A bis(pyridyl)-N-alkylamine/Cu(i) catalyst system for aerobic alcohol oxidation†
Abstract
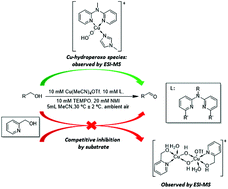
Herein a bis(pyridyl)-N-alkylamine/CuI/TEMPO/NMI catalyst system is reported for aerobic oxidation of a variety of primary alcohols to the corresponding aldehydes using readily available reagents, at room temperature and ambient air as the oxidant. ESI-MS analysis of the reaction showed the formation of a [(L1)(NMI)CuII-OOH]+ species, which is a key intermediate in the alcohol oxidation reaction. Evaluation of the effect of reaction parameters on the initial rate of the reaction allowed us to obtain the optimum conditions for catalytic activity. The careful choice of reaction solvent allowed for the oxidation of 4-hydroxybenzyl alcohol, a substrate which proved problematic in previous studies. In the case of 2-pyridinemethanol as substrate, experimental evidence shows that catalytic activity is diminished due to competitive inhibition of the catalyst by the alcohol substrate.
- This article is part of the themed collection: Mechanistic Aspects of Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...