An achievement of over 12 percent efficiency in an organic dye-sensitized solar cell†
Kenji
Kakiage
ab,
Yohei
Aoyama
a,
Toru
Yano
*a,
Takahiro
Otsuka
a,
Toru
Kyomen
c,
Masafumi
Unno
c and
Minoru
Hanaya
*c
aEnvironmental & Energy Materials Laboratory, ADEKA CORPORATION, 7-2-35 Higashiogu, Arakawa, Tokyo 116-8554, Japan. E-mail: yanotoru@adeka.co.jp
bHuman Resource Cultivation Center (HRCC), Gunma University, 1-5-1 Tenjin-cho, Kiryu, Gunma 376-8515, Japan
cDivision of Molecular Science, Faculty of Science and Technology, Gunma University, 1-5-1 Tenjin-cho, Kiryu, Gunma 376-8515, Japan. E-mail: mhanaya@gunma-u.ac.jp
First published on 25th April 2014
Abstract
Dye-sensitized solar cells fabricated by using a novel metal-free alkoxysilyl carbazole as a sensitizing dye and a Co3+/2+-complex redox electrolyte exhibited light-to-electric energy conversion efficiencies of over 12% with open-circuit photovoltages higher than 1 V by applying a hierarchical multi-capping treatment to the photoanode.
Dye-sensitized solar cells (DSSCs), composed of mesoporous nanocrystalline-TiO2 films modified via sensitizing dyes as photoelectrodes, redox electrolytes and counter electrodes, have become a promising alternative photovoltaic technology of the next generation to conventional inorganic solar cells because of their ease of fabrication and potential low costs.1–3 Towards the realization of higher photovoltaic performance and durability in DSSCs, the development of the sensitizing dyes is one of the most important approaches. However, the investigation of the sensitizing dyes has been mostly limited to those including carboxy groups as the anchor moiety for chemical bonding to the surface of the TiO2 electrodes.1–5
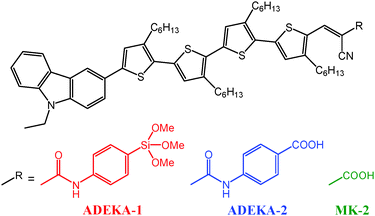
Organosilicon compounds such as alkoxysilanes and silanols have high bonding ability to metal-oxide surfaces by forming firm Si–O–metal bonds,6–9 and alkoxysilyl dyes have been demonstrated as potential materials for sensitizing dyes by a higher electron-transfer efficiency from the light-excited dye to the TiO2 electrode through Si–O–Ti (titanosiloxane) bonds and a higher photovoltage of the cell than that using the carboxy analog.10 Recently, metal-free MK dyes of carbazole/alkyl-functionalized oligothiophene/cyanoacrylic acid type compounds were reported by Koumura and Hara.11–13 The DSSCs sensitized with those organic dyes were demonstrated to possess high photostability and showed relatively high light-to-electric energy conversion efficiencies (η) of about 8%,11,12 and 9–10% efficiencies have been obtained by using a cobalt(III/II) tris(2,2′-bipyridine) complex ([Co(bpy)3]3+/2+) as the redox electrolyte in the cells.13,14 By the application of anchoring with titanosiloxane bonds to this type of dyes and by employing Co3+/2+-complex redox electrolytes, it would be possible to produce efficient DSSCs. In this work, therefore, we designed and synthesized an alkoxysilyl derivative of MK-2, ADEKA-1 (Fig. 1), and succeeded in achieving the η value of 12.5% in a DSSC sensitized by the dye.
ADEKA-1 and ADEKA-2, which is a benzoic-acid derivative of ADEKA-1 synthesized as a reference dye, exhibited similar UV-visible absorption spectra to MK-2 in toluene solutions; their major absorption bands assignable to the π–π* transition were observed in the visible region between 400 and 600 nm (Fig. S1, ESI†). The maximum molar absorption coefficients (εmax) at λmax were evaluated to be 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 (498 nm), 42
200 (498 nm), 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 (504 nm) and 37
700 (504 nm) and 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 (489 nm) for ADEKA-1, ADEKA-2 and MK-2, respectively. Slight red shifts of λmax and increments of εmax in ADEKA-1 and ADEKA-2 compared with MK-2 are considered to be due to the introduction of the phenyl-amide moieties. The oxidation potentials (Eox: approximately the energy levels of HOMO) of these dyes were determined by means of cyclic voltammetry (Fig. S2, ESI†). ADEKA-1 and ADEKA-2 introduced phenyl-amide moieties showed more positive Eox of 0.99 V and 1.01 V vs. NHE, respectively, compared to that of MK-2 (0.95 V). The values of Eox are more positive than that of the redox potential of 0.56 V vs. NHE in [Co(bpy)3]3+/2+,15 thus providing thermodynamic driving forces for the dye regeneration reactions by the electron transfer from the [Co(bpy)3]3+/2+ redox.
400 (489 nm) for ADEKA-1, ADEKA-2 and MK-2, respectively. Slight red shifts of λmax and increments of εmax in ADEKA-1 and ADEKA-2 compared with MK-2 are considered to be due to the introduction of the phenyl-amide moieties. The oxidation potentials (Eox: approximately the energy levels of HOMO) of these dyes were determined by means of cyclic voltammetry (Fig. S2, ESI†). ADEKA-1 and ADEKA-2 introduced phenyl-amide moieties showed more positive Eox of 0.99 V and 1.01 V vs. NHE, respectively, compared to that of MK-2 (0.95 V). The values of Eox are more positive than that of the redox potential of 0.56 V vs. NHE in [Co(bpy)3]3+/2+,15 thus providing thermodynamic driving forces for the dye regeneration reactions by the electron transfer from the [Co(bpy)3]3+/2+ redox.
The adsorptions of carboxy dyes such as ADEKA-2 and MK-2 on the TiO2 electrodes are understood to proceed from the formation of ester-like and/or bridging linkages between the carboxy groups and the hydroxy groups on the surface of the TiO2 electrode.3,11 On the other hand, ADEKA-1, which has a trimethoxysilyl group as the anchor moiety to the TiO2 electrode, is considered to adsorb on the electrode with the formation of three titanosiloxane (Si–O–Ti) bonds (Fig. S3, ESI†).7,9,10 To examine the durability of the linkages between the dyes and the TiO2 electrodes, we soaked the dye-adsorbed TiO2 electrodes into a mixed solvent of 3-methoxypropionitrile and water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
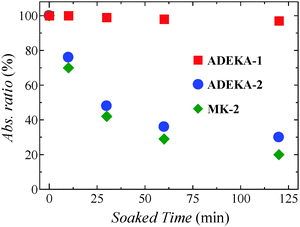
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume) at 85 °C in the dark and traced the time dependence of the light absorption due to the dyes on the TiO2 electrodes (Fig. 2, Fig. S4 and S5, ESI†). As shown in Fig. 2, although 70–80% of adsorbed dye molecules were observed to eliminate from the electrodes after 120 min soaking in the cases of ADEKA-2 and MK-2, more than 95% of ADEKA-1 molecules were confirmed to be on the TiO2 electrode. These results evidence the robustness of the linkage by the titanosiloxane bonds.
1 in volume) at 85 °C in the dark and traced the time dependence of the light absorption due to the dyes on the TiO2 electrodes (Fig. 2, Fig. S4 and S5, ESI†). As shown in Fig. 2, although 70–80% of adsorbed dye molecules were observed to eliminate from the electrodes after 120 min soaking in the cases of ADEKA-2 and MK-2, more than 95% of ADEKA-1 molecules were confirmed to be on the TiO2 electrode. These results evidence the robustness of the linkage by the titanosiloxane bonds.
The photovoltaic properties of the cells fabricated using the three carbazole dyes as sensitizers are listed in Table 1. The photovoltaic parameters, short-circuit photocurrent density (Jsc), open-circuit photovoltage (Voc), fill factor (FF) and η, were assessed from the photocurrent–voltage (I–V) curves under the simulated AM-1.5G solar irradiation with the intensity of 100 mW cm−2 (Fig. S6, ESI†). Entries 1–3 are the cells using the [Co(bpy)3]3+/2+ redox electrolyte. Among the cells, the ADEKA-1-sensitized cell exhibited slightly higher Jsc and Voc values than the cells sensitized by the carboxy dyes of ADEKA-2 and MK-2, and a higher η over 10% was evaluated than those for the cells with ADEKA-2 and MK-2. The higher η is considered to be mainly due to the higher Voc caused by the linkage of the dye by the titanosiloxane bonds on the surface of the TiO2 electrode (Fig. S3, ESI†).10
| Entry | Dye | Redox/electrolytea | Cappingb | J sc (mA cm−2) | V oc (V) | FF | η (%) |
|---|---|---|---|---|---|---|---|
| a Electrolyte A: 0.25 M Co2+ + 0.035 M Co3+ + 0.10 M LiClO4 + 0.50 M TBP in acetonitrile, electrolyte B: 0.25 M [Co(Cl-phen)3]2+ + 0.035 M [Co(Cl-phen)3]3+ + 0.07 M LiClO4 + 0.02 M NaClO4 + 0.03 M TBAPF + 0.01 M TBPPF + 0.01 M HMImPF + 0.30 M TBP + 0.10 M TMSP + 0.10 M MP in acetonitrile. b Single: the single-capping with heptanoic acid, multi: the hierarchical multi-capping. c The results for the cell attached with an antireflection film on the surface of the photoanode. d The value is the average of the results of the three cells examined (Table S2, ESI). | |||||||
| 1 | ADEKA-1 | [Co(bpy)3]3+/2+/A | Single | 16.1 | 0.848 | 0.762 | 10.4 |
| 2 | ADEKA-2 | [Co(bpy)3]3+/2+/A | Single | 15.1 | 0.821 | 0.752 | 9.32 |
| 3 | MK-2 | [Co(bpy)3]3+/2+/A | Single | 15.8 | 0.814 | 0.753 | 9.68 |
| 4 | ADEKA-1 | [Co(Cl-phen)3]3+/2+/A | Single | 15.6 | 0.897 | 0.764 | 10.7 |
| 5 | MK-2 | [Co(Cl-phen)3]3+/2+/A | Single | 8.8 | 0.811 | 0.752 | 5.37 |
| 6 | ADEKA-1 | [Co(Cl-phen)3]3+/2+/A | Multi | 15.8 | 0.958 | 0.748 | 11.3 |
| 7 | ADEKA-1 | [Co(Cl-phen)3]3+/2+/B | Multi | 14.9 | 1.034 | 0.777 | 12.0 |
| 8c | ADEKA-1 | [Co(Cl-phen)3]3+/2+/B | Multi | 15.6d | 1.036d | 0.774d | 12.5d |
The maximum photovoltage (Vmax) obtained in the DSSC is attributed to the energy gap between the quasi-Fermi level [approximately the energy level of the conduction-band edge (EC.B.)] of the TiO2 [−0.5 V vs. NHE for anatase2,3] and the redox potential of the electrolyte. To obtain an even higher photovoltage and to improve the η value further in the cells, therefore, we employed a cobalt(III/II) tris(5-chloro-1,10-phenanthroline) complex ([Co(Cl-phen)3]3+/2+) as the redox electrolyte, which has a higher redox potential of 0.72 V vs. NHE than [Co(bpy)3]3+/2+.15 By using the [Co(Cl-phen)3]3+/2+ redox electrolyte in the ADEKA-1-sensitized cell (entry 4), the Voc value was increased to 0.897 V from that in the cell with the [Co(bpy)3]3+/2+ redox electrolyte of 0.848 V. The η was actually improved by an increment of the Voc value, although the Jsc value decreased slightly by a sluggish diffusion of [Co(Cl-phen)3]3+/2+ compared to [Co(bpy)3]3+/2+ due to the bulkiness.15–17 On the other hand, a significant lowering of η was observed in the MK-2-sensitized cell using the [Co(Cl-phen)3]3+/2+ redox electrolyte (entry 5). An energy gap between the Eox of MK-2 and the redox potential of [Co(Cl-phen)3]3+/2+ was estimated to be 0.23 eV, which is smaller than 0.27 eV for ADEKA-1. The low η value observed is considered to be the result of the lack of the thermodynamic driving force for the dye regeneration reaction, which proceeds through the electron transfer from the Co2+ complex to the dye in the oxidized state, by the small energy gap.15–18
The Voc value in the ADEKA-1-sensitized cell was increased by using [Co(Cl-phen)3]3+/2+ redox instead of [Co(bpy)3]3+/2+. However the increment and the observed Voc were much lower than the expected value from the estimated Vmax of the cells, which are 1.22 V and 1.06 V in the cases of [Co(Cl-phen)3]3+/2+ and [Co(bpy)3]3+/2+, respectively (Fig. S7, ESI†). The reason for the lower Voc in comparison with the Vmax is believed to be because the cells employing Co3+/2+-complex redox electrolytes with more positive redox potentials are exposed to the faster recombination reaction, that is, the undesirable electron transfer from the TiO2 electrode to the electrolyte, and the reaction can be suppressed by restricting the contact of the Co3+ complex to the naked surface of the TiO2 electrode between the adsorbed sensitizing dye molecules.16–22 Therefore, we examined a hierarchical multi-capping treatment of the ADEKA-1-adsorbed TiO2 electrode using eight molecules with various alkyl-chain lengths and with three kinds of anchor moieties, i.e. carboxy, phosphonic and silyl groups (Fig. S8, ESI†). In the treatment, we performed the capping by larger agents, which need larger space for the adsorption, to smaller ones, which need only small space, and tried to form an ‘alkyl-thicket’ structure on the surface of the TiO2 electrode between the adsorbed sensitizing dye molecules for an effective passivation of the electrode (Fig. S9, ESI†). The hierarchical multi-capping treatment worked properly in the ADEKA-1-sensitized cell with the [Co(Cl-phen)3]3+/2+ redox electrolyte, and the Voc value was improved from 0.897 V to 0.958 V resulting in a higher conversion efficiency of 11.3% (entry 6; Table S1, ESI†).
In DSSCs, photovoltaic performances are well known to be affected by the compositions of the electrolyte solutions.23–25 This was also true in the ADEKA-1-sensitized cell, and the η value was improved up to 12.0% with the Voc of over 1 V by using an experimentally optimized electrolyte (entry 7; Fig. S10, ESI†). In order to harvest the incident light more efficiently, we finally attached an antireflection film to the cell in entry 7 (entry 8). The I–V curve and incident monochromatic photon-to-current conversion efficiency (IPCE) spectrum of the cell are shown in Fig. 3. A maximum IPCE value was reached to 85%, and we succeeded in obtaining an η value of 12.5%. This efficiency is the highest among those reported so far for DSSCs with organic dyes.2–4,14,22,26 Such a high photovoltaic performance of the cell is based on the high stability of titanosiloxane bonds formed between ADEKA-1 and the surface of the TiO2 electrodes. The stability allowed the formation of the ‘alkyl-thicket’ structure on the TiO2 electrode by using the hierarchical multi-capping treatment.
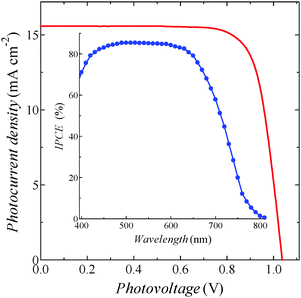 | ||
| Fig. 3 Typical I–V properties of the ADEKA-1-sensitized solar cell with the highest efficiency of 12.5% (entry 8 in Table 1) under the simulated one sun irradiation (AM-1.5G, 100 mW cm−2). Inset shows the IPCE spectrum of the cell. | ||
The achievement of the η value of 12.5% under the one sun condition in the cell with the metal-free dye of ADEKA-1 as a single sensitizer indicates the high potential of organic dyes with organosilicon tethers for binding the metal oxides as the photosensitizers for DSSCs, and the present results provide a fertile base for further improving the photovoltaic performance of DSSCs. The combination of the co-sensitization using plural sensitizing dyes with organosilyl anchor moieties and the hierarchical multi-capping in the cells with Co3+/2+-complex redox electrolytes is one of the approaches for achieving a higher efficiency of over 13%, and such a challenge is currently underway in our group.
This work was partly supported by the “Element Innovation” Project by Ministry of Education, Culture, Sports, Science & Technology in Japan. We also thank the Human Resource Cultivation Center, Gunma University for a research fund.
Notes and references
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef CAS.
- M. Grätzel, Acc. Chem. Res., 2009, 42, 1788 CrossRef PubMed.
- A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595 CrossRef CAS PubMed.
- S. Ahmad, E. Guillén, L. Kavan, M. Grätzel and M. K. Nazeeruddin, Energy Environ. Sci., 2013, 6, 3439 CAS.
- S. Mathew, A. Yella, P. Gao, R. Humphry-Baker, B. F. E. Curchod, N. Ashari-Astani, I. Tavernelli, U. Rothlisberger, M. K. Nazeeruddin and M. Grätzel, Nat. Chem., 2014, 6, 242 CrossRef CAS PubMed.
- M. Unno, K. Kakiage, M. Yamamura, T. Kogure, T. Kyomen and M. Hanaya, Appl. Organomet. Chem., 2010, 24, 247 CrossRef CAS.
- K. Kakiage, M. Yamamura, E. Ido, T. Kyomen, M. Unno and M. Hanaya, Appl. Organomet. Chem., 2011, 25, 98 CrossRef CAS.
- K. Kakiage, T. Tokutome, S. Iwamoto, T. Kyomen and M. Hanaya, Chem. Commun., 2013, 49, 179 RSC.
- C. Baik, D. Kim, M.-S. Kang, S. O. Kang, J. Ko, M. K. Nazeeruddin and M. Grätzel, J. Photochem. Photobiol., A, 2009, 201, 168 CrossRef CAS PubMed.
- K. Kakiage, M. Yamamura, E. Fujimura, T. Kyomen, M. Unno and M. Hanaya, Chem. Lett., 2010, 39, 260 CrossRef CAS.
- Z.-S. Wang, N. Koumura, Y. Cui, M. Takahashi, H. Sekiguchi, A. Mori, T. Kubo, A. Furube and K. Hara, Chem. Mater., 2008, 20, 3993 CrossRef CAS.
- R. Katoh, A. Furube, S. Mori, M. Miyashita, K. Sunahara, N. Koumura and K. Hara, Energy Environ. Sci., 2009, 2, 542 CAS.
- T. Uchiyama, T. N. Murakami, N. Yoshii, Y. Uemura, N. Koumura, N. Masaki, M. Kimura and S. Mori, Chem. Lett., 2013, 42, 453 CrossRef CAS.
- W. Xiang, W. Huang, U. Bach and L. Spiccia, Chem. Commun., 2013, 49, 8997 RSC.
- S. M. Feldt, G. Wang, G. Boschloo and A. Hagfeldt, J. Phys. Chem. C, 2011, 115, 21500 CAS.
- J. Cong, X. Yang, L. Kloo and L. Sun, Energy Environ. Sci., 2012, 5, 9180 CAS.
- T. W. Hamann, Dalton Trans., 2012, 41, 3111 RSC.
- S. M. Feldt, P. W. Lohse, F. Kessler, M. K. Nazeeruddin, M. Grätzel, G. Boschloo and A. Hagfeldt, Phys. Chem. Chem. Phys., 2013, 15, 7087 RSC.
- Y. Cao, N. Cai, Y. Wang, R. Li, Y. Yuan and P. Wang, Phys. Chem. Chem. Phys., 2012, 14, 8282 RSC.
- M. Zhang, J. Zhang, Y. Fan, L. Yang, Y. Wang, R. Li and P. Wang, Energy Environ. Sci., 2013, 6, 2939 CAS.
- Y. Liu, J. R. Jennings, X. Wang and Q. Wang, Phys. Chem. Chem. Phys., 2013, 15, 6170 RSC.
- M. Liang and J. Chen, Chem. Soc. Rev., 2013, 42, 3453 RSC.
- K. Kakiage, T. Tsukahara, T. Kyomen, M. Unno and M. Hanaya, Chem. Lett., 2012, 41, 895 CrossRef CAS.
- S. R. Raga, E. M. Barea and F. Fabregat-Santiago, J. Phys. Chem. Lett., 2012, 3, 1629 CrossRef CAS.
- T. Kanzaki, S. Nakade, Y. Wada and S. Yanagida, Photochem. Photobiol. Sci., 2006, 5, 389 CAS.
- M. Zhang, Y. Wang, M. Xu, W. Ma, R. Li and P. Wang, Energy Environ. Sci., 2013, 6, 2944 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4cc02192d |
| This journal is © The Royal Society of Chemistry 2014 |