Electrochemical ion transfer mediated by a lipophilic Os(ii)/Os(iii) dinonyl bipyridyl probe incorporated in thin film membranes†
Abstract
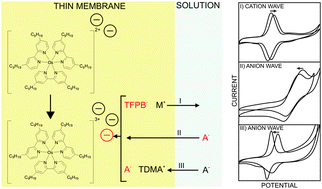
A new lipophilic dinonyl bipyridyl Os(II)/Os(III) complex successfully mediates ion transfer processes across voltammetric thin membranes. An added lipophilic cation-exchanger may impose voltammetric anion or cation transfer waves of Gaussian shape that are reversible and repeatable. The peak potential is found to shift with the ion concentration in agreement with the Nernst equation. The addition of tridodecylmethylammonium nitrate to the polymeric film dramatically reduces the peak separation from 240 mV to 65 mV, and the peak width to a near-theoretical value of 85 mV, which agrees with a surface confined process. It is suggested that the cationic additive serves as a phase transfer catalyst.


 Please wait while we load your content...
Please wait while we load your content...