Amino-functionalized ordered mesoporous silica SBA-15, a rapid and efficient adsorbent for the adsorption of (−)-epigallocatechin gallate from green tea extract†
Guicen Ma,
Jianyang Zhang,
Liyan Chen,
Ting Liu,
Liangzi Yu,
Xin Liu* and
Chengyin Lu*
Tea Research Institute, Chinese Academy of Agricultural Sciences, Hangzhou, China. E-mail: liuxin@tricaas.com; lchy@tricaas.com; Tel: +86-571-8665-1650
First published on 30th July 2014
Abstract
Ordered mesoporous silica SBA-15 with different pore size and organic groups (–SH, –NH2, –COOH) were synthesized. The adsorption of (−)-epigallocatechin gallate (EGCG) from green tea extract (GTE) was conducted on these functional ordered mesoporous silica SBA-15, which were studied as a class of potential adsorbents. The adsorption performance of EGCG was correlated with the surface chemistry and pore size. Amino-functionalized SBA-15 (SBA-15-NH2) with a large pore size (10.7 nm) exhibited extremely rapid rate as well as high capacity of 287 mg g−1 for adsorbing EGCG at 25 °C, which is much higher than that of other adsorbent previously reported. EGCG rather than CAF were preferred to adsorb onto SBA-15-140 °C-NH2 since the adsorption capacity of EGCG was almost 19 times as high as that of CAF (287 mg g−1 vs. 15 mg g−1). The kinetic data were well fitted with pseudo-second-order kinetics based on an evaluation of the adsorption kinetics. Furthermore, the Langmuir and Freundlich isotherms provided additional information for better understanding of the adsorption process. Hydrogen bonding and electrostatic interaction were the main driving forces for the adsorption of EGCG on the SBA-15-NH2s. Considering the excellent adsorption performance, it is expected that mesoporous silica materials are attractive for the separation and enrichment of EGCG.
Introduction
EGCG as the most important component and the highest concentration of tea catechins, is widely used in the fields of food, medicine, health products, and cosmetics due to their many perfect health benefits, such as anticancer, anti-inflammatory effects, strong antimicrobial activity, preventing HIV-1 infection, and antiviral effects, etc.1 However, EGCG extracted from tea by common extraction technology is always contaminated with other components, such as caffeine (CAF).2 Excessive intake of CAF may have adverse effects, including sleep deprivation, increased risk of cardiovascular disease, reduced fertility rates and increased incidence of miscarriages.3 Therefore, it is important to develop an efficient method to obtain highly enriched EGCG and to decrease the content of CAF as far as possible prior to applications in pharmaceuticals, functional foods and beverages.Several strategies have been attempted to separate EGCG from CAF of tea extracts, such as extraction with organic solvent,4 supercritical carbon dioxide extraction,5 microbial degradation6 and genetically modified caffeine-free tea plants.7 However, there are significant disadvantages in these separation methods, such as the potential carcinogenic effects of the residual organic solvents, expensive equipment and unavoidably loss of a large amount of catechin in supercritical carbon dioxide extraction. In contrast, adsorption is regarded as a simple and efficient technology for the separation and enrichment of EGCG. Activated carbon,8 tea stalk lignocellulose,9 fir sawdust lignocelluloses grafted with N-vinylpyrrolidone,10 poly(acrylamide-co-ethyleneglycoldimethacry-late),11 poly(vinylpolypyrrolidone)12 and macroporous resins functionalized with phenylamino13 were all reported as adsorbents. Scientists never stop exploring the novel desirable adsorbents with high adsorption capacity and perfect selectivity for EGCG.
It is generally accepted that adsorbate-adsorbent interactions plays an important role in the adsorption process. The effective interactions include van der Waals force, hydrogen bonding, hydrophobic interaction, and electrostatic interaction.13 EGCG has the general structure of C6–C3–C6 with two aromatic rings and several hydroxyl groups, while CAF is a N-containing fused heterocycle system with two carbonyl groups (Fig. 1). The adsorption of EGCG is based on the hydrogen-bonding, whereas the adsorption of CAF is mainly driven by hydrophobic interactions.14 Therefore, the design synthesis of adsorbent with different surface chemistry, which shows different interactions to EGCG and CAF, is very important for their high efficient separation and enrichment. Adsorbents with hydrogen-bonding groups (amine, amide, hydroxyl or carboxyl) could effectively adsorb EGCG, but not CAF based on the interaction mechanisms.
Compared to conventional adsorbents, ordered mesoporous silica materials with high surface area, large pore volume and controllable pore size are considered promising candidates for applications in adsorption and separation.15,16 Moreover, the surface of mesoporous silica can be easily grafted with desirable organic groups by post-modification without any change in the mesostructure, which can further tailor their adsorption performance.17 A variety of organic chelating groups were modified inside the mesopore channels, such as amino, carboxyl and thiol groups. The modified hybrid materials have been widely used as adsorbents for environmental clean-up, removal of heavy metals and basic dye stuffs.18–22 However, the adsorption of EGCG and CAF on functionalized ordered mesoporous silica has not been reported.
Herein, a detailed study of EGCG and CAF adsorption on mesoporous silica (SBA-15) functionalized with different organic groups is presented. We clarified the influence of surface chemistry (amino, carboxyl and thiol groups) on the adsorption performance of EGCG and CAF from GTE. The effects of the pore size of amino-functionalized SBA-15 on the adsorption behavior of EGCG and CAF was further explored. Furthermore, using amino functionalized SBA-15 as a model, the dynamic and thermodynamics adsorption behaviors were also determined to achieve a comprehensive understanding of the adsorption process of EGCG and CAF on mesoporous materials. Our study expanded the application of functionalized ordered mesoporous silica in the field of enrichment of active ingredients from the extracts of natural products and it is expected that mesoporous silica materials are attractive for the separation and enrichment of EGCG.
Results and discussion
Characterization of SBA-15s
Ordered mesoporous silica SBA-15s were synthesized using EO20PO70EO20 (P123) as the template under acid conditions. SBA-15s with different organic groups were obtained by grafting with organosilane in toluene (see ESI†). The synthesized mesoporous silica SBA-15s was characterized by small angle powder X-ray diffraction (XRD), transmission electron microscopy (TEM), N2 sorption and Fourier transform infrared (FTIR) spectroscopy to identify their structures and physiochemical properties. The XRD patterns (Fig. 2a) exhibited three well-resolved diffraction peaks, associated with the {100}, {110} and {200} reflections, indicating the formation of a highly ordered 2D mesostructure with a space group of p6mm. The ordered mesostructure was maintained after the incorporation of a –NH2 group for SBA-15-100 °C-NH2, which was also confirmed by the TEM images (ESI Fig., S1†). The N2 adsorption/desorption isotherms of SBA-15s functionalized with –NH2 group are type IV isotherms with H1 hysteresis indicating the existence of highly uniform channel-like mesopores (Fig. 2b). Furthermore, the volume adsorption at higher relative pressure P/P0 showed a sharp increase with increasing hydrothermal temperature. The pore sizes of SBA-15-NH2s increased from 4.8 nm to 7.4 nm and then to 10.7 nm as the hydrothermal temperature was increased from 40 °C to 100 °C and then to 140 °C, respectively (see pore size distribution from Fig. S2†). The physiochemical parameters of SBA-15s used in the selective adsorption of GTE are listed in Table S1 (ESI†).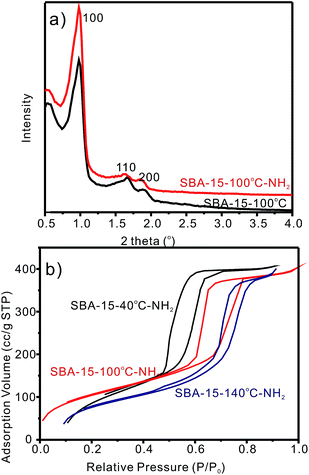 | ||
| Fig. 2 (a) Small angle XRD pattern of SBA-15-100 °C before and after NH2 functionalized; (b) N2 sorption isothermals of SBA-15-NH2s with different pore size. | ||
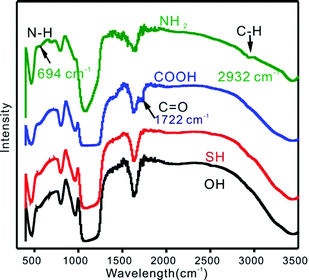
The presence of functional groups on the SBA-15 surface was confirmed by FTIR and element analysis. The C–H stretching band at 2932 cm−1 and N–H stretching vibrations band at 694 cm−1 clearly confirmed the grafting of aminopropyl anchored to the surface of the mesoporous support23 (Fig. 3). The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration at about 1722 cm−1 confirmed the modification of the carboxylic species.24 Owing to the aggregation of mercapto groups and the hydrogen binding effects, the characteristic peaks of SBA-15-SH sample did not show up.21 The greatly increased content of C and the existence of N by element analysis further confirmed the successful grafting of organic groups on SBA-15s (Table S2†).
O stretching vibration at about 1722 cm−1 confirmed the modification of the carboxylic species.24 Owing to the aggregation of mercapto groups and the hydrogen binding effects, the characteristic peaks of SBA-15-SH sample did not show up.21 The greatly increased content of C and the existence of N by element analysis further confirmed the successful grafting of organic groups on SBA-15s (Table S2†).
Influence of the surface chemistry
In order to better understand the adsorption behaviors of EGCG and CAF on the ordered mesoporous silica and establish the relationship between surface chemistry and the adsorption ability, four samples with different surface groups were used in our study. They are denoted as SBA-15-SH, SBA-15-NH2, SBA-15-COOH and the as-synthesized SBA-15 sample (hydrothermal at 40 °C) with many –OH groups, which is called SBA-15-OH. The adsorption test was conducted by adding 0.1 g SBA-15s into 10 mL of a GTE solution and stirring for 3 h (see ESI† for details). Fig. 4 shows the adsorption capacities of these SBA-15 samples functionalized with different organic groups. Under the same experimental conditions, SBA-15-OH shows low adsorption capacity of EGCG (17.8 mg g−1) or CAF (4.1 mg g−1). Both SBA-15-SH and SBA-15-COOH showed no significant improvement on the adsorption capacity of EGCG or CAF. However, SBA-15-NH2 exhibits much higher adsorption of EGCG, which is 10 times that of SBA-15-OH (170 mg g−1 vs. 17.8 mg g−1). The modification of amino groups on SBA-15 greatly improved the adsorption performance of EGCG.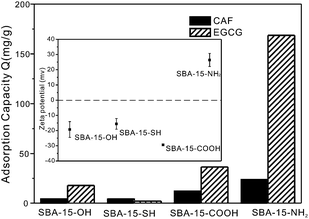 | ||
| Fig. 4 Adsorption capacity of EGCG and CAF on SBA-15-40 °C with different organic groups at 15 °C and the zeta potential of various organic groups functionalized SBA-15s. | ||
Zeta potential was employed to determine the change in surface charge before and after grafting with organic groups (Fig. 4 inset). The surface of mesoporous silica SBA-15-OH has a negative surface charge (−19.3 mV). The surface charges of SBA-15 did not show any significant change after being functionalized with –COOH or –SH groups. However, the modification of –NH2 makes the negative charged surface turns positive (26.4 mV). The change in the surface charge resulted in great improvement of the adsorption capacity of EGCG on SBA-15-NH2. The positive surface charge prefers the adsorption of EGCG due to surface electrostatic interactions.
The influence of the grafting amount of amino group of SBA-15-NH2 on the adsorption capacity was also studied. The results show that the adsorption capacity of EGCG was greatly increased (78.8 mg g−1 vs. 159.3 mg g−1) as the grafting amount increased from 5% to 10% (Table 1). However, the adsorption capacity of EGCG was only slightly improved when the grafting amount increased from 10% to 18.9%.
| Samples | N% (cal.) | N% (exp.) | EGCG (mg g−1) | CAF (mg g−1) |
|---|---|---|---|---|
| a Adsorption test is carried at 15 °C.b Adsorption test was carried at 25 °C. | ||||
| SBA-15-40 °C-NH2a | 1.07 | 1.20 | 78.8 | 4.2 |
| SBA-15-40 °C-NH2a | 1.97 | 2.02 | 159.3 | 8.2 |
| SBA-15-40 °C-NH2a | 3.41 | 3.23 | 168.6 | 23.7 |
| SBA-15-40 °C-NH2b | 3.41 | 3.23 | 224.6 | 16.5 |
| SBA-15-100 °C-NH2b | 3.41 | 2.03 | 229.7 | 27.6 |
| SBA-15-140 °C-NH2b | 3.41 | 1.71 | 286.9 | 14.8 |
Influence of the pore size
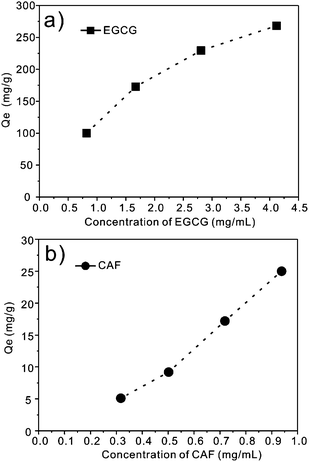
The pore size of the mesoporous silica adsorbents is an important factor for the adsorption of EGCG. It is generally known that EGCG has the structure of C6–C3–C6 with two aromatic rings and several hydroxyl groups. Its large structure requires a much larger pore size to be adsorbed into the channel of SBA-15. Three SBA-15-NH2s with different pore sizes were synthesized by adjusting the hydrothermal treatment temperature (see ESI†). It can be directly observed that the adsorption capacity of EGCG on the SBA-15-NH2s correspondingly improves with increasing pore size from 4.8 nm (SBA-15-40 °C-NH2) to 10.7 nm (SBA-15-140 °C-NH2) at 25 °C (Table 1). The mesopore diameter plays a key role in the enhancement of the adsorption capacity since the SBA-15-140 °C-NH2 sample shows the highest adsorption capacity of EGCG (287 mg g−1, 14.7 mg for CAF), which is higher than that of other adsorbent previously reported.12,13 The larger pore size of SBA-15-NH2 facilitates the rapid diffusion of EGCG into the inner part of the pore channels thus results in a high adsorption capacity. It is noteworthy that the adsorption amount of EGCG was 19 times as high as that of CAF (287 mg g−1 for EGCG vs. 14.7 mg g−1 for CAF), which implies that SBA-15-140 °C-NH2 favors the adsorption of EGCG rather than CAF.Adsorption kinetics of EGCG and CAF on the SBA-15-NH2s
Mesoporous silica SBA-15-100 °C-NH2 with a pore size of 7.4 nm was employed as a model for further research on the dynamics of the adsorption process. The kinetics curves describe the adsorption behavior of EGCG and CAF onto SBA-15-100 °C-NH2 at different contact times (Fig. 5a). The adsorption capacity of EGCG sharply increased during the first 3 min after the addition of SBA-15-100 °C-NH2 into the GTE solution and almost reached equilibrium within 30 min. The adsorption of CAF was extremely rapid at the first 3 min and there was a slight decrease during the following contact times (Fig. 5a). It is important to note that a rapid adsorption rate is crucial for practical applications. Such a rapid adsorption rate is the most valuable characteristic of mesoporous silica SBA-15, which is remarkably faster than that of other adsorbent previously reported.12,13 Furthermore, the adsorption capacity of EGCG on SBA-15-100 °C-NH2 was studied at different temperatures. It was shown that the adsorption amount improves with increasing temperature (Fig. 5b). The equilibrium adsorption amount (Qe) of EGCG was 177 mg g−1 at 15 °C and increased to 258 mg g−1 at 35 °C.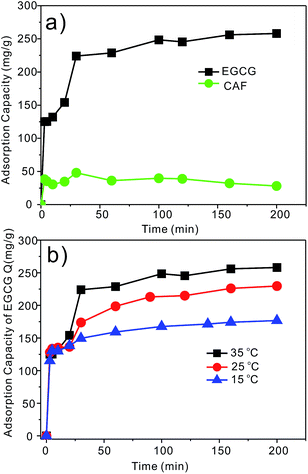 | ||
| Fig. 5 (a) Adsorption kinetics curves of EGCG and CAF on SBA-15-100 °C-NH2 at 35 °C and (b) the kinetic curves of EGCG at different temperatures. | ||
The pseudo-first-order and pseudo-second-order kinetics models were applied to illustrate the adsorption mechanisms of EGCG and CAF onto the SBA-15-NH2s. The best-fit model was selected on the basis of the linear regression correlation coefficient values (r2). After model fitting, the rate constant k, Qe and r2 along with the relative error of Qe between the experiment and calculation from the two models are summarized in Table 2. The equilibrium adsorption capacities (Qe.cal) of EGCG predicted from the pseudo-second order model were much closer to the experimental values (Qe.exp) (relative error 0.9–7.1%) than those from the pseudo-first-order model (relative error 47.2–68.6%), and the r2 value of the pseudo-second-order model (r2 > 0.997) was also higher than that of the pseudo-first-order model. Thus, the adsorption of EGCG onto the SBA-15-NH2s fitted the pseudo-second order model better. The rate constant (k2) of the pseudo-second-order model, an index of adsorption efficiency, indicates the adsorbent amount (g) for adsorption of 1 mg of adsorbate in 1 min. A smaller k2 value suggests the higher efficiency of adsorption. The k2 value of EGCG substantially decreased with increasing adsorption temperature (0.00128 at 15 °C vs. 0.00045 at 35 °C). The results imply that EGCG is more efficiently adsorbed by SBA-15-100 °C-NH2 at higher temperatures. The crucial step influencing the adsorption rate is the in-pore diffusion step, and the migration of EGCG into the inner part of mesopores is an endothermic process in order to break the static interaction between SBA-15-100 °C-NH2 and EGCG. Therefore, high temperature facilitates the adsorption of EGCG into the channel of SBA-15-100 °C-NH2.
| EGCG | Pseudo-first-order equation | ||||
|---|---|---|---|---|---|
| k1 (min−1) | Qe (mg g−1) | r2 | Rel error (%) | ||
| SBA-15-100 °C-NH2 | 15 °C | 0.0196 | 55.5 | 0.9795 | 68.6 |
| 25 °C | 0.0212 | 115.3 | 0.9666 | 49.8 | |
| 35 °C | 0.0240 | 136.3 | 0.9370 | 47.2 | |
| EGCG | Pseudo-second-order equation | ||||
|---|---|---|---|---|---|
| K2 (g mg−1 min−1) | Qe (mg g−1) | r2 | Rel error (%) | ||
| SBA-15-100 °C-NH2 | 15 °C | 0.00128 | 178.3 | 0.9991 | 0.91 |
| 25 °C | 0.00051 | 235.9 | 0.9967 | 2.69 | |
| 35 °C | 0.00045 | 276.4 | 0.9976 | 7.09 | |
| CAF | Pseudo-second-order equation | ||||
|---|---|---|---|---|---|
| k2 (g mg−1 min−1) | Qe (mg g−1) | r2 | Rel error (%) | ||
| SBA-15-100 °C-NH2 | 15 °C | 0.0287 | 30.5 | 0.9984 | 0.53 |
| 25 °C | −0.0091 | 29.4 | 0.9834 | 6.52 | |
| 35 °C | −0.0048 | 30.4 | 0.9672 | 8.11 | |
For CAF, the pseudo-first-order kinetics model is not suitable to explain the experiment data since the r2 values of pseudo-first-order model is lower than 0.8 (not shown). Although k2 values of CAF were negative in the prediction of the pseudo-second-order model, the model may still be suitable for describing the adsorption process of CAF onto SBA-15-NH2s as the predicted Qe was close to the experimental data (relative error 0.5–8.8%) (Table 2). This phenomenon is similar to CAF adsorbed on PVPP previously reported.12 It is suggested that CAF is probably not adsorbed on the surface of SBA-15-NH2s directly, which may be attached to EGCG during the adsorption process.
In the porous adsorbent, the intraparticle diffusion model was employed to investigate the possibility of EGCG being transported within the channels of SBA-15-NH2s. The intraparticle diffusion model was adopted according to literature.25 After model fitting, the plots of intraparticle diffusion of EGCG onto SBA-15-NH2s with different pore size are shown in Fig. S3.† The plots suggest that the adsorption process of EGCG include external surface adsorption, intraparticle diffusion and a fixation on the active binding sites steps.26 In the first stage, the adsorption process of EGCG is very fast, which was considered to be external surface adsorption or instantaneous adsorption stage. Further, EGCG molecular gradually adsorbed into the channels of SBA-15-NH2s where the intraparticle diffusion is rate limited. The linear plots of SBA-15-NH2s did not pass through the origin, which indicates that intraparticle diffusion was not the only rate controlling step.27
Adsorption isotherms of EGCG and CAF on the SBA-15-100 °C-NH2
The adsorption isotherm is another important parameter to identify the mechanism of the adsorption process. The plots of equilibrium adsorption capacity (Qe) of EGCG and CAF at different equilibrium concentrations (Ce) from a GTE solution onto the sample of SBA-15-100 °C-NH2 were obtained at 25 °C, as shown in Fig. 6. It was obvious that the adsorption capacities of EGCG increased with increasing the equilibrium concentration of EGCG. Although the Qe value of CAF also increased with Ce, it changed in a different way from EGCG (Fig. 6b). The results confirmed that CAF may not be directly adsorbed onto the SBA-15-100 °C-NH2 surface. The adsorption data was simulated by the Langmuir isotherm and the Freundlich isotherm. The results are shown in Table 3. The Langmuir isotherm model is usually used to describe the monolayer adsorption with constant heat onto the adsorbent surface with a finite number of homogeneous sites. As shown in Table 3, the rL2 value of EGCG was higher than 0.99 for SBA-15-100 °C-NH2, which indicated a very good mathematical fit. The essential characteristics of the Langmuir isotherm were represented according to a dimensionless constant, called the separation factor or equilibrium parameter (RL). The value of RL gives an indication of the shape of isotherm to be either unfavorable (RL > 1), linear (RL = 1), favorable (0 < RL < 1), or irreversible (RL = 0).28 In the present study, it was obvious that the RL value of EGCG was in the range of 0 < RL < 1, as shown in Table 3. The RL value of CAF was larger than 1, implying it is unfavorable for the adsorption of CAF on SBA-15-100 °C-NH2. According to the Langmuir model, the maximum adsorption amounts (Qm) of EGCG were predicted up to 454 mg g−1, which were much higher than that of other reported adsorbents.| Isothermal parameters | EGCG | CAF |
|---|---|---|
| Langmuir | ||
| KL (mg mL−1) | 2.813 | −1.79 |
| Qm (mg g−1) | 454.5 | −24.1 |
| rL2 | 0.9947 | 0.9486 |
| RL | 0.32 | 2.14 |
| Freundlich | ||
| 1/n | 0.6152 | 1.4873 |
| KF(mg(n−1)/n g−1 mL1/n) | 118.1 | 27.33 |
| rF2 | 0.9748 | 0.9941 |
The Freundlich isothermal model was employed to describe heterogeneous systems. A comparison of the rF2 values of EGCG and CAF given in Table 3 showed that the experimental data of CAF adsorption on the SBA-15-100 °C-NH2 fitted better than that of EGCG, which meant that the adsorption of CAF onto the SBA-15-100 °C-NH2 was a multiple layer adsorption. The value of 1/n was a measure of the adsorption intensity or surface heterogeneity, which indicated that the adsorption of CAF on the SBA-15-100 °C-NH2 was a co-operative adsorption (1/n > 1).13 The result is in agreement with the data indicating that CAF may be attached to EGCG instead of being directly adsorbed by SBA-15-100 °C-NH2. The value of 1/n of EGCG is less than 1, suggesting that EGCG molecules are favorably adsorbed by SBA-15-100 °C-NH2.
Adsorption mechanisms of EGCG and CAF on the SBA-15-NH2
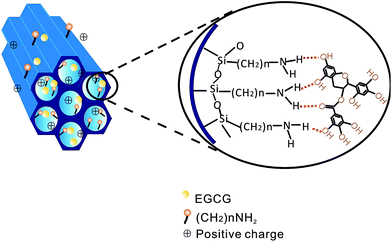
The adsorption mechanisms of EGCG and CAF on SBA-15-NH2s were deduced on the above analysis. Two kinds of interactions between EGCG and SBA-15-NH2s resulted in its high adsorption capacity. (1) Hydrogen bonding was formed between EGCG and mesoporous silica SBA-15-NH2 because EGCG has many hydroxyl groups. (2) Strong electrostatic interactions occurred between EGCG and SBA-15-NH2s since the surface of SBA-15-NH2 possesses positive surface charge (Scheme 1). The kinetic data of EGCG and CAF were fitted to a pseudo-second-order kinetic model well. On the other hand, CAF may be directly attached to EGCG instead of being absorbed by SBA-15-NH2s.Conclusions
A highly efficient adsorbent of SBA-15-NH2s for the adsorption of EGCG from GTE was fabricated. It was revealed that the pore size and the surface grafting groups play critical roles in the high efficient and selective adsorption of EGCG. The amino-functionalized SBA-15 mesoporous silica shows an extremely rapid adsorption rate as well as high adsorption capacity of EGCG (287 mg g−1) compared to other adsorbent previously reported. The adsorption amount of EGCG is 19 times as high as that of CAF on SBA-15-NH2, indicating its high selectivity. It was demonstrated that the kinetic data were fitted well using a pseudo-second-order kinetics model. The adsorption data of EGCG fitted the Langmuir isothermal model better than Freundlich isothermal model. Considering the excellent adsorption performance, it is expected that mesoporous silica materials will find applications in the separation and enrichment of EGCG.Acknowledgements
We are grateful for financial support from Modern Agro–Industry Technology Research System (nycytx-26) and the tea quality and risk assessment of innovation team of science and technology innovation project in Chinese Academy of Agricultural Sciences.Notes and references
- N. Khan and H. Mukhtar, Tea polyphenols for health promotion, Life Sci., 2007, 81(7), 519–533 CrossRef CAS PubMed.
- Q. V. Vuong, J. B. Golding, M. Nguyen and P. D. Roach, Extraction and isolation of catechins from tea, J. Sep. Sci., 2010, 33(21), 3415–3428 CrossRef CAS PubMed.
- P. Nawrot, S. Jordan, J. Eastwood, J. Rotstein, A. Hugenholtz and M. Feeley, Effects of caffeine on human health, Food Addit. Contam., 2003, 20(1), 1–30 CrossRef CAS PubMed.
- A. Perva-Uzunalic, M. Skerget, Z. Knez, B. Weinreich, F. Otto and S. Gruner, Extraction of active ingredients from green tea (Camellia sinensis): Extraction efficiency of major catechins and caffeine, Food Chem., 2006, 96(4), 597–605 CrossRef CAS PubMed.
- S. Lee, M. K. Park, K. H. Kim and Y. S. Kim, Effect of supercritical carbon dioxide decaffeination on volatile components of green teas, J. Food Sci., 2007, 72(7), S497–S502 CrossRef CAS PubMed.
- S. S. Dash and S. N. Gummadi, Catabolic pathways and biotechnological applications of microbial caffeine degradation, Biotechnol. Lett., 2006, 28(24), 1993–2002 CrossRef CAS PubMed.
- M. Kato, K. Mizuno, A. Crozier, T. Fujimura and H. Ashihara, Plant biotechnology-Caffeine synthase gene from tea leaves, Nature, 2000, 406(6799), 956–957 CrossRef CAS PubMed.
- J.-H. Ye, Y.-R. Liang, J. Jin, H.-L. Liang, Y.-Y. Du, J.-L. Lu, Q. Ye and C. Lin, Preparation of partially decaffeinated instant green tea, J. Agric. Food Chem., 2007, 55(9), 3498–3502 CrossRef CAS PubMed.
- J. H. Ye, J. Jin, H. L. Liang, J. L. Lu, Y. Y. Du, X. Q. Zheng and Y. R. Liang, Using tea stalk lignocellulose as an adsorbent for separating decaffeinated tea catechins, Bioresour. Technol., 2009, 100(2), 622–628 CrossRef CAS PubMed.
- J. H. Ye, J. J. Dong, J. L. Lu, X. Q. Zheng, J. Jin, H. Chen and Y. R. Liang, Effect of graft copolymerization of fir sawdust lignocellulose with N-vinylpyrrolidone on adsorption capacity to tea catechins, Carbohydr. Polym., 2010, 81(2), 441–447 CrossRef CAS PubMed.
- J.-L. Lu, M.-Y. Wu, X.-L. Yang, Z.-B. Dong, J.-H. Ye, D. Borthakur, Q.-L. Sun and Y.-R. Liang, Decaffeination of tea extracts by using poly(acrylamide-co-ethylene glycol dimethylacrylate) as adsorbent, J. Food Eng., 2010, 97(4), 555–562 CrossRef CAS PubMed.
- Z.-B. Dong, Y.-R. Liang, F.-Y. Fan, J.-H. Ye, X.-Q. Zheng and J.-L. Lu, Adsorption Behavior of the Catechins and Caffeine onto Polyvinylpolypyrrolidone, J. Agric. Food Chem., 2011, 59(8), 4238–4247 CrossRef CAS PubMed.
- Y. Liu, Q. Bai, S. Lou, D. Di, J. Li and M. Guo, Adsorption Characteristics of (−)-Epigallocatechin Gallate and Caffeine in the Extract of Waste Tea on Macroporous Adsorption Resins Functionalized with Chloromethyl, Amino, and Phenylamino Groups, J. Agric. Food Chem., 2012, 60(6), 1555–1566 CrossRef CAS PubMed.
- N. Ma, P. Wang, X. Kong, R. Shi, Z. Yuan and C. Wang, Selective removal of caffeine from tea extracts using macroporous crosslinked polyvinyl alcohol adsorbents, J. Sep. Sci., 2012, 35(1), 36–44 CrossRef CAS PubMed.
- Z. Wu and D. Zhao, Ordered mesoporous materials as adsorbents, Chem. Commun., 2011, 47(12), 3332–3338 RSC.
- K. Ariga, A. Vinu, M. Miyahara, J. P. Hill and T. Mori, One-Pot Separation of Tea Components through Selective Adsorption on Pore-Engineered Nanocarbon, Carbon Nanocage, J. Am. Chem. Soc., 2007, 129(36), 11022–11023 CrossRef CAS PubMed.
- F. Hoffmann, M. Cornelius, J. Morell and M. Froeba, Silica-based mesoporous organic–inorganic hybrid materials, Angew. Chem., Int. Ed., 2006, 45(20), 3216–3251 CrossRef CAS PubMed.
- A. Benhamou, M. Baudu, Z. Derriche and J. P. Basly, Aqueous heavy metals removal on amine-functionalized Si-MCM-41 and Si-MCM-48, J. Hazard. Mater., 2009, 171(1–3), 1001–1008 CrossRef CAS PubMed.
- J. Aguado, J. M. Arsuaga, A. Arencibia, M. Lindo and V. Gascon, Aqueous heavy metals removal by adsorption on amine-functionalized mesoporous silica, J. Hazard. Mater., 2009, 163(1), 213–221 CrossRef CAS PubMed.
- Z. A. Alothman and A. W. Apblett, Metal ion adsorption using polyamine-functionalized mesoporous materials prepared from bromopropyl-functionalized mesoporous silica, J. Hazard. Mater., 2010, 182(1–3), 581–590 CrossRef CAS PubMed.
- G. Li, Z. Zhao, J. Liu and G. Jiang, Effective heavy metal removal from aqueous systems by thiol functionalized magnetic mesoporous silica, J. Hazard. Mater., 2011, 192(1), 277–283 CAS.
- T. Phuong Tran Thi, D. Hang Tran, P. Hung Nguyen, V. Nga Nguyen Thi, S. J. Kim and V. Vien, Synthesis, characterization and phenol adsorption of carbonyl-functionalized mesoporous silicas, J. Porous Mater., 2012, 19(3), 295–300 CrossRef.
- X. Wang, K. S. K. Lin, J. C. C. Chan and S. Cheng, Direct Synthesis and Catalytic Applications of Ordered Large Pore Aminopropyl-Functionalized SBA-15 Mesoporous Materials, J. Phys. Chem. B, 2005, 109(5), 1763–1769 CrossRef CAS PubMed.
- M. C. Bruzzoniti, A. Prelle, C. Sarzanini, B. Onida, S. Fiorilli and E. Garrone, Retention of heavy metal ions on SBA-15 mesoporous silica functionalised with carboxylic groups, J. Sep. Sci., 2007, 30(15), 2414–2420 CrossRef CAS PubMed.
- L. J. Kennedy, J. J. Vijaya, K. Kayalvizhi and G. Sekaran, Adsorption of phenol from aqueous solutions using mesoporous carbon prepared by two-stage process, Chem. Eng. J., 2007, 132(1–3), 279–287 CrossRef CAS PubMed.
- A. E. Ofomaja, Kinetics and mechanism of methylene blue sorption onto palm kernel fibre, Process Biochem., 2007, 42(1), 16–24 CrossRef CAS PubMed.
- B. H. Hameed, J. M. Salman and A. L. Ahmad, Adsorption isotherm and kinetic modeling of 2,4-D pesticide on activated carbon derived from date stones, J. Hazard. Mater., 2009, 163(1), 121–126 CrossRef CAS PubMed.
- X.-j. Hu, J.-s. Wang, Y.-g. Liu, X. Li, G.-m. Zeng, Z.-l. Bao, X.-x. Zeng, A.-w. Chen and F. Long, Adsorption of chromium(VI) by ethylenediamine-modified cross-linked magnetic chitosan resin: Isotherms, kinetics and thermodynamics, J. Hazard. Mater., 2011, 185(1), 306–314 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of experimental procedures of preparation and equations of adsorption models and other data. See DOI: 10.1039/c4ra06922f |
| This journal is © The Royal Society of Chemistry 2014 |