An infrared study of CO2 activation by holmium ions, Ho+ and HoO+†
Abstract
We report a combined experimental and computational study of carbon dioxide activation at gas-phase Ho+ and HoO+ centres. Infrared action spectra of Ho(CO2)n+ and [HoO(CO2)n]+ ion–molecule complexes have been recorded in the spectral region 1700–2400 cm−1 and assigned by comparison with simulated spectra of energetically low-lying structures determined by density functional theory. Little by way of activation is observed in Ho(CO2)n+ complexes with CO2 binding end-on to the Ho+ ion. By contrast, all [HoO(CO2)n]+ complexes n ≥ 3 show unambiguous evidence for formation of a carbonate radical anion moiety, 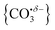 . The signature of this structure, a new vibrational band observed around 1840 cm−1 for n = 3, continues to red-shift monotonically with each successive CO2 ligand binding with net charge transfer from the ligand rather than the metal centre.
. The signature of this structure, a new vibrational band observed around 1840 cm−1 for n = 3, continues to red-shift monotonically with each successive CO2 ligand binding with net charge transfer from the ligand rather than the metal centre.

- This article is part of the themed collection: Stability and properties of new-generation metal and metal-oxide clusters down to subnanometer scale


 Please wait while we load your content...
Please wait while we load your content...