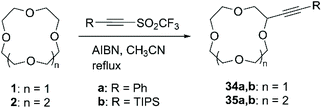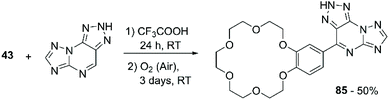Open Access Article
Open Access ArticleDirect synthetic routes to functionalised crown ethers
Federico
Nicoli
 ab,
Massimo
Baroncini
ab,
Massimo
Baroncini
 ac,
Serena
Silvi
ac,
Serena
Silvi
 ad,
Jessica
Groppi
ad,
Jessica
Groppi
 *a and
Alberto
Credi
*a and
Alberto
Credi
 *ab
*ab
aCLAN-Center for Light Activated Nanostructures, Istituto ISOF-CNR via Gobetti 101, 40129 Bologna, Italy. E-mail: alberto.credi@unibo.it; jessica.groppi@unibo.it
bDipartimento di Chimica Industriale “Toso Montanari”, Università di Bologna, viale del Risorgimento 4, 40136 Bologna, Italy
cDipartimento di Scienze e Tecnologie Agro-alimentari, Università di Bologna, viale Fanin 44, 40127 Bologna, Italy
dDipartimento di Chimica “G. Ciamician”, Università di Bologna, via Selmi 2, 40126 Bologna, Italy
First published on 1st July 2021
Abstract
Crown ethers are macrocyclic hosts that can complex a wide range of inorganic and organic cations as well as neutral guest species. Their widespread utilization in several areas of fundamental and applied chemistry strongly relies on strategies for their functionalisation, in order to obtain compounds that could carry out multiple functions and could be incorporated in sophisticated systems. Although functionalised crown ethers are normally synthesised by templated macrocyclisation using appropriately substituted starting materials, the direct addition of functional groups onto a pre-formed macrocyclic framework is a valuable yet underexplored alternative. Here we review the methodologies for the direct functionalisation of aliphatic and aromatic crown ethers sporadically reported in the literature over a period of four decades. The general approach for the introduction of moieties on aliphatic crown ethers involves a radical mediated cross dehydrogenative coupling initiated either by photochemical or thermal/chemical activation, while aromatic crown ethers are commonly derivatised via electrophilic aromatic substitution. Direct functionalization routes can reduce synthetic effort, allow the later modification of crown ether-based architectures, and disclose new ways to exploit these versatile macrocycles in contemporary supramolecular science and technology.
1. Introduction
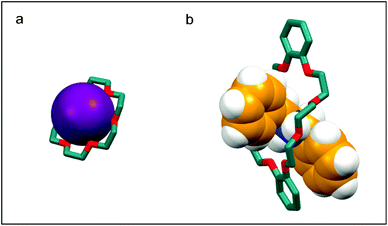
Crown ethers (CE), in their most common form, are macrocyclic oligomers of ethylene oxide characterised by selective binding abilities toward cationic and neutral species. Although cyclic polyethers had been known since the early 1900s,1 Charles J. Pedersen was the first in 1967 to discover an efficient strategy to prepare macrocyclic polyethers, and to systematically study their binding properties towards metal cations.2 Pedersen named “crown ethers” this new class of compounds, inspired by the appearance of the molecular models of the complexes formed with cations, in which the ether host seems to crown the metal guest (Fig. 1).3 His seminal work led Pedersen, along with Donald J. Cram and Jean-Marie Lehn, to receive the Nobel Prize in Chemistry in 1987, for “the development and use of molecules with structure-specific interactions of high selectivity”.3–5 CE have been instrumental for the advancement of the field of supramolecular chemistry6,7 and have found widespread application,8 so much so that currently many of them are commercially available.CE can be divided into two groups: aliphatic and aromatic (Fig. 2). The most common aliphatic CE are cyclic oligomers of ethylene oxide, in which from four to a maximum of ten –(CH2CH2O)– repeating units are linked together; one or more ethylene groups of the macrocycle may be part of an aliphatic moiety such as cyclohexyl. Aromatic CE are characterised by the presence of endocyclic aromatic rings in their structure. Although a wide range of aromatic moieties has been incorporated in aromatic CE,9 the benzene ring represents by far the most common instance, giving rise to benzocrown (BCE) and dibenzocrown (DBCE) ethers. Crown ethers are conventionally indicated as x-crown-y (abbreviated xCy) in which x is the number of atoms in the macrocyclic cavity and y is the number of these atoms that are oxygen. Thus, 18-crown-6, or 18C6, is a crown ether with a cavity of 18 atoms, 6 of which are oxygen.
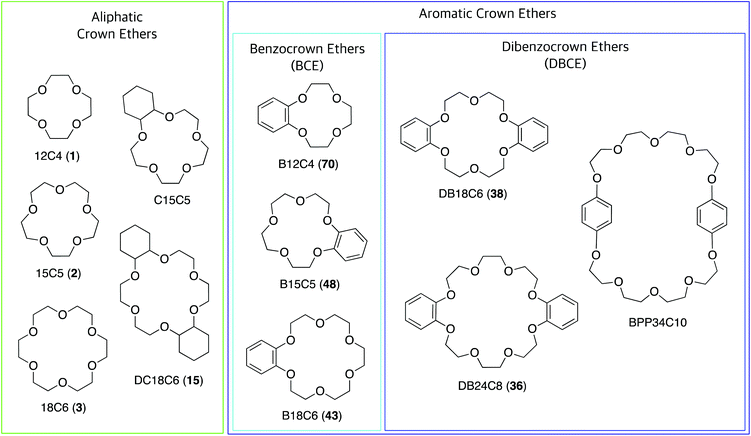 | ||
| Fig. 2 Structure of commonly used crown ethers, most of which are commercially available. The numbers in parentheses are the compound labels used in this article. | ||
The peculiar characteristics of CEs originate from the graceful interaction between the chemically stable and conformationally flexible ethylene units, and the nucleophilic oxygen atoms symmetrically arranged around the ring. This combination generates an electron rich cavity able to accommodate suitable guests, as well as to endow the compounds with a relatively amphiphilic nature.3,10 Early complexation studies were focused on the interaction between CE and metal ions: it was demonstrated that the process is driven by several factors, including the relative sizes of the ether host and the cationic guest, the charge of the cation, the number and location of the oxygen atoms, the steric hindrance on the ring, and the competition by the solvent.2,3,6,7
Indeed, CE are able to selectively bind alkali and alkaline earth ions (Fig. 1a), forming complexes which, owing to the lipophilic character, are soluble in non-polar solvents, thus acting as carriers of the charged species. For this reason, CE have been extensively used in phase transfer catalysis11 and ion extraction;12 more recently, they have been exploited as ion translocators and as components for synthetic channels through lipidic membranes.13 In the last three decades, it has been demonstrated that the complexing ability of CE is not limited to metal cations, as CE can generate stable supramolecular complexes with a wide variety of organic guests in aprotic solvents (Fig. 1b). Arenediazonium salts, which are readily complexed and stabilised by 18C6 and 21C7, were one of the first reported examples of non-metallic guests.14 Aromatic CEs, by virtue of their π-electron rich aromatic groups, act as hosts for different π-electron poor guests such as bipyridinium,15 diazapyrenium,16 bis-(pyridinium)ethane,17 imidazolium,18 and triazolium.19 Besides electrostatic effects, both donor–acceptor π–π interactions and strong hydrogen bonds contribute to the formation and stability of these complexes.
The complexes of primary alkylammonium ions (RNH3+) with DB18C6 have been extensively studied. The host and guest interact in a face-to-face orientation, with the formation of three alternating hydrogen bonds between the oxygen atoms of the macrocycle and the ammonium group, forming very stable supramolecular entities. A consequence of this binding motif is that the stability of complexes formed with secondary and tertiary ammonium ions is much lower.20
Stoddart and co-workers first demonstrated that crown ethers with a larger cavity, such as DB24C8, form complexes with secondary alkylammonium ions in which the cation “pierces through” the cavity of the macrocycle (Fig. 1b).21 Secondary ammonium guests designed to have an axle-type structure are therefore able to thread appropriately sized crown ethers22 to generate pseudorotaxanes which are not only interesting per se,23 but also useful supramolecular precursors to mechanically interlocked molecules (MIMs). CE, given their versatility as host molecules, have nowadays become a staple in the construction of MIMs and have been included in the design of hundreds of rotaxane architectures and catenane topologies.24 A direct consequence of the inclusion of CE as components of MIMs, is their application in the development of artificial molecular machines:24–26 pH, redox, or light activated mechanical switches27 and molecular pumps28 are just a few examples of molecular devices incorporating CE. The interest in functionalising CE has grown in parallel to the number of their areas of application. Crown ethers have been derivatised in order to couple the ability to form complexes with other functions such as catalysis,29 self-assembly30 and sensing,31 and to be introduced in dendrimers,32 polymers,33 and covalent34 or metal organic frameworks.35 In the field of MIMs, functionalised CE have been used for the construction of interlocked polymers36 and higher order structures,37 molecular transporters,38 mechanically planar chiral rotaxanes,39 and dynamic crystalline materials.40
Despite the extensive application of functionalised CE and the astounding number of reports dealing with the properties and uses of this class of macrocycles, to our best knowledge the only review article on the synthesis of modified CE was published by Bradshaw et al. in 1980.41 Homberg et al. recently described in a minireview an alternative approach to templated ring closure for the synthesis of chiral polyether macrocycles.42 To the present day, only one publication by Pluzhnik-Gladyr describes methodologies for the direct halogenation of CE.43
This review will focus on direct synthetic routes to functionalised CE, encompassing publications until April 2021; the literature cited in previous reviews will not be included, unless it is necessary to better assess newer protocols. After a brief excursus regarding the main synthetic strategies for the preparation of functionalised crown ethers and their pro and cons, the discussion will move to direct functionalisation protocols and it will be organized according to the class of the CE substrate, either aliphatic or aromatic.
2. Synthetic approaches to functionalised crown ethers
The synthesis of functionalised crown ethers is performed following two main approaches: templated macrocyclisation, using pre-functionalised starting materials, or direct functionalisation of previously formed CE. Although templated macrocyclisation is the most used approach for the synthesis of functionalised CE, the introduction of substituents on pre-formed macrocycles represents a valid and, under some aspects, superior alternative. First of all, direct functionalisation routes are obviously the only option available when the CE to be functionalised is irreversibly embedded in a more extended architecture (e.g., a MIM). Hence, identifying procedures to perform selective reactions on CE is important to enable the modification of multicomponent species via post-synthetic pathways.The protocols for the synthesis and purification of non-functionalised crown ethers have been optimized for large scale production, and a number of CE are currently commercially available at reasonable prices. Therefore, the direct functionalisation of a purchased macrocycle may drastically reduce the synthetic effort necessary to prepare ad hoc CE. This is particularly true for aliphatic CE, for which the preparation of the derivatised precursors usually requires a significant number of steps, potentially resulting in a waste of time and resources compared to a one-step direct functionalisation. Moreover, in templated approaches, the introduced functional groups must be very stable under the macrocyclisation reaction conditions. Specifically, they must be able to withstand basic conditions and heating, and they must be inert towards nucleophilic addition as well as not being nucleophiles themselves; if this is not the case, parasite processes will likely lead to low yields and challenging purification procedures. This stringent limitation is clearly overcome by performing the functionalisation directly as the final step.
The principal limitation of direct approaches is indeed the lack of efficient, rigorously described and verified protocols and procedures. For example, direct reactions for mono-functionalisation of DBCE are characterised by poor yields, while those for bis-functionalisation suffer from scarce regioselectivity. Another drawback of direct functionalisation protocols is that an excess of the starting CE (or CE-containing substrate) must usually be employed to reach high yields of the final product; in most instances, however, the unreacted material can be recovered during purification. Surely, albeit direct functionalisation approaches are still underexplored, they can represent an advantageous and expedient synthetic strategy that deserves to be considered while confronting challenging CE-based synthetic targets.
Templated strategies rely on the size complementarity between the cavity of the target macrocycle and an added metal cation. The latter acts as a template by pre-organising the polyether chain precursor into the correct conformation required for efficient cyclisation, hence minimising the formation of polymeric side products.7,10Thus, Li+ templates the macrocyclisation of 12-membered CE, whereas Na+, K+ and Cs+ favour 15-, 18- and 24-membered cycles, respectively.44–47
Aliphatic CE can be obtained either by closure of the ether macrocycle by nucleophilic addition of a functionalised diol to an n-ethyleneglycol-ditosylate (Scheme 1a, top)44 or by a two-step procedure involving the reaction between an n-ethyleneglycol and a derivatised epoxide to produce the open functionalised (n + 1)-ethyleneglycol chain. The latter is then converted to the final crown ether via a one-pot tosylation-macrocyclisation reaction (Scheme 1a, bottom).45 The yield of the isolated products could vary significantly according to the final ring size and the nature of the functional group, ranging from 12% to 72%.
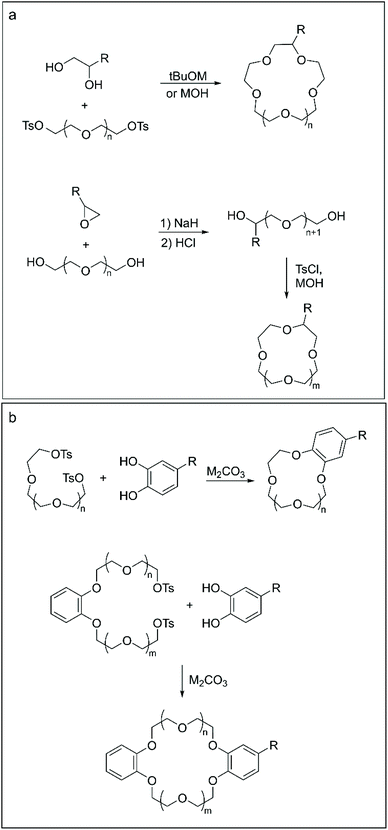 | ||
| Scheme 1 Methodologies for the templated synthesis of functionalised aliphatic (a) and aromatic (b) crown ethers. | ||
Templated macrocyclisation represents the go-to approach for the synthesis of BCE and DBCE presenting functional groups on the aromatic portion of their structure. In a typical procedure, a functionalised catechol is reacted with either an n-ethyleneglycol-ditosylate (or di-halogen) chain, to obtain BCE (Scheme 1b, top),46 or with a benzo-di-n-ethyleneglycol-ditosylate precursor, in the case of DBCE47 (Scheme 1b, bottom). Aromatic CE are routinely obtained in 40%–50% yield following such synthetic routes.
The methodologies for direct functionalisation will be thoroughly discussed in the upcoming sections of this review. In brief, functional groups can be introduced on aliphatic CE mainly via radical-mediated cross dehydrogenative coupling (CDC), while aromatic CE are generally functionalised via electrophilic substitution reactions of the aromatic moieties.
3. Methodologies for direct functionalisation
3.1. Aliphatic crown ethers
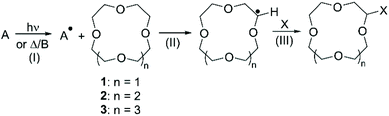
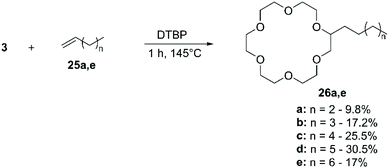
As introduced in the previous paragraph, most of the literature describing the direct functionalisation of aliphatic CE relies on the use of CDC for the introduction of a variety of functional groups: the selective oxidation of the α-C(sp3)–H bond to α-C(sp3)–X (X = C, N, F) bond is achieved via radical intermediate mechanism.48 The process can be divided in three distinct steps: (I) generation of an electrophilic radical; (II) hydrogen abstraction from the CE by the electrophilic radical, with formation of a radical at the electron-rich α-carbon; (III) reaction of the radical with the coupling partner to yield the functionalised crown (Scheme 2).The routes for direct functionalisation of aliphatic CE can be divided in two groups, according to the method used for the generation of the electrophilic radical (step I). As the latter can be produced by either photochemical or thermal/chemical activation, this section will be organised accordingly. Peculiarities will be dealt with in due course, but some general remarks can be made. All the examples found report mono-functionalisation, although a large excess of the starting CE is usually needed. Reactions are normally performed under inert atmosphere and the purification of the derivatives is typically straightforward, achieved either by fractional distillation or chromatography. Finally, higher yields are reported for procedures that rely on photochemical radical generation compared to thermal/chemical one. The implications related to the generation of one or more chiral centres on the crown ether upon introduction of functional groups are frequently overlooked. Indeed, the functionalization of aliphatic CE leads to the formation of enantiomers, or diastereomeric pairs if a chiral centre is also present on the grafted functional group. Although such stereoisomers could be separated and might exhibit different recognition properties, they are usually isolated and characterized as mixtures (e.g. racemates).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
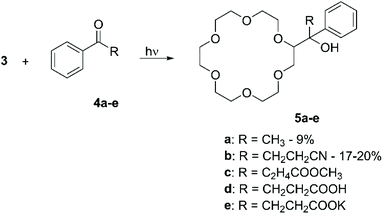
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 3 and a ketone for several hours produced the corresponding mono-functionalised derivatives in yields comprised between 7% and 21%, according to the nature of the R group present on the keto moiety. It is interesting to note that higher yields were obtained for those ketones able to generate complexes with 3, i.e.4b and 4e (Scheme 3). Similarly, aliphatic CE were directly functionalised with 1,4-benzoquinones by photoirradiation.50
1 mixture of 3 and a ketone for several hours produced the corresponding mono-functionalised derivatives in yields comprised between 7% and 21%, according to the nature of the R group present on the keto moiety. It is interesting to note that higher yields were obtained for those ketones able to generate complexes with 3, i.e.4b and 4e (Scheme 3). Similarly, aliphatic CE were directly functionalised with 1,4-benzoquinones by photoirradiation.50
More recently, Kamijo et al. exploited the ability of photochemically generated oxyl radicals to abstract a hydrogen atom by homolytic C–H bond cleavage, to develop a general methodology for the conversion of electron-rich C(sp3)–H into C(sp3)–C bonds. In this instance the ketone only acts as a photosensitiser in the process and the coupling partner is added as a tosyl derivative. Such an approach (Scheme 4) allowed to introduce cyano,51 alkynyl,52 alkenyl53 and aldoxime (as formyl precursor)54 functional groups on alkanes, benzylic compounds, ethers, alcohols and amines, generating derivatives suitable for conversion to synthetically relevant synthons. 15-crown-5 (2) was included in the substrate scope: irradiation with a Hg lamp of a mixture containing an excess of 2, the ketone (1 equiv.) and the coupling partner (1 equiv.) at room temperature for periods that depend on the coupling partner (Table 1) leads to mono-functionalisation in high yield, despite the presence of multiple reactive sites on the molecule (Scheme 4).
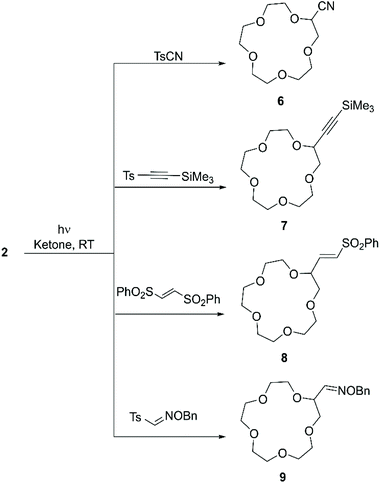 | ||
| Scheme 4 Functional groups introduced on 15C5 (2) according to the methodology reported by Kamijo et al. | ||
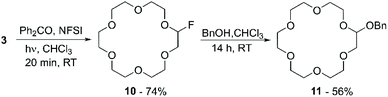
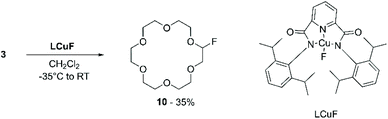
This work motivated Beniazza et al. to apply a similar approach to the mono-fluorination of cyclic ethers to obtain derivatives suitable for further modification by nucleophilic substitution.55 The optimised procedure, consisting of a two-step one-pot protocol, was applied to the functionalisation of 3 with benzyl alcohol. First, a CHCl3 solution containing 3 (1 equiv.), a catalytic amount of benzophenone (2% mol), and N-fluorobis(phenyl)sulfonimide (NFSI) (0.5 equiv.) as a fluorine source, is irradiated at 320–390 nm for 20 minutes at room temperature. This process generates a transient monofluorinated macrocycle (10) in 74% yield, which, by addition of benzyl alcohol in the second step of the reaction, undergoes nucleophilic substitution affording product 11 in 56% yield (Scheme 5). The transient nature of the monofluorinated adduct was explained by the formation of sulfonimide acid as a side product, which catalyses fluoride elimination.
Compound 10 was also reported by Bower et al.56 In this work, a formally copper(III) fluoride complex (LCuF) performed both the hydrogen atom abstraction and radical capture roles. Upon mixing 3 and LCuF in a 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in dichloromethane, product 10 was obtained in 35% yield (Scheme 6). The reactivity of the monofluorinated crown ether, however, was not further investigated.
1 ratio in dichloromethane, product 10 was obtained in 35% yield (Scheme 6). The reactivity of the monofluorinated crown ether, however, was not further investigated.
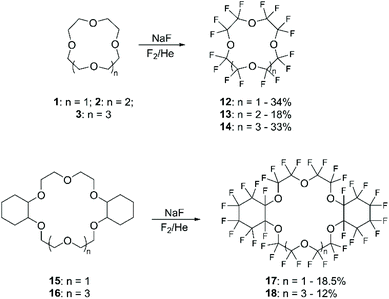
Previous to these recent studies on direct mono-fluorination, only per-fluorination of aliphatic CE, including cyclohexano derivatives, had been carried out.57 The general procedure consisted in coating NaF with the chosen CE followed by exposing the solid mixture to a flow of fluorine gas in a cryogenic fluorination reactor under inert conditions (Scheme 7). The yields, that range between 12% and 35%, were highly affected by several parameters, including fluorination conditions, the exposed surface area of the starting materials, as well as the initial purity of the reagents.
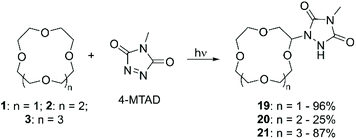
Risi et al. studied the photochemical coupling reaction between cyclic ethers and 4-methyl-1,2,4-triazoline-3,5-dione (4-MTAD).58 The interest in such a specific functionalisation originates from the wide application of triazolinedione compounds in the fields of polymer science and click chemistry.59 Slow addition of 4-MTAD to an excess of CE 12-crown-4 (1), 2 or 3 under continuous laser irradiation at λ = 514.5 nm for several days, allowed for the formation of the corresponding α-urazolyl derivatives. While in the case of 2 and 3 only the mono-functionalised product was isolated, for 1 also the bis-urazoyl compound was obtained, for which the authors suggest either a symmetrical 1,3 or 1,7 substitution pattern according to the simple 1H NMR and 13C NMR spectra. (Scheme 8). The reaction mechanism, elucidated by ESR and computational calculations, involves the photoreduction of 4-MTAD to generate an urazolyl radical, followed by abstraction of a hydrogen atom from the polyether, and terminated in the coupling of the two intermediates. The reaction yields were excellent for products 19 and 21 while only 25% was reported for compound 20; no further investigation was carried out to elucidate the reason behind the different reactivities of the three CE.
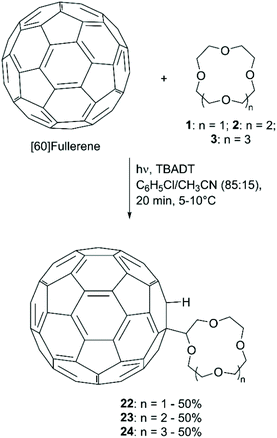
The covalent attachment of CE to [60]fullerene (C60) has attracted attention for the photophysical and electrochemical properties of the resulting adducts and their potential applications in ion sensing and fluorescence switching devices. Tzirakis et al. first described the direct coupling of CE to C60, achieved photochemically using tetrabutylammonium decatungstate [(nBu4N)4W10O32, TBADT] as a photosensitiser (Scheme 9).60 The procedure, applied to the functionalisation of 1, 2 and 3, consisted in the irradiation with a xenon lamp of a mixture of the CE (100 equiv.), C60 (1 equiv.) and TBADT (0.5 equiv.) in C6H5Cl/CH3CN (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) for 20 minutes at 5–10 °C. The yield was reported to be 50% for all the three isolated products. The authors also mentioned that the more common photosensitiser benzophenone was tested, but lower yields (20%) were obtained despite the use of 100 equiv. of ketone.
15) for 20 minutes at 5–10 °C. The yield was reported to be 50% for all the three isolated products. The authors also mentioned that the more common photosensitiser benzophenone was tested, but lower yields (20%) were obtained despite the use of 100 equiv. of ketone.
In a recent publication, compound 23 was synthesised with the aim to study its reactivity toward sodium and bis(arene)chromium compounds.61 The coupling between C60 and 15C5 (2) was performed photochemically in presence of benzophenone at 50 °C, irradiating a solution of the reagents in DCB for 5 hours. The reported yield was 30%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) olefin
olefin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTBP in 10
DTBP in 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio led to very low conversion of 3 and formation of telomers, highlighting the poor control over the process. On the other hand, the slow addition of peroxide and olefin to a very large excess of 3 (3
1 molar ratio led to very low conversion of 3 and formation of telomers, highlighting the poor control over the process. On the other hand, the slow addition of peroxide and olefin to a very large excess of 3 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) olefin
olefin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTBP = 20
DTBP = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) led to a slight improvement of the yield, reaching values up to 30%. Nonetheless, the methodology has been applied to the functionalisation of 1, 2 and 3 with allyl chloride for the introduction of 8-hydroxyquinoline63 and to synthesise alkyl-CE for the development of antifungal agents.64
1) led to a slight improvement of the yield, reaching values up to 30%. Nonetheless, the methodology has been applied to the functionalisation of 1, 2 and 3 with allyl chloride for the introduction of 8-hydroxyquinoline63 and to synthesise alkyl-CE for the development of antifungal agents.64
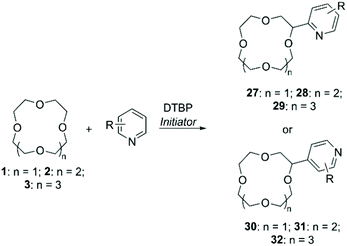
DTBP in presence of radical initiators was also used as a hydrogen abstractor in protocols for the functionalisation of CE with N-aromatic bases (Scheme 11). Early procedures relied on the use of metals such as Fe(II) and Ti(III) to generate the peroxyl radical,65 while in a recent more “green” approach DTBP was activated by photoirradiation with a higher control on the regioselection of the final products (Table 2).66 In all cases, functionalised CE were obtained in good yields.
| CE | N-aromatic base | Init. | Solv. | t (h) | T (°C) | Yield (%) [Prod]ref |
|---|---|---|---|---|---|---|
| 3 | 2-Methylquinoline | FeSO4 | DMSO | 1 | RT | 70–90 [32]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65a 65a |
| 3 | Quinoxaline | TiCl3 | H2O | — | RT | — [29]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65b 65b |
| 3 | 2-Methylquinoline | TiCl3 | H2O | — | RT | — [32]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65b 65b |
| 3 | 2,6-Lutidine | TiCl3 | H2O | — | RT | — [32]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65b 65b |
| 3 | 4-Methylquinoline | TiCl3 | H2O | — | RT | — [29]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65b 65b |
| 3 | Quinoline | FeSO4 | DMSO | 0.5 | RT | 20 [19]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65c 30 [32] 65c 30 [32]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65c 65c |
| 3 | Quinoxaline | FeSO4 | DMSO | 0.5 | RT | 85 [29]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65c 65c |
| 3 | 2-Methylquinoline | FeSO4 | DMSO | 1 | RT | 65 [32]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65d 65d |
| 1 | 2-Methylquinoline | FeSO4 | DMSO | 1 | RT | 69 [30]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65d 65d |
| 2 | 2-Methylquinoline | FeSO4 | DMSO | 1 | RT | 65 [31]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65d 65d |
| 3 | 4-Methylquinoline | hν | EDC | 23 | 40 | 71 [29]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 66 66 |
| 2 | 4-Methylquinoline | hν | EDC | 23 | 40 | 61 [28]66 |
| 3 | Isoquinoline | hν | EDC | 23 | 40 | 68 [29]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 66 66 |
| 2 | Isoquinoline | hν | EDC | 23 | 40 | 61 [28]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 66 66 |
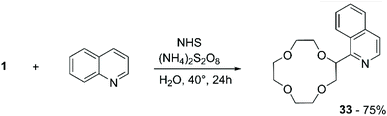
An alternative to peroxyl radicals for the direct attachment of N-aromatic bases to CE is represented by N-hydroxysuccinimide (NHS)-mediated Cα-heteroarylation.67 The reaction consists in the formation of a reactive N-radical form of NHS by oxidation with ammonium persulfate (APS); the generated radical carries out hydrogen abstraction on the ether, which in turn proceeds to attack the heteroaromatic base in a regioselective fashion (Scheme 12). Following this method, 1 was selectively introduced onto position 1 of isoquinoline to obtain compound 33 in 75% yield.
The serendipitous discovery that acetylenic triflones spontaneously react with THF at room temperature to afford the α-alkynylated product in almost quantitative yields brought Fuchs et al. to establish a methodology which exploits the reactivity of the trifluoromethyl radical towards C–H hydrogen abstraction for the introduction of alkyne functional group on several classes of compounds.68 Subsequently, the reaction was successfully applied for the direct alkynylation of 1 and 2 (Scheme 13).69 Heating an excess of CE and acetylenic triflone (1 equiv.) in CH3CN in presence of a catalytic amount of azobisisobutyronitrile (AIBN) (20% mol) as the radical initiator, led to the conversion to the corresponding mono-alkynylated product in good yields (Table 3).
| Product | CE equiv. | t (h) | Yield (%) |
|---|---|---|---|
| 34a | 10 | 3 | 70 |
| 34b | 5 | 6 | 70 |
| 35a | 10 | 2 | 80 |
| 35b | 5 | 5 | 70 |
3.2. Aromatic crown ethers
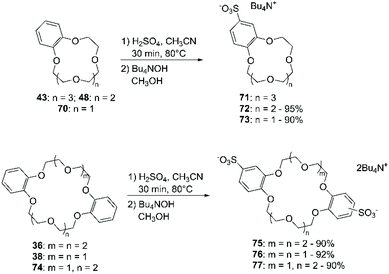
The optimisation of synthetic protocols for production upscaling and the straightforward access to functionalised catechols enabled the preparation of a large variety of aromatic CE characterised by a wide range of properties. Moreover, the ease of handling and the solubility in most organic solvents brought this class of crown ethers at the center of many basic and applied investigations. The direct functionalisation of BCE and DBCE is usually achieved by electrophilic substitution on the aromatic portion. Hence, this part of the review will be organised according to the introduced functional groups. Albeit procedures have been adapted from general methodologies used for the derivatisation of aromatic compounds, the outcome can be affected by electronic and steric effects related to ring size and conformation. The weak points of this approach are the lack of control on the number of groups introduced in the crown ether framework (specifically, mono-functionalised adducts are obtained in low yields, since bis-functionalisation frequently proceeds in parallel) and, relatively to DBCE, poor regioselectivity (both syn and anti disubstituted products are obtained and their separation is not straightforward).The reported methodologies were applied only for the direct addition of chlorine, bromine and iodine to BCE and DBCE. The author pointed out the lack of relevant literature focused on the direct fluorination of aromatic crown ethers.43 In general, direct bromination of BCE is the most successful reaction among the protocols mentioned above, with yields that can exceed 90%; chlorination and iodination are less performing due to either selectivity or stability reasons. Indeed, the most challenging aspect of the direct halogenation of DBCE is selectivity: the mono-substituted derivative is usually obtained in low yields since the reaction leads to the formation of mixtures of mono- and di-substituted species. Moreover, di-substituted derivatives could be obtained both as anti and syn isomers which can only be separated by fractional crystallisation. Higher yields (80–90%) could be achieved in the preparation of the tetra-substituted derivatives.
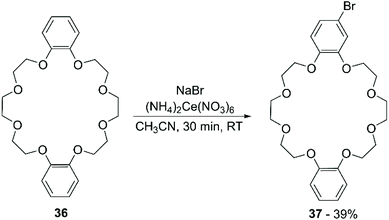
Recently, Mobian et al. reported the bromination of DB24C8 (36) using sodium bromide (NaBr) and cerium(IV) ammonium nitrate [(NH4)2Ce(NO3)6] in acetonitrile (Scheme 14).70 The reaction proceeds fast at room temperature and both mono- and di-functionalised adducts are formed. A yield of 39% was only reported for the mono-brominated derivative 37.
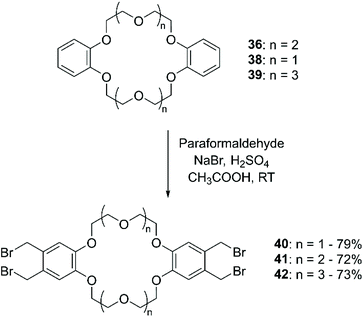
The addition of (halogen)methyl groups is briefly described in the review cited at the beginning of this section,43 where the approach reported by Biernat et al.,71 involving the use of paraformaldehyde and hydrogen bromide in CH3COOH, is given as an example for the bromomethylation of BCE and DBCE. An alternative procedure to obtain the tetra-bromomethyl- functionalised DBCE was reported by Nakamura et al., substituting HBr with a combination of sodium bromide (NaBr) and H2SO4 (Scheme 15).72 Both methodologies led to yields that routinely exceeded 70%.
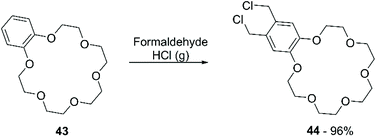
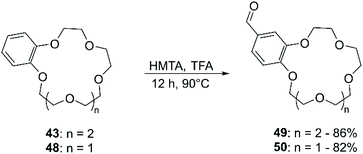
Chloromethylation has been performed in a similar way, using formaldehyde and gaseous HCl (Scheme 16). Following this procedure, a 90% yield of bis-chloromethylated B18C6 (43) was reported,73 while for DB18C6 a conversion of 70% was obtained, with the formation of 80% of the mono-chloromethylated derivative and 20% of the bis-chloromethylated product.74
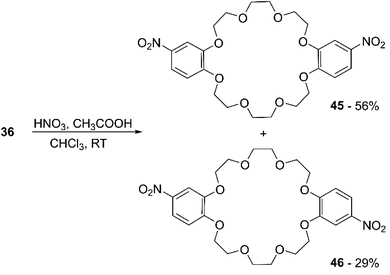
Slight variations of this protocol were adopted to convert B12C4, B15C5 and B18C6 into their mono-nitro76 and di-nitro77 derivatives in decent yields. Duggan et al. reported the synthesis of tetra-nitro derivatives of various DBCE in excellent yields, using H2SO4 instead of CH3COOH in the nitrating solution.78 Grebenyuk et al. described an alternative method for the nitration of BCE and DBCE with KNO3 and polyphosphoric acid (PPA).79 The process was carried out using a large excess of PPA, and different stoichiometric ratios between crown ether and KNO3 were tested; however, mixtures of mono- and di-nitro derivatives were obtained in all cases.
Nitro derivatives are important compounds since they can be easily and quantitatively reduced to amino compounds, which in turn can be converted to other functional groups either for further derivatisation with chromophores or to be introduced in polymers.75–77 It is worth noting, however, that amino functionalised CE undergo fast oxidation, particularly the di- and tetra-modified derivatives; thus, they must be handled under a controlled atmosphere and cannot be stored for a long time.77,78
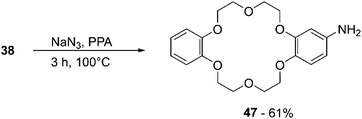
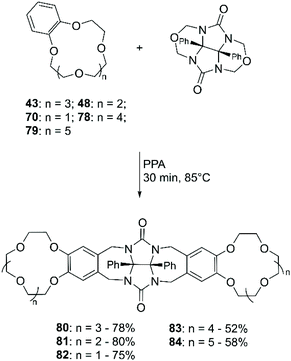
A methodology for the direct amination of DB18C6 (38) was reported by Lyakhovnenko et al. using sodium azide (NaN3) in PPA.80 Heating at 100 °C of an equimolar mixture of 38 and NaN3 in neat PPA led to the formation of the mono-functionalised derivative (47) in 61% yield (Scheme 18).
Formylbenzocrowns represent useful intermediates, since they can be easily converted to a wide range of functional groups. CEs modified according to Wada's procedure have been used for the synthesis of molecular tweezers containing cyclidene-Ni complexes83 and BODIPY,84 and derivatised with benzimidazole,85 benzothiazolyl-86 and porphyrins.87
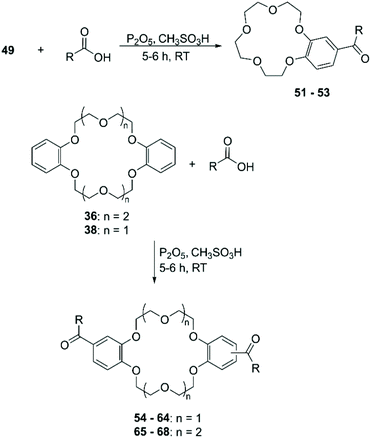
| Prod. | R | Yield (%) | Prod. | R | Yield (%) |
|---|---|---|---|---|---|
| 51 | CH3 | 63 | 60 | CH3(CH2)7 | 84 |
| 52 | CH3(CH2)5 | 55 | 61 | CH3(CH2)8 | 75 |
| 53 | CH3(CH2)12 | 26 | 62 | CH3(CH2)10 | 79 |
| 54 | CH3 | 86 | 63 | CH(CH3)2 | 88 |
| 55 | CH3CH2 | 66 | 64 | C(CH3)3 | 85 |
| 56 | CH3(CH2)2 | 72 | 65 | CH3(CH2)3 | 66 |
| 57 | CH3(CH2)3 | 100 | 66 | CH3(CH2)8 | 84 |
| 58 | CH3(CH2)4 | 78 | 67 | CH3(CH2)10 | 53 |
| 59 | CH3(CH2)5 | 86 | 68 | CH3(CH2)12 | 85 |
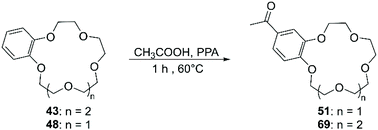
The functionalisation using PPA for the generation of the electrophile is the most frequently adopted methodology and reported yields are comparable with the ones obtained using Eaton's reagent (Scheme 21).89 Saifullina et al. resorted to acetates as the source of acetyl group for the derivatisation of DBCE. It was demonstrated that there is a relation between the size of the counter cation of the acetate and the rate and regioselectivity of the reaction, due to the complexation between crown ether and cation.90 Acyl derivatives, analogously to formyl ones, are suitable precursors for several functional groups including vinyl,91 ester92 and α,β-unsaturated ketones.93 Although acylated CE can be reduced in order to obtain alkyl derivatives, different procedures have been devised for the direct alkylation of BCE and DBCE. Alcohols in combination with PPA94 or sulfuric acid (H2SO4)95 have been used for the introduction of alkyl groups on aromatic CE. The outcome of the reaction proved to be dependent on the nature of the alcohol used and the size of the CE. Moreover, in addition to the formation of regioisomers, these routes exhibited a poor control on the number of functional groups added to the CE substrate.
An alternative approach to sulfonation is based on the use of potassium sulfate in PPA.98 This methodology afforded sulfonated BCE and DBCE in good yields. Sulfonated CE can be readily converted by treatment with sulfonyl chloride to their chlorosulfonate derivative, which in turn can be reduced to produce thiol-functionalised BCE.99 A reported case of a good yielding direct chloro-sulfonation of aromatic CE involves the use of chlorosulfonic acid in chloroform.100
Gorbunov et al. developed a synthetic methodology for the introduction of aromatic ethers and thiophene onto H-azolo[1,5-a][1–3]triazolo[4,5-e]pyrimidines, analogues of 8-azapurines, aiming to develop compounds that present enhanced biological activity by the combination of multiple pharmacophore fragments.103 The process involves a nucleophilic displacement of hydrogen (SNH) reaction characterised by a two-step addition-oxidation mechanism. B18C6 was considered for the scope of the reaction. Mixing the CE with the pyrimidine derivative in trifluoroacetic acid (CF3COOH) allows the formation of the addition product, which is then oxidised to 85 in 50% yield by exposure to atmospheric oxygen over a three-day period (Scheme 24).
Conclusions
Crown ethers (CE) are a most prominent class of macrocyclic compounds in supramolecular science and technology. Their versatility as host molecules for cationic and neutral species allowed for their application in a wide range of fields including catalysis, separation and purification, transport and delivery of ionic species, mechanically interlocked molecules and materials, and molecular machines. The growing complexity of systems of this kind and the interest in enriching the behaviour of CE as host compounds with other functions, has led to a growing need for the development of methodologies for the synthesis of derivatised macrocyclic polyethers that could be easily inserted into elaborated architectures.Functionalised CE can be obtained either by templated macrocyclisation, using appropriately substituted starting materials, or through synthetic routes for the direct introduction of moieties on pre-formed macrocycles. Direct functionalisation of aliphatic CE is generally performed using cross dehydrogenative coupling (CDC) reactions: the process follows a radical mechanism initiated photochemically or thermally. Mono-functionalised aliphatic CE are thus obtained in good yields, albeit a large excess of the starting substrate is required. A wide range of functional groups has been introduced on BCE and DBCE via electrophilic aromatic substitution. Control over the number of moieties added on the product and regioselectivity represent the principal challenges in this case.
Despite the significant potential in the context of contemporary chemistry, the procedures dealing with the direct functionalisation of CE are quite scattered in the literature, and some of them are mere adaptations of general protocols. A focused optimisation of reaction conditions to fit the properties and structural characteristics of CE would allow a full exploitation of the opportunities offered by this approach, particularly with regard to the post-synthetic modification of sophisticated CE-based architectures. Indeed, the direct functionalisation of crown ethers could provide a valuable alternative to templated macrocyclisation, by reducing the synthetic effort and opening new routes to exploit this ceaselessly fascinating class of macrocycles.
Author Contributions
F. Nicoli: conceptualization, analysis, writing-original draft; M. Baroncini: supervision, writing-review and editing; S. Silvi: writing-review and editing; J. Groppi: conceptualization, analysis, writing-original draft; A. Credi: supervision, funding acquisition, writing-review and editing.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
Financial support from the European Union's H2020 Research and Innovation Program (FET-OPEN “MAGNIFY” No. 801378 and ERC AdG “LEAPs” No. 692981) is gratefully acknowledged.Notes and references
-
(a) L. H. Cretcher and W. H. Pittenger, Syntheses with β, β’-dichloro-diethyl ether, J. Am. Chem. Soc., 1925, 47, 163–166 CrossRef CAS
; (b) G. F. Zellhoefer, Solubility of halogenated hydrocarbon refrigerants in organic solvents, Ind. Eng. Chem., 1937, 29, 548–551 CrossRef CAS
; (c) J. L. Down, J. Lewis, B. Moore and G. Wilkinson, The solubility of alkali metals in ethers, J. Chem. Soc., 1959, 3767–3773 RSC
.
-
(a) C. J. Pedersen, Cyclic polyethers and their complexes with metal salts, J. Am. Chem. Soc., 1967, 89, 7017–7036 CrossRef CAS
; (b) C. J. Pedersen, New macrocyclic polyethers, J. Am. Chem. Soc., 1970, 92, 391–394 CrossRef CAS
.
- C. J. Pedersen, The discovery of crown ethers (Nobel lecture), Angew. Chem., Int. Ed. Engl., 1988, 27, 1021–1027 CrossRef
.
- J.-M. Lehn, Supramolecular chemistry—scope and perspectives molecules, supermolecules, and molecular devices (Nobel Lecture), Angew. Chem., Int. Ed. Engl., 1988, 27, 89–112 CrossRef
.
- D. J. Cram, The design of molecular hosts, guests, and their complexes (Nobel lecture), Angew. Chem., Int. Ed. Engl., 1988, 27, 1009–1020 CrossRef
.
-
(a)
J.-M. Lehn, Supramolecular Chemistry – Concepts and Perspectives, WileyVCH, Weinheim, 1995 CrossRef
; (b) J. W. Steed and J. L. Atwood, Supramolecular Chemistry, Wiley, Chichester, 2nd edn, 2009 Search PubMed
.
-
(a)
G. W. Gokel, Crown Ethers and Cryptands, RSC Publishing, Cambridge, 1991 Search PubMed;
(b)
G. W. Gokel, S. Negin and R. Cantwell, in Comprehensive Supramolecular Chemistry II, eds. J. L. Atwood, G. W. Gokel and L. J. Barbour, Elsevier, Amsterdam, 2017, vol. 3, pp. 3–48 Search PubMed
.
-
(a) G. W. Gokel, W. M. leevy and M. E. Weber, Crown ethers: sensors for ions and molecular scaffolds for materials and biological models, Chem. Rev., 2004, 104, 2723–2750 CrossRef CAS PubMed
; (b) B. Zheng, F. Wang, S. Dong and F. Huang, Supramolecular polymers constructed by crown ether-based molecular recognition, Chem. Soc. Rev., 2012, 41, 1621–1636 RSC
; (c) S. Shirakawa and K. Maruoka, Recent developments in asymmetric phase–transfer reactions, Angew. Chem., Int. Ed., 2013, 52, 4312–4348 CrossRef CAS PubMed
; (d) I. V. Kolesnichenko and E. V. Anslyn, Practical applications of supramolecular chemistry, Chem. Soc. Rev., 2017, 46, 2385–2390 RSC
; (e) J. Li, D. Yim, W.-D. Jang and J. Yoon, Recent progress in the design and applications of fluorescence probes containing crown ethers, Chem. Soc. Rev., 2017, 46, 2437–2458 RSC
; (f) H.-W. Schmidt and F. Würthner, A periodic system of supramolecular elements, Angew. Chem., Int. Ed., 2020, 59, 8766–8775 CrossRef CAS PubMed
.
-
(a) P. R. Ashton, E. J. T. Chrystal, J. P. Mathias, K. P. Parry, A. M. Z. Slawin, N. Spencer, J. F. Stoddart and D. J. Williams, Complexation of diquat and paraquat by macrocyclic polyethers incorporating two dibydroxynaphthalene residues, Tetrahedron Lett., 1987, 28, 6367–6370 CrossRef
; (b) O. Š. Miljanić and J. F. Stoddart, Dynamic donor–acceptor [2]catenanes, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 12966–12970 CrossRef PubMed
; (c) A. Gumus, S. Karadeniz, H. I. Ugras, M. Bulut, U. Cakir and A. C. Goren, Synthesis, complexation, and biological activity studies of 4−aminomethyl–7,8−dihydroxy coumarines and their crown ether derivatives, J. Heterocycl. Chem., 2010, 47, 1127–1133 CrossRef CAS
; (d) G. Barin, A. Coskun, D. C. Friedman, M. A. Olson, M. T. Colvin, R. Carmielli, S. K. Dey, O. A. Bozdemir, M. R. Wasielewski and J. F. Stoddart, A multistate switchable [3]rotacatenane, Chem. – Eur. J., 2011, 17, 213–222 CrossRef CAS PubMed
; (e) H. Maeda, K. Nakamura, T. Furuyama and M. Segi, (1,3)pyrenophanes containing crown ether moieties as fluorescence sensors for metal and ammonium ions, Photochem. Photobiol. Sci., 2019, 18, 2397–2410 CrossRef CAS PubMed
; (f) M. Gaedke, F. Witte, J. Anhauser, H. Hupatz, H. V. Schroder, A. Valkonen, K. Rissanen, A. Lutzen, B. Paulus and C. A. Schalley, Chiroptical inversion of a planar chiral redox-switchable rotaxane, Chem. Sci., 2019, 10, 10003–10009 RSC
.
- G. W. Gokel and H. D. Durst, Principles and synthetic applications in crown ether chemistry, Synthesis, 1976, 168–184 CrossRef CAS
.
-
(a) P. L. Anelli, B. Czech, F. Montanari and S. Quici, Reaction mechanism and factors influencing phase-transfer catalytic activity of crown ethers bonded to a polystyrene matrix, J. Am. Chem. Soc., 1984, 106, 861–869 CrossRef CAS
; (b) G. Pozzi, S. Quici and R. H. Fish, Fluorous phase transfer catalysts: From onium salts to crown ether, J. Fluor. Chem., 2008, 129, 920–929 CrossRef CAS
; (c) R. Schettini, M. Sicignano, F. De Riccardis, I. Izzo and G. Della Sala, Macrocyclic hosts in asymmetric phase-transfer catalyzed reactions, Synthesis, 2018, 4777–4795 CAS
.
-
(a) A. H. Bond, M. L. Dietz and R. Chiarizia, Incorporating size selectivity into synergistic solvent extraction: a review of crown ether-containing systems, Ind. Eng. Chem. Res., 2000, 39, 3442–3464 CrossRef CAS
; (b) F. W. van Leeuwen, W. Verboom and D. N. Reinhoudt, Selective extraction of naturally occurring radioactive Ra2+, Chem. Soc. Rev., 2005, 34, 753–761 RSC
; (c) J. Wang and S. Zhuang, Cesium separation from radioactive waste by extraction and adsorption based on crown ethers and calixarenes, Nucl. Eng. Technol., 2020, 52, 328–336 CrossRef CAS
.
-
(a) G. W. Gokel and M. M. Dashbach, Coordination and transport of alkali metal cations through phospholipid bilayer membranes by hydraphile channels, Coord. Chem. Rev., 2008, 252, 886–902 CrossRef CAS PubMed
; (b) N. Sakai and S. Matile, Synthetic ion channels, Langmuir, 2013, 29, 9031–9040 CrossRef CAS PubMed
; (c) S.-P. Zheng, L.-B. Huang, Z. Sun and M. Barboiu, Self–assembled artificial ion–channels toward natural selection of functions, Angew. Chem., Int. Ed., 2021, 60, 566–597 CrossRef CAS PubMed
.
-
(a) J. Krane and T. Skjetne, Studies of crown ether complexes aryldiazonium ion complexes, Tetrahedron Lett., 1980, 21, 1775–1778 CrossRef CAS
; (b) J. R. Beadle, D. M. Dishong, R. K. Khanna and G. W. Gokel, Syntheses and properties of arenediazonium and anilinium cation lariat ether complexes: an “ostrich molecule” complex and evidence for intramolecular sidearm-macroring interaction, Tetrahedron, 1984, 40, 3935–3944 CrossRef CAS
; (c) H. Nakazumi, T. Kitao and H. Zollinger, Dediazoniation of arenediazonium ions. 24. Dual and triple substituent parameter evaluation of competitive heterolytic and homolytic dediazoniations of diazonium ions complexed with 18-crown-6 ether, J. Org. Chem., 1987, 52, 2825–2830 CrossRef CAS
.
-
(a) L. Pospisil and R. Fuoco, Complex formation of methyl viologen and diquat with macrocyclic ether dibenzo-24-crown-8, J. Electroanal. Chem., 1988, 240, 105–116 CrossRef CAS
; (b) A. B. Braunschweig, C. M. Ronconi, J.-Y. Han, F. Aricò, S. J. Cantrill, J. F. Stoddart, S. I. Khan, A. J. P. White and D. J. Williams, Pseudorotaxanes and rotaxanes formed by viologen derivatives, Eur. J. Org. Chem., 2006, 1857–1866 CrossRef CAS
.
- P. R. Ashton, S. E. Boyd, A. Brindel, S. J. Langford, S. Menzer, L. Perez-Garcia, J. A. Preece, F. M. Raymo, N. Spencer, J. F. Stoddart, A. J. P. White and D. J. Williams, Diazapyrenium-containing catenanes and rotaxanes, New J. Chem., 1999, 23, 587–602 RSC
.
- S. J. Loeb and J. A. Wisner, A new motif for the self–assembly of [2]pseudorotaxanes; 1,2−bis(pyridinium) ethane axles and 24-crown–8 ether wheels, Angew. Chem., Int. Ed., 1998, 37, 2838–2840 CrossRef CAS PubMed
.
-
(a) S. Kiviniemi, M. Nissen, M. T. Lamsa, J. Jalonen, K. Rissanen and J. Pursiainen, Complexation of planar, organic, five-membered cations with crown ethers, New J. Chem., 2000, 24, 47–52 RSC
; (b) N. Noujeim, K. Zhu, N. Vukotich and S. J. Loeb, [2]pseudorotaxanes from T-shaped benzimidazolium axles and 24-crown-8 wheels, Org. Lett., 2012, 14, 2484–2487 CrossRef CAS PubMed
.
- G. Ragazzon, A. Credi and B. Colasson, Thermodynamic insights on a bistable acid–base switchable molecular shuttle with strongly shifted co–conformational equilibria, Chem. – Eur. J., 2017, 23, 2149–2156 CrossRef CAS PubMed
.
- R. M. Izatt, J. D. Lamb, N. E. Izatt, B. E. Rossiter, Jr., J. J. Christensen and B. L. Haymore, A calorimetric titration study of the reaction of several organic ammonium cations with 18-crown-6 in methanol, J. Am. Chem. Soc., 1979, 101, 6273–6276 CrossRef CAS
.
- P. R. Ashton, E. J. T. Chrystal, P. T. Glink, S. Menzer, C. Schiavo, N. Spencer, J. F. Stoddart, P. A. Tasker, A. J. P. White and D. J. Williams, Pseudorotaxanes formed between secondary dialkylammonium salts and crown ethers, Chem. – Eur. J., 1996, 2, 709–728 CrossRef CAS
.
- J. Groppi, L. Casimiro, M. Canton, S. Corra, M. Jafari-Nasab, G. Tabacchi, L. Cavallo, M. Baroncini, E. Fois and A. Credi, Precision molecular threading/dethreading, Angew. Chem., Int. Ed., 2020, 59, 14825–14834 CrossRef CAS PubMed
.
- See, e.g.: E. Ishow, A. Credi, V. Balzani, F. Spadola and L. Mandolini, A molecular–level plug/socket system: electronic energy transfer from a binaphthyl unit incorporated into a crown ether to an anthracenyl unit linked to an ammonium ion, Chem. – Eur. J., 1999, 5, 984–989 CrossRef CAS
.
-
C. J. Bruns and J. F. Stoddart, The Nature of the Mechanical Bond: From Molecules to Machines, Wiley, Hoboken, 2016 Search PubMed
.
-
V. Balzani, A. Credi and M. Venturi, Molecular Devices and Machines – Concepts and Perspectives for the Nanoworld, Wiley-VCH, Weinheim, 2008 Search PubMed
.
-
(a) S. Erbas-Cakmak, D. A. Leigh, C. T. McTernan and A. L. Nussbaumer, Artificial molecular machines, Chem. Rev., 2015, 115, 10081–10206 CrossRef CAS PubMed
; (b) M. Baroncini, S. Silvi and A. Credi, Photo-and redox-driven artificial molecular motors, Chem. Rev., 2020, 120, 200–268 CrossRef CAS PubMed
.
- Recent representative examples:
(a) G. Ragazzon, C. Schäfer, P. Franchi, S. Silvi, B. Colasson, M. Lucarini and A. Credi, Remote electrochemical modulation of pKa in a rotaxane by co-conformational allostery, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 9385–9390 CrossRef CAS PubMed
; (b) Q. Zhang, S.-J. Rao, X. Tao, X. Li, T.-Y. Xu, D.-W. Li, D.-H. Qu, Y.-T. Long and H. Tian, Muscle-like artificial molecular actuators for nanoparticles, Chem, 2018, 4, 2670–2684 CrossRef CAS
; (c) B. Riss-Yaw, C. Clavel, P. Laurent, P. Waeles and F. Coutrot, The importance of length and flexibility of macrocycle–containing molecular translocators for the synthesis of improbable [2]rotaxanes, Chem. – Eur. J., 2018, 24, 13659–13666 CrossRef CAS PubMed
; (d) J.-J. Yu, L.-Y. Zhao, Z.-T. Shi, Q. Zhang, G. London, W.-J. Liang, C. Gao, M.-M. Li, X.-M. Cao, H. Tian, B. L. Feringa and D.-H. Qu, Pumping a ring-sliding molecular motion by a light-powered molecular motor, J. Org. Chem., 2019, 84, 5790–5802 CrossRef CAS PubMed
; (e) H. W. Schroeder, F. Stein, J. M. Wollschlager, S. Sobottka, M. Gaedke, B. Sarkar and C. A. Schalley, Accordion–like motion in electrochemically switchable crown ether/ammonium oligorotaxanes, Angew. Chem., Int. Ed., 2019, 58, 3496–3500 CrossRef CAS PubMed
; (f) G. Baggi, L. Casimiro, M. Baroncini, S. Silvi, A. Credi and S. J. Loeb, Threading-gated photochromism in [2]pseudorotaxanes, Chem. Sci., 2019, 10, 5104–5113 RSC
; (g) Y. Sagara, M. Karman, A. Seki, M. Pannipara, N. Tamaoki and C. Weder, Rotaxane-based mechanophores enable polymers with mechanically switchable white photoluminescence, ACS Cent. Sci., 2019, 5, 874–881 CAS
.
-
(a) G. Ragazzon, M. Baroncini, S. Silvi, M. Venturi and A. Credi, Light-powered autonomous and directional molecular motion of a dissipative self-assembling system, Nat. Nanotechnol., 2015, 10, 70–75 CrossRef CAS PubMed
; (b) S. Erbas-Cakmak, S. D. P. Fielden, U. Karaca, D. A. Leigh, C. T. McTernan, D. J. Tetlow and M. R. Wilson, Rotary and linear molecular motors driven by pulses of a chemical fuel, Science, 2017, 358, 340–343 CrossRef CAS PubMed
.
-
(a) C. Yoo, H. M. Dodge and A. J. M. Miller, Cation-controlled catalysis with crown ether-containing transition metal complexes, Chem. Commun., 2019, 55, 5047–5059 RSC
; (b) S. Di Stefano, G. Capocasa and L. Mandolini, Supramolecular catalysts featuring crown ethers as recognition units, Eur. J. Org. Chem., 2020, 3340–3350 CrossRef CAS
; (c) M. Zuo, K. Velmurugan, K. Wang, X. Tian and K.-Y. Hu, Insight into functionalized-macrocycles-guided supramolecular photocatalysis, Beilstein J. Org. Chem., 2021, 17, 139–155 CrossRef CAS PubMed
.
-
(a) K. Ghosh, H.-B. Yang, B. H. Northrop, M. M. Lyndon, Y.-R. Zheng, D. C. Muddiman and P. J. Stang, Coordination-driven self-assembly of cavity-cored multiple crown ether derivatives and poly [2]pseudorotaxanes, J. Am. Chem. Soc., 2008, 130, 5320–5334 CrossRef CAS PubMed
; (b) S. Schneider, E.-D. Licsandru, I. Kocksis, A. Gilles, F. Dumitru, E. Moulin, J. Tan, J.-M. Lehn, N. Giuseppone and M. Barboiu, Columnar self-assemblies of triarylamines as scaffolds for artificial biomimetic channels for ion and for water transport, J. Am. Chem. Soc., 2017, 139, 3721–3727 CrossRef CAS PubMed
; (c) S. Dong, L. Wang, J. Wu, L. Jin, Y. Ge, Z. Qi and C. Wu, Thermosensitive phase behavior of benzo-21-crown-7 and its derivatives, Langmuir, 2017, 33, 13861–13866 CrossRef CAS PubMed
; (d) S. Dong, J. Leng, Y. Feng, M. Liu, C. J. Stackhouse, A. Schönhals, L. Chiappisi, L. Gao, W. Chen, J. Shang, L. Jin, Z. Qi and C. A. Schalley, Structural water as an essential comonomer in supramolecular polymerization, Sci. Adv., 2017, 3, 1–8 Search PubMed
; (e) A. Abdelfatah, M. Mohammed and H.-C. Lin, The crown ether size and stereochemistry affect the self-assembly, hydrogelation, and cellular interactions of crown ether/peptide conjugates, J. Mater. Chem. B, 2020, 8, 9961–9970 RSC
.
-
(a) A. V. Tsukanov, A. D. Dubonosov, V. A. Bren and V. I. Minkin, Organic chemosensors with crown-ether groups, Chem.
Heterocycl. Compd., 2008, 44, 899–923 CrossRef CAS
; (b) I. Moczar and P. Huszthy, Optically active crown ether–based fluorescent sensor molecules: A mini–review, Chirality, 2019, 31, 97–109 CrossRef CAS PubMed
; (c) R. M. Kakhki and M. Rakhshanipour, Application of nanoparticle modified with crown ether in colorimetric determinations, Arabian J. Chem., 2019, 12, 3096–3107 CrossRef
; (d) K. Sambath, X. Liu, Z. Wan, L. Hutnik, K. D. Belfield and Y. Zhang, Potassium ion fluorescence probes: structures, properties and bioimaging, ChemPhotoChem, 2021, 5, 317–325 CrossRef CAS
.
-
(a) V. Percec, G. Johansson, G. Ungar and J. Zhou, Fluorophobic effect induces the self-assembly of semifluorinated tapered monodendrons containing crown ethers into supramolecular columnar dendrimers which exhibit a homeotropic hexagonal columnar liquid crystalline phase, J. Am. Chem. Soc., 1996, 118, 9855–9866 CrossRef CAS
; (b) H. W. Gibson, N. Yagamuci, L. Hamilton and J. W. Jones, Cooperative self-assembly of dendrimers via pseudorotaxane formation from a homotritopic guest molecule and complementary monotopic host dendrons, J. Am. Chem. Soc., 2002, 124, 4653–4665 CrossRef CAS PubMed
; (c) G. M. Dykes and D. K. Smith, Supramolecular dendrimer chemistry: using dendritic crown ethers to reversibly generate functional assemblies, Tetrahedron, 2003, 59, 3999–4009 CrossRef CAS
; (d) M. Tanaka, Y. Higuci, N. Adachi, Y. Shibutani, S. A. Ahmed, S. Kado, M. Nakamura and K. Kimura, Crowned dendron: Ion-responsive flexibility of macromolecules induced by integrated crown ether moieties, Tetrahedron, 2005, 61, 8159–8166 CrossRef CAS
; (e) D. Alivertis, V. Theodorou, G. Paraskevopoulos and K. Skobridis, Searching for new host compounds: synthesis and characterization of novel crown ether-functionalized dendrimers, Tetrahedron Lett., 2007, 48, 4091–4095 CrossRef CAS
.
-
(a) J. Smid, Ion-binding properties of crown ether and cryptand containing polymers, Ind. Eng. Chem. Prod. Res. Dev., 1980, 19, 364–371 CrossRef CAS
; (b) U. Tunca and Y. Yagci, Crown ether-containing polymers, Prog. Polym. Sci., 1994, 19, 233–286 CrossRef CAS
; (c) S. D. Alexandratos and C. L. Stine, Synthesis of ion-selective polymer-supported crown ethers: a review, React. Funct. Polym., 2004, 60, 3–16 CrossRef CAS
; (d) D. Xia, P. Wang, X. Ji, N. M. Khashab, J. L. Sessler and F. Huang, Functional supramolecular polymeric networks: the marriage of covalent polymers and macrocycle-based host–guest interactions, Chem. Rev., 2020, 120, 6070–6123 CrossRef CAS PubMed
.
-
(a) C. Yuan, S. Fu, K. Yang, B. Hou, Y. Liu, J. Jiang and Y. Cui, Crystalline C—C and C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond-linked chiral covalent organic frameworks, J. Am. Chem. Soc., 2021, 143, 369–381 CrossRef CAS PubMed
C bond-linked chiral covalent organic frameworks, J. Am. Chem. Soc., 2021, 143, 369–381 CrossRef CAS PubMed ; (b) S. An, Q. Xu, Z. Ni, J. Hu, C. Peng, L. Zhai, Y. Guo and H. Liu, Construction of covalent organic frameworks with crown ether struts, Angew. Chem., Int. Ed., 2021, 60, 9959–9963 CrossRef CAS PubMed
.
- W. Gong, W. Zhang, F. A. Son, K. Yang, Z. Chen, X. Chen, J. Jiang, Y. Liu, O. K. Farha and Y. Cui, Topological strain-induced regioselective linker elimination in a chiral Zr(IV)-based metal-organic framework, Chem, 2021, 7, 190–201 CAS
.
-
(a) M. Zhang, D. Xu, X. Yan, J. Chen, S. Dong, B. Zheng and F. Huang, Self–healing supramolecular gels formed by crown ether based host–guest interactions, Angew. Chem., Int. Ed., 2012, 51, 7011–7015 CrossRef CAS PubMed
; (b) F. Ishiwari, K. Nakazono, Y. Koyama and T. Takata, Induction of single–handed helicity of polyacetylenes using mechanically chiral rotaxanes as chiral Sources, Angew. Chem., Int. Ed., 2017, 56, 14858–15862 CrossRef CAS PubMed
; (c) L. Wang, L. Cheng, G. Li, K. Liu, Z. Zhang, P. Li, S. Dong, W. Yu, F. Huang and X. Yan, A self-cross-linking supramolecular polymer network enabled by crown-ether-based molecular recognition, J. Am. Chem. Soc., 2020, 142, 2051–2058 CrossRef CAS PubMed
; (d) T. Takata, Switchable polymer materials controlled by rotaxane macromolecular switches, ACS Cent. Sci., 2020, 6, 129–143 CrossRef CAS PubMed
.
-
(a) X. Yan, B. Zheng and F. Huang, Integrated motion of molecular machines in supramolecular polymeric scaffolds, Polym. Chem., 2013, 4, 2395–2399 RSC
; (b) H.-Y. Zhou, Q.-S. Zong, Y. Han and C.-F. Chen, Recent advances in higher order rotaxane architectures, Chem. Commun., 2020, 56, 9916–9936 RSC
; (c) N. H. Perez and J. E. M. Lewis, Synthetic strategies towards mechanically interlocked oligomers and polymers, Org. Biomol. Chem., 2020, 18, 6757–6780 RSC
; (d) C.-S. Kwan and K. C.-F. Leung, Development and advancement of rotaxane dendrimers as switchable macromolecular machines, Mater. Chem. Front., 2020, 4, 2825–2844 RSC
.
- C. Schäfer, G. Ragazzon, B. Colasson, M. La Rosa, S. Silvi and A. Credi, An artificial molecular transporter, ChemistryOpen, 2016, 5, 120–124 CrossRef PubMed
.
-
(a) E. M. G. Jamieson, F. Modicom and S. M. Goldup, Chirality in rotaxanes and catenanes, Chem. Soc. Rev., 2018, 47, 5266–5311 RSC
; (b) S. Corra, C. de Vet, J. Groppi, M. La Rosa, S. Silvi, M. Baroncini and A. Credi, Chemical on/off switching of mechanically planar chirality and chiral anion recognition in a [2]rotaxane molecular shuttle, J. Am. Chem. Soc., 2019, 141, 9129–9133 CrossRef CAS PubMed
; (c) C. Tian, S. D. P. Fielden, B. Perez-Saavedra, I. J. Vitorica-Yrezabal and D. A. Leigh, Single-step enantioselective synthesis of mechanically planar chiral [2]rotaxanes using a chiral leaving group strategy, J. Am. Chem. Soc., 2020, 142, 9803–9808 CrossRef CAS PubMed
; (d) A. Imayoshi, B. V. Lakshimi, Y. Ueda, T. Yoshimura, A. Matayoshi, T. Furuta and T. Kawabata, Enantioselective preparation of mechanically planar chiral rotaxanes by kinetic resolution strategy, Nat. Commun., 2021, 12(404)), 1–7 Search PubMed
.
-
(a) W. Li, W. Zhang, O. Š. Miljanić, C.-H. Sue, Y.-L. Zhao, L. Liu, C. B. Knobler, J. F. Stoddart and O. M. Yaghi, Docking in metal-organic frameworks, Science, 2009, 325, 855–859 CrossRef PubMed
; (b) K. Zhu, C. A. O'Keefe, V. N. Vukotic, R. W. Shurko and S. J. Loeb, A molecular shuttle that operates inside a metal–organic framework, Nat. Chem., 2015, 7, 514–519 CrossRef CAS PubMed
; (c) B. H. Wilson and S. J. Loeb, Integrating the mechanical bond into metal-organic frameworks, Chem, 2020, 6, 1604–1612 CrossRef CAS
.
- J. S. Bradshaw and P. E. Stott, Preparation of derivatives and analogs of the macrocyclic oligomers of ethylene oxide (Crown compounds), Tetrahedron, 1980, 36, 461–510 CrossRef CAS
.
- A. Homberg and J. Lacour, From reactive carbenes to chiral polyether macrocycles in two steps–synthesis and applications made easy?, Chem. Sci., 2020, 11, 6362–6369 RSC
.
- S. M. Pluzhnik-Gladyr, Halogen derivatives of benzo-and dibenzocrown ethers: synthesis, structure, properties and application, Russ. Chem. Rev., 2016, 86, 172–203 CrossRef
.
-
(a) B. R. Bowsher and A. J. Rest, Template synthesis and ionophorous properties of substituted crown ethers towards alkali-metal ions, J. Chem. Soc. Dalton Trans., 1984, 1421–1425 RSC
; (b) B. Czech, Alternative, simple route to hydroxymethyl crown ethers, Tetrahedron Lett., 1980, 21, 4197–4198 CrossRef CAS
; (c) H. Maeda, T. Kikui, Y. Nakatsuji and M. Okahara, Synthesis of aminomethyl crown ethers, J. Org. Chem., 1982, 47, 5167–5171 CrossRef CAS
.
-
(a) I. Ikeda, S. Yamamura, Y. Nakatsuji and M. Okahara, Synthesis of substituted crown ethers from oligoethylene glycols, J. Org. Chem., 1980, 45, 5355–5358 CrossRef CAS
; (b) S. J. Jungk, J. A. Moore and R. D. Gandour, Efficient syntesis of C-pivot lariat ethers. 2-(alkyloxymethyl)-1,4,7,10,13,16-hexaoxacyclooctadecanes, J. Org. Chem., 1983, 48, 1116–1120 CrossRef CAS
.
-
(a) N. Yamaguci, L. M. Hamilton and H. W. Gibson, Dendritic pseudorotaxanes, Angew. Chem., Int. Ed., 1998, 37, 3275–3279 CrossRef
; (b) F. Camps, J. Coll and S. Ricart, Synthesis of crown-ether precocenes, J. Heterocycl. Chem., 1983, 20, 249–250 CrossRef CAS
; (c) G. W. Buchanan, A. Moghimi and C. I. Ratcliffe, Molecular motion in crown ethers. Application of 13C and 2H NMR to the study of 4-carboxybenzo-24-crown-8 ether and its KNCS complex in solution and in the solid phase, Can. J. Chem., 1996, 74, 1437–1446 CrossRef CAS
.
-
(a) P. R. Ashton, I. Baxter, S. J. Cantrill, M. C. T. Fyfe, P. T. Glink, J. F. Stoddart, A. J. P. White and D. J. Williams, Supramolecular daisy chains, Angew. Chem., Int. Ed., 1998, 37, 1294–1296 CrossRef CAS
; (b) F. Dietrich, L. Echegoyen, M. Gomez-Lopez, R. Kessinger and J. F. Stoddart, The self-assembly of fullerene-containing [2]pseudorotaxanes: formation of a supramolecular C60 dimer, J. Chem. Soc., Perkin Trans. 2, 1999, 1577–1586 RSC
.
-
(a) T. Newhouse and P. S. Baran, If C-H bonds could talk: selective C-H bond oxidation, Angew. Chem., Int. Ed., 2011, 50, 3362–3374 CrossRef CAS PubMed
; (b) S. A. Girard, T. Knauber and C.-J. Li, The cross dehydrogenative coupling of Csp3-H bonds: a versatile strategy for C-C bond formations, Angew. Chem., Int. Ed., 2014, 53, 74–100 CrossRef CAS PubMed
; (c) A. M. Faisca Philips and A. J. L. Pombeiro, Recent developments in transition metal-catalized cross dehydrogenative coupling reactions of ethers and thioethers, ChemCatChem, 2018, 10, 3354–3383 CrossRef
; (d) A. K. Bagdi, M. Rahman, D. Bhattacherjee, G. V. Zyryanov, S. Ghosh, O. N. Chupakhin and A. Hajra, Visible light pronoted cross-dehydrogenative coupling: a decade update, Green Chem., 2020, 22, 6632–6681 RSC
.
- M. Tada and H. Hirano, Photochemistry of host-guest complex I, Photochemical reaction of alkyl aryl ketones with 18-crown-6, Tetrahedron Lett., 1978, 51, 5111–5114 CrossRef
.
-
(a) V. I. Porkhun, L. N. Rygalov and B. D. Sviridov, Mechanism of the photolysis of 2,6-diphenyl-1,4-benzoquinone in a medium of oxygen containing heterocycles, Russ. J. Gen. Chem., 1988, 58, 2121–2124 Search PubMed
; (b) V. I. Porkhun and A. I. Rakhimov, Mechanism of the photochemical reactions of substituted benzoquinones, Russ. J. Gen. Chem., 2011, 81, 890–912 CrossRef
.
-
(a) S. Kamijo, T. Hoshikawa and M. Inoue, Photochemically induced radical transformation of C(sp3)-H bonds to C(sp3)-CN bonds, Org. Lett., 2011, 13, 5928–5931 CrossRef CAS PubMed
; (b) T. Hoshikewa, S. Yoshioka, S. Kamijo and M. Inoue, Photoinduced direct cyanation of C(sp3)-H bonds, Synthesis, 2013, 874–887 Search PubMed
.
- T. Hoshikawa, S. Kamijo and M. Inoue, Photochemically induced radical alkynylation of C(sp3)-H bonds, Org. Biomol. Chem., 2013, 11, 164–167 RSC
.
- Y. Amaoka, M. Nagatomo, M. Watanabe, K. Tao, S. Kamijo and M. Inoue, Photochemically induced readical alkenylation of C(sp3)-H bonds, Chem. Sci., 2014, 5, 4339–4345 RSC
.
- S. Kamijo, G. Takao, K. Kamijo, M. Hirota, K. Tao and T. Murafuji, Photo-induced substitutive introduction of the aldoxime functional group to carbon chains: a formal formylation of non-acidic C(sp3)-H bonds, Angew. Chem., Int. Ed., 2016, 55, 9695–9699 CrossRef CAS PubMed
.
- R. Beniazza, B. Abadie, L. Remisse, D. Jardel, D. Lastécouères and J.-M. Vincent, Light-promoted metal-free cross dehydrogenative couplings on ethers mediated by NFI: reactivity and mechanistic studies, Chem. Commun., 2017, 53, 12708–12711 RSC
.
- J. K. Bower, A. D. Cypcar, B. Henriquez, S. C. E. Stieber and C. Zhang, C(sp3)-H fluorination with a copper(II)/(III) redox couple, J. Am. Chem. Soc., 2010, 142, 8514–8521 CrossRef PubMed
.
-
(a) W.-H. Lin, W. I. Bailey, Jr. and R. J. Lagow, The first perfluoro crown ethers, J. Chem. Soc., Chem. Commun., 1985, 1350–1352 RSC
; (b) T.-Z.- Lin, W.-H. Lin, W. D. Clark, R. J. Lagow, S. B. Larson, S. H. Simonsen, V. M. Lynch, J. S. Brodbelt, Si. D. Maleknia and C.-C. Liou, Synthesis and chemistry of perfluoro macrocycles, J. Am. Chem. Soc., 1994, 116, 5172–5179 CrossRef CAS
.
- F. Risi, A.-M. Alstanei, E. Volanschi, M. Carles, L. Piazzala and J.-P. Aycard, Photoaddition of aliphatic ethers to 4-methyl-1,2,4-triazoline-3,5-dione: application to the synthesis of functionalized crown ethers and mechanistm, Eur. J. Org. Chem., 2000, 617–626 CrossRef CAS
.
- K. De Bruycker, S. Billiet, H. A. Houck, S. Chattopadhyay, J. M. Winne and F. E. Du Prez, Triazolinediones as highly enabling synthetic tools, Chem. Rev., 2016, 116, 3919–3974 CrossRef CAS PubMed
.
- M. D. Tzirakis and M. Orfanopoulos, photochemical addition of ethers to C60: synthesis of the simplest [60]fullerene/crown ether conjugates, Angew. Chem., Int. Ed., 2010, 49, 5891–5893 CrossRef CAS PubMed
.
- G. V. Markin, S. Y. Ketkov, M. A. Lopatin, V. A. Kuropatov, A. S. Shavyrin and A. A. Belikov, Preparation of sodium and bisarenechromium fullerides containing esters of ethylene glycol, diethyleneglycol, crown ethers, methoxyarenes, and N-ethyl-phenylbenzamide, Russ. Chem. Bull. Int. Ed., 2020, 69, 751–757 CrossRef CAS
.
- J. B. Zeleconok, V. V. Orlovskij, S. S. Zlotskij and D. L. Rachmankulov, Synthesis of substituted crownethers by radical addition of 18-crown-6, J. Prakt. Chem., 1990, 5, 719–722 CrossRef
.
- Y. B. Zelechonok, V. V. Orlocskii, S. F. Zelechonok, S. S. Zlotskii and D. L. Rakhmankulov, Synthesis of a complexone containing 8-hydroxyquinoline and crown ether fragments, Chem. Heterocycl. Compd., 1990, 26, 121 CrossRef
.
- R. M. Khafizova, V. V. Orlocskii, B. I. Pantukh, S. S. Zlotskii and D. L. Rakhmankulov, Antifungal properties of alkycrown ethers based on 18-crown-6, Russ. J. Appl. Chem., 1991, 64, 211–213 Search PubMed
.
-
(a) Y. B. Zelechonok, V. V. Zorin, S. S. Zlotskii and D. L. Rakhmankulov, Homolytic alkylation of 2-methylquinoline with 18-crown-6, Chem. Heterocycl. Compd., 1985, 21, 1056 CrossRef
; (b) Y. B. Zelechonok, L. P. Ivanova, V. V. Zorin, S. S. Zlotskii and D. L. Rakhmankulov, Homologous alkylation of aromatic bases with 1,4-dioxane and its macrocyclic analogs, Dokl. Chem., 1987, 297, 494–496 Search PubMed
; (c) Y. B. Zelechonok, L. P. Ivanova, V. V. Zorin, S. A. Kotlyar, S. S. Zlotskii and D. L. Rakhmankulov, homolityc alkylation of quinaldine and quinoxaline with 18-crown-6, Chem. Heterocycl. Compd., 1988, 24, 64 CrossRef
; (d) Y. B. Zelechonok, V. V. Zorin, M. C. Klyavlin, S. S. Zlotskii, D. L. Rakhmankulov, M. Bartok and A. Molnar, homolytic substitution of 2-methylquinoline by crown ethers, Tetrahedron Lett., 1988, 29, 5037–5038 CrossRef CAS
.
- N. Okugawa, K. Moriyama and H. Togo, Introduction of ether groups onto electron-deficient nitrogen containing heteroaromatics using radical chemistry under transition-metal-free conditions, Eur. J. Org. Chem., 2015, 4973–4981 CrossRef CAS
.
- S. Liu, A. Liu, Y. Zhang and W. Wang, Direct Cα-heteroarylation of structurally diverse ethers via a mild N-hydroxysuccinimide mediated cross-dehydrogenative coupling reaction, Chem. Sci., 2017, 8, 4044–4050 RSC
.
- J. Gong and P. L. Fuchs, Alkynylation of C-H bonds via reaction with acetylenic triflones, J. Am. Chem. Soc., 1996, 118, 4486–4487 CrossRef CAS
.
- J. Xiang, W. Jiang and P. L. Fuchs, Scope and limitations of functionalized acetylenic triflones in the direct alkynylation of C-H bonds, Tetrahedron Lett., 1997, 38, 6635–6638 CrossRef CAS
.
- P. Mobian, N. Banerji, G. Bernardinelli and J. Lacour, Towards the stereoselective synthesis of inherently chiral pseudorotaxanes, Org. Biomol. Chem., 2006, 4, 224–231 RSC
.
- A. Cygan, J. F. Biernat and H. Chadzynski, Macrocyclic polyfunctional lewis-bases .3. Electrophoretic behavior of macrocyclic ethers, Pol. J. Chem., 1979, 53, 929–933 CAS
.
- Y. Nakamura, A. Asami, T. Ogawa, S. Inokuma and J. Nishimura, Regioselective synthesis and properties of novel [60]fullerene bisadducts containing a dibenzocrown ether moiety, J. Am. Chem. Soc., 2002, 124, 4329–4335 CrossRef CAS PubMed
.
- J. Otsuki, K. C. Russell and J.-M. Lehn, A bis-crown ether derivative of triaminotriazine: synthesis and behavior of the ion-selective and hydrogen bonding responsive rotamers, Bull. Chem. Soc. Jpn., 1997, 70, 671–679 CrossRef CAS
.
- I. A. Stempnevskaya and A. K. Tashmukhamedova, Synthesis of derivatives of dibenzo-18-crown-6 with a chloromethyl group in the side chain, Chem. Nat. Compd., 1982, 635–636 CrossRef
.
- W. M. Feigenbaum and R. H. Michel, Novel polyamides from macrocyclic ethers, J. Polym. Sci., Part A-1: Polym. Chem., 1971, 9, 817–820 CrossRef CAS
.
-
(a) G. E. Pacey, Y. P. Wu and B. P. Bubnis, Synthesis of a lithium specific chromogenic crown ether, Synth. Commun., 1981, 11, 323–328 CrossRef CAS
; (b) T. Liu, C. Bao, H. Wang, Y. Lin, H. Jia and L. Zhu, Light-controlled ion channels formed by amphiphilic small molecules regulate ion conduction via cis-trans photoisomerization, Chem. Commun., 2013, 49, 10311–10313 RSC
; (c) D. R. Patil, D. R. Chendam, A. G. Mulik, S. D. Jagdale, P. P. Patil and M. B. Deshmukh, Synthesis and cation recognition study of novel benzo crown ether functionalized enamine derivatives, Synth. Commun., 2015, 45, 1902–1911 CrossRef CAS
.
-
(a) J. D. Pike, D. T. Rosa and D. Coucouvanis, Lipophilic metal-salicylideneimine-crown ether hybrids – ditopic carriers in the facilitated transport of amphiphilic molecules across bulk liquid membranes, Eur. J. Inorg. Chem., 2001, 761–777 CrossRef CAS
; (b) G. J. Kirkovits, R. S. Zimmerman, M. T. Huggins, V. M. Lynch and J. L. Sessler, Synthesis, structural characterization and complexation properties of the first ‘'crowned” dipyrrolylquinoxalines, Eur. J. Org. Chem., 2002, 3768–3778 CrossRef CAS
.
- S. A. Duggan, G. Fallon, S. J. Langford, V.-L. Lau, J. F. Satchell and M. N. Paddon-Row, Crown-linked porphyrin systems, J. Org. Chem., 2001, 66, 4419–4426 CrossRef CAS PubMed
.
- A. D. Grebenyuk, A. A. Andreev, I. A. Stempnevskaya, M. G. Levkovich and A. K. Tashmukhamedova, Nitration of benzo crown ethers with potassium nitrate in polyphosphoric acid, Chem. Heterocycl. Compd., 2000, 36, 1449–1456 CrossRef CAS
.
- A. S. Lyakhovenko, A. V. Aksenov and M. M. Kugutov, An original approach
to the amination of crown ethers, Chem. Heterocycl. Compd., 2010, 46, 1138–1139 CrossRef
.
- E. M. Hyde, B. L. Shaw and I. Shepherd, Complexes of platinum metals with crown ethers containing tertiary phosphine-substituted benzo groups, J. Chem. Soc. Dalton Trans.,, 1978, 1696–1705 RSC
.
- F. Wada, H. Hirayama, H. Namiki, k. Kikukawa and T. Matsuda, New application of crown ethers. II. Synthesis of 4′-formylbenzocrown ethers, Bull. Chem. Soc. Jpn., 1980, 53, 1473–1474 CrossRef CAS
.
- O. P. Kryatova, A. G. Kolchinsky and E. V. Rybak-Akimova, Metal-containing ditopic receptors for molecular recognition of diammonium cations, Tetrahedron, 2003, 59, 231–239 CrossRef CAS
.
- S. Shao, H. B. Gobeze, P. A. Karr and F. D'Souza, ‘'Two-point” self-assembly and photoinduced electron transfer in meso-donor-carrying bis(styryl crown ether)-BODIPY-bis(alkylammonium)fullerene donor-acceptor conjugates, Chem. - Asian J., 2017, 12, 2258–2270 CrossRef CAS PubMed
.
- D. Patil, D. Chandam, A. Mulik, P. Patil, S. Sankapal and M. Deshmukh, Novel dibenzo-18-crown-6 ether functionalized bis-benzimidazole derivatives: synthesis and antifungal evaluation, Res. Chem. Intermed., 2016, 42, 2449–2459 CrossRef CAS
.
- S. D. Jagadale, A. D. Sawant and M. B. Deshmukh, Synthesis of dibenzothiazolyldibenzo-18-crown-6 and its applications in colorimetric recognition of palladium and as antimicrobial agent, J. Heterocycl. Chem., 2017, 54, 161–164 CrossRef CAS
.
- A. Shinohara, C. Pan, L. Wang and H. Shinmori, Acid-base controllable singlet oxygen generation in supramolecular porphyrin-gold nanoparticle composites tethered by rotaxane linkers, J. Porphyr. Phtalocya., 2019, 23, 1–10 CrossRef
.
-
(a) W. W. Parish, P. E. Stott and C. W. McCausland, Modified crown ether catalysts. 1. Synthesis of alkanoyl-, aroyl-, and α-hydroxyalkylbenzo crown ethers, J. Org. Chem., 1978, 43, 4577–4581 CrossRef CAS
; (b) P. E. Stott and J. S. Bradshaw, Modified crown ether catalysts. 2. Synthesis of alkanoyl-, aroyl-, α-hydroxyalkylbenzo and alkylbenzo and alkylcyclohexano crown ethers, J. Org. Chem., 1980, 45, 4716–4720 CrossRef CAS
.
-
(a) H. Hirano, K. Kuruyama and M. Tada, The photochemistry of the host-guest complex. V. The effect of the sodium ion on the photoreaction of benzil derivatives with a crown ether moiety, Bull. Chem. Soc. Jpn., 1981, 54, 2708–2711 CrossRef CAS
; (b) D. Vazquez-Garcia, A. Fernandez, M. Lopez-Torres, A. Rodriguez, A. Varela, M. T. Pereira, J. M. Vila and J. J. Fernandez, Functionalized palladacycles with crown ether rings derived from terdentate [C,N,N] ligands. Crystal and molecular structure of the dinuclear palladium/silver complex [Pd{3,4-(AgC10H20O6)C6H2C(Me) = NN(H)(4′-ClC4H2N2)}(PPh3)][CF3SO3]2, Organometallics, 2011, 30, 396–404 CrossRef CAS
; (c) C. Zhang, J. Pu, H. Wu, S. Cheng, R. Zhang, A. Zhang and M. Zhang, The synthesis and mesophases studies of a novel discotic compound containing two triphenylene cores linked by crown ether, Mol. Cryst. Liq. Cryst., 2011, 542, 99–105 CAS
.
- N. Z. Saifullina and A. K. Tashmukhamedova, Acylation of dibenzo-18-crown-6 with alkali-metal acetates in polyphosphoric acid, Chem. Heterocycl. Compd., 2001, 41, 973–976 CrossRef
.
- A. G. Talma, H. van Vossen, E. J. R. Sudholter, J. van Eerden and D. N. Reinhoudt, Synthesis of 4′-vinylbenzo-3n-crown-n ethers (4≤n≤10), Synthesis, 1986, 680–683 CrossRef CAS
.
-
(a) F. Wada, R. Arata, T. Goto, K. Kikuyawa and T. Matsuda, New application of crown ethers. III. Synthesis of 4′-hydroxy-benzocrown ethers and their bis(benzocrown ether)s linked by poy(oxyethylene) chain, Bull. Chem. Soc. Jpn., 1980, 53, 2061–2063 CrossRef CAS
; (b) L. Zhao, X. Cheng, F. Guo, B. Gou, C. Yang and W. Xia, Luminescent properties and logic nature of a crown Schiff base responding to sodium ion and zinc ion, J. Lumin., 2014, 145, 486–491 CrossRef CAS
.
- K. Ver Heyen, E. Cielen, A. Tahri, A. Saleh, N. Boens and G. J. Hoornaert, Synthesis and characterization of new fluorescent Na+ and K+ indicators, Tetrahedron, 1999, 55, 5207–5226 CrossRef
.
-
(a) A. K. Tashmukhamedova, I. A. Stempnevskaya, N. Z. Saifullina and M. G. Levkovich, Alkylation of benzo- and dibenzocrown ethers by various alcohols, Chem. Heterocycl. Compd., 1986, 22, 1461 CrossRef
; (b) E. Luboch, A. Cygan and J. F. Biernat, The synthesis of some aromatic crown ether derivatives and their ion-selective electrode properties, Tetrahedron, 1990, 46, 2461–2472 CrossRef CAS
.
- S. A. Kotlyar and E. I. Klimova, Alkylation of benzocrown ethers by certain alcohols, Chem. Heterocycl. Compd., 1990, 26, 1313–1314 CrossRef
.
- T. Sasaki, S. Umentani, M. Masui, S. Tsurubou, T. Kimura and Z. Yoshida, Complex formation of lanthanide ions with sulfonated crown ethers in aqueous solution, Bull. Chem. Soc. Jpn., 1998, 71, 371–377 CrossRef CAS
.
- D. J. Hoffart, J. Tiburcio, A. de la Torre, L. K. Knight and S. J. Loeb, Cooperative ion-ion interactions in the formation of interpenetrated molecules, Angew. Chem., Int. Ed., 2008, 47, 97–101 CrossRef CAS PubMed
.
- A. D. Grebenyuk, L. V. Zotova and A. K. Tashmukhamedova, Sulfonation of benzocrown ethers by potassium sulfate in polyphosphoric acid, Chem. Heterocycl. Compd., 2001, 37, 822–826 CrossRef CAS
.
- Y. Sun, X. Lu, R. Wang, L. Shi and B. Yang, Short and efficient synthesis of thiol group functionalized crown ethers, Synth. Commun., 2007, 37, 2995–3002 CrossRef CAS
.
-
(a) L. N. Markovskii, D. M. Rudkevich and V. I. Kal'chenko, Chlorosulfonyl derivatives of monobenzo- and dibenzo-crown ethers, Russ. J. Org. Chem., 1989, 25, 1995–2000 CAS
; (b) M. D. Barboiou, N. D. Hovnanian, C. Luca and L. Cot, Functionalized derivatives of benzocrown-ethers, V multiple molecular recognition of zwitterionic phenylalanine, Tetrahedron, 1999, 55, 9221–9232 CrossRef
.
- R. P. Sijbesma and R. J. Nolte, Synthesis of concave receptors derived from diphenylglycoluril, Recl. Trav. Chim. Pays-Bas, 1993, 112, 643–647 CrossRef CAS
.
- T. Y. Bogashenko, A. Y. Lyapunov, L. S. Kikot, A. V. Mazepa, M. M. Botoshansky, M. S. Fornari and T. I. Kirichenko, Synthesis, crystal structure, and alkali metal picrate extraction capabilities of molecular clips based on diphenylglycouril and benzocrown ethes, Tetrahedron, 2012, 4757–4764 CrossRef
.
- E. B. Gorbunov, G. L. Rusinov, E. N. Ulomsky, V. L. Rusinov, V. N. Charusin and O. N. Chupakhin, C-H functionalization of triazolo[α]-annulated 8-azapurines, Tetrahedron Lett., 2016, 57, 2303–2305 CrossRef CAS
.
| This journal is © the Partner Organisations 2021 |