Fused heterocycle-based energetic materials (2012–2019)
Haixiang
Gao
 *a,
Qinghua
Zhang
*a,
Qinghua
Zhang
 *b and
Jean'ne M.
Shreeve
*b and
Jean'ne M.
Shreeve
 *c
*c
aDepartment of Applied Chemistry, China Agricultural University, Beijing 100193, China. E-mail: hxgao@cau.edu.cn
bInstitute of Chemical Materials, China Academy of Engineering Physics, Mianyang 621900, China. E-mail: qinghuazhang@caep.cn
cDepartment of Chemistry, University of Idaho, Moscow, Idaho 83844-2343, USA. E-mail: jshreeve@uidaho.edu
First published on 6th February 2020
Abstract
Fused cyclic energetic materials, a unique class of large conjugate structures which contain two or more rings that share two atoms and the bond between the rings, have been identified as promising contenders to traditional energetic materials. With a coplanar polycyclic structure, fused heterocyclic ring-based energetic materials feature considerably higher heats of formation (HOF) and ring-strain energy stored in the molecules. These result in attractive features of good energetic performance, enhanced thermal stability and low sensitivity toward destructive mechanical stimuli, which increases the safety of the synthesis, transfer, and storage of high-energy density compounds. This review addresses the chemistry of fused heterocyclic compounds, which provide valuable scaffolds for more powerful and less sensitive eco-friendly energetic materials. The reactions that are selected and discussed illustrate the versatility of 64 different fused heterocycles designed as building blocks for the synthesis of a wide range of high-performance energetic materials.
Introduction
Among energetic materials, traditional chemical explosives, such as black powder, potassium nitrate, ammonium perchlorate, nitroglycerine, and 2,4,6-trinitrotoluene (TNT), specifically represent the most influential materials in human history and have gained much attention in the chemical sciences. Trinitrotoluene (TNT) was first synthesized in 1863, which makes it one of the oldest and most commonly used explosives for industrial, mining and military applications. It is valued partly because of its insensitivity to mechanical stimuli; however, it is less powerful than the alternatives hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) and 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane (HMX) (Table 1). TNT is considered the standard measure of strength for other explosives and RDX and HMX are considered as powerful chemical explosives and the benchmark for current HDEMs (High Density Energetic Materials) (Table 1). 2,4,6-Triamino-1,3,5-trinitrobenzene (TATB), a powerful explosive (somewhat more powerful than TNT but less than RDX), and one which is extremely insensitive to shock, vibration, fire, or impact. Hexanitrohexaazaisowurtzitane, also known as HNIW or CL-20, is more powerful than traditional HMX-based propellants, and is widely superior to conventional high-energy propellants and explosives.The chemistry of energetic materials has been successfully explored. In the synthesis of energetic compounds, diverse backbones, such as aromatic, aliphatic, caged carbon and heterocyclic frameworks, have been selected. Usually, the backbone is used as fuel, and has no explosive properties. Explosophoric groups (e.g., –NO2, –ONO2, N3, –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–, N-oxide and NF2, etc.) and moieties (e.g., NHNO2, –C(NO2)3, –C(NO2)2H, and N(O)
N–, N-oxide and NF2, etc.) and moieties (e.g., NHNO2, –C(NO2)3, –C(NO2)2H, and N(O)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNO2, among others) that can be covalently or ionically attached to the backbone of a molecule provide the energy.2 With these groups, the oxidation of the C and H atoms by the O atoms in the molecules generates carbon mono- or dioxide and water to release the chemical energy that is stored in the core.3
NNO2, among others) that can be covalently or ionically attached to the backbone of a molecule provide the energy.2 With these groups, the oxidation of the C and H atoms by the O atoms in the molecules generates carbon mono- or dioxide and water to release the chemical energy that is stored in the core.3
Although many of the classical energetic materials are still effective, given the growing demand for high energy density materials (HEDMs) as propellants, explosives, and pyrotechnics with improved detonation performance for military and civilian applications, the design and synthesis of new HEDMs that contain a backbone with highly energetic functionalities are a key goal in their discovery and development.4,5
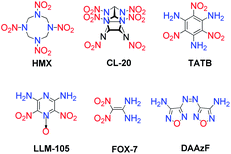
In general, the various strategies developed for introducing more of the highly energetic functional groups are capable of improving the detonation properties of the target molecules. However, in addition to detonation properties, molecular stability is also a primary criterion for evaluating the overall performance of energetic materials. Energetic properties (detonation velocity and pressure), stability and sensitivity of HEDMs are often observed to be inversely related. Modern HEDMs still have several drawbacks, including low stability and high sensitivity because of their high energy content. For example, HMX and CL-20 are high-performance explosives, but they are more sensitive than and lack the stability of well-known insensitive explosives such as TATB, 2,6-diamino-3,5-dinitropyrazine-1-oxide (LLM-105), 1,1-diamino-2,2-dinitroethene (FOX-7) and 3,3′-diamino-4,4′-azofurazan (DAAzF) (Scheme 1). Therefore, an accurate understanding of the relationship between molecular structure and energetic and physical properties and of the trade-off between the high energetic performance of an explosive and its good stability is essential for designing and developing effective methods to synthesize the next generation of HEDMs.
To achieve an ideal combination of high detonation pressure and velocity, good stability (high thermal stability and low sensitivity) and environmentally friendly decomposition gases, chemists around the world have made significant efforts that have resulted in the generation of superior HEDMs via the construction of strained ring or cage systems, nitrogen-rich compounds, energetic salts, cocrystals, metal–organic frameworks and molecular perovskite high-energetic materials, among others.3,5–7
Recently, fused cyclic energetic materials, a unique class of large conjugate structures containing two or more rings that share two atoms and the bond between the rings, have been identified as promising contenders to traditional energetic materials. Fused heterocyclic ring-based energetic materials with a coplanar polycyclic structure exhibit considerably higher heats of formation (HOF) and ring-strain energy stored in the molecules and show obvious characteristics of good energetic performance, enhanced thermal stability and low sensitivities toward destructive mechanical stimuli which increases the safety of the synthesis, transfer, and storage of HEDMs.8,9 Thus far, the limited number of fused cyclic backbones available for the preparation of different molecules has hindered their expansion in the field of HEDMs. Therefore, this research area is attracting attention, since it is particularly interesting to study these systems in order to develop various types of new HEDMs with satisfactory detonation properties and sensitivity properties.
This review covers the most recent progress in the field of fused cyclic energetic materials since 2012. The fused ring systems are divided into four parts based on the number and type of rings found in the backbone. Five reference compounds are used for comparison purposes (TNT, TATB, RDX, HMX, CL-20), all the data (density, heat of formation, detonation pressure and detonation velocity) (Table 1) are from the EXPLO 5 (Version 6.01).1 It is hoped that the review will provide an overview of the fundamental methodology of building fused heterocyclic rings and introducing “explosophoric” groups into them to design and synthesize new HEDMs while concomitantly addressing aspects such as stability, detonation performance and providing a comprehensive comparison and discussion that will certainly stimulate further studies.
| No. | T m , °C | T d , °C | d , g cm−3 | D , m s−1 | P , GPa | HOFf, kJ mol−1/kJ g−1 | OBg, % | ISh, J | FSi, N | Ref.j |
|---|---|---|---|---|---|---|---|---|---|---|
| a Melting point. b Decomposition temperature (onset). c Density. d Detonation velocity (calculated). e Detonation pressure (calculated). f Heat of formation (calculated). g Oxygen balance (based on CO2) for CaHbOcNd, 1600 (c–a–b)/MW; MW = molecular weight. h Impact sensitivity. i Friction sensitivity. j Reference. All the quantity symbols are used in all the tables in this review. | ||||||||||
| TNT | 81 | 295 | 1.65 | 7303 | 21.3 | −59.3/−0.26 | −73.97 | 15 | 353 | 1 |
| TATB | 350 | ∼360 | 1.94 | 8544 | 32.1 | −154.2/−0.60 | −55.78 | 50 | 360 | 1 |
| RDX | 205 | 210 | 1.80 | 8795 | 34.9 | 70.7/0.32 | −21.61 | 7.5 | 120 | 1 |
| HMX | — | 280 | 1.91 | 9144 | 39.2 | 74.8/0.25 | −21.61 | 7 | 120 | 1 |
| CL-20 | — | 210 | 2.04 | 9706 | 45.2 | 397.8/0.91 | −10.95 | 4 | 94 | 1 |
[5,5]-Bicyclic heterocyclic energetic materials
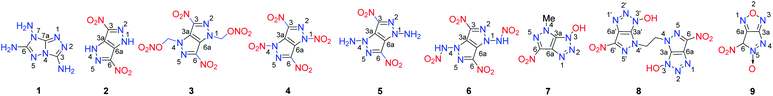
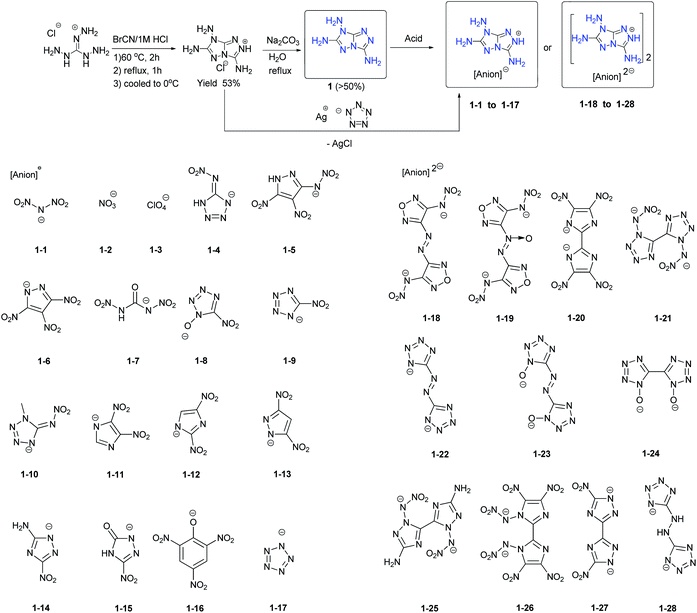
[5,5]-Bicyclic heterocycles are the simplest fused rings for building molecules of energetic materials. In recent years, several [5,5]-bicyclic heterocycle-fused energetic materials were synthesized (Scheme 2). As is the case for other fused ring compounds, the construction of the backbone is the first step in obtaining new energetic materials. 3,6,7-Triamino-7H-[1,2,4]triazolo[4,3-b][1,2,4]triazole (1) is a bis-triazole fused heterocyclic ring with three amino groups on its backbone which is synthesized readily through the cascade cyclization of cyanogen bromide with triaminoguanidinium chloride followed by alkalization with Na2CO3 (Scheme 3). Recently, by reaction with selected energetic acids, a series of energetic salts containing the 3,5,7-triamino-7H-s-triazolo[5,1-c]-s-triazolium cation were prepared.8–10 The calculated detonation velocities and pressures of 1 and its salts fall in the range of 8052–9477 m s−1 and 24.1–33.9 GPa, respectively (Table 2). Most of their performance levels are comparable to those of RDX with respect to thermal stability, detonation properties and sensitivity. Their performance arises mainly because the three amino groups on the rings facilitate the formation of extensive intermolecular hydrogen bonds between the cation and anion in its salts.| No. | T m, °C | T d, °C | d, g cm−3 | D, m s−1 | P, GPa | HOF, kJ mol−1/kJ g−1 | OB, % | IS, J | FS, N | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | — | 251 | 1.73 | 8580 | 25.9 | 470.5/3.06 | −93.43 | 40 | 360 | 13 |
| — | 245 | 1.73 | 9385 | 29.7 | 446.7/2.90 | 40 | 360 | 8 | ||
| 1-1 | — | 199 | 1.82 | 9090 | 33.9 | 463.3/1.78 | −33.7 | 6 | 160 | 13 |
| 1-2 | — | 279 | 1.78 | 8765 | 29.7 | 274.5/1.26 | −47.89 | 40 | 360 | 13 |
| — | 280 | 1.78 | 9005 | 30.2 | 261.5/1.20 | 40 | 360 | 8 | ||
| 1-3 | — | 257 | 1.77 | 8216 | 28.1 | 325.9/1.28 | −31.42 | 15 | 240 | 13 |
| — | 264 | 1.78 | 8312 | 28.1 | 314.6/1.24 | 9 | 216 | 8 and 13 | ||
| 1-4 | — | 226 | 1.73 | 8618 | 27.9 | 763.7/2.69 | −56.30 | 10 | 240 | 13 |
| 1-5 | — | 211 | 1.80 | 8704 | 31.3 | 666.0/1.79 | −42.99 | 25 | 240 | 13 |
| 1-6 | — | 208 | 1.74 | 8365 | 28.3 | 582.9/1.63 | −42.55 | 40 | 360 | 13 |
| 1-7 | — | 167 | 1.81 | 8881 | 32.3 | 424.4/1.39 | −36.82 | 6 | 120 | 13 |
| 1-8 | — | 222 | 1.73 | 8814 | 29.0 | 691.7/2.43 | −47.69 | 25 | 360 | 8 |
| 1-10 | — | 237 | 1.65 | 8760 | 26.9 | 783.9/2.63 | −69.74 | 8 | 360 | 8 |
| 1-11 | — | 207 | 1.71 | 8189 | 24.7 | 446.8/1.43 | −61.49 | 22 | — | 14 |
| 1-12 | 152 | 226 | 1.70 | 8113 | 24.1 | 422.4/1.35 | −61.49 | >40 | — | 14 |
| 1-13 | 120 | 246 | 1.72 | 8286 | 25.4 | 490.0/1.57 | −61.49 | 35 | — | 14 |
| 1-14 | 218 | 232 | 1.68 | 8549 | 25.6 | 561.9/1.98 | −70.62 | >40 | — | 14 |
| 1-15 | — | 256 | 1.70 | 8232 | 24.2 | 353.0/1.24 | −61.93 | >40 | — | 14 |
| 1-16 | — | 278 | 1.79 | 8162 | 25.9 | 191.6/0.50 | −64.71 | 15 | — | 14 |
| 1-17 | — | 121 | 1.62 | 7791 | 24.6 | 853.8/3.79 | −71.1 | >40 | >360 | 15 |
| 1-18 | — | 243 | 1.73 | 8052 | 24.0 | 867.7/1.46 | −56.53 | 10 | 120 | 13 |
| 1-19 | — | 246 | 1.80 | 8457 | 27.6 | 918.8/1.51 | −52.42 | 8 | 120 | 13 |
| 1-21 | — | 224 | 1.76 | 9242 | 32.0 | 1845.5/3.26 | −53.67 | 7.5 | 108 | 8 |
| 1-22 | — | 200 | 1.72 | 9360 | 30.7 | 1811.8/3.82 | −77.57 | 20 | 360 | 8 |
| 1-23 | — | 210 | 1.73 | 9289 | 31.5 | 1834.1/3.62 | −66.35 | 30 | 360 | 8 |
| 1-25 | 185 | 200 | 1.71 | 8977 | 29.0 | 1674.0/2.82 | −67.29 | 30 | 360 | 10 |
| 1-26 | — | 203 | 1.85 | 8899 | 33.8 | 1455.2/1.96 | −40.95 | 10 | 120 | 16 |
| 1-27 | — | 311 | 1.75 | 8718 | 27.7 | 1208.2/2.26 | −68.86 | 30 | — | 14 |
| 1-28 | — | 219 | 1.74 | 9477 | 31.4 | 1138.0/3.53 | −80.60 | >40 | — | 14 |
Among these molecules, compounds 1-21, 1-22 and 1-27 are the most promising candidates for potential applications as HEDMs. These salts combine good stability with excellent explosive properties (D > 9200 m s−1 and P > 30 GPa), all of which meet the required criteria for mechanical (IS = 7.5 J, FS > 120 N) or thermal (Td > 200 °C) stimuli. These studies demonstrate that 1 and its energetic salts involve a ready synthetic route, good stability toward thermal and mechanical stimuli, and high energetic performance making them promising energetic building blocks for designing new HEDMs.
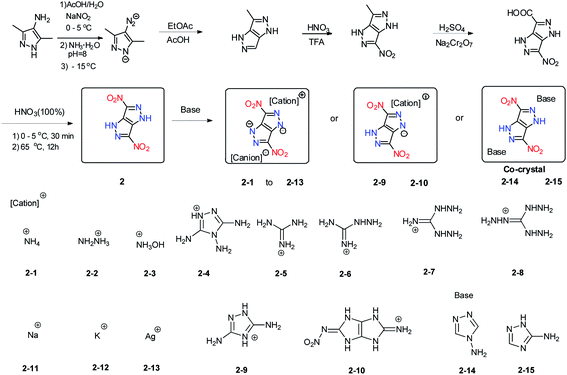
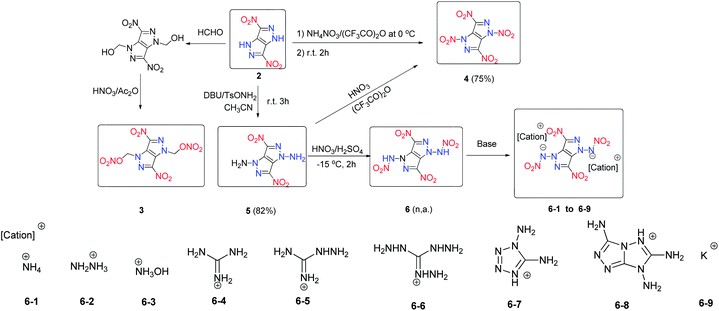
3,6-Dinitropyrazolo[4,3-c]pyrazole (2) is an attractive energetic material with low sensitivity to external mechanical stimuli and favourable thermal stability because it has a stable fused ring backbone. It was synthesized through a modified synthesis process with enhanced efficiency and reproducibility (Scheme 4).11 Due to the acidity of 2, it is straightforward to synthesize a series of its energetic salts (2-1 to 2-13) through either neutralization or metathesis reactions (Scheme 4).11,12 Compound 2 and its salts exhibit outstanding thermal stabilities (209–395 °C). The densities of the salts of 2 fall in the range between 1.66 and 2.20 g cm−3, which places them in a class of relatively dense HEDMs. Their detonation velocities (7948–9005 m s−1) and detonation pressures (22.5–35.4 GPa) are comparable to those of TNT and RDX. Most of the salts have acceptable friction sensitivities (80 to 360 N) and impact sensitivities (12 to >40 J) (Table 3). In studying the reactions of 2 with 3-amino-1,2,4-triazole and 4-amino-1,2,4-triazole, two energetic cocrystals composed of 2 and 3-amino-1,2,4-triazole (2-14) or 4-amino-1,2,4-triazole (2-15), respectively, with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 were obtained.11 The amino groups in 3-amino-1,2,4-triazole and 4-amino-1,2,4-triazole are able to provide sufficient hydrogen bond donor character driving them to cocrystallization with 2. Compared to 2, cocrystals 2-14 and 2-15 have comparable explosive power and low sensitivities to impact and friction. These features make the salts and cocrystals of 2 potentially useful candidates for further investigation as thermally stable and insensitive HEDMs.
2 were obtained.11 The amino groups in 3-amino-1,2,4-triazole and 4-amino-1,2,4-triazole are able to provide sufficient hydrogen bond donor character driving them to cocrystallization with 2. Compared to 2, cocrystals 2-14 and 2-15 have comparable explosive power and low sensitivities to impact and friction. These features make the salts and cocrystals of 2 potentially useful candidates for further investigation as thermally stable and insensitive HEDMs.
| No. | T m, °C | T d, °C | d, g·cm−3 | D, m·s−1 | P, GPa | HOF, kJ mol−1/kJ g−1 | OB, % | IS, J | FS, N | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | — | 336 | 1.85 | 8250 | 27.4 | 322.6/1.94 | −40.38 | 15 | 160 | 12 |
| 2-1 | 160 | 328 | 1.69 | 8212 | 25.4 | 133.7/0.38 | −55.13 | > 40 | 360 | 12 |
| 2-2 | 174 | 327 | 1.82 | 9005 | 35.4 | 550.9/1.92 | −54.92 | 29 | 360 | 12 |
| 2-3 | — | 247 | 1.72 | 8860 | 30.3 | 467.0/2.04 | −36.34 | 16 | 160 | 12 |
| 2-4 | — | 287 | 1.71 | 8036 | 24.5 | 595.2/1.87 | −71.31 | > 40 | 360 | 12 |
| 2-5 | — | 289 | 1.67 | 8230 | 24.6 | 423.1/1.20 | −70.83 | > 40 | 360 | 12 |
| 2-6 | 318 | 324 | 1.68 | 7948 | 22.5 | 738.9/1.93 | −69.31 | > 40 | 360 | 12 |
| 2-7 | 213 | 222 | 1.69 | 8400 | 25.6 | 531.2/1.38 | −68.03 | > 40 | 360 | 12 |
| 2-8 | — | 209 | 1.71 | 8732 | 28.0 | 454.7/1.04 | −66.94 | > 40 | 360 | 12 |
| 2-9 | 208 | 215 | 1.76 | 8814 | 29.9 | 692.9/1.49 | −51.14 | 12 | 80 | 12 |
| 2-10 | — | 238 | 1.79 | 8355 | 27.9 | 1144.8/2.17 | −56.66 | 23 | 160 | 12 |
| 2-11 | — | 395 | 2.14 | — | — | 1683.3/3.25 | −26.44 | 14 | 160 | 12 |
| 2-12 | — | 365 | 2.20 | — | — | 1599.5/2.56 | −23.33 | > 40 | 160 | 12 |
| 2-13 | — | 327 | 3.27 | — | — | 152.9/0.43 | −15.54 | 29 | 160 | 12 |
| 2-14 | 252 | 282 | 1.70 | 8024 | 23.9 | 322.6/1.94 | −74.26 | >40 | >360 | 11 |
| 2-15 | 231 | 284 | 1.68 | 8234 | 25.6 | 133.7/0.38 | −74.26 | >40 | >360 | 11 |
In addition to the production of energetic salts from 2, modification of the compounds is also a facile way to generate new energetic materials. A series of derivatives of 2 were synthesized by using versatile N-functionalization strategies (Scheme 4).17 In that study, treating 2 with formaldehyde under acidic conditions gave the N,N′-dihydroxymethyl intermediate, and after nitration by using fuming HNO3 and acetic anhydride 3,6-dinitropyrazolo[4,3-c]pyrazole-1,4-dinitrate (3) was obtained. N-nitration of 2 by NH4NO3 in trifluoroacetic anhydride gave 1,3,4,6-tetranitro-1,4-dihydropyrazolo[4,3-c]pyrazole and Table 2 (4) was obtained in good yield by the amination of 2 with O-tosylhydroxylamine (TsONH2). Nitration of 5 gave 1,3-dinitramino-4,6-dinitro-1,4-dihydropyrazolo-[4,3-c]pyrazole (6) (Scheme 5).17 In addition, a series of energetic salts (6-1–6-9) was prepared by treating 6 with a variety of bases. Compounds 4, 6, and 6-3 exhibit high densities and excellent detonation properties (Table 4), which surpass those of HMX. Compared with 2, compounds 4 and 6 also have higher oxygen balances and densities, highlighting their application potential as energetic oxidizers. The potassium salt 6-9 is a competitive compound as a green primary explosive since it is very sensitive but has good density and thermal stability (Table 4). All of these compounds exhibit good physical and detonation properties and can be variously classified as green primary explosives, fuel-rich propellants, secondary explosives, or propellant oxidizers.
| No. | T d, °C | d, g cm−3 | D, m s−1 | P, GPa | HOF, kJ mol−1/kJ g−1 | OB, % | IS, J | FS, N | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 2 | 336 | 1.85 | 8250 | 27.4 | 322.6/1.94 | −16.2 | 15 | 160 | 17 |
| 3 | 206 | 1.82 | 8674 | 33.1 | 133.7/0.38 | −18.38 | 10 | 120 | 17 |
| 4 | 145 | 1.95 | 9460 | 40.9 | 550.9/1.92 | 0.00 | 3 | 20 | 17 |
| 5 | 230 | 1.84 | 8864 | 33.9 | 467.0/2.04 | −42.08 | 7 | 120 | 17 |
| 6 | 128 | 1.93 | 9507 | 41.8 | 595.2/1.87 | −5.03 | 2 | 20 | 17 |
| 6-1 | 181 | 1.81 | 8977 | 35.9 | 423.1/1.20 | −18.17 | 10 | 120 | 17 |
| 6-2 | 174 | 1.85 | 9399 | 39.5 | 738.9/1.93 | −20.93 | 5 | 60 | 17 |
| 6-3 | 170 | 1.88 | 9495 | 41.3 | 531.2/1.38 | −8.33 | 7 | 120 | 17 |
| 6-4 | 190 | 1.68 | 8295 | 26.9 | 454.7/1.04 | −36.67 | 35 | 360 | 17 |
| 6-5 | 153 | 1.71 | 8612 | 29.3 | 692.9/1.49 | −37.74 | 30 | 360 | 17 |
| 6-6 | 141 | 1.70 | 8884 | 30.8 | 1144.8/2.17 | −39.52 | 10 | 80 | 17 |
| 6-7 | 163 | 1.78 | 9166 | 36.0 | 1683.3/3.25 | −27.78 | 5 | 60 | 17 |
| 6-8 | 203 | 1.83 | 8993 | 33.1 | 1599.5/2.56 | −48.53 | 10 | 120 | 17 |
| 6-9 | 208 | 2.11 | 8306 | 31.2 | 152.9/0.43 | 0.00 | 2 | 20 | 17 |
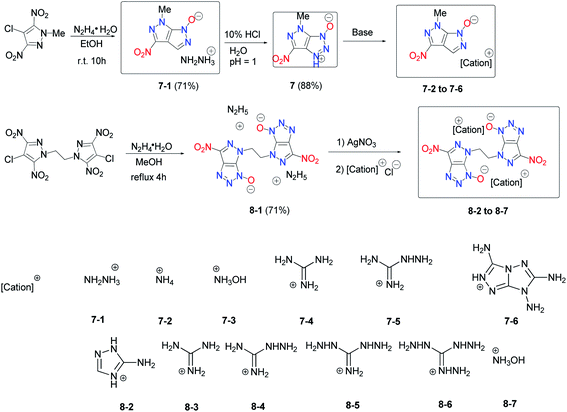
A novel fused backbone, pyrazolo[3,4-d][1,2,3]triazole, was synthesized by treating a chloro-substituted nitropyrazole with hydrazine hydrate in a one-pot procedure and then 3-hydroxy-4-methyl-6-nitro-pyrazolo[3,4-d][1,2,3]triazole (7) (Scheme 6) can be obtained accordingly.18 Although dinitropyrazole derivatives are considered attractive energetic materials due to their good physical and detonation properties, molecules comprised of a triazole ring fused with a pyrazole ring are relatively rare. In a further study of this reaction, 1,2-bis(4-chloro-3,5-dinitro-1H-pyrazol-1-yl)ethane was chosen as the precursor, and 4,4′-(ethane-1,2-diyl)bis(6-nitropyrazolo[3,4-d][1,2,3]triazole) (8) was synthesized via a similar cyclization reaction as 7. In addition, the energetic salts 7-1 to 7-6 and 8-1 to 8-7 were prepared through metathesis reactions. These salts exhibit good detonation properties with relatively low sensitivities. The overall energetic evaluation highlights the fused pyrazolo[3,4-d][1,2,3]triazole ring as a useful framework for building advanced HEDMs (Table 5).
| No. | T d, °C | d, g cm−3 | D, m s−1 | P, GPa | HOF, kJ mol−1/kJ g−1 | OB, % | IS, J | FS, N | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 7 | 101 | 1.70 | 8183 | 27.6 | 414.4/2.25 | −60.83 | 6 | — | 18 |
| 7-1 | 146 | 1.71 | 8724 | 30.9 | 549.6/2.54 | −85.51 | 8 | — | 18 |
| 7-2 | 144 | 1.67 | 8341 | 26.3 | 393.1/1.96 | −87.93 | 15 | 120 | 9 |
| 7-3 | 112 | 1.76 | 8859 | 31.9 | 439.4/2.02 | −74.02 | 8 | 120 | 9 |
| 7-4 | 192 | 1.62 | 7911 | 22.5 | 371.0/1.53 | −92.49 | 40 | 360 | 9 |
| 7-5 | 181 | 1.64 | 8196 | 24.3 | 485.3/1.88 | −90.20 | 25 | 240 | 9 |
| 7-6 | 188 | 1.74 | 8519 | 27.5 | 916.4/2.71 | −87.77 | 15 | 160 | 9 |
| 8-1 | 122 | 1.69 | 8570 | 28.2 | 1096.3/2.55 | −63.24 | 6 | 80 | 9 |
| 8-2 | 85 | 1.70 | 8115 | 24.9 | 1254.9/2.35 | −74.85 | 20 | 160 | 9 |
| 8-3 | 155 | 1.63 | 7865 | 22.4 | 774.4/1.27 | −72.67 | 40 | 360 | 9 |
| 8-4 | 162 | 1.61 | 7979 | 23.0 | 1021.4/1.99 | −71.54 | 15 | 240 | 9 |
| 8-5 | 143 | 1.65 | 8344 | 25.6 | 1257.7/2.31 | −70.53 | 25 | 360 | 9 |
| 8-6 | 168 | 1.66 | 8526 | 26.7 | 1452.0/2.53 | −69.63 | 30 | 360 | 9 |
| 8-7 | 101 | 1.75 | 8698 | 31.3 | 881.9/2.04 | −51.82 | 10 | 160 | 9 |
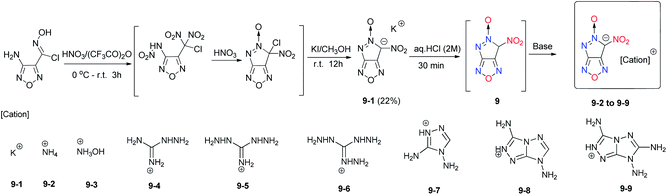
Compounds 1 to 8 are all azole-based [5,5]-bicyclic heterocycles. The addition of a furazan group to the fused ring should improve the oxygen balance and increase the heat of formation of the energetic materials. To date, limited furazan-containing [5,5]-bicyclic heterocyclic energetic materials were reported. By nitration of adjacent chlorohydroximoyl and amino groups followed by KI reduction, the potassium salt of 6-nitro-6H-pyrazolo[3,4-c]furazan-5-oxide (9-1) was synthesized(Scheme 7).19 Neutral compound 9 containing the fused pyrazolo[3,4-c]furazan N-oxide was obtained in situ (not isolated) by acidifying 9-1. Subsequently, the energetic salts 9-2 to 9-9 were obtained with excellent detonation properties (D = 9174 m s−1 and P = 39.1 GPa) but are highly sensitive to impact and friction. Therefore, 9-3 is a potential candidate for use as a primary explosive. Additionally, 9-6 and 9-9 are promising with respect to their detonation properties, thermal stability and sensitivity to impact (Table 6).
| No. | T m, °C | T d, °C | d, g cm−3 | D, m s−1 | P, GPa | HOF, kJ mol−1/kJ g−1 | OB, % | IS, J | FS, N | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 9-1 | — | 175 | 2.04 | 7973 | 28.6 | 159.0/0.76 | −15.30 | 2 | 40 | 19 |
| 9-2 | — | 179 | 1.80 | 8777 | 33.6 | 285.9/1.52 | −34.02 | 3 | 60 | 19 |
| 9-3 | — | 152 | 1.87 | 9174 | 39.1 | 336.8/1.65 | −23.52 | 2 | 40 | 19 |
| 9-4 | — | 163 | 1.69 | 8343 | 27.2 | 375.1/1.53 | −48.95 | 12 | 120 | 19 |
| 9-5 | 106 | 131 | 1.75 | 8741 | 30.0 | 478.7/1.84 | −49.20 | 10 | 120 | 19 |
| 9-6 | 119 | 141 | 1.76 | 8957 | 31.6 | 591.7/2.15 | −49.42 | 15 | 240 | 19 |
| 9-7 | 151 | 153 | 1.77 | 8545 | 29.2 | 351.6/1.22 | −53.30 | 18 | 240 | 19 |
| 9-8 | 105 | 164 | 1.76 | 8540 | 29.1 | 630.2/1.92 | −56.74 | 15 | 240 | 19 |
| 9-9 | — | 186 | 1.81 | 8741 | 30.8 | 809.7/2.49 | −56.58 | 20 | 360 | 19 |
[5,6]-Bicyclic and [6,6]-bicyclic heterocyclic energetic materials
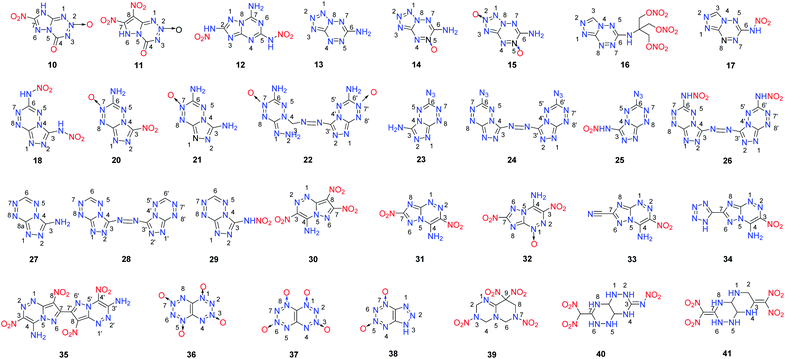
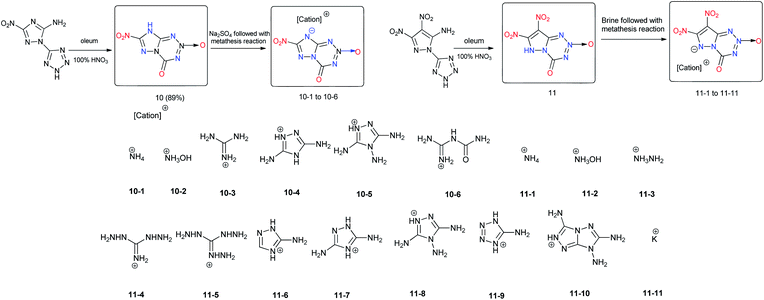
In contrast to [5,5]-bicyclic heterocyclic energetic materials, energetic materials with [5,6]-bicyclic and [6,6]-bicyclic heterocyclic backbones feature a 6-membered ring that can either increase the nitrogen content or provide positions where additional energetic functional groups can be introduced. Approximately 30 [5,6]-bicyclic and [6,6]-bicyclic heterocyclic energetic materials were found (Scheme 8).A promising approach for the synthesis of 7-nitro-4-oxo-4,8-dihydro-[1,2,4]triazolo[5,1-d][1,2,3,5]tetrazine 2-oxide (10) was suggested.20 The proposed pathway for the formation of the fused ring of [1,2,4]triazolo[5,1-d][1,2,3,5]tetrazine involves the cleavage of a tetrazole ring and the cyclization of a C–N bond with a nitro group (Scheme 9). Compound 10 is highly hygroscopic, but this limitation was overcome by transforming it into its energetic salts 10-1 to 10-6 through neutralization reactions. The densities of these salts fall between 1.77 to 1.97 g cm−3.
These energetic salts are thermally stable, most of them with decomposition temperatures above 230 °C, which tend to be insensitive to external stimuli. Their detonation pressures and velocities are within the ranges 25.2 to 39.5 GPa and 7856 to 9069 m s−1, respectively. The hydroxylammonium salt 10-2 exhibits satisfactory density (1.97 g cm−3), high thermal stability (Td = 197 °C), low sensitivities (IS > 40 J, FS = 324 N), and excellent detonation velocity (9069 m s−1) and pressure (39.5 GPa), making it superior to RDX (Table 7). The combination of high performance and low sensitivity of 10-2 highlights its potential to be used as an HEDM. This strategy of building fused [1,2,4]triazolo[5,1-d][1,2,3,5]tetrazine rings provides an efficient approach for designing stable HEDMs with low sensitivities. 7,8-Dinitro-4-oxo-4,6-dihydropyrazolo[5,1-d][1,2,3,5]tetrazine 2-oxide (11) was prepared in a one-pot reaction via N-nitration followed by a ring closure reaction, which is similar to 10. In addition, a series of salts 11-1 to 11-11 was prepared. Comparing the structures of 10 and 11 reveals that the only difference is that the pyrazole ring in 11 is replaced by the triazole ring in compound 10, which makes it possible to introduce one more nitro group into compounds and to give 11 a higher oxygen balance and density. Among the energetic salts of 11, the hydroxylammonium salt 11-2 exhibits the best detonation properties (D = 9228 m s−1 and P = 39.4 GPa) due to high density (1.92 g cm−3), and low sensitivity (IS = 19 J, FS = 360 N) derived from the coplanar structure of its fused ring system (Scheme 9).21
| No. | T m, °C | T d, °C | d, g cm−3 | D, m s−1 | P, GPa | HOF, kJ mol−1/kJ g−1 | OB, % | IS, J | FS, N | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 10-1 | — | 249 | 1.77 | 8252 | 29.0 | 180.6/0.84 | −29.61 | >40 | 324 | 20 |
| 10-2 | — | 197 | 1.97 | 9069 | 39.5 | 218.6/0.94 | −20.68 | >40 | 324 | 20 |
| 10-3 | 266 | 269 | 1.78 | 8113 | 27.1 | 151.8/0.59 | −43.38 | >40 | 324 | 20 |
| 10-4 | — | 252 | 1.89 | 8463 | 30.7 | 350.8/1.18 | −48.29 | >40 | 360 | 20 |
| 10-5 | 213 | 237 | 1.81 | 8374 | 29.2 | 475.6/1.52 | −48.53 | >40 | 324 | 20 |
| 10-6 | 208 | 241 | 1.80 | 7856 | 25.2 | −55.6/−0.18 | −45.16 | >40 | >360 | 20 |
| 11 | — | 243 | 2.02 | 8515 | 33.2 | 131.8/0.50 | −16.45 | 2.5 | 144 | 21 |
| 11-1 | — | 237 | 1.88 | 8836 | 35.0 | 180.1/0.69 | −24.60 | 7 | 360 | 21 |
| 11-2 | — | 171 | 1.95 | 9228 | 39.4 | 226.2/0.82 | −17.38 | 19 | 360 | 21 |
| 11-3 | — | 207 | 1.79 | 8674 | 32.7 | 336.8/1.22 | −26.17 | 6.5 | 168 | 21 |
| 11-4 | — | 201 | 1.68 | 8157 | 26.2 | 364.6/1.10 | −38.53 | 18 | 252 | 21 |
| 11-5 | — | 183 | 1.68 | 8293 | 26.9 | 475.2/1.37 | −39.17 | 8 | 216 | 21 |
| 11-6 | — | 217 | 1.74 | 8149 | 27.0 | 394.4/1.20 | −41.57 | 9 | 360 | 21 |
| 11-7 | — | 212 | 1.82 | 8467 | 29.9 | 353.5/1.03 | −42.08 | 25 | 360 | 21 |
| 11-8 | — | 207 | 1.80 | 8387 | 28.6 | 475.2/1.33 | −42.55 | 18 | 360 | 21 |
| 11-9 | — | 184 | 1.76 | 8499 | 30.2 | 564.3/1.72 | −29.25 | 4 | 240 | 21 |
| 11-10 | — | 232 | 1.80 | 8485 | 29.4 | 698.0/1.76 | −46.32 | 15 | 360 | 21 |
| 11-11 | — | 315 | 1.97 | 8008 | 28.0 | 34.7/0.12 | −11.38 | 10 | 240 | 21 |
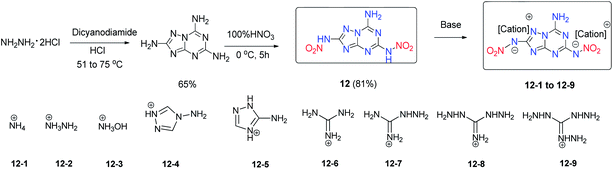
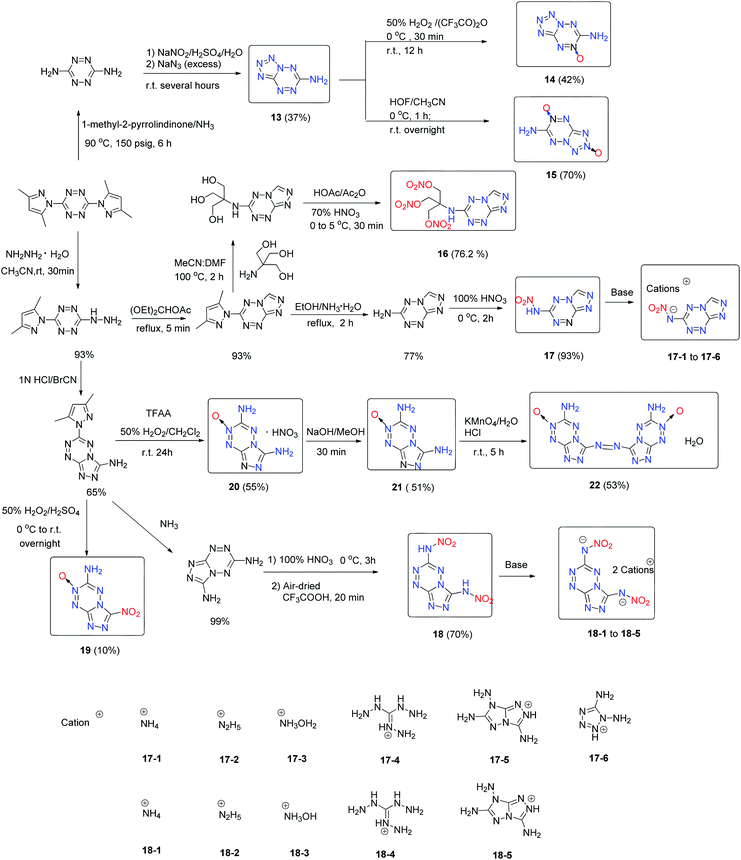
2,5-Dinitramide-7-amino-[1,2,4]triazolo[1,5-a][1,3,5]triazine (12), which contains a fused framework of a triazolo-triazine ring with one amino and two nitramino groups (Scheme 10), was obtained.22 Its nitrogen-rich energetic salts (12-1 to 12-9) were prepared through neutralization and metathesis reactions. Featuring a planar structure, a conjugated system and hydrogen bond interactions originating from the amino groups in the fused ring framework, most of the energetic salts of 12 have high thermal stability and with good detonation performance. In particular, compounds 12-2 and 12-3 exhibit high detonation performance (D = 9313, and 9088 m s−1; P = 33.9, and 34.1 GPa) comparable to that of the high explosive HMX and with low sensitivity (IS > 40 J; FS > 360 N) similar to that of TATB, causing them to stand out as some of the few examples featuring a good balance between high energy level and stability (Table 8). Thus, 12-2 and 12-3 are promising candidates as insensitive HEDMs. Additionally, the good stabilities and detonation properties of these amino-nitramino fused heterocycles indicate a potential design strategy for insensitive HEDMs. The 1,2,4,5-tetrazine ring has a high nitrogen content and has been used as a building block in the design and synthesis of energetic materials. However, the tetrazine-fused ring system has been relatively unexploited for use in energetic materials. Recently, the groups of Shreeve and Chavez have synthesized and characterized the oxidation products of tetrazolo[1,5-b][1,2,4,5]-tetrazine-6-amine (13). The synthesis of 6-amino-tetrazolo[1,5-b]-1,2,4,5-tetrazine-5-oxide (14) through the oxidation of 6-amino-[1,5-b]tetrazolo-1,2,4,5-tetrazine (13) by the H2O2/(CF3CO)2O system was realized (Scheme 11).23 Following the study of the oxidation of 13, treatment of 13 with hypofluorous acid (HOF), which is a stronger oxidant than the H2O2/(CF3CO)2O system, was carried out, and it was found that in addition to 14, 6-amino-[1,5-b]tetrazolo-1,2,4,5-tetrazine-2,5-dioxide (15) was also formed.24 In terms of detonation properties, 14 (D = 9326 m s−1 and P = 36.4 GPa) is superior to RDX and 15 (D = 9600 m s−1 and P = 41.3 GPa) is superior to HMX (Table 9). For 15, the addition of the second N-oxide group makes its impact sensitivity slightly higher than that of 14. Compound 15 exhibits a good detonation velocity of 9600 m s−1 and a detonation pressure of 41.3 GPa. Because of the fused ring system and the N-oxide group in the molecules, the N-oxide derivatives of tetrazines 14 and 15 exhibit more promising energetic performances and better sensitivities than some of their known derivatives such as 3,6-diazido-1,2,4,5-tetrazine, 2,4,6-tri(azido)-1,3,5-triazine, and 4,4′,6,6′-tetra(azido)azo-1,3,5-triazine. This result suggests the potential application of 14 and 15 as HEDMs. Accordingly, several N-oxide derivatives with fused tetrazine rings were synthesized recently, which include 3-nitro-6-amino-1,2,4-triazolo[4,3-b][1,2,4,5]tetrazine-7-N-oxide (19),25 3,6-diamino-1,2,4-triazolo[4,3-b][1,2,4,5]tetrazine-7-N-oxide nitrate (20),25 3,6-diamino-1,2,4-triazolo[4,3-b][1,2,4,5]tetrazine-7-N-oxide (21)25 and 6,6′-diamino-3,3′-azo-1,2,4-triazolo[4,3-b][1,2,4,5]tetrazine-7,7′-N,N-dioxide (22) (Scheme 11).25
| No. | T d, °C | d, g cm−3 | D, m s−1 | P, GPa | HOF, kJ mol−1/kJ g−1 | OB, % | IS, J | FS, N | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 12 | 189 | 1.84 | 8787 | 34.2 | 544.9/2.13 | −37.48 | >40 | >360 | 22 |
| 12-1 | 165 | 1.77 | 8791 | 29.7 | 386.1/1.33 | −49.62 | >40 | >360 | 22 |
| 12-2 | 194 | 1.78 | 9313 | 33.9 | 754.6/2.36 | −49.96 | >40 | >360 | 22 |
| 12-3 | 199 | 1.79 | 9088 | 34.1 | 528.8/1.64 | −34.76 | >40 | >360 | 22 |
| 12-4 | 170 | 1.70 | 8445 | 27.0 | 1245.3/2.93 | −67.87 | >40 | >360 | 22 |
| 12-5 | 221 | 1.60 | 7823 | 22.1 | 1002.3/2.36 | −67.87 | >40 | >360 | 22 |
| 12-6 | 197 | 1.61 | 7824 | 21.5 | 396.5/1.06 | −64.12 | >40 | >360 | 22 |
| 12-7 | 209 | 1.67 | 8500 | 26.3 | 797.6/1.97 | −63.32 | >40 | >360 | 22 |
| 12-8 | 176 | 1.64 | 8440 | 25.6 | 854.8/1.97 | −62.62 | >40 | >360 | 22 |
| 12-9 | 196 | 1.63 | 8546 | 26.4 | 1085.0/2.34 | −62.02 | >40 | >360 | 22 |
| No. | T d, °C | d, g cm−3 | D, m s−1 | P, GPa | HOF, kJ mol−1/kJ g−1 | OB, % | IS, J | FS, N | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 13 | 200 | 1.68 | 8449 | 27.0 | 686.2/4.97 | −57.93 | 1.5 | 15 | 23 |
| 14 | 185 | 1.87 | 9326 | 36.4 | 631.4/4.09 | −41.53 | 10 | 60 | 23 |
| 15 | 150 | 1.93 | 9600 | 41.3 | 576.0/3.39 | −28.22 | 6 | 109 | 24 |
| 16 | 155 | 1.79 | 8290 | 29.4 | 246.0/0.65 | −38.28 | 3.9 | 94 | 26 |
| 17 | 148 | 1.76 | 8621 | 30.4 | 675.1/3.71 | −43.93 | 20 | >240 | 27 |
| 17-1 | 193 | 1.76 | 8937 | 31.6 | 649.1/3.26 | −52.23 | 20 | >240 | 27 |
| 17-2 | 133 | 1.77 | 9216 | 33.9 | 802.1/3.75 | −52.30 | 12 | 240 | 27 |
| 17-3 | 156 | 1.82 | 9276 | 36.1 | 698.1/3.24 | −40.91 | 28 | >240 | 27 |
| 17-4 | 152 | 1.79 | 8748 | 28.4 | 959.5/3.35 | −61.49 | 38 | >360 | 27 |
| 17-5 | 155 | 1.76 | 9085 | 32.8 | 1233.6/3.67 | −66.62 | 17 | >360 | 27 |
| 17-6 | 248 | 1.80 | 8916 | 30.9 | 1176.4/4.17 | −51.03 | >40 | >360 | 27 |
| 18 | 138 | 1.91 | 9301 | 38.3 | 740.9/3.06 | −19.82 | 3 | >5 | 27 |
| 18-1 | 213 | 1.84 | 9301 | 35.4 | 543.5/1.97 | −34.76 | 32 | >360 | 27 |
| 18-2 | 154 | 1.82 | 9625 | 37.9 | 871.8/2.85 | −36.58 | 13 | >240 | 27 |
| 18-3 | 154 | 1.92 | 9712 | 42.9 | 638.9/2.07 | −20.77 | 25 | >360 | 27 |
| 18-4 | 150 | 1.70 | 9061 | 30.8 | 1301.6/2.89 | −53.29 | 30 | >360 | 27 |
| 18-5 | 244 | 1.76 | 8792 | 29.8 | 1780.9/3.24 | −61.05 | >40 | >360 | 27 |
| 19 | 220 | 1.86 | 9384 | 39.1 | 744.0/3.76 | −32.31 | 25 | 240 | 25 |
| 20 | 191 | 1.81 | 8808 | 30.1 | 502.3/2.76 | −31.15 | >40 | >40 | 25 |
| 21 | 196 | 1.82 | 9008 | 34.7 | 492.0/2.13 | −66.62 | >40 | >360 | 25 |
| 22·H2O | 210 | 1.82 | 8542 | 28.8 | 1062/2.99 | −57.79 | 22 | 40 | 25 |
| 23 | 165 | 1.74 | 8581 | 28.5 | 913.6/5.13 | −62.88 | 30 | >45 | 28 |
| 24 | 183 | 1.77 | 8690 | 30.2 | 2194.6/6.23 | −54.51 | 1 | <5 | 28 |
| 25 | 150 | 1.85 | 9236 | 36.3 | 951.8/4.27 | −32.27 | 1 | >40 | 28 |
| 25-1 | 180 | 1.77 | 9149 | 34.3 | 1055.6/4.40 | −39.98 | 1 | >160 | 28 |
| 25-2 | 170 | 1.76 | 8879 | 31.0 | 1026.0/3.64 | −51.03 | 13 | >160 | 28 |
| 25-3 | 156 | 1.79 | 9289 | 36.8 | 1106.3/4.32 | −31.23 | 2 | >100 | 28 |
| 25-4 | 129 | 1.74 | 9180 | 32.7 | 1352.6/4.13 | −51.34 | 1 | >40 | 28 |
| 26 | 170 | 1.85 | 9500 | 39.8 | 2037.8/5.22 | −36.90 | 14 | <5 | 27 |
| 26-1 | 240 | 1.84 | 9566 | 38.8 | 2014.7/4.75 | −28.35 | 10 | >40 | 27 |
| 26-2 | 185 | 1.99 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 233 233 |
48.6 | 2099.3/4.60 | −35.07 | 14 | >10 | 27 |
| 28 | 305 | 1.91 | 9200 | 34.8 | 1525.2/5.64 | −76.99 | 16 | >360 | 28 |
| 29 | 132 | 1.81 | 8880 | 33.2 | 647.8/3.56 | −43.93 | 31 | >240 | 28 |
| 29-1 | 193 | 1.79 | 9129 | 33.4 | 705.0/3.54 | −52.23 | 33 | >360 | 28 |
| 29-2 | 150 | 1.83 | 9408 | 37.8 | 751.2/3.49 | −40.91 | 43 | >360 | 28 |
| 29-3 | 280 | 1.79 | 8932 | 30.7 | 678.6/2.81 | −63.03 | 50 | >360 | 28 |
| 29-4 | 123 | 1.79 | 9416 | 35.7 | 858.4/4.01 | −52.30 | 32 | >360 | 28 |
| 29-5 | 156 | 1.78 | 8486 | 28.0 | 1009.7/3.10 | −61.49 | 8 | >240 | 28 |
Through using nitrate as a functional group to modify the fused tetrazine backbone, 6-[tris(hydroxymethyl)aminomethane trinitrate]-1,2,4-triazolo-[4,3-b]-1,2,4,5-tetrazine (16) was prepared by the nitration of the intermediate 6-[tris(hydroxymethyl)-aminomethane]-1,2,4-triazolo[4,3-b]1,2,4,5-tetrazine (Scheme 11).26 Intermolecular hydrogen bonding with the N–H group in 16 leads to a relatively high density (1.79 g cm−3). However, low thermal, impact and friction stabilities were observed because of the trinitrate group in the molecule (Table 9), which limits its practical application as an HEDM.26
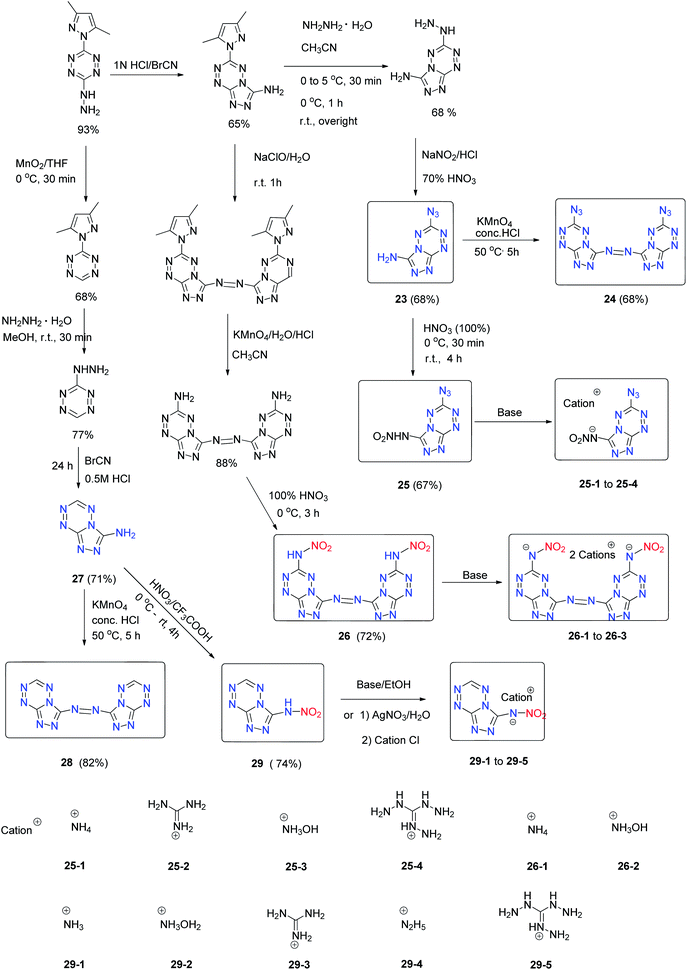
Azide and nitramino groups were used to modify the fused tetrazine ring as well. 3-Amine-6-azido-1,2,4-triazolo[4,3-b][1,2,4,5]tetrazine (23),26 6,6′-diazide-3,3′-azo-1,2,4-triazolo[4,3-b][1,2,4,5]tetrazine (24),26 and 3-nitroamino-6-azido-[1,2,4]triazolo[4,3-b][1,2,4,5]tetrazine (25)26 were synthesized. 6-Nitroamino-1,2,4-triazolo[4,3-b][1,2,4,5]tetrazine (17) (Scheme 11), 3,6-dinitramino-1,2,4-triazolo[4,3-b][1,2,4,5]tetrazine (18) (Scheme 11), 6,6′-dinitramino-3,3′-azo-1,2,4-triazolo[4,3-b][1,2,4,5]tetrazine (26) and 3-nitroamino-1,2,4-triazolo[4,3-b][1,2,4,5]tetrazine (29) and their energetic salts were prepared (Scheme 12).27
Some of these nitroamino-functionalized 1,2,4-triazolo[4,3-b][1,2,4,5]-tetrazine fused rings exhibit excellent detonation properties; for example, the sensitivities of all the hydroxylammonium salts are lower than those of their neutral precursors, such as compound 18 (3 J, >5 N) and compound 18-3 (25 J, 360 N) (Table 9). The detonation properties of 18-3 (D = 9712 m s−1, P = 43 GPa) and 26-2 (D = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 233 m s−1; P = 49 GPa) exceed those of the present high explosive benchmarks, such as HMX and CL-20. This result indicates that the fused 1,2,4-triazolo[4,3-b][1,2,4,5]tetrazine ring system provides a balance between desirable detonation performances and acceptable stabilities and offers a promising approach to the design of low-sensitivity high-energy materials.
233 m s−1; P = 49 GPa) exceed those of the present high explosive benchmarks, such as HMX and CL-20. This result indicates that the fused 1,2,4-triazolo[4,3-b][1,2,4,5]tetrazine ring system provides a balance between desirable detonation performances and acceptable stabilities and offers a promising approach to the design of low-sensitivity high-energy materials.
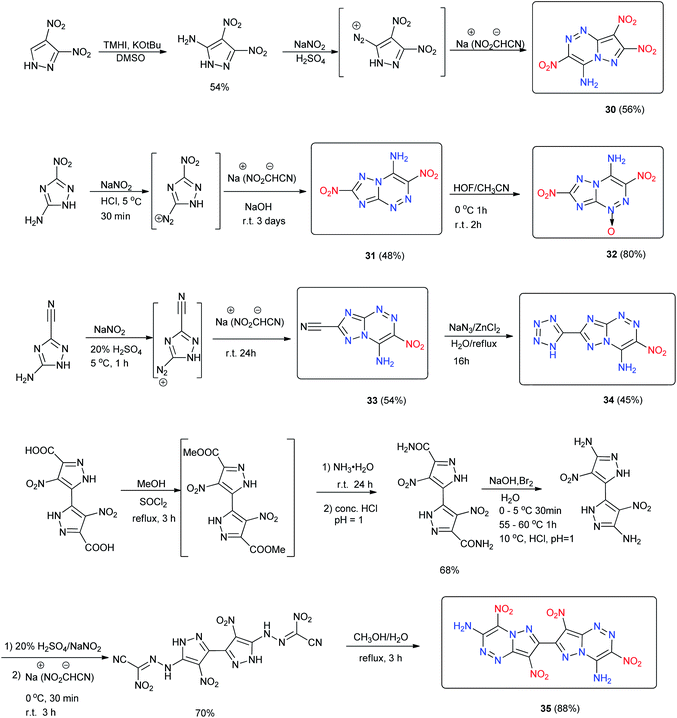
Similar to 24 and 26, 1,2-bis([1,2,4]triazolo[4,3-b][1,2,4,5]tetrazin-3-yl)diazene (28) was synthesized with an azo-bridge (Scheme 12). Compound 28 has a high measured density of 1.91 g cm−3, an excellent thermal stability (Td = 305 °C), and a very good calculated detonation performance (D = 9200 m s−1 and P = 34.8 GPa), which outperforms all current heat-resistant explosives. It has significant potential as a heat-resistant explosive. Azocoupling of diazopyrazole with nucleophiles is an efficient way to generate a fused ring and was studied in detail. This approach has proven to be an efficient way to generate amino-nitro fused systems with a pyrazolo-[5,1-c][1,2,4]triazine backbone for designing energetic materials.27 4-Amino-3,7,8-trinitropyrazolo-[5,1-c][1,2,4]triazine (30) was obtained (Scheme 13).29 The crystal density of compound 30 is 1.95 g cm−3 and it exhibits HMX-like performance with low sensitivity and good thermal stability, mainly due to the intra- and intermolecular hydrogen bonding in its structure. The notable combination of high calculated performance and low sensitivity makes 30 a promising explosive candidate (Table 10).29
| No. | T d, °C | d, g cm−3 | D, m s−1 | P, GPa | HOF, kJ mol−1/kJ g−1 | OB, % | IS, J | FS, N | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 30 | 246 | 1.95 (300 K) | 8998 | 36.0 | 370.0/1.37 | −29.62 | 58.4 | 324–360 | 29 |
| 31 | 232 | 1.86 | 8700 | 32 | 387.0/1.71 | −35.38 | 29 | >360 | 30 |
| 272 | 1.82 | 8580 | 31.2 | 403.5/1.78 | >60 | >360 | 31 | ||
| 32 | 138 | 1.90 (predicted) | 8970 | 35.4 | 378.0/1.56 | −26.43 | 10.3 | 258 | 30 |
| 33 | 229 | 1.81 | 7763 | 23.1 | 533.5/3.25 | −69.86 | — | — | 32 |
| 34 | 305 | 1.82 | 8312 | 27.0 | 752.7/3.02 | −61.01 | >80 | >360 | 32 |
| 35 | 315 | 1.85 | 8572 | 31.4 | 899.0/2.01 | −49.97 | >60 | >360 | 33 |
Following the synthesis of 30, 4-amino-3,7-dinitrotriazolo-[5,1-c][1,2,4]triazine (31) was obtained by reacting nitroacetonitrile with 5-amino-3-nitro-1H-1,2,4-triazole,23,26 and further study by the oxidation of 31 with hypofluorous acid led to its oxidation product, 4-amine-3,7-dinitro-[1,2,4]triazolo[5,1-c][1,2,4]triazine-1-oxide (32) (Scheme 13). Compounds 31 and 32 display similar or better performance than RDX, and also show excellent insensitivity toward mechanical stimuli (Table 10).
4-Amino-3-nitro-[1,2,4]triazolo[5,1-c][1,2,4]triazine-7-carbo-nitrile (33) was discovered by the diazotization of 3-amino-5-cyano-1,2,4-triazole followed by reaction with nitroacetonitrile (Scheme 13). The reaction of 33 with sodium azide resulted in the cyclization of the carbonitrile group to give 3-nitro-7-(1H-tetrazol-5-yl)-[1,2,4]triazolo[5,1-c][1,2,4]triazin-4-amine (34) (Scheme 13). Compound 34 is a thermally stable compound (Td > 305 °C) with a density of 1.819 g cm−3. It has good detonation properties (D = 8312 m s−1, P = 27.04 GPa), and low sensitivity to impact, friction, and electrical discharge, making it an attractive candidate for replacing TATB (Scheme 13).
Using a similar strategy, 7,7′-bridged-bis-(4-amino-3,8-dinitropyrazolo-[5,1-c][1,2,4]triazine) (35) was synthesized from diethyl oxalate and acetone.33 Compound 35 exhibits excellent thermal stability (Td = 315 °C), high density (1.85 g cm−3), good detonation properties and insensitivity to impact and friction with potential as a highly thermally stable HEDM.
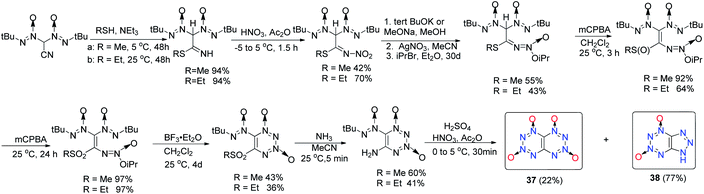
Compared with [5,6]-bicyclic heterocyclic energetic materials, [6,6]-bicyclic heterocyclic energetic materials are limited, but earlier theoretical studies predict that [1,2,3,4]tetrazino[5,6-e][1,2,3,4]tetrazine-1,3,5,7-tetraoxide (36) and [1,2,3,4]tetrazino[5,6-e][1,2,3,4]tetrazine-1,3,6,8-tetraoxide (37) will match or substantially outperform CL-20 (Scheme 8).34 Compound 37 has excellent theoretical detonation properties (D = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 m s−1 and P = 60.0 GPa). As a result, their syntheses were desirable targets for chemists and have attracted considerable interest (Scheme 14).35
900 m s−1 and P = 60.0 GPa). As a result, their syntheses were desirable targets for chemists and have attracted considerable interest (Scheme 14).35
Compound 37 was prepared through a ten step series of reactions with 2,2-bis(tert-butyl-NNO-azoxy)acetonitrile as the starting material (Scheme 14).36,37 1H-1,2,3-triazolo[4,5-e][1,2,3,4]tetrazine-5,7-dioxide (38) was also found as an unexpected byproduct, which lowered the yield of compound 37 to <1%. Compound 37 has acceptable thermal stability with a melting point between 183–186 °C (decomposition); however, its low hydrolytic stability may limit its potential application as a HEDM.37
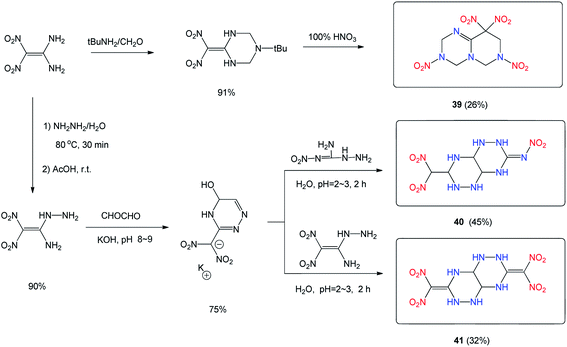
Apart from its excellent detonation and safety properties and great promise for applications, 1,1-diamino-2,2-dinitroethene (FOX-7) also shows exciting and unpredictable reaction chemistry in building fused ring energetic materials. In 2019, Zhang's group synthesized a novel fused-ring polynitro compound 3,4,6,7,8,9-hexahydro-3,7,9,9-tetranitro-3,7,9,9-2H-pyrimido[1,6-a]-1,3,5-triazine (39) which features dinitromethylene through two-step reactions from FOX-7 (Scheme 15).38 Compound 39 exhibits a high crystal density of 1.86 g cm−3, a good thermal decomposition temperature of 176 °C, high detonation performances comparable to RDX, and low mechanical sensitivities.38
The condensation of the hydrazine derivative of FOX-7, (1-amino-1-hydrazino-2,2-dinitroethene, H-FOX) with glyoxal in aqueous KOH solution gives the 3-(dinitromethyl)-1,2,4-triazine potassium salt, which when reacted with 1-nitro-3-aminoguanidine, forms the 3-nitrimino-7-dinitromethylene-octahydro-[1,2,4]triazino-[6,5-e][1,2,4]triazine (40).39 A similar reaction of 3-(dinitromethyl)-1,2,4-triazine potassium salt with 1-amino-1-hydrazino-2,2-dinitroethene led to 3,7-bis(dinitromethylene)-octahydro-[1,2,4]triazino-[6,5-e][1,2,4]triazine (41),39 which exhibits better detonation properties (density 1.85 g cm3; pressure, 35.2 GPa, velocity, 8862 m s−1, IS = 7.5 J) than 40, and compares favorably with (RDX), and thus may have potential as a HEDM (Table 11).
[5,6,5]- or [5,7,5]-Tricyclic heterocycle-based energetic materials
Tricyclic heterocycle-based energetic materials have a greater scope in selecting different rings in their backbones. Usually, the generation of the third ring through a ring closure reaction is the main method in building the tricyclic backbones of these compounds, which makes most of them symmetric (Scheme 16). 4,4′,5,5′-Tetranitro-2H,2′H-3,3′-bipyrazole, 5,5′-dinitro-2H,2′H-3,3′-bi-1,2,4-triazole,4,4′,5,5′-tetranitro-2H,2′H-bisimidazole and their derivatives were synthesized as energetic materials. Recently, these compounds have become attractive building blocks for fused tricyclic energetic compounds containing the 1,2,3,4-tetrazine unit.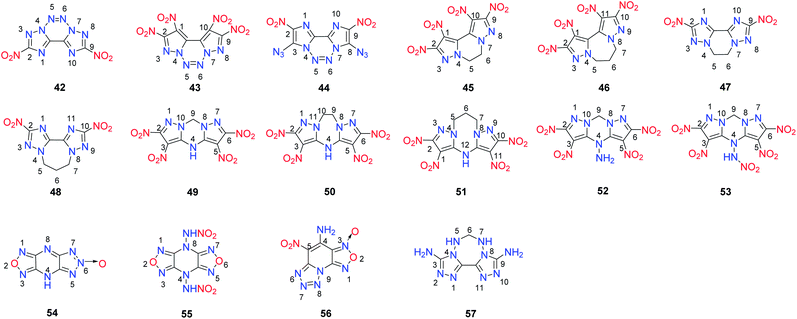 | ||
| Scheme 16 [5,6,5]-Tricyclic, [5,7,5]-tricyclic and [5,8,5]-tricyclic heterocyclic energetic materials. | ||
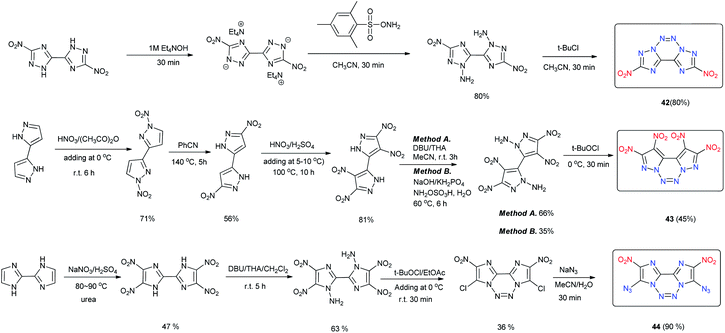
The fused tricyclic compound, 2,9-dinitro-bis[1,2,4]triazolo[1,5-d:5′,1′-f][1,2,3,4]tetrazine (42) was synthesized through the amination of 5,5′-dinitro-2H,2′H-3,3′-bi-1,2,4-triazole followed by N-azo coupling by oxidizing the product with tert-butyl hypochlorite (t-BuOCl) (Scheme 17).40 Calculations indicate that 42 is a highly energetic material that is similar to RDX with respect to its sensitivity properties. However, its low thermal stability (Td = 138 °C) limits its practical application (Table 12). The low thermal stability of 42 may be the result of the conjugation of the fused backbone and the nature of the pyrazole ring. Using a similar strategy as that employed for 42, 4,4′,5,5′-tetranitro-2H,2′H-3,3′-bipyrazole was converted into 1,2,9,10-tetranitro-dipyrazolo[1,5-d:5′,1′-f][1,2,3,4]tetrazine (43) (Scheme 17).41 Compound 43 exhibited good thermal stability (Td = 233 °C) and high density (d = 1.96 g cm−3). Its detonation properties (D = 9631 m s−1; P = 44.0 GPa) are superior to those of RDX and HMX and comparable to those of CL-20. Furthermore, 42 is more stable toward impact and friction than CL-20. The excellent properties of 43 indicate that it is a superior HEDM, and the method for building the backbone of 43 provides a potential design concept for introducing the 1,2,3,4-tetrazine moiety into a fused ring system. Attempts to design and synthesize different conjugated tricyclic fused energetic molecules by using 4,4′,5,5′-tetranitro-2H,2′H-bisimidazole as the starting material have been made. In contrast to the synthesis of 43, the oxidation reaction of 4,4′,5,5′-tetranitro-2,2′-diamino-bisimidazole with tBuOCl led to a dichloro-substituted tricyclic fused ring product with an N-azo structure.42 When the chlorine atom was replaced with an azide group, 3,8-diazido-2,9-dinitrodiimidazo[1,2-d:2′,1′-f]-[1,2,3,4]tetrazine (44) was formed (Scheme 17). It exhibits high density (1.89 g cm−3) and excellent detonation properties (D = 9256 m s−1; P = 36.7 GPa). The high sensitivities of 44 toward impact and friction suggest it as a promising primary explosive.
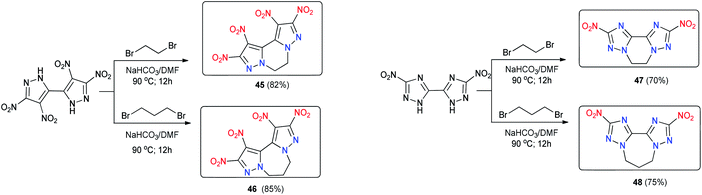
The tricyclic energetic molecules 1,2,9,10-tetranitro-5,6-dihydro-dipyrazolo[1,5-a:50,10-c]pyrazine (45), 1,2,10,11-tetranitro-6,7-dihydro-5H-dipyrazolo[1,5-a:5′,1′-c][1,4]diazepine (46), 2,9-dinitro-5,6-dihydro-bis([1,2,4]triazolo)[1,5-a:5′,1′-c]pyrazine (47), and 2,10-dinitro-6,7-dihydro-5H-bis([1,2,4]triazolo)[1,5-a:50,10-c][1,4]diazepine (48) were prepared through the ring closure of the sodium salts of 4,4′,5,5′-tetranitro-2H,2′H-3,3′-bipyrazole and 5,5′-dinitro-2H,2′H-3,3′-bi-1,2,4-triazole with an N,N′-ethylene bridge (45 and 47) or an N,N′-propylene bridge (46 and 48) (Scheme 18).
The new compounds have excellent thermal stabilities, with 48 having the highest decomposition temperature at 328 °C (Table 13). The incorporation of an N,N′-ethylene/propylene bridge into polynitroazoles improves their thermal stabilities. These compounds exhibit better detonation performance than TNT, and the performance of 45 is comparable to that of TATB. Their sensitivities toward impact and friction are also better than TNT. Their good detonation performance and, remarkable high thermal stability and “green” nitrogen-rich backbone make them potential candidates in the field of HDEMs. This ring closure strategy may open a route to the design of highly thermally stable energetic compounds.
| No. | T m, °C | T d, °C | d, g cm−3 | D, m s−1 | P, GPa | HOF, kJ mol−1/kJ g−1 | OB, % | IS, J | FS, N | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 45 | 233 | 261 | 1.76 | 8135 | 28.1 | 326.6/0.96 | −47.04 | 15 | >360 | 44 |
| 46 | 278 | 280 | 1.68 | 7700 | 24.1 | 280.1/0.79 | −58.72 | 28 | >360 | 44 |
| 47 | 304 | 307 | 1.73 | 7838 | 24.1 | 345.3/1.37 | −63.45 | 22 | >360 | 44 |
| 48 | 325 | 328 | 1.63 | 7336 | 19.7 | 307.2/1.15 | −78.14 | 30 | >360 | 44 |
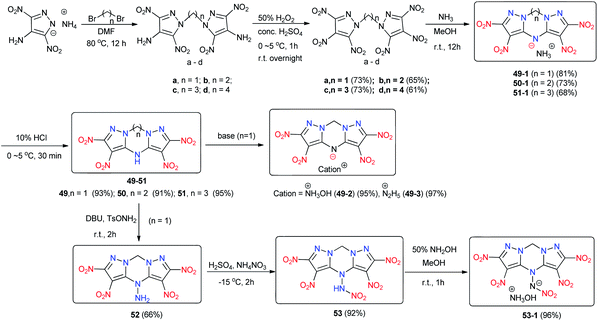
A concise methodology for constructing the fused compounds 2,3,5,6-tetranitro-4H,9H-dipyrazolo[1,5-a:5′,1′-d][1,3,5]triazine (49), 2,3,5,6-tetranitro-9,10-dihydro-4H-dipyrazolo[1,5-a:5′,1′-d][1,3,5]triazepine (50) and 1,2,10,11-tetranitro-6,7-dihydro-5H,12H-dipyrazolo[1,5-a:5′,1′-d][1,3,5]triazocine (51) was reported.43 These compounds contain tricyclic frameworks of dipyrazolo-1,3,5-triazinane, dipyrazolo-1,3,5-triazepane, and dipyrazolo-1,3,5-triazocane, respectively (Scheme 19) and were easily obtained by treating the corresponding N,N′-alkylene-bridged polynitropyrazoles with ammonia–methanol solution followed by acidification with hydrochloric acid. This approach is a facile and concise cyclization strategy for building the seven-membered 1,3,5-triazepane and eight-membered 1,3,5-triazocane rings. The deprotonation of 49 in 50% hydroxylamine solution or hydrazine monohydrate solution yields hydroxylammonium 2,3,5,6-tetranitro-9H-dipyrazolo[1,5-a:5′,1′-d][1,3,5]triazin-4-ide (49-2) or hydrazinium 2,3,5,6-tetranitro-9H-dipyrazolo[1,5-a:5′,1′-d][1,3,5]triazin-4-ide (49-3), respectively. The amination of 49 gave 2,3,5,6-tetranitro-4-amine-4H,9H-dipyrazolo[1,5-a:5′,1′-d][1,3,5]triazine (52), which was nitrated to obtain 4-nitroamino-2,3,5,6-tetranitro-4H,9H-dipyrazolo[1,5-a:5′,1′-d][1,3,5]triazine (53). Similar to 50, the deprotonation of 53 was carried out to yield the hydroxylammonium salt 53-1.
Among these new energetic tricyclic compounds, 50 and 53 exhibit high densities (1.90 and 1.94 g cm−3) comparable to that of HMX. These high densities arise because the dipyrazolo-1,3,5-triazinane backbone has a nearly planar structure that enhances the density and thermal stability of 50 and its derivatives (Table 14). Regarding detonation performance, some representative compounds (49-2, D = 8893 m s−1, P = 35.9 GPa; 53, D = 9226 m s−1, P = 38.8 GPa; 53-1, D = 9034 m s−1, P = 37.1 GPa) are comparable to RDX or HMX. Most of the compounds exhibit favorable thermal stability, and their Td values range from 207 to 307 °C, except for the nitramine 53 (Td = 117 °C) and its hydroxylammonium salt 53-1 (Td = 138 °C). In addition, 53 and 53-1 exhibit sensitivity toward mechanical stimuli, limiting their application as HEDMs. Considering both detonation properties and sensitivity, 49-2 is characterized by a promising overall energetic performance (49-2, D = 8893 m s−1, P = 35.9 GPa, IS = 35 J, FS = 360 N) exceeding that of the benchmark explosive RDX.
| No. | T d, °C | d, g cm−3 | D, m s−1 | P, GPa | HOF, kJ mol−1/kJ g−1 | OB, % | IS, J | FS, N | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 49 | 261 | 1.90 | 8792 | 34.3 | 375.3/1.10 | −35.17 | 15 | 240 | 43 |
| 50 | 307 | 1.80 | 8257 | 29.2 | 301.9/0.85 | −47.30 | 25 | 240 | 43 |
| 51 | 276 | 1.76 | 8051 | 27.2 | 299.1/0.81 | −58.50 | 30 | 360 | 43 |
| 49-1 | 220 | 1.82 | 8518 | 31.7 | 347.4/0.97 | −40.20 | 40 | 360 | 43 |
| 49-2 | 221 | 1.86 | 8893 | 35.9 | 392.9/1.05 | −34.21 | 35 | 360 | 43 |
| 49-3 | 207 | 1.83 | 8690 | 33.2 | 496.4/1.33 | −40.73 | 25 | 360 | 43 |
| 52 | 242 | 1.79 | 8356 | 29.6 | 341.9/0.96 | −35.94 | 15 | 160 | 43 |
| 53 | 117 | 1.94 | 9226 | 38.8 | 521.5/1.30 | −21.94 | 3 | 20 | 43 |
| 53-1 | 138 | 1.87 | 9034 | 37.1 | 573.1/1.32 | −22.11 | 10 | 80 | 43 |
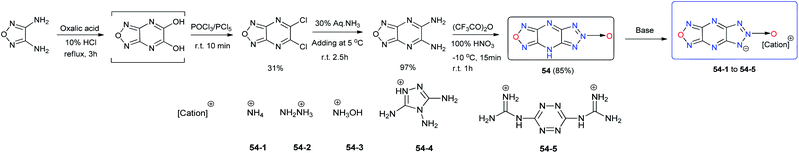
Furazano-[3,4-b]pyrazine is considered an attractive fragment for the construction of planar polycondensed molecules to produce polynitrogen compounds with unique energy characteristics. 1H-[1,2,5]oxadiazolo[3,4-b][1,2,3]triazolo[4,5-e]pyrazine, 6-oxide (54) was prepared using a straightforward method starting from the diamino furazan (Scheme 20),45 and its energetic salts 54-1 to 54-5 were prepared by neutralization reactions. As seen in Table 16, the introduction of an N-oxide into the fused 1,2,3-triazolo[4,5-e]furazano[3,4-b]pyrazine ring results in an increase in the density of 54 (d = 1.85 g cm−3), which contributes to the high performance of 54 and its energetic salts. Compared to their precursor, most of the energetic salts of 54 are less sensitive to impact and exhibit prominent physical and detonation properties, such as high thermal stability, good density, and good detonation properties, which are comparable to those of explosives such as TNT, and RDX. These results suggest that they might have potential as insensitive nitrogen-rich HEDMs for future applications.
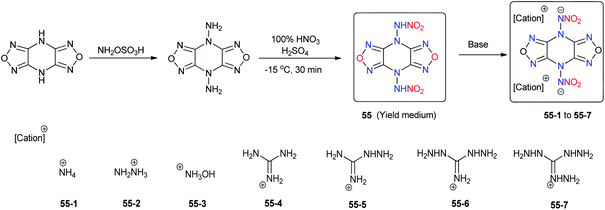
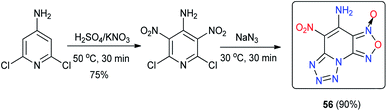
In contrast to 54, which has an asymmetric structure, 4,8-dinitraminodifurazano[3,4-b,e]pyrazine (55) was obtained by treating 4,8-dihydrodifurazano[3,4-b,e]pyrazine with NH2OSO3H followed by nitration with HNO3 (100%) (Scheme 21).46 A series of new energetic salts (55-1 to 55-7) based on 55 were further synthesized by neutralization or metathesis reactions. The introduction of highly energetic nitramino moieties into the nitrogen-rich 4,8-dihydrodifurazano[3,4-b,e]pyrazine backbone obviously resulted in significant detonation properties. Among these compounds, energetic salts 55-1, 55-2, 55-3 and 55-7 exhibited satisfactory detonation performances (8921 m s−1 < D < 9413 m s−1, 32.8 GPa < P < 36.8 GPa), which are close to or exceed that of RDX. However, these salts are highly sensitive (Table 15). Notably, the detonation velocity of 55-6 is superior to that of RDX, while the mechanical stability is similar to that of RDX. The prominent detonation performance along with moderate sensitivity indicates that compound 55-6 has the potential to compete with traditional high explosives and serve as a new !synthesis of 4-amino-5-nitro-[1,2,5]oxadiazolo-[3,4-e]tetrazolo[1,5-a]pyridine-3-oxide (56) from 4-amino-2,6-dichloropyridine in a short reaction time using a step-economical fashion (Scheme 22) has been reported.47 Compound 56 is a tricyclic fused energetic material containing vicinal amino and nitro moieties. The facile synthetic approach was shown to be a successful method to build tricyclic heterocyclic backbones with adequate purity, easy operation, and mild reaction conditions and allows further scale-up to mass production level. It has a high density (1.92 g cm−3), which is much higher than those of known polynitro energetic materials, such as TNT, RDX and TATB. As shown in Table 16, the high density of 56 is associated with high detonation properties (P = 36.01 GPa, D = 8838 m s−1), which are superior to those of TNT. In addition to the detonation properties, 56 with an impact sensitivity of 3 J and a friction sensitivity of 100 N is more sensitive than RDX and HMX (7.4 J, 120 N). It also has a low thermal stability decomposing at 134 °C.
| No. | T d, °C | d, g cm−3 | D, m s−1 | P, GPa | HOF, kJ mol−1/kJ g−1 | OB, % | IS, J | FS, N | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 54 | 281 | 1.85 | 8532 | 32.4 | 597/3.3 | −58.07 | 32 | — | 45 |
| 54-1 | 270 | 1.73 | 8079 | 26.3 | 476/2.4 | −65.26 | >40 | — | 45 |
| 54-2 | 141 | 1.76 | 8378 | 30.0 | 641/3.0 | −64.41 | 38 | — | 45 |
| 54-3 | 157 | 1.74 | 8518 | 30.3 | 542/2.5 | −52.80 | 35 | — | 45 |
| 54-4 | 274 | 1.70 | 7972 | 25.0 | 794/2.7 | −73.67 | >40 | — | 45 |
| 54-5 | 301 | 1.69 | 7871 | 24.0 | 1070/2.8 | −72.15 | >40 | — | 45 |
| 55-1 | 208 | 1.76 | 8921 | 32.8 | 558.6/1.75 | −29.98 | 2 | 42 | 46 |
| 55-2 | 189 | 1.78 | 9378 | 36.0 | 882.8/2.52 | −31.98 | 1 | 24 | 46 |
| 55-3 | 155 | 1.80 | 9156 | 36.8 | 680.0/1.93 | −18.17 | 1 | 28 | 46 |
| 55-4 | 232 | 1.61 | 8135 | 24.1 | 607.0/1.50 | −47.49 | 8 | 120 | 46 |
| 55-5 | 190 | 1.64 | 8509 | 26.4 | 828.0/1.91 | −47.89 | 10 | 112 | 46 |
| 55-6 | 187 | 1.72 | 9166 | 31.4 | 1045.5/2.25 | −48.24 | 8 | 108 | 46 |
| 55-7 | 207 | 1.71 | 9413 | 33.0 | 1380.5/2.79 | −48.55 | 3 | 54 | 46 |
| No. | T d, °C | d, g cm−3 | D, m s−1 | P, GPa | HOF, kJ mol−1/kJ g−1 | OB, % | IS, J | FS, N | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 56 | 134 | 1.92 | 8838 | 36.0 | 908.0/3.81 | −47.04 | 3 | 100 | 47 |
| 57-1 | 203 | 1.79 | 8073 | 29.6 | 222.8/0.67 | 16 | 360 | 48 | |
| 57-2 | 278 | 1.92 | 8622 | 34.6 | 305.4/0.75 | 12 | 240 | 48 | |
| 57-3 | 142 | 1.85 | 8942 | 37.4 | 715.5/1.40 | 10 | 160 | 48 | |
| 57-4 | 206 | 1.77 | 7694 | 27.9 | 705.2/1.06 | 22 | 300 | 48 | |
| 57-5 | 262 | 1.76 | 7862 | 26.9 | 890.0/2.04 | 28 | 360 | 48 | |
| 57-7 | 100 | 7615 | 23.6 | 1341.2/3.83 | 10 | 120 | 49 and 50 | ||
| 58 | 400 | 1.69 | 7032 | 19.9 | 695.0/0.9 | −92.65 | 19.0 | — | 51 |
| 59 | 356 | 1.77 | 7160 | 19.4 | 463.0/2.3 | −107.38 | 18.0 | — | 51 |
| 59-1 | 162 | 2.10 | — | — | — | −71.88 | 7.5 | — | 51 |
| 59-2 | 283 | 2.41 | — | — | — | −60.87 | 5.0 | — | 51 |
| 59-3 | 391 | 2.80 | — | — | — | −36.80 | 3.5 | — | 51 |
| 60 | 38 | 1.82 | 8376 | 31.2 | 710.0/2.1 | −28.56 | 3.0 | — | 51 |
| 61 | 207 | 1.80 | 6234 | 13.7 | 711.0/2.3 | −55.18 | 5.0 | — | 51 |
| 62·2H2O | 129 | 1.79 | 7946 | 26.9 | 618.0/2.1 | −46.71 | 5.5 | — | 51 |
| 63 | 128 | 1.96 | — | — | — | −9.60 | 11 | 130 | 52 |
| 64 | 157 | 1.97 | — | — | — | −21.52 | 14 | 190 | 52 |
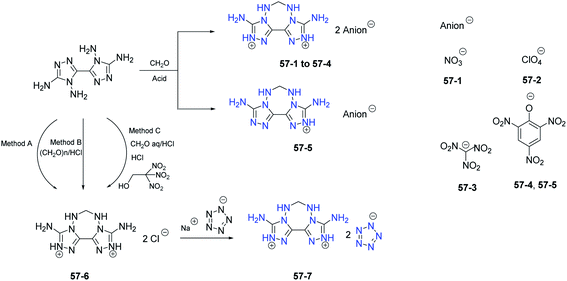
Most of the above tricyclic heterocycle-based energetic materials are neutral compounds, some of them have a tendency to lose one or two protons and their negative ions (anions) were prepared.43,45,46 However, none of them exhibits a strong tendency to gain a proton to form a cation. In 2018, through the cyclization condensation of the 4,4′,5,5′-tetraamino-3,3′-bi-1,2,4-triazole with 37% HCHO solution, a new family of 3,9-diamino-6,7-dihydro-5H-bis([1,2,4]triazolo)[4,3-e:3′,4′-g][1,2,4,5]tetrazepine-2,10-diium salts with a fused tricyclic backbone in the cation was synthesized. Through reaction with a variety of energetic acids or metathesis reactions, a series of energetic salts were obtained (Scheme 23).48–50 The fused tricyclic energetic salts 57-2 (d: 1.92 g cm−3; D: 8622 m s−1; P: 34.6 GPa) and 57-3 (d: 1.85 g cm−3; D: 8942 m s−1; P: 37.4 GPa) display interesting thermal behaviour and are predicted to be high-performance energetic materials. This work highlights ([1,2,4]triazolo)[4,3-e:3′,4′-g][1,2,4,5]tetrazepine-2,10-diium cation as a promising energetic building block that may provide new inspiration for fused heterocyclic chemistry.
Tetracyclic heterocycle-based energetic materials
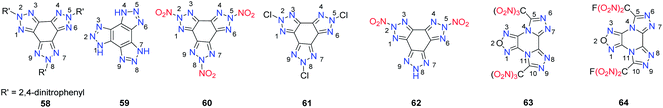
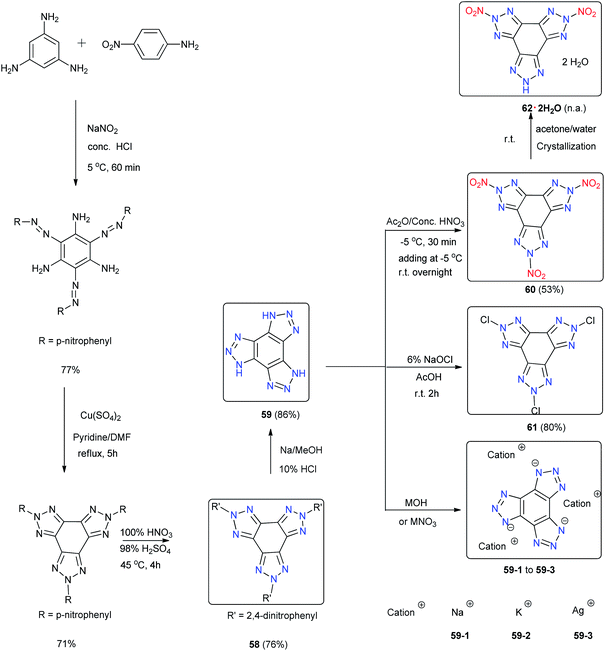
Compared with bicyclic or tricyclic heterocycle-based energetic materials, tetracyclic heterocycle-based energetic materials are more difficult to synthesize, and few backbones containing tetracyclic heterocycles have been reported in recent years (Scheme 24). 1,4,7-Trihydro-benzo[1,2-d:3,4-d′:5,6-d′]tris[1,2,3]triazole (59) is a fused ring system with three triazole units on one benzene ring. An improved synthesis of 59 and its derivatives from 1,3,5-triaminobenzene trihydrochloride, an easily accessible starting material, by using reported methods of treating the 2,5,8-tris(2,4-dinitrophenyl)-5,8-dihydro-2H-benzo[1,2-d:3,4-d′:5,6-d′′]tris[1,2,3]triazole (58) with sodium followed with acidification produced 59.51 Metal salts (59-1, 59-2 and 59-3) and 2,5,8-trinitro-benzo[1,2-d:3,4-d′:5,6-d′′]tris[1,2,3]triazole (60) and 2,5,8-trichloro-benzo[1,2-d:3,4-d′:5,6-d′′]tris[1,2,3]triazole (61) were synthesized with good yields (Scheme 25). Attempts to crystallize the trinitro compound 60 in acetone/water led to single crystals of the hydrate of 5,8-dihydro-2,5-dinitro-2H-benzo[1,2-d:3,4-d′:5,6-d′′]tris[1,2,3]triazole (62·2H2O). The trinitro compound 59 could not be recrystallized because it decomposed and only 62·2H2O was obtained. The single-crystal X-ray analysis shows that the structure of 62·2H2O is influenced by strong hydrogen bonds between H2O and 62 with the triazole rings being nearly planar. Because tris(triazolo) benzene has the advantage of having high thermal tolerance and being rich in nitrogen, these derivatives exhibit good physical and detonation properties, including high stabilities, high densities, and high HOF. These explosives exhibit calculated detonation values comparable to those of TNT and PETN, which suggests that they might be of interest for future applications as environmentally friendly HDEMs (Table 16). Compound 61 was found to be a powerful hypergolic oxidizer with various commonly used fuels, resulting in better ignition delay times than those with white fuming nitric acid (WFNA).Another example of a tetracyclic heterocycle-based energetic material is a one-pot method utilized for the synthesis of 5,10-bis(trinitromethyl)furazano[3,4-e]di([1,2,4]triazolo)[4,3-a:3′,4′-c]pyrazine (63) and its fluorinated analogue 5,10-bis(fluorodinitromethyl) furazano[3,4-e]di([1,2,4]triazolo)[4,3-a:3′,4′-c]pyrazine (64). The synthesis was performed at low temperature, and the product was obtained in only two steps (Scheme 26).52,53 Compounds 62 and 63 are tetracyclic hybrids of the dense 1,2,4-triazole and furazano[3,4-b]pyrazine backbones, which ensures good density and acceptable thermal stability (Scheme 26). These compounds are much less sensitive to impact and friction than HMX and are predicted to be HEDMs (Table 16). The trinitromethyl groups in 63 are on the structurally rigid backbone. The oxygen-rich character of 63 makes it useful in the processing of energetic formulations.
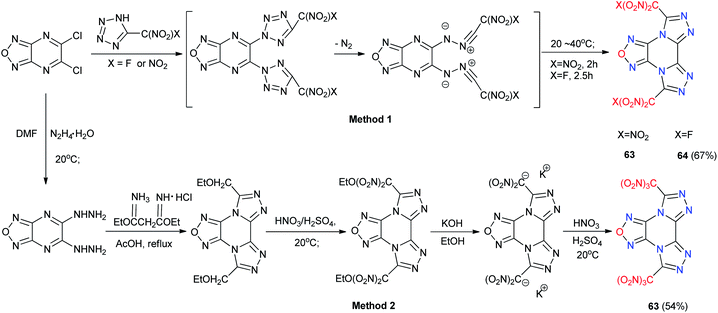 | ||
| Scheme 26 Synthesis of 5,10-bis(trinitromethyl)furazano[3,4-e]di([1,2,4]triazolo)[4,3-a:3′,4′-c]pyrazine (63) and 5,10-bis(fluorodinitromethyl)furazano[3,4-e]di([1,2,4]triazolo)[4,3-a:3′,4′-c]pyrazine (64).52 | ||
Conclusion
The chemistry of fused heterocyclic compounds which represent valuable scaffolds for constructing more powerful and less sensitive eco-friendly energetic materials is reviewed. The reactions that are presented and discussed illustrate the versatility of 64 different fused heterocycles designed as building blocks for the synthesis of a wide range of high-performance energetic materials. The key contributions of these fused backbones, which strongly regulates their energetic performance, are enhanced thermal stability and low sensitivity toward destructive mechanical stimuli. Additionally, these results exemplify the rational design and discovery of new fused heterocycle-based explosive molecules via the construction of the fused ring skeleton and subsequent improvements in their energetic performance by adding energetic groups to the backbones. These lead to novel energetic compounds with a combination of features that enhance performance (high density and good oxygen balance) while preserving molecular stability (hydrogen bonding, planar geometries, and π–π interactions). Thus far, some of the processes have been found to be straightforward, practical and easily scalable, such as those for compounds 1, 10, 11, 12 and 55; however, the design and syntheses of the backbones of most fused heterocyclic energetic materials are difficult. The authors believe that a more reasonable procedure for searching for fused heterocyclic energetic materials with the desired detonation properties includes two steps. The first step is the selection of possible backbones to ensure the necessary properties of the target molecules, where developing a more efficient way of building the backbones should be key. The second step is the estimation of properties for the selected backbones and selection of the energetic groups to modify these moieties, which then become the targets of a subsequent synthetic search.It is necessary to clarify that the heat of formation and detonation performance of all the compounds in this review are obtained by theoretical calculations, and it is practically used in current research of design and synthesis of new energetic materials.54 The main reason for using this method is that the synthesis of new energetic materials is difficult and risky. Usually, the amount of the new energetic materials is limited, and, in most cases, there is only enough to measure spectral data (infrared, nuclear magnetic resonance), elemental analysis, decomposition temperature, and single crystal diffraction data, etc. To obtain the standard molar formation enthalpy and detonation performance by experimental methods, a larger amount (few grams or even few hundred grams) is required. Considering that not all compounds have excellent detonation performance, in order to avoid a large number of synthetic experiments, potential risk of explosion and cost reduction must be considered. We found that the combination of empirical and theoretical calculations are powerful tools in predicting HOF of energetic materials. In addition, the studies show that the difference between the calculated detonation performance (D, P) by EXPLO5 (ref. 1 and 55) and experimental values of is less than 5% (for D) and 10% (for P), respectively. Different theoretical models are effective methods to study the new energetic materials using this model of theoretical calculation; they have time-saving capabilities and they can reduce costs and inherent danger of synthesis associated with tests.56
From the above results, it is shown clearly that considerable effort has been devoted to the research and development of fused energetic materials over the past several years. The number of publications relating to fused energetic materials studies reveals that this field is an interesting area that requires further exploration because it is particularly relevant to the target-oriented synthesis of energetic materials featuring integrated properties. This research area will become a major focus in the pursuit of the next generation of HEDMs.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the support of the Science Challenge Project (TZ2018004).Notes and references
- M. Sućeska, EXPLO5, Version 6.01, Brodarski Institute, Zagreb, Croatia, 2013 Search PubMed.
- T. Brinck, Green Energetic Materials, John Wiley & Sons, New York, NY, 2014, vol. 1, pp. 1–13 Search PubMed.
- Q. Zhang and J. M. Shreeve, Chem. Rev., 2014, 114, 10527–10574 CrossRef CAS PubMed.
- Y. Qu and S. P. Babailov, J. Mater. Chem. A, 2018, 6, 1915–1940 RSC.
- P. He, J.-G. Zhang, X. Yin, J.-T. Wu, L. Wu, Z.-N. Zhou and T.-L. Zhang, Chem.–Eur. J., 2016, 22, 7670–7685 CrossRef CAS PubMed.
- R. P. Singh, H. Gao, D. T. Meshri and J. M. Shreeve, Struct. Bonding, 2007, 125, 35–83 CrossRef CAS.
- S. Zhang, Q. Yang, X. Liu, X. Qu, Q. Wei, G. Xie, S. Chen and S. Gao, Coord. Chem. Rev., 2016, 307, 292–312 CrossRef CAS.
- T. M. Klapötke, P. C. Schmid, S. Schnell and J. Stierstorfer, Chem.–Eur. J., 2015, 21, 9219–9228 CrossRef PubMed.
- P. Yin, C. He and J. M. Shreeve, J. Mater. Chem. A, 2016, 4, 1514–1519 RSC.
- T. M. Klapötke, M. Leroux, P. C. Schmid and J. Stierstorfer, Chem.–Asian J., 2016, 11, 844–851 CrossRef PubMed.
- J. Zhang, D. A. Parrish and J. M. Shreeve, Chem. Commun., 2015, 51, 7337–7340 RSC.
- J. Zhang, D. A. Parrish and J. M. Shreeve, Chem.–Asian J., 2014, 9, 2953–2960 CrossRef CAS PubMed.
- P. Yin, J. Zhang, D. A. Parrish and J. M. Shreeve, J. Mater. Chem. A, 2015, 3, 8606–8612 RSC.
- Q. Ma, G. Fan, L. Liao, H. Lu, Y. Chen and J. Huang, ChemPlusChem, 2017, 82, 474–482 CrossRef CAS PubMed.
- Y. Xu, L. Tian, D. Li, P. Wang and M. Lu, J. Mater. Chem. A, 2019, 7, 12468–12479 RSC.
- P. Yin, C. He and J. M. Shreeve, Chem.–Eur. J., 2016, 22, 2108–2113 CrossRef CAS PubMed.
- P. Yin, J. Zhang, L. A. Mitchell, D. A. Parrish and J. M. Shreeve, Angew. Chem., Int. Ed., 2016, 55, 12895–12897 CrossRef CAS PubMed.
- C. He, J. Zhang, D. A. Parrish and J. M. Shreeve, J. Mater. Chem. A, 2013, 1, 2863–2868 RSC.
- Y. Tang, C. He and J. M. Shreeve, J. Mater. Chem. A, 2017, 5, 4314–4319 RSC.
- C. Bian, X. Dong, X. Zhang, Z. Zhou, M. Zhang and C. Li, J. Mater. Chem. A, 2015, 3, 3594–3601 RSC.
- B.-J. Zhao, P. Wang, W. Fu, C. Li and Z.-M. Zhou, ChemistrySelect, 2018, 3, 4797–4803 CrossRef CAS.
- J. Ma, G. Cheng, X. Ju, Z. Yi, S. Zhu, Z. Zhang and H. Yang, Dalton Trans., 2018, 47, 14483–14490 RSC.
- H. Wei, J. Zhang and J. M. Shreeve, Chem.–Asian J., 2015, 10, 1130–1132 CrossRef CAS PubMed.
- D. E. Chavez, D. A. Parrish, L. Mitchell and G. H. Imler, Angew. Chem., Int. Ed., 2017, 56, 3575–3578 CrossRef CAS PubMed.
- L. Hu, P. Yin, G. H. Imler, D. A. Parrish, H. Gao and J. M. Shreeve, Chem. Commun., 2019, 55, 8979–8982 RSC.
- T. W. Myers, C. J. Snyder, D. E. Chavez, R. J. Scharff and J. M. Veauthier, Chem.–Eur. J., 2016, 22, 10590–10596 CrossRef CAS PubMed.
- L. Hu, P. Yin, G. Zhao, C. He, G. H. Imler, D. A. Parrish, H. Gao and J. M. Shreeve, J. Am. Chem. Soc., 2018, 140, 15001–15007 CrossRef CAS PubMed.
- Y. Liu, G. Zhao, Y. Tang, J. Zhang, L. Hu, G. H. Imler, D. A. Parrish and J. M. Shreeve, J. Mater. Chem. A, 2019, 7, 7875–7884 RSC.
- M. C. Schulze, B. L. Scott and D. E. Chavez, J. Mater. Chem. A, 2015, 3, 17963–17965 RSC.
- D. G. Piercey, D. E. Chavez, B. L. Scott, G. H. Imler and D. A. Parrish, Angew. Chem., Int. Ed., 2016, 55, 15315–15318 CrossRef CAS PubMed.
- D. Kumar, G. H. Imler, D. A. Parrish and J. M. Shreeve, Chem.–Eur. J., 2017, 23, 1743–1747 CrossRef CAS PubMed.
- C. J. Snyder, T. W. Myers, G. H. Imler, D. E. Chavez, D. A. Parrish, J. M. Veauthier and R. J. Scharff, Propellants, Explos., Pyrotech., 2017, 42, 238–242 CrossRef CAS.
- Y. Tang, C. He, G. H. Imler, D. A. Parrish and J. M. Shreeve, Chem. Commun., 2018, 54, 10566–10569 RSC.
- P. Politzer, P. Lane and J. S. Murray, Cent. Eur. J. Energ. Mater., 2013, 10, 37–52 CAS.
- K. O. Christe, D. A. Dixon, M. Vasiliu, R. I. Wagner, R. Haiges, J. A. Boatz and H. L. Ammon, Propellants, Explos., Pyrotech., 2015, 40, 463–468 CrossRef CAS.
- M. S. Klenov, O. V. Anikin, A. M. Churakov, Y. A. Strelenko, I. V. Fedyanin, I. V. Ananyev and V. A. Tartakovsky, Eur. J. Org. Chem., 2015, 2015, 6170–6179 CrossRef CAS.
- M. S. Klenov, A. A. Guskov, O. V. Anikin, A. M. Churakov, Y. A. Strelenko, I. V. Fedyanin, K. A. Lyssenko and V. A. Tartakovsky, Angew. Chem., Int. Ed., 2016, 55, 11472–11475 CrossRef CAS PubMed.
- C. Yan, X. Qi, K. Wang, Y. Jin, G. Cheng, T. Liu, H. Yang and Q. Zhang, Chem. Commun., 2019, 55, 3497–3500 RSC.
- H. Gao and J. M. Shreeve, Angew. Chem., Int. Ed., 2015, 54, 6335–6338 CrossRef CAS PubMed.
- D. E. Chavez, J. C. Bottaro, M. Petrie and D. A. Parrish, Angew. Chem., Int. Ed., 2015, 54, 12973–12975 CrossRef CAS PubMed.
- Y. Tang, D. Kumar and J. M. Shreeve, J. Am. Chem. Soc., 2017, 139, 13684–13687 CrossRef CAS PubMed.
- Y. Tang, C. He, P. Yin, G. H. Imler, D. A. Parrish and J. M. Shreeve, Eur. J. Org. Chem., 2018, 2018, 2273–2276 CrossRef CAS.
- P. Yin, J. Zhang, G. H. Imler, D. A. Parrish and J. M. Shreeve, Angew. Chem., Int. Ed., 2017, 56, 8834–8838 CrossRef CAS PubMed.
- Y. Tang, C. He, G. H. Imler, D. A. Parrish and J. M. Shreeve, J. Mater. Chem. A, 2018, 6, 8382–8387 RSC.
- V. Thottempudi, P. Yin, J. Zhang, D. A. Parrish and J. M. Shreeve, Chem.–Eur. J., 2014, 20, 542–548 CrossRef CAS PubMed.
- W. Li, J. Tian, X. Qi, K. Wang, Y. Jin, B. Wang and Q. Zhang, ChemistrySelect, 2018, 3, 849–854 CrossRef CAS.
- C. Ma, Y. Pan, J. Jiang, Z. Liu and Q. Yao, New J. Chem., 2018, 42, 11259–11263 RSC.
- Y. Xu, Z. Zhu, C. Shen, Q. Lin and M. Lu, Propellants, Explos., Pyrotech., 2018, 43, 595–601 CrossRef CAS.
- Y. Xu, Q. Lin, P. Wang and M. Lu, Chem.–Asian J., 2018, 13, 924–928 CrossRef CAS PubMed.
- Y. Xu, L. Tian, P. Wang, Q. Lin and M. Lu, Cryst. Growth Des., 2019, 19, 1853–1859 CrossRef CAS.
- V. Thottempudi, F. Forohor, D. A. Parrish and J. M. Shreeve, Angew. Chem., Int. Ed., 2012, 51, 9881–9885 CrossRef CAS PubMed.
- A. B. Sheremetev, V. L. Korolev, A. A. Potemkin, N. S. Aleksandrova, N. V. Palysaeva, T. H. Hoang, V. P. Sinditskii and K. Y. Suponitsky, Asian J. Org. Chem., 2016, 5, 1388–1397 CrossRef CAS.
- D. B. Lempert and A. B. Sheremetev, Russ. Chem. Bull., 2018, 67, 2065–2072 CrossRef CAS.
- H. Gao, C. Ye, C. Piekarski and J. M. Shreeve, J. Phys. Chem. C, 2007, 111, 10718–10731 CrossRef CAS.
- M. Sućeska, Mater. Sci. Forum, 2004, 325–330 Search PubMed.
- H. Gao and J. M. Shreeve, Chem. Rev., 2011, 111, 7377–7436 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |