Open Access Article
Open Access ArticleA flexible semitransparent photovoltaic supercapacitor based on water-processed MXene electrodes†
Leiqiang
Qin‡
 *a,
Jianxia
Jiang‡
ab,
Quanzheng
Tao
a,
Chuanfei
Wang
*a,
Jianxia
Jiang‡
ab,
Quanzheng
Tao
a,
Chuanfei
Wang
 c,
Ingemar
Persson
a,
Mats
Fahlman
c,
Ingemar
Persson
a,
Mats
Fahlman
 c,
Per O. Å.
Persson
c,
Per O. Å.
Persson
 a,
Lintao
Hou
a,
Lintao
Hou
 b,
Johanna
Rosen
*a and
Fengling
Zhang
b,
Johanna
Rosen
*a and
Fengling
Zhang
 *ab
*ab
aDepartment of Physics, Chemistry and Biology (IFM), Linköping University, Linköping, SE-58183, Sweden. E-mail: leiqiang.qin@liu.se; johanna.rosen@liu.se; fengling.zhang@liu.se
bGuangzhou Key Laboratory of Vacuum Coating Technologies and New Energy Materials, Physics Department, Jinan University, Guangzhou, 510632, PR China
cLaboratory of Organic Electronics, ITN, Linkoping University, SE-60174 Norrkoping, Sweden
First published on 3rd March 2020
Abstract
Solar energy, although it has the highest power density available in terms of renewable energy, has the drawback of being erratic. Integrating an energy harvesting and storage device into photovoltaic energy storage modules is a viable route for obtaining self-powered energy systems. Herein, an MXene-based all-solution processed semitransparent flexible photovoltaic supercapacitor (PSC) was fabricated by integrating a flexible organic photovoltaic (OPV) with Ti3C2Tx MXene as the electrode and transparent MXene supercapacitors with an organic ionogel as the electrolyte in the vertical direction, using Ti3C2Tx thin film as a common electrode. In the quest for a semitransparent flexible PSC, Ti3C2Tx MXene was first used as a transparent electrode for OPV with a high power conversion efficiency of 13.6%. The ionogel electrolyte-based transparent MXene supercapacitor shows a high volumetric capacitance of 502 F cm−3 and excellent stability. Finally, a flexible PSC with a high average transmittance of over 33.5% was successfully constructed by all-solution processing and a remarkable storage efficiency of 88% was achieved. This strategy enables a simple route for fabricating MXene based high-performance all-solution-processed flexible PSCs, which is important for realizing flexible and printable electronics for future technologies.
1. Introduction
Various energy harvesting concepts are currently under investigation for the conversion of renewable sources into electrical energy, with the goal of providing long-term off-grid power for portable electronic devices and sensors.1–4 Organic photovoltaics (OPVs), as the most promising technology for long-term renewable energy production, have attracted enormous attention due to their great possibilities of high flexibility, light weight, low cost, and printing or roll-to-roll manufacturing.5–8 However, PVs have a major drawback in their intermittent nature, i.e. the power output depends strongly on the fluctuation in light intensity caused by the diurnal cycle, weather, and season, among other factors, which results in an inability to maintain a constant and continuous electricity supply for electronic devices driven by PVs.9 Efficient energy storage devices are therefore needed as one potential solution to deal with fluctuating energy production. Therefore, integrating PVs with energy storage devices such as supercapacitors (SCs) offers a promising opportunity to store surplus available energy for later use during periods of non-generation or low power generation and to provide electricity reliably for long-term off-grid and commercial applications.10–12Recently, several attempts have been made to combine energy harvesting and storage into photovoltaic energy storage modules (PESM) for self-powered systems.13–15 However, external circuits are commonly used as interconnections between the PVs and the charge storage part of the integrated devices, which results in low surface area utilization due to planar interconnection and is not compatible with roll-to-roll printing on flexible substrates. It is a challenge to explore devices with high mechanical flexibility and optical transparency to meet the needs of future ubiquitous electronics, including wearable devices and interactive systems.16,17 The ultimate objective of the field is to develop highly efficient, flexible, transparent, and low-cost PESMs in the vertical direction via printing or roll-to-roll manufacturing.18,19 Therefore, an all-solution-processed flexible PESM realized at low temperature is very suitable for implementation of upscaling and also for cost-effectiveness.
A commonly used transparent electrode in PV devices is indium tin oxide (ITO), which can offer high transmittance with low sheet resistance. However, ITO is mechanically brittle and experiences conductivity issues on plastic substrates.20,21 In addition, metal oxide films are commonly processed via vacuum sputtering at high temperatures, which is incompatible with printing and roll-to-roll manufacturing. Several emerging materials have been explored as transparent electrode materials, such as conductive polymers, graphene, carbon nanotubes, random networks of metal nanowires (Ag/Cu NWs), and hybrid films. In 2011, a new class of 2D materials emerged, made up of transition metal carbides/nitrides or carbonitrides.22 They were called MXenes, being of the general formula Mn+1XnTx (n = 1–3), where M represents an early transition metal, X is carbon and/or nitrogen, and T stands for the surface terminations O, OH, or F. Ti3C2Tx was the first MXene reported in 2011 and has been intensively studied.22–27 Ti3C2Tx possesses many attractive properties, including excellent electronic conductivity (up to 9880 S cm−1, surpassing other solution-processed 2D materials),28 high transmittance (transmitting >97% of visible light per nanometer),29 and good flexibility. In addition, the hydrophilic surface of the Ti3C2Tx MXene allows it to be processed with various solutions or inks, such as spin/spray coating, blading, printing, and roll-to-roll manufacturing.30–32 Altogether, these interesting properties make Ti3C2Tx MXene a promising candidate for several energy conversion and storage applications, including supercapacitors, batteries, electrocatalysis, and photocatalysis. Yet MXenes are comparatively unexplored in the field of optoelectronics.
Inspired by the electrical properties of MXenes, we have herein fabricated Ti3C2Tx MXene-based all-solution-processed, semitransparent, and flexible solid-state photovoltaic supercapacitors (PSCs) via vertical stacking. Through spin-casting of colloidal solutions of Ti3C2Tx nanosheets, highly conducting and transparent flexible films were obtained, made up of Ti3C2Tx nanoflakes aligned parallel to the substrates. Consequently, the OPV models were constructed by solution processing at lower temperatures on the Ti3C2Tx electrodes. To the best of our knowledge, this is the first report of pristine Ti3C2Tx as transparent electrodes for solution-processed flexible OPVs. Assisted by the transfer-printing method, the transparent Ti3C2Tx films were used as both the top electrode of the OPVs and the bottom electrode of the energy storage units. Finally, the transparent electrolyte layer and the other Ti3C2Tx electrodes of the supercapacitors were constructed using lamination (Scheme S1†). The all-solution-processed PSCs constructed at low temperatures exhibited high transparency, great flexibility, and excellent cycling stability, etc., which make them suitable for printing, roll-to-roll manufacturing, and blading/shearing for making high-efficiency PSCs.
2. Results and discussion
Fabrication of the MXene transparent electrodes
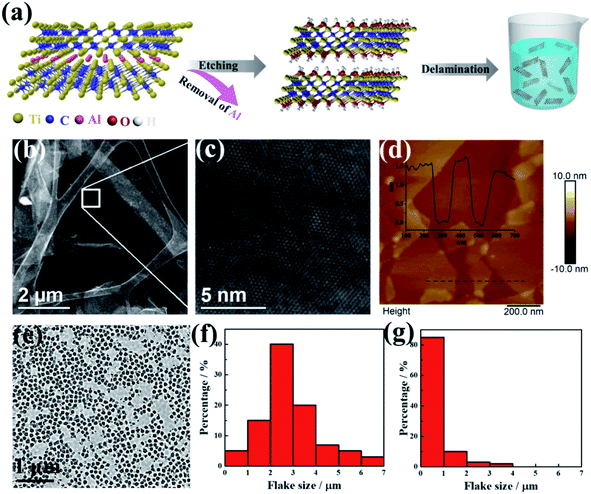
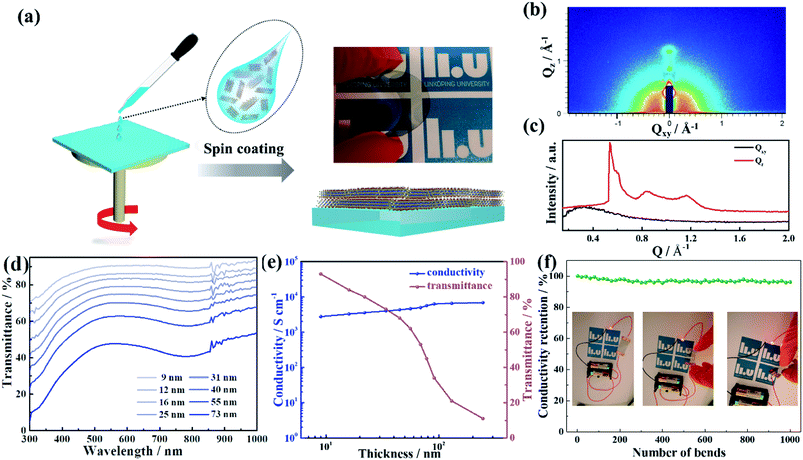
The Ti3C2Tx MXene has shown excellent electronic conductivity and electrochemical performance, which are ideal for fabricating transparent electrodes and powerful transparent supercapacitors. We first prepared the Ti3C2Tx MXene by selective etching of Al from Ti3AlC2 MAX phase following the minimally intensive layer delamination (MILD) method (see Fig. 1a and the Experimental section in the ESI†).33 This protocol offers delamination of the etched powder to form a colloidal solution of Ti3C2Tx flakes via manual shaking in deionized water. The synthesized Ti3C2Tx colloidal dispersion exhibits the Tyndall effect, manifesting inherent colloidal stability caused by negative surface charge (Fig. S1†). The colloidal solution resulting from this method contains Ti3C2Tx single sheets with a lateral size of a few micrometers (Fig. 1b). High-magnification transmission electron microscopy (TEM) images (Fig. 1c) reveal the typical appearance of a single sheet of Ti3C2Tx MXene.34 In addition, the thickness of the flakes is around 1.5 nm, according to the atomic force microscopy (AFM) measurements in Fig. 1d, in good agreement with a previous report.35 Furthermore, the sizes of the Ti3C2Tx flakes can be reduced to smaller than 0.5 μm by probe sonication, as shown in the scanning electron microscope images (SEM, Fig. 1e). The lateral size distribution of the Ti3C2Tx flakes before sonication is concentrated in the range 1–4 μm (Fig. 1f), but the majority of the Ti3C2Tx flakes after sonication are smaller than 1 μm (Fig. 1g), which is consistent with dynamic light scattering (DLS, Fig. S1c†).The surface functional groups of MXene make it hydrophilic and enable the solution processability of MXene to obtain highly conductive thin films through a variety of techniques, including vacuum assisted filtration, and spray, spin, and dip coating.36–38 In order to meet the requirements of transparent electrodes for OPVs, small-sized MXene flakes obtained by spin-coating were chosen for the current study, which could yield smoother, flexible transparent films on any substrate (Fig. 2a, S2 and S3†). The two-dimensional GIWAXS patterns of the Ti3C2Tx films prepared by spin-coating exhibit visible arcs of diffracted intensity (Fig. 2b), implying the formation of regular orientations. One-dimensional intensity distribution curves, produced by integrating along the horizontal and vertical directions, were used to investigate the textured stacking structures in detail. The out-of-plane diffraction signals at q = 0.55 Å−1 (corresponding to layer spacings of 11.6 Å, and also consistent with the interlayer distances of Ti3C2Tx flakes) are significantly stronger than the in-plane signals, suggesting the high degree of alignment of the flakes parallel to the substrate plane under the effect of the shear force.39,40 The smooth films produced by this parallel-aligned stacking are a necessary condition for Ti3C2Tx as electrodes of OPVs.
To understand the optoelectronic properties of the Ti3C2Tx, the evolution of the transmittance spectra with the thickness of films was measured (Fig. 2d). The spectra show a broad peak in the visible region and the transmittance decreases as the films become thicker. The transmittance at 550 nm and the conductivity of the spin-coated Ti3C2Tx films as a function of film thickness were plotted and are shown in Fig. 2e. The high transmittance coupled with extraordinary conductivity give Ti3C2Tx excellent optoelectronic properties compared to most other transparent conductive materials. For example, at T = 86%, the conductivity of Ti3C2Tx (3352 S cm−1) is much higher than that of PEDOT:PSS (500 S cm−1)41 or P3-SWCNT (263 S cm−1).42 In addition to outstanding optoelectronic performances in the visible range, the Ti3C2Tx films possess other important advantages: namely, good mechanical flexibility and durability under bending stress. The conductivity of the electrodes on the flexible PET substrates was measured as a function of bending cycles (Fig. 2f). The Ti3C2Tx film exhibits an excellent mechanical stability with a nearly constant resistance after continuous bending cycles, whereas the resistance of the ITO electrode is drastically increased.19 The Ti3C2Tx films with high transmittance, conductivity, and excellent mechanical stability motivated us to explore the MXene films as transparent flexible electrodes for high-performance all-solution-processed flexible OPVs.
MXene electrode-based OPVs
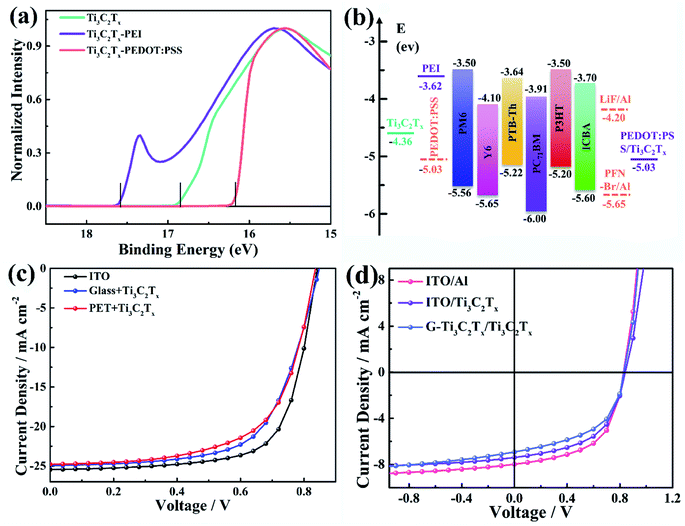
Apart from the excellent optical and electrical characteristics of the Ti3C2Tx films, the work function (WF) of the MXene film can be easily modified, which is a very important condition for electrode materials. Here, ultraviolet photoelectron spectroscopy (UPS) was used to probe the WF values of as-caste Ti3C2Tx film and Ti3C2Tx film modified with poly(3,4-ethylenedioxythiophene):poly-(styrenesulfonate) (PEDOT:PSS) or polyethylenimine (PEI), as shown in Fig. 3a. From the position of the secondary electron cut-off, the spectra reveal that the WF of Ti3C2Tx is reduced from 4.36 to 3.62 eV after PEI modification, while the WF increases from 4.63 to 5.03 eV after PEDOT:PSS modification, meaning that the Ti3C2Tx film can be used as both hole and electron collecting electrodes of OPVs, depending on the choice of overlayer.With the combined high-performance features of Ti3C2Tx films, wider tests and studies were carried out on a series of OPVs with different types of donors and acceptors (PM6, PTB7-Th, P3HT, Y6,43 PC71BM, ICBA) in the active layers (Fig. S6†), to prove their potential application in flexible organic electronics. The detailed fabrication procedure can be found in the Experimental section. The device architecture and corresponding energy level diagram are presented in Fig. 3b, S7 and ESI,† respectively. Based on Ti3C2Tx/glass substrates, we fabricated OPVs with different thicknesses of Ti3C2Tx films. For comparison, control devices with ITO (∼110 nm)/glass were fabricated. The current density–voltage (J–V) characteristics of the OPVs were measured under standard 1 sun simulated solar illumination using Air Mass 1.5 global (AM 1.5G) conditions and an irradiation intensity of 100 mW cm−2 (Fig. S8 and S9†). All the photovoltaic parameters of the devices are listed in Table S1.† With increasing thickness of Ti3C2Tx film, the power conversion efficiency (PCE) of the device increases at first and then decreases. This should reflect the compromise between conductivity and transmittance. The PCE reaches 13.62% (for PM6:Y6) and 7.76% (for PTB7-Th:PC71BM) with a 17 nm Ti3C2Tx film, which are comparable values to those of the control devices with ITO/glass substrates (Fig. 3c). In addition, the flexible devices yield high PCE values of 13.15% (PM6:Y6) and 7.37% (PTB7-Th:PC71BM) with Ti3C2Tx/PET substrates. The Ti3C2Tx based devices show slightly lower external quantum efficiency (EQE) compared with ITO based devices in the wavelength range 300–500 nm (Fig. S10 and S11†), which may be caused by the slightly higher absorption by Ti3C2Tx films at short wavelengths.
To further evaluate the charge collection capability of the Ti3C2Tx MXene transparent electrodes, OPVs with MXene films as both bottom and top electrodes were prepared. In this work, to fabricate semitransparent flexible OPVs by solution processing, a patterned Ti3C2Tx film modified by a thin layer of PEI was used as the bottom electrode. Based on UPS results, PEI modification can significantly reduce the WF of the underlying Ti3C2Tx films and it has been proven to improve the electron collection and performance of various types of solar cells.44,45 The poly(3-hexylthiophene) (P3HT):indene-C60 bisadduct (ICBA) or PTB7-Th:PC71BM and PEDOT:PSS are used as the active layer and hole transport layer, respectively, and are prepared by spin-coating. Finally, the transparent Ti3C2Tx top electrode is prepared by film-transfer lamination. For reference, control devices with ITO (∼110 nm)/glass were fabricated. The photovoltaic parameters are summarized in Table 1. As shown in Fig. 3d, good efficiency can be achieved when Ti3C2Tx is used as the bottom electrode, top electrode and even as both the top and the bottom electrode for flexible semi-transparent OPVs. The efficiency is comparable to that of an ITO electrode. Considering that the route to fabrication of Ti3C2Tx based flexible semi-transparent OPVs is facile, cost-effective, and scalable, it is reasonable to assume that Ti3C2Tx-based OPVs meet the requirements for energy harvesting units for semitransparent flexible photovoltaic supercapacitors.
| Device configuration | J sc [mA cm−2] | V oc [V] | FF [%] | PCEb [%] | AVTc [%] |
|---|---|---|---|---|---|
| a J sc, short-circuit current density; Voc, open-circuit voltage; FF, fill factor. b Average PCEs are based on 20 independent devices. c AVT is the average visible transmittance in the range 380–780 nm. d Photovoltaic performance of a flexible PSC with light from the OPV direction. e Photovoltaic performance of flexible PSC with light from the SC direction. | |||||
| Glass/ITO/PEDOT:PSS/PM6:Y6/PFN-Br/Al | 25.46 | 0.84 | 70.3 | 15.07 (14.97 ± 0.11) | — |
| Glass/Ti3C2Tx/PEDOT:PSS/PM6:Y6/PFN-Br/Al | 24.97 | 0.84 | 64.9 | 13.62 (13.45 ± 0.21) | — |
| PET/Ti3C2Tx/PEDOT:PSS/PM6:Y6/PFN-Br/Ag | 24.78 | 0.83 | 0.64 | 13.15 (13.01 ± 0.24) | — |
| Glass/ITO/PEDOT:PSS/PTB7-Th:PC71BM/LiF/Al | 14.95 | 0.79 | 69.79 | 8.24 (8.11 ± 0.10) | — |
| Glass/Ti3C2Tx/PEDOT:PSS/PTB7-Th:PC71BM/LiF/Al | 14.91 | 0.79 | 65.61 | 7.76 (7.58 ± 0.12) | — |
| PET/Ti3C2Tx/PEDOT:PSS/PTB7-Th:PC71BM/LiF/Ag | 14.21 | 0.79 | 65.32 | 7.37 (7.13 ± 0.09) | — |
| PET/Ti3C2Tx/PEI/PTB7-Th:PC71BM/PEDOT:PSS/Ti3C2Tx | 14.43 | 0.75 | 48.73 | 5.26 (5.10 ± 0.14) | 31.7 |
| Glass/ITO/PEI/P3HT:ICBA/MoO3/Al | 7.98 | 0.83 | 56.05 | 3.70 (3.42 ± 0.21) | — |
| Glass/ITO/PEI/P3HT:ICBA/PEDOT:PSS/Ti3C2Tx | 7.39 | 0.84 | 53.71 | 3.34 (3.19 ± 0.11) | 51.7 |
| Glass/Ti3C2Tx/PEI/P3HT:ICBA/PEDOT:PSS/Ti3C2Tx | 6.91 | 0.84 | 51.53 | 2.96 (2.75 ± 0.19) | 42.3 |
| PET/Ti3C2Tx/PEI/P3HT:ICBA/PEDOT:PSS/Ti3C2Tx | 6.28 | 0.82 | 50.84 | 2.62 (2.41 ± 0.12) | 41.5 |
| PET/Ti3C2Tx/PEI/P3HT:ICBA/PEDOT:PSS/Ti3C2Tx/ionogel/Ti3C2Tx/PDMS | 6.89d | 0.80 | 45.32 | 2.50 (2.21 ± 0.22) | 33.5 |
| 6.45e | 0.80 | 36.59 | 1.89 (1.73 ± 0.13) | 33.5 | |
Electrochemical performance of transparent MXene supercapacitors
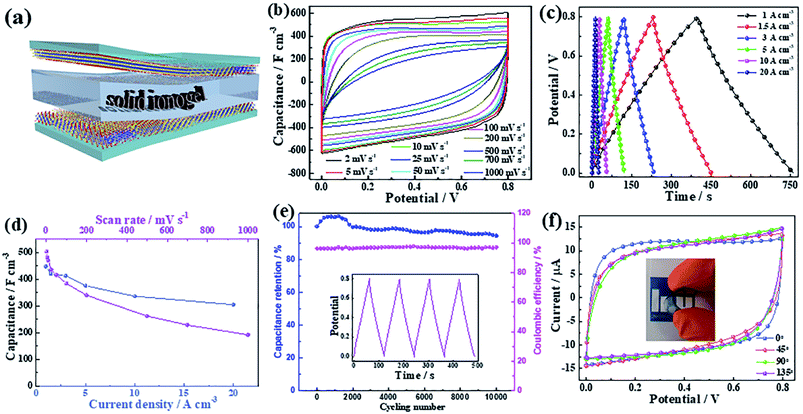
For semitransparent photovoltaic supercapacitors, not only does the energy conversion part require higher transmittance, but the energy storage part also requires high transmittance. Therefore, high conductivity, high transmission, and excellent capacitance performance are prerequisites for potential candidate materials. Ti3C2Tx MXene has shown significant energy-storage capability with excellent flexibility, high conductivity, and optical transparency, suggesting that Ti3C2Tx is suitable for our purpose.28,46,47 Considering the choice of electrolyte, a sulfuric acid based electrolyte, which is commonly used for Ti3C2Tx based supercapacitors, can penetrate through the electrode of the capacitor, which would damage the photovoltaic conversion part for vertically integrated energy conversion and storage modules. Based on this consideration, a transparent, flexible solid supercapacitor was constructed using a solid organic ionogel electrolyte instead of a sulfate-based electrolyte (Fig. 4a).The performance of the transparent, flexible all-solid-state supercapacitor was investigated based on CV curves (Fig. 4b). The shapes of the CV curves are pseudorectangular, even at very high scan rates (500 mV s−1), which indicate highly capacitive behavior and excellent power handling properties in the device. Fig. 4c shows the galvanostatic charging/discharging (GCD) curves of the transparent device. The symmetric sloping shapes of these curves indicate good coulombic efficiency of the charging/discharging process, which is in good agreement with the CV curves shown in Fig. 4b. The volumetric capacitances of the transparent solid-state supercapacitors are calculated from the CVs and GCD profiles and are presented in Fig. 4d. The capacitance reach 450 F cm−3 at 1 A cm−3 (502 F cm−3 at 2 mV s−1) and maintains 68% of initial capacitance as the current density is increased by 20 times, indicating the high-rate capability of supercapacitors based on an MXene electrode and an organic ionogel electrolyte, which is comparable with that of a sulfuric acid based electrolyte.28 To show further the merits of supercapacitors based on an MXene electrode and an organic ionogel electrolyte, the electrochemical impedance spectra (EIS) of the devices are shown in Fig. S17.† The curve shows a large slope close to 90° at the low frequency of the complex plane plots, which indicates fast ion diffusion between the organic ionogel electrolyte and the Ti3C2Tx thin films. At the high frequency, see inset, it shows a small charge-transport semicircle, which is consistent with the high conductivity of the Ti3C2Tx films. The lifetime of the flexible transparent device was evaluated through GCD measurements (Fig. 4e). 95% of initial capacitance was retained after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. In addition, the device also exhibits high stability under different bending angles (Fig. 4f). The high volumetric capacitances, long lifetime, and high transmittance make the organic ionogel electrolyte based Ti3C2Tx flexible supercapacitor the best choice for the energy storage modules of flexible semi-transparent photovoltaic supercapacitors.
000 cycles. In addition, the device also exhibits high stability under different bending angles (Fig. 4f). The high volumetric capacitances, long lifetime, and high transmittance make the organic ionogel electrolyte based Ti3C2Tx flexible supercapacitor the best choice for the energy storage modules of flexible semi-transparent photovoltaic supercapacitors.
The performance of PSCs
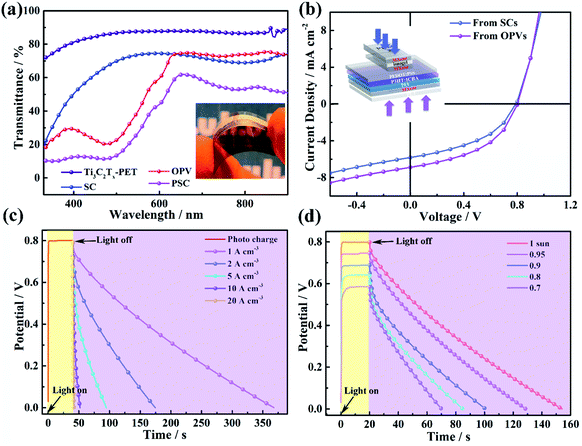
In order to achieve simultaneous energy harvesting and storage in a single device, a flexible semi-transparent photovoltaic supercapacitor was fabricated by vertically integrating energy harvesting and energy storage units by employing Ti3C2Tx transparent thin film as a common electrode. The procedure is briefly summarized as follows (more details in the Experimental section, ESI†): based on the transparent flexible energy harvesting part, the organic ionogel electrolyte and Ti3C2Tx thin films are used as the separator and active electrode of supercapacitor, respectively, and laminated on the transparent top electrode of the OPV to form the energy storage device. This means that the middle Ti3C2Tx thin layer acts not only as the top electrode for the OPV, but also acts as the active electrode for the supercapacitor. As shown in Fig. 5a, the transmittance spectra of the single electrode and the symmetric device show that the transmittance of the single electrode is around 90% and 70% can be achieved for a device, which is higher than the transmittance of a reported supercapacitor.28 The average visible transmittance (AVT) of the flexible transparent OPV is 42%. Finally, the flexible transparent PSC exhibits an AVT of over 33.5%. Fig. 5b shows the J–V characteristics of a flexible transparent PSC illuminated from the OPV and the supercapacitor side, respectively. When illuminated from the OPV device, the PESM shows Voc = 0.81 V, Jsc = 6.89 mA cm−2, and F = 0.45, yielding an average PCE of 2.5%. When illuminated from the supercapacitor device, a lower Jsc value is obtained due to the lower transmittance of the top energy storage device. The photocharging and discharging principle (Fig. S19†) and performance (Fig. 5c) of the PSC were investigated under an illumination of AM 1.5 (100 mW cm−2). In the charging process (yellow section), the PSC reaches a capacitor voltage (Vcap) of 0.8 V within a very short charging time of 2 s. Under continuous illumination, the Vcap value remains the same at the saturated values of 0.8 V. The discharge behavior of the PSCs under different current densities after shutting down the light source was evaluated in Fig. 5c (purple section). The specific capacitance at a discharge current density of 1 A cm−3 exhibits 410 F cm−3, which is close to the maximum performance of an un-integrated Ti3C2Tx-based flexible transparent supercapacitor, indicating the successful integration of the energy storage unit into the energy harvesting device. Furthermore, the photo charging and discharging performance of the PSC were evaluated as a function of light irradiance. As shown in Fig. 5d, with decreasing light intensity, the photo charging time increases and the light saturated Vcap decreases. This is due to the decrease in Jsc and Voc at low irradiance in the energy harvesting device. The overall photoelectric conversion and storage efficiency versus photocharging time were calculated. As shown in Fig. S20,† a self-power pack with a flexible semi-transparent PSC had maximum ηstorage and ηoverall values of up to 88% and 2.2%, respectively. Considering that the efficiency of the energy storage module is high, the overall efficiency is strongly dependent on the energy harvesting part. Further optimization can be expected by utilizing efficient and stable organic photovoltaic materials.3. Conclusions
In summary, MXene-based all-solution-processed flexible semi-transparent PSCs were fabricated with a vertically integrated Ti3C2Tx-electrode-based transparent flexible photovoltaic device and Ti3C2Tx-based transparent supercapacitor. Ti3C2Tx transparent films were produced, aligned parallel to the substrate and demonstrating outstanding flexibility, transmittance, and conductivity. These properties make the films efficient transparent electrodes for OPV with an obtained high PCE of 13.6%, comparable with that of ITO electrodes, and as the active material for supercapacitors with a high volumetric capacitance of 502 F cm−3. The long cycling stability is based on a high-performance organic solid ionogel electrolyte. The organic solid electrolyte effectively solves the issue of the leakage of water-based electrolyte from the energy storage device for the energy harvesting device during vertical integration. Finally, the semi-transparent flexible PSC with Ti3C2Tx thin films as the common electrodes exhibits an AVT of 33.5%, and maximum ηstorage and ηoverall values of up to 88% and 2.2%, respectively. Undoubtedly, through further optimization of the efficiency of the energy harvesting part, the overall efficiency of the PSC devices could be further improved. This all-solution-processing semitransparent flexible PSC is suitable for blading, printing, and roll-to-roll manufacturing, which is promising for the production of cost-efficient flexible PSCs to satisfy the increasing energy demands for portable, wearable and miniature electronic devices.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was financed by the Swedish Energy Agency (EM 42033-1), the Swedish Government Strategic Research Area in Material Science on Functional Materials at Linköping University (Faculty Grant SFO-Mat-LiU No 200900971) and the Swedish Research Council (2017-04123), the SSF Research Infrastructure Fellow Program no. RIF 14-0074 and the SSF Synergy Program EM16-0004, and by the Knut and Alice Wallenberg (KAW) Foundation through a Fellowship Grant, a Project Grant (KAW 2015.0043), and for support of the electron microscopy laboratory and the device physics lab in Linköping. Support from the National Natural Science Foundation of China (61774077), the Open Fund of the State Key Laboratory of Luminescent Materials and Devices (2018-skllmd-12) and the Fundamental Research Funds for the Central Universities are also acknowledged.References
- S. Chu and A. Majumdar, Nature, 2012, 488, 294–303 CrossRef CAS PubMed.
- Y. Fu, H. Wu, S. Ye, X. Cai, X. Yu, S. Hou, H. Kafafy and D. Zou, Energy Environ. Sci., 2013, 6, 805–812 RSC.
- S. Park, S. W. Heo, W. Lee, D. Inoue, Z. Jiang, K. Yu, H. Jinno, D. Hashizume, M. Sekino, T. Yokota, K. Fukuda, K. Tajima and T. Someya, Nature, 2018, 561, 516–521 CrossRef CAS PubMed.
- G. Li, R. Zhu and Y. Yang, Nat. Photonics, 2012, 6, 153–161 CrossRef CAS.
- Y. Liu, J. Zhao, Z. Li, C. Mu, W. Ma, H. Hu, K. Jiang, H. Lin, H. Ade and H. Yan, Nat. Commun., 2014, 5, 5293 CrossRef CAS PubMed.
- Z. He, B. Xiao, F. Liu, H. Wu, Y. Yang, S. Xiao, C. Wang, T. P. Russell and C. Yong, Nat. Photonics, 2015, 9, 174–179 CrossRef CAS.
- Y.-B. Cheng, A. Pascoe, F. Huang and Y. Peng, Nature, 2016, 539, 488–489 CrossRef CAS PubMed.
- F. Zhang, M. Johansson, M. R. Andersson, J. C. Hummelen and O. Inganäs, Adv. Mater., 2002, 14, 662–665 CrossRef CAS.
- J. L. Smith, Science, 1981, 212, 1472–1478 CrossRef CAS PubMed.
- Y. Jin, Z. Li, L. Qin, X. Liu, L. Mao, Y. Wang, F. Qin, Y. Liu, Y. Zhou and F. Zhang, Adv. Mater., 2017, 4, 1700704 Search PubMed.
- G. Wee, T. Salim, Y. M. Lam, S. G. Mhaisalkar and M. Srinivasan, Energy Environ. Sci., 2011, 4, 413–416 RSC.
- S. Pan, J. Ren, X. Fang and H. Peng, Adv. Energy Mater., 2016, 6, 1501867 CrossRef.
- Z. Zhang, X. Chen, P. Chen, G. Guan, L. Qiu, H. Lin, Z. Yang, W. Bai, Y. Lou and H. Peng, Adv. Mater., 2014, 26, 466–470 CrossRef CAS PubMed.
- W. Guo, X. Xue, S. Wang, C. Lin and Z. Wang, Nano Lett., 2012, 12, 2520–2523 CrossRef CAS PubMed.
- J. Xu, H. Wu, S. Wang, C. Lin and Z. Wang, Adv. Funct. Mater., 2013, 24, 1840–1846 CrossRef.
- K. Nomura, H. Ohta, A. Takagi, T. Kamiya, M. Hirano and H. Hosono, Nature, 2004, 432, 488–492 CrossRef CAS PubMed.
- T. Georgiou, R. Jalil, B. D. Belle, L. Britnell, R. V. Gorbachev, S. V. Morozov, Y.-J. Kim, A. Gholinia, S. J. Haigh and O. Makarovsky, Nat. Nanotechnol., 2013, 8, 100–103 CrossRef CAS PubMed.
- H. Kang, G. Kim, J. Kim, S. Kwon, H. Kim and K. Lee, Adv. Mater., 2016, 28, 7821–7861 CrossRef CAS PubMed.
- H. Kang, S. Jung, S. Jeong, G. Kim and K. Lee, Nat. Commun., 2015, 6, 6503 CrossRef CAS PubMed.
- S. Ye, A. R. Rathmell, Z. Chen, I. E. Stewart and B. J. Wiley, Adv. Mater., 2014, 26, 6670–6687 CrossRef CAS PubMed.
- N. Espinosa, R. Garcia-Valverde and F. C. Krebs, Energy Environ. Sci., 2011, 4, 1547–1557 RSC.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed.
- B. Anasori, M. R. Lukatskaya and Y. Gogotsi, Nat. Rev. Mater., 2017, 2, 16098 CrossRef CAS.
- M. Ghidiu, M. R. Lukatskaya, M.-Q. Zhao, Y. Gogotsi and M. W. Barsoum, Nature, 2014, 516, 78–81 CrossRef CAS PubMed.
- L. Qin, Q. Tao, X. Liu, M. Fahlman, J. Halim, P. Persson, J. Rosen and F. Zhang, Nano Energy, 2019, 60, 734–742 CrossRef CAS.
- M. R. Lukatskaya, O. Mashtalir, C. E. Ren, Y. Dall'Agness, P. Rozier, P. L. Taberna, M. Naguib, P. Simon, M. W. Barsoum and Y. Gogotsi, Science, 2013, 341, 1502–1505 CrossRef CAS PubMed.
- Y. Xia, T. S. Mathis, M.-Q. Zhao, B. Anasori, A. Dang, Z. Zhou, H. Cho, Y. Gogotsi and S. Yang, Nature, 2018, 557, 409–412 CrossRef CAS PubMed.
- C. Zhang, B. Anasori, A. Seral-Ascaso, S.-H. Park, N. McEvoy, A. Shmeliov, G. S. Duesberg, J. N. Coleman, Y. Gogotsi and V. Nicolosi, Adv. Mater., 2017, 29, 1702678 CrossRef PubMed.
- A. D. Dillon, M. J. Ghidiu, A. L. Krick, J. Griggs, S. J. May, Y. Gogotsi, M. W. Barsoum and A. T. Fafarman, Adv. Funct. Mater., 2016, 26, 4162–4168 CrossRef CAS.
- C. Zhang, S.-H. Park, A. Seral-Ascaso, S. Barwich, N. McEvoy, C. S. Boland, J. N. Coleman, Y. Gogotsi and V. Nicolosi, High capacity silicon anodes enabled by MXene viscous aqueous ink, Nat. Commun., 2019, 10, 849 CrossRef PubMed.
- C. Zhang, L. McKeon, M. P. Kremer, S.-H. Park, O. Ronan, A. Seral-Ascaso, S. Barwich, C. O. Coileain, N. McEvoy, H. C. Nerl, B. Anasori, J. N. Coleman, Y. Gogotsi and V. Niclolsi, Nat. Commun., 2019, 10, 1795 CrossRef PubMed.
- G. S. Gund, J. H. Park, R. Harpalsinh, M. Kota, J. H. Shin, T. Kim, Y. Gogotsi and H. S. Park, Joule, 2019, 3, 1–13 CrossRef.
- M. Alhabeb, K. Maleski, B. Anasori, P. Lelyukh, L. Clark, S. Sin and Y. Gogotsi, Chem. Mater., 2017, 29, 7633–7644 CrossRef CAS.
- L. H. Karlsson, J. Birch, J. Halim, M. W. Barsoum and P. O. Å. Persson, Nano Lett., 2015, 15, 4955–4960 CrossRef CAS PubMed.
- W. Tian, A. VahidMohammadi, Z. Wang, L. Ouyang, M. Beidaghi and M. M. Hamedi, Nat. Commun., 2019, 10, 2558 CrossRef PubMed.
- Q. Jiang, N. Kurra, M. Alhabeb, Y. Gogotsi and H. N. Alshareef, Adv. Energy Mater., 2018, 8, 1703043 CrossRef.
- P. Salles, E. Quain, N. Kurra, A. Sarycheva and Y. Gogotsi, Small, 2018, 14, 1802864 CrossRef PubMed.
- Y. Peng, B. Akuzum, N. Kurra, M.-Q. Zhao, M. Alhabeb, B. Anasori, E. C. Kumbur, H. N. Alshareef, M. DerGer and Y. Gogotsi, Energy Environ. Sci., 2016, 9, 2847–2854 RSC.
- A. D. Dillon, M. J. Ghidiu, A. L. Krick, J. Griggs, S. J. May, Y. Gogotsi, M. W. Barsoum and A. T. Fafarman, Adv. Funct. Mater., 2016, 26, 4162–4168 CrossRef CAS.
- E. Pomerantseva and Y. Gogotsi, Nat. Energy, 2017, 2, 17089 CrossRef CAS.
- Z. Tang, A. Elfwing, A. Melianas, J. Bergqvist, Q. Bao and O. Inganäs, J. Mater. Chem. A, 2015, 3, 24289–24296 RSC.
- P. J. King, T. M. Higgins, S. De, N. Nicoloso and J. N. Coleman, ACS Nano, 2012, 6, 1732–1741 CrossRef CAS PubMed.
- J. Yuan, Y. Zhang, L. Zhou, G. Zhang, H.-L. Yip, T.-K. Lau, X. Lu, C. Zhu, H. Peng, P. A. Johnson, M. Leclerc, Y. Cao, J. Ulanski, Y. Li and Y. Zou, Joule, 2019, 3, 1140–1151 CrossRef CAS.
- Y. Zhou, C. Fuentes-Hernandez, J. W. Shim, J. Meyer, A. J. Giordano, H. Li, P. Winget, T. Papadopoulos, H. Cheun, J. Kim, M. Fenoll, A. Dindar, W. Haske, E. Najafabadi, T. M. Khan, H. Sojoudi, S. Barlow, S. Graham, J.-L. Brédas, S. R. Marder, A. Kahn and B. Kippelen, Science, 2012, 336, 327–332 CrossRef CAS PubMed.
- Y. Zhou, C. Fuentes-Hernandez, J. W. Shim, T. M. Khan and B. Kippelen, Energy Environ. Sci., 2012, 5, 9827–9832 RSC.
- M.-Q. Zhao, C. Ren, Z. Ling, M. R. Lukatskaya, C. Zhang, K. L. Van Aken, M. W. Barsoum and Y. Gogotsi, Adv. Mater., 2015, 27, 339–345 CrossRef CAS PubMed.
- M. R. Lukatskaya, S. Kota, Z. Lin, M.-Q. Zhao, N. Shpigel, M. D. Levi, J. Halim, P.-L. Taberna, M. W. Barsoum, P. Simon and Y. Gogotsi, Nat. Energy, 2017, 2, 17105 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ta00687d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |