Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Sculpturing the future of water-soluble cyclodextrin branched polymers in pharmaceutical applications
Marco
Agnes
*a,
Arianna
Mazza†
a,
Milo
Malanga
b and
Ilse
Manet
 *a
*a
aConsiglio Nazionale delle Ricerche (CNR), Istituto per la Sintesi Organica e la Fotoreattività (ISOF), Via Gobetti 101, 40129 Bologna, Italy. E-mail: ilse.manet@isof.cnr.it; marco.agnes@isof.cnr.it
bCarboHyde Zrt., Berlini str., 47-49, Budapest, 1045, Hungary
First published on 24th July 2024
Abstract
Water-soluble polymers of cyclodextrins (CyD) can be easily obtained in alkaline media following polycondensation of the naturally occurring monomers in the presence of a crosslinking agent. They can be further modified to customize specifically functionalized architectures. Compared to other macromolecules natural and not, the CyD polymers are endowed with a unique feature, the cone-shaped cavities where they can host guests of various nature. This element has sollicited interest in this class of molecules for a wide range of applications including the biomedical field, in particular drug delivery. The CyD polymers display excellent behavior in terms of water solubility and solubilizing power towards drugs and therapeutic agents that are incompatible with biological fluids. Moreover, they can load more than one type of therapeutic agent in a single system thus allowing to implement combination therapy. In spite of some very promising results as delivery systems, their potentialities remain limited by some intrinsic hurdles. Herein, we comment on their limits mainly related to the production process and the possible solutions to overcome them, giving an outlook on their assets for innovation in disease treatment.
Introduction
Cyclodextrins (CyDs) are natural products enzymatically derived from starch using CyD glycosyltransferases. They are water soluble, macrocyclic oligosaccharides, made of α-D-glucopyranose units joined by α(1–4) linkages (Fig. 1A).1 The most common α, β and γCyDs present 6, 7 or 8 glucopyranose units, respectively. CyDs have the shape of a truncated cone with the height of a glucose molecule, whereas the number of glucopyranoses determines the size of the cavity.2 This cavity has slightly hydrophobic character, while the external faces are more hydrophilic.3 This feature allows CyDs to host hydrophobic molecules within their cavity upon formation of supramolecular complexes, enabling solubilization and stabilization in aqueous media of otherwise insoluble substances. Technologies based on this principle have been exploited in the formulation of pharmaceutical active substances, in analytical separations, in food and cosmetic products, in agricultural treatments, in textile and environmental technologies, in wastewater treatments, etc.1,4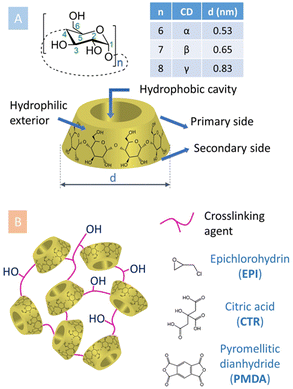 | ||
| Fig. 1 (A) General and structural details regarding CyDs. (B) Schematic representation of CyD-polymers obtained using different crosslinking agents. | ||
CyD derivatives can be tailored exploiting the different reactivity of the hydroxyl groups in positions 2, 3 and 6 of the glucopyranose units affording hydrophilic, hydrophobic and ionizable CyDs. They can be selectively, randomly or fully substituted with one or more functional groups, resulting in very versatile substrates.5
Thanks to their exceptional tunability, the interest has grown well beyond monomeric CyD derivatives and scientists have moved towards systems incorporating various CyDs as one of the building blocks and in literature many reviews are available.6 Among them, CyD-based polymers (pCyD, Fig. 1B) obtained upon a polycondensation reaction of the natural CyDs with a crosslinking agent in water have received considerable interest. They usually display:
• Enhanced CyD solubility: dissolution of pCyDs in water affords significantly higher CyD concentration compared to monomers: the polymerization process disrupts the network of intramolecular H-bonds in the monomers allowing more interactions between the –OH groups and the surrounding water molecules. Moreover, the chains connecting different CyD monomers often contain hydrophilic moieties, i.e. –OH, –COOH, etc.
• Enhanced hosting ability: thanks to the presence of many hydrophobic micro-environments, hydrophobic guests can reach higher concentrations with respect to the corresponding complexes with CyD monomers;
• Ability of pCyDs to self-organize in small nanoparticles (NPs) upon dissolution in aqueous/physiological environment.
Natural and synthetic CyD monomers have found applications in many fields, especially in drug development thanks to their good safety profile and ability to host lipophilic drugs in their cavity.7 Differently, pCyDs have only been proposed at lab level for biomedical applications in spite of their performance that is often superior to the monomers. In this perspective article, we give a concise overview on the state-of-the-art regarding the preparation, and drug delivery applications. We have selected few examples illustrated in detail to highlight the strengths and potential of this class of polymers. Moreover, we comment on the main hurdles blocking their progress and the main future perspectives in the field of pharmaceutical development.
Strengths and limits of straightforward synthesis
The first syntheses of pCyDs date back to 1960s.8 Since then, polycondensation in alkaline aqueous environment in the presence of a multifunctional crosslinking agent (CLA) has become the method of election to produce both water-soluble pCyDs as well as water-insoluble hydrogels. Discrimination among the two is achieved by attentively monitoring diverse reaction parameters: the initial concentration and molar ratio of reagents, the type of CyD, the reaction temperature and time, the base amount, the presence and quantity of catalyst, etc.9 All these parameters are relevant to rigorously control the overall molecular weight (MW) and degree of substitution (DS, number of substituents per CyD unit) which are ultimately essential to grant batch-to-batch reproducibility and the general quality and properties of the materials. Moreover, they also affect the availability of CyD cavities since, inevitably, the polycondensation reaction can occur on both rims of CyDs, involving different hydroxyl functionalities in position 2, 3 and 6 of the substrates at the same time. Thus, it is not trivial to obtain hyperbranched pCyDs with reproducible physico-chemical characteristics.The most frequently used CLAs can be grouped in three main categories (Fig. 1B):
• Bifunctional reactive species, e.g. epichlorohydrin (EPI)
• Molecules bearing more than two carboxylic acid groups, e.g. citric acid (CTR)
• Di-/poly anhydrides, e.g. pyromellitic dianhydride (PMDA).
EPI is the most widely used CLA in the synthesis of water-soluble pCyDs, thanks to its high reactivity, commercial availability and low price, overcoming its unfavourable toxicity profile. Polycondensation between EPI and CyDs takes place by heating the reagents in aqueous basic conditions allowing to obtain after proper work-up neutral pCyDs with a CyD content up to 70% w/w and relatively high MW (Fig. 2A).10 The seminal work of E. Renard et al. in 1997 reported a detailed analysis of the synthetic parameters enabling control over the production of water-soluble EPI-pCyDs when DS < 10, avoiding gelation occurring for DS > 12. The mean MW determined by the authors was 44 kDa for a 3 hours reaction time. For longer reaction times MW of the polymer can increase up to 400 kDa.10
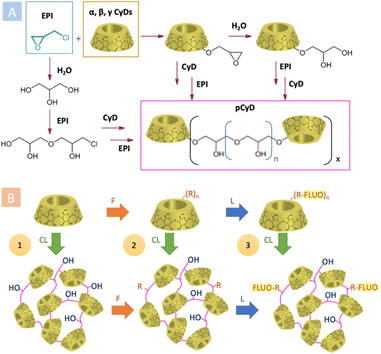 | ||
| Fig. 2 (A) General synthetic scheme for the preparation of pCyDs using EPI as CLA; (B) synthetic strategies for pCyDs functionalizations (CL = crosslinking, F = functionalization, L = labelling). | ||
The use of CLAs other than EPI came across more recently. The group of B. Martel published11 and patented12 a new synthesis for water-soluble pCyDs obtained with tri- and polycarboxylic acids, above all, citric acid (CTR, Fig. 1B). They proposed a greener method in the dry state involving also a catalyst (i.e. phosphate salts) producing CTR-pCyDs with biodegradable ester linkages and overall negative charge. Due to the solid-state reaction requiring high temperature and the high number of esterifiable groups present on each CyD unit, it is very difficult to avoid gel formation and have strict control over batch-to-batch reproducibility. Hence, industrial scale up is more challenging compared to EPI-pCyDs.
Also di- and polyanhydrides have been exploited as CLAs for the preparation of negatively charged pCyDs under physiological conditions. Trotta et al. optimized a synthesis based on pyromellitic dianhydride (PMDA) to obtain either water-soluble (up to 800 mg mL−1) over a wide pH range and water-insoluble materials (named CyD-nanosponges) by modulating the PMDA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CyD ratio.13 Also in this case the limit of gelation during the reaction at room temperature is very subtle, thus complicating a scaled-up production.
CyD ratio.13 Also in this case the limit of gelation during the reaction at room temperature is very subtle, thus complicating a scaled-up production.
Many other crosslinking agents have been tested, some of them showing promising results. However, being the use of EPI as straightforward as tunable, the neutral EPI-pCyDs are the only water-soluble ones currently commercially available and their industrial production reached the sub kg scale.
The pCyDs can also be further modified in order to afford materials with auxiliary features, like net charges or optical properties. These functionalizations can be achieved following three different strategies (Fig. 2B):14
1. Post-crosslinking functionalizations.
2. Pre-crosslinking functionalizations.
3. Sequential crosslinking and functionalizations.
In this context, an important Achilles heel concerns the modification of CyDs as it often affords a mixture of isomers that are very hard to purify and separate. This has several consequences: (i) proper characterization of the material according to GMP and ICH guidelines is challenging, (ii) identifying by-products, degradation products and metabolites is demanding, (iii) in vivo and/or side effects cannot be unambiguously linked to a single component or degradation product. Moreover, not all of synthetic approaches fit upscaling as, on large scales, they are too costly and/or hazardous, thus not compatible with GMP standards for drug production. So, it is necessary to face the challenges in the chemistry of ad hoc functionalized CyDs as single isomers creating new knowledge on sustainable, synthetic protocols for pCyDs polycondensation and functionalization, especially when targeting clinical applications.
pCyDs for the simultaneous delivery of multiple therapeutic agents
The first steps in the use of pCyDs as drug carriers date back to the 1980s’ when Szejtli et al. highlighted the significantly higher concentration of CyDs obtained when dissolving the polymers and the better performance of the pCyDs in solubilizing guests also enhanced by the cooperativity of adjacent cavities in guest encapsulation.15Later, a key work was carried out by P. Couvreur exploring the combination of EPI-pβCyD and a lauryl-modified dextran polymer as delivery system (Fig. 3).16 EPI-pβCyD alone was able to dissolve up to 0.7 mM of drug compared to 0.15 mM for βCyD thanks to the much higher amounts of βCyD dissolved in water as polymer: 70 mg mL−1 βCyD for 100 mg mL−1 polymer, compared to 17.5 mg mL−1 for the monomeric βCyD. Mixing the two polymers dissolved in high amounts allowed to obtain a gel used to load tamoxifen (TMX), a drug used in breast cancer treatment. The system showed at 37 °C a linear time-dependent release of TMX in vitro.17 The same group hypothesized the gel formation was due to inclusion of the hydrophobic alkyl chains in the CyD cavities and they demonstrated that the mixing of the same polymers in lower ratios could afford soluble nanoparticles (NPs) with dimension <200 nm and size stability over 20 days.16,18 In the case of the NP system they proved the crucial role played by the CyD cavity in 1H-NMR experiments showing the inclusion of the lauryl moieties of the dextran polymer in the CyD cavities, yet leaving ≈50% of the cavities empty. The remaining CyD units could then be exploited for TMX loading in dosage consistent with clinical application or benzophenone, a model compound, whose presence inside the CyD cavities was again proven by 1H-NMR experiments.16 Their findings represented a first proof-of-principle that, differently from the monomers, pCyDs enable the contemporary encapsulation of two or more components in a unique system. They gave further credit to this hypothesis when testing the successful co-encapsulation of TMX and benzophenone (BP), into pβCyD NPs.19
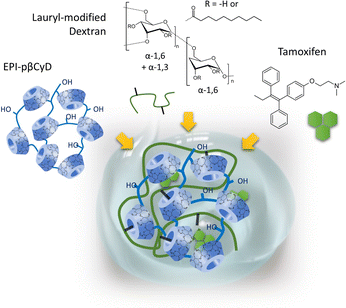 | ||
| Fig. 3 Self-assembling of lauryl-modified dextran and EPI-pβCyD affords hydrogels for the sustained delivery of Tamoxifen. | ||
The contemporary delivery of multiple therapeutic agents was further pursued by Gref et al. who performed an in-depth investigation of the use of EPI-pβCyD for the loading of ethionamide (ETH), a second line drug for tuberculosis, poorly soluble in water and inclined to crystallization, with a booster, an organic hydrophobic molecule that strongly increase ETH efficacy when co-administered (Scheme 1).20 Inclusion in EPI-pβCyD NPs increased the water solubility up to 25 mM for ETH and 1 mM for the booster, compared to 4 and 0,1 mM respectively for monomeric βCyD. Co-encapsulation of ETH with the booster, was achieved within the EPI-pβCyD NP confirming the same drug amounts as for single drug inclusion and ETH was found to reside not only in βCyD cavities, but also in other domains of the NPs.
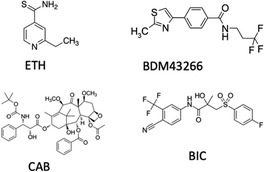 | ||
| Scheme 1 Structure of ethionamide (ETH), booster BDM43266, cabazitaxel (CAB) and bicalutamide (BIC). | ||
The efficacy of this formulation was evaluated in vitro and in vivo in TB infected macrophages and mice, respectively. The suspensions of loaded NPs could be administered directly into mice lungs using a Microsprayer® system leading to a 3-log decrease of the pulmonary mycobacterial load after 6 administrations compared to untreated mice (Fig. 4). The authors also discovered that unloaded EPI-pβCyD NPs (i) internalizes by means of phagocytosis in alveolar macrophages known as mycobacteria host impeding bacterial proliferation, (ii) is not causing cytotoxic effects in murine macrophages up to 5 mg mL−1, (iii) neither induces gene toxicity in human macrophages nor solicits immune response and (iv) reduced the mycobacterial load, thus launching the polymeric CyD platform itself as most promising antibiotic agent.21
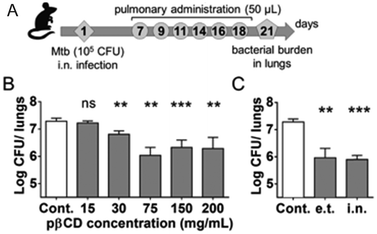 | ||
| Fig. 4 (A) Mice were i.n. inoculated with 105 CFU of Mtb H37Rv strain in 20 μL of PBS. At days 7, 9, 11, 14, 16, and 18, mice received administrations of 50 μL of suspensions via the e.t. route using a microsprayer delivering aerosol directly into the lungs. At day 21, lungs were harvested for determination of bacterial burden by CFU counting. (B) Bacterial load at day 21 postchallenge after 6 inoculations of 50 μL of different EPI-pβCyD concentrations by the e.t. route. (C) Comparison of the impact of EPI-pβCyD (6 × 50 μL at 150 mg mL−1) on Mtb pulmonary load administrated by the i.n. route or by the e.t. route after i.n. infection. Symbols ** and *** denote p < 0.01 and p < 0.001, respectively. Reprinted from ref. 21 with permission from American Chemical Society. | ||
Most recently the superior ability of the polymer in drug solubilization and co-encapsulation was demonstrated by different groups using pCyD platforms for the contemporary delivery of three therapeutic agents to implement combination therapy, in particular chemotherapy with phototherapy.
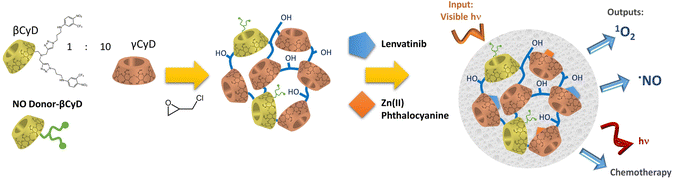
S. Sortino et al. investigated pβCyD platforms for bimodal light-activated therapies of cancer and bacterial infections. They combined a nitroaniline NO donor activated upon blue light irradiation triggering NO radical release with a photosensitizer (PS), such as Zn(II) phthalocyanine, for the formation of 1O2 upon NIR irradiation.22 Both the NO radical and 1O2 are cytotoxic species generated locally and attacking multiple targets, not suffering from any kind of resistance mechanism. Noteworthy, conservation of the light-activated properties of the NO donor and PS when confined in a restricted space is not trivial, as self-quenching can occur. Clear-cut evidence was provided of a synergistic mechanism in killing neoplastic cells by comparison of the performance of the NPs loading both NO donor and PS with the NPs containing a single component.23 Encouraged by these positive findings they recently used a mixed β and γCyD polymer to implement the loading of 3 different therapeutic agents in NPs: the NO donor in this case was covalently linked to the pCyD, and the functionalized polymer was subsequently loaded with Zn(II) phthalocyanine as PS and lenvatinib (LVB) as chemotherapeutic drug (Fig. 5). The NP system maintained high water solubility, increased the solubility of all therapeutic agents and preserved the photophysical properties of both NO donor and PS as well as the anticancer activity of LVB. In particular, this mixed polymer increases the solubility of LVB 30-fold compared to the free drug and 2-fold compared to a similar polymer with only βCD.24
Recently, Pancani et al. reported an EPI-pβCyD system also loaded with 3 agents for combination therapy with: (i) cabazitaxel as chemotherapeutic agent; (ii) bicalutamide as androgen receptor; (iii) chlorin e6 as PS for photodynamic therapy. Combination of cabazitaxel with bicalutamide was proposed as the androgen receptor can reverse resistance acquired by the tumor to the taxane (Scheme 1).25 Stable NPs were obtained while increasing significantly the solubility of both drugs in water, with PS properties being intact in the presence of the other two agents.
Noticeably, in an era where multidrug resistance (MDR) is advancing rapidly in the treatment of cancer as well as microbial infections compromising the output of conventional therapies, the potential of the pCyD platforms to load multiple agents is of utmost importance and fostering further research to bring them to the clinics.
Hurdles on the way to the clinics
Looking for CyD applications in the clinics we immediately take note that we are looking at a signboard “Work in progress”. While the potential of CyD monomers as excipients in pharmaceutics is widely accepted and many CyD-based substances have been approved for drug formulations, only few CyDs have been unveiled as active pharmaceutical ingredient (API). Sugammadex was approved as antidote for rocuronium, a neuromuscular blocking agent used as anaesthetics, working upon selective encapsulation of the target.26 2-Hydroxypropyl-βCyD (HPβCD) was found effective for the treatment of Niemann Pick type-C diseases.27 These discoveries triggered several researches aimed at developing CyD-based therapies in different lysosomal storage and neurodegenerative diseases, cardiovascular dysfunctions, infections and various types of cancer. Most recently, M. Anderson et al. patented a βCyD dimer for the selective capture of the metabolite 7-ketocholesterol considered responsible for plaque formation in arteries,28 while M. Agnes et al. patented metal-binding CyDs as potentiators of antibiotics.29Despite their huge potential, at the moment there are no pCyDs tested or commercialized as API or delivery tools and their clinical applications are not taking off. We discern several reasons for this gridlock. The polymers have not yet been approved by drug and food agencies for clinical applications. This is subject to strict product quality requirements and full toxicologic screening at high costs, a risk that pharmaceutical companies have not run yet. Few research groups have addressed the latter topic with encouraging findings suggesting a lack of cytotoxicity in normal cells, but the available data need to be integrated.21 Additionally, the analytical challenges associated with quantifying any potential reactive groups remaining post-synthesis that might pose risks by alkylating and binding to proteins or DNA in the body, further complicates the development process and increases the associated costs for complying with the necessary quality requirements and safety parameters.21 If pCyDs will be formally approved, an escalation of their clinical use is expected thanks to the possibility to use them in combination with already FDA approved drugs targeting different diseases. Moreover, the search for new sustainable production processes of amphiphilic CyDs, proposed at lab level for gene delivery, is also expected to drive innovation as gene delivery is a very hot topic for treatment innovation in many pathological conditions.
While the release kinetics of the loaded cargo has been addressed by several groups, less information is available on premature leakage of the cargo in vivo that may cause undesired side effects. In this frame we need to keep in mind that this issue is not always pertinent as it depends on the type of administration. When local treatment applies, as in the case of long infections and skin diseases, evidence is already available on the feasibility of pCyD systems for in situ pulmonary application by means of microsprayer systems and gel preparations with antibiotic activity, respectively. Differently, when systemic administration is required, the retainment of the cargo is crucial and some authors addressed this topic by mixing pCyDs with other polymers such as the hydrophobic dextrans to form more stable NPs.16,30
Similarly, another aspect still to be better explored is the target specificity of the polymeric system. Again, this feature strongly depends on the route of administration. Addition of a targeting ligand is feasible and expected to become straightforward being available several synthetic routes to functionalize pCyDs. As to pβCyDs, an example is available where folic acid, specifically recognized by the folate receptor present on the cell membrane of many tumors, was covalently linked to the βCyD units. Unfortunately, the approach did not afford an improvement of the IC50 value of the loaded cis-Pt prodrug compared to the system lacking the folic acid functionalization.31 Another interesting example concerns the functionalization of pβCyDs with histidine or carcinine (β-alanyl-histamine) moieties, both ligands with important physiological roles in Cu(II) metabolism in mammals. The functionalized pβCyDs formed Cu(II) complexes in situ thus simulating superoxide dismutase (SOD) activity and protecting cells from oxidative damage.32 Note that a critical point for further deployment remains the presence of single functionalized βCyD isomers in the polymers.
Translation to the clinics also implies use of sterile products. The correct timing of the sterilization phase needs to be defined as it can occur at two stages, either before or after drug loading. In a very informative paper Bilensoy et al. compared gamma radiation, micro-filtration and autoclaving to sterilize formulations of amphiphilic CyDs organizing in NPs.33 Sterilization by gamma irradiation appeared the best as particle size, drug loading and release profile were not altered. Filtration was no option as the NPs were too large and autoclaving caused NP aggregation. Few reports on sterilization of the CyD polymers object of this paper are available up to now. Von Recum et al. investigated the effect of autoclaving at 120 °C, gamma radiation and ethylene oxide on crosslinked polymers obtained with βCyD and diisocyanate.34 Ethylene oxide or gamma radiation sterilized, antibiotic-loaded polymers displayed the same bactericidal activity compared to non-sterilized polymers. Autoclavation was discarded as lower drug amounts were loaded due to crosslinking to higher extents. While autoclave sterilization with heating is feasible for pCyDs, in the case of loaded pCyDs the production of by-products can occur and needs to be checked.35 Gref et al. proposed microfiltration with 0.22 μm filters, a very common method, to sterilize pβCyDs loading ETH and booster assessing with HPLC the full recovery of the NPs measuring few tens of nm.20b Couvreur et al. performed sterilization by means of a high hydrostatic pressure system particularly suited for soft materials such as the mixed gel of pβCyD and alkyl-modified dextran.36 Finally, UV sterilization is feasible, with little risk for the unmodified pCyDs that are totally transparent to UV light, but the cargo may be light-responsive thus requiring a check on the photostability of the loading.37 Whatever method is selected for sterilization, a rigorous control of the effects of the method used on the formulation composition and behaviour is mandatory in all cases.
Conclusions/outlook
In conclusion, pCyDs stimulated great interest for many applications and the more they got used and known, the more their profiles could be adopted for specific targets, pharmacological and not.Enzymatic starch digestion is a sustainable process that affords the natural monomers as building blocks in very large amounts at inexpensive costs. Further research is expected to provide new knowledge on sustainable, synthetic protocols for CyD functionalization. The actual polycondensation procedure has high atom economy and requires a very low number of reagents with accessible costs keeping the synthesis cheap. Further exploration of CLAs other than EPI and systematic investigation of the reaction conditions to optimize parameters is expected to boost sustainable pCyDs production on large scale.
Translation of the pCyDs to the clinics as drug delivery systems or API implies however much more than sustainable synthesis at accessible costs with mandatory batch-to-batch reproducibility. In-depth inquiries need to be performed on the toxicological profile of each new pCyD-based product proposed for the clinics. Also, as more information is becoming available on the ability of pCyD-based systems to interact with different biological players, not only the identified target, we need more research to unveil their biological profile. In this context, actual research and innovation activity carried out worldwide on this class of polymers is of utmost importance to foster further progress.
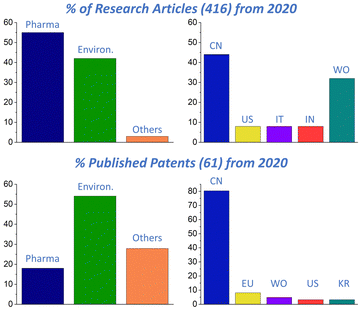
An inquiry on Web of Science (WoS) and Espacenet (EN) confirms the great interest in pCyDs for many different fields of applications which is a driving force in the development of these materials and their conquest of concrete industrial and clinical applications. A comparison between the research articles (Web of Science®) and patents (Espacenet®) containing “cyclodextrin polymer” in the title and published from 2020 (Fig. 6) afforded some interesting information. While the published research articles are more in the field of medical and pharmaceutical applications, the patents are mostly about environmental applications. In both cases, China is the place where most of the research outputs are produced. The gap between research and patents regarding pharmacological applications is probably due to the limitations mentioned above. Hopefully, thanks to the wide number of developing patents regarding other uses of pCyDs, we expect increasing interest in these materials and this will attract more investments enabling to fulfil the gap on missing toxicological and regulatory information regarding pCyDs.
Author contributions
IManet: funding acquisition, conceptualization, writing original draft, and review, supervision; MAgnes: conceptualization, writing original draft, and review, supervision; MMalanga: writing original draft, and review, literature analysis; AMazza: literature analysis and editing.Data availability
Representing all contributing authors, I declare that no primary research results, software or code have been included and no new data were generated or analysed as part of this Perspective.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Authors acknowledge funding from the European Union's Horizon Europe Research and Innovation Programme under the Marie Sklodowska-Curie grant agreement Bicyclos no. 101130235 and H2020 Hypocyclo no. 894942 grant. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union. The European Union cannot be held responsible for them. Authors acknowledge the CNR project Cyclodel funded in the frame of progetti@CNR 2020 call. Open Access Funding provided by Consiglio Nazionale delle Ricerche within the CRUI-CARE Agreement. The NextGeneration EU Programme is also acknowledged for supporting the Ecosystem for Sustainable Transition in Emilia-Romagna (ECOSISTER). Image for the Table of Content was downloaded and then further modified from pixabay.com.References
- P. Jansook, N. Ogawa and T. Loftsson, Int. J. Pharm., 2018, 535, 272–284 CrossRef CAS PubMed.
- J. Szejtli, Chem. Rev., 1998, 98, 1743–1754 CrossRef CAS PubMed.
- S. Li and W. C. Purdy, Chem. Rev., 1992, 92, 1457–1470 CrossRef CAS.
- (a) G. Crini, Chem. Rev., 2014, 114, 10940–10975 CrossRef CAS PubMed; (b) A. G. Pereira, M. Carpena, P. G. Oliveira, J. C. Mejuto, M. A. Prieto and J. S. Gandara, Int. J. Mol. Sci., 2021, 22 Search PubMed; (c) N. Morin-Crini, S. Fourmentin, E. Fenyvesi, E. Lichtfouse, G. Torri, M. Fourmentin and G. Crini, Environ. Chem. Lett., 2021, 19, 2581–2617 CrossRef CAS; (d) S. S. Braga, J. S. Barbosa, N. E. Santos, F. El-Saleh and F. A. A. Paz, Pharmaceutics, 2021, 13, 409 CrossRef CAS PubMed; (e) A. Matencio, S. Navarro-Orcajada, F. Garcia-Carmona and J. M. Lopez-Nicolas, Trends Food Sci. Technol., 2020, 104, 132–143 CrossRef CAS.
- L. Jicsinszky, E. Fenyvesi, H. Hashimoto and A. Ueno, in Comprehensive Supramolecular Chemistry, ed. J. Szejtli and T. Osa, Elsevier, Oxford, 1996, vol. 3 Search PubMed.
- (a) Y. Zhao, Z. Zheng, C.-Y. Yu and H. Wei, J. Mater. Chem. B, 2024, 12, 39–63 RSC; (b) Z. Liu, L. Ye, J. Xi, J. Wang and Z.-G. Feng, Prog. Polym. Sci., 2021, 118, 101408 CrossRef CAS.
- (a) T. Loftsson, J. Pharm. Sci., 2021, 110, 654–664 CrossRef CAS PubMed; (b) S. R. Gandhi, J. D. S. S. Quintans, G. R. Gandhi, A. A. D. S. Araújo and L. J. Quintans Júnior, Expert Opin. Drug Delivery, 2020, 17, 1069–1080 CrossRef CAS PubMed; (c) O. Adeoye and H. Cabral-Marques, Int. J. Pharm., 2017, 531, 521–531 CrossRef CAS PubMed.
- É. Fenyvesi, J. Inclusion Phenom., 1988, 6, 537–545 CrossRef.
- M. Agnes, E. Pancani, M. Malanga, E. Fenyvesi and I. Manet, Macromol. Biosci., 2022, 22, 2200090 CrossRef CAS PubMed.
- E. Renard, A. Deratani, G. Volet and B. Sebille, Eur. Polym. J., 1997, 33, 49–57 CrossRef CAS.
- B. Martel, D. Ruffin, M. Weltrowski, Y. Lekchiri and M. Morcellet, J. Appl. Polym. Sci., 2005, 97, 433–442 CrossRef CAS.
- M. Weltrowski, M. Morcellet and B. Martel, vol. WO/2000/047630, 2000.
- B. Rossi, S. Caponi, F. Castiglione, S. Corezzi, A. Fontana, M. Giarola, G. Mariotto, A. Mele, C. Petrillo, F. Trotta and G. Viliani, J. Phys. Chem. B, 2012, 116, 5323–5327 CrossRef CAS PubMed.
- (a) F. Trotta, F. Caldera, R. Cavalli, A. Mele, C. Punta, L. Melone, F. Castiglione, B. Rossi, M. Ferro, V. Crupi, D. Majolino, V. Venuti and D. Scalarone, Beilstein J. Org. Chem., 2014, 10, 2586–2593 CrossRef PubMed; (b) M. Malanga, M. Balint, I. Puskas, K. Tuza, T. Sohajda, L. Jicsinszky, L. Szente and E. Fenyvesi, Beilstein J. Org. Chem., 2014, 10, 3007–3018 CrossRef PubMed.
- (a) J. Szeman, E. Fenyvesi, J. Szejtli, H. Ueda, Y. Machida and T. Nagai, J. Inclusion Phenom., 1987, 5, 427–431 CrossRef CAS; (b) J. Szejtli, E. Fenyveasi, B. Zsadon, M. Szilasi and L. Décsei, vol. EP0119453A2 (Ed.: E. P. Office), 1983.
- R. Gref, C. Amiel, K. Molinard, S. Daoud-Mahammed, B. Sébille, B. Gillet, J.-C. Beloeil, C. Ringard, V. Rosilio, J. Poupaert and P. Couvreur, J. Controlled Release, 2006, 111, 316–324 CrossRef CAS PubMed.
- S. Daoud-Mahammed, P. Couvreur, C. Amiel, M. Besnard, M. Appel and R. Gref, J. Drug Delivery Sci. Technol., 2004, 14, 51–55 CrossRef CAS.
- S. Daoud-Mahammed, C. Ringard-Lefebvre, N. Razzouq, V. Rosilio, B. Gillet, P. Couvreur, C. Amiel and R. Gref, J. Colloid Interface Sci., 2007, 307, 83–93 CrossRef CAS PubMed.
- S. Daoud-Mahammed, P. Couvreur, K. Bouchemal, M. Chéron, G. Lebas, C. Amiel and R. Gref, Biomacromolecules, 2009, 10, 547–554 CrossRef CAS PubMed.
- (a) J. Wankar, G. Salzano, E. Pancani, G. Benkovics, M. Malanga, F. Manoli, R. Gref, E. Fenyvesi and I. Manet, Int. J. Pharm., 2017, 568–576 CrossRef CAS PubMed; (b) G. Salzano, J. Wankar, S. Ottani, B. Villemagne, A. R. Baulard, N. Willand, P. Brodin, I. Manet and R. Gref, Int. J. Pharm., 2017, 531, 577–587 CrossRef CAS PubMed.
- A. Machelart, G. Salzano, X. Li, A. Demars, A.-S. Debrie, M. Menendez-Miranda, E. Pancani, S. Jouny, E. Hoffmann, N. Deboosere, I. Belhaouane, C. Rouanet, S. Simar, S. Talahari, V. Giannini, B. Villemagne, M. Flipo, R. Brosch, F. Nesslany, B. Deprez, E. Muraille, C. Locht, A. R. Baulard, N. Willand, L. Majlessi, R. Gref and P. Brodin, ACS Nano, 2019, 13, 3992–4007 CrossRef CAS PubMed.
- A. Fraix, C. Parisi, M. Seggio and S. Sortino, Chem. – Eur. J., 2021, 27, 12714–12725 CrossRef CAS PubMed.
- A. Fraix, N. Kandoth, I. Manet, V. Cardile, A. C. E. Graziano, R. Gref and S. Sortino, Chem. Commun., 2013, 49, 4459–4461 RSC.
- F. Laneri, M. Seggio, C. Parisi, S. Béni, A. Fraix, M. Malanga and S. Sortino, ACS Appl. Polym. Mater., 2023, 5, 7918–7926 CrossRef CAS PubMed.
- E. Pancani, D. Veclani, M. Agnes, A. Mazza, A. Venturini, M. Malanga and I. Manet, RSC Adv., 2023, 13, 10923–10939 RSC.
- A. Abad-Gurumeta, J. Ripollés-Melchor, R. Casans-Francés, A. Espinosa, E. Martínez-Hurtado, C. Fernández-Pérez, J. M. Ramírez, F. López-Timoneda and J. M. Calvo-Vecino, Anaesthesia, 2015, 70, 1441–1452 CrossRef CAS PubMed.
- (a) C. D. Davidson, N. F. Ali, M. C. Micsenyi, G. Stephney, S. Renault, K. Dobrenis, D. S. Ory, M. T. Vanier and S. U. Walkley, PLoS One, 2009, 4, e6951 CrossRef PubMed; (b) Mandos LLC, 2021, Clinical Trial: VTS-270 to Treat Niemann-Pick Type C1 (NPC1) Disease https://clinicaltrials.gov/ct2/show/NCT02534844.
- A. M. Anderson, I. Manet, M. Malanga, D. M. Clemens, K. Sadrerafi, Á. Piñeiro, R. García-Fandiño and M. S. O'Connor, Carbohydr. Polym., 2024, 323, 121360 CrossRef CAS PubMed.
- M. Agnes, E. M. Kasimati, M. Inclán, A. Thanassoulas, G. Miliotis, M. Malanga, G. Benkovics, G. Nounesis, E. García-España, P. Bouziotis, Y. G. Lazarou, V. Miriagou, I. M. Mavridis and K. Yannakopoulou, Carbohydr. Polym., 2023, 321, 121323 CrossRef CAS PubMed.
- N. Kandoth, J. Mosinger, R. Gref and S. Sortino, J. Mater. Chem. B, 2013, 1, 3458 RSC.
- V. Giglio, V. Oliveri, M. Viale, R. Gangemi, G. Natile, F. P. Intini and G. Vecchio, ChemPlusChem, 2015, 80, 536–543 CrossRef CAS PubMed.
- N. Bognanni, F. Bellia and G. Vecchio, ChemMedChem, 2023, 18, e202300035 CrossRef CAS PubMed.
- E. Memisoglu-Bilensoy and A. A. Hincal, Int. J. Pharm., 2006, 311, 203–208 CrossRef CAS PubMed.
- J. M. Halpern, C. A. Gormley, M. A. Keech and H. A. von Recum, J. Mater. Chem. B, 2014, 2, 2764–2772 RSC.
- Y. Hu, K. Xing, X. Li, S. Sang, D. J. McClements, L. Chen, J. Long, A. Jiao, X. Xu, J. Wang, Z. Jin and C. Qiu, npj Sci. Food, 2023, 7, 20 CrossRef PubMed.
- S. Daoud-Mahammed, J. L. Grossiord, T. Bergua, C. Amiel, P. Couvreur and R. Gref, J. Biomed. Mater. Res., Part A, 2008, 86A, 736–748 CrossRef CAS PubMed.
- K. Khoshgard, N. Ahmadi and M. Jaymand, Carbohydr. Polym. Technol. Appl., 2023, 6, 100369 CAS.
Footnote |
| † Arianna Mazza is currently employed by U-Series in Bologna. |
| This journal is © The Royal Society of Chemistry 2024 |