Open Access Article
Open Access ArticleStrategic Ni-doping improved electrocatalytic H2 production by Bi3O4Br in alkaline water†
Manodip
Pal
a,
Rathindranath
Biswas
 a,
Sanmitra
Barman
*b and
Arnab
Dutta
a,
Sanmitra
Barman
*b and
Arnab
Dutta
 *acd
*acd
aChemistry Department, Indian Institute of Technology Bombay, Powai, Maharashtra 400076, India. E-mail: arnab.dutta@iitb.ac.in
bCenter for Advanced Materials and Devices (CAMD), BML Munjal University, Haryana-122413, India. E-mail: sanmitra.barman@bmu.edu.in
cNational Center of Excellence, CCU, Indian Institute of Technology Bombay, Powai, Maharashtra 400076, India
dInterdisciplinary Program in Climate Studies, Indian Institute of Technology Bombay, Powai, Maharashtra 400076, India
First published on 24th May 2024
Abstract
Establishing a cost-effective and efficient electrocatalytic pathway for the hydrogen evolution reaction (HER) is the key to our quest for a carbon-neutral energy landscape. We report a simple and straightforward approach to synthesize an efficient, stable, and low-cost noble metal-free Bi3O4Br electrocatalyst. Tactical doping of Ni ions into Bi3O4Br effectively enhanced the conductivity, accelerated the charge transfer process, and provided more catalytic active sites to significantly boost the alkaline electrochemical HER performance of Bi3O4Br. This Ni-doped Bi3O4Br exhibited a lower overpotential of 662 mV compared to that of Bi3O4Br (736 mV) at a higher current density (50 mA cm−2). Additionally, the HER kinetics were also enhanced in terms of Tafel slope for this doped material (159 mV dec−1) compared to the pristine Bi3O4Br (245 mV dec−1), which coincides with a significant improvement in the mass activity (52 A g−1 to 98 A g−1). Notably, the overpotential of Ni-doped Bi3O4Br was further reduced to 614 mV at the same current density of 50 mA cm−2 during photoelectrochemical HER performance testing, and the faradaic efficiency was improved from 79% to 87%. Finally, an enhanced durability of the material was observed for Bi3O4Br following the Ni-doping. Hence, this strategy highlights the importance of unravelling upgraded catalytic behaviour for abundant materials with rational doping.
Introduction
Hydrogen is a popular choice of future energy material for transducing renewable energy to practically usable electricity with minimal greenhouse gas emissions.1–4 In this flow of events, the hydrogen evolution reaction (HER) from abundant water is reckoned as a key step. Water electrolysis is looked upon as the primary pathway for industrial-scale HER, which is required to be executed at a rapid rate with low overpotential requirements. Over the last few decades, extensive research efforts have been made to develop an electrocatalyst that can produce hydrogen via the electrocatalytic splitting of water at a lower overpotential with appreciable rates.5–9 Electrocatalytic water splitting can be carried out in both acidic and alkaline media, and the lack of acid-tolerant counter electrode materials is a bottleneck for the production of multimillion tons of H2via acidic electrolyzers.10–12 Therefore, major emphasis has been given to the quest for a stable and highly active catalyst for alkaline HER in the past few decades.7,13 Moreover, similar efforts are being made to develop compatible, stable, and efficient electrocatalysts for the other half-cell reaction of water electrolysis: the complementary oxygen evolution reaction (OER) under similar conditions. The appropriate assembly of HER and OER electrocatalysts is vital for a fully functional alkaline water electrolyzer.14–19 Here, the challenge is to generate an electrocatalyst composed of earth-abundant elements that can display good stability and high efficiency while executing the HER in an alkaline medium.20–22 There has been a plethora of electrocatalysts containing earth-abundant transition metals developed recently for electrocatalytic water splitting.23–25Bismuth oxyhalides (BiOX, where X = Cl, Br or I), classified as a ternary compound, have a layered structure consisting of repetitive units of [Bi2O2]2+ and X−, which are typically held together by van der Waals forces of attraction. These materials have emerged as a serious contender for practical electrocatalytic applications owing to their ease of preparation, excellent photocatalytic properties, relative nontoxicity, and low cost. These materials are promising photocatalysts due to their inherent wide bandgap that broadens their absorption profile through the ultra-violet and visible region. Nevertheless, these materials exhibit facile charge recombination, leading to low catalytic responses in the absence of any charge-trapping centers.26,27 Tactical incorporation of surface defects in these nanomaterials is reckoned as a key approach for stabilizing the charge-separated holes and electrons, which in turn proceeds to effective catalysis. Enriching the bismuth oxyhalide framework with additional bismuth ions is found to produce promising results.28 These bismuth-rich bismuth oxyhalides are formulated as BimOnXp, where X = Cl, Br or I, and m, n, and p are various stoichiometric proportions.
Among these bismuth-rich bismuth oxyhalides, Bi3O4Br is one of the popular choices due to the ease of synthesis, tuneable morphology with controllable stoichiometry, and enhanced visible light absorption properties.29–31 It is reported that Bi3O4Br has a layer structure composed of [Bi3O4]1+ units attached to the Br− by van der Waals force of attraction. Two bismuth coordination environments were reported for this material. In the first coordination geometry, the bismuth ion is ligated to three oxygen atoms and one bromine atom. In the second environment, one bismuth atom is attached to four oxygen atoms in the unit cell of Bi3O4Br. To further tailor the catalytic properties of Bi3O4Br, the bismuth is substituted with a transition metal, such as Co, which was reported to be an effective strategy. Recently, single atom (Co)-doped Bi3O4Br was also reported that illustrated remarkable photo-electrocatalytic activity for carbon dioxide conversion.29 Jiang et al. developed Ag-doped Bi3O4Br for the efficient photodegradation of bisphenol-A.32 Wang et al. fabricated an Ni-doped CsPbBr3/Bi3O4Br heterostructured material for efficient photocatalytic CO2 conversion following a Z-scheme of electron transfer.33 Hence, Bi3O4Br-based materials have been widely used in the field of photochemical applications.
In this work, we have synthesized a Ni-doped (0.4 atomic %) Bi3O4Br material and probed its reactivity as an electrocatalyst in an alkaline aqueous medium (pH ∼14.0). The inclusion of Ni 3d-bands into the Bi 4f orbitals reduced the original bandgap of the Bi3O4Br, which was supported by the X-ray photoelectron spectroscopy (XPS) data. During the assembly of the electrocatalytic material, polyvinylpyrrolidone (PVP) was added to prevent any lateral growth of Bi3O4Br nanocrystals. The PVP directly interacts with Bi3O4Br, as a passivation layer is created around the crystal core through the strong interaction between the Bi3+ and the oxygen and nitrogen atoms present on the pyrrolidone ring, resulting in the formation of 2D-plate-shaped nanocrystals. The catalyst is fully characterized by an array of analytical techniques, such as XRD, XPS, UV-vis, FTIR, and Raman spectroscopy. For comparison, we have also synthesized pristine Bi3O4Br by following the same protocol and compared its electrocatalytic HER properties with those of the Ni-doped Bi3O4Br in an aqueous alkaline (1.0 M KOH, pH 14.0) medium.
Experimental procedures
Reagents and materials
All the chemicals mentioned are of analytical grade unless otherwise mentioned, which were used as procured without further purification. Bi(NO3)3·5H2O (98%), KCl, KBr, HNO3 (70%), Ni(II)(CH3COO)2·4H2O (98%), polyvinyl pyrrolidone (PVP), mannitol, ethanol (absolute), and NaOH (>97%) were purchased from Sigma Aldrich, India. Milli Q water (resistivity 18.2 MΩ cm) was used throughout for all the experiments. The pH value was maintained by using buffer capsules (Merck, India).Synthesis of Bi3O4Br and Ni-doped Bi3O4Br
Bi3O4Br and Ni-doped Bi3O4Br nanoparticles were prepared by the hydrothermal method under similar experimental conditions as reported in the literature.29 In summary, 0.5 mmol of Bi(NO3)3·5H2O was added to 0.2 g polyvinyl pyrrolidone (PVP) and 0.1 M mannitol solution in 15.0 mL of Millipore water. 0.005 mmol of Ni(II)(CH3COO)2·4H2O was added to the above mixture with vigorous stirring. In another beaker, 0.5 mmol of KBr was dissolved in 0.1 M mannitol solution (5 mL) in water. The KBr solution was added dropwise to the Bi(NO3)3·5H2O solution with vigorous stirring (1000 rpm). The reaction mixture was stirred for 30 minutes, and thereafter, the pH was adjusted to 11.5 with the addition of 1.0 M NaOH. The solution was heated at 160 °C for 20 hours in an autoclave and thereafter cooled down to room temperature gradually. The reaction mixture was washed repeatedly with distilled water and ethanol. The off-white color with greenish taint powder compound was isolated by centrifugation and dried at 50 °C overnight. Bi3O4Br was prepared following the same protocol without’ added nickel salt during the preparation.Characterization techniques
Crystal structure and phase identification studies were carried out through X-ray diffractometer (XRD, Pananalytical) using a Cu Kα source with wavelength, λ = 1.54 Å. The Raman scattering measurements were conducted using an electrically cooled CCD camera coupled with an Olympus BX41 Raman spectrometer by WITEC with laser lines at 532 nm. The absorption of light in the wavelength range 300–800 nm was studied by UV-vis diffuse reflectance spectroscopy (DRS) (UV-vis Cary series, Agilent Technologies). A Metrohm Auto lab PGSTAT 204 potentiostat galvanostat was used for all electrochemical measurements with a conventional three-electrode system at room temperature (298 K) using Nova 2.1.6 software. Transmission electron microscopy (TEM) analysis was performed by a Thermo Scientific, Themis 300 G3 instrument. The sample was drop cast on a carbon coated 300 mesh Cu grid (Ted Pella, Inc.) for TEM analysis. Fourier transform infrared spectroscopy (FTIR) (Vertex 70v, Bruker) was performed to understand the bonding structure. XPS was performed with a PHI-5702 X-ray photoelectron spectrometer.Electrochemical measurements
All the electrochemical studies were performed by using the Metrohm Auto lab potentiostat with a conventional three-electrode system at room temperature (298 K). The pH of the solutions was adjusted using an ORION STAR A111 (Thermo Scientific) or LMPH-9 (Labman Scientific) pH Meter. A spiral platinum electrode (Pt) and an Ag/AgCl electrode submerged in 3.0 M KCl were taken as the counter and reference electrodes, respectively. The catalyst ink was then prepared by taking 3.0 mg of catalyst in the solution of 120 μL of HPLC grade IPA, 80 μL of millipore water (18.2 MΩ cm at 298 K), and 30 μL of 5 wt% Nafion. The prepared catalyst was deposited on carbon fiber paper (with approximate mass loading of 1.04 mg cm−2) and directly used as the working electrode. 1.0 M KOH solution (pH 14.0) was utilized as the electrolyte solution. Before performing the linear sweep voltammetry (LSV) and electrochemical impedance spectroscopy (EIS), 100 cycles of cyclic voltammetry (CV) were performed with a 50 mV s−1 scan rate to obtain a steady state of the electrocatalysts. The Nyquist plots were measured by the application of a sinusoidal wave with an AC amplitude of 10 mV from the 105 Hz to 0.1 Hz operating frequency range. The potential values were measured against the Ag/AgCl reference electrode, which was then converted to a reversible hydrogen electrode (RHE) by using this equation.| ERHE (V) = EAg/AgCl (V) + 0.059pH + Eapplied |
Results and discussion
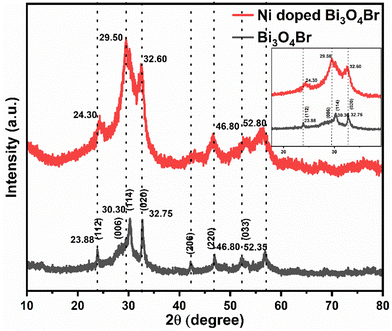
The pristine Bi3O4Br and Ni-doped Bi3O4Br were synthesized via the hydrothermal process. The crystalline structures and associated crystal phases of Bi3O4Br and Ni-doped Bi3O4Br were identified by deploying powder X-ray diffraction (XRD). All the peaks for the Bi3O4Br were indexed with the already existing literature that implied the presence of an orthorhombic unit cell (Fig. 1).29,34 The XRD signals broadened following the Ni-doping, suggesting that the inclusion of Ni in the structure reduces the crystallinity of Bi3O4Br. Ni-doping possibly creates bismuth and oxygen vacancies in the matrix due to the size mismatch to induce a change in the crystallinity of Bi3O4Br. The influence of the Ni-doping was specifically vivid with a shift observed for the peaks associated with the (112) and (114) planes, while the rest of the peaks remained constant.The X-ray photoelectron spectroscopic (XPS) study was performed next to further confirm the chemical composition, electronic structure, and valence state of each element present in Bi3O4Br and Ni-doped Bi3O4Br (Fig. 2). All the XPS spectra were corrected for specimen charging by referencing the C1s peak to 284.60 eV. Two high-intensity peaks in the high-resolution XPS spectra of Bi are observed at 158.48 eV and 163.81 eV that are assigned as Bi 4f7/2 and Bi 4f5/2, respectively, with a splitting energy Δ = 5.33 eV (Fig. 2a).35 On the contrary, the Bi 4f7/2 and Bi 4f5/2 peaks for the Ni-doped Bi3O4Br were red-shifted by 0.2 eV compared to the pristine Bi3O4Br. This low-energy shift indicates that the electronic environment around the bismuth atom has changed slightly after nickel doping. The two high-intensity peaks denote the +3 oxidation state of Bi in the Bi–O bonding inside the crystal lattice, and the slight shift towards lower binding energy (by 0.2 eV) following the Ni-addition possibly suggests an elongated Bi–O bonding in the Ni-doped Bi3O4Br. It is important to mention that the very low-intensity shoulders around 156.2 eV and 161.4 eV are due to the Bi 4f7/2 and Bi 4f5/2, respectively, arising from the Bi3+ sites of the Bi–Br bonding. O 1s showed two peaks with the binding energy values of 529.02 eV and 530.22 eV in Bi3O4Br with a splitting energy Δ = 1.2 eV. These two peaks are assigned to oxygen present in the vicinity of oxygen defects and the lattice oxygen (bound to metal, Bi), respectively (Fig. 2b).35 Similar peaks were observed at slightly higher energies of 529.92 eV and 531.80 eV with a splitting energy Δ = 1.88 eV. Such a shift in binding energy presumably appeared due to the redistribution of the electronic charge around the O atoms subsequent to the Ni-addition. The oxygen defect peak is relatively higher in intensity in the case of Ni-doped Bi3O4Br compared to pristine Bi3O4Br. Hence, the XPS data insinuates that the Ni-doped Bi3O4Br is more defect-rich in terms of oxygen vacancies than the pristine Bi3O4Br. Similarly, two Br peaks were observed at 67.6 eV and 68.70 eV and were allocated to Br 3d5/2 and Br 3d3/2, respectively (Fig. 2c).30 A Ni 2p3/2 peak is observed at 854.88 eV for the Ni-doped sample, which can be assigned to the +2 oxidation state of Ni (Fig. 2d).36,37 Lastly, the valence band XPS spectra show that the Ni doping shifts the valence band (VB) towards a more (0.05 eV) positive side in the energy scale with higher oxidizing ability than the pristine Bi3O4Br. From the XPS peak area analysis, the presence of Ni was observed as ca ∼ 0.4 atomic %.
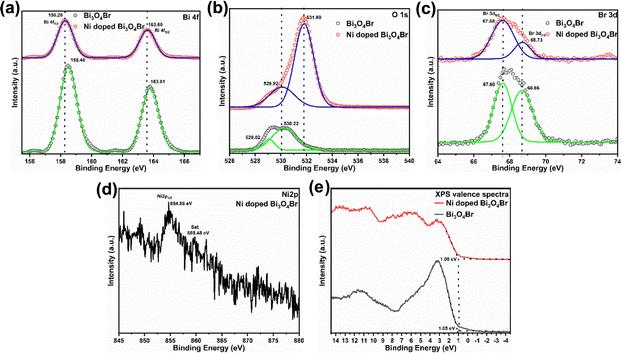 | ||
| Fig. 2 High resolution XPS spectra of (a) Bi 4f, (b) O 1s, (c) Br 3d, (d) Ni 2p, and (e) valence spectra for Bi3O4Br and Ni-doped Bi3O4Br. | ||
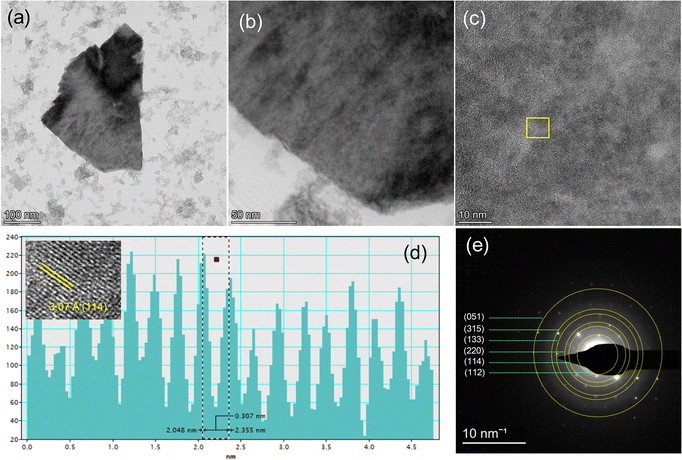
A transmission electron microscopy (TEM) study was performed for the morphological investigation of the materials. The TEM images of pure Bi3O4Br and Ni-doped Bi3O4Br represent the 2D-plate-like morphology (Fig. 3, and Fig. S1, ESI†). The fast Fourier transform (FFT) is used to convert the raw image data obtained from the transmission electron microscope into a frequency domain representation. This permits the analysis of the spatial frequency content of the image, which can provide information about the crystal structure, defects, and other features of the sample being studied. The FFT is particularly useful for analysing diffraction patterns, which can provide valuable information about the atomic arrangement within a material. The obtained FFT contains diffraction spots (reciprocal space pattern), which can be further used to create images of the region of interest using the inverse FFT method. HR-TEM image and inverse fast Fourier transition (FFT) analysis confirmed the presence of the (114) plane on the surface of the Ni-doped Bi3O4Br nanostructure (Fig. 3c and d). It is worth mentioning that this (114) plane displayed a distinct shift following the Ni-doping to Bi3O4Br. Selected area electron diffraction (SAED) is a crystallographic experimental technique performed using a transmission electron microscope. In the SAED pattern, each obtained spot corresponds to a satisfied diffraction condition. For any crystalline nanomaterial, SAED patterns typically provide an image composed of single spots (dots) only if it is a single crystal, and a ring pattern for a polycrystalline material. This feature of SAED is useful for distinguishing crystalline nanomaterials from their amorphous counterparts. Herein, the selected area electron diffraction (SAED) pattern of Ni-doped Bi3O4Br reveals the formation of a polycrystalline nanostructured material (Fig. 3e). The high-angle annular dark-field scanning transmission electron microscopy image and corresponding elemental mapping confirm the uniform distribution of Bi, O, Br, and Ni elements in the Ni-doped Bi3O4Br nanostructures (Fig. 4 and Fig. S2, ESI†). Analogous data was also recorded for pristine Bi3O4Br (Fig. S3, ESI†).
 | ||
| Fig. 4 (a) HAADF image of Ni-doped Bi3O4Br for the elemental mapping of (b) Bi, (c) Br, (d) O, (e) Ni, and (f) mixed elements of Bi, O, and Ni. | ||
The optical absorption properties of Bi3O4Br and Ni-doped Bi3O4Br were investigated by UV-vis absorption and diffuse reflectance spectra (DRS). A significant optical absorption band for the Ni-doped Bi3O4Br was extended beyond 500 nm. However, the analogous band terminates around 450 nm for the Bi3O4Br (Fig. S4, ESI†).31,38,39 This shows that Ni doping shifts the bandgap of Bi3O4Br towards the visible region. For crystalline semiconductor materials, the optical absorption near the band edge follows the equation: αhv = A(hv − Eg)n/2, where α, ν, A, and Eg are the absorption coefficient, frequency of light, proportionality constant, and energy bandgap, respectively. The quantity of “n” depends on the characteristics of transition. For a direct bandgap semiconductor, n is taken as 1, and for an indirect bandgap semiconductor, n is taken as 4. Since Bi3O4Br is known as an indirect bandgap semiconductor, n is taken as 4. By plotting (αhv)1/2vs. energy (eV), we have obtained the bandgaps of the Bi3O4Br and Ni-doped Bi3O4Br as 2.49 eV and 2.36 eV, respectively. From the XPS valence spectra and the diffuse reflectance spectra (DRS), we have obtained the positions of the valence band (VB) and conduction band (CB) in Bi3O4Br and Ni-doped Bi3O4Br (Fig. 2e and Fig. S4, ESI†). The VB after Ni doping becomes less positive. Hence, the Ni-doped Bi3O4Br is a better oxidizing catalyst than the pristine Bi3O4Br. On the other hand, the pristine Bi3O4Br is a better reducing catalyst than the Ni-doped Bi3O4Br as the position of the CB is more negative for the Bi3O4Br than the Ni-doped Bi3O4Br.
To further characterize Bi3O4Br and Ni-doped Bi3O4Br, we also studied Raman and FTIR analysis, as depicted in Fig. S5 (ESI†). The Raman bands centered at 108.9 and 151.34 cm−1 belong to the A1g internal and Eg internal Bi–Br stretching modes, respectively (Fig. S5a, ESI†).39,40 An increased peak intensity and broadening of the signals was observed for Ni-doped Bi3O4Br compared to pristine Bi3O4Br. This can be attributed to the difference in electron–phonon coupling in the single-unit cell regime for the two compounds. The distinct signatures that appeared in the FTIR spectrum of Bi3O4Br at 1450–1190 cm−1 can be ascribed to the asymmetric and symmetric stretching vibrations of Bi–Br bonds, while the peak at 524 cm−1 is assigned to the Bi–O vibration modes (Fig. S5b, ESI†).39 A broad band at 3442 cm−1 and a peak at 1638 cm−1 are associated with the stretching and bending vibration modes of H2O molecules adsorbed on the surface of Bi3O4Br during FTIR analysis.41 Similar characteristic peaks were also observed in the FTIR spectrum of the Ni-doped Bi3O4Br material.
Electrochemical and photoelectrochemical HER performance
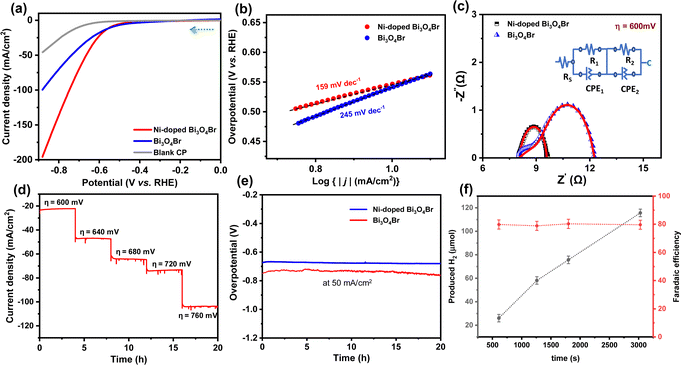
The electrocatalytic HER performances of Bi3O4Br and Ni-doped Bi3O4Br were studied by a series of electrochemical experiments in 1.0 M KOH (pH ∼14.0) with a standard three-electrode system. Linear sweep voltammetry (LSV) measurements showed that Ni-doped Bi3O4Br exhibited an overpotential of 545 and 662 mV to achieve a conventional current density of 10 and 50 mA cm−2, respectively. On the other hand, pristine Bi3O4Br showed an overpotential of 547 and 736 mV to attain the same current density (Fig. 5a). Hence, there is a significant deterioration in overpotential at higher current density after the Ni-doping into Bi3O4Br. The reaction kinetics of the catalysts were examined by Tafel slope analysis of the catalysts (Fig. 5b). A relatively low Tafel slope of 159 mV dec−1 was exhibited by Ni-doped Bi3O4Br compared to the pristine Bi3O4Br (245 mV dec−1), which indicated a better HER kinetics for the Ni-doped sample in an alkaline medium. Electrochemical impedance spectroscopy (EIS) was carried out at an applied overpotential of 600 mV to have a better insight into the charge transfer resistance (Rct) at the electrode and electrolyte interface. The obtained EIS spectra and corresponding circuit diagrams are represented in Fig. 5c, where Rs is the resistance of the electrolyte, and CPE is a constant phase element. The lower Rct value of Ni-doped Bi3O4Br (1.55 Ω) further confirms higher electron transfer ability across the electrode and electrolyte interface than that of Bi3O4Br (5.12 Ω) during the HER process. Therefore, it can be concluded that Ni-doping substantially improves the background HER activity of Bi3O4Br.To explore the enhanced activity, double layer capacitance (Cdl) values of the catalysts were measured, followed by the electrochemically active surface area (ECSA) from CV measurements in a non-faradaic potential region (Fig. S6, ESI†). A higher ECSA value (40.5 cm2) was observed for the Ni-doped Bi3O4Br catalyst in comparison to the precursor Bi3O4Br (31.5 cm2). This data implies that Ni-doped Bi3O4Br possesses more active sites, which is beneficial for the adsorption of water molecules and close contact with the electrolyte during HER catalysis. The LSV results of the materials are normalized with these obtained electrochemically active surface area values to provide the intrinsic effect of Ni-doping in the Bi3O4Br template (Fig. S7, ESI†). The ECSA normalized LSV plots represent that the HER activity trend is maintained, as it is in the geometric area normalized polarization curve for Ni-doped Bi3O4Br and Bi3O4Br catalysts. Moreover, the mass activity of Bi3O4Br was improved drastically from 52 to 98 A g−1 after incorporation of Ni in the pristine nanostructure (Fig. S8, ESI†).
In addition to showcasing the HER performance, long-term stability is another crucial factor for a promising electrocatalyst that can be translated to industrial applications. A potential-dependent chronoamperometry stability test was performed with 4.0 hour intervals (Fig. 5d). The chronoamperometry stability test demonstrated consistent current densities at different applied overpotential values. In addition, we have studied the chronopotentiometry stability of the Ni-doped Bi3O4Br and Bi3O4Br catalysts at the current density of 50 mA cm−2 which exhibited an enhancement in the overpotential of 13 and 17 mV after 20 hours, respectively (Fig. 5e). Therefore, Ni-doped Bi3O4Br demonstrates superior durability in a strongly alkaline medium while displaying HER activity. Interestingly, the pristine Bi3O4Br catalyst exhibited a significant loss of 19% current density after an analogous 20 hours chronoamperometry stability test (Fig. S9, ESI†). Notably, the incorporation of Ni ions into Bi3O4Br not only enhanced the HER activity but also improved the durability of the catalyst. Faradaic efficiency is an important parameter to measure the efficiency of charge transfer in an electrochemical reaction. It was measured by comparing the theoretically and experimentally obtained amount of H2, which was evaluated using a gas chromatography (GC) instrument equipped with a TCD detector and 5 Å molecular sieve column for separation of the gases with argon as the carrier gas (CIC Dhruva). The GC instrument was calibrated using three standard gas samples containing 1%, 2%, and 5% H2, respectively. The Bi3O4Br and Ni-doped Bi3O4Br catalyst exhibited a faradaic efficiency of ∼65 and 79% for the HER in an alkaline medium (pH ∼14.0) with constant growth in accumulated H2 during the chronoamperometric experiment (Fig. 5f and Fig. S10 and S11, ESI†).
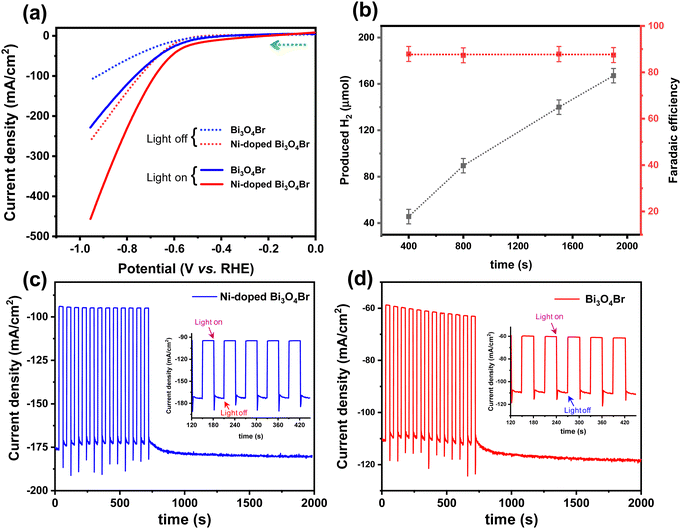
Moreover, we have also studied the photoelectrochemical HER performance under UV-vis light irradiation. LSV measurements displayed that Bi3O4Br exhibited an overpotential of 614 and 668 mV to achieve a conventional current density of 10 and 50 mA cm−2, whereas the same current density was exhibited by Ni-doped Bi3O4Br at the overpotential of 418 and 614 mV, respectively (Fig. 6a). Therefore, a significant improvement in overpotential was observed in the HER performance under UV-vis light irradiation. Furthermore, a substantial increase in photocurrent response by 60% was observed for the Ni-doped Bi3O4Br compared to that of Bi3O4Br in the PEC measurements, indicating that enhanced charge separation occurred due to the incorporation of Ni in the Bi3O4Br catalyst (Fig. 6c and d). The faradaic efficiency of Ni-doped Bi3O4Br was calculated to be 87% during the photoelectrocatalytic HER, which was ∼33% and 10% superior to that of Bi3O4Br, and Ni-doped Bi3O4Br during the electrocatalytic HER process, respectively (Fig. 6b and Fig. S11, ESI†).
We have studied the post-electrocatalytic ECSA of the Ni-doped Bi3O4Br catalyst, which was found to be 1.51 cm2 (Fig. S14, ESI†). The 6.79% decrease in electrochemically active surface area may be due to the leaching out of Ni, which is one of the effective active areas. This statement can also be supported by the before and post-catalytic ICP-AES analysis of the Ni-doped Bi3O4Br catalyst as described in the ESI.† Moreover, we have deeply focused on the stability of the catalyst after the long-term electrochemical HER performance test. The structural stability studied was determined using a powder XRD pattern (Fig. S15, ESI†); on the other hand the morphological stability of the catalyst was investigated by TEM and bright field elemental mapping (Fig. S16 and S17, ESI†). No such morphological and structural deformation was observed after the long-term electrocatalytic HER performance test of the catalyst.
Conclusions
This specific cation doping approach is certainly one of the best ways to explicitly enhance the electrocatalytic HER performance under alkaline conditions with highly exposed active sites. The doping of Ni ions in Bi3O4Br facilitated the reduction of the overpotential and improved the kinetics during the HER activity. The electrochemically active surface area and mass activity of Bi3O4Br were improved drastically by 29% and 88%, respectively, after the incorporation of Ni ions in the pristine nanostructure. Substantial improvement in the HER performance and faradaic efficiency were also observed under UV-vis light irradiation that contributes to the accelerated evolution of hydrogen in the photocatalysis system. The durability of the Bi3O4Br template was also enhanced following the Ni-doping, which was evident from the stable current density and negligible change in overpotential during a 20-hour stability test at an applied current density of 50 mA cm−2. The present work not only demonstrates a facile approach to fabricate cost-effective HER catalysts but also explores a new route to develop a sustainable and highly efficient robust electrocatalyst derived from the family of transition metal-doped semiconductor materials. Ni-doped Bi3O4Br nanostructured materials can further be used in the field of sensors, supercapacitors, and photochemical small molecule activation reactions, aiding the journey towards a carbon-neutral landscape.Author contributions
SB and AD conceptualized the project; MP, RB, and SB performed the experiments; MP, RB, SB, and AD analyzed the data; RB, SB and AD wrote the manuscript; SB and AD revised and edited the manuscript; AD supervised the project.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors would like to thank the experimental facility and financial support provided by the Indian Institute of Technology Bombay (IITB). A. D. acknowledges the funding support from the DST-SERB (CRG/2020/001239). SB would like to thank the Center for Advanced Materials and Devices (CAMD), BML Munjal University for the utilization of the facilities.References
- S. Chu, Y. Cui and N. Liu, Nat. Mater., 2017, 16, 16–22 CrossRef PubMed.
- I. A. Liyanage, H. Barmore and E. G. Gillan, Energy Adv., 2023, 2, 1831–1842 RSC.
- M. N. I. Salehmin, T. Husaini, J. Goh and A. B. Sulong, Energy Convers. Manage., 2022, 268, 115985 CrossRef CAS.
- G. Srividhya, C. Viswanathan and N. Ponpandian, Energy Adv., 2023, 2, 1464–1475 RSC.
- H. Lin, Z. Shi, S. He, X. Yu, S. Wang, Q. Gao and Y. Tang, Chem. Sci., 2016, 7, 3399–3405 RSC.
- Z. Chen, W. Gong, S. Cong, Z. Wang, G. Song, T. Pan, X. Tang, J. Chen, W. Lu and Z. Zhao, Nano Energy, 2020, 68, 104335 CrossRef CAS.
- B. H. Honnappa, S. Mohan, M. Shanmugam, A. Augustin, P. J. Sagayaraj, C. Chuaicham, S. Rajendran, T. K. A. Hoang, K. Sasaki and K. Sekar, Energy Adv., 2022, 1, 738–760 RSC.
- S. Das, C. Das, N. A. Shah, S. Ghorai, P. Majumder and A. Dutta, Chem. Commun., 2023, 59, 7243–7246 RSC.
- R. T. Parayil, S. K. Gupta, M. Pal, A. Dutta, D. Tyagi, K. Sudarshan and M. Mohapatra, RSC Adv., 2023, 13, 31101–31111 RSC.
- S. Mansingh, K. K. Das and K. Parida, Sustainable Energy Fuels, 2021, 5, 1952–1987 RSC.
- R. Biswas, I. Ahmed, P. Manna, P. Mahata, R. S. Dhayal, A. Singh, J. Lahtinen and K. K. Haldar, ChemPlusChem, 2023, 88, e202200320 CrossRef CAS PubMed.
- A. P. Murthy, J. Madhavan and K. Murugan, J. Power Sources, 2018, 398, 9–26 CrossRef CAS.
- W. Yang and S. Chen, Chem. Eng. J., 2020, 393, 124726 CrossRef CAS.
- K. Zhang and R. Zou, Small, 2021, 17, 2100129 CrossRef CAS PubMed.
- A. Banerjee, M. K. Awasthi, P. Maji, M. Pal, S. T. Aziz, G. K. Lahiri and A. Dutta, ChemElectroChem, 2023, 10, e202201098 CrossRef CAS.
- R. Biswas, P. Thakur, G. Kaur, S. Som, M. Saha, V. Jhajhria, H. Singh, I. Ahmed, B. Banerjee and D. Chopra, Inorg. Chem., 2021, 60, 12355–12366 CrossRef CAS PubMed.
- J. S. Kim, B. Kim, H. Kim and K. Kang, Adv. Energy Mater., 2018, 8, 1702774 CrossRef.
- S. T. Aziz, M. Ummekar, I. Karajagi, S. Riyajuddin, K. Siddhartha, A. Saini, A. Potbhare, R. G. Chaudhary, V. Vishal and P. C. Ghosh, Cell Rep. Phys. Sci., 2022, 3, 101106 Search PubMed.
- K. S. Sairam, S. T. Aziz, I. Karajagi, A. Saini, M. Pal, P. C. Ghosh and A. Dutta, Int. J. Hydrogen Energy, 2023, 48, 10521–10531 CrossRef.
- J. Yu, T. A. Le, N. Q. Tran and H. Lee, Chem. – Eur. J., 2020, 26, 6423–6436 CrossRef CAS PubMed.
- J. Mohammed-Ibrahim and X. Sun, J. Energy Chem., 2019, 34, 111–160 CrossRef.
- V. Pundir, A. Gaur, R. Kaur and V. Bagchi, Energy Adv., 2023, 2, 321–327 RSC.
- V. Manjunath, S. Bimli, R. Biswas, P. N. Didwal, K. K. Haldar, M. Mahajan, N. G. Deshpande, P. A. Bhobe and R. S. Devan, Int. J. Hydrogen Energy, 2022, 47, 39018–39029 CrossRef CAS.
- R. Biswas, A. Kundu, M. Saha, V. Kaur, B. Banerjee, R. S. Dhayal, R. A. Patil, Y.-R. Ma, T. Sen and K. K. Haldar, New J. Chem., 2020, 44, 12256–12265 RSC.
- J. Ran, J. Zhang, J. Yu, M. Jaroniec and S. Z. Qiao, Chem. Soc. Rev., 2014, 43, 7787–7812 RSC.
- X. Wei, M. U. Akbar, A. Raza and G. Li, Nanoscale Adv., 2021, 3, 3353–3372 RSC.
- J. Di, J. Xia, H. Li, S. Guo and S. Dai, Nano Energy, 2017, 41, 172–192 CrossRef CAS.
- J. Xiong, P. Song, J. Di and H. Li, J. Mater. Chem. A, 2020, 8, 21434–21454 RSC.
- J. Di, C. Chen, S.-Z. Yang, S. Chen, M. Duan, J. Xiong, C. Zhu, R. Long, W. Hao and Z. Chi, Nat. Commun., 2019, 10, 2840 CrossRef PubMed.
- F. Zhang, X. Xiao and Y. Xiao, J. Alloys Compd., 2022, 923, 166417 CrossRef CAS.
- J. Wang, Y. Yu and L. Zhang, Appl. Catal., B, 2013, 136, 112–121 CrossRef.
- X. Jiang, X. He, H. Huang, Y. Li, J. Yang, J. Mei and S. Cui, J. Alloys Compd., 2023, 963, 171221 CrossRef CAS.
- X. Wang, Z. Wang, Y. Li, J. Wang and G. Zhang, Appl. Catal., B, 2022, 319, 121895 CrossRef CAS.
- Y. Zhang, F. Guo, K. Wang, J. Di, B. Min, H. Zhu, H. Chen, Y.-X. Weng, J. Dai and Y. She, Chem. Eng. J., 2023, 465, 142663 CrossRef CAS.
- J. Hu, F. Chen, K. Mu, J. Zhang and J. Lu, Sep. Purif. Technol., 2022, 286, 120416 CrossRef CAS.
- R. Biswas, P. Thakur, I. Ahmed, T. Rom, M. S. Ali, R. A. Patil, B. Kumar, S. Som, D. Chopra and A. K. Paul, Energy Fuels, 2022, 37, 604–613 CrossRef.
- I. Ahmed, R. Biswas, S. G. Dastider, H. Singh, S. Mete, R. A. Patil, M. Saha, A. K. Yadav, S. N. Jha and K. Mondal, Energy Fuels, 2022, 36, 12160–12169 CrossRef CAS.
- K.-L. Li, W. W. Lee, C.-S. Lu, Y.-M. Dai, S.-Y. Chou, H.-L. Chen, H.-P. Lin and C.-C. Chen, J. Taiwan Inst. Chem. Eng., 2014, 45, 2688–2697 CrossRef CAS.
- R. Li, J. Feng, X. Zhang, F. Xie, J. Liu, C. Zhang, Y. Wang, X. Yue and C. Fan, Sep. Purif. Technol., 2020, 247, 117007 CrossRef CAS.
- A.-H. Lee, Y.-C. Wang and C.-C. Chen, J. Colloid Interface Sci., 2019, 533, 319–332 CrossRef CAS PubMed.
- R. Biswas, S. Mete, M. Mandal, B. Banerjee, H. Singh, I. Ahmed and K. K. Haldar, J. Phys. Chem. C, 2020, 124, 3373–3388 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ya00228h |
| This journal is © The Royal Society of Chemistry 2024 |