative electrodes, which are separated by a flammable organic electrolyte, can trigger thermal runaway and fire explosion accidents. When using large-capacity/high-voltage positive electrodes, another important requirement is an electrolyte with high stability against oxidation. As the EV industry must achieve a high energy density and should simultaneously reduce the costs, the potential candidates for chemical components of positive-electrode materials, electrolytes, and binders are severely limited. For example, reducing the content of expensive and scarce cobalt is a primary requirement, whereas a long calendar life should also be obtained. Under these multiple severe restrictions, only an atomistic understanding of the degradation mechanisms of each component and their interfaces/interphases can enable the rational electrode developments and improvements.
Although several complementary reviews have summarized the practical limitations associated with some positive-electrode materials and their technical solutions,22–34 in this review, we aim to establish a multiscale (micro/meso/macroscopic) overview of large-capacity/high-voltage positive-electrode materials. A machine-learning technique is applied to analyse and obtain the most efficient technical approaches towards higher energy density with minimal trade-offs.
2. Origins of large capacity/high voltage
Let us consider an electrode reaction:| nLi+ + ne− + A → LinA, |
where F is the Faraday constant, M is the molecular weight of A, εF is the Fermi energy of A, ΔμLi+ is the difference between the lithium-ion chemical potentials of A and LinA, and D(ε) is the density of states of A, respectively. The average reaction voltage, Eav [vs. Li/Li+], is given as follows:
where μLi+ and μe− are the lithium-ion and electron chemical potentials of LinA, respectively. According to these expressions, using electrode materials with a large D(ε) for εF > ε>εF − FΔE + ΔμLi+) achieves a large capacity, whereas those with low μLi+ or low μe− achieves a high voltage.
One of the most promising positive electrode materials for achieving high energy density is a nickel-rich layered oxide, i.e. LiNixTM1−xO2 (TM: Mn, Co).12,13,35–37 For example, LiNi0.8Co0.1Mn0.1O2 (NCM811) typically delivers a large specific capacity of approximately 200 mA h g−1 at an average voltage of 3.8 V vs. Li/Li+ (Fig. 1b; operation voltage: 3.0–4.5 V vs. Li/Li+).33,34 In general, increasing the nickel content by replacing cobalt increases the specific capacity (e.g. LiCoO2vs. LiNiO2, LiNi1/3Co1/3Mn1/3O2 (NCM333) vs. NCM811) (Fig. 1b).5,12,38–41 As the frontier energy level of Ni eg–O 2p antibonding bands is higher (i.e. higher μe−) than that of Co t2g nonbonding bands, the reaction voltage of LiNiO2 (3.8 V vs. Li/Li+) is lower than that of LiCoO2 (4.1 V vs. Li/Li+) (Fig. 1b). Consequently, nickel-rich layered oxides exhibit a large amount of (de)lithiation, even under a low upper cut-off voltage (larger integrated D(ε) for εF > ε > εF − FΔE + ΔμLi+). Meanwhile, replacing manganese with nickel (altering Mn3+ to Mn4+0.5Ni2+0.5, then to Ni3+) decreases the number of strong Jahn–Teller Mn3+ ions,42,43 thereby suppressing large structural distortions and enabling a large capacity with somewhat better reversibility.
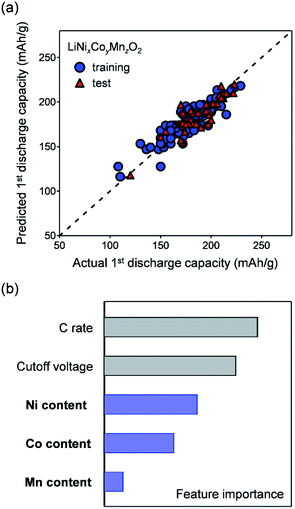
To confirm the influence of the nickel content on the specific capacity, we applied a machine-learning technique to a data-set assembled from the reported capacities of nickel-rich layered oxides.38,41,44–112 The supervised learning of the reported discharge capacities during the first cycle was conducted using a random forest regression with five simple features, i.e. Ni, Co, and Mn contents, upper cut-off voltage, and charge/discharge C rate. Fig. 2a compares the predicted and actual discharge capacities (R2 = 0.82 and 0.70 for the training and test data, respectively), suggesting the reasonable generalization performance of the model, even with only five simple features. As shown in Fig. 2b, except for the extrinsic experimental conditions (upper cut-off voltage and charge/discharge C rate), Ni content is the most important feature for explaining the trend in the specific capacity of nickel-rich layered oxides (Fig. 3a), which supports the above discussion. Conversely, the Co content is a negative explanatory variable for the discharge capacity.
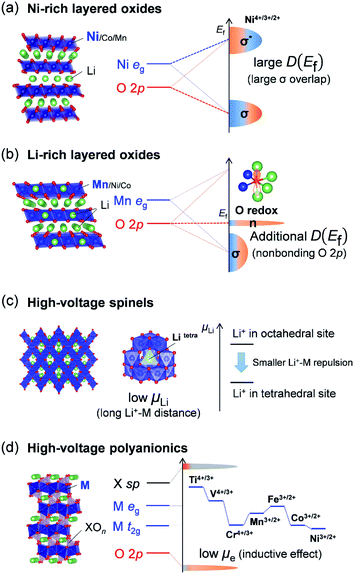 | ||
| Fig. 3 Origins of the high performance of (a) nickel-rich layered oxides, (b) lithium-rich layered oxides, (c) high-voltage spinels, and (d) high-voltage polyanionic compounds. | ||
Another important class of layered oxides capable of a large storage capacity is lithium-rich layered oxides, Li1+xTM1−xO2.15–17,25–27,113 For example, Li1.2Ni0.13Co0.13Mn0.54O2 and Li1.2Ni0.2Mn0.6O2 deliver specific capacities greater than 250 mA h g−1 (Fig. 1c).114,115 As the transition metals are partially replaced by lithium ions, the coordination environment of oxide ions involves a specific axis of Li–O–Li, along which an oxygen 2p orbital remains nonbonding near εF to contribute to the charge compensation for (de)lithiation (additional D(ε), Fig. 3b).116–118 Although the chemical state of oxidized oxygen after delithiation has long been debated,119–124 accumulative redox reactions by both the transition metals (cationic redox) and oxide ions (anionic redox or oxygen redox) provide large specific capacities.125 Unlike nickel-rich layered oxides, a machine-learning technique applied to the reported capacities of lithium-rich layered oxides with simple features (such as Li, Mn, Co, and Ni contents, upper cut-off voltage, or charge/discharge C rate) could not provide a reasonable prediction model with good generalization performance. Presumably, other complicated factors, such as particle morphology, carbon additive, electrolyte composition, and initial activation process, which were not considered in the supervised learning, could influence the capacities of the lithium-rich layered oxides.
LiNi0.5Mn1.5O4 is a representative high-voltage spinel oxide for positive electrodes, wherein Mn3+ in LiMn2O4 is replaced by Ni (Mn3+ → Mn0.54+Ni0.52+), and the two-electron redox reaction of Ni4+/Ni3+/Ni2+ occurs.18,126,127 Unstable Jahn–Teller Ni3+ increases the reaction voltage of Ni3+/Ni2+, while lowering the reaction voltage of Ni4+/Ni3+, thereby leading to a continuous potential profile for the two-electron redox capacity. Lithium ions occupy tetrahedral 8a sites, which are located away from the transition metals in octahedral 16d sites. The coulombic repulsion between lithium ions and transition metals is lower than that of layered oxides (where lithium ions occupy octahedral 3a sites near the transition metals in octahedral 3b sites), which in turn yields a lower μLi+, resulting in a high voltage of approximately 4.8 V vs. Li/Li+ (Fig. 3c). Indeed, similar high reaction voltages have been reported for other spinel oxides LiTM0.5Mn1.5O4 (TM: Co, Cr) with little dependence on TM.127–129
Polyanionic compounds such as LiCoPO4 are another family of high-voltage positive-electrode materials.20,130 Transition metals in polyanionic compounds are mainly coordinated by the oxygen atoms of oxyanions (XOnm−, X: P, S, B, and Si), wherein the oxygen atom predominantly donates the electrons to X atom (a covalent X–O bond), resulting in a highly ionic TM–O bond. The energy levels of TM–O antibonding states composed of predominantly TM 3d orbitals under ionic coordination environments (small TM–O bonding–antibonding splitting) are much lower (i.e. lower μe−) than those under more covalent coordination environments, such as those of transition-metal oxides.10,131 In addition, the large interstitial spaces for lithium ions decrease lithium-ion chemical potential (low μLi+). Owing to these combined effects, polyanionic compounds generally exhibit high reaction voltages (Fig. 3d). In particular, as the redox couple of Co3+/Co2+ has a significantly low μe− owing to the electronic configuration of high-spin Co3+ (t42ge2g), LiCoPO4 operates at a high average voltage of approximately 4.8 V vs. Li/Li+. The redox couples of Ni3+/Ni2+ (stable Ni2+ (t62ge2g)) and Cr4+/Cr3+ (stable Cr3+ (t32g)) are also expected to have low μe−, leading to high reaction voltages.132–137 In addition, incorporating a highly ionic fluoride ion into the coordination environment of transition metals (e.g. Li2CoPO4F) further lowers μe− and raises the reaction voltage.138–143
3. Oxidation stability of electrolytes
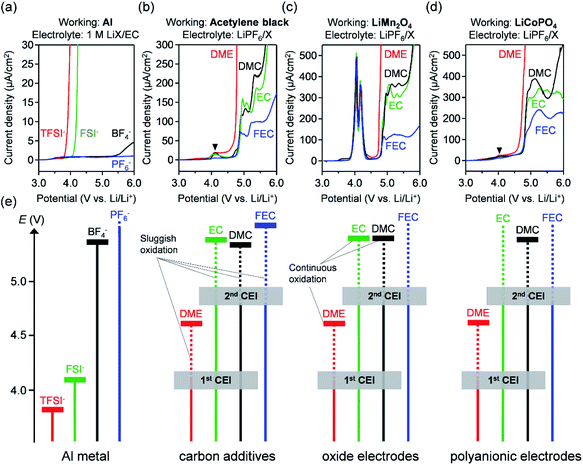
The oxidation stability of electrolytes is crucial for the stable operation of large-capacity/high-voltage positive electrodes.144Fig. 4 shows systematic linear sweep voltammetry results of various electrolytes with an aluminium current collector, conductive carbon (acetylene black), oxide electrode (LiMn2O4), and polyanionic electrode (LiCoPO4).The first requisite for electrolytes is to suppress aluminium corrosion at high voltages. Imide anions (such as bis(fluorosulfonyl)imide (FSI−) and bis(trifluoromethanesulfonyl)imide (TFSI−)) severely corrode aluminium below 4.2 V vs. Li/Li+ because of the formation of soluble Al(FSI)3 and Al(TFSI)3 complexes (Fig. 4a).145,146 Although employing a high salt concentration can hinder the dissolution of these complexes to suppress aluminium corrosion,147 imide anions may not be a primary option for large-capacity/high-voltage electrodes. In contrast, the BF4− anion does not exhibit anodic current flow below 5.3 V vs. Li/Li+,148 which is compatible with large-capacity positive electrodes. As the PF6− anion possesses the best oxidation stability up to 6.0 V vs. Li/Li+,148,149 it is the best anion for large-capacity/high-voltage positive-electrode materials. For both BF4− and PF6− anions, insoluble AlF3 forms, which passivates the current collector surface.150
The second requisite is to suppress the parasitic oxidation of the electrolyte components. Conductive carbon additives in large-capacity/high-voltage positive electrodes possess a large specific surface area, which generally results in nontrivial parasitic oxidation. Fig. 4b shows that a conductive carbon (acetylene black) electrode exhibits a small anodic current flow at 4.1 V vs. Li/Li+ with conventional electrolytes. This current flow is attributed to the oxidation of electrolyte solvents rather than that of the conductive carbon,151–153 because the current does not flow with an oxidation-durable fluorinated solvent, i.e. fluoroethylene carbonate (FEC). However, the further oxidation of carbonate electrolytes is suppressed up to 4.7 V vs. Li/Li+, suggesting the formation of a cathode-electrolyte interphase (CEI) at approximately 4.1 V vs. Li/Li+.155 In contrast, continuous anodic current above 4.1 V vs. Li/Li+ for ether electrolytes, such as dimethyl ether (DME), indicates their incompatibility with large-capacity/high-voltage electrodes.154 As identical results were obtained for an oxide electrode (LiMn2O4) containing a carbon additive (Fig. 4c), the active site for CEI formation should be the electrophilic surface of the conductive carbon, which abstracts the electrons from solvent molecules. In particular, the electron abstraction of carbonates generates the radical cations of alkyl carbonates (R+–OCOO˙; R = alkyl group), forming CEIs via their polymerization.150 Owing to this protective CEI formation, carbonate electrolytes are compatible with large-capacity electrodes (typically up to 4.4 and 4.7 V vs. Li/Li+ for nickel-rich layered oxides and lithium-rich layered oxides, respectively). Indeed, the major electrolytes for large-capacity electrodes are 1.0–1.2 M LiPF6 in a mixture of cyclic and linear carbonates.
To stably operate high-voltage electrodes, an electrolyte should be electrochemically stable above 4.8 V vs. Li/Li+. However, even relatively oxidation-resistant linear/cyclic carbonates exhibit sluggish but continuous oxidation above 4.7 V vs. Li/Li+ (Fig. 4d). As fluorinated carbonates, such as FEC, slightly suppress the anodic oxidation, perhaps owing to their low highest occupied molecular orbital (HOMO) levels and/or the formation of fluorine-rich CEI, the fluorination of electrolyte solvents can be a practical option for improving the reversibility and capacity retention of high-voltage electrodes.88,156 For example, an electrolyte consisting solely of fluorinated solvents (FEC/methyl(2,2,2-trifluoroethyl)carbonate (FEMC)/1,1,2,2-tetrafluoroethyl-2′,2′,2′-trifluoroethyl ether (HFE)) was proposed for high-voltage electrodes.88 However, achieving an intrinsic oxidation stability above 4.7 V vs. Li/Li+ is still a major challenge (Fig. 5a).
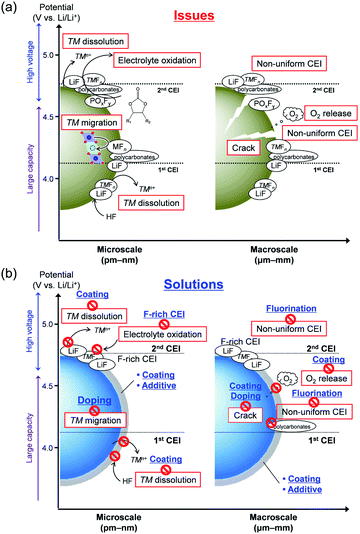 | ||
| Fig. 5 (a) Issues with large-capacity/high-voltage positive electrodes, and (b) solutions to these issues. | ||
4. Issues and technical solutions
4.1. Intrinsic degradation
Cations (lithium and transition-metal ions) in a positive-electrode material occupy interstitial (e.g. octahedral/tetrahedral) sites in an array of anions (oxide ions or oxyanions), and lithium ions are deintercalated to generate lithium-ion vacancies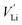 (Kröger–Vink notation) upon charge as follows:
(Kröger–Vink notation) upon charge as follows:where attractive forces between
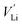 and
and 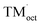 tend to drive the migration of TM to lithium-ion vacancies (cation migration).157–160 A large capacity generates a large amount of
tend to drive the migration of TM to lithium-ion vacancies (cation migration).157–160 A large capacity generates a large amount of 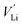 upon charging, whereas high voltage forms vacant sites with low μLi+ (i.e. an unstable vacancy), both of which accelerate the migration of TM. Because TM ions at lithium-ion sites hinder lithium-ion diffusion, cation migration is a common atomistic degradation mechanism in most positive electrode materials (Fig. 5a).
upon charging, whereas high voltage forms vacant sites with low μLi+ (i.e. an unstable vacancy), both of which accelerate the migration of TM. Because TM ions at lithium-ion sites hinder lithium-ion diffusion, cation migration is a common atomistic degradation mechanism in most positive electrode materials (Fig. 5a).
From a meso/macroscopic viewpoint, layered oxides comprise alternating stackings of Li+ and TMO2 slabs. Although a pristine layered structure is stabilized by Li+–O2− coulombic attractions, significantly weaker van der Waals force between the TMO2 slabs becomes dominant in deeply delithiated (charged) states, thereby triggering a damaging phase transition (e.g. hexagonal 2 phase (H2) → hexagonal 3 phase (H3)), during which the unit cell volume considerably changes by over 7%.161 This large volume change, along with gliding of the TMO2 slabs upon deep charging, can delaminate the TMO2 slabs and crack, fracture, and/or pulverization LiTMO2 particles, which severely degrades the electrode composite (Fig. 5a).157,161
For lithium-rich layered oxides, a large capacity is achieved by the additional redox reaction of oxide ions. An oxidized oxide ion has an unstable electronic configuration (2p5) and may easily dimerize, for instance, to form a peroxide-like ion (O22−).119,122,162 Such dimerized ions should be further oxidized to O2 at a high voltage, resulting in a large amount of oxygen vacancies, which predominantly form on the particle surface. In turn, these vacancies cause the formation of cation-densified phases that are electrochemically inactive (Fig. 5a).116,123,163
High-voltage spinel oxides possess a three-dimensional framework of TM2O4, through which lithium ions diffuse three-dimensionally. Because this framework is rigid, the unit cell volume changes by less than 6% during delithiation,164,165 limiting crack formation. Therefore, the migration of TM is the major intrinsic mode of degradation in high-voltage spinel oxides.165,166
Generalizing the structural features and degradation mechanisms of a wide range of polyanionic compounds is difficult, but changes in their unit cell volume are usually large (>6%) during delithiation owing to the ionic nature of the TM–O bonds, i.e. approximately 7% for high-voltage olivine Li1−xCoPO4.167 Particularly in the bi-phasic electrode reactions that proceed with phase boundary movement, large mechanical strain arises in the region between lithium-rich and lithium-poor phases, occasionally causing cracks during (de)lithiation (Fig. 5a).168
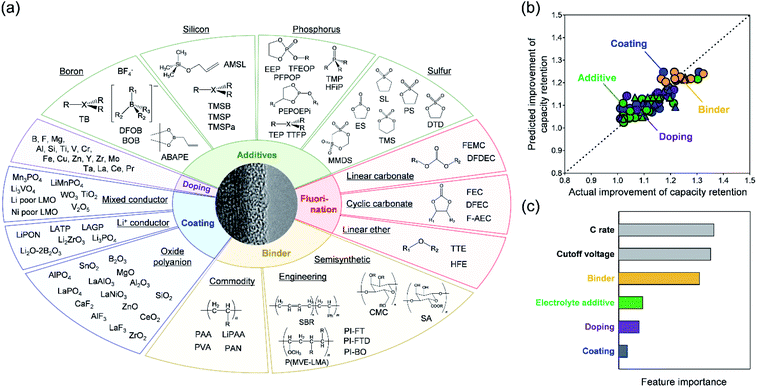
In any cases, an effective way to suppress cation migration and cracks upon cycling is to use dopants that are electrochemically inactive at the expense of the specific capacity in three scenarios: (i) redox-inactive dopants typically suppress over-delithiation at high voltages, (ii) while also disturbing the cooperative behaviors (e.g. phase transition) of the delithiated states. In parallel, (iii) heterodoping can modulate the morphology of secondary particles. Scenario (i) should decelerate cation migration, whereas scenarios (ii) and (iii) may relieve mechanical strain at domain boundaries (Fig. 5b). Because the redox-inactive dopants prevent the over-oxidation of oxide ions in lithium-rich layered oxides, the release of O2 gas can also be suppressed. Indeed, various cations and anions (such as B, F, Mg, and Al) have been investigated as dopants for positive-electrode materials with high energy density, all of which improved capacity retention during charge/discharge cycling.71–77,92,93,102,103,106–109,169–173 For example, doping B into a nickel-rich layered oxide was reported to increase capacity retention after 100 cycles from 83% to 92% by suppressing crack formation.92
4.2. Interfacial degradation
Synergistic degradation occurs at the interface between a positive-electrode material and electrolyte (Fig. 5a). For example, hydrofluoric acid, which is generated by H2O contamination or anodic dissociation, such as PF6− ↔ F˙ + PF5 + e−, reacts with most positive-electrode materials to generate LiF and TMFn.153 Then, TMn+ dissolves into the electrolyte and migrates to the negative electrode. Finally, TMn+ is reduced and deposits on the negative electrode, where it causes unfavorable catalytic decomposition of the electrolyte and increases the internal resistance of the cell.174–178 Subsequently, TM dissolution generates active pits on the positive-electrode surface, causing the additional oxidation of the electrolytes.175 In parallel, oxidized electrolyte solvents have been postulated to accelerate TM dissolution.180Analogously to the solid-electrolyte interphases (SEI) of negative electrodes, the formation of a CEI is a natural way to suppress interfacial degradation via TM dissolution. The chemically and electrochemically stable CEI that uniformly forms on positive electrodes is expected to passivate the electrode surface from damaging chemical reagents such as HF.84,181,182 However, the naive CEI of high-energy-density positive electrodes with conventional carbonate electrolytes cannot effectively protect the electrode surface. Intensive research has revealed the complex components of CEI in high-energy-density positive electrodes, such as Li2CO3 (mainly from an initial contaminant), polycarbonates (from oxidation of linear/cyclic carbonates), POxFy (from oxidation of PF6−), TMFn (from HF attack), and LiF (from PF6− dissociation).169,171,183–185 Importantly, the CEI composition depends on the electrode potential (i.e. electrochemically unstable).40,186,187 In addition, a non-uniform CEI is formed by damage (cracks or pits) on the surface of positive-electrode materials. Meso/macroscopic cracks generate new reactive surfaces that cause parasitic oxidation of electrolytes.179,188 All these drawbacks associated with naive CEIs make the effective passivation of the positive electrodes difficult, thus calling for technical solutions.
To address the aforementioned interfacial issues, coating positive-electrode materials is a popular approach (Fig. 5b) for (i) suppressing electrophilic attack from an electrode to an electrolyte while allowing ion transfer, (ii) protecting them from damaging chemical reagents such as HF, thus preventing TM from dissolving to the electrolyte, and (iii) forming a rigid shell to restrict crack formation upon cycling. Indeed, several materials, including ionic conductors,50,51,54,66,67,189 mixed conductors (active materials),46,56–58,60,190–192 organic compounds,58,60,193,194 and electrochemically inactive oxides/polyanionic compounds44,45,47–49,52,53,55,59,61–65,68–70,195–201 have been investigated as coatings for passivating the active surface of large-capacity/high-voltage electrode materials. For example, a B2O3-coated nickel-rich layered oxide retained 85% of its capacity after 200 cycles, significantly more than that of a pristine compound (68%).70
Another effective approach for the formation of a CEI is to utilize the oxidative decomposition of electrolytes or its additives that contain specific elements, such as boron,41,78–82,202–207 silicon,203,206,208–212 phosphorous,83–86,207,209–211,213–221 sulfur,87,204,205,211,222–233 and fluorine.41,78,82,83,85,86,88,89,156,182,188,205,207,213–215,217,219–221,234,235 Additives that are vulnerable to oxidation and contain nucleophilic B, Si, P and S elements are expected to preferentially oxidize to form a uniform CEI at the positive-electrode surface. In the presence of fluorine-containing components or additives, a fluorine-rich, electrochemically stable, ion-conducting surface film forms, composed of complex deposits of Li–X–O/F (X = B, Si, P, and S) that effectively behave as a CEI. The mechanisms underlying its functionality are as follows: (i) XOxFyn− scavenges oxidized radicals and hydrofluoric acid, (ii) microporous/amorphous Li–X–O/F enables fast Li+ transfer while (iii) hindering interfacial electron transfer. For instance, when 0.6 M LiTFSI + 0.4 M lithium bis(oxalato)borate (LiBOB) + 0.05 M LiPF6 in ethylene carbonate (EC)/EMC (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 by weight) was used as an electrolyte for LiNi0.76Mn0.14Co0.1O2, a low impedance CEI comprising ion-conducting/electronic-insulating components (polycarbonates, Li–B–O, and Li2CO3) formed that achieved a capacity retention greater than 80% after 1000 cycles.81
6 by weight) was used as an electrolyte for LiNi0.76Mn0.14Co0.1O2, a low impedance CEI comprising ion-conducting/electronic-insulating components (polycarbonates, Li–B–O, and Li2CO3) formed that achieved a capacity retention greater than 80% after 1000 cycles.81
4.3. Electrode disintegration
Using a functional polymer as a binder also effectively improves the electrochemical properties of large-capacity/high-voltage positive electrodes, as its functional groups, such as carboxyl and hydroxyl groups, scavenge damaging radicals or hydrofluoric acid. In addition, adhesive polymers acting as an artificially deposited uniform CEI could mechanically suppress crack formation and particle disintegration in positive-electrode materials, while simultaneously preventing TM from dissolving into the electrolyte. Typical examples include engineering polymers such as polyimides,90,91,236–239 commodity polymers such as polyacrylates,240–242 and semisynthetic polymers such as styrene–butadiene rubber/carboxymethyl cellulose,242–245etc.246–249 For example, polyimides possess heterocyclic imide rings that strongly interact with the surface of positive electrodes, thus forming a uniform protective film. These rigid imide rings also provide mechanical strength to composite electrodes for minimizing their disintegration due to the pulverization of the active materials. Using a polyimide as a binder for a NCM811 electrode increased the capacity retention after 100 cycles from 30% to 79%.90 Furthermore, the fluorination of the polyimide binder improved both oxidation and thermal stability, enabling the stable operation of lithium-rich layered oxide Li1.13Mn0.463Ni0.203Co0.203O2.2405. Technical priority analysis
As positive-electrode materials with high energy density suffer from severe performance degradation arising from both bulk (i.e. cation migration and cracking) and interfacial (i.e. TM dissolution and non-uniform CEI formation) issues, several technical solutions including doping, coating, electrolyte additives, electrolyte fluorination, and functional binders have been intensively investigated (Fig. 6a), as explained in previous sections. Although each technical solution was demonstrated to improve the electrode performance, hierarchizing their effectiveness is important for prioritizing technical solutions. We applied a machine-learning technique to the reported capacity retentions of large-capacity/high-voltage positive-electrode materials.38,41,44–112,169–173,189–249 The supervised learning of capacity retention after 50 cycles was conducted using a random forest regression with six simple features, i.e. coating, doping, electrolyte additives, functional binders, cut-off voltage, and C-rate. Target data (capacity retention) was transformed to its improvement index (the ratio of the capacity retentions with/without coating, doping, electrolyte additives, and functional binders).Fig. 6b compares the predicted and actual improvements in cycle stability (R2 = 0.75 and 0.52 for the training and test data, respectively), which suggests a relatively high variance in the model due to the limited number of data. However, the prediction model clearly indicates that the use of functional binders is the most effective strategy for improving the capacity retention after 50 cycles (Fig. 6c). Functional binders can form uniform artificial CEIs, which offer multiple functions (HF scavenging, protection from TM dissolution, and mechanical integration of the composite) in retaining the electrode performance. Fig. 6c also indicates that coating does not significantly contribute towards improving the capacity retention, relative to doping and electrolyte additives. Thus, if a coating process is costly, it may not be the first choice for enhancing electrode performance.
6. Summary and perspectives
This review summarizes the issues associated with large-capacity/high-voltage positive-electrode materials for high-energy-density LIBs and their technical solutions. Both a large capacity (large D(ε) for εF > ε > εF − FΔE + ΔμLi+) and high voltage (low μLi+ or low μe−) are intrinsically prone to accelerating performance degradation in the bulk and at the interface. Several technical solutions have been proposed to address these issues. Electrode materials have been typically doped to resolve bulk issues, whereas electrode coating, electrolyte additives, and functional binders have been used to mitigate interfacial issues. However, the machine-learning analyses of reported data clearly indicate that the use of functional binders is most effective for improving capacity retention, and hence, the further exploration of novel binders that can retain either a large capacity or high voltage should be prioritized.In addition to the above-mentioned technical solutions, a less explored approach for achieving a longer calendar life is the development of novel large-capacity or high-voltage positive-electrode materials, which possess functionalities that stabilize the electrode during cycling. For example, O2-type layered oxides LiTMO2 and Li1+xTM1−xO2 (O2: lithium ions occupy octahedral sites in a close-packed oxide-ion array of ABCBA) mitigate TM migration due to TM–TM coulombic repulsion, which suppresses capacity fade and voltage decay during cycling.250–252 In addition, a layered structure self-organizes upon cycling via coulombic attractions between ions and vacancies in Na2−xRuO3, which can effectively retain a large capacity.253,254 Transition-metal vacancies can generate nonbonding O 2p states, leading to a highly reversible and stable oxygen-redox capacity, as reported for Na2−xMn3O7.255 Essentially, precisely controlling the arrangement of transition metals, transition-metal vacancies, and oxide ions could yield novel functionalities that ameliorate the unsatisfactory performance of the present high-energy-density positive electrodes.
As for other important performance such as safety, rate capability, and coulombic efficiency, the amount of reported data is limited; therefore, identifying the most effective technical solution is difficult at this point. For example, the use of functional binders, which most effectively helps in retaining a large capacity, should degrade the rate performance because the artificial CEI of the binders decelerates interfacial ion transfer. However, the trade-off between rate performance degradation and capacity retention is not clearly mentioned in most reports, making systematic data analysis futile. Another consideration missing from this work is that the performance of the positive-electrode materials can be highly susceptible to the counter electrode; the data analysed in this work were only from the results of half cells. In addition, several important features, such as separator, electrolyte composition, and temperature, were not considered. Thus, although the present machine-learning techniques provide a firm proof of concept, they are still not enough to provide an overall protocol for accurate predictions together with hierarchical features behind.
Notably, owing to the limited amount of experimental data, which is far smaller than the usual amount used in ‘big data’ analyses, it is unrealistic to develop an accurate prediction model with multiple features that fully describe the chemical structures and properties of battery components. For instance, rationally designed fluorinated cyclic phosphate serves as a fire-extinguishing solvent for highly safe electrolytes, as organic phosphates can trap hydrogen radicals and prevent combustion.256–259 Although such molecular design is a promising approach for high-energy-density batteries, it is highly challenging to choose informative features from their complex chemical structure and properties for predicting and evaluating additional functionalities. Thus, carefully selecting discriminating features and target functionalities is crucial for reliable data analysis and prediction.
After collecting systematic experimental data, machine learning plays an essential role in data analysis. Indeed, various machine-learning techniques have recently been applied to predict battery performance metrics, e.g. voltage, cycle life, and state of charge.260–266 Once feature importance is identified with hierarchical and quantitative accuracy and reliability, the underlying chemical/physical relationships between electrode performance and several controllable parameters can also be clarified. This comprehensive understanding would enable the rational development of optimal large-capacity/high-voltage positive electrodes.267
7. Methods
Electrolytes were prepared by dissolving lithium salts in solvents in an Ar-filled glove box. Lithium bis(fluorosulfonyl)imide (LiFSI) and lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) salts were provided by Nippon Shokubai. Other salts (LiBF4 and LiPF6) and solvents (ethylene carbonate (EC), dimethyl carbonate (DMC), 1,2,-dimethoxyethane (DME), fluoroethylene carbonate (FEC)) were purchased from Kishida Chemicals. LiMn2O4 was purchased from Hohsen. LiCoPO4 was synthesized using a sol–gel process. Specifically, LiNO3, Co(NO3)2·6H2O, NH4H2PO4, and citric acid were dissolved in distilled water at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The mixture was stirred at 80 °C for 30 min and then dried at 120 °C overnight in an oven. LiCoPO4 was obtained by heating the resultant brown gel at 350 °C for 3 h and then 800 °C for 12 h. For the carbon coating, LiCoPO4 powder was ball-milled with acetylene black (Li400, Denka) in a weight ratio of 9
2. The mixture was stirred at 80 °C for 30 min and then dried at 120 °C overnight in an oven. LiCoPO4 was obtained by heating the resultant brown gel at 350 °C for 3 h and then 800 °C for 12 h. For the carbon coating, LiCoPO4 powder was ball-milled with acetylene black (Li400, Denka) in a weight ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, followed by heating at 400 °C for 1 h under flowing Ar. All the chemicals were purchased from Wako. For the carbon electrodes, acetylene black and polyvinylidene difluoride (PVdF, Kureha) binder were mixed in N-methylpyrrolidone (NMP, Wako) in a weight ratio of 50
1, followed by heating at 400 °C for 1 h under flowing Ar. All the chemicals were purchased from Wako. For the carbon electrodes, acetylene black and polyvinylidene difluoride (PVdF, Kureha) binder were mixed in N-methylpyrrolidone (NMP, Wako) in a weight ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50. For the LiMn2O4 and LiCoPO4 electrodes, the active materials, acetylene black, and PVdF binder were mixed in a weight ratio of 80
50. For the LiMn2O4 and LiCoPO4 electrodes, the active materials, acetylene black, and PVdF binder were mixed in a weight ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 in NMP. The prepared slurries were cast onto Al foil (Fuchikawa Rare Metal) and dried at 80 °C in an oven. The loading level for LiMn2O4, LiCoPO4, and carbon electrodes were controlled to be 1.8–2.1, 2.9–3.0, and 0.3–0.4 mg cm−2, respectively. The oxidation durability of electrolytes with Al, carbon, LiMn2O4, and LiCoPO4 electrodes was evaluated via linear sweep voltammetry from open circuit potential to 6 V (vs. Li/Li+) at a scan rate of 0.1 mV s−1 at 30 °C using VMP3 potentiostat (BioLogic) and 2032-type coin cell with Li metal as the counter electrode.
10 in NMP. The prepared slurries were cast onto Al foil (Fuchikawa Rare Metal) and dried at 80 °C in an oven. The loading level for LiMn2O4, LiCoPO4, and carbon electrodes were controlled to be 1.8–2.1, 2.9–3.0, and 0.3–0.4 mg cm−2, respectively. The oxidation durability of electrolytes with Al, carbon, LiMn2O4, and LiCoPO4 electrodes was evaluated via linear sweep voltammetry from open circuit potential to 6 V (vs. Li/Li+) at a scan rate of 0.1 mV s−1 at 30 °C using VMP3 potentiostat (BioLogic) and 2032-type coin cell with Li metal as the counter electrode.
All the machine learning was conducted using scikit-learn on Python. A random forest regression was selected as the prediction model based on nested cross-validation (k-fold cross-validation and grid search for hyperparameter optimization) of several regression models. The determination coefficient (R2) was calculated as follows:
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan; Grant-in-Aid for Specially Promoted Research No. 15H05701 and Scientific Research (S) No. 20H05673. M. O. was financially supported by JSPS KAKENHI Grant Numbers 19H05816, 18K19124, 18H03924, and the Asahi Glass Foundation.References
- IEA, Global EV Outlook 2019, IEA, Paris, 2019, https://www.iea.org/reports/global-ev-outlook-2019 Search PubMed.
- US Advanced Battery Consortium, USABC Goals for Advanced High-Performance Batteries for Electric Vehicle (EV) Applications, http://www.uscar.org/guest/article_view.php?articles_id=85 Search PubMed.
- D. Larcher and J. M. Tarascon, Nat. Chem., 2015, 7, 19–29 CrossRef CAS PubMed.
- X. Zeng, M. Li, D. A. El-Hady, W. Alshitari, A. S. Al-Bogami, J. Lu and K. Amine, Adv. Energy Mater., 2019, 9, 1900161 CrossRef.
- K. Mizushima, P. C. Jones, P. J. Wiseman and J. B. Goodenough, Mater. Res. Bull., 1980, 15, 783–789 CrossRef CAS.
- M. M. Thackeray, P. J. Johnson, L. A. de Picciotto, P. G. Bruce and J. B. Goodenough, Mater. Res. Bull., 1984, 19, 179–187 CrossRef CAS.
- A. K. Padhi, K. S. Nanjundaswamy and J. B. Goodenough, Journal of the Electrochemical Society, 1997, 144, 1188–1194 CrossRef CAS.
- A. Manthiram, Nat. Commun., 2020, 11, 1550 CrossRef CAS PubMed.
- M. S. Whittingham, Chem. Rev., 2004, 104, 4271–4302 CrossRef CAS PubMed.
- C. Masquelier and L. Croguennec, Chem. Rev., 2013, 113, 6552–6591 CrossRef CAS PubMed.
- A. Yamada, S. C. Chung and K. Hinokuma, J. Electrochem. Soc., 2001, 148, A224–A229 CrossRef CAS.
- J. Dahn, U. von Sacken and C. Michal, Solid State Ionics, 1990, 44, 87 CrossRef CAS.
- Z. Liu, A. Yu and J. Y. Lee, J. Power Sources, 1999, 81–82, 416 CrossRef CAS.
- F. Schipper, E. M. Erickson, C. Erk, J. Y. Shin, F. F. Chesneau and D. Aurbach, J. Electrochem. Soc., 2017, 164, A6220–A6228 CrossRef CAS.
- Z. Lu, D. D. MacNeil and J. Dahn, ECS Solid State Lett., 2001, 4, A191–A194 CrossRef CAS.
- Z. Lu and J. Dahn, J. Electrochem. Soc., 2002, 149, A815–A822 CrossRef CAS.
- G. Assat and J. M. Tarascon, Nat. Energy, 2018, 3, 373–386 CrossRef CAS.
- Q. Zhong, A. Bonakdarpour, M. Zhang, Y. Gao and J. R. Dahn, J. Electrochem. Soc., 1997, 144, 205–213 CrossRef CAS.
- W. Li, B. Song and A. Manthiram, Chem. Soc. Rev., 2017, 46, 3006–3059 RSC.
- K. Amine, H. Yasuda and M. Yamachi, Electrochem. Solid-State Lett., 2000, 3, 178 CrossRef CAS.
- M. Zhang, N. Garcia-Araez and A. L. Hector, J. Mater. Chem. A, 2018, 6, 14483–14517 RSC.
- A. Chakraborty, S. Kunnikuruvan, S. Kumar, B. Markovsky, D. Aurbach, M. Dixit and D. T. Major, Chem. Mater., 2020, 32, 915–952 CrossRef CAS.
- H. H. Sun, H. H. Ryu, U. H. Kim, J. A. Weeks, A. Heller, Y. K. Sun and C. B. Mullins, ACS Energy Lett., 2020, 5, 1136–1146 CrossRef CAS.
- H. M. K. Sari and X. Li, Adv. Energy Mater., 2019, 9, 1901597 CrossRef.
- E. M. Erickson, F. Schipper, T. R. Penki, J. Y. Shin, C. Erk, F. F. Chesneau, B. Markovsky and D. Aurbach, J. Electrochem. Soc., 2017, 164, A6341–A6348 CrossRef CAS.
- P. Rozier and J. M. Tarascon, J. Electrochem. Soc., 2015, 162, A2490–A2499 CrossRef CAS.
- S. Hy, H. Liu, M. Zhang, D. Qian, B. J. Hwang and Y. S. Meng, Energy Environ. Sci., 2016, 9, 1931–1954 RSC.
- H. Xu, H. Zhang, J. Ma, G. Xu, T. Dong, J. Chen and G. Cui, ACS Energy Lett., 2019, 4, 2871–2886 CrossRef CAS.
- M. Gauthier, T. J. Carney, A. Grimaud, L. Giordano, N. Pour, H. H. Chang, D. P. Fenning, S. F. Lux, O. Paschos, C. Bauer, F. Maglia, S. Lupart, P. Lamp and Y. Shao-Horn, J. Phys. Chem. Lett., 2015, 6, 4653–4672 CrossRef CAS PubMed.
- A. M. Haregewoin, A. S. Wotango and B. J. Hwang, Energy Environ. Sci., 2016, 9, 1955–1988 RSC.
- T. Kim, W. Song, D. Y. Son, L. K. Ono and Y. Qi, J. Mater. Chem. A, 2019, 7, 2942–2964 RSC.
- H. Zhang, H. Zhao, M. A. Khan, W. Zou, J. Xu, L. Zhang and J. Zhang, J. Mater. Chem. A, 2018, 6, 20564–20620 RSC.
- J. Duan, X. Tang, H. Dai, Y. Yang, W. Wu, X. Wei and Y. Huang, Electrochem. Energy Rev., 2020, 3, 1–42 CrossRef CAS.
- T. Li, X. Z. Yuan, L. Zhang, D. Song, K. Shi and C. Bock, Electrochem. Energy Rev., 2020, 3, 43–80 CrossRef.
- E. Rossen, C. D. W. Jones and J. R. Dahn, Solid State Ionics, 1992, 57, 311–318 CrossRef CAS.
- T. Ohzuku, A. Ueda, M. Nagayama, Y. Iwakoshi and H. Komori, Electrochim. Acta, 1993, 38, 1159–1167 CrossRef CAS.
- H. J. Noh, S. Youn, C. S. Yoon and Y. K. Sun, J. Power Sources, 2013, 233, 121–130 CrossRef CAS.
- H. Q. Pham, E. H. Hwang, Y. G. Kwon and S. W. Song, Chem. Commun., 2019, 55, 1256–1258 RSC.
- N. Yabuuchi and T. Ohzuku, J. Power Sources, 2003, 119–121, 171–174 CrossRef CAS.
- J. Zhang, Q. Li, Y. Wang, J. Zheng, X. Yu and H. Li, Energy Storage Materials, 2018, 14, 1–7 CrossRef.
- T. Deng, X. Fan, L. Cao, J. Chen, S. Hou, X. Ji, L. Chen, S. Li, X. Zhou, E. Hu, D. Su, X. Q. Yang and C. Wang, Joule, 2019, 3, 2550–2564 CrossRef CAS.
- A. R. Armstrong and P. G. Bruce, Nature, 1996, 381, 499–500 CrossRef CAS.
- A. Yamada, K. Miura, K. Hinokuma and M. Tanaka, J. Electrochem. Soc., 1995, 142, 2149–2156 CrossRef CAS.
- Z. Gan, G. Hu, Z. Peng, Y. Cao, H. Tong and K. Du, Appl. Surf. Sci., 2019, 481, 1228–1238 CrossRef CAS.
- Z. Chen, Y. Liu, Z. Lu, R. Hu, J. Cui, H. Xu, Y. Ouyang, Y. Zhang and M. Zhu, J. Alloys Compd., 2019, 803, 71–79 CrossRef CAS.
- U. H. Kim, H. H. Ryu, J. H. Kim, R. Mucke, P. Kaghazchi, C. S. Yoon and Y. K. Sun, Adv. Energy Mater., 2019, 9, 1803902 CrossRef.
- Y. Xu, X. Li, Z. Wang, H. Guo, W. Peng and W. Pan, Electrochim. Acta, 2016, 219, 49–60 CrossRef CAS.
- J. Duan, C. Wu, Y. Cao, K. Du, Z. Peng and G. Hu, Electrochim. Acta, 2016, 221, 14–22 CrossRef CAS.
- W. Cho, S. M. Kim, K. W. Lee, J. H. Song, Y. N. Jo, T. Yim, H. Kim, J. S. Kim and Y. J. Kim, Electrochim. Acta, 2016, 198, 77–83 CrossRef CAS.
- H. Liang, Z. Wang, H. Guo, J. Wang and J. Leng, Appl. Surf. Sci., 2017, 423, 1045–1053 CrossRef CAS.
- M. Wang, R. Zhang, Y. Gong, Y. Su, D. Xiang, L. Chen, Y. Chen, M. Luo and M. Chu, Solid State Ionics, 2017, 312, 53–60 CrossRef CAS.
- Y. C. Li, W. M. Zhao, W. Xiang, Z. G. Wu, Z. G. Yang, C. L. Xu, Y. D. Xu, E. H. Wang, C. J. Wu and X. D. Guo, J. Alloys Compd., 2018, 766, 546–555 CrossRef CAS.
- S. H. Lee, G. J. Park, S. J. Sim, B. S. Jim and H. S. Kim, J. Alloys Compd., 2019, 791, 193–199 CrossRef CAS.
- M. Du, P. Yang, W. He, S. Bie, H. Zhao, J. Yin, Z. G. Zou and J. Liu, J. Alloys Compd., 2019, 805, 991–998 CrossRef CAS.
- H. Tong, P. Dong, J. Zhang, J. Zheng, W. Yu, K. Wei, B. Zhang, Z. Liu and D. Chu, J. Alloys Compd., 2018, 764, 44–50 CrossRef CAS.
- Y. Lee, H. Kim, T. Yim, K. Y. Lee and W. Choi, J. Power Sources, 2018, 400, 87–95 CrossRef CAS.
- B. Zhang, P. Dong, H. Tong, Y. Yao, J. Zheng, W. Yu, J. Zhang and D. Chu, J. Alloys Compd., 2017, 706, 198–204 CrossRef CAS.
- W. Luo and B. Zhang, Appl. Surf. Sci., 2017, 404, 310–317 CrossRef CAS.
- L. A. Riley, S. V. Atta, A. S. Cavanagh, Y. Yan, S. M. George, P. Liu, A. C. Dilon and S. H. Lee, J. Power Sources, 2011, 196, 3317–3324 CrossRef CAS.
- S. Chen, T. He, Y. Su, Y. Lu, L. Bao, L. Chen, Q. Zhang, J. Wang, R. Chen and F. Wu, ACS Appl. Mater. Interfaces, 2017, 9, 29732–29743 CrossRef CAS PubMed.
- D. Becker, M. Borner, R. Nolle, M. Diehl, S. Klein, U. Todehorst, R. Schmuch, M. Winter and T. Placke, ACS Appl. Mater. Interfaces, 2019, 11, 18404–18414 CrossRef CAS PubMed.
- Y. Wu, H. Ming, M. Li, J. Zhang, W. Wahyudi, L. Xie, X. He, J. Wang, Y. Wu and J. Ming, ACS Energy Lett., 2019, 4, 656–665 CrossRef CAS.
- Y. Wu, M. Li, W. Wahyudi, G. Sheng, X. Miao, T. D. Anthopoulos, K. W. Huang, Y. Li and Z. Lai, ACS Omega, 2019, 4, 13972–13980 CrossRef CAS PubMed.
- S. W. Lee, M. S. Kim, J. H. Jeong, D. H. Kim, K. Y. Chung, K. C. Roh and K. B. Kim, J. Power Sources, 2017, 360, 206–214 CrossRef CAS.
- B. C. Park, H. B. Kim, S. T. Myung, K. Amine, I. Belharouak, S. M. Lee and Y. K. Sun, J. Power Sources, 2008, 178, 826–831 CrossRef CAS.
- Y. S. Park, K. H. Choi, H. K. Park and S. M. Lee, J. Electrochem. Soc., 2010, 157, A850–A853 CrossRef CAS.
- G. Song, H. Zhong, Z. Wang, Y. Dai, X. Zhou and J. Yang, ACS Appl. Energy Mater., 2019, 2, 7923–7932 CrossRef CAS.
- H. J. Song, S. H. Jiang, J. Ahn, S. H. Oh and T. Yim, J. Power Sources, 2019, 416, 1–8 CrossRef CAS.
- S. H. Jang, K. J. Lee, J. Mun, Y. K. Han and T. Yim, J. Power Sources, 2019, 410–411, 15–24 CrossRef CAS.
- Q. Xie, W. Li, A. Dolocan and A. Manthiram, Chem. Mater., 2019, 31, 8886–8897 CrossRef CAS.
- Z. Qiu, Z. Liu, X. Fu, J. Liu and Q. Zeng, J. Alloys Compd., 2019, 806, 136–145 CrossRef CAS.
- Y. Zhang, T. Ren, J. Zhang, J. Duan, X. Li, Z. Zhou, P. Dong and D. Wang, J. Alloys Compd., 2019, 805, 1288–1296 CrossRef CAS.
- H. H. Ryu, N. Y. Park, J. H. Seo, Y. S. Yu, M. Sharma, R. Mucke, P. Kaghazchi, C. S. Yoon and Y. K. Sun, Mater. Today, 2020, 36, 73–82 CrossRef CAS.
- S. J. Sim, S. H. Lee, B. S. Jin and H. S. Kim, Sci. Rep., 2019, 9, 8952 CrossRef PubMed.
- A. M. Hashem, A. E. Abdel-Ghany, M. Scheuermann, S. Indris, H. Ehrenberg, A. Mauger and C. M. Julien, Materials, 2019, 12, 2899 CrossRef CAS PubMed.
- C. Lv, J. Yang, Y. Peng, X. Duan, J. Ma, Q. Li and T. Wang, Electrochim. Acta, 2019, 297, 258–266 CrossRef CAS.
- H. Xu, L. Ai, J. Yan, G. Yan and W. Zhang, Ceram. Int., 2019, 45, 23089–23096 CrossRef CAS.
- X. Zuo, C. Fan, J. Liu, X. Xiao, J. Wu and J. Nan, J. Electrochem. Soc., 2013, 160, A1199–A1204 CrossRef CAS.
- Z. Wang, L. Xing, J. Li, B. Li, M. Xu, Y. Liao and W. Li, Electrochim. Acta, 2015, 184, 40–46 CrossRef CAS.
- L. Zhang, Y. Ma, X. Cheng, P. Zuo, Y. Cui, T. Guan, C. Du, Y. Gao and G. Yin, Solid State Ionics, 2014, 263, 146–151 CrossRef CAS.
- W. Zhao, J. Zheng, L. Zou, H. Jia, B. Liu, H. Wang, M. H. Engelhard, C. Wang, W. Xu, Y. Yang and J. G. Zhang, Adv. Energy Mater., 2018, 8, 1800297 CrossRef.
- G. Lan, H. Zhou, L. Xing, J. Chen, Z. Li, R. Guo, Y. Che and W. Li, J. Energy Chem., 2019, 39, 235–243 CrossRef.
- C. C. Su, M. He, C. Peebles, L. Zeng, A. Tornheim, C. Liao, L. Zhang, J. Wang, Y. Wang and Z. Zhang, ACS Appl. Mater. Interfaces, 2017, 9, 30686–30695 CrossRef CAS PubMed.
- D. Gao, J. B. Xu, M. Lin, Q. Xu, C. F. Ma and H. F. Xiang, RSC Adv., 2015, 5, 17566–17571 RSC.
- J. Chen, L. Xing, X. Yang, X. Liu, T. Li and W. Li, Electrochim. Acta, 2018, 290, 568–576 CrossRef CAS.
- L. Wang, Y. Ma, Q. Li, Y. Cui, P. Wang, X. Cheng, P. Zuo, C. Du, Y. Gao and G. Yin, Electrochim. Acta, 2017, 243, 72–81 CrossRef CAS.
- P. Dong, D. Wang, Y. Yao, X. Li, Y. Zhang, J. Ru and T. Ren, J. Power Sources, 2017, 344, 111–118 CrossRef CAS.
- X. Fan, L. Chen, O. Borodin, X. Ji, J. Chen, S. Hou, T. Deng, J. Zheng, C. Yang, S. C. Liou, K. Amine, K. Xu and C. Wang, Nat. Nanotechnol., 2018, 13, 715–722 CrossRef CAS PubMed.
- Y. M. Lee, K. M. Nam, E. H. Hwang, Y. G. Kwon, D. H. Kang, S. S. Kim and S. W. Song, J. Phys. Chem. C, 2014, 118, 10631–10639 CrossRef CAS.
- H. Q. Pham, J. Lee, H. M. Jung and S. W. Song, Electrochim. Acta, 2019, 317, 711–721 CrossRef CAS.
- J. H. Park, J. H. Cho, S. B. Kim, W. S. Kim, S. Y. Lee and S. Y. Lee, J. Mater. Chem., 2012, 22, 12574–12581 RSC.
- H. H. Ryu, N. Y. Park, D. R. Yoon, U. H. Kim, C. S. Yoon and Y. K. Sun, Adv. Energy Mater., 2020, 10, 2000495 CrossRef CAS.
- F. Xin, H. Zhou, X. Chen, M. Zuba, N. Chernova, G. Zhou and M. S. Whittingham, ACS Appl. Mater. Interfaces, 2019, 11, 34889–34894 CrossRef CAS PubMed.
- W. Lee, S. Muhammad, T. Kim, H. Kim, E. Lee, M. Jeong, S. Son, J. H. Ryou and W. S. Yoon, Adv. Energy Mater., 2018, 8, 1701788 CrossRef.
- C. H. Jo, D. H. Cho, H. J. Noh, H. Yashiro, Y. K. Sun and S. T. Myung, Nano Res., 2015, 1, 1464–1479 CrossRef.
- Z. Cao, M. Hashinokuchi, T. Doi and M. Inaba, J. Electrochem. Soc., 2019, 166, A82–A88 CrossRef CAS.
- B. Song, W. Li, S. M. Oh and A. Manthiram, ACS Appl. Mater. Interfaces, 2017, 9, 9718–9725 CrossRef CAS PubMed.
- O. Srur-Lavi, V. Miikkulainen, B. Markovsky, J. Grinblat, M. Talianker, Y. Fleger, G. Cohen-Taguri, A. Mor, Y. Tal-Yosef and D. Aurbach, J. Electrochem. Soc., 2017, 164, A3266–A3275 CrossRef CAS.
- K. Meng, Z. Wang, H. Guo, X. Li and D. Wang, Electrochim. Acta, 2016, 211, 822–831 CrossRef CAS.
- L. Wang, Y. Ma, P. Wang, S. Lou, X. Cheng, P. Zuo, C. Du, Y. Gao and G. Yin, J. Electrochem. Soc., 2017, 164, A1924–A1932 CrossRef CAS.
- X. Guo, L. N. Cong, Q. Zhao, L. H. Tai, X. L. Wu, J. P. Zhang, R. S. Wang, H. M. Xie and L. Q. Sun, J. Alloys Compd., 2015, 651, 12–18 CrossRef CAS.
- D. Wang, X. Li, Z. Wang, H. Guo, Y. Xu and Y. Fan, Electrochim. Acta, 2016, 196, 101–109 CrossRef CAS.
- Y. D. Xu, J. Zhang, Z. G. Wu, C. L. Xu, Y. C. Li, W. Xiang, Y. Wang, Y. J. Zhong, X. D. Guo and H. Chen, Energy Technology, 2020, 8, 1900498 CrossRef CAS.
- S. S. Zhang, J. Chen and C. Wang, J. Electrochem. Soc., 2019, 166, A487–A492 CrossRef CAS.
- Y. Li, S. Deng, Y. Chen, J. Gao, J. Zhu, L. Xue, T. Lei, G. Cao, J. Guo and S. Wang, Electrochim. Acta, 2019, 300, 26–35 CrossRef CAS.
- L. Qiu, W. Xiang, W. Tian, C. L. Xu, Y. C. Li, Z. G. Wu, T. R. Chen, K. Jia, D. Wang D, F. R. He and X. D. Guo, Nano Energy, 2019, 63, 103818 CrossRef CAS.
- X. Yang, Y. Tang, G. Shang, J. Wu, Y. Lai, J. Li, Y. Qu and Z. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 32015–32024 CrossRef CAS.
- F. Schipper, H. Bouzaglo, M. Dixit, E. M. Erickson, T. Weigel, M. Talianker, J. Grinblat, L. Burstein, M. Schmidt, J. Lampert and C. Erk, Adv. Energy Mater., 2018, 8, 1701682 CrossRef.
- H. Yang, H. H. Wu, M. Ge, L. Li, Y. Yuan, Q. Yao, J. Chen, L. Xia, J. Zheng, Z. Chen and J. Duan, Adv. Funct. Mater., 2019, 29, 1808825 CrossRef.
- A. Yasmin, M. A. Shehzad, J. Wang, X. D. He, X. Ding, S. Wang, Z. Wen and C. Chen, ACS Appl. Mater. Interfaces, 2020, 12, 826–835 CrossRef CAS.
- R. Zhao, J. Liang, J. Huang, R. Zeng, J. Zhang, H. Chen and G. Shi, J. Alloys Compd., 2017, 724, 1109–1116 CrossRef CAS.
- Q. Liu, G. Yang, S. Liu, M. Han, Z. Wang and L. Chen, ACS Appl. Mater. Interfaces, 2019, 11, 17435–17443 CrossRef CAS PubMed.
- M. M. Thackeray, S. H. Kang, C. S. Johnson, J. T. Vaughey, R. Benedek and S. A. Hackney, J. Mater. Chem., 2007, 17, 3112–3125 RSC.
- S. L. Cui, Y. Y. Wang, S. Liu, G. R. Li and X. P. Gao, Electrochim. Acta, 2019, 328, 135109 CrossRef CAS.
- K. Luo, M. R. Roberts, N. Guerrini, N. Tapia-Ruiz, R. Hao, F. Massel, D. M. Pickup, S. Ramos, Y. S. Liu, J. Guo, A. V. Chadwick, L. C. Duda and P. G. Bruce, J. Am. Chem. Soc., 2016, 138, 11211–11218 CrossRef CAS PubMed.
- K. Luo, M. R. Roberts, R. Hao, N. Guerrini, D. M. Pickup, Y. S. Liu, K. Edström, J. H. Guo, A. V. Chadwick, L. C. Duda and P. G. Bruce, Nat. Chem., 2016, 8, 684–691 CrossRef CAS PubMed.
- D. H. Seo, J. Lee, A. Urban, R. Malik, S. Y. Kang and G. Ceder, Nat. Chem., 2016, 8, 692–697 CrossRef CAS PubMed.
- M. Okubo and A. Yamada, ACS Appl. Mater. Interfaces, 2017, 9, 36463–36472 CrossRef CAS PubMed.
- E. McCalla, A. M. Abakumov, M. Saubanère, D. Foix, E. J. Berg, G. Rousse, M. L. Doublet, D. Gonbeau, P. Novák, G. Van Tedeloo, R. Dominko and J. M. Tarascon, Science, 2015, 350, 1516–1521 CrossRef CAS PubMed.
- N. Yabuuchi, M. Nakayama, M. Takeuchi, S. Komaba, Y. Hashimoto, T. Mukai, H. Shiiba, K. Sato, Y. Kobayashi, A. Nakao, M. Yonemura, K. Yamanaka, K. Mitsuhara and T. Ohta, Nat. Commun., 2016, 7, 13814 CrossRef CAS PubMed.
- B. Mortemard de Boisse, S. Nishimura, E. Watanabe, L. Lander, A. Tsuchimoto, J. Kikkawa, E. Kobayashi, D. Asakura, M. Okubo and A. Yamada, Adv. Energy Mater., 2018, 8, 1800409 CrossRef.
- J. Hong, W. E. Gent, P. Xiao, K. Lim, D. H. Seo, J. Wu, P. M. Csernia, C. J. Takacs, D. Nordlund, C. J. Sun, K. H. Stone, D. Passerello, W. L. Yang, D. Prendergast, G. Ceder, M. F. Toney and W. C. Chueh, Nat. Mater., 2019, 8, 256–265 CrossRef.
- R. A. House, U. Maitra, M. A. Perez-Osorio, J. G. Lozano, L. Jin, J. W. Somerwille, L. C. Dude, A. Nag, A. Walters, K. J. Zhou, M. R. Roberts and P. G. Bruce, Nature, 2020, 577, 502–508 CrossRef CAS PubMed.
- T. Sudayama, K. Uehara, T. Mukai, D. Asakura, X. M. Shi, A. Tsuchimoto, B. Mortemard de Boisse, T. Shimada, E. Watanabe, Y. Harada, M. Nakayama, M. Okubo and A. Yamada, Energy Environ. Sci., 2020, 13, 1492–1500 RSC.
- H. Koga, L. Croguennec, M. Menetrier, K. Douhil, S. Belin, L. Bourgeois, E. Suard, F. Weil and C. Delmas, J. Electrochem. Soc., 2013, 160, A786–A792 CrossRef CAS.
- T. Zheng and J. R. Dahn, Phys. Rev. B: Condens. Matter Mater. Phys., 1997, 56, 3800–3805 CrossRef CAS.
- T. Ohzuku, S. Takeda and M. Iwanaga, J. Power Sources, 1999, 81–82, 90–94 CrossRef CAS.
- C. Sigala, D. Guyomard, A. Verbaere, Y. Piffard and M. Tournoux, Solid State Ionics, 1995, 81, 167–170 CrossRef CAS.
- H. Kawai, M. Nagata, H. Tukamoto and A. R. West, J. Mater. Chem., 1998, 8, 837–839 RSC.
- S. Okada, S. Sawa, M. Egashira, J. Yamaki, M. Tabuchi, H. Kageyama, T. Konishi and A. Yoshino, J. Power Sources, 2001, 97–98, 430–432 CrossRef CAS.
- A. Manthiram and J. B. Goodenough, J. Power Sources, 1989, 26, 403–408 CrossRef CAS.
- F. Zhou, M. Cococcioni, C. A. Marianetti, D. Morgan and G. Ceder, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 235121 CrossRef.
- F. Zhou, M. Cococcioni, K. Kang and G. Ceder, Electrochem. Commun., 2004, 6, 1144–1148 CrossRef CAS.
- M. Herklotz, F. Scheiba, R. Glaum, E. Mosymow, S. Oswald, J. Eckert and H. Ehrenberg, Electrochim. Acta, 2014, 139, 356–364 CrossRef CAS.
- K. Kawai, W. Zhao, S. Nishimura and A. Yamada, ACS Appl. Energy Mater., 2018, 1, 928–931 CrossRef CAS.
- J. Zhang, Y. C. Liu, X. D. Zhao, L. H. He, H. Liu, Y. Z. Song, S. D. Sun, Q. Li, X. R. Xing and J. Chen, Adv. Mater., 2020, 32, 1906348 CrossRef CAS PubMed.
- K. Kawai, D. Asakura, S. Nishimura and A. Yamada, Chem. Commun., 2019, 44, 13717–13720 RSC.
- S. Okada, M. Ueno, Y. Uebou and J. Yamaki, J. Power Sources, 2005, 146, 565–569 CrossRef CAS.
- S. C. Yin, P. Subramanya Herle, A. Higgins, N. J. Taylor, Y. Makimura and L. F. Nazar, Chem. Mater., 2006, 18, 1745–1752 CrossRef CAS.
- J. Baker, M. Y. Saidi and J. L. Swoyer, J. Electrochem. Soc., 2003, 150, A1394–A1398 CrossRef.
- T. Mueller, G. Hautier, A. Jain and G. Ceder, Chem. Mater., 2011, 23, 3854–3862 CrossRef CAS.
- B. L. Ellis, R. M. Makahnouk, Y. Makiura, K. Toghill and L. F. Nazar, Nat. Mater., 2007, 6, 749–753 CrossRef CAS PubMed.
- N. Recham, J. N. Chotard, L. Dupont, C. Delacourt, W. Walker, M. Armand and J. M. Tarascon, Nat. Mater., 2010, 9, 68–74 CrossRef CAS PubMed.
- S. Tan, Y. J. Ji, Z. R. Zhang and Y. Yang, ChemPhysChem, 2014, 15, 1956–1969 CrossRef CAS PubMed.
- L. J. Krause, W. Lamanna, J. Summerfield, M. Engle, G. Korba, R. Loch and R. Atanasoski, J. Power Sources, 1997, 68, 320–325 CrossRef CAS.
- H. Yang, K. Kwon, T. M. Devine and J. W. Evans, J. Electrochem. Soc., 2000, 147, 4399–4407 CrossRef CAS.
- Y. Yamada, C. H. Chiang, K. Sodeyama, J. Wang, Y. Tateyama and A. Yamada, ChemElectroChem, 2015, 2, 1687–1694 CrossRef CAS.
- M. Ue, M. Takeda, M. Takehara and S. Mori, J. Electrochem. Soc., 1997, 144, 2684–2688 CrossRef CAS.
- J. M. Tarascon and D. Guyomard, Solid State Ionics, 1994, 69, 293–305 CrossRef CAS.
- K. Kanamura, T. Okagawa and Z. Takehara, J. Power Sources, 1995, 57, 119 CrossRef CAS.
- S. Ko, Y. Yamada, L. Lander and A. Yamada, Carbon, 2020, 158, 766–771 CrossRef CAS.
- Y. Zhang, Y. Katayama, R. Tatara, L. Giordano, Y. Yu, D. Fraggedakis, J. G. Sun, F. Maglia, R. Jung, M. Z. Bazant and Y. Shao-Horn, Energy Environ. Sci., 2020, 13, 183–199 RSC.
- O. Borodin, W. Behl and T. R. Jow, J. Phys. Chem. C, 2013, 117, 8661–8682 CrossRef CAS.
- V. R. Koch, J. Electrochem. Soc., 1979, 126, 181 CrossRef CAS.
- K. Xu, S. P. Ding and T. R. Jow, J. Electrochem. Soc., 1999, 146, 4172–4178 CrossRef CAS.
- Z. Zhang, L. Hu, H. Wu, W. Wang, M. Koh, P. C. Redfern, L. A. Curtiss and K. Amine, Energy Environ. Sci., 2013, 6, 1806–1810 RSC.
- H. Wang, Y. I. Jang, B. Huang, D. R. Sadoway and Y. M. Chiang, J. Electrochem. Soc., 1999, 146, 473–480 CrossRef CAS.
- C. Pouillerie, L. Croguennec and C. Delmas, Solid State Ionics, 2000, 132, 15–29 CrossRef CAS.
- R. V. Chebiam, F. Prado and A. Manthiram, J. Electrochem. Soc., 2001, 148, A49–A53 CrossRef CAS.
- S. Choi and A. Manthiram, J. Electrochem. Soc., 2002, 149, A1157–A1163 CrossRef CAS.
- H. H. Ryu, K. J. Park, C. S. Yoon and Y. K. Sun, Chem. Mater., 2018, 30, 1155–1163 CrossRef CAS.
- M. Sathiya, G. Rousse, K. Ramesha, C. P. Laisa, H. Vezin, M. T. Sougrati, M. L. Doublet, D. Foix, D. Gonbeau, W. Walker, A. S. Prakash, M. Ben Hassine, L. Dupont and J. M. Tarascon, Nat. Mater., 2013, 12, 827–835 CrossRef CAS PubMed.
- P. Yan, J. Zheng, Z. K. Tang, A. Devaraj, G. Chen, K. Amine, J. G. Zhang, L. M. Liu and C. Wang, Nat. Nanotechnol., 2019, 14, 602–608 CrossRef CAS PubMed.
- R. Alcantara, M. Jaraba, P. Lavela and J. L. Tirado, Electrochim. Acta, 2002, 47, 1829–1835 CrossRef CAS.
- J. H. Kim, C. S. Yoon, S. T. Myung, J. Prakash and Y. K. Sun, Electrochem. Solid-State Lett., 2004, 7, A216–A220 CrossRef CAS.
- Y. Gong, Y. Chen, Q. Zhang, F. Meng, J. A. Shi, X. Liu, X. Liu, J. Zhang, H. Wang, J. Wang, Q. Yu, Z. Zhang, Q. Xu, R. Xiao, Y. S. Hu, L. Gu, H. Li, X. Huang and L. Chen, Nat. Commun., 2018, 9, 3341 CrossRef PubMed.
- N. N. Bramnik, K. Nikolowski, C. Baehtz, K. G. Bramnik and H. Ehrenberg, Chem. Mater., 2007, 19, 908–915 CrossRef CAS.
- J. Wolfenstine, B. Poese and J. Allen, J. Power Sources, 2004, 138, 281–282 CrossRef CAS.
- S. Yamamoto, H. Noguchi and W. Zhao, J. Power Sources, 2015, 278, 76–86 CrossRef CAS.
- W. Zhao, L. Xiong, Y. Xu, X. Xiao, J. Wang and Z. Ren, J. Power Sources, 2016, 330, 37–44 CrossRef CAS.
- T. Weigel, F. Schipper, E. M. Erickson, F. A. Susai, B. Markovsky and D. Aurbach, ACS Energy Lett., 2019, 4, 508–516 CrossRef CAS.
- F. A. Susai, D. Kovacheva, A. Chakraborty, T. Kravchuk, R. Ravikumar, M. Talianker, J. Grinblat, L. Burstein, Y. Kauffmann, D. T. Major, B. Markovsky and D. Aurbach, ACS Appl. Energy Mater., 2019, 2, 4521–4534 CrossRef CAS.
- F. Nobili, F. Croce, R. Tossici, I. Meschini, P. Reale and R. Marassi, J. Power Sources, 2012, 197, 276–284 CrossRef CAS.
- D. Aurbach, J. Power Sources, 2000, 89, 206–218 CrossRef CAS.
- D. Aurbach, B. Markovsky, G. Salitra, E. Markevich, Y. Talyossef, M. Koltypin, L. Nazar, B. Ellis and D. Kovacheva, J. Power Sources, 2007, 165, 491–499 CrossRef CAS.
- W. Li, A. Dolocan, P. Oh, H. Celio, S. Park, J. Cho and A. Manthiram, Nat. Commun., 2018, 8, 14589 CrossRef PubMed.
- J. Li and A. Manthiram, Adv. Energy Mater., 2019, 9, 1902731 CrossRef CAS.
- Y. K. Sun, K. J. Hong, J. Prakash and K. Amine, Electrochem. Commun., 2002, 4, 344–348 CrossRef CAS.
- J. Zheng, M. Gu, J. Xiao, B. J. Polzin, P. Yan, X. Chen, C. Wang and J. G. Zhang, Chem. Mater., 2014, 26, 6320–6327 CrossRef CAS.
- D. H. Jang, Y. J. Shin and S. M. Oh, J. Electrochem. Soc., 1996, 143, 2204–2211 CrossRef CAS.
- S. Jiao, X. Ren, R. Cao, M. H. Engelhard, Y. Liu, D. Hu, D. Mei, J. Zheng, W. Zhao, Q. Li, N. Liu, B. D. Adams, C. Ma, J. Liu, J. G. Zhang and W. Xu, Nat. Energy, 2018, 3, 739–746 CrossRef CAS.
- D. Sun, Q. Wang, J. Zhou, Y. Lyu, Y. Liu and B. Guo, J. Electrochem. Soc., 2018, 165, A2032–A2036 CrossRef CAS.
- K. Edstrom, T. Gustafsson and J. O. Thomas, Electrochim. Acta, 2004, 50, 397–403 CrossRef.
- D. Chen, M. A. Mahmoud, J. H. Wang, G. H. Waller, B. Zhao, C. Qu, M. A. El-Sayed and M. Liu, Nano Lett., 2019, 19, 2037–2043 CrossRef CAS PubMed.
- H. Duncan, Y. Abu-Lebdeh and I. J. Davidson, J. Electrochem. Soc., 2010, 157, A528–A535 CrossRef CAS.
- Z. W. Lebens-Higgins, S. Sallis, N. V. Faenza, F. Badway, N. Pereira, D. M. Halat, M. Wahila, C. Schlueter, T. L. Lee, W. Yang, C. P. Grey, G. G. Amatucci and L. F. Piper, Chem. Mater., 2018, 30, 958–969 CrossRef CAS.
- S. Liu, L. Wang, C. Zhang, B. Chu, C. Wang, T. Huang and A. Yu, J. Power Sources, 2019, 438, 226979 CrossRef CAS.
- X. Ren, L. Zou, X. Cao, M. H. Engelhard, W. Liu, S. D. Burton, H. Lee, C. Niu, B. E. Matthews, Z. Zhu, C. Wang, B. W. Arey, J. Xiao, J. Liu, J. G. Zhang and W. Xu, Joule, 2019, 3, 1662–1676 CrossRef CAS.
- W. Liu, P. Oh, X. Liu, S. Myeong, W. Cho and J. Cho, Adv. Energy Mater., 2015, 5, 1500274 CrossRef.
- X. Ding, D. Luo, J. Cui, H. Xie, Q. Ren and Z. Lin, Angew. Chem., Int. Ed., 2020, 132, 7852–7856 CrossRef.
- Y. K. Sun, S. T. Myung, B. C. Park, J. Prakash, I. Belharouak and K. Amine, Nat. Mater., 2009, 8, 320–324 CrossRef CAS PubMed.
- Z. Zhu, D. Yu, Y. Yang, C. Su, Y. Huang, Y. Dong, I. Waluyo, B. Wang, A. Hunt, X. Yao, J. Lee, W. Xue and J. Li, Nat. Energy, 2019, 4, 1049–1058 CrossRef CAS.
- J. Z. Kong, L. P. Xu, C. L. Wang, Y. X. Jiang, Y. Q. Cao and F. Zhou, J. Alloys Compd., 2017, 719, 401–410 CrossRef CAS.
- P. Oh, M. Ko, S. Myeong, Y. Kim and J. Cho, Adv. Energy Mater., 2014, 4, 1400631 CrossRef.
- M. Si, D. Wang, R. Zhao, D. Pan, C. Zhang, C. Yu, X. Lu, H. Zhao and Y. Bai, Adv. Sci., 2019, 1902538 Search PubMed.
- G. Xu, J. Li, Q. Xue, Y. Dai, H. Zhou, X. Wang and F. Kang, Electrochim. Acta, 2014, 117, 41–47 CrossRef CAS.
- F. Wu, Q. Li, L. Bao, Y. Zheng, Y. Lu, Y. Su, J. Wang, S. Chen, R. Chen and J. Tian, Electrochim. Acta, 2018, 260, 986–993 CrossRef CAS.
- K. Park, J. H. Park, B. Choi, J. H. Kim, S. G. Hong and H. N. Han, Electrochim. Acta, 2017, 257, 217–223 CrossRef CAS.
- K. A. Walz, C. S. Johnson, J. Genthe, L. C. Stoiber, W. A. Zeltner, M. A. Anderson and M. M. Thackeray, J. Power Sources, 2010, 195, 4943–4951 CrossRef CAS.
- D. Chen, F. Zheng, L. Li, M. Chen, X. Zhong, W. Li and L. Lu, J. Power Sources, 2017, 341, 147–155 CrossRef CAS.
- M. Bettge, Y. Li, B. Sankaran, N. D. Rago, T. Spila, R. T. Haasch, I. Petrov and D. P. Abraham, J. Power Sources, 2013, 233, 346–357 CrossRef CAS.
- Z. Luo, H. Zhang, L. Yu, D. Huang and J. Shen, J. Electroanal. Chem., 2019, 833, 520–526 CrossRef CAS.
- J. Li, L. Xing, R. Zhang, M. Chen, Z. Wang, M. Xu and W. Li, J. Power Sources, 2015, 285, 360–366 CrossRef CAS.
- H. Zhou, D. Xiao, C. Yin, Z. Yang, K. Xiao and J. Li, J. Electroanal. Chem., 2018, 808, 293–302 CrossRef CAS.
- L. Dong, F. Liang, D. Wang, C. Zhu, J. Liu, D. Gui and C. Li, Electrochim. Acta, 2018, 270, 426–433 CrossRef CAS.
- Y. Meng, G. Chen, L. Shi, H. Liu and D. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 45108–45117 CrossRef CAS PubMed.
- B. Liu, H. Zhou, C. Yin, H. Guan and J. Li, Electrochim. Acta, 2019, 321, 134690 CrossRef CAS.
- J. Chen, H. Zhang, M. Wang, J. Liu, C. Li and P. Zhang, J. Power Sources, 2016, 303, 41–48 CrossRef CAS.
- Y. K. Han, J. Yoo and T. Yim, Electrochim. Acta, 2016, 215, 455–465 CrossRef CAS.
- H. Rong, M. Xu, B. Xie, W. Huang, X. Liao, L. Xing and W. Li, J. Power Sources, 2015, 274, 1155–1161 CrossRef CAS.
- L. Ma, J. Xia and J. R. Dahn, J. Electrochem. Soc., 2015, 162, A1170–A1174 CrossRef CAS.
- K. Kim, D. Hwang, S. Kim, S. O. Park, H. Cha, Y. S. Lee, J. Cho, S. K. Kwak and N. S. Choi, Adv. Energy Mater., 2020, 2000012 CrossRef CAS.
- M. Xu, Y. Liu, B. Li, W. Li, X. Li and S. Hu, Electrochem. Commun., 2012, 18, 123–126 CrossRef CAS.
- J. Pires, A. Castets, L. Timperman, J. Santos-Pena, E. Dumont, S. Levasseur, C. Tessier, R. Dedryvere and M. Anouti, J. Power Sources, 2015, 296, 413–425 CrossRef CAS.
- J. Liu, X. Song, L. Zhou, S. Wang, W. Song, W. Liu, H. Long, L. Zhou, H. Wu, C. Feng and Z. Guo, Nano Energy, 2018, 46, 404–414 CrossRef CAS.
- N. N. Sinha, J. C. Burns and J. R. Dahn, J. Electrochem. Soc., 2014, 161, A1084–A1089 CrossRef CAS.
- M. He, C. C. Su, C. Peebles, Z. Feng, J. G. Connell, C. Liao, Y. Wang, I. A. Shkrob and Z. Zhang, ACS Appl. Mater. Interfaces, 2016, 8, 11450–11458 CrossRef CAS PubMed.
- Z. D. Li, Y. C. Zhang, H. F. Xiang, X. M. Ma, Q. F. Yuan, Q. S. Wang and C. H. Chen, J. Power Sources, 2013, 240, 471–475 CrossRef CAS.
- A. von Cresce and K. Xu, J. Electrochem. Soc., 2011, 158, A337–A342 CrossRef CAS.
- S. Tan, Z. Zhang, Y. Li, Y. Li, J. Zhang, Z. Zhou and Y. Yang, J. Electrochem. Soc., 2013, 160, A285–A292 CrossRef CAS.
- N. von Aspern, D. Diddens, T. Kobayashi, M. Borner, O. Stubbmann-Kazakova, V. Kozel, G. V. Roschenthaler, J. Smiatek, M. Winter and I. Cekic-Laskovic, ACS Appl. Mater. Interfaces, 2019, 11, 16605–16618 CrossRef CAS PubMed.
- X. Zuo, C. Fan, X. Xiao, J. Liu and J. Nan, J. Power Sources, 2012, 219, 94–99 CrossRef CAS.
- X. Cui, H. Zhang, S. Li, Y. Zhao, L. Mao, W. Zhao, Y. Li and X. Ye, J. Power Sources, 2013, 240, 476–485 CrossRef CAS.
- T. Zhang, I. de Meatza, X. Qi and E. Paillard, J. Power Sources, 2017, 356, 97–102 CrossRef CAS.
- J. Alvarado, M. A. Schroeder, M. Zhang, O. Borodin, E. Gobrogge, M. Olguin, M. S. Ding, M. Gobet, S. Greenbaum, Y. S. Meng and K. Xu, Mater. Today, 2018, 21, 341–353 CrossRef CAS.
- X. Zheng, T. Huang, G. Fang, Y. Pan, Q. Li and M. Wu, ACS Appl. Mater. Interfaces, 2019, 11, 36244–36251 CrossRef CAS PubMed.
- R. Wang, X. Li, Z. Wang, H. Guo and J. Wang, Electrochim. Acta, 2015, 180, 815–823 CrossRef CAS.
- L. Madec, J. Xia, R. Petibon, K. J. Nelson, J. P. Sun, I. G. Hill and J. R. Dahn, J. Phys. Chem. C, 2014, 118, 29608–29622 CrossRef CAS.
- P. Janssen, R. Schmitz, R. Muller, P. Isken, A. Lex-Balducci, C. Schreiner, M. Winter, I. Cekic-Laskovic and R. Schmitz, Electrochim. Acta, 2014, 125, 101–106 CrossRef CAS.
- J. Xia, J. E. Harlow, R. Petibon, J. C. Burns, L. P. Chen and J. R. Dahn, J. Electrochem. Soc., 2014, 161, A547–A553 CrossRef CAS.
- L. Madec, R. Petibon, K. Tasaki, J. Xia, J. P. Sun, I. G. Hill and J. R. Dahn, Phys. Chem. Chem. Phys., 2015, 17, 27062–27076 RSC.
- J. Xia, N. N. Sinha, L. P. Chen, G. Y. Kim, D. J. Xiong and J. R. Dahn, J. Electrochem. Soc., 2014, 161, A84–A88 CrossRef CAS.
- M. H. Park, Y. S. Lee, H. Lee and Y. K. Han, J. Power Sources, 2011, 196, 5109–5114 CrossRef CAS.
- M. C. Smart, B. V. Ratnakumar, V. S. Ryan-Mowrey, S. Surampudi, G. K. S. Prakash, J. Hu and I. Cheung, J. Power Sources, 2003, 119–121, 359–367 CrossRef CAS.
- M. Hekmatfar, I. Hasa, R. Eghbal, D. V. Carvalho, A. Moretti and S. Passerini, Adv. Mater. Interfaces, 2019, 1901500 Search PubMed.
- H. Q. Pham, G. Kim, H. M. Jung and S. W. Song, Adv. Funct. Mater., 2018, 28, 1704690 CrossRef.
- J. H. Cho, J. H. Park, M. H. Lee, H. K. Song and S. Y. Lee, Energy Environ. Sci., 2012, 5, 7124–7131 RSC.
- G. Qian, L. Wang, Y. Shang, X. He, S. Tang, M. Liu, T. W. Li, G. Zhang and J. Wang, Electrochim. Acta, 2016, 187, 113–118 CrossRef CAS.
- J. Zhang, Q. Lu, J. Fang, J. Wang, J. Yang and Y. NuLi, ACS Appl. Mater. Interfaces, 2014, 6, 17965–17973 CrossRef CAS PubMed.
- N. P. W. Pieczonka, V. Borgel, B. Ziv, N. Leifer, V. Dargel, D. Aurbach, J. H. Kim, Z. Liu, X. Huang, S. A. Krachkovskiy, G. R. Goward, I. Halalay, B. R. Powell and A. Manthiram, Adv. Energy Mater., 2015, 5, 1501008 CrossRef.
- J. Yang, P. Li, F. Zhong, X. Feng, W. Chen, X. Ai, H. Yang, D. Xia and Y. Cao, Adv. Energy Mater., 2020, 1904264 CrossRef CAS.
- S. Hitomi, K. Kubota, T. Horiba, K. Hida, T. Matsuyama, H. Oji, S. Yasuno and S. Komaba, ChemElectroChem, 2019, 6, 5070–5079 CrossRef CAS.
- Z. Han, H. Zhan and Y. Zhou, Mater. Lett., 2014, 114, 48–51 CrossRef CAS.
- S. Zhang, H. Gu, H. Pan, S. Yang, W. Du, X. Li, M. Gao, Y. Liu, M. Zhu, L. Ouyang, D. Jian and F. Pan, Adv. Energy Mater., 2017, 7, 1601066 CrossRef.
- N. Yabuuchi, Y. Kinoshita, K. Misaki, T. Matsuyama and S. Komaba, J. Electrochem. Soc., 2015, 162, A538–A544 CrossRef CAS.
- T. Dong, H. Zhang, Y. Ma, J. Zhang, X. Du, C. Lu, X. Shangguan, J. Li, M. Zhang, J. Yang, X. Zhou and G. Cui, J. Mater. Chem. A, 2019, 7, 24594–24601 RSC.
- T. Zhang, J. T. Li, J. Liu, Y. P. Deng, Z. G. Wu, Z. W. Yin, D. Guo, L. Huang and S. G. Sun, Chem. Commun., 2016, 52, 4683 RSC.
- F. Wu, W. Li, L. Chen, Y. Lu, Y. Su, W. Bao, J. Wang, S. Chen and L. Bao, J. Power Sources, 2017, 359, 226–233 CrossRef CAS.
- P. P. Prosini, M. Carewska and A. Masci, Solid State Ionics, 2015, 274, 88–93 CrossRef CAS.
- B. Mortemard de Boisse, J. Jang, M. Okubo and A. Yamada, J. Electrochem. Soc., 2018, 165, A3630–A3633 CrossRef CAS.
- D. Eum, B. Kim, S. J. Kim, H. Park, J. Wu, S. P. Cho, G. Yoon, M. H. Lee, S. K. Jung, W. Yang, W. M. Seong, K. Ku, O. Tamwattana, S. K. Park, I. Hwang and K. Kang, Nat. Mater., 2020, 19, 419–427 CrossRef CAS PubMed.
- C. Cui, X. Fan, X. Zhou, J. Chen, Q. Wang, L. Ma, C. Yang, E. Hu, X. Q. Yang and C. Wang, J. Am. Chem. Soc., 2020, 142, 8918–8927 CrossRef CAS PubMed.
- B. Mortemard de Boisse, M. Reynaud, J. Ma, J. Kikkawa, S. Nishimura, M. Casas-Cabanas, C. Delmas, M. Okubo and A. Yamada, Nat. Commun., 2019, 10, 2185 CrossRef PubMed.
- B. Mortemard de Boisse, G. Liu, J. Ma, S. Nishimura, S. C. Chung, H. Kiuchi, Y. Harada, J. Kikkawa, Y. Kobayashi, M. Okubo and A. Yamada, Nat. Commun., 2016, 7, 11397 CrossRef CAS PubMed.
- B. Mortemard de Boisse, S. Nishimura, E. Watanabe, L. Lander, A. Tsuchimoto, J. Kikkawa, E. Kobayashi, D. Asakura, M. Okubo and A. Yamada, Adv. Energy Mater., 2018, 8, 1800409 CrossRef.
- Q. Zheng, Y. Yamada, R. Shang, S. Ko, Y. Y. Lee, K. Kim, E. Nakamura and A. Yamada, Nat. Energy, 2020, 5, 291–298 CrossRef CAS.
- N. von Aspern, D. Diddens, T. Kobayashi, M. Borner, O. Stubbmann-Kazakova, V. Kozel, G. V. Roschenthaler, J. Smiatek, M. Winter and I. Cekic-Laskovic, ACS Appl. Mater. Interfaces, 2019, 11, 16605 CrossRef CAS PubMed.
- C. C. Su, M. He, C. Peebles, L. Zeng, A. Tornheim, C. Liao, L. Zhang, J. Wang, Y. Wang and Z. Zhang, ACS Appl. Mater. Interfaces, 2017, 9, 30686–30695 CrossRef CAS PubMed.
- J. Wang, Y. Yamada, K. Sodeyama, E. Watanabe, K. Takada, Y. Tateyama and A. Yamada, Nat. Energy, 2018, 3, 22–29 CrossRef CAS.
- C. Chen, Y. Zuo, W. Ye, X. Li, Z. Deng and S. P. Ong, Adv. Energy Mater., 2020, 10, 1903242 CrossRef CAS.
- Z. Jiang, J. Li, Y. Yang, L. Mu, C. Wei, X. Yu, P. Pianetta, K. Zhao, P. Cloetens, F. Lin and Y. Liu, Nat. Commun., 2020, 11, 2310 CrossRef CAS PubMed.
- N. Kireeva and V. S. Pervov, Batteries Supercaps, 2020, 3, 427–438 CrossRef CAS.
- A. Killic, Ç. Odabasi, R. Yildirim and D. Eroglu, Chem. Eng. J., 2020, 390, 124117 CrossRef.
- R. P. Joshi, J. Eickholt, L. Li, M. Fornari, V. Barone and J. E. Peralta, ACS Appl. Mater. Interfaces, 2019, 11, 18494–18503 CrossRef CAS PubMed.
- K. A. Severson, P. M. Attia, N. Jin, N. Perkins, B. Jiang, Z. Yang, M. H. Chen, M. Aykol, P. K. Herring, D. Fraggedakis, M. Z. Bazant, S. J. Harris, W. C. Chueh and R. D. Braatz, Nat. Energy, 2019, 4, 383–391 CrossRef.
- A. D. Sendek, E. D. Cubuk, E. R. Antoniuk, G. Cheon, Y. Cui and E. J. Reed, Chem. Mater., 2019, 31, 342–352 CrossRef CAS.
- S. Ko, Y. Yamada and A. Yamada, Batteries Supercaps, 2020, 3, 910–916 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |