Open Access Article
Open Access ArticleState-of-the-art and challenges towards a Molecular Solar Thermal (MOST) energy storage device
Alberto
Giménez-Gómez†
,
Lucien
Magson†
,
Cecilia
Merino-Robledillo
,
Sara
Hernáez-Troya
,
Nil
Sanosa
,
Diego
Sampedro
 * and
Ignacio
Funes-Ardoiz
* and
Ignacio
Funes-Ardoiz
 *
*
Instituto de Investigación Química de la Universidad de La Rioja (IQUR), Departamento de Química, Universidad de La Rioja, Madre de Dios, 53, Logroño, 26006, Spain. E-mail: diego.sampedro@unirioja.es; ignacio.funesa@unirioja.es
First published on 9th May 2024
Abstract
The current global energy scenario calls for the urgent replacement of fossil fuels for alternative, environmentally affordable, abundant and cheap energy sources. Among the different options available, MOlecular Solar Thermal (MOST) systems have emerged in the last few years as a promising alternative. While this technology has already shown great potential under lab conditions, some difficulties remain to be dealt with when it comes to its application in real devices. In this minireview, we briefly summarize the basic concepts of MOST systems and we focus on the critical problems yet to be solved to turn this technology into a real alternative for energy generation and storage.
Introduction
In recent years, the energy demand has risen briskly and it is predicted to double in the next 40 years due to the growing world population and the increase in energy consumption in developing countries.1 On top of this, the current dependence on fossil fuels poses significant concerns in terms of energy security and environmental sustainability.2There is a significant need to mitigate the negative impacts associated with the extraction and combustion of these resources, along with the urgency to reduce greenhouse gas emissions. In particular, the 2015 United Nations Climate Change Conference (COP21) Paris Agreement on climate change states explicitly that the greenhouse gas emissions must be reduced by 60% prior to 2050.3 This complex scenario underscores the critical importance of developing new sustainable energy solutions. These technologies should not only have the potential to address imbalances in energy supply and demand, but they should also catalyze the transition towards a cleaner and more efficient energy system.
In the last few decades, different alternative energy sources have been considered. Among them, solar energy exploitation has been widely investigated, experiencing a remarkable increase of more than 900% in installed capacity.3 This demonstrates its rapid expansion highlighting its growing prominence as an emerging energy source. The International Energy Association (IEA) foresees that solar energy might deliver over 25% of the global energy needs around the deadline of the aforementioned Paris Agreement. Albeit being the most powerful renewable energy available, with an average of 342 W m−2 solar energy falling upon Earth over an entire year,4 its intermittent nature and the implicit load levelling challenge of this source have hindered its extensive use. To solve this, an effective storage solution of solar energy is primordial for the progress towards the widespread use of this energy source.
Whilst the logistics of fossil fuels are incorporated in our societies, problems related to energy storage and transport are common to many different alternative sources. In the quest for effective energy storage solutions, different innovative approaches to use and store solar energy have been explored in the last few years. One technology that has been benefited by the rise in this research field has been electric batteries, including applications in real life devices such as electric vehicles. Nevertheless, the use and development of improved batteries have been proved to have environmental concerns for solar energy storage due to their low recyclability and limited life-span.5
Among other effective storage solutions, one promising avenue is the development of artificial photosynthesis, a process that mimics natural photosynthesis to convert solar energy into high-value molecules that can be stored and used as fuel, such as hydrogen or hydrocarbons. Artificial photosynthesis is a clever green alternative to energy use and storage, not only because sunlight is the energy source, but also because carbon dioxide can be trapped along the process, thus reducing the presence of this greenhouse gas in the atmosphere. Natural photosynthesis converts sunlight into stored chemical energy with an efficiency lying around the 3–6% mark.6 In contrast, current artificial systems struggle to achieve similar efficiencies at comparable costs.7–10 However, artificial photosynthesis can produce more dense energy fuels like methane, instead of the metabolites prepared by its natural counterpart. Besides, the use of man-made catalysts compared to natural enzymes could imply potentially faster reactions in the future. Although there is still plenty of room for improvement, artificial photosynthesis emerges as a possible sustainable solution to solve some of the solar energy storage limitations.
A significant drawback of artificial photosynthesis is that it involves a high number of optimizable reaction steps. These include the solar spectrum match and high absorption of the photosensitizer, the complex electron transfer, the water oxidation and the carbon dioxide reduction that produces the final fuel.
To solve this, different technologies have tried to build a conceptually much simpler situation. In MOlecular Solar Thermal (MOST) systems,11 a parent molecule is photoconverted upon light excitation into a high-energy metastable isomer, which can release the energy stored on demand in the form of heat.12,13 The simplicity of this technology relies on being a closed system where no reagents are consumed and no by-products are formed, thus implying a much cleaner solution for solar energy storage. Although having some promising features, this technology is not free from drawbacks. In this review, we explore the limitations of the current state-of-the-art and associated challenges, highlighting the imperative need to drive research and innovation in the field of molecular solar energy storage to advance towards a more sustainable energy future.
MOlecular Solar Thermal (MOST) systems
Photoswitches as MOST systems
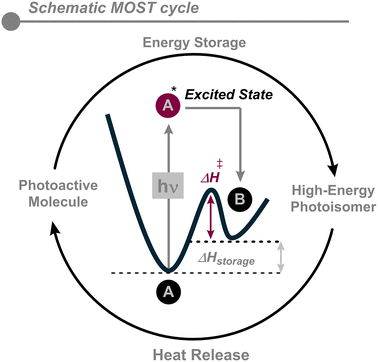
Photoswitches are organic or organometallic compounds that react through a reversible chemical transformation upon absorbing light. These molecules can undergo a reversible change in their geometry and molecular properties upon photon absorption.14 Among the myriad of applications of photoswitches, the exploitation of sunlight has become relevant in the last few years. If one isomer of the photoswitch is significantly less stable than the other, the photoisomerization from the parent to the metastable isomer transforms some of the absorbed photon energy into stored chemical energy. The conversion of the metastable isomer back to the parent isomer then allows this energy to be released as heat (Fig. 1).Photoswitches for solar thermal energy storage are based on this energy difference between the isomers, first absorbing sunlight and then releasing heat on demand. This energy can be related to the ability to store solar energy and should be maximized without compromising the thermal stability of the metastable isomer or the quantum yield of its photochemical generation. This may be achieved by optimizing different properties such as energy storage density, solar spectrum match, the quantum yield of photoconversion, and the lifetime of the metastable isomer as some of the required features of MOST systems, as discussed below.15,16
The pioneering work by Cargill17 in the sixties and the subsequent approaches developed by Yoshida in 1985,18 Bren et al. in 1991,19 and Dubonosov et al. in 200220 established the foundation for the current development of these systems. Different chemical structures have been proposed, involving different reactivities: valence isomerization, as in norbornadiene–quadricyclane or nitrone–oxazirine; geometric Z/E isomerization, as in indigo derivatives; geometric isomerization followed by an intramolecular rearrangement as in the case of N-acylated aminovinyl ketones; or the thermally reversible photodimerization reactions, found in anthracene derivatives.21 Unfortunately, in spite of the intensive research in the field during the last few decades, the perfect molecule to be of practical use in this technology remains elusive, due to the long list of requirements that has to be fulfilled.
The ideal MOST candidate
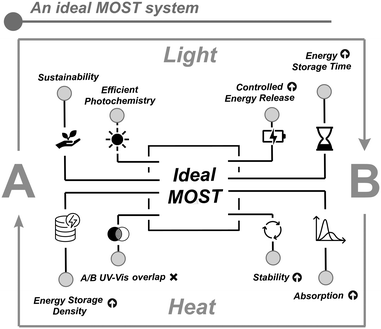
In general, the parent unsubstituted core structures for all the reported MOST candidates are not efficient in storing solar energy. Thus, it is necessary to optimize several factors for successful implementation (Fig. 2). This comprises a list of criteria for an optimal MOST system that can be classified into the following categories:18,21–24(i) Absorption: the molecule must effectively absorb a significant portion of the solar spectrum corresponding to ultraviolet and visible light to make use of the widest significant portion of incoming sunlight.
(ii) Quantum yield: as a measure of the ability of the molecule to undergo photoisomerization after light absorption. Ideally, to efficiently use the absorbed photons from the solar spectrum, the photoisomerization reaction should be triggered with a quantum yield as close to one as possible.
(iii) Energy storage time: the half-life of the high-energy metastable isomer must demonstrate long-term stability. This is quantified by its storage half-time (t1/2) that correlates with the free energy barrier (ΔG‡), required for the metastable isomer to back-convert to the parent isomer. This value should be long enough to allow chemical energy storage for long periods of time (days to years).
(iv) Energy storage density: the molecule should have the highest possible energy density. This value is related to the energy difference between isomers and their molecular weight.
(v) Energy release: at the right time and place, the system should be able to release the stored energy as heat during the back-conversion to the parent isomer. This process could be triggered by various external stimuli, such as catalysis, electrochemistry, heat or light.
(vi) Stability: the system should be robust enough to allow operation through extended periods of time. At the molecular scale, this implies that the molecule could be involved in numerous cycles of photoisomerization and back-conversion without showing degradation.
(vii) Non-overlapping spectra: both the parent isomer and the high-energy isomer should not absorb light in the same region of the solar spectrum to prevent competition for photon absorption between the two compounds and should also try to avoid the presence of photostationary states (PSSs). The composition of these PSSs is critical to define the stored energy density, and thus, the overall efficiency of the process.
(viii) Sustainability: MOST systems, as greener alternatives to fossil fuels, should be environmentally friendly and non-toxic.
Norbornadiene/quadricyclane as the “MOST” studied candidate
Various MOST systems have been explored for a wide range of applications, highlighting norbornadienes, azobenzenes, stilbenes, anthracenes, and dihydroazulenes. These families of compounds are involved in different isomerization reactions with two main categories: double bond isomerization and electrocyclic reactions. The electrocyclic reactions, as represented by the norbornadiene (NBD)/quadricyclane (QC) couple, show promise for solar thermal storage due to their high storage enthalpy, low molecular weight, and availability.25–27 Again, in this system, the absorbed photon can trigger an electronic transition from the parent isomer in the ground state to the high-energy state, causing a [2 + 2] intramolecular cycloaddition. Then, the parent NBD molecule could be eventually recovered releasing heat.13While the unsubstituted NBD has some limitations such as poor matching with the solar spectrum and a low quantum yield, NBD derivatives have gathered significant interest due to the combination of features: high quantum yields, high energy storage density, long half-life times and wide chemical availability.28–32 One notable advantage of NBD/QC systems compared to other photoswitches is the occurrence of a blue-shift upon absorption. As a result, the QC molecule usually absorbs at shorter wavelengths in an irrelevant region of the solar spectrum. This lack of overlap between isomers avoids the occurrence of photostationary states, as happens with azobenzene derivatives.15
The most successful strategy for shifting NBD absorption towards longer wavelengths (entering the visible region) involves the introduction of electron-donating and electron-withdrawing groups to create a ‘push–pull’ effect. This makes the system capable of absorbing photons from the solar spectrum beyond 400 nm.12 A long list of NBD derivatives have been prepared and tested for this application.33,34
Charging step: the photoisomerization reaction
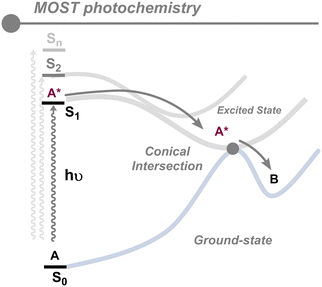
The solar energy is captured by the absorbing compound and transformed into chemical energy through a photoisomerization reaction yielding the high-energy metastable isomer. This is the first half of the whole MOST cycle. As illustrated in Fig. 3, the photoisomerization reaction implies adequate sunlight absorption and an efficient photoreaction. The low-energy isomer should absorb a photon inducing a vertical electronic transition from the fundamental electronic state (S0) to the excited electronic state (Sn). Then, the excited molecule should undergo a photoconversion process to attain the metastable isomer in its ground state. This process usually involves an S1/S0 conical intersection, enabling non-radiative vibrational relaxation from the excited state to the ground state while forming the photoisomer. The quantum yield (φiso) reflects the probability of obtaining the metastable isomer once the stable isomer absorbs a photon and serves as an efficiency unitless indicator for the photoconversion process. To maximize efficiency, the quantum yield must approach one, thereby circumventing competitive processes that could hamper the formation of the metastable isomer and, thus, the conversion of solar energy into chemical energy. The stored energy is the difference in energy between the metastable isomer and the parent isomer.15As described in the previous section, several factors need to be optimized in an ideal MOST system. With respect to the photochemical half-cycle, it is essential to consider specific parameters: (i) energy storage capacity, (ii) quantum yield, and (iii) solar spectrum match. Optimizing these factors is not straightforward, as there exists a large degree of interdependence between them which implies that optimization of some parameters may lead to a worsening of others.
The energy storage capacity is linked to storage density, defined as the energy stored per unit mass. To optimize energy storage capacity, it is necessary to increase stored energy while minimizing the molecular mass of the compound.35 Although it is related to the back-conversion reaction (see below), this property strongly depends on the MOST molecular structure and can be tuned by design. To do so, two different approaches have been proposed: on the one hand, either stabilizing the isomer or destabilizing the metastable isomer can enhance the energy difference. However, this often requires adding substituents to the system, which is detrimental as it increases the molecular weight. On the other hand, molecular weight can be reduced by combining multiple isomers to form dimers or trimers of the starting molecules, enhancing storage density.16
Another parameter to be optimized is the solar spectrum match.36 To achieve the formation of the metastable isomer, it is necessary for the low-energy isomer to absorb a photon. Depending on the energy difference between the HOMO and LUMO of the starting compound, the system will be able to absorb in a specific region of the solar spectrum. To attain the ideal MOST, it is crucial that the stable isomer absorbs the maximum number of photons in the UV-visible region (300–700 nm) since this is the region of maximum solar light intensity. Additionally, the high-energy isomer should not absorb in the same region as the parent isomer to minimize by-product formation and competitive photochemical reactions. In this case, the optimal approach to enhance absorption is to increase the conjugation of the molecule, as this reduces the energy gap between the two electronic states and produces a red-shifting in the absorption. The incorporation of “push–pull” substituents (combining acceptor and donor substituents to enhance homoconjugation) is an effective technique to increase the conjugation of the system.37 However, it is important to note that while this approach enhances absorption and the solar match, it is clearly detrimental to the energy density as it increases the molecular weight.24,38 Recent studies reported by Libuda and co-workers presented photoelectrochemical infrared reflection absorption spectroscopy (PEC-IRRAS), facilitating the real-time tracking of the full energy storage and release cycle via in situ vibrational spectroscopy.39 Overall, the photoconversion parameters can be condensed in the concept of solar conversion efficiency, developed by Moth-Poulsen and co-workers recently.41–43
For practical implementation of this technology, it is required that a significant amount of energy could be stored through the photochemical reaction to be released as heat afterwards. To do so, an important aspect to highlight is the concentration effect. For MOST to be effective, the device must operate at high concentrations to achieve large energy density. However, under these conditions, the photochemical step may be hampered as elevated concentrations may lead to increased intermolecular reactions44 or competitive absorption,23 which will eventually lead to poor performance. In this regard, MOST molecules can be dissolved in a solvent or exist in a liquid state and the properties of the system will vary greatly depending on the solvent used.45 Liquids utilizing the NBD/QC couple have been formulated with promising storage densities but face challenges related to intermolecular degradation reactions.46
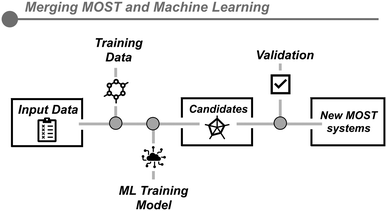
The complex task of designing molecules that could fulfill the long list of requirements for a compound to be used as MOST has been lately addressed by machine learning techniques (Fig. 4). This enables the study of different substituted systems and allows information on relevant parameters to be extracted as energy densities and absorption spectra through quantum chemistry-based calculations, despite the multireference character of the transition states, which considerably makes this task very challenging.47,48 After the chemical space is chosen (the core molecule with a range of different substituents), machine learning tools allow for the generation of species within the chemical space. The most stable conformers are optimized and their properties (electron density, absorption spectrum, and back-conversion energy barrier) are predicted.14 Through this approach, groups of up to 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 molecules were successfully examined. From this study, 15 molecules were selected as promising candidates for further investigation, five of which have shown energy densities greater than 0.45 MJ kg−1. The limitation in these strategies lies in the capacity to generate adequate training databases to instruct the models in identifying the most optimal MOST systems. Additionally, the algorithm is overly restrictive concerning the molecules under study and the results do not show any candidate with an outstanding energy density. Also, as this approach does not include the synthetic viability of the molecules in the algorithm, the output reflects quite complex chemical structures, which hinders the experimental production of the desired molecules.43
000 molecules were successfully examined. From this study, 15 molecules were selected as promising candidates for further investigation, five of which have shown energy densities greater than 0.45 MJ kg−1. The limitation in these strategies lies in the capacity to generate adequate training databases to instruct the models in identifying the most optimal MOST systems. Additionally, the algorithm is overly restrictive concerning the molecules under study and the results do not show any candidate with an outstanding energy density. Also, as this approach does not include the synthetic viability of the molecules in the algorithm, the output reflects quite complex chemical structures, which hinders the experimental production of the desired molecules.43
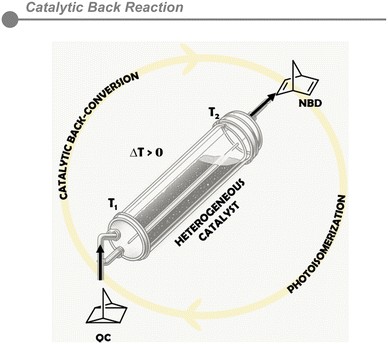
Controlled release of energy – catalytic back-conversion
In MOST systems, the energy release process is crucial to close the cycle around the reversible photoisomerization of the selected molecular switches. The subsequent catalytic or thermal relaxation of the excited molecule towards its original parent molecule releases the stored energy in the form of heat. Controlling this back-reaction process is key to implementing this technology in real devices and therefore, the catalytic back-conversion is far more interesting than the corresponding thermal one, due to the actual kinetic control of the process (Fig. 5).In an ideal MOST-system tailored towards long-term energy storage applications, the activation enthalpy typically exceeds room temperature energy levels and requires a suitable catalyst to facilitate the back-conversion. Regardless of that, many systems still face limitations, primarily due to their half-life time, which limit their functionality as batteries to some extent.
However, there are applications diverging from the conventional battery-like prototypes where catalysts are unnecessary. Take, for instance, a study led by Moth-Poulsen and his team, which integrated NBDs into polymers for climate control in window laminates.40 Here, a lower activation energy is required to facilitate the thermal back-conversion of the corresponding QC, exhibiting half-lifetimes ranging between 4 and 8 hours. This process liberates heat steadily and efficiently without relying on costly catalysts. Nevertheless, this review predominantly emphasizes long term energy storage to address the intermittent nature of solar energy. Therefore, some form of catalytic control becomes imperative.
While photoisomerization of MOST systems has been vastly studied, the back-conversion is still underexplored. However, recent efforts in this regard have been made to expand the understanding of this catalytic process to achieve real implementation in a device.12
In the initial states of MOST development,19 homogeneous catalysis was used to provide understanding of the back-conversion reaction due to the easy control of the catalyst structure, the high degree of interaction between the catalyst and MOST molecule, without mass-transport limitations, and the easy screening of the catalyst due to the similar solubility to MOST molecules. However, heterogeneous catalysis has recently emerged as the most viable option for real devices due to its easy implementation in flow systems, its physical separation from the charged NBD/QC solution and its higher effectivity in most of the cases. This makes the catalysts easier to recover, reuse and recycle once they are inactive, thus enhancing the overall feasibility of a solar energy storage device.
Regarding the heat release process, Moth-Poulsen and co-workers reached a milestone in the field, detecting up to 63 °C temperature gradient when using a norbornadiene derivative that can store up to 89 kJ mol−1 (396 kJ kg−1).49 In that case, a 1.5 M QC solution was pumped at 5 ml h−1 through a small catalytic bed of a CoPc/C catalyst. However, this methodology is still far from real conditions, where large amounts of QC solution should be tested and the integration of both the photoisomerization and the back-conversion reaction should run at similar reaction rates.
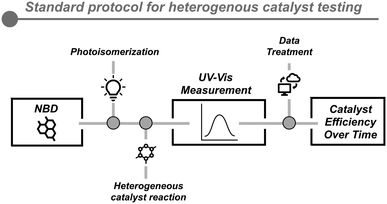
The comparability of different heterogeneous catalysts is not trivial and it requires a series of validated protocols to extract valuable information about the kinetics of the back-conversion reaction. Our group recently reported a standardized protocol to compare different catalysts, independent of their properties (metal loading, particle size, etc.).12 This method allows quick and robust assessment of the efficiency of a large series of heterogeneous catalysts using UV-VIS spectroscopy as the analytical method to rapidly characterize the NBD/QC ratio. Using minimal amounts of QC (10 mg) solution and catalyst (1 mg), the evaluation of the efficiency over time is quantified by the amount of NBD generated in the back-conversion reaction (Fig. 6). In this first study, a commercial Pt/C catalyst was found as the most active one in the field, defining the state-of-the-art of the back-conversion process at that point. Also, this methodology could be potentially implemented as an in line measurement in a potential MOST device.
Following this methodology, a series of heterogeneous catalysts based on Pt metal as the reference and first-row transition metals (Ni and Cu) were synthesized using both an oxidative and a reductive method. The idea was to identify what features of the catalyst affect the back-conversion kinetics and to identify potential candidates based on non-noble metal catalysts.50 In this study, when the synthesis was carried out under an oxidative atmosphere, the metal on carbon catalysts showed a larger efficiency compared to the corresponding reductive conditions. Also, the Cu/C catalyst has a comparable efficiency to Pt/C, both synthesized under the same oxidative conditions. This can be crucial to reduce the cost of a real device and facilitate the implementation of MOST systems in solar-energy capture devices.
Integrated system – designing a real device
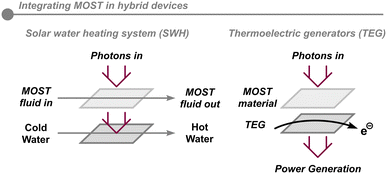
The application of MOST technology in real-life scenarios requires a proof-of-concept of the integration of both the photoisomerization and the back-conversion reaction in a single device. Recently, the design of various lab-scale devices, which store solar energy, has been undertaken,51 many of which are conceived on the milligram scale, which is not realistic for a real-life application. Thus, it can be challenging to predict potential problems when scaling up these devices.Also, due to the limited amount of energy that is stored in the highest performing NBD molecules, different combinations of MOST systems and alternative energy technologies have been proposed, called hybrid MOST systems. In the first example, a two-layer device is comprised of a top layer containing MOST fluid and a bottom layer with a thermal water heating system (Fig. 7).46 In this strategy, the NBD is converted to QC in the upper part of the system whilst the remaining solar energy is transmitted and harvested by water flowing through the lower part. The top layer can store solar energy as chemical energy at an efficiency of 1.1%, whereby the combined device has a total efficiency of 80%.
Alternatively, by integrating an NBD/QC pairing into a MOST–TEG (thermoelectric generator) system, a functional solar-to-electrical battery was conceived.52 These devices were able to generate power densities up to 1.3 W m−3, demonstrating the off-grid potential of solar energy and power storage. This is a unique demonstration which extends the versatility of the energy release in MOST systems from the conventional form of heat on demand to electrical power.
Finally, a MOST phase-change storage material was proposed, where both thermophysical and thermochemical storage strategies are employed to deliver energy during day and night.53 The concept of the hybrid system was to complement both the advantages and disadvantages of each energy storage method. For instance, the MOST system used has high energy densities of 0.4 MJ kg−1,49 whilst the localized phase change material has low energy densities. On the other hand, MOST systems often show material fatigue, having far shorter lifetimes than thermophysical energy storage media which usually exhibit high thermal stabilities.
A case study on the application of MOST hybrid technology in a family household scenario is yet to be projected, but considering the current state-of-the-art of MOST molecules, the application of this technology is still not realistic. According to Eurostat, space heating and water heating utilities account for a total of 78.9% of energy consumption in EU households.54 From a case study encompassing 38 households based in Northern Germany, an average consumption per family household with 2.38 inhabitants is 2829 kW h from May 2018 to the end of 2020.55 The maximum daily consumption reached is at 108.5 kW h per household wherein 85.6 kW h is required for space and water heating purposes. To apply MOST systems into a domestic setting, a few assumptions need to be acknowledged. First, the seasonal, temporal, and geographic disparities are disregarded, plus the NBD/QC system, which has an energy density of 0.4 MJ kg−1 and concentrations of up to 1.5 M, can be irradiated. The daily household energy demand of 85.6 kW h, or 308.16 MJ, can be met if 770.4 kg of NBD is dissolved in approximately 2300 liters of fluid. Another key assumption to consider is that about 5% of incoming solar photons should be efficiently photoisomerized in the collector setup, plus from an engineering perspective, the MOST collector should be moderately sized and ideally located on the roof to collect solar rays. Alternatively, conversion of the energy release to electricity could be a potential solution to these limitations.39 Also, the overall price should be reasonable for house installation, and several factors such as the pumping system or MOST fluid refilling should be considered in the device design.
Current limitations of the MOST technology
In this next section, we discuss the current chemical and practical challenges MOST systems must overcome to be implemented into engineering devices, plus the diverse engineering and chemical approaches which have been proposed to solve these problems.Photoisomerization efficiency in highly concentrated solutions
The major limitation of MOST systems at this point is the efficient photoisomerization of highly concentrated to neat solutions. Indeed, most of the reported studies work at low concentrations (10−4–10−3 M) and very few examples have been reported with realistic concentrations, but without a detailed analysis of the photoisomerization reaction.49 In one study, a droplet dispersed as a thin film was fully photoisomerized, although an uncontrollable thermal back-conversion was triggered likely due to local heat generation. Hence, systematically controlling the energy storage and release remains out of reach.56 This has been recently improved by designing a polymer film device with very good properties.57 Also, in many MOST systems, the photoisomerization reaction is unimolecular and increasing the concentration can potentially lead to undesired reactivity, such as intermolecular reactivity (i.e., polymerization).Many of these hybrid devices are strongly limited by their overall efficiency to convert solar irradiance into chemical fuels. Recently, a proposed solution involved stacking on top of each other several MOST photoswitches, whereby each photoswitch is separated into layers with counter directional flows.58 In this way, each MOST photoswitch contains a distinct absorption spectrum, which absorb different parts of the solar sunlight (Fig. 8). However, despite the larger theoretical maximum efficiency, the experimental measurements showed a low value overall (∼0.01–0.02%).
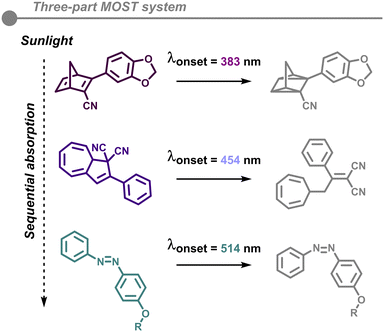 | ||
| Fig. 8 A hybrid device for solar energy capture comprised of a three-part MOST system with counter directional flows. | ||
In fact, the rate of photoisomerization of each photoswitch is independent of each other. Moreover, the intensity of solar irradiance required to convert the parent isomer to the metastable one varies vastly per MOST photoswitch, with differences in rates becoming even more prominent at higher concentrations. To attain full photoisomerization conversion at high concentrations over shorter times, a far more intense light source would be required. However, the metastable isomer may be prone to revert to the original parent isomer or undergo photothermal degradation.
Energy density & heat release
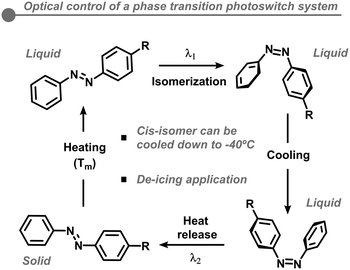
Few methods have been successful in increasing the energy storage density of MOST systems. As previously mentioned, the most common manner of increasing energy densities of MOST systems is by lowering their molecular weight or adding more photoactive units. Alternatively, a radically unique method to increase energy densities involves taking. One radically different design of MOST systems includes taking advantage of the substantial structural and polarity changes photoswitch systems undergo upon photoisomerization. In some cases, a solid to liquid phase transition occurs; this strategy is an effective way to increase the number of photoactive units gravimetrically or volumetrically.59 Recent examples using azobenzene and arylazopyrazole derivatives can store energy and release thermal heat through the following process in Fig. 9.59,60In the first step, the trans-azobenzene form is in the solid state; upon heating, a solid to liquid phase transition occurs approximately at the melting temperature. Then, the transition from the trans to cis form is triggered optically by UV irradiation. Intriguingly, the absorption band of the azobenzene derivative matches the emission spectra of indoor fluorescent light, opening the possibility of recycling wasted photons. A crucial element to this system is that the cis form of the solution can be cooled down to −40 °C, its glass transition temperature, yet remarkably retaining the stored solar energy. Thus, the heat release can be triggered optically at sub-zero temperatures, in which a crystallization process occurs transforming the liquid back to the solid state. These systems offer great opportunity as a de-icing application. Yet, there are two critical limitations to this strategy. First, very few cis isomers can maintain liquid states below 0 °C, relying on a supercooling behavior to do so.60–62 Second, to avoid the unnecessary initial heating step, a spontaneous transformation from the solid trans state to the liquid cis state would be desired; however, few cis isomers have melting point values lower than ambient temperatures.63
Also, energetically dense systems need to be achieved for MOST systems to be practically viable. If this objective is met, moving to higher concentrations poses problems not only in the photochemical transformation but also in the reverse catalytic transformation between isomers. The catalytically triggered heat release from more viscous MOST solutions needs to be efficiently managed. In a fixed bed reactor scenario, catalyst particles are filled into a chamber and offer a high active surface area for the reaction. The residence times of the MOST fluid and the hydrodynamic/thermodynamic behavior can be optimized to achieve full conversions and the maximum thermal output.64 However, little testing has been performed on a large scale, thus material fatigue and catalytic deactivation pathways have barely been explored yet.
Solvent for MOST systems
In the early stages of MOST development, all the research efforts were focused on discovering potential candidates and evaluating the distinct parts of the system (photoisomerization and back-conversion) individually. However, for integrating the system in a prototype device, there are engineering requirements as well as safety concerns that force to transfer the MOST technology to potentially scalable solvents.In fact, the solvent role is not only to dissolve the photoswitch, which immediately causes a lowering of the energy density of the system, but also to facilitate the reversible reactivity, which can be sensitive towards solvent properties. Longer half-lives were found for more polar solvents due to the norbornadiene donor–acceptor structure. Thus, more polar solvents can reorganise and stabilise better the excited state promoting a more efficient crossing across the conical intersection to the metastable quadricyclane.65 The heat release originating from a MOST system is primarily dictated by the extent of solubility of the photoswitch pairing in the respective solution and the associated energy density. Very few comprehensive studies have been conducted to ascertain the effect of the solvent on the catalytic activity of MOST systems.
Currently, the state-of-the-art of MOST systems is based on liquid-based toluene solutions66 as they are photochemically stable, do not interfere with the targeted absorption band of an idyllic parent isomer and have been implemented successfully in solid–liquid catalytic reactions.50 However, toluene does not have the correct physical properties to be safely implemented into engineering devices. The solvent used to dissolve the MOST photoswitch should not be prone to auto combustion especially at low temperatures. Plus, despite the MOST concept being a closed system, governmental safety regulations will prohibit the deployment of MOST systems in toxic solvents, especially in a household scenario.
Also, the solvent needs to fulfill the following thermochemical properties. First, low heat capacities and high thermal conductivities are required to enable efficient heat transfer from solution to heat transfer fluid. Plus, for storing MOST solutions during colder seasons, the solvent ideally needs to have sub-zero melting point temperatures to avoid solidification.
Unlike toluene, water-based MOST systems have recently been proposed, displaying long half-lives and moderately high storage densities, but with poor photochemical properties and at very low concentrations.67 The solar spectrum match is in the energetic UV-B range, plus the solubilities of the MOST derivatives are very low, far from ideal for a MOST system.
Outlook
During the last few years, the MOST concept has been developed and the system has demonstrated unique potential to store sunlight energy into chemical bonds. Also, the closed character of the MOST cycle makes this technology very promising due to the expected reduced waste production and the possibility of being installed in many different places, such as houses or portable devices. However, the current potential of the technology has been only proven at the laboratory scale and several factors prevent the advance towards real devices, both from chemical and engineering points of view.In the following years, the research community in the field should focus on the crucial aspects to scale up the process, which are related to the solar conversion efficiency, the energy density, the possibility of using highly concentrated (or even neat) MOST solutions and the essential transition towards sustainable systems, especially concerning the solvent that acts as the MOST carrier.
Regarding the back-conversion reaction, the recent advances in heterogeneous catalysis are facilitating real implementation in a reactor and will be key for the development of a suitable device. Also, there is still an urgent need for integrating the solar conversion and the back-reaction in a unified system, to manage the kinetics of both processes, which will require to be comparable. A MOST system should not be only reversible, but both forward and reverse reactions should also require similar reaction rates.
In conclusion, the extrapolation of laboratory MOST systems to real devices is not an easy task but the research community is pushing forward the limits of this technology and we expect to have promising advances in the near future.
Author contributions
D. S. and I. F.-A.: conceptualization; supervision; writing – review and editing; funding acquisition. A. G. G., L. M., S. H.-T., C. M.-R.: writing – original draft. N. S.: visualization; writing – review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
D. S. and I. F. A. acknowledge the MICIU/AEI/https://doi.org/10.13039/501100011033 and the European Union (NextGenerationEU/PRTR) for funding the project TED 2021-131896B-I00. Also, D. S. thanks the MICIU/AEI/https://doi.org/10.13039/501100011033 and FEDER, UE for financial support (project PID2021-126075NB-I00). I. F. A. thanks the MICIU/AEI/https://doi.org/10.13039/501100011033 and FSE+ for the Ramon y Cajal (RYC2022-035776-I) funding. N. S. thanks the University of La Rioja/https://doi.org/10.13039/501100018811 “Becas Santander/Contratos Predoctorales 2023” program for a predoctoral fellowship.Notes and references
- N. S. Lewis and D. G. Nocera, Powering the planet: chemical challenges in solar energy utilization, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15729–15735 CrossRef CAS PubMed.
- BP Statistical Review of World Energy 2022 (71st edition), Retrieved from: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2022-full-report.pdf (Accessed February 2024).
- United Nations, Adoption of the Paris Agreement, 2015, Retrieved from, https://unfccc.int/process-and-meetings/the-paris-agreement (accessed February 2024).
- The Balance of Power in the Earth-Sun System, NASA Facts, https://eospso.nasa.gov/publications/balance-power-earth-sun-system (accessed February 2024).
- M. S. E. Houache, C.-H. Yim, Z. Karkar and Y. Abu-Lebdeh, On the Current and Future Outlook of Battery Chemistries for Electric Vehicles—Mini Review, Batteries, 2020, 8, 70 CrossRef.
- A. Machín, M. Cotto, J. Ducongé and F. Márquez, Artificial Photosynthesis: Current Advancements and Future Prospects, Biomimetics, 2023, 8, 298 CrossRef PubMed.
- Y. H. Hong, Y.-M. Lee, W. Nam and S. Fukuzumi, Molecular Photocatalytic Water Splitting by Mimicking Photosystems I and II, J. Am. Chem. Soc., 2022, 144, 695–700 CrossRef CAS PubMed.
- L. Shen, X. Yang, J. An, L. Zhang, K. Yang and Z. Deng, Effect of Different Site Trifluoromethylbenzoic Acid Organic Photosensitizer for Dye-sensitized Solar Cells, ChemistrySelect, 2021, 6, 4645–4650 CrossRef CAS.
- L. Liao, Q. Zhang, Z. Su, Z. Zhao, Y. Wang, Y. Li, X. Lu, D. Wei, G. Feng, Q. Yu, X. Cai, J. Zhao, Z. Ren, H. Fang, F. Robles-Hernandez, S. Baldelli and J. Bao, Efficient solar water-splitting using a nanocrystalline CoO photocatalyst, Nat. Nanotechnol., 2014, 1, 69–73 CrossRef PubMed.
- E. O. Eren and S. Özkar, Recent advances in heterogeneous catalysts for the effective electroreduction of carbon dioxide to carbon monoxide, J. Power Sources, 2021, 506, 230215 CrossRef CAS.
- Z. Wang, H. Hölzel and K. Moth-Poulsen, Status and challenges for molecular solar thermal energy storage system based devices, Chem. Soc. Rev., 2022, 51, 7313–7326 RSC.
- A. Gimenez-Gomez, B. Rollins, A. Steele, H. Hölzel, N. Baggi, K. Moth-Poulsen, I. Funes-Ardoiz and D. Sampedro, Unveiling the Potential of Heterogeneous Catalysts for Molecular Solar Thermal Systems, Chem. – Eur. J., 2024, 1, e202303230 CrossRef PubMed.
- A. Gimenez-Gomez, L. Magson, B. Peñin, N. Sanosa, J. Soilán, R. Losantos and D. Sampedro, A Photochemical Overview of Molecular Solar Thermal Energy Storage, Photochem, 2022, 2, 694–716 CrossRef CAS.
- C.-L. Sun, C. Wang and R. Boulatov, Applications of Photoswitches in the Storage of Solar Energy, ChemPhotoChem, 2019, 3, 268–283 CrossRef CAS.
- A. Lennartson, A. Roffey and K. Moth-Poulsen, Designing photoswitches for molecular solar thermal energy storage, Tetrahedron Lett., 2015, 56, 1457–1465 CrossRef CAS.
- J. Orrego-Hernández, A. Dreos and K. Moth-Poulsen, Engineering of Norbornadiene/Quadricyclane Photoswitches for Molecular Solar Thermal Energy Storage Applications, Acc. Chem. Res., 2020, 53, 1478–1487 CrossRef PubMed.
- W. G. Dauben and R. L. Cargill, Photochemical transformations—VIII: The isomerization of Δ2,5-bicyclo[2.2.1]heptadiene to quadricyclo[2.2.1.02,6·03,5]heptane (quadricyclene), Tetrahedron, 1961, 15, 197–201 CrossRef CAS.
- Z.-I. Yoshida, New molecular energy storage systems, J. Photochem., 1985, 29, 27–40 CrossRef CAS.
- V. A. Bren, A. D. Dubonosov, V. I. Minkin and V. A. Chernoivanov, Norbornadiene–quadricyclane-an effective molecular system for the storage of solar energy, Russ. Chem. Rev., 1991, 60, 451–469 CrossRef.
- A. D. Dubonosov, V. A. Bren and V. A. Chernoivanov, Norbornadiene-quadricyclane as an abiotic system for the storage of solar energy, Russ. Chem. Rev., 2002, 71, 917–927 CrossRef CAS.
- A. M. Kolpak and J. C. Grossman, Azobenzene-functionalized carbon nanotubes as high-energy density solar thermal fuels, Nano Lett., 2011, 11, 3156–3162 CrossRef CAS PubMed.
- R. Losantos and D. Sampedro, Design and Tuning of Photoswitches for Solar Energy Storage, Molecules, 2021, 26, 3796 CrossRef CAS PubMed.
- M. Mansø, A. U. Petersen, Z. Wang, P. Erhart, M. B. Nielsen and K. Moth-Poulsen, Molecular solar thermal energy storage in photoswitch oligomers increases energy densities and storage times, Nat. Commun., 2018, 9, 1945 CrossRef PubMed.
- Z. Wang, P. Erhart, T. Li, Z.-Y. Zhang, D. Sampedro, Z. Hu, H. A. Wegner, O. Brummel, J. Libuda, M. B. Nielsen and K. Moth-Poulsen, Storing energy with molecular photoisomers, Joule, 2021, 5, 3116–3136 CrossRef CAS.
- J. Fang, Q. Liu, S. Guo, J. Lei and H. Jin, Spanning solar spectrum: A combined photochemical and thermochemical process for solar energy storage, Appl. Energy, 2019, 247, 116–126 CrossRef CAS.
- F. Hemauer, H.-P. Steinrück and C. Papp, The Norbornadiene/Quadricyclane Pair as Molecular Solar Thermal Energy Storage System: Surface Science Investigations, ChemPhysChem, 2024, e202300806 CrossRef CAS PubMed.
- C. Schuschke, C. Hohner, M. Jevric, A. U. Petersen, Z. Wang, M. Schwarz, M. Kettner, F. Waidhas, L. Fromm, C. J. Sumby, A. Görling, O. Brummel, K. Moth-Poulsen and J. Libuda, Solar energy storage at an atomically defined organic-oxide hybrid interface, Nat. Commun., 2019, 10, 2384 CrossRef PubMed.
- J. L. Elholm, A. E. Hillers-Bendtsen, H. Hölzel, K. Moth-Poulsen and K. V. Mikkelsen, High throughput screening of norbornadiene/quadricyclane derivates for molecular solar thermal energy storage, Phys. Chem. Chem. Phys., 2022, 24, 28956–28964 RSC.
- M. Mansø, L. Fernandez, Z. Wang, K. Moth-Poulsen and M. B. Nielsen, Donor-Acceptor Substituted Benzo-, Naphtho- and Phenanthro-Fused Norbornadienes, Molecules, 2020, 2, 322 CrossRef PubMed.
- S. Ghasemi, L. Ornago, Z. Liasi, M. B. Johansen, T. J. Von Buchwald, A. E. Hillers-Bendtsen, S. Van Der Poel, H. Hölzel, Z. Wang, F. M. A. Noa, L. Öhrström, K. V. Mikkelsen, H. S. J. Van Der Zant, S. Lara-Avila and K. Moth-Poulsen, Exploring the impact of select anchor groups for norbornadiene/quadricyclane single-molecule switches, J. Mater. Chem. C, 2023, 11, 15412–15418 RSC.
- A. Dreos, J. Ge, F. Nájera, B. E. Tebikachew, E. Pérez-Inestrosa, K. Moth-Poulsen, K. Blennow, H. Zetterberg and J. Hanrieder, Investigating New Applications of a Photoswitchable Fluorescent Norbornadiene as a Multifunctional Probe for Delineation of Amyloid Plaque Polymorphism, ACS Sens., 2023, 8, 1500–1509 CrossRef CAS PubMed.
- K. Jorner, A. Dreos, R. Emanuelsson, O. E. Bakouri, I. F. Galván, K. Börjesson, F. Feixas, R. Lindh, B. Zietz, K. Moth-Poulsen and H. Ottosson, Unraveling factors leading to efficient norbornadiene–quadricyclane molecular solar-thermal energy storage systems, J. Mater. Chem. A, 2017, 5, 12369–12378 RSC.
- J. Orrego-Hernández, H. Hölzel, M. Quant, Z. Wang and K. Moth-Poulsen, Scalable Synthesis of Norbornadienes via in situ Cracking of Dicyclopentadiene Using Continuous Flow Chemistry, Eur. J. Org. Chem., 2021, 2021, 5337 CrossRef.
- N. Baggi, H. Hözel, H. Schomacker, K. Moreno and K. Moth-Poulsen, Flow-Integrated Preparation of Norbornadiene Precursors for Solar Thermal Energy Storage, ChemSusChem, 2024, 17, 2 Search PubMed.
- I. Gur, K. Sawyer and R. Prasher, Engineering, Searching for a better thermal battery, Science, 2012, 335, 1454–1455 CrossRef PubMed.
- K. Börjesson, A. Lennartson and K. Moth-Poulsen, Efficiency Limit of Molecular Solar Thermal Energy Collecting Devices, ACS Sustainable Chem. Eng., 2015, 1, 585–590 CrossRef.
- R. J. Salthouse and K. Moth-Poulsen, Multichromophoric photoswitches for solar energy storage: from azobenzene to norbornadiene, and MOST things in between, J. Mater. Chem., 2024, 12, 3180–3208 RSC.
- M. J. Kuisma, A. M. Lundin, K. Moth-Poulsen, P. Hyldgaard and P. Erhart, Comparative Ab-Initio Study of Substituted Norbornadiene-Quadricyclane Compounds for Solar Thermal Storage, J. Phys. Chem. C, 2016, 120, 3635–3645 CrossRef CAS PubMed.
- O. Brummel, F. Waidhas, U. Bauer, Y. Wu, S. Bochmann, H. Steinrück, C. Papp, J. Bachmann and J. Libuda, Photochemical Energy Storage and Electrochemically Triggered Energy Release in the Norbornadiene–Quadricyclane System: UV Photochemistry and IR Spectroelectrochemistry in a Combined Experiment, J. Phys. Chem. Lett., 2017, 8, 2819–2825 CrossRef CAS PubMed.
- Z. Wang, R. Losantos, D. Sampedro, M. Morikawa, K. Börjesson, N. Kimizuka and K. Moth-Poulsen, Demonstration of an azobenzene derivative based solar thermal energy storage system, J. Mater. Chem. A, 2019, 7, 15042–15047 RSC.
- K. Börjesson, A. Lennartson and K. Moth-Poulsen, Efficiency Limit of Molecular Solar Thermal Energy Collecting Devices, ACS Sustainable Chem. Eng., 2013, 1, 585–590 CrossRef.
- M. Kuisma, A. Lundin, K. Moth-Poulsen, P. Hyldgaard and P. Erhart, Comparative Ab-Initio Study of Substituted Norbornadiene-Quadricyclane Compounds for Solar Thermal Storage, J. Phys. Chem. C, 2016, 120, 3635–3645 CrossRef CAS PubMed.
- A. E. Hillers-Bendtsen, J. L. Elholm, O. B. Obel, H. Hölzel, K. Moth-Poulsen and K. V. Mikkelsen, Searching the Chemical Space of Bicyclic Dienes for Molecular Solar Thermal Energy Storage Candidates, Angew. Chem., 2023, 62, e202309543 CrossRef CAS PubMed.
- A. Kjaersgaard, H. Hölzel, K. Moth-Poulsen and M. B. Nielsen, Photolytic Studies of Norbornadiene Derivatives under High-Intensity Light Conditions, J. Phys. Chem. A, 2022, 126, 6849–6857 CrossRef CAS PubMed.
- I. L. H. Kjeldsen, J. F. Høvring, T. J. von Buchwald, A. E. Hillers-Bendtsen and K. V. Mikkelsen, The effects of solvation on the back reaction and storage capabilities of solar thermal energy storage systems, Phys. Chem. Chem. Phys., 2022, 24, 5564–5577 RSC.
- A. Dreos, K. Börjesson, Z. Wang, A. Roffey, Z. Norwood, D. Kushnir and K. Moth-Poulsen, Exploring the potential of a hybrid device combining solar water heating and molecular solar thermal energy storage, Energy Environ. Sci., 2017, 10, 728–734 RSC.
- N. Ree, M. Koerstz, K. V. Mikkelsen and J. H. Jensen, Virtual screening of norbornadiene-based molecular solar thermal energy storage systems using a genetic algorithm, J. Chem. Phys., 2021, 155, 184105 CrossRef CAS PubMed.
- O. Christensen, R. D. Schlosser, R. B. Nielsen, J. R. Johansen, M. Koerstz, J. H. Jensen and K. V. Mikkelsen, A Neural Network Approach for Property Determination of Molecular Solar Cell Candidates, J. Phys. Chem. A, 2022, 155, 184105 Search PubMed.
- Z. Wang, A. Roffey, R. Losantos, A. Lennartson, M. Jevric, A. U. Petersen, M. Quant, A. Dreos, X. Wen, D. Sampedro, K. Börjesson and K. Moth-Poulsen, Macroscopic heat release in a molecular solar thermal energy storage system, Energy Environ. Sci., 2019, 12, 187–193 RSC.
- L. Magson, H. Hölzel, A. S. Aslam, S. Henninger, G. Munz, K. Moth-Poulsen, M. Knaebbeler-Buss, I. Funes-Ardoiz and D. Sampedro, Synthesis and Characterization of Carbon-Based Heterogeneous Catalysts for Energy Release of Molecular Solar Thermal Energy Storage Materials, ACS Appl. Mater. Interfaces, 2024, 16, 7211–7218 CrossRef CAS PubMed.
- K. Moth-Poulsen, D. Ćoso, K. Börjesson, N. Vinokurov, S. K. Meier, A. Majumdar, K. P. C. Vollhardt and R. A. Segalman, Molecular solar thermal (MOST) energy storage and release system, Energy Environ. Sci., 2012, 5, 8534–8537 RSC.
- Z. Wang, Z. Wu, Z. Hu, J. Orrego-Hernández, E. Mu, Z.-Y. Zhang, M. Jevric, Y. Liu, X. Fu, F. Wang, T. Li and K. Moth-Poulsen, Chip-scale solar thermal electrical power generation, Cell Rep. Phys. Sci., 2022, 3, 100789 CrossRef CAS.
- V. Kashyap, S. Sakunkaewkasem, P. Jafari, M. Nazari, B. Eslami, S. Nazifi, P. Irajizad, M. D. Marquez, T. R. Lee and H. Ghasemi, Full Spectrum Solar Thermal Energy Harvesting and Storage by a Molecular and Phase-Change Hybrid Material, Joule, 2019, 3, 3100–3111 CrossRef CAS.
- Eurostat, Energy consumption in households, Eurostat, 2020, https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Energy_consumption_in_households (accessed March 2024).
- M. Schlemminger, T. Ohrdes, E. Schneider and M. Knoop, Dataset on electrical single-family house and heat pump load profiles in Germany, Sci. Data, 2022, 9, 56 CrossRef PubMed.
- A. Dreos, Z. Wang, J. Udmark, A. Strom, P. Erhart, K. Borjesson, M. B. Nielsen and K. Moth-Poulsen, Liquid Norbornadiene Photoswitches for Solar Energy Storage, Adv. Energy Mater., 2018, 8, 1703401 CrossRef.
- A. U. Petersen, A. I. Hofmann, M. Fillols, M. Mansø, M. Jevric, Z. Wang, C. J. Sumby, C. Müller and K. Moth-Poulsen, Solar Energy Storage by Molecular Norbornadiene–Quadricyclane Photoswitches: Polymer Film Devices, Adv. Sci., 2019, 6, 2198–3844 Search PubMed.
- Z. Wang, H. Moïse, M. Cacciarini, M. B. Nielsen, M.-A. Morikawa, N. Kimizuka and K. Moth-Poulsen, Liquid-Based Multijunction Molecular Solar Thermal Energy Collection Device, Adv. Sci, 2021, 8, 2103060 CrossRef CAS PubMed.
- A. Gonzalez, E. S. Kengmana, M. V. Fonseca and G. G. D. Han, Solid-state photoswitching molecules: structural design for isomerization in condensed phase, Mater. Today Adv., 2020, 6, 100058 CrossRef.
- M. A. Gerkman, R. S. L. Gibson, J. Calbo, Y. Shi, M. J. Fuchter and G. G. D. Han, Arylazopyrazoles for Long-Term Thermal Energy Storage and Optically Triggered Heat Release below 0 °C, J. Am. Chem. Soc., 2020, 142, 8688–8695 CrossRef PubMed.
- Y. Shi, M. A. Gerkman, Q. Qiu, S. Zhang and G. G. D. Han, Sunlight-activated phase change materials for controlled heat storage and triggered release, J. Mater. Chem. A, 2021, 9, 9798–9808 RSC.
- Z.-Y. Zhang, Y. He, Z. Wang, J. Xu, M. Xie, P. Tao, D. Ji, K. Moth-Poulsen and T. Li, Photochemical Phase Transitions Enable Coharvesting of Photon Energy and Ambient Heat for Energetic Molecular Solar Thermal Batteries That Upgrade Thermal Energy, J. Am. Chem. Soc., 2020, 142, 12256–12264 CrossRef CAS PubMed.
- W.-C. Xu, S. Sun and S. Wu, Photoinduced Reversible Solid-to-Liquid Transitions for Photoswitchable Materials, Angew. Chem., Int. Ed., 2019, 58, 9712–9740 CrossRef CAS PubMed.
- M. Chen, Q. Li, L. Cheng, X. Wang, C. Lyu and Q. Fan, A Study to Investigate the Viscosity Effect on Micro-Confined Fluids Flow in Tight Formations Considering Fluid–Solid Interaction, Front. Earth Sci., 2021, 9, 795842 CrossRef.
- M. Quant, A. Hamrin, A. Lennartson, P. Erhart and K. Moth-Poulsen, Solvent Effects on the Absorption Profile, Kinetic Stability, and Photoisomerization Process of the Norbornadiene-Quadricyclanes System, J. Phys. Chem. C, 2019, 123, 7081–7087 CrossRef CAS.
- P. Lorenz, F. Wullschläger, A. Rüter, B. Meyer and A. Hirsch, Tunable Photoswitching in Norbornadiene (NBD)/Quadricyclane (QC) – Fullerene Hybrids, Chem. – Eur. J., 2021, 2714501 Search PubMed.
- F. Castro, J. S. Gancheff, J. C. Ramos, G. Seoane, C. Bazzicalupi, A. Bianchi, F. Ridi and M. Savastano, A Norbornadiene-Based Molecular System for the Storage of Solar–Thermal Energy in an Aqueous Solution: Study of the Heat-Release Process Triggered by a Co(II)-Complex, Molecules, 2023, 28, 7270 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |