A reflection on ‘Self-assembly of aligned rutile@anatase TiO2 nanorod@CdS quantum dots ternary core–shell heterostructure: cascade electron transfer by interfacial design’
Xian
Yan
a,
Jiao-Nan
Yuan
a,
Fang-Xing
Xiao
*a and
Bin
Liu
*b
aCollege of Materials Science and Engineering, Fuzhou University, New Campus, Fuzhou 350108, China. E-mail: fxxiao@fzu.edu.cn
bDepartment of Materials Science and Engineering, City University of Hong Kong, Hong Kong SAR 999077, China. E-mail: bliu48@cityu.edu.hk
First published on 30th September 2024
Abstract
Interface design plays a pivotal role in optimizing the carrier separation and migration kinetics in artificial photosystems by meticulously aligning energy levels to facilitate the smooth flow of photogenerated charge carriers. In 2014, Liu et al., reported a facile and efficient layer-by-layer (LbL) self-assembly strategy for the preparation of a rutile@anatase TiO2 NRs@CdS QDs ternary core–shell heterostructure, in which an in situ formed monodispersed anatase TiO2 layer is intimately sandwiched in-between rutile TiO2 nanorods (NRs) and CdS quantum dots (QDs), achieving efficient charge separation (F.-X. Xiao, J. Miao and B. Liu, Mater. Horiz., 2014, 1, 259–263, https://doi.org/10.1039/C3MH00097D). Here, we briefly review the subsequent research on LbL assembly mediated interface design, triggered by this work, aiming to explore in depth the key role of the interface engineering afforded by this booming strategy in finely regulating the charge transport and separation for solar energy conversion.
Overview
Among the diverse strategies for solar energy conversion, photoelectrochemical (PEC) water splitting and photocatalysis1–3 have been arousing interest since the groundbreaking work performed by Honda and Fujishima.4 The artificial photosystems employ semiconductor materials as the light absorbers to facilitate the charge generation, separation, and transport, as well as to drive surface catalytic reactions.5,6 However, the critical issue in photocatalysis and photoelectrocatalysis is predominantly centered on the ultra-fast recombination of photoinduced charge carriers and low solar energy conversion efficiency especially in the visible or near-infrared spectrum.7,8 In 2014, Xiao et al., (https://doi.org/10.1039/C3MH00097D) reported a facile and efficient self-assembly strategy for the preparation of a rutile@anatase TiO2 NRs@CdS QDs ternary core–shell heterostructure, in which an in situ formed monodispersed anatase TiO2 layer was intimately sandwiched in-between rutile TiO2 nanorods (NRs) and CdS quantum dots (QDs).9 This well-defined nanoarchitecture ingeniously optimized the energy level alignment among the building blocks at the interface, facilitating the smooth flow of photocarriers, ultimately achieving efficient charge separation. This unique interface configuration design substantially enhanced the PEC water oxidation and photocatalytic selective organic transformation performances. This endeavor indicates that precise control over the structure, composition, and interface plays a pivotal role in customizing multifarious high-performance functional photoelectrodes or photocatalysts, which flexibly and harmoniously contribute to the optimization of diverse artificial photosystems.Inspired by this work, in the following years, Xiao et al., continued to develop and harness the layer-by-layer (LbL) assembly technique to craft a large variety of novel spatially hierarchical heterostructured photoelectrodes/photocatalysts for solar energy conversion.10,11 By fine-tuning the type and deposition sequence of tailor-made building blocks, versatile structure and composition are harmoniously integrated within one multilayer photosystem.11 For example, in 2018, Xiao and co-workers utilized TiO2 nanorod arrays (TiO2 NRs) as the framework, onto which positively charged Ag+ ion-coordinated poly ethyleneimine (PEI-Ag+) complexes were integrated with negatively charged graphene quantum dots (GQDs) using an LbL assembly approach at ambient conditions.12 In this way, PEI-Ag+ and GQDs were alternatively and intimately assembled in a face-to-face stacking mode on the surface of TiO2 NRs. Under light irradiation, the coordinated Ag+ ions anchored on the molecular chain of PEI were in situ reduced to the plasmonic Ag nanoparticles (NPs). The in situ formed plasmonic Ag NPs facilitated the generation of hot electrons, and simultaneously the GQDs acted as an efficient charge transfer medium, initiating a cascade hot electron transfer pathway from plasmonic Ag NPs to TiO2. This significantly prolonged the lifetime of plasmonic hot charge carriers and considerably enhanced the solar water oxidation performance of the TiO2 NRs@Ag@GQDs ternary heterostructure. Benefiting from the merits of the LbL assembly strategy in precisely customizing interface configuration and mediating the interfacial charge transfer pathway, we push forward the rapid, facile, and exquisite construction of robust and novel artificial photosystems by rationally selecting the applicable building blocks. Moreover, it should be emphasized that the relatively tedious repeated procedures of the LbL assembly strategies reported in various works have been surmounted by our self-designed instrument, which is able to monitor the LbL assembly process automatically and efficiently.
Discussion
Non-conjugated polyelectrolytes (PEs) have been widely utilized as surface charge modifiers.13,14 In the LbL assembly buildup, non-conjugated PEs can act as self-assembly initiators, inducing coupling of building blocks with opposite surface charges via molecular interactions to form well-defined nanoarchitectures.15,16 Noteworthily, in traditional cognition, non-conjugated PEs are often considered unfavorable for interfacial charge transfer due to lack of π-conjugated repeating units in the backbone of the molecular chain, and thus they can only act as “molecular glue” between the coupling interfaces of building blocks.17 Consequently, in previous works, calcination has been frequently applied to forcibly remove these polymers to obtain a clean interface to enhance the interfacial charge transfer efficiency. In 2020, Xiao et al., unveiled for the first time the unexpected charge transport capability of the solid-state non-conjugated polymer, poly(diallyl-dimethylammonium chloride) (PDDA).18 In this scenario, oppositely charged PDDA and metal nanoclusters (NCs) as building blocks, were alternately deposited on the WO3 substrate via the LbL assembly strategy, giving rise to the spatially multilayered (metal NCs/PDDA)n/WO3 photoanodes (Fig. 1). The unique face-to-face integration ensured the maximum interfacial contact between metal NCs and PDDA. It was unveiled that the ultra-thin PDDA interim layer played a pivotal role in electron relay, which facilitated electron migration from metal NCs to WO3.19 Considering the multilayered structure, the periodically stacked (metal NCs/PDDA) bi-layer repeating units functioned as integrated light-harvesting antennas and charge transfer mediators, synergistically contributing to the tandem charge flow from metal NCs to WO3 substrate and endowing the WO3/(PDDA-metal NCs)n heterostructures with significantly enhanced PEC water oxidation performances under simulated and visible light irradiation. Moreover, the researchers have also demonstrated that this long-range cascade electron transport channel, mediated by PDDA, is universal for various metal oxides (e.g., TiO2 and Fe2O3) and metal NCs (e.g., Au25 and Agx NCs).20 Consequently, this work not only reveals the unexpected electron-withdrawing capability of PDDA in accelerating interfacial charge transfer, but also provides new insights for improving the solar water oxidation performances of photoelectrodes through judicious interface engineering, laying a solid foundation for unleashing the unanticipated function of non-conjugated insulating polymers and diversifying the construction of insulating polymer based artificial photosystems. Xiao et al., also ascertained that LbL assembly could provide a powerful platform to stabilize atomically precise metal NCs by encapsulating them in-between non-conjugated polymer layers, resulting in the robust and stable solar water oxidation performance.21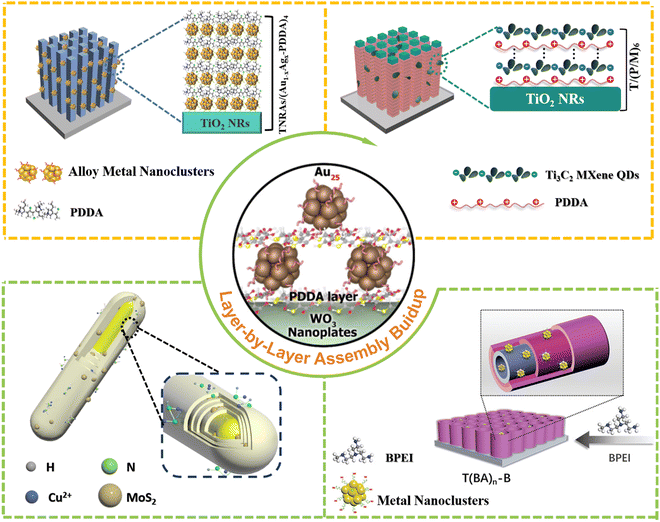 | ||
| Fig. 1 Some typical works of interface engineering using the LbL assembly tactic.6,18,21–23 | ||
In subsequent work, Xiao et al., took full advantage of the electron-withdrawing PDDA in conjunction with the LbL assembly tactic to design novel photoelectrodes. For instance, oppositely charged PDDA and Ti3C2 MXene quantum dots (MQDs) were utilized as the buildings blocks that were periodically deposited on the TiO2 NRs for fabricating multilayered TiO2/(PDDA/MQDs)n heterostructured photoanodes (Fig. 1).22 Given that Ti3C2 MQDs demonstrate metal-like properties, and the PDDA interim layer has electron-withdrawing ability, directional electron transfer from TiO2 substrate to each Ti3C2 MQD layer relayed by the ultra-thin PDDA interim layer is significantly stimulated. Noteworthily, the strategic intercalation of PDDA at the interface plays a critical role in enhancing the charge transfer efficiency, which not only facilitated the favorable energy level alignment between TiO2 and Ti3C2 MQDs, but also reduced the interfacial barriers. This precise control over the interface architecture is crucial for optimizing interfacial charge separation. The unique interface configuration endowed by the LbL assembly and the pivotal role of the non-conjugated polymer as an interfacial charge transport mediator, synergistically contribute to the spatial charge transport route for significantly boosted PEC water oxidation.19 This work not only consolidates the core role of PDDA in promoting interfacial charge transfer and separation, but also provides a new perspective to improve solar energy conversion through exquisite interface control. Apart from MXene, transition metal chalcogenide quantum dots (TMC QDs) can also function as building blocks and cooperate synergistically with non-conjugated polymers to construct TMC QDs-based composite photosystems, which enables high-efficiency electron tunnelling and tandem charge transport.24 On the other hand, Xiao et al. also made enormous efforts to study the synergy of insulating polymer and LbL assembly in heterogeneous photocatalysis. For instance, a simple thermal reduction method was developed to achieve in situ anchoring of electron-trapping Pt NPs on WO3via LbL assembly.16 This significantly accelerated the separation of photogenerated charge carriers, achieving highly efficient photocatalytic degradation of organic pollutants under visible light irradiation. Alternatively, Xiao and co-workers have discovered that the non-conjugated polymer of polyethyleneimine (BPEI) can serve as an efficient hole transport medium to boost the interfacial charge separation in photocatalytic reactions (Fig. 1). Tailor-made MoS2 QDs and BPEI assembly units were selected as the electron acceptor and hole mediator, respectively, which were alternately assembled on the CdS framework to construct the CdS(MoS2/BPEI)n multilayered heterostructure (Fig. 1).25 MoS2 could effectively capture electrons, while BPEI acted as a hole transport layer, creating spatial dual charge transfer channels that accelerated the unidirectional flow of electrons and holes. This endeavor introduces a revolutionary solution to the precise regulation of charge separation and transfer.
Over the past decade, a wave of related research efforts has been inspired by the proposed interface design strategy. For example, Ryu and coworkers developed a multilayer hematite composite photoelectrode that integrates GO and Co-POM by a LbL assembly approach to improve the PEC conversion efficiency.26 This multilayer photoelectrode was precisely optimized by adjusting the number of assembly building blocks. Also, Ryu and coworkers employed the LbL assembly strategy to deposit cationic polyelectrolyte (PDDA) and anionic polyelectrolyte (PSS) on the surface of Fe2O3 and TiO2 photoanodes, aiming to construct an interfacial dipole layer to regulate the charge separation efficiency of the photoanodes for solar water oxidation.27 Interestingly, charge separation efficiency of multilayer photoanodes can be finely modulated by tuning their magnitude and direction, which in turn can be achieved by controlling the number of (PDDA/PSS) bilayers and type of terminal polyelectrolytes, respectively.27 In the following work, the same group utilized multilayer LbL-assembled thin-film electrodes to reveal the dependence of electrochemistry on the thickness and architecture of multilayer electrodes, the competition between mass and charge transfer, and the control over ion permeation selectivity and interfacial dipole moments in multilayer electrodes.28 These efforts have deepened our understanding on the critical role of interface design in optimizing the performance of multilayer electrodes. They provide new directions for the fabrication of promising materials by enabling more efficient ion transport and charge storage through the precise control of electrode thickness, structure, and interface. Based on these advancements, other research groups have also made significant strides in interface design. For instance, in the field of organic photovoltaics (OPVs), Chen and coworkers added an asymmetric electron acceptor, BTP-S2, to the binary donor–acceptor system of PM6:BO-4Cl during the LbL assembly process, which led to the formation of a vertical phase distribution in the OPV devices, with donor enrichment at the anode and acceptor enrichment at the cathode.29 The formation of vertical phase distribution not only reduces charge complexation, but also promotes charge collection, which improves the photocurrent and fill factor of LbL-type ternary OPVs.29 It is noteworthy that the OPVs designed in this way exhibit one of the highest values reported so far for OPVs. Alternatively, Min and coworkers constructed organic solar cells (OSCs) with a controllable “p–i–n” morphology through interface design, which possess unique advantages such as good charge transport and extraction performances, as well as versatility.30,31 These works inspired by Xiao et al. 's work, have significantly advanced the field of interface design, leading to innovative strategies for enhancing the performance of various PEC or photovoltaic systems. The exploration of spatially multilayered photosystems and optimization of charge separation efficiencies over photocatalysts, photoelectrodes and photovoltaic devices have provided valuable insight into the interplay between photosystem architecture and performance. Collectively, these efforts underscore the critical role of interface design in optimizing material performance, paving the way for future innovations in energy conversion and storage technologies.
Outlook
The endeavors of Xiao et al., in the fields of photoelectrocatalysis and heterogeneous photocatalysis significantly reinforce the fundamental understanding of precise interface engineering for creating robust, stable, and diverse artificial photosystems via LbL assembly. They challenge the traditional cognition, reveal the unexpected charge transfer ability of non-conjugated insulating polymers such as PDDA and BPEI, and judiciously harness these insulating polymers as interfacial charge transport mediators for LbL assembly of multifarious photocatalysts and photoelectrodes. These works provide important theoretical and practical insights for optimizing charge transfer pathways using a smart interface configuration design towards solar energy conversion. Meanwhile, apart from photocatalysis, other research groups inspired by the work of Xiao et al., have also made significant progress in their respective fields, especially in the field of organic photovoltaics. These works will not only drive the commercialization of solar energy technology, but also push forward the prosperity of renewable energy. It can be foreseen that future research will focus on precise interface design to foster the construction of high-efficiency and stable artificial photosystems, solving the global energy shortage problem and promoting the sustainable development of solar energy conversion.Author contributions
Xian Yan, Jiao-Nan Yuan, Fang-Xing Xiao and Bin Liu wrote the manuscript.Conflicts of interest
The authors declare no competing interests.Acknowledgements
This work was financially supported by the Minjiang Scholar Professorship, the National Natural Science Foundation of China (No. 21703038, 22072025), the State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Science (No. 20240018), City University of Kong Hong startup fund (9020003) and ITF–RTH – Global STEM Professorship (9446006).References
- H. Huang, Z. Li, Z. Li, B. Khan, K.-W. Huang, Z. Lai and J.-H. He, Nano Energy, 2023, 115, 108683 CrossRef CAS.
- Y. Song, Y. Ren, H. Cheng, Y. Jiao, S. Shi, L. Gao, H. Xie, J. Gao, L. Sun and J. Hou, Angew. Chem., Int. Ed., 2023, 62, e202306420 CrossRef CAS PubMed.
- K. Song, H. Hou, D. Zhang, F. He and W. Yang, Appl. Catal., B, 2023, 330, 122630 CrossRef CAS.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- X.-C. Dai, M.-H. Huang, Y.-B. Li, T. Li, S. Hou, Z.-Q. Wei and F.-X. Xiao, J. Phys. Chem. C, 2020, 124, 4989–4998 CrossRef CAS.
- X. Zhang, X. Wang, D. Wang and J. Ye, ACS Appl. Mater. Interfaces, 2018, 11, 5623–5631 CrossRef PubMed.
- K. Lee, A. Mazare and P. Schmuki, Chem. Rev., 2014, 114, 9385–9454 CrossRef CAS PubMed.
- Z.-Q. Wei, X.-C. Dai, S. Hou, Y.-B. Li, M.-H. Huang, T. Li, S. Xu and F.-X. Xiao, J. Mater. Chem. A, 2020, 8, 177–189 RSC.
- F.-X. Xiao, J. Miao and B. Liu, Mater. Horiz., 2014, 1, 259–263 RSC.
- B.-J. Liu, Q. Chen, Q.-L. Mo and F.-X. Xiao, Coord. Chem. Rev., 2023, 493, 215285 CrossRef CAS.
- F.-X. Xiao, M. Pagliaro, Y.-J. Xu and B. Liu, Chem. Soc. Rev., 2016, 45, 3088–3121 RSC.
- Z. Zeng, T. Li, Y.-B. Li, X.-C. Dai, M.-H. Huang, Y. He, G. Xiao and F.-X. Xiao, J. Mater. Chem. A, 2018, 6, 24686–24692 RSC.
- C. Dai and B. Liu, Energy Environ. Sci., 2020, 13, 24–52 RSC.
- S. Hou, X.-C. Dai, Y.-B. Li, M.-H. Huang, T. Li, Z.-Q. Wei, Y. He, G. Xiao and F.-X. Xiao, J. Mater. Chem. A, 2019, 7, 22487–22499 RSC.
- S. Xu, M.-H. Huang, T. Li, Z.-Q. Wei, X. Lin, X.-C. Dai, S. Hou, X.-Y. Fu and F.-X. Xiao, J. Mater. Chem. A, 2020, 8, 8360–8375 RSC.
- H.-J. Lin, S. Xu, X.-Y. Fu, Z.-Q. Wei, M.-H. Huang, X. Lin, Y. He, G. Xiao and F.-X. Xiao, Inorg. Chem., 2020, 59, 4129–4139 CrossRef CAS PubMed.
- H.-J. Lin, T. Li, M.-H. Huang, X.-C. Dai, Y.-B. Li and F.-X. Xiao, J. Phys. Chem. C, 2019, 123, 28066–28080 CrossRef CAS.
- Z.-Q. Wei, S. Hou, X. Lin, S. Xu, X.-C. Dai, Y.-H. Li, J.-Y. Li, F.-X. Xiao and Y.-J. Xu, J. Am. Chem. Soc., 2020, 142, 21899–21912 CrossRef CAS PubMed.
- Y. Xiao, Q.-L. Mo, G. Wu, K. Wang, X.-Z. Ge, S.-R. Xu, J.-L. Li, Y. Wu and F.-X. Xiao, J. Mater. Chem. A, 2023, 11, 2402–2411 RSC.
- B. Tang, S.-C. Zhu, H. Liang, S. Li, B.-J. Liu and F.-X. Xiao, J. Mater. Chem. A, 2022, 10, 4032–4042 RSC.
- Q. L. Mo, X. C. Dai and F. X. Xiao, Small, 2023, 19, 2302372 CrossRef CAS.
- S. Li, Q.-L. Mo, S.-C. Zhu, Z.-Q. Wei, B. Tang, B.-J. Liu, H. Liang, Y. Xiao, G. Wu, X.-Z. Ge and F.-X. Xiao, Adv. Funct. Mater., 2022, 32, 2110848 CrossRef CAS.
- P. Su, B. Tang and F. X. Xiao, Small, 2024, 20, 2307619 CrossRef CAS.
- S. Hou, Q. L. Mo, S. C. Zhu, S. Li, G. Xiao and F. X. Xiao, Inorg. Chem., 2022, 61, 1188–1194 CrossRef CAS.
- Y.-B. Li, T. Li, X.-C. Dai, M.-H. Huang, S. Hou, X.-Y. Fu, Z.-Q. Wei, Y. He, G. Xiao and F.-X. Xiao, ACS Appl. Mater. Interfaces, 2020, 12, 4373–4384 CrossRef CAS PubMed.
- Y. Choi, D. Jeon, Y. Choi, D. Kim, N. Kim, M. Gu, S. Bae, T. Lee, H.-W. Lee, B.-S. Kim and J. Ryu, ACS Nano, 2018, 13, 467–475 CrossRef PubMed.
- S. Bae, D. Kim, H. Kim, M. Gu, J. Ryu and B. S. Kim, Adv. Funct. Mater., 2019, 30, 1908492 CrossRef.
- M. Gu and B.-S. Kim, Acc. Chem. Res., 2020, 54, 57–69 CrossRef PubMed.
- L. Zhan, S. Li, X. Xia, Y. Li, X. Lu, L. Zuo, M. Shi and H. Chen, Adv. Mater., 2021, 33, 2007231 CrossRef CAS PubMed.
- R. Sun, Q. Wu, J. Guo, T. Wang, Y. Wu, B. Qiu, Z. Luo, W. Yang, Z. Hu, J. Guo, M. Shi, C. Yang, F. Huang, Y. Li and J. Min, Joule, 2020, 4, 407–419 CrossRef CAS.
- R. Sun, J. Cuo, C. K. Sun, T. Wang, Z. H. Luo, Z. H. Zhang, X. C. Jiao, W. H. Tang, C. L. Yang, Y. F. Li and J. Min, Energy Environ. Sci., 2019, 12, 384–395 RSC.
| This journal is © The Royal Society of Chemistry 2024 |
