Open Access Article
Open Access ArticleInnovative approaches in skin therapy: bionanocomposites for skin tissue repair and regeneration
Ayça
Bal-Öztürk
 abc,
Emine
Alarçin
d,
Gökçen
Yaşayan
e,
Meltem
Avci-Adali
f,
Arezoo
Khosravi
g,
Atefeh
Zarepour
h,
Siavash
Iravani
abc,
Emine
Alarçin
d,
Gökçen
Yaşayan
e,
Meltem
Avci-Adali
f,
Arezoo
Khosravi
g,
Atefeh
Zarepour
h,
Siavash
Iravani
 *i and
Ali
Zarrabi
*i and
Ali
Zarrabi
 *jk
*jk
aDepartment of Analytical Chemistry, Faculty of Pharmacy, Istinye University, 34010 Istanbul, Türkiye
bInstitute of Health Sciences, Department of Stem Cell and Tissue Engineering, Istinye University, 34010 Istanbul, Türkiye
cStem Cell and Tissue Engineering Application and Research Center (ISUKOK), Istinye University, Istanbul, Türkiye
dDepartment of Pharmaceutical Technology, Faculty of Pharmacy, Marmara University, 34854 Istanbul, Türkiye
eDepartment of Pharmaceutical Technology, Faculty of Pharmacy, Yeditepe University, 34755 Istanbul, Türkiye
fDepartment of Thoracic and Cardiovascular Surgery, University Hospital Tübingen, 72076 Tübingen, Germany
gDepartment of Genetics and Bioengineering, Faculty of Engineering and Natural Sciences, Istanbul Okan University, 34959 Istanbul, Türkiye
hDepartment of Research Analytics, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai 600 077, India
iIndependent Researcher, W Nazar ST, Boostan Ave, Isfahan, Iran. E-mail: siavashira@gmail.com
jDepartment of Biomedical Engineering, Faculty of Engineering and Natural Sciences, Istinye University, Sariyer, 34396, Istanbul, Türkiye. E-mail: alizarrabi@gmail.com
kGraduate School of Biotechnology and Bioengineering, Yuan Ze University, Taoyuan 320315, Taiwan
First published on 30th May 2024
Abstract
Bionanocomposites (BNCs) have gained significant attention in the field of biomaterials, particularly for their potential applications in skin tissue repair and regeneration. Advantages of these biomaterials in skin care and wound healing/dressings include their ability to provide a suitable environment for tissue regeneration. They can mimic the extracellular matrix, supporting cellular interactions and promoting the formation of new tissue. They can also be engineered to have controlled release properties, allowing for the localized and sustained delivery of bioactive molecules, growth factors, or antimicrobial agents to the wound site. BNCs can be used as scaffolds or matrices for bioprinting, enabling the fabrication of complex structures that closely resemble native tissue. BNC-based films, hydrogels, and dressings can serve as protective barriers, promoting an optimal wound healing environment and preventing infection. These materials can also be incorporated into advanced wound care products, such as smart dressings, which can monitor wound healing progress and provide real-time feedback to healthcare professionals. This review aims to provide a comprehensive overview of the current trends, advantages, challenges, and future directions in this rapidly evolving field. The current trends in the field are deliberated, including the incorporation of natural polymers, such as silk fibroin, hyaluronic acid, collagen, gelatin, chitosan/chitin, alginate, starch, bacterial cellulose, among others. These BNCs offer biocompatibility/biodegradability, enhanced mechanical strength, and the ability to promote cell adhesion and proliferation. However, crucial challenges such as biocompatibility optimization, mechanical property tuning, and regulatory approval need to be addressed. Furthermore, the future directions and emerging research areas are deliberated, including the development of biomimetic BNCs that mimic the native tissue microenvironment in terms of composition, structure, and bioactive cues. Furthermore, the integration of advanced fabrication techniques, such as 3D bioprinting and electrospinning, and the incorporation of nanoparticles and bioactive molecules hold promise for enhancing the therapeutic efficacy of BNCs in skin tissue repair and regeneration.
1. Introduction
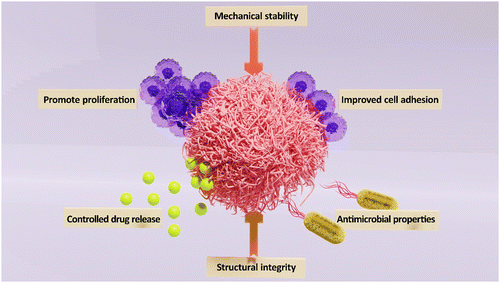
In recent years, the field of tissue engineering and regenerative medicine has witnessed tremendous advancements, particularly in the area of skin tissue repair and regeneration. In this context, bionanocomposites (BNCs) have shown promising potential in developing wound care and tissue regeneration (Fig. 1).1 Typically, they are materials composed of a biopolymer matrix and nano-sized fillers, such as nanoparticles (NPs) or nanofibers. The biopolymer matrix provides structural integrity and mechanical stability, while the NPs offer enhanced functionalities, such as improved cell adhesion, controlled drug release, and antimicrobial properties. The synergistic combination of these components makes BNCs an ideal choice for skin tissue repair and regeneration.2,3 One of the key advantages of BNCs is their ability to promote cell adhesion and proliferation. The surface properties of nanomaterials, such as their high surface-to-volume ratio and unique surface chemistry, facilitate cell attachment and spreading. This promotes the formation of new tissue and accelerates the healing process. For instance, soft BNCs composed of polyampholyte and nanosilicate exhibited tunable mechanical, cell adhesion, and degradation properties, serving as suitable scaffolds for tissue engineering.4 Furthermore, BNCs can be designed to mimic the natural extracellular matrix (ECM), providing a favorable microenvironment for cell growth and differentiation.5 Another significant benefit of BNCs is their potential for controlled drug delivery.6,7 By incorporating therapeutic agents or NPs with drug-loading capabilities into the biopolymer matrix, BNCs can release therapeutic agents in a controlled manner. This allows for targeted drug delivery to the site of injury, minimizing side effects and optimizing the healing process;6,8 the release kinetics can be tailored to match the specific requirements of the wound, ensuring optimal treatment outcomes. Notably, infections are a common complication in skin tissue repair and regeneration. BNCs can address this issue by incorporating antimicrobial agents/NPs, such as silver, TiO2, or zinc oxide NPs (ZnO), into the biopolymer matrix.9 These NPs exhibit potent antimicrobial activity, effectively preventing bacterial colonization and promoting wound healing. The use of BNCs with antimicrobial properties can significantly reduce the risk of infection and improve patient outcomes.9,10In the field of skin wound repair and regeneration, various materials and techniques are utilized to enhance the healing process, including traditional approaches such as the use of polymer fibers-based wound dressings containing therapeutic agents or NPs. The challenges such as limited efficacy and potential adverse reactions have led to the exploration of more advanced techniques. One advanced technique is the development of Janus-type biomimetic fabrics with antigravity liquid transport properties.11 Innovative Janus-type biomimetic fabrics, designed with antigravity unidirectional liquid transport capabilities, play a pivotal role in enhancing wound healing processes. These fabrics facilitate the removal of biofluid from the wound bed while preserving an optimal moisture level. Drawing inspiration from the transpiration mechanism observed in plants, where water is transported from the roots to the leaves and subsequently evaporates, these fabrics mimic nature's efficiency. The distinct feature of asymmetric wettability in Janus fabric, with one side being hydrophilic and the other hydrophobic, generates a compelling force for directing unidirectional liquid transport.11,12 By harnessing this unique property, researchers aim to create wound dressings that can effectively manage exudate and promote faster healing. The incorporation of such cutting-edge technologies holds great promise for improving outcomes in skin wound repair and regeneration. In one study, a novel biomimetic fabric, drawing inspiration from natural transpiration mechanisms in plants, was unveiled.13 This fabric ingeniously merged a commercial polyethylene terephthalate fabric with asymmetrical growth of one-dimensional rutile titanium dioxide (TiO2) micro/nanostructures. It exhibited essential plant characteristics, such as hierarchically porous networks and hydrophilic water conduction channels. This unique structure granted the fabric unparalleled antigravity wicking-evaporation capabilities. The fabric showcased a remarkable 780% one-way transport capacity and a water evaporation rate of 0.75 g h−1, far exceeding that of traditional moisture-wicking textiles. Notably, the integration of one-dimensional rutile TiO2 micro/nanostructures introduced solar-light triggered antibacterial properties, crucial for dismantling and eliminating wound biofilms. The biomimetic transpiration fabric proved instrumental in draining exudate and eradicating biofilms in Staphylococcus aureus-infected wounds. It demonstrated a substantially faster infection eradication potential in comparison to the commonly used ciprofloxacin irrigation method.13 These significant findings paved the way for the development of high-performance textile-based wound dressings. These dressings offered efficient clinical solutions to combat biofilms associated with chronic wounds, marking a notable advancement in wound care technology.
When it comes to BNC scaffolds, the design principles revolve around incorporating nanomaterials into biocompatible matrices to enhance the scaffold's mechanical properties, bioactivity, and biodegradability.6,14,15 By combining nanomaterials such as NPs, nanofibers, or nanotubes with natural or synthetic polymers, researchers aim to create scaffolds that closely mimic the extracellular matrix of tissues. These BNC scaffolds offer a supportive structure for cell attachment, proliferation, and differentiation in tissue engineering applications. The design process typically involves optimizing the composition, morphology, and distribution of nanomaterials within the scaffold to achieve desired mechanical strength, porosity, and bioactive properties. Notably, surface modifications can be implemented to promote cell adhesion and tissue regeneration. By carefully tailoring the nanocomposite scaffold's properties, researchers can create a biomimetic environment that facilitates the regeneration of damaged tissues.9 BNCs, composed of natural polymers such as silk fibroin, hyaluronic acid, collagen, gelatin, chitosan/chitin, alginate, starch, bacterial cellulose, and more, exhibit a wide range of properties that make them highly suitable for skin tissue repair and regeneration.16 Their unique characteristics, such as mechanical strength, biodegradability, moisture retention, cellular interactions, antimicrobial properties, gel-forming capabilities, and fibrous structure, contribute to their versatility and potential in the field of tissue engineering and regenerative medicine.17,18 For instance, silk fibroin is renowned for its remarkable mechanical strength, biocompatibility, and biodegradability.19 Incorporating silk fibroin into BNCs not only imparts structural integrity and support to the regenerated tissue but also ensures gradual degradation of the BNC scaffold, allowing the regenerated tissue to assume its functionality. Silk fibroin exhibits exceptional absorbent properties, and its versatility enables the formation of diverse materials at different scales, including macro, micro, and nano, such as nanofibers, NPs, hydrogels, and microspheres. The incorporation of inorganic NPs with silk fibroin has gained significant attention, offering enhanced functionalities and value addition. BNCs incorporating silk fibroin have been extensively investigated for tissue replacement applications, such as tendon, corneal stroma, bone, and dermis, owing to their exceptional biocompatibility.19 In addition, hyaluronic acid, a naturally occurring polysaccharide, is known for its exceptional water-binding capacity and biocompatibility. When integrated into BNCs, hyaluronic acid provides excellent hydration and lubrication properties, making it beneficial for wound healing and tissue regeneration. Its ability to create a favorable microenvironment for cell growth and migration is also advantageous in promoting skin regeneration.18,20
Collagen and gelatin, derived from animal sources, are widely recognized for their biocompatibility and bioactivity.21 These proteins provide a favorable environment for cell adhesion, proliferation, and tissue regeneration. When incorporated into BNCs, collagen and gelatin enhance the biocompatibility of scaffolds, promoting cellular interactions and facilitating the regeneration of skin tissue.22 Furthermore, chitosan and chitin, derived from crustacean shells, possess antimicrobial properties that can prevent infection during the healing process.23 They exhibit excellent wound healing properties, promoting cell growth and angiogenesis. When combined with NPs, chitosan/chitin BNCs offer a dual-action approach, protecting against bacterial colonization and accelerating the healing of skin tissue.23,24 In addition, alginate, starch, and bacterial cellulose are polysaccharides known for their biocompatibility and biodegradability. When utilized in BNCs, they provide structural integrity, promote cell attachment, and regulate the release of bioactive molecules. These properties are essential for skin tissue repair and regeneration, as they assist in wound healing, neovascularization, and extracellular matrix formation.16,25
BNCs have shown immense potential in promoting angiogenesis by incorporating NPs that can release growth factors or stimulate the expression of angiogenic proteins. They can create a favorable microenvironment that encourages the growth of new blood vessels, thereby enhancing the supply of oxygen and nutrients to the healing tissue.16,25 The skin barrier function is essential for protecting the body from external pathogens and maintaining proper hydration.26 BNCs can play a pivotal role in restoring and improving the skin barrier function through their unique properties. By modulating the composition and structure of the biopolymer matrix, BNCs can mimic the natural skin barrier and regulate transdermal water loss. This helps to prevent excessive moisture loss and maintain optimal hydration levels, facilitating the healing process. For instance, the integration of chitin nanofibrils into poly(lactic acid) (PLA)-based BNCs exhibited significant antimicrobial activity, rendering them suitable for the development of skin-compatible films aimed at wound healing applications.27 Besides, BNCs offer a platform for enhanced cellular interactions during the repair and regeneration process. The incorporation of NPs with bioactive properties, such as cell adhesion molecules, can facilitate cell attachment and communication. This fosters the integration of regenerated cells with the surrounding tissue, enabling seamless healing and minimizing the risk of complications.14,15 While BNCs hold immense potential for skin tissue repair and regeneration, several challenges need to be overcome. Addressing issues of biocompatibility, mechanical properties, degradation rate, biomimicry, scalability, stability, regulatory approval, and clinical translation are essential for the successful application of BNCs in this field. Developing scalable and cost-effective manufacturing methods for BNCs is a significant hurdle. Ensuring consistent quality, reproducibility, and scale-up production of BNCs is necessary for their widespread use in skin tissue repair and regeneration. Notably, bridging the gap between laboratory research and clinical implementation is essential. In this context, conducting rigorous preclinical and clinical studies, addressing ethical considerations, and ensuring affordability are crucial for successful clinical translation.
Herein, this review aims to provide a comprehensive overview of the current trends, advantages, challenges, and future directions in the field of BNCs for skin tissue repair and regeneration. By consolidating existing knowledge and highlighting advancements made in the development and application of these BNCs, this review seeks to shed light on their potential in promoting skin tissue repair. It explores the different types of BNCs and the unique properties that make them advantageous for tissue regeneration. In addition, the review addresses the challenges faced in terms of biocompatibility, mechanical properties, and regulatory approval, while also focusing on potential future directions and emerging research areas. While there have been previous reviews on this topic, this review presents the latest research findings, emerging trends, and recent advancements in the field. Furthermore, it highlights the potential future directions and identifies research gaps that need to be addressed to further advance the field. By providing a fresh perspective and incorporating the latest research, this review offers a novel contribution to the existing body of knowledge on BNCs for skin tissue repair and regeneration.
2. Wound repair and regeneration: mechanisms, signaling, and translation
After an injury, rapid wound closure and regeneration of the damaged skin are crucial for restoring its protective function. Wound healing is a complex but finely-tuned process that begins immediately after an injury and can continue for months or even years.28 Effective wound repair is precisely orchestrated and regulated at multiple levels and requires communication and interaction between various cell types. The wound healing process can be broadly categorized into four sequential but overlapping phases: hemostasis, inflammation, proliferation, and remodeling.29 Deregulation of any of these phases leads to impaired healing, such as the development of chronic, difficult-to-heal ulcers, or excessive scarring (Fig. 2 (A)).5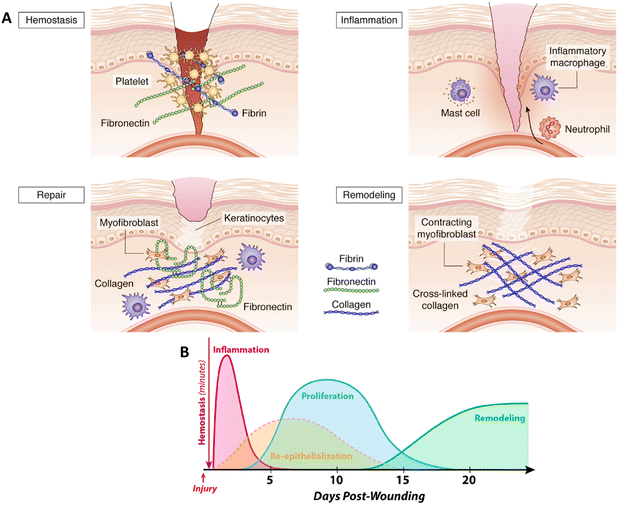 | ||
| Fig. 2 Mechanisms of wound healing: (A) different phases of wound healing. Reprinted from5 under the terms of the Creative Commons Attribution License (CC BY). (B) Timeline of cutaneous wound healing. Reprinted from30 under the terms of CC BY license. | ||
2.1. Hemostasis
Immediately after an injury, the damaged endothelium releases endothelin-1, which leads to vasoconstriction.31 In addition, the contact of platelets with the exposed components of the sub-endothelial vascular matrix, e.g. collagen, results in platelet activation.32 The primary aim of these rapid responses is to prevent blood loss and to initiate the early phases of the coagulation process. Platelets adhere via receptors such as glycoprotein VI (GPVI) and integrin α2β1 to proteins of the ECM, especially to collagen of the injured blood vessel wall.33 In addition, the plasma von Willebrand Factor (vWF) binds to the exposed collagen, which undergoes a shear-induced conformational change. The immobilized vWF then initiates the adhesion of platelets via binding to glycoprotein (GP)Ib-IX complex (CD42) on the platelets34 followed by triggering the platelet activation by thrombin that leads to a conformational change, and the release of alpha and dense granules containing cytokines and growth factors (like platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β), basic fibroblast growth factor (bFGF), insulin-like growth factor-1 (IGF-1), and vascular endothelial growth factor (VEGF)) along with coagulation factors that promote coagulation and platelet aggregation.35This process concludes with the formation of an insoluble clot known as the eschar, which is composed of platelets, fibrin, fibronectin, vitronectin, and thrombospondin,36 and effectively seals the wound, prevents further bleeding, and serves as a provisional matrix. Beyond its hemostatic role, the eschar also prevents bacterial invasion, serves as a scaffold for cell migration, and as a reservoir for cytokines and growth factors released by activated platelets to guide the behavior of wound cells during the early stages of repair.37 The released cytokines attract circulating inflammatory cells to the side of the wound, trigger tissue movements for re-epithelialization and connective tissue contraction, and stimulate the angiogenic response of the wound.
2.2. Inflammation
The second phase of wound healing is the inflammatory phase, which begins immediately after hemostasis and lasts around 4–6 days (Fig. 2(B)).30 This phase can be divided into two separate phases, the early inflammatory phase and the late inflammatory phase.38Upon activation, mast cells release granules containing enzymes, notably histamine, resulting in vasodilation and increasing vascular permeability.40 In addition, the release of pro-inflammatory cytokines and chemokines induces the expression of endothelial adhesion molecules, such as intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), P- and E-selectin, on the surface of blood vessels.41 This, in turn, enables the adhesion of circulating neutrophils and monocytes to the inflamed endothelium and promotes their extravasation into the affected tissue. Previous studies in which E- and P-selectin were blocked led to a significant impairment of immune cell infiltration and wound healing process and demonstrated the significance of selectins for the recruitment of immune cells.42,43
Neutrophils are recruited by chemoattractants, such as leukotriene B4 or LTB4, the chemokines (C-X-C motif) ligand-5 (CXCL5) and CXCL8, the complement anaphylatoxins C3a and C5a, and the bacteria-derived formly peptides N-formyl-Met-Leu-Phe (fMLF), into the wound site.44 In response to pro-inflammatory signals, neutrophils, along with other cells present at the wound site, release cytokines such as interleukin 1β (IL-1β), IL-6, and tumor necrosis factor-alpha (TNF-α).45 This initiates the activation of inflammatory signaling pathways, such as nuclear factor kappa B (NF-κB), which in turn, further recruits and activates fibroblasts and epithelial cells in the wound healing process.46 Furthermore, the neutrophils recruited to the wound site play a crucial role in the elimination of damaged cells and pathogens by various mechanisms, like phagocytosis.47 These cells also deploy an array of weapons, such as the release of reactive oxygen species (ROSs), antimicrobial peptides, eicosanoids, and proteases e.g. neutrophil elastase and collagenase.37 These proteases remove components of the ECM damaged by the initial injury. In addition, neutrophils trap and kill pathogens in an extruded web of DNA–histone complexes coated with antimicrobial peptides and proteins released by activated neutrophils, known as neutrophil extracellular traps (NETs).48,49 Typically, the presence of neutrophils in the wound diminishes after 2 to 3 days due to the process of apoptosis, and they are subsequently replaced by attracted monocytes into the tissue.35 Apoptotic neutrophils are removed by tissue-resident macrophages via efferocytosis.50
2.4. Proliferation
The proliferation phase starts 3 days after the injury and lasts about 2 weeks.38 During this phase, the provisional fibrin matrix is replaced by a new matrix of collagen fibers, enriched in proteoglycans, and fibronectin, to restore the structure and function of the tissue.53 Crucial events in this phase include the formation of granulation tissue, re-epithelialization, and angiogenesis to replace previously damaged vessels and restore circulation.2.5. Remodeling phase
The final phase of the healing process is the remodeling phase, which begins two to three weeks after the injury and can last one year or longer.65 During the remodeling phase, the granulation tissue matures into a scar, and the tensile strength of the tissue is increased.66 This maturation process is accompanied by a decrease in the number of capillaries via aggregation into larger vessels. In addition, the cell density and metabolic activity in the granulation tissue are reduced. The initially produced type III collagen in the ECM is replaced by type I collagen,67 which is the dominant fibrillar collagen in the skin and has a higher tensile strength. This replacement is regulated by MMPs.68 Over several months or longer, alterations in collagen organization within the repaired tissue slowly increase the tensile strength to a maximum of about 80% of normal tissue.693. BNCs in skin tissue engineering and regenerative medicine
One of the areas that tissue engineering and regenerative medicine focuses on is creation as well as regeneration of tissues and organs. For this purpose, use of biopolymers has attracted attention due to their significant advantages over synthetic polymers. They are biocompatible, biodegradable, could have additional functionalities based on the biopolymer, and perform well in applications due to hydrophilic characteristics.16 Their benefits encompass the replication of natural pathways for tissue regeneration, and restoration of physiological functions. Some biopolymers, such like collagen, are inherent to the ECM structure, and some could participate in natural healing mechanisms by supporting cell migration, adhesion, proliferation, and cell differentiation, and directing cell alignment. Moreover, some biopolymers have important intrinsic properties like antimicrobial, hemostatic, anti-inflammatory properties that further support the material properties.16,70–72Nanomaterials could improve the solubility of drugs, reduce doses, enable targeting, minimize toxicity, and enhance bioavailability. They include a diverse class of materials with different compositions such like metallic NPs, engineered therapeutic agents like oligonucleotides, or nano-sized polymeric structures.73 Developments in nanotechnology in the last decades also have paved the way for new approaches in tissue engineering; there is a significant focus on nanotechnology within the realm of biopolymer matrix-based BNCs across diverse applications. BNC materials has been a subject that has been studied extensively in recent years.74 By expanding the scope of biocomposites to include nanostructured hybrid materials, the term ‘bionanocomposites’ can be defined in two distinct ways. Firstly, it can refer to nanocomposites crafted from renewable NPs in conjunction with petroleum-derived polymers. Secondly, BNCs is defined as nanocomposites derived from biopolymers paired with synthetic or inorganic nanofillers. Nano-elements in BNCs could support skin repair mainly either by unique properties of the particles or by encapsulation of functional elements that promote cellular growth and repair.75 NPs could be added to the BCS by different methods. A method is in situ formation of NPs within the polymer matrix. An example of this method is chemical reduction of silver ions and formation of Ag-NPs in situ. In ex situ procedure, NPs are prepared and later added to the polymer matrix.76,77 Surface modification is another approach in preparation of the functional dressings. In a study, Harandi et al. make surface modification of wound dressing material NPs incorporating Lactobacillus strains. In the study two hybrids Lactobacillus plantarum and Lactobacillus acidophilus-green Fe2O3 NPs were used for surface-coating of electrospun wound dressing enhanced antimicrobial and antibiofilm activity.78
Creation of a moisty environment is an important aspect of wound healing to support healing. However, overhydration could cause delayed healing and may cause undesired effects such like maceration that could lead softening and breaking down of the surrounding skin as well as infections.79 Also, addition of NPs could be useful in surface hydrophilic/hydrophobic regulation. Some studies suggest that addition of NPs to films changed water vapor permeability (WVP) of the films. Generally, a decrease in WVP was observed in compatible polymers, while an increase is observed in nanoclusters due to increased water diffusion through the dressing, or loose packaging of hydrophilic and hydrophobic components within the NC that allows WVP.80,81 In this part, BNCs in skin tissue engineering and regenerative medicine is discussed, and the recent studies in this field have been reviewed.
3.1. Natural polymers
SF nanofibrous scaffolds, a compelling type of SF-derived skin substitute, are created using electrospinning to establish a pseudo 3D network for optimal cell adhesion and growth.85 The pores formed between the nanofibers not only facilitate oxygen exchange but also effectively prevent the infiltration of external fluids and bacteria. This unique combination of properties creates an ideal microenvironment for promoting cell proliferation and skin regeneration.86 Sheikh et al. identified 3D SF nanofiber scaffolds as an excellent choice for artificial skin reconstruction, emphasizing their potential for scarless regeneration of fetal skin.87 In another study by Wang et al., a silk-based construct incorporating hyaluronic acid (HA), SF, and silk nanofiber was developed to mimic the ECM and improve the healing process. The introduction of HA resulted in remarkable water-binding potential, high-porosity, and mechanical stability. The incorporation of silk nanofibers demonstrated a benefical impact on hindering scar formation. This innovative avenue, leveraging an ECM-like SF scaffold, holds great potential for biomaterial configuration and applications in skin tissue engineering.88 In another study, Du et al., created a nanofiber composite of SF–gelatin enriched with propolis, demonstrating favorable physical properties. They incorporated varying amounts of propolis into the nanofiber to enhance its healing and antibacterial capabilities. The findings revealed that the inclusion of propolis caused changes in the physicochemical features of SF–gelatin nanofibers, resulting in a significant enhancement of their antimicrobial features and effective promotion of healing. The nanofibers containing SF–gelatin–propolis extract show considerable promise for applications in skin tissue engineering and wound treatment.21 In another study, Gao et al., employed electrospinning to create a periplaneta americana extract-implemented SF nanofiber mat. This mat displayed a considerably higher wound closure rate in the mouse model of full-thickness wounds compared to commercial products. Histomorphological assessments revealed that the periplaneta americana extract-implemented SF nanofiber mat promoted re-epithelialization, angiogenesis, and collagen deposition at wound sites, facilitating the overall wound healing process.89
Double-layered wound dressings with multifunctional features can be appealing for efficient skin regeneration. Farshi et al., innovatively designed a double-layered wound dressing, combining electrospun SF for enhanced mechanical strength in the upper layer and a carboxymethyl cellulose (CMC)/gelatin composite film as the skin-interacting substrate. In wound dressing applications, CMC exhibits limited mechanical stability and low cellular activity. To address these shortcomings, a suggested approach involves combining CMC with SF to create a more effective dressing. The mechanical properties of the resulting double-layered wound dressing significantly improved with the incorporation of SF. In addition, the dressing promotes increased cell proliferation, attributed to the cytocompatible characteristics of SF. Consequently, results showed the capacity of this developed structure as an encouraging choice for skin tissue regeneration.90 In another work, Liu et al., created SF sponges with nanopore-containing walls derived from SF NPs produced during autoclaving of SF solutions (Fig. 3(A)). These nanopores proved beneficial for wound healing by improving exudate absorption, maintaining moisture, facilitating nutrient and gas diffusion, and enhancing cell adhesion (Fig. 3(B)). In a rat skin wound model, SF sponges with nanopores exhibited superior performance in speeding up wound healing, promoted by increased collagen accumulation (Fig. 3(D)), vascularization, and epidermal thickness (Fig. 3(C)) over 21 days, compared to samples without nanopores. This biomaterial, featuring a hierarchical multi-scale pore shape, offers a novel strategy for designing and engineering high-performing skin tissue constructs that can independently support vascularization, cell migration, and tissue regeneration without the addition of growth factors.22
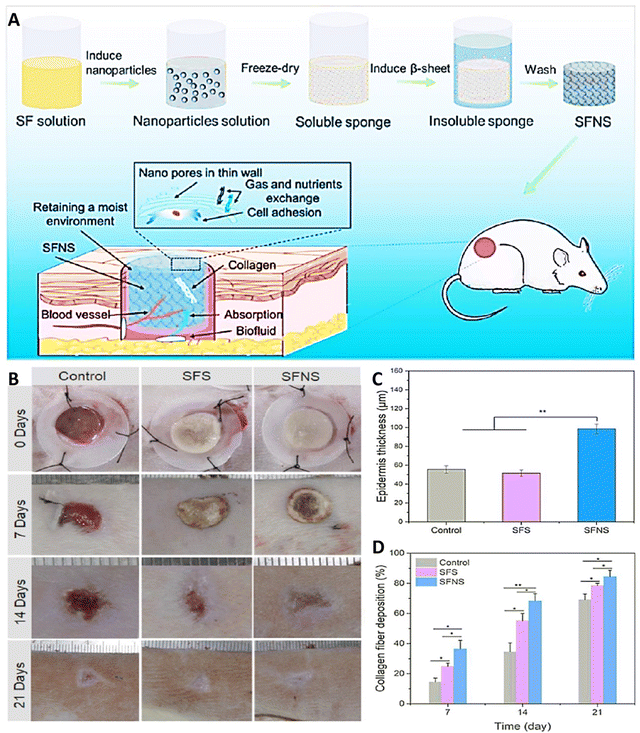 | ||
| Fig. 3 (A) Schematic of SF sponges with nanopore-containing walls derived from SF NPs supporting wound repair through a number of mechanisms. (B) Image of wound healing results for 21 days. Results of thickness of the epidermis (C) and collagen accumulation (D) after exposing with different treatment (*p < 0.05, **p < 0.01). Reprinted with permission from.22 Copyright 2023, American Chemical Society. | ||
The limited clinical applications of HA-based scaffolds are often attributed to their poor mechanical properties. To overcome this challenge, the implementation of supporting elements such as nanocrystalline cellulose (CNC) into HA-based wound dressings has shown promise in improving mechanical properties. Furthermore, enhancing the healing process can be achieved through the controlled delivery of growth factors to the wound area using NPs. In research done by Dehkordi et al., a new wound dressing was developed, consisting of CNC-reinforced HA-based nanocomposites. This innovative dressing also incorporated chitosan NPs loaded with granulocyte macrophage colony stimulating factor proteins (GM-CSF). Chitosan NPs loaded with GM-CSF were synthesized using the ionic gelation method, resulting in particles with a size of about 366.9 ± 9.15 nm. The controlled release of GM-CSF over a 48-h period from the composite wound dressings was achieved, offering a more favorable healing environment compared to the rapid release of this growth factor. Wound dressings incorporating GM-CSF-loaded chitosan NPs demonstrated superior effects on wound healing when compared to pristine dressings (completely healed the wound in 13 days in rats treated with CNC-HA/GM-CSF-Chi-NPs composite, while in control cases 70% wound size reduction was seen). Release of GM-CSF in the structure of this dressing led to reducing the inflammation, enhancing the granulation tissue formation, and accelerating re-epithelization as compared with hydrogels without it.94
Li et al., developed a HA BNC using aldehyde-functionalized sodium hyaluronate, hydrazide-functionalized sodium hyaluronate, and aldehyde-functionalized cellulose nanocrystals (oxi-CNC). Platelet-rich plasma (PRP) was implemented into the HA nanocomposite constructs to synergistically support skin wound healing. The fabricated formulation provided the capability of sustained release of PRP from the hydrogel, resulted from the interactions between hydrogel network and PRP, that enhanced the therapeutic effects of growth factors incorporated inside PRP on the wound site. In animal experiments, the PRP-loaded HA nanocomposite hydrogels significantly enhanced full-thickness skin wound healing by promoting tissue granulation (1147.43 ± 31.07 μm), simplifying collagen accumulation (63.93 ± 1.45%), and ascending neovascularization and re-epithelialization. It showed accelerated wound closer (95.28 ± 0.56%) compared to rats exposed with the only gel (92.05 ± 2.13%) and the control group (90.29 ± 1.68%).95 Hu et al. (2022) addressed the challenge of abdominal wall injuries, often complicated by serious infections, by developing a multifunctional hydrogel.96 This construct on the basis of dopamine-functionalized HA, gelatin, and AgNPs, aimed to advance the repair of abdominal wall damages. In rat full-thickness skin injury model and rat full-thickness abdominal wall injury model, the developed construct demonstrated the ability to speed up the healing function. It achieved this by decreasing wound inflammation, enhancing wet adhesion, and supporting the generation of granulation tissues and angiogenesis. The developed system exhibited great potential for the full-thickness abdominal wall defect therapy.96
A versatile wound dressing was created by incorporating graphene oxide/copper nanocomposites with HA/chitosan constructs. This innovative approach represents an encouraging choice for successful therapeutic intervention in bacteria-infected wound healing, especially in the context of plastic surgery clinics. The fabricated formulation showed a sustainable pH responsive release behavior so that the amounts of released copper NPs were increased by decreasing pH (about 60.3%, 76.2% and 88.1% under alkaline, neutral, and acidic conditions, respectively, after 14 days). Presence of Cu and its release from this formulation provided high bactericidal and antibiofilm abilities for the hydrogel and confirmed its effectiveness in treatment of infected wound. The developed multifunctional constructs significantly accelerated wound healing (wound closer area of about 93.1 ± 1.2%, 61.7 ± 3.7%, 39.2 ± 8.0%, and 18.5 ± 2.1% for the C/H/GO/Cu, C/H/GO, C/H, and control groups, respectively), featuring regulated inflammatory infiltration, and enhanced angiogenesis in granulation tissues. Besides, there was no bacterial infection in cases treated with the C/H/GO, C/H scaffold that could be due to the presence of GO/Cu nanocomposites, along with the CuNPs, and Cu2+ released from the dressing scaffolds throughout the wound healing process. Importantly, no pathological injury was observed in the tissue constructs of examined organs, including the liver, lung, kidney and heart.97 Zhou et al. (2021) created a 3D printing platform to mimic full-thickness skin by mixing catechol–hyaluronic acid (HACA) with sodium alginate and calcium chloride, and gelatin with horseradish peroxidase and hydrogen peroxide.98 The developed bioink was first printed using 3D-printing method and then thrombin-free fibrinogen, combined with human dermal fibroblasts, was applied to induce the gelation of bio-printed scaffolds. After removing the gelatin, human HaCaTs keratinocytes were placed on the printed scaffold to grow and generate skin-like networks. The printed-construct, demonstrated high elasticity, promoted the creation of a double-layered, and cell-laden skin-like network. The findings indicate that this 3D printing system establishes a groundwork for skin regeneration.98
Chang et al. (2023) engineered an enzyme-crosslinked HA–tyramine based hydrogel wound dressing that incorporates antioxidants and photothermal AgNPs (HTA) (Fig. 4). AgNPs capped with natural antioxidant tannic acids were synthesized, utilizing tannic acids as stabilizing and reducing agents in the process.99 The NPs had a size of 149 nm, a zeta potential of 26.4 mV, and a polydispersity index (PDI) of 0.24, indicating their relative stability. Encapsulation of AgNPs capped with TA significantly enhanced the hydroxylic radical scavenging ability due to the presence of TA, a natural polyphenol small molecule. Moreover, these TA could interact with metal ions and concentrated Ca2+ (coagulation factor IV) that led to an enhanced hemostatic performance. Utilizing near infrared (NIR) light led to a stable photothermal effect dependent on the concentration of AgNPs@TA. This photothermal ability was used to improve the antibacterial activity of hydrogel so that by increasing the time of NIR irradiation from 1 to 3 min, the amounts of bacterial colony reached to zero (Fig. 4(A)). Besides, utilizing NIR led to expanding the inhibition zone of HTA0.4 hydrogel against both Gram-positive and Gram-negative bacteria (Fig. 4(B)). Presence of AgNPs@TA in the structure of this hydrogel induced antioxidant activity in it so that cells treated with hydrogels contained NPs had high antioxidant activity (Fig. 4(C) and (D)). The combination use of NIR and HTA0.4 hydrogel accelerated the healing ratio of wound in animal model (Fig. 4(E)) via improving collagen deposition, re-epithelilization, and tissue regeneration.99
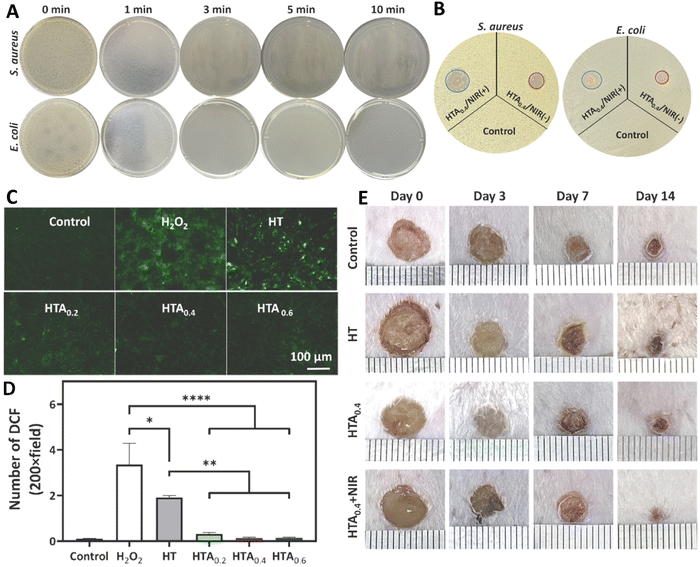 | ||
| Fig. 4 Antibacterial activity against S. aureus and E. coli using (A) clony counting assay after 0, 1, 3, 5, 10 min exposing with near infrared (NIR) light and hyaluronic acid–tyramine (HT) loaded AgNPs (HTA) hydrogel, and (B) inhibition zone assay in the presence of HTA with/without NIR. (C) and (D) The antioxidant results of different samples (control, H2O2, HT, HT + 0.4 mg ml−1 of AgNPs@TA (HTA0.4), and HTA0.6) using qualitative and quantitative methods. (E) Wound healing results of mice model after exposing with different treatments. Reprinted with permission from.99 Copyright 2022, Elsevier. | ||
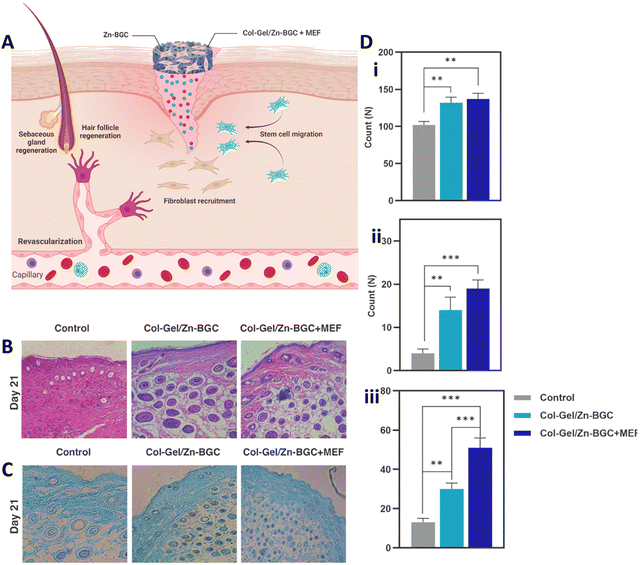 | ||
| Fig. 5 (A) Supportive function of collagen–gelatin/Zn-BGC BNC construct encapsulated with mouse embryonic fibroblasts for cutaneous wound healing by promoting revascularization, regenerating hair follicles, recruiting fibroblasts, and facilitating re-epithelialization. (B) Hematoxylin–eosin (H&E) staining on day 21. (C) Masson's Trichrome staining on day 21. (D) Quantification of fibroblasts (i), blood vessels (ii), and hair follicles (iii) in skin wounds for both the control and test groups on day 21. (Statistically significant at **: p < 0.01, ***: p < 0.001). Reprinted with permission from.104 Copyright 2023, Elsevier. | ||
ZnO–curcumin implemented collagen BNC was fabricated for efficient scarless skin regeneration in acute burn injuries. The ZnO–curcumin nanocomposite in the hybrid structure up-regulated angiogenesis and TGF-β3 expression thereby reduced the scar formation. It was shown that the native collagen had antioxidant activity which was significantly improved by the addition of ZnO–curcumin nanocomposite. The hybrid of ZnO and curcumin also led to decreasing the inflammatory effect via down-regulating the expression of tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1). Evaluation of the newly regenerated epidermis thickness and relative scar elevation index (SEI) value in animals treated with the hybrid construct indicated scarless wound healing. Curcumin could also accelerate proliferative stage via improving the proliferation and migration of fibroblasts. On the other hand, Zn2+ ions enhanced the migration of keratinocyts via acting as cofactor for enzymes like zinc dependent matrix metalloproteinases. All of these led to improved wound healing effect that was confirmed by enhancing collagen deposition, neovascularization, and re-epithelialization. Results showed wound closer percentage of about 82 and 91% for collagen–ZnO (Coll/OTSP/ZnO 5%) and collagen–curcumin (Coll/OTSP/Cur 5%) scaffold compared to 65 and 80% for untreated and native collagen scaffold.106 In another work, Khadivar et al. fabricated a biocompatible collagen-based nanoscaffold using collagen, carboxymethylated diethyl aminoethyl cellulose, and wood nanocellulose for skin tissue engineering applications. In vitro studies conducted on MSCs and keratinocytes demonstrated the biocompatibility of the nanocomposite scaffold. In vivo studies were carried out to assess potential immunogenic or allergic behaviors of the nanocomposite scaffold. Transforming growth factor β (TGF-β), a multifunctional cytokine associated with inflammation, was screened on day 3 to evaluate any immune reaction triggered by the scaffold. The lower level of TGF-β expression in wounds treated with scaffold proved that the nanocomposite scaffold did not induce significant allergic or immune reactions. On day 21 of treatment, cytokeratin 18 (CK18) and collagen deposition were examined to assess the extent of skin reconstruction. The developed nanocomposite scaffold facilitated the wound regeneration process and accelerated overall healing. The scaffold, with its moisture-absorbing capability, support for cell adhesion, high zeta potential, and satisfactory stability properties, emerged as a potent film for wound healing and skin tissue engineering.107
Collagen nanofibers were developed through electrospinning, incorporating nanophase hydroxyapatite crystals for the purpose of skin regeneration and repair. The release of calcium ions from the nano-hydroxyapatite improved cellular growth and proliferation rates, while preventing the pathogenic bacteria adhesion appeared in human skin flora. Implantation of the collagen/nano-hydroxyapatite BNC in rat subcutaneous tissue demonstrated the absence of adverse reactions. Consequently, this mechanically robust composite membrane holds significant potential for utilization either as a scaffold for cell transplantation in skin wound regeneration or as a wound dressing biomaterial in applications such as burn therapy, plastic surgery, or addressing various skin diseases.108
The development of multifunctional hydrogel adhesives that can inhibit infections and enable electrical stimulation for tissue repair is extremely desired for healing surgical wounds and other skin damages.109 In a recent work, Gu et al. developed a collagen-based self-healing and injectable hydrogel composed of collagen/chitosan/oxidation-modified Konjac glucomannan hydrogel matrix (COL–CS–OKGM) with multifunctional capabilities for the regeneration of infected wounds. OKGM was used as crosslinker to build dynamic Schiff-base bonds. Collagen and chitosan were chosen for their strong synergistic compatibility, aiming to create a complex that mimics the composition of the ECM. The integration of AgNPs into the hydrogel resulted in significantly enhanced antibacterial activity stemmed from the synergistic effect between the released Ag+ ions and the mild photothermal potential of AgNPs. Indeed, utilizing NIR irradiation enhanced the release of Ag+ ions which were then interacting with the sulfhydryl groups on the surface of bacteria, destroying their membrane, affecting the nucleic acid of bacteria that led to bacterial death. It also enhanced local capillary blood circulation in the wound site and facilitated the wound healing procedure. The COL–CS–OKGM hydrogel, with self-healing behaviors, facilitates the dressing to conform seamlessly to irregular wound surfaces, establishing an optimal moist environment for the healing process. The COL–CS–OKGM–AgNPs hydrogel demonstrated antibacterial activity and quickest healing process among the animal models, with the regenerated skin closely resembling normal skin resulted from ref. 110.
Nanofibrous nanocomposite structure was developed by incorporating graphene nanosheets into electrospun gelatin/chitosan matrices.112 The introduction of graphene nanosheets significantly enhanced the porosity of the construct, reaching levels of up to 90% of their volume. This heightened porosity is deemed advantageous for the fabrication of wound dressings, facilitating optimal oxygen and nutrient perfusion within the designated wound-healing region. Furthermore, the incorporation of graphene nanosheets was observed to enhance the antibacterial efficacy of the fabricated nanofiber composite. In this system, gelatin was chosen for its high biocompatibility, biodegradability and hydrophilicity, and low irritability, immunogenicity, and antigenicity behaviors. Combining chitosan and gelatin resulted in the formation of a promising blend composite biomaterial. The inclusion of hydrophilic gelatin enhanced the overall hydrophilicity of chitosan and promoted cell spreading and adhesion on the fabricated biomaterial surface. The synergistic impact of these all components positions the fabricated material as a potent antibacterial wound bandage, capable of safeguarding the wound against potential complications or contamination during the healing process.112
Nazarnezhad et al. (2022) fabricated a set of nanofibrous constructs that mimic the ECM, comprising gelatin and polycaprolactone (PCL)-based nanofibers.113 These nanofibers were enriched with platelet lysate, showcasing potential applications in the regeneration of the epidermis. The developed system exhibited extended degradation over a period of 28 days without inducing any cell-toxicity. The poor cell adhesion and proliferation associated with the lack of cell-recognition sites and the hydrophobic nature of PCL was overcome by blending PCL with gelatin. The incorporation of biological molecules, specifically blood derivatives rich in growth factors, not only enhanced cell survival but also demonstrated a protective response against bacteria. Notably, this result could be attributed to electrostatic interactions between amine groups of platelet lysate and chitosan with the surface negative charge of bacteria. This characteristic positions the construct as a viable option for preventing infections during healing process.113
Aboomeirah et al. (2022) engineered one-dose bio-replicating skin substitute by preparing wet electrospun nanofibers reinforced with a gelatin/alginate-based nanocomposite.114 The nanocomposite was crosslinked using N-(3-(dimethylamino)propyl)-N′-ethylcarbodiimide hydrochloride (EDC) and strengthened by incorporating fragmented trans-ferulic acid (FA)-implemented cellulose acetate/polycaprolactone nanofibers. The fabricated ECM-replicating skin alternatives exhibited effective free-radical scavenging, notable antibacterial potential, porosity, water absorption capability, cytocompatibility, and favorable biodegradability. In vivo application of these ECM-replicating skin alternatives demonstrated remarkable wound-healing efficacy, decreasing the wound diameter to 0.95 mm following 15 days of treatment, highlighting their appropriateness for one-time therapy of deep wounds. Furthermore, histological examination of the wound area revealed that the applied skin alternatives not only accelerated the wound healing process but also contributed to enhancing the characteristics of the regenerated skin in the treated region. This was achieved by facilitating the regeneration and deposition of collagen fibers, indicating a positive impact on the structural integrity and overall quality of the healed skin.114
Studies with application of chitin demonstrate that chitin-based dressings support wound healing in various formulations such like mats, membranes, hydrogel formulations as filling agents or wound dressings in cases like surgical tissue defects, trauma cases and in herniorrhaphy.121,122 Chitin/silk fibroin/TiO2 bio-nanocomposite was prepared as a biocompatible wound dressing bandage. They prepared an interconnected microporous membrane with antibacterial activity (against Escherichia coli (E. coli), Staphylococcus aureus (S. aureus), and Candida albicans (C. albicans)), blood clotting properties, as well as good mechanical properties. In vitro evaluation of the dressings were carried using normal human dermal fibroblast cells, and it was found that the dressings are cytocompatible, and support cell viability, proliferation and attachment. The controlled release of TiO2 NPs from the hydrogel that was accelerated by the swelling behavior of the hydrogel provided the antibacterial activity for the hydrogel.123 Chitosan is a more extensively studied polysaccharide used in skin tissue engineering and regenerative medicine, compared to chitin, due to its superior properties such as supporting cell growth, absorbing exudate, minimizing scar formation, and promoting platelet adhesion and aggregation. It is an inexpensive material that could be easily produced, while having properties like hemostatic ability (helps control bleeding) and antibacterial activity (eliminates bacterial infections).124–127 Degradation products of chitosan are also useful in wound management. Chitooligosaccharides obtained via enzymatic or chemical degradation of chitosan, could promote fibroblast proliferation through regulation of mitogen-activated protein kinase pathway and hepatocyte growth factor.128 Due to these advantages, both polymers are frequently used for the fabrication of BNCs that could be used for skin tissue engineering and regenerative medicine.
In a study, Masud et al., designed a BNC-based wound healing material composed of chitosan–zinc oxide (ZnO)/poly(ethylene glycol) (PEG) crosslinked by sodium tripolyphosphate. Afterwards, an antibiotic, gentamicin sulphate, was loaded within the BNC, and then its wound healing capability was evaluated. ZnO NPs were about 50 nm in diameter and incorporated to enhance antimicrobial properties of the dressing. It is demonstrated that the dressing could provide a moist environment to the wound site. In vitro evaluation of the dressings was conducted on the BHK-21 (baby hamster kidney fibroblast cells) and vero cells, and according to the results, the dressings were found to have good biocompatibility. The combination of drug and ZnO NPs provide enhanced antibacterial activity against E. coli and S. enterica. In vivo studies were carried using male nude mice to evaluate wound healing efficacy of the BNC. Drug-loaded BNC, drug-free BNC, and gauze were used in the studies, and contraction, re-epithelialization, and morphology of the wound was evaluated. The antibiotic gentamicin sulphate loaded samples were found to achieve complete wound closure without any scars, and a smoother new skin formation and less scabby. It was while, the nanocomposite without drug and the conventional gauze (used as control) showed 80% and 50% wound closer after 10 days. According to overall results, dressings have a good loading efficiency of drug (76%), as well as good biocompatibility, enhanced antibacterial activity, wound healing properties, without any scar formation compared to commercial wound dressing.129
Carboxymethyl chitosan/gelatin-based films were developed with mesoporous silica NPs. Myrtus communis L. (Myrtle) aqueous extract was loaded within the NPs that have several phenolic groups and has free radical scavenging ability. According to results, mesoporous silica NPs were found to have many functions such as increasing the tensile strength as well as swelling ratio, and oxygen and water vapor permeability. Also, delayed release of the extract from the NPs resulted in reduced fibroblast cell toxicity. According to histological data in mice models, collagen percentage and fibroblast migration was found to be increased in the wound area after application of the BNC dressing containing 5% mesoporous silica NPs, which suggest dressings accelerate wound healing in mice model, and may find applications in wound treatment.130
An asymmetric wound dressing membrane made of chitosan/polyvinylpyrrolidone (PVP)/nanocellulose was fabricated, featuring a hydrophobic side and a hydrophilic side. The hydrophobic side was achieved through a coating layer of 3% stearic acid. The hydrophobic surface of the BNC material had porous structure, while hydrophilic surface was smooth. It was found that hydrophilic surface showed antibacterial activity against bacterial pathogens, and the hydrophobic side had water repellent and antiadhesion properties against E. coli. Chitosan was selected due to its desirable properties such as biocompatibility, biodegradability, intrinsic antimicrobial, and hemostatic property. Poor mechanical properties of chitosan were hindered by preparation of BNCs, where blended materials also serve specific functions in the dressings. For instance, it was found that addition of nanocellulose increased the physicochemical properties of the dressing and also enhanced the antibacterial activity along with chitosan. In vivo tests demonstrate that the dressing supports re-epithelialization and wound contraction compared with control studies and compared with the samples without nanocellulose so that the chitosan/PVP/nanocellulose 3%-stearic acid (CPNC3%-S) BNC showed about 100% healing rate after 21 days, while the chitosan/PVP–stearic acid (CP–S) and control group had healing rate of about 80% and 75%, respectively.131
Nanocomposite sponges of sodium alginate/graphene oxide/polyvinyl alcohol were developed for wound healing applications (Fig. 6).139 The dressings were formulated as sponges, and the obtained sponges had a uniform pore structure as well as flexibility. They found that porous and interconnected network structure was obtained, and the formulation with 1% graphene oxide had more homogeneous pores. The porous structure of the sponges could promote cell growth, ensure breathability, and improve water absorption capacity of the dressing. When a norfloxacin was encapsulated within the formulation for treatment of bacterial infections, sustained release of the drug was achieved that led to a strong inhibitory effect against both E. coli and S. aureus (Fig. 6(A)). Wound-healing rate of the samples were assessed on in vivo mice model, that showed the fabricated formulation with 1% graphene oxide encapsulating norfloxacin had enhanced healing ability (Fig. 6(B)) and prevented the inflammation in wound area without any toxic effects compared to the BNCs without norfloxacin and the gauze treated wounds. In here, alginate was preferred due to its ability to absorb wound exudate, provide moist environment, and its hemostatic activity, and indeed the wound closure was higher in the BNCs due to lower WVTR rate which is crucial for creating a moist environment in healing. In vivo results also demonstrated that drug loaded nanocomposites accelerate wound closure (about 90% wound closer compared with 65% in control), and the wounds were smooth without significant scars (Fig. 6(C) and (D)). The wounds treated with the complete composite showed better skin regeneration via exhibiting more generation of hair follicles and blood vessels compared to sodium alginate/graphene oxide (GO)/polyvinyl alcohol (SPG) sponge and control group.139
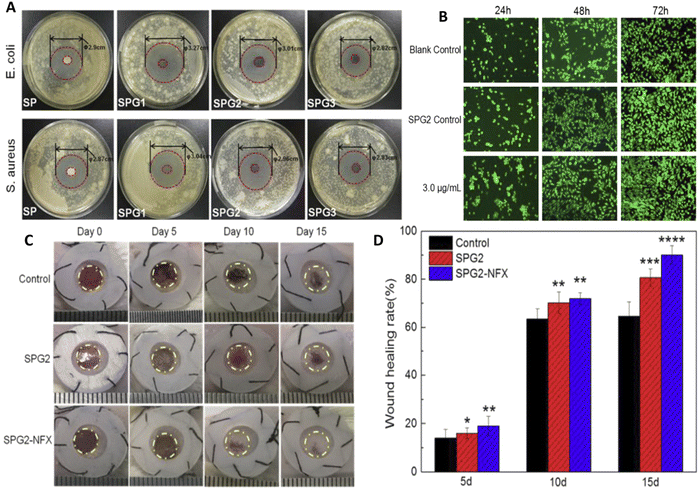 | ||
| Fig. 6 Preparation and characterization of nanocomposite sponges of sodium alginate/graphene oxide/polyvinyl alcohol (SA/GO/PVA) for wound healing applications. Different amounts of graphene oxide (GO) were used in the studies, and the sponges were referred to as SP, SPG1, SPG2, and SPG3 depending on the GO levels (as 0, 0.5, 1.0 and 2.0 wt% of total solids contents, respectively). (A) In vitro antibacterial activity of the samples against E. coli and S. aureus. (B) NIH 3T3 fibroblast growth cultured using extracts of blank control, SPG2, and SPG2-NFX with different NFX contents for 24, 48, 72 h. (C) Wound-healing image of mice exposed with different treatment. (D) Result of wound healing rate during time and after exposure to different formulation. Reprinted with permission from.139 Copyright 2019, Elsevier. | ||
Crosslinked alginate/chitosan nanocomposite sponges were developed using green synthesized carbon dots. Alginate was selected because of its highly water-absorbent, non-stick, cost-effective properties. Beside these, the anionic property of this polymer enables preparation of composites effectively with positively charged chitosan through electrostatic interaction. Carbon dots were selected in preparation of the BNCs; carbon dots less than 10 nm found applications in many areas due to their biocompatibility, hydrophilicity, low cytotoxicity, antibacterial properties, good mechanical properties as well as easy manufacturing. In the study, carbon dots were synthesized, and then used in the fabrication of nanocomposite sponges with chitosan and sodium alginate. The fabricated alginate/chitosan wound dressing showed many advantages like enhancing red blood cell and platelet formation and blood clot production. Also, ECM secretion and tissue granulation were supported by alginate/chitosan dressing. This dressing could absorb the exudate of the wound site, and its antibacterial property supports wound healing. Besides, the incorporation of carbon dots enhanced the porosity of nanocomposite sponge as well as its water absorption and transmission rate, hydrophilicity, and mechanical properties. The sponge transformed into a semi-swelling viscous colloid, potentially leading to capillary blockage. Cytocompatibility tests were carried out with L929 cells, and the results indicated no apparent cytotoxic effects from the nanocomposite sponge. Nanocomposite sponges found hemocompatible, and having hemostatic potential in increasing CD concentrations.140
A pH-sensitive magnetic starch-based nanocomposite hydrogel was introduced for wound healing applications.147 The composite hydrogel was synthesized by graft copolymerizing itaconic acid onto starch in the presence of Fe3O4 NPs. A model analgesic and anti-inflammatory drug, Guaifenesin, was loaded into the hydrogels, and pH and magnetic field-triggered release of the drug from hydrogels was studied. According to the results, the release of drug from hydrogel network was achieved in a controlled and pH-dependent manner. Guaifenesin release was accelerated by the increase of starch content. When an external magnetic field applied, the percentage of the drug release was significantly enhanced. Cytotoxicity studies showed that HUVEC cell viability was found to be above 88%. In vivo studies were carried using healthy male white mice, and wound healing was studied on a full-thickness circular wound model. The study was carried out for 20 days, and it was found that the wound of drug-loaded nanocomposite was healed after 10 days. Also, during the study wound area of nanocomposites were smaller compared to control studies, blank hydrogels, blank nanocomposites, and drug loaded hydrogels. It is assumed that strong adhesion of the starch-based nanocomposite material and the anti-inflammatory property of the drug could be the reason of fast closure of the wounds.147
A BC–GO composite film was recently developed using a biological blending self-growth method that naturally incorporated GO during the production of a bacterial cellulose film. This film was then functionalized with dopamine and silver, resulting in the generation of an Ag-pDA (rGO) composite film with good micro-current conductivity and heat generation following applied voltage. The composite film also exhibited excellent antibacterial activity against E. coli, with a bactericidal rate of over 84% for more than 72 h, indicating a prolonged antibacterial effect. Moreover, the Ag-pDA/BC (rGO) composite film demonstrated excellent biocompatibility according to in vitro cytotoxicity tests. In the live/dead staining test, BC-treated NIH3T3 cells exhibited almost completely green color because of the excellent biocompatibility of BC film alone. Nevertheless, BC(GO) and PDA/BC(GO) slightly inhibited the proliferation NIH3T3 cells (about 2% inhibition). On the surface of wound, presence of AgNPs and so release of Ag+ ions provided antibacterial and antifungal activity. Presence rGO in the structure of this composite provided photoelectric properties for this film that enable it to treat wound via accelerating migration of skin cells in different parts of wound that enhance the healing rate.153 Similarly, Khan et al., synthesized curcumin-loaded GO-functionalized-BC (GO-f-BC) hydrogel using hydrothermal method. BC and gelatin were chemically crosslinked by tetraethyl orthosilicate (TEOS) as a crosslinker. According to Franz diffusion test, the hydrogels with GO exhibited a slower drug delivery rate than the GO-free hydrogels, while displaying sufficient antibacterial efficacy due to the synergistic effect of the polymer and GO. GO could adhere to bacterial membranes through electrostatic interactions, while the polymeric part of the hydrogels interacted with bacterial phospholipids and lipopolysaccharides to hinder bacterial growth. Both of the hydrogels, with and without GO, exhibited a negligible hemolysis rate (less than 5%) and were found to be hemocompatible. The slight hemolysis observed in GO-included hydrogels was attributed to the twisting effect of GO, which may electrostatically interact with blood cells.154
Wan et al.,155 developed BNC hydrogels composed of BC and silver nanowires (AgNWs) through a step-by-step in situ biosynthesis process. It showed antibacterial activity against Gram-positive and Gram-negative bacteria via affecting cellular membrane of these bacteria. Interestingly, this hydrogel had a better effect on Gram-negative bacteria that was due to the protective effect of lipopolysaccharides, proteins, and lipids that are presented in the structure of Gram-negative. The BC/AgNW dressing with 38.4 wt% AgNWs showed superior expression of cytokeratin-10 and integrin-β4, higher keratinocytes proliferation and epithelial tissues formation, which has been shown to enhance skin regeneration in a mouse model of circular excisional full-thickness wounds.155 Another study by Gupta et al., utilized a green chemistry approach to produce silver NPs (AgNPs) using an aqueous solution of curcumin and hydroxypropyl-β-cyclodextrin (CUR:HPβCD). These AgNPs were then loaded into biosynthetic BC hydrogels to create hydrogel dressings. The CUR:HPβCD inclusion complex was formed to prevent the hydrophobicity of curcumin, a natural polyphenolic compound with wound-healing properties. The resulting BC hydrogels with CUR:HPβCD demonstrated high cytocompatibility and hemocompatibility, as well as antibacterial activity against three common wound-infecting pathogens: S. aureus, Pseudomonas aeruginosa, and Candida auris.156
3.2. Synthetic polymers
Crosslinked composite membranes were fabricated by combining PVA, chitosan-loaded AgNO3 NPs, and vitamin E via gamma irradiation. After exposing an aqueous solution of PVA to ionizing gamma radiation, free radicals were generated along the polymer chains due to hydrogen abstraction. The –OH and –NH2 groups on the chitosan backbone were potential sites for the reaction, and the formation of radical sites at oxygen and nitrogen atoms was dependent on a high amount of energy to break the O–H and N–H bonds. Hence, radical sites at oxygen and nitrogen atoms were generated by gamma irradiation. The copolymerization of PVA and chitosan radicals resulted in the formation of crosslinked (PVA/Cs) hydrogel membrane. The gelation of these membranes increased significantly with PVA composition, irradiation dose, and glycerol content up to 20%, whereas it decreased with the presence of AgNP due to increased viscosity. The swelling ratio decreased with increasing radiation dose and AgNP amount due to the effects of AgNP and reduced crosslinking degree of the membranes. These BNC membranes exhibited potent antimicrobial activity against Streptococcus mutans compared to other bacterial and fungal microorganisms, attributed to the presence of AgNPs via affecting the bacterial membrane as well as some of the bacterial enzymes and proteins.162
Song and colleagues prepared AgNPs-incorporated PVA/BC hydrogels as wound dressings using a freeze–thaw method. The microstructure of PVA/BC hydrogel exhibited uniform honeycomb pores with diameters of 1–2 μm, while the PVA/BC–Ag composite hydrogels showed more irregularly serrated and wedge-shaped structure with pore diameters of 3–5 μm, providing better oxygen exchange, moisture retention, and cell adhesion and growth. The mechanical properties of PVA/BC–Ag hydrogels were improved compared to PVA/BC. Moreover, the water absorption capacity of PVA/BC–Ag (approximately 1604.9 ± 58.2%) was higher than PVA/BC hydrogel due to larger pores and higher water-holding capacity of AgNP-incorporated hydrogels. Antibacterial tests demonstrated that PVA/BC–Ag showed remarkable antibacterial activity against both Gram-negative (E. coli) and Gram-positive (S. aureus) bacteria, with bactericidal rates of 99.72 ± 0.14% and 99.38 ± 0.48%, respectively. All hydrogels exhibited high biocompatibility on L929 mouse skin fibroblasts. Moreover, after 15 days, PVA/BC–Ag groups demonstrated more mature, flat, and thickened epidermis layers with more glands and blood vessels, so that the percentage of reducing wound size in group treated with PVA/BC–Ag3, PVA/BC, and control was about 97.89 ± 0.97, 61.13 ± 5.37%, 47.74 ± 4.13%, respectively.163
Zheng et al.,109 designed an electro-conductive hydrogel composed of β-cyclodextrin-embedded Ag NPs (CDAgNPs) containing PVA matrix incorporated free β-cyclodextrin (CD) and β-glucan grafted with hyaluronic acid (HAG) (PVA/CD/HAG/CDAgNP) (Fig. 7(A)). They applied electrical stimulation (ES) to induce tissue reparation. PVA/CD/HAG hydrogel showed low antibacterial efficacy against E. coli and S. aureus with a killing ratio below 15%, whereas PVA/CD/HAG with CDAgNP killed ≥99.99% of both bacterial strain within 1 and 4 h, respectively (Fig. 7(B)). In vitro fibroblast proliferation of PVA/CD/HAG and PVA/CD/HAG/CDAgNP was evaluated with and without ES of 100 mV mm−1 over 1 h per day. According to the CCK-8 assay, fibroblasts proliferated well on both materials after 5 days, in the absence of ES (p > 0.05). After 3 days, ES accelerated the cell proliferation with both materials, indicating a favorable effect of exogenous electric fields on the physiology and metabolism of fibroblasts. However, higher electroconductivity of PVA/CD/HAG/CDAgNP led to significantly higher cell growth upon ES than PVA/CD/HAG. PVA/CD/HAG/CDAgNP also promoted in vivo wound healing and hemostasis in the rat model (Fig. 7(C)). ES improved the healing process, and the PVA15/CD/HAG and PVA15/CD/HAG/CDAgNP groups led to complete skin injury recovery after 10 and 6 days, respectively. This was attributed to the higher electroconductivity of PVA15/CD/HAG/CDAgNP upon ES, which enables better signal transmission, controls cell activities, and accelerates healing. When ES was applied, more developed hair follicle and sebaceous gland systems, the highest volume of collagen fibers, and more efficient epidermal regeneration in the basal, spinous, and granular strata were achieved with the AgNP-containing nanocomposite hydrogel (Fig. 7(D)).109
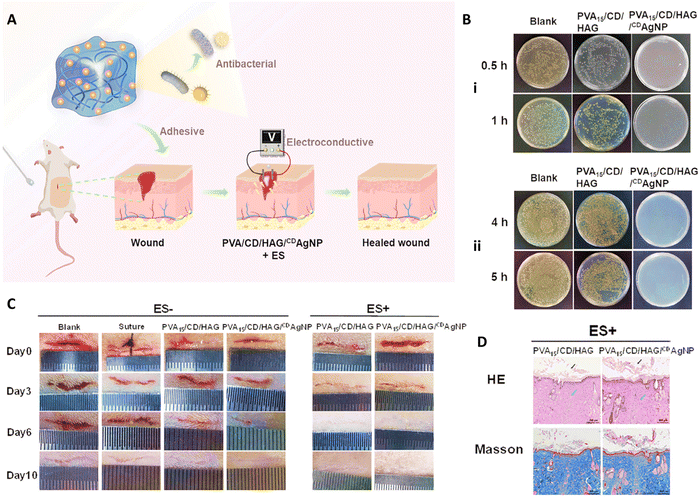 | ||
| Fig. 7 (A) Schematic image of combination use of PVA/CD/HAG/CDAgNP and electrical stimulation applied for healing wound. (B) Antibacterial activity of PVA15/CD/HAG/CDAgNP against (i) E. coli and (ii) S. aureus. (C) Wound healing results of different treatment in the presence and absence of ES for 10 days. (D) Histological results of wound after 10 days exposure to different samples. Reprinted with permission from.109 Copyright 2023, Wiley. | ||
Liu et al.,164 prepared mupirocin (MP) and/or cerium oxide NPs (CeNPs) incorporated PVA/CS nanofiber membrane wound dressing using electrostatic spinning (Fig. 8). The study reported the controlled release of MP from the PVA/CS nanofiber dressing, resulting in the fast and sustained antibacterial activity against both methicillin-sensitive Staphylococcus aureus (MSSA) and methicillin-resistant Staphylococcus aureus (MRSA) strains. In addition, the CeNPs displayed a slower release profile, providing favorable reactive oxygen species (ROS) scavenging efficiency and maintaining a normal physiological level of local ROS. All hydrogels exhibited high cell viability (>90%) after days 3 and 5 on L929 cells, with no significant difference in cell spreading area among the different hydrogels. The efficacy of PVA–CS, PVA–CS–CeNPs, PVA–CS–MP, and PVA–CS–CeNPs–MP electrospun dressings was evaluated in a diabetic wound rat model. PVA–CS–CeNPs–MP showed superior wound closure after 14 days (near 90%), followed by the PVA–CS–MP and PVA–CS–CeNPs groups (about 75% and 70%, respectively). Moreover, PVA–CS–CeNPs–MP demonstrated greater skin regeneration and neovascularization, as seen in Masson's trichrome staining.164
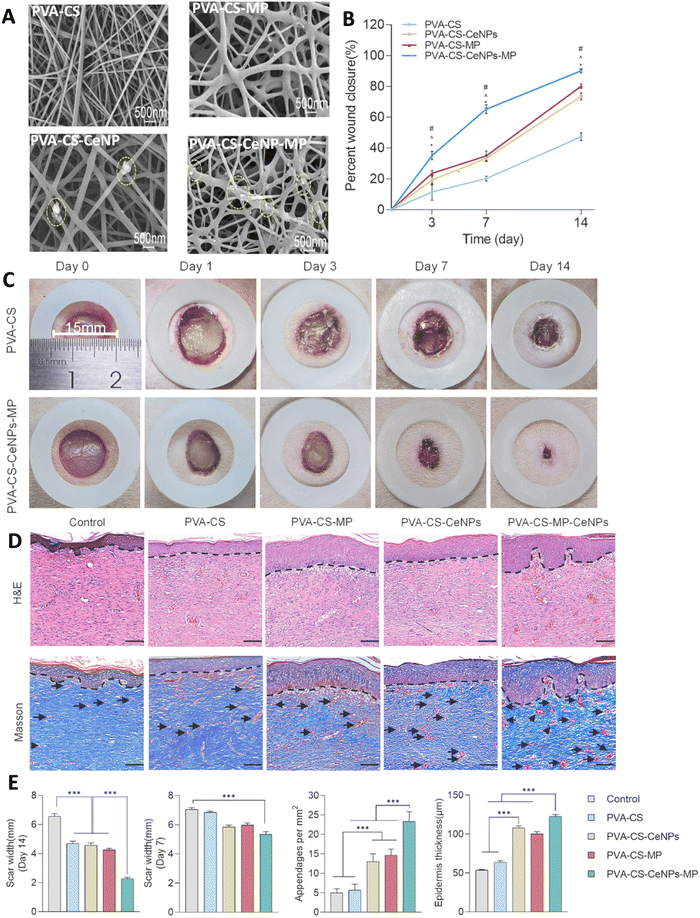 | ||
| Fig. 8 (A) The SEM image of the PVA–CS, PVA–CS–MP, PVA–CS–CeNPs, and PVA–CS–CeNPs–MP nanofiber dresses; circles show CeNPs. Quantitative analyses (B) and image (C) of wound closure on the wound bed in the CS–PVA, and CS–PVA–CeNPs–MP groups at the predefined time intervals. (D) Representative images of H&E and Masson's trichrome staining. Dashed black lines and black arrows show epidermis and the blood vessels, respectively. (E) The scar widths and blood vessels in the wound areas, and the thicknesses of the epidermal layers of healed wounds treated with the various PVA–CS-based membranes (*P < 0.05, ***P < 0.001). Reprinted with permission from.164 Copyright 2023, Elsevier. | ||
Electrospun scaffolds were prepared using PVA, gelatin, and ZnO NPs (0%, 0.5%, and 1%). The addition of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wt% ZnO to PVA/gelatin hydrogel slightly increased the water contact angle from 48.874° to 66.282°, but it remained hydrophilic (WCA < 90°). After 14 days of incubation in PBS, the water uptake capacity of PVA/gelatin, PVA/gelatin/ZnO 0.5
wt% ZnO to PVA/gelatin hydrogel slightly increased the water contact angle from 48.874° to 66.282°, but it remained hydrophilic (WCA < 90°). After 14 days of incubation in PBS, the water uptake capacity of PVA/gelatin, PVA/gelatin/ZnO 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wt%, and PVA/gelatin/ZnO 1
wt%, and PVA/gelatin/ZnO 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wt% were 2057%, 1485%, and 1291%, respectively. Mesenchymal stem cells adhered and spread well on PVA/gelatin/ZnO 1
wt% were 2057%, 1485%, and 1291%, respectively. Mesenchymal stem cells adhered and spread well on PVA/gelatin/ZnO 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wt% hydrogel. The survival rate was highest for the control group and PVA/gelatin scaffold possessed greater survival rate than scaffolds containing ZnO NPs. These nanocomposite scaffolds directly inhibited bacterial growth in a dose-dependent manner. PVA/gelatin without ZnO exhibited no inhibition zone, whereas the inhibition zone ranged from 5.5
wt% hydrogel. The survival rate was highest for the control group and PVA/gelatin scaffold possessed greater survival rate than scaffolds containing ZnO NPs. These nanocomposite scaffolds directly inhibited bacterial growth in a dose-dependent manner. PVA/gelatin without ZnO exhibited no inhibition zone, whereas the inhibition zone ranged from 5.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mm to 1
mm to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm with the presence of 0.5% and 1% ZnO. In here, ZnO NPs could disrupt bacterial membrane via electrostatic interactions with the cell wall as well as production of ROSs, and also exhibiting photocatalytic activity that led to producing ROSs and disturbing vital compounds of bacteria.165
cm with the presence of 0.5% and 1% ZnO. In here, ZnO NPs could disrupt bacterial membrane via electrostatic interactions with the cell wall as well as production of ROSs, and also exhibiting photocatalytic activity that led to producing ROSs and disturbing vital compounds of bacteria.165
Hybrid nanofibers were designed using PLA and keratin (K)/PVA with the incorporation of natural nanofibrillated chitosan (CHNF)/ZnO NPs (ZnONPs) (CSZ) as the nanofiller. To fabricate nanofibers, PLA solution from one syringe and K/PVA from another one with incorporation of CHNF, CSZ (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), (1
1), (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and (1
1) and (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) were electrospun and nanofibers were collected on the rotating collector. The diameter of the K/PVA nanofiber was around 380.48 ± 24 nm, whereas the presence of CHNF/ZnONPs (2
2) were electrospun and nanofibers were collected on the rotating collector. The diameter of the K/PVA nanofiber was around 380.48 ± 24 nm, whereas the presence of CHNF/ZnONPs (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), (1
1), (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and (1
1) and (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) resulted in the 376.34 ± 29 nm, 369.13 ± 33 nm and 363.68 ± 34 nm in nanofiber diameter, respectively. The recommended scaffold for wound healing was K/PVA
2) resulted in the 376.34 ± 29 nm, 369.13 ± 33 nm and 363.68 ± 34 nm in nanofiber diameter, respectively. The recommended scaffold for wound healing was K/PVA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CSZ (2
CSZ (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)–PLA
1)–PLA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CSZ (2
CSZ (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) nanofiber with a diameter of 352.50 ± 31 nm, a contact angle of 48 ± 3°, and a tensile strength of 0.96 ± 0.18 MPa. The addition of CHNF to the K/PVA/CS-PLA/CS nanofiber improved cell proliferation significantly than K/PVA-PLA nanofiber (P ≤ 0.05). The antibacterial activity of K/PVA
1) nanofiber with a diameter of 352.50 ± 31 nm, a contact angle of 48 ± 3°, and a tensile strength of 0.96 ± 0.18 MPa. The addition of CHNF to the K/PVA/CS-PLA/CS nanofiber improved cell proliferation significantly than K/PVA-PLA nanofiber (P ≤ 0.05). The antibacterial activity of K/PVA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CSZ (2
CSZ (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)–PLA
1)–PLA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CSZ (2
CSZ (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) against Gram positive and Gram negative bacteria was greater (with inhibition zones of about 33.13 ± 0.67 mm and 25.06 ± 0.24 mm against S. aureus and E. coli bacteria, respectively) than other scaffolds due to the effect of CHNF and ZnONPs.173
1) against Gram positive and Gram negative bacteria was greater (with inhibition zones of about 33.13 ± 0.67 mm and 25.06 ± 0.24 mm against S. aureus and E. coli bacteria, respectively) than other scaffolds due to the effect of CHNF and ZnONPs.173
Huang et al.,178 incorporated ZnO NPs with PLGA/SF nanofibers for sustained release of NPs on the wound area. Based on SEM images, the nanofibers were generally smooth, and the fiber diameters increased with the increasing ZnO amounts. PLGA/SF nanofibers with ZnO concentration of 0%, 1%, 2%, and 3% showed a stress of 3.21, 4.43, 4.93, and 7.5 MPa, and strain as 102.63, 46.31, 30.56, and 35.7%, respectively. According to CCK8 assay on L929 cells, all membranes were biocompatible, but cell proliferation slightly decreased with increasing ZnO concentration. The nanofibers exhibited mild antioxidant activity which was significantly increased with increasing time. The antioxidant activity is mainly attributed to the amino acids and phenolic side chain of SF. The BNC nanofibers showed antibacterial activity against E. coli and S. aureus dependent on ZnO concentration. In the rat model, PLGA/SF nanofibers with 3% ZnO facilitated earlier wound closure and provided superior re-epithelialization, granulation tissue formation, collagen deposition, and angiogenesis compared to PLGA/SF nanofibers without ZnO (about 87% wound closer in the case of PLGA/SF nanofibers with 3% ZnO compared with about 71% and 66% closer in the case of PLGA/SF nanofibers and control groups, respectively).178
A bifunctional composite scaffold was fabricated consisting of black phosphorus nanosheets (BPNSs) containing collagen sponge with a PLGA knitted mesh (PLGA–collagen–BPNS) for the treatment of melanoma and skin regeneration. BPNSs demonstrated the ability to initiate photothermal ablation of melanoma cells both in vitro and in vivo by increasing temperature under near-infrared laser irradiation. The scaffold also accelerated fibroblast proliferation and enhanced the expression of angiogenesis-related genes and genes of ECM components for new skin formation. The porosity and nanostructure of these nanocomposite meshes supported cellular adhesion, migration, proliferation, and cell–cell interactions, while biodegradable components of the BPNSs promoted dermal fibroblast proliferation, angiogenesis, and further skin wound healing.179
Kouser et al.,185 utilized solution casting technique to functionalize the surface of halloysite nanotubes (HNTs) with sodium alginate and reinforce them with PCL, resulting in nanocomposite structures with increased thermal stability, mechanical strength, and crystallinity with an increasing amount of nanofiller. The nanocomposite films showed high cytocompatibility with NIH3T3 mouse fibroblast cells through cell proliferation and adhesion. The PCL film with 5% sodium alginate–HNTs demonstrated the highest cell proliferation, which exhibited a concentration-dependent trend. This was mainly due to the fact that HNTs have a negative charge on their surface and a positive charge in their inner lumen, resulting in significant surface functionalization. HNTs also improved cell adhesion and exhibited superior anti-inflammatory activity compared to pristine PCL films. The scratch assay confirmed the wound closure effect of the nanocomposite films on the rate of cell migration, where 5% nanocomposite films demonstrated the highest migration, indicating enhanced wound closure. The addition of sodium alginate and HNTs in the PCL matrix significantly accelerated cell migration in a concentration-dependent manner. The in vitro anti-inflammatory activity of the nanocomposite films was analyzed using human red blood corpuscles membrane stabilization (HRBCMS) method, which showed superior anti-inflammatory activity for nanocomposite compared to pristine PCL films. Based on these results, the nanocomposite films were hemocompatible and suitable for wound healing applications.185 Some of the other samples are summarized in Table 1.
| Polymer | Nanostructure | Preparation/method | Cells/microorganisms | Application | Main findings | Ref. |
|---|---|---|---|---|---|---|
| Silk fibroin/poly(ε-caprolactone) | Nanofibrous scaffold | Custom-designed cold plate electrospinning and automatic magnet agitation system. | NIH 3T3 fibroblasts | Artificial dermis | – The rate of healing wounds and the deposition of collagen were found to enhance proportionally with the inclusion of silk fibroin in the formulation. | 186 |
| – Desirable spatial cues, surface topography, and surface chemistry for the native cells to infiltrate into the scaffolds. | ||||||
| – The wound healing capability of the nanofibrous scaffold is comparable to that of the commercially available Matriderm® artificial dermis. | ||||||
| Silk fibroin/poly(glycerol sebacate)/poly(ε-caprolactone) poly(ε-caprolactone) | Electrospun fiber mat | The nozzle-free electrospinning technique | Human dermal fibroblasts | Artificial skin-like platform | — Favorable in vitro fibroblast attachment patterns and optimal growth | 187 |
| — A novel promising platform for advancing the development of skin substitute products. | ||||||
| Collagen/silk fibroin | TiO2 NPs | Membranes/freeze drying | – S. aureus and E. coli. | Skin tissue regeneration | – Antibacterial behavior increased with increasing concentration of TiO2. | 188 |
| – NIH3T3 fibroblast cells | – Good biocompatibility and cell proliferation on fibroblasts. | |||||
| Melatonin-loaded collagen/aminated xanthan gum | Bio-capped AgNPs | Bio-hybrid hydrogel scaffold/freeze drying | – Escherichia coli, Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa and multidrug-resistant (MDR) Klebsiella pneumoniae | – Accelerated tissue regeneration in skin defects | – Rapid tissue regeneration, collagen accumulation and angiogenesis at the wound site predominantly due to the synergistic effect of AgNPs and melatonin. | 189 |
| – NIH 3T3 fibroblast cells | – Effective care for various ailments, including infected chronic wounds. | |||||
| Gelatin/hyaluronic acid/polycaprolactone | Hydroxyapatite/atorvastatin-loaded nanostructured lipid carriers | Membranes | Human dermal fibroblasts | Skin tissue engineering | – Mechanical stability (approximately 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MPa elastic modulus) more than two-fold of tensile modulus of normal human skin. MPa elastic modulus) more than two-fold of tensile modulus of normal human skin. |
190 |
– Degraded up to 98% after 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) days days |
||||||
– Drug release efficiency was in a range of 75–79% for 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) days days |
||||||
| Chitosan | Halloysite Nanotubes | Pour powder | – Normal human dermal fibroblasts (NHDF) from juvenile foreskin | Chronic wound healing | – Better skin reepithelization and reorganization than halloysite nanotubes or chitosan oligosaccharide separately. | 191 |
| – Murine model | – Presence of granulation tissue after treatment | |||||
| – Overall skin structure was recovered after 18 days | ||||||
| Chitosan, poly(N-vinylpyrrolidone) | Titanium dioxide (TiO2) NPs | Films | – NIH3T3 and L929 fibroblast cells | Wound healing | – Addition of NPs increases the strength of the composite | 192 |
| – Albino rat model | – Antimicrobial efficacy and good biocompatibility in cell culture studies | |||||
| – Pathogenic bacteria (a loopful of bacterial strains) | – Accelerated healing of open excision type wounds in albino rat model compared to gauze, ointment and chitosan treated groups | |||||
| Chitin | Nano ZnO | Micro-porous composite bandages | – Human dermal fibroblast | Wound Healing | – Enhanced swelling, blood clotting and antibacterial activity | 193 |
| – Cells attached and penetrated into the interior of the bandages | ||||||
| Alginate | Pitavastatin nanovesicles | Hydrogel | – Mongrel dogs | Wound Healing | – High water uptake | 194 |
| – Sustained drug release up to 7 days | ||||||
| – Promoting wound healing and wound closure | ||||||
| – Fast and proper rejuvenation of damaged skin layers compared to plain hydrogel and drug suspension | ||||||
| Alginate | Eudragit NPs containing edaravone | Hydrogel | – Diabetic mice model | Chronic wound healing | – Protection and sustained the release of edaravone was achieved. | 195 |
| – Promotion of wound healing in a dose-dependent way. | ||||||
| Polyvinylidene fluoride and starch mats | Ciprofloxacin loaded on TiO2 NPs | Electrospun mats | – Escherichia coli and Staphylococcus aureus | Wound healing | – Fiber diameters thickened by increasing starch | 196 |
| – L929 mouse fibroblast cell line | – Sustained drug release and antibacterial activity were achieved | |||||
| – Dressings found to be biocompatible | ||||||
| Bacterial cellulose | Graphene quantum dots | Hydrogel | – Staphylococcus aureus, Streptococcus agalactiae, Methicillin-resistant Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa. | Wound healing | – Approximately 11.7 wt% of GQDs loaded in the BC | 197 |
| – Human fibroblasts | – High water uptake | |||||
| – Dressings exhibited significant inhibition S. aureus and S. agalactiae and bactericidal effect on Methicillin-resistant S. aureus, E. coli, and P. aeruginosa. | ||||||
| – Upregulated expression of endothelial nitric oxide synthase, vascular endothelial growth factor A, matrix metallopeptidase 9, and Vimentin gene in fibroblasts were achieved, enhancing angiogenesis. | ||||||
| PVA | Copper oxide, ZnO, and Au NPs | Film | – E. coli and S. aureus | Wound healing | – An antibacterial efficacy of 98.7% against E. coli and 97.5% against S. aureus was achieved under red laser irradiation, with photothermal and photodynamic effect, and released Zn2+ and Cu2+. | 198 |
| – Mouse embryonic fibroblast (NIH 3T3) cells | – Fast healing with minimum scarring was shown with film dressings in full-thickness circular rat wound model. | |||||
| PCL | Quercetin loaded graphene oxide NPs | Nanofiber | – S. aureus | Wound healing | – NIH/3 T3 fibroblast cells showed 95% viability on scaffolds | 199 |
| – NIH/3 T3 fibroblast cells | – Cell attachment and proliferation on scaffolds was observed by FESEM | |||||
| PLGA | Silver NPs | Nanofiber | – K. pneumonia, E. coli, and P. aeruginosa and S. saprophyticus | Wound healing | – Fiber diameters ranged between 487 and 781 nm. | 200 |
| – Human dermal fibroblast | – Integration of AgNPs within the fibers was accelerated the mechanical properties comparable to the mechanical properties of the human skin. | |||||
| – Nanofibers demonstrated minimal cytotoxicity. | ||||||
| – Antimicrobial activity was shown using three different strains of Gram-positive and Gram-negative bacteria. | ||||||
| PLA, PCL | ZnO NPs | Bilayer nanofiber Capparis spinosa L. extract loaded PLA/ZnO loaded PCL | – Staphylococcus aureus and Escherichia coli | Wound healing | – Bilayer nanofiber membranes showed satisfactory wettability with water contact angle of 130° for outside layer, 72.4° for inner layer. | 201 |
| – L929 cells (mouse fibroblast cell line) | – Nanofibers was not break until reaching 10.69 MPa, much higher than the tensile strengths of the individual layers. | |||||
| – Bilayer nanofiber membranes significantly prevented bacterial growth. | ||||||
| – They highly promoted cell growth. |
Natural polymers, such as alginate, hyaluronic acid, chitosan, collagen, gelatin, and silk fibroin, possess unique features that make them effective in enhancing tissue healing due to their similarities to native skin. These polymers offer specific protein-binding sites in their backbone, which facilitates cellular interaction and promotes cellular migration, adhesion, proliferation, and differentiation. In addition, their high biocompatibility, biodegradability, unique texture, and porosity further enhance cellular interactions and re-epithelialization. However, natural polymers also have some drawbacks, such as insufficient mechanical properties, batch-to-batch variations, and the risk of immune responses. In contrast, synthetic polymer-based materials can be designed to meet the structural and mechanical requirements of native ECM. However, the main limitation of using synthetic polymers is the absence of bioactive sites for cellular interactions. To address these issues, a crucial strategy is to use both natural and synthetic polymers with the incorporation of nanosized materials to create multifunctional materials that overcome the shortcomings of both natural and synthetic polymers.
4. Challenges and future prospects
Despite their advantages, BNCs have some limitations that could affect their applications. Controllable blending and stabilization of dispersion are essential to produce BNCs. A primary challenge encountered during the processing of these composites is NPs tendency to aggregation, which is attributed to the unique combination of specific surface area and volume leading to inadequate scattering within the selected formulations. Moreover, increasing the amount of nanofiller leads to a reduction in the toughness and strength of the BNC, resulting in production of a more brittle material. In addition, the viscosities of BNCs also elevate with increasing the nanofiller content, posing difficulties in the manufacturing process. Therefore, it is critical to optimize the properties of NPs and its amounts first, and then use it for the preparation of hydrogels. For instance, NPs could be covered with some polymers to prevent their aggregation, first, and then be applied in the structure of the BNC. Or some techniques like agitation or sonication could be applied for enhancing their dispersion during preparation step. The important points that should be considered here is that these modification and techniques should not have any effect on the properties of the NPs or nanocomposite.The other challenge is related to the long-term safety of nanomaterials used in the structure of BNCs. Indeed, several types of nanomaterials are widely used for fabrication of different types of BNCs in recent research that could impact cell viability and tissue interactions. Achieving a delicate balance between promoting wound healing and avoiding adverse effects requires extensive research to understand the intricate dynamics of these materials within the biological environment. Overcoming these biocompatibility challenges is imperative to ensure the safety and efficacy of BNCs in wound healing applications and so substantial efforts are needed to generate new knowledge about their impact on human health before establishing regulatory frameworks in this field.202
In the case of natural polymers, the variability in their source, probability of contamination, lack of proper mechanical stability, uncontrollable degradation rate, and difficulty in processing are challenges that could affect the performance of BNCs.203 To overcome these limitations, it is critical to introduce and use standard methods for the extraction and purification of natural polymers to ensure their quality and reduce any possibility of contamination. Moreover, cross linking or combination use these polymers with other compounds could enhance their mechanical and degradation properties. On the other hand, synthetic polymers have challenges in their cost, environmental impact, and toxicity, that could be related to their byproduct. These also make it important to develop new cost-effective methods for producing these polymers and use of non-toxic monomers to eliminate the probability of producing toxic byproducts. Besides, these polymers could be combined and modified with other materials to reducing their effect on environment as well as modifying their degradation ability.
In the case of a complete BNCs, one of the main challenges is related to tuning their mechanical properties of BNCs due to their effectiveness in wound healing process. Indeed, skin, like other tissues in the body, have its specific mechanical properties such as elasticity, tensile strength, and stiffness and the BNCs used in wound healing need to match these properties to ensure proper integration and functionality. It should maintain the structural integrity of wound dressings during application and throughout the healing process. If the material is too weak, it may break down prematurely and compromising the healing environment. It also could ensure that the dressing is not too stiff or too loose, enhancing patient comfort. Moreover, tuning the mechanical properties could affects the degradation profile, ensuring the material provides support as long as needed and then safely degrades without causing harm. Mechanical property of BNCs also can influence cell behavior, including attachment, proliferation, and differentiation. Optimal mechanical properties can create a conducive environment for cells to grow and heal the wound effectively.
Scalability and cost-effectiveness pose challenges for the widespread adoption of BNCs in wound care. The production of these materials often involves sophisticated processes and specialized equipment, leading to higher production costs. This limits their accessibility, especially in resource-constrained environments, where cost-effective solutions are essential. Addressing scalability issues and finding economically viable production methods are crucial steps in enhancing the practical utility of BNCs for wound healing on a larger scale. However, due to the possibility of using diverse materials for the fabrication of BNCs, they could be a good candidate for the fabrication of scaffolds that have the capability of mimicking the ECM of skin resembling the natural microenvironment, fostering cell adhesion, proliferation, and differentiation. In addition, the incorporation of bioactive NPs can further enhance the regenerative potential by promoting angiogenesis and modulating immune responses. This personalized approach holds the promise of accelerating wound healing and minimizing scarring, ultimately leading to more effective and aesthetically pleasing outcomes for patients undergoing skin regeneration procedures. They could be fabricated using intelligent materials with the ability of responding to the specific physiological triggers, enabling dynamic interactions with the surrounding tissue. Smart BNCs may incorporate sensors for real-time monitoring of the healing process, allowing for timely adjustments to the therapeutic strategy. Moreover, the controlled release of growth factors or drugs from these materials can be precisely regulated, optimizing the healing trajectory, and minimizing potential side effects. On the other hand, there is a growing emphasis on sustainability in materials selection. Researchers are increasingly exploring the integration of eco-friendly and renewable resources in the fabrication of these advanced bionanomaterials. The shift towards sustainable materials not only aligns with global environmental goals but also addresses concerns related to the biocompatibility and long-term effects of synthetic polymers. Utilizing biodegradable and naturally sourced components for BNCs not only reduces the environmental impact but also ensures that the materials are compatible with the body's natural processes. By incorporating sustainable elements, the future of skin regeneration technologies aims not only to promote tissue healing but also to contribute to a more environmentally conscious and ethical approach in the development of next-generation biomedical solutions.204–206
As research in BNCs progresses, the convergence of nanotechnology and regenerative medicine holds immense potential for transforming the landscape of skin repair and regeneration. Designing BNCs that closely mimic the natural extracellular matrix is crucial for effective tissue regeneration. The composition, structure, and bioactive cues of the BNCs must be carefully tailored to promote cell adhesion, migration, and differentiation. Controlling the degradation rate of BNCs is critical to match the healing timeline of the injured tissue. An excessively slow degradation rate may hinder tissue regeneration, while rapid degradation can compromise the scaffold's ability to provide mechanical support. Notably, clinical translation studies provide valuable insights into the real-world performance of BNCs, helping to validate their effectiveness and safety. Conducting rigorous preclinical and clinical studies, addressing ethical considerations, and ensuring affordability are crucial for successful clinical translation. These studies also contribute to optimizing product formulation, dosing, and delivery methods.
Another crucial challenge that needs to be addressed is regulatory approval procedures. Understanding the regulatory landscape for BNCs in wound healing applications involves a precise, multifaceted approval process to ensure safety and efficacy. This process is overseen by key regulatory bodies, such as the FDA (dood and drug administration) and EMA (European medicines agency). The journey begins with extensive preclinical testing, including cytotoxicity, hemocompatibility, and animal studies, to assess biocompatibility, toxicity, and therapeutic potential. Successful preclinical outcomes pave the way for multi-phase clinical trials to evaluate safety, dosage, efficacy, and side effects in progressively larger patient cohorts. Adhering to good manufacturing practices (GMP) and precise quality control is critical, involving comprehensive documentation and consistent batch testing. The conclusion of this data in a detailed regulatory report, encompassing technical files, clinical results, and risk management analyses, is submitted for approval. Post-market surveillance ensures long-term safety and efficacy through adverse event reporting and periodic safety updates. By precisely addressing these regulatory challenges, developers can advance BNCs from lab to clinic, offering innovative, safe, and effective solutions for wound healing and skin regeneration.
5. Conclusion
In this review, we have discussed the application of different types of BNCs for wound healing and skin regeneration. In this context, first different phases of wound healing were discussed; then, BNCs based on the type of their biopolymer were introduced. They mostly composed of a biopolymeric hydrogel used alone or in combination with other polymers that were applied to improve the physicochemical properties of the hydrogel, and a nanomaterial with intrinsic antimicrobial or antioxidant ability that prevented the infections in the site of wound and accelerated healing process via managing inflammatory phase. These composites also accelerated the healing process of wound in comparison to their control groups via enhancing re-epithelialization, promoting tissue granulation and collagen deposition, and inducing neovascularization that finally led to the tissue regeneration. However, like other materials, these substances face several challenges. Among the most significant concerns are those related to the application of nanomaterials and scalability issues, that make it crucial to conduct more research to fully understand these materials and their applications. Overall, BNCs offer exciting prospects in advanced wound healing and smart wound dressings. These materials, formed by combining biopolymers and NPs, possess unique properties that can develop wound healing and smart wound dressings. They can be used in the development of smart dressings with sensors and drug delivery systems, enabling real-time monitoring and targeted treatment.Conflicts of interest
Authors declare no conflict of interest.References
- J. Bramhill, S. Ross and G. Ross, Int. J. Environ. Res. Public Health, 2017, 14, 66 CrossRef PubMed.
- L. Suamte, A. Tirkey and P. J. Babu, Smart Mater. Med., 2023, 4, 243–256 CrossRef.
- A. Das, T. Ringu, S. Ghosh and N. Pramanik, Polym. Bull., 2023, 80, 7247–7312 CrossRef CAS PubMed.
- M. Jain and K. Matsumura, J. Biomed. Mater. Res., Part A, 2016, 104, 1379–1386 CrossRef CAS PubMed.
- L. Moretti, J. Stalfort, T. H. Barker and D. Abebayehu, J. Biol. Chem., 2022, 298, 101530 CrossRef CAS PubMed.
- H. H. C. de Lima, V. L. Kupfer, M. P. Moisés, M. R. Guilherme, J. D. Rinaldi, S. L. Felisbino, A. F. Rubira and A. W. Rinaldi, Carbohydr. Polym., 2018, 196, 126–134 CrossRef CAS PubMed.
- M. Rahim, M. R. H. Mas Haris and N. U. Saqib, Biophys. Rev., 2020, 12, 1223–1231 CrossRef PubMed.
- N. Kučuk, M. Primožič, Ž. Knez and M. Leitgeb, Int. J. Mol. Sci., 2023, 24, 3188 CrossRef PubMed.
- M. Alavi and A. Nokhodchi, Carbohydr. Polym., 2020, 227, 115349 CrossRef CAS PubMed.
- Z. Zhang, J. Ma, T. Xu, T. Wang, X. Jia, J. Lin, C. Lv, L. Cao, Y. Ying, L. Ji, S. Wang and C. Fu, Adv. Healthcare Mater., 2024, 2401005 CrossRef PubMed.
- B. Xu, A. Li, R. Wang, J. Zhang, Y. Ding, D. Pan and Z. Shen, Adv. Funct. Mater., 2021, 31, 2105265 CrossRef CAS.
- J. Liu, M. Cao, L. Li, X. Xu, J. Zheng, W. Yao and X. Hou, Giant, 2022, 10, 100100 CrossRef.
- Z. Zhang, J. Ma, T. Xu, T. Wang, X. Jia, J. Lin, C. Lv, L. Cao, Y. Ying, L. Ji, S. Wang and C. Fu, Adv. Healthcare Mater., 2024, 2401005 CrossRef PubMed.
- A. M. Diez-Pascual, Polymers, 2021, 9, 260 CrossRef PubMed.
- M. Râpă, L. M. Stefan, T. Zaharescu, A.-M. Seciu, A. A. Turcanu, E. Matei, A. M. Predescu, I. Antoniac and C. Predescu, Appl. Sci., 2020, 10, 2265 CrossRef.
- S. Sharma, A. Malik and P. Gupta, in Bionanocomposites in Tissue Engineering and Regenerative Medicine, ed. S. Ahmed and Annu, Woodhead Publishing, 2021, pp. 507–532 DOI:10.1016/B978-0-12-821280-6.00021-0.
- M. R. Alam, S. Alimuzzaman, M. A. Shahid, F.-E. Karim and M. E. Hoque, J. Biomater. Sci., Polym. Ed., 2023, 34, 1517–1538 CrossRef CAS PubMed.
- C. Oliveira, D. Sousa, J. A. Teixeira, P. Ferreira-Santos and C. M. Botelho, Front. Bioeng. Biotechnol., 2023, 11, 1136077 CrossRef PubMed.
- C. Belda Marín, V. Fitzpatrick, D. L. Kaplan, J. Landoulsi, E. Guénin and C. Egles, Front. Chem., 2020, 8, 604398 CrossRef PubMed.
- M. A. Shalaby, M. M. Anwar and H. Saeed, J. Polym. Res., 2022, 29, 91 CrossRef CAS.
- P. Du, X. Chen, Y. Chen, J. Li, Y. Lu, X. Li, K. Hu, J. Chen and G. Lv, Heliyon, 2023, 9, e13506 CrossRef CAS PubMed.
- J. Liu, X. Xie, T. Wang, H. Chen, Y. Fu, X. Cheng, J. Wu, G. Li, C. Liu and H. Liimatainen, ACS Appl. Mater. Interfaces, 2023, 15, 12696–12707 CrossRef CAS PubMed.
- P. Feng, Y. Luo, C. Ke, H. Qiu, W. Wang, Y. Zhu, R. Hou, L. Xu and S. Wu, Front. Bioeng. Biotechnol., 2021, 9, 650598 CrossRef PubMed.
- H. L. Loo, B. H. Goh, L.-H. Lee and L. H. Chuah, Asian J. Pharm. Sci., 2022, 17, 299–332 CrossRef PubMed.
- F. Leone, M. Firlak, K. Challen, W. Bonnefin, B. Onida, K. L. Wright and J. G. Hardy, J. Funct. Biomater., 2019, 10, 50 CrossRef CAS PubMed.
- H. Moustafa, H. E. Nasr and A. M. Youssef, Biomass Convers. Biorefin., 2022, 1–13, DOI:10.1007/s13399-022-03403-2.
- M.-B. Coltelli, L. Aliotta, A. Vannozzi, P. Morganti, L. Panariello, S. Danti, S. Neri, C. Fernandez-Avila, A. Fusco, G. Donnarumma and A. Lazzeri, J. Funct. Biomater., 2020, 11, 21 CrossRef CAS PubMed.
- M. Rodrigues, N. Kosaric, C. A. Bonham and G. C. Gurtner, Physiol. Rev., 2019, 99, 665–706 CrossRef CAS PubMed.
- N. X. Landén, D. Li and M. Ståhle, Cell. Mol. Life Sci., 2016, 73, 3861–3885 CrossRef PubMed.
- H. E. desJardins-Park, S. Mascharak, M. S. Chinta, D. C. Wan and M. T. Longaker, Front. Physiol., 2019, 10, 322 CrossRef PubMed.
- A. Kowalczyk, P. Kleniewska, M. Kolodziejczyk, B. Skibska and A. Goraca, Arch. Immunol. Ther. Exp., 2015, 63, 41–52 CrossRef CAS PubMed.
- A. Scridon, Int. J. Mol. Sci., 2022, 23, 12772 CrossRef CAS PubMed.
- B. P. Nuyttens, T. Thijs, H. Deckmyn and K. Broos, Thromb. Res., 2011, 127, S26–S29 CrossRef CAS PubMed.
- M. E. Quach, W. Chen, Y. Wang, H. Deckmyn, F. Lanza, B. Nieswandt and R. Li, J. Thromb. Haemostasis, 2021, 19, 2044–2055 Search PubMed.
- G. S. Schultz, G. A. Chin, L. Moldawer and R. F. Diegelmann, Mechanisms of vascular disease: a reference book for vascular specialists, 2011, p.423 Search PubMed.
- P. Martin, Science, 1997, 276, 75–81 CrossRef CAS PubMed.
- H. N. Wilkinson and M. J. Hardman, Open Biol., 2020, 10, 200223 CrossRef CAS PubMed.
- T. Velnar, T. Bailey and V. Smrkolj, J. Int. Med. Res., 2009, 37, 1528–1542 CrossRef CAS PubMed.
- J. S. Roh and D. H. Sohn, Immune Netw., 2018, 18, e27 CrossRef PubMed.
- K. Ashina, Y. Tsubosaka, T. Nakamura, K. Omori, K. Kobayashi, M. Hori, H. Ozaki and T. Murata, PLoS One, 2015, 10, e0132367 CrossRef PubMed.
- S. Čejková, I. Králová-Lesná and R. Poledne, Cor et Vasa, 2016, 58, e419–e425 CrossRef.
- T. Yukami, M. Hasegawa, Y. Matsushita, T. Fujita, T. Matsushita, M. Horikawa, K. Komura, K. Yanaba, Y. Hamaguchi and T. Nagaoka, J. Leukoc. Biol., 2007, 82, 519–531 CrossRef CAS PubMed.
- M. Subramaniam, S. Saffaripour, L. Van De Water, P. S. Frenette, T. N. Mayadas, R. O. Hynes and D. D. Wagner, Am. J. Pathol., 1997, 150, 1701 CAS.
- M. Metzemaekers, M. Gouwy and P. Proost, Cell. Mol. Immunol., 2020, 17, 433–450 CrossRef CAS PubMed.
- S. A. Eming, P. Martin and M. Tomic-Canic, Sci. Transl. Med., 2014, 6, 265sr266 Search PubMed.
- T. Liu, L. Zhang, D. Joo and S.-C. Sun, Signal Transduction Targeted Ther., 2017, 2, 1–9 Search PubMed.
- J. G. Filep and A. Ariel, Am. J. Physiol.: Cell Physiol., 2020, 319, C510–C532 CrossRef PubMed.
- M. T. Masucci, M. Minopoli, S. Del Vecchio and M. V. Carriero, Front. Immunol., 2020, 11, 1749 CrossRef CAS PubMed.
- F. H. Pilsczek, D. Salina, K. K. Poon, C. Fahey, B. G. Yipp, C. D. Sibley, S. M. Robbins, F. H. Green, M. G. Surette and M. Sugai, J. Immunol., 2010, 185, 7413–7425 CrossRef CAS PubMed.
- Y. Ge, M. Huang and Y.-M. Yao, Front. Cell Dev. Biol., 2022, 10, 839248 CrossRef PubMed.
- A. Hassanshahi, M. Moradzad, S. Ghalamkari, M. Fadaei, A. J. Cowin and M. Hassanshahi, Cells, 2022, 11, 2953 CrossRef CAS PubMed.
- B. A. Shook, R. R. Wasko, G. C. Rivera-Gonzalez, E. Salazar-Gatzimas, F. López-Giráldez, B. C. Dash, A. R. Muñoz-Rojas, K. D. Aultman, R. K. Zwick and V. Lei, Science, 2018, 362, eaar2971 CrossRef PubMed.
- P. Olczyk, Ł. Mencner and K. Komosinska-Vassev, BioMed Res. Int., 2014, 2014, 1–8 Search PubMed.
- R. Goldman, Adv. Skin Wound Care, 2004, 17, 24–35 CrossRef PubMed.
- I. A. Darby, B. Laverdet, F. Bonté and A. Desmoulière, Clin., Cosmet. Invest. Dermatol., 2014, 301–311 Search PubMed.
- F. Cialdai, C. Risaliti and M. Monici, Front. Bioeng. Biotechnol., 2022, 10, 958381 CrossRef PubMed.
- X. Li, Y. Yang, B. Zhang, X. Lin, X. Fu, Y. An, Y. Zou, J.-X. Wang, Z. Wang and T. Yu, Signal Transduction Targeted Ther., 2022, 7, 305 CrossRef CAS PubMed.
- P. Bao, A. Kodra, M. Tomic-Canic, M. S. Golinko, H. P. Ehrlich and H. Brem, J. Surg. Res., 2009, 153, 347–358 CrossRef CAS PubMed.
- G.-H. Fong, J. Mol. Med., 2009, 87, 549–560 CrossRef CAS PubMed.
- S. Ramakrishnan, V. Anand and S. Roy, J. Neuroimmune Pharmacol., 2014, 9, 142–160 CrossRef CAS PubMed.
- A. Weidemann and R. Johnson, Cell Death Differ., 2008, 15, 621–627 CrossRef CAS PubMed.
- M. L. Usui, J. N. Mansbridge, W. G. Carter, M. Fujita and J. E. Olerud, J. Histochem. Cytochem., 2008, 56, 687–696 CrossRef CAS PubMed.
- I. Pastar, O. Stojadinovic, N. C. Yin, H. Ramirez, A. G. Nusbaum, A. Sawaya, S. B. Patel, L. Khalid, R. R. Isseroff and M. Tomic-Canic, Adv. Wound Care, 2014, 3, 445–464 CrossRef PubMed.
- N. Akombaetwa, A. Bwanga, P. A. Makoni and B. A. Witika, Polymers, 2022, 14, 2931 CrossRef CAS PubMed.
- A. C. d O. Gonzalez, T. F. Costa, Z. d A. Andrade and A. R. A. P. Medrado, An. Bras. Dermatol., 2016, 91, 614–620 CrossRef PubMed.
- F. V. Borbolla-Jiménez, S. I. Peña-Corona, S. J. Farah, M. T. Jiménez-Valdés, E. Pineda-Pérez, A. Romero-Montero, M. L. Del Prado-Audelo, S. A. Bernal-Chávez, J. J. Magaña and G. Leyva-Gómez, Pharmaceutics, 2023, 15, 1914 CrossRef PubMed.
- D. Singh, V. Rai and D. K. Agrawal, Cardiol. Cardiovasc. Med., 2023, 7, 5 Search PubMed.
- R. B. Diller and A. J. Tabor, Biomimetics, 2022, 7, 87 CrossRef CAS PubMed.
- A. E. Rivera and J. M. Spencer, Clin. Dermatol., 2007, 25, 39–48 CrossRef PubMed.
- H. A. Wallace, B. M. Basehore and P. M. Zito, Wound Healing Phases, StatPearls Publishing, Treasure Island (FL), 2019 Search PubMed.
- P. Aramwit, Introduction to biomaterials for wound healing, Elsevier Ltd, 2016 Search PubMed.
- G. Yaşayan, E. Alarçin, A. Bal-Öztürk and M. Avci-Adali, in Studies in Natural Products Chemistry, ed. R. Atta ur, Elsevier, 2022, 74, pp. 367–441 Search PubMed.
- B. Mordorski and T. Prow, Curr. Dermatol. Rep., 2016, 5, 278–286 CrossRef.
- B. Arora, R. Bhatia and P. Attri, in New Polymer Nanocomposites for Environmental Remediation, ed. C. M. Hussain and A. K. Mishra, Elsevier, 2018, pp. 699–712 DOI:10.1016/B978-0-12-811033-1.00027-5.
- X. Wang, J. Chang and C. Wu, Appl. Mater. Today, 2018, 11, 308–319 CrossRef.
- I. Gholamali and M. Yadollahi, Regener. Eng. Transl. Med., 2021, 7, 129–146 CrossRef CAS.
- V. G. Bhat, S. P. Masti, S. S. Narasagoudr, R. B. Chougale, P. Kumar and A. B. Vantamuri, Chem. Data Collect., 2023, 44, 101009 CrossRef CAS.
- F. N. Harandi, A. C. Khorasani, S. A. Shojaosadati and S. Hashemi-Najafabadi, Surf. Interfaces, 2022, 28, 101592 CrossRef CAS.
- L. Qi, K. Ou, Y. Hou, P. Yuan, W. Yu, X. Li, B. Wang, J. He, S. Cui and X. Chen, Mater. Des., 2021, 201, 109461 CrossRef CAS.
- M. Heydari-Majd, B. Ghanbarzadeh, M. Shahidi-Noghabi, A. Abdolshahi, S. Dahmardeh and M. Malek Mohammadi, Polym. Bull., 2022, 79, 97–119 CrossRef CAS.
- Z. Chu, T. Zhao, L. Li, J. Fan and Y. Qin, Materials, 2017, 10, 659 CrossRef PubMed.
- A. Dhasmana, L. Singh, P. Roy and N. C. Mishra, Mater. Sci. Eng., C, 2019, 97, 313–324 CrossRef CAS PubMed.
- P. Dam, S. Altuntas, R. Mondal, J. R. V. Baudrit, A. Kati, S. Ghorai, A. Sadat, D. Gangopadhyay, S. Shaw and O. L. Franco, Mater. Lett., 2022, 327, 133024 CrossRef CAS.
- B.-M. Min, G. Lee, S. H. Kim, Y. S. Nam, T. S. Lee and W. H. Park, Biomaterials, 2004, 25, 1289–1297 CrossRef CAS PubMed.
- M. Gholipourmalekabadi, S. Sapru, A. Samadikuchaksaraei, R. L. Reis, D. L. Kaplan and S. C. Kundu, Adv. Drug Delivery Rev., 2020, 153, 28–53 CrossRef CAS PubMed.
- K. Chen, Y. Li, Y. Li, W. Pan and G. Tan, Macromol. Biosci., 2023, 23, 2200380 CrossRef CAS PubMed.
- F. A. Sheikh, H. W. Ju, J. M. Lee, B. M. Moon, H. J. Park, O. J. Lee, J.-H. Kim, D.-K. Kim and C. H. Park, Nanomedicine, 2015, 11, 681–691 CrossRef CAS PubMed.
- Q. Wang, S. Zhou, L. Wang, R. You, S. Yan, Q. Zhang and M. Li, Composites, Part B, 2021, 224, 109165 CrossRef CAS.
- S. Gao, X. Xiong, H. Xie, P. Li, F. Kong, Y. Fan, S. Meng, J. Yuan and Q. Jiang, Process Biochem., 2023, 134, 218–231 CrossRef CAS.
- P. Farshi, R. Salarian, M. Rabiee, S. Alizadeh, M. Gholipourmalekabadi, S. Ahmadi and N. Rabiee, Polym. Eng. Sci., 2022, 62, 2741–2749 CrossRef CAS.
- X. Yang, S. He, J. Wang, Y. Liu, W. Ma, C.-Y. Yu and H. Wei, Int. J. Biol. Macromol., 2023, 242, 124872 CrossRef CAS PubMed.
- Y. Fu, J. Zhang, H. Lin and A. Mo, Mater. Sci. Eng., C, 2021, 118, 111367 CrossRef CAS PubMed.
- R. Cassano, F. Curcio, R. Sole and S. Trombino, Polysaccharide Hydrogels for Drug Delivery and Regenerative Medicine, Elsevier, 2024, pp.35–46 Search PubMed.
- N. K. Dehkordi, M. Minaiyan, A. Talebi, V. Akbari and A. Taheri, Biomed. Mater., 2019, 14, 035003 CrossRef CAS PubMed.
- S. Li, Q. Dong, X. Peng, Y. Chen, H. Yang, W. Xu, Y. Zhao, P. Xiao and Y. Zhou, ACS Nano, 2022, 16, 11346–11359 CrossRef CAS PubMed.
- J. Hu, M. Tao, F. Sun, C. Chen, G. Chen and G. Wang, Int. J. Biol. Macromol., 2022, 222, 55–64 CrossRef CAS PubMed.
- Y. Yang, Z. Dong, M. Li, L. Liu, H. Luo, P. Wang, D. Zhang, X. Yang, K. Zhou and S. Lei, Int. J. Nanomed., 2020, 8231–8247 CrossRef CAS PubMed.
- Y. Zhou, Y. Fan, Z. Chen, Z. Yue and G. Wallace, Biofabrication, 2021, 14, 015004 CrossRef PubMed.
- R. Chang, D. Zhao, C. Zhang, K. Liu, Y. He, F. Guan and M. Yao, Int. J. Biol. Macromol., 2023, 226, 870–884 CrossRef CAS PubMed.
- R. Kashimoto, Y. Kamei, S. Nonaka, Y. Kondo, S. Yamamoto, S. Furukawa, A. Ohashi and A. Satoh, Dev. Biol., 2023, 498, 14–25 CrossRef CAS PubMed.
- A. A. Chaudhari, K. Vig, D. R. Baganizi, R. Sahu, S. Dixit, V. Dennis, S. R. Singh and S. R. Pillai, Int. J. Mol. Sci., 2016, 17, 1974 CrossRef PubMed.
- C. Valentino, B. Vigani, G. Zucca, M. Ruggeri, C. Boselli, A. I. Cornaglia, L. Malavasi, G. Sandri and S. Rossi, Int. J. Biol. Macromol., 2023, 242, 125000 CrossRef CAS PubMed.
- M. R. Alam, M. A. Shahid, S. Alimuzzaman and A. N. Khan, Adv. Biomed. Eng., 2022, 100064 CrossRef.
- E. Sharifi, S. Yousefiasl, N. Laderian, N. Rabiee, P. Makvandi, S. Pourmotabed, M. Ashrafizadeh, F. Familsattarian and W. Fang, Int. J. Biol. Macromol., 2023, 251, 125898 CrossRef CAS PubMed.
- C. Kalirajan and T. Palanisamy, Adv. Healthcare Mater., 2020, 9, 2000247 CrossRef CAS PubMed.
- C. Kalirajan and T. Palanisamy, J. Mater. Chem. B, 2019, 7, 5873–5886 RSC.
- P. Khadivar, S. Khajeniazi and A. Karimi, J. Mater. Res. Technol., 2022, 19, 3966–3979 CrossRef CAS.
- N. Ribeiro, A. Sousa, C. Cunha-Reis, A. L. Oliveira, P. L. Granja, F. J. Monteiro and S. R. Sousa, Nanomedicine, 2021, 33, 102353 CrossRef CAS PubMed.
- W. Zheng, W. Yang, W. Wei, Z. Liu, P. L. Tremblay and T. Zhang, Adv. Healthcare Mater., 2023, 2303138 Search PubMed.
- H. Gu, H. Li, L. Wei, J. Lu and Q. Wei, Regener. Biomater., 2023, 10, rbad018 CrossRef CAS PubMed.
- H. Singh, I. Yadav, W. M. Sheikh, A. Dan, Z. Darban, S. A. Shah, N. C. Mishra, S. Shahabuddin, S. Hassan and S. M. Bashir, Int. J. Biol. Macromol., 2023, 251, 126349 CrossRef CAS PubMed.
- I. H. Ali, A. Ouf, F. Elshishiny, M. B. Taskin, J. Song, M. Dong, M. Chen, R. Siam and W. Mamdouh, ACS Omega, 2022, 7, 1838–1850 CrossRef CAS PubMed.
- S. Nazarnezhad, F. Kermani, V. R. Askari, S. A. Hosseini, A. Ebrahimzadeh-Bideskan, A. Moradi, R. K. Oskuee, S. Mollazadeh and S. Kargozar, J. Pharm. Sci., 2022, 111, 2531–2539 CrossRef CAS PubMed.
- A. A. Aboomeirah, W. A. Sarhan, E. A. Khalil, A. Abdellatif, A. S. Abo Dena and I. M. El-Sherbiny, ACS Appl. Bio Mater., 2022, 5, 3678–3694 CrossRef CAS PubMed.
- M. Kumar, M. Rajput, T. Soni, V. Vivekanand and N. Pareek, Front. Chem., 2020, 8, 469 CrossRef CAS PubMed.
- D. Elieh-Ali-Komi and M. R. Hamblin, Int. J. Adv. Res., 2016, 4, 411 CAS.
- A. Anitha, S. Sowmya, P. S. Kumar, S. Deepthi, K. Chennazhi, H. Ehrlich, M. Tsurkan and R. Jayakumar, Prog. Polym. Sci., 2014, 39, 1644–1667 CrossRef CAS.
- M. E. Abd El-Hack, M. T. El-Saadony, M. E. Shafi, N. M. Zabermawi, M. Arif, G. E. Batiha, A. F. Khafaga, Y. M. Abd El-Hakim and A. A. Al-Sagheer, Int. J. Biol. Macromol., 2020, 164, 2726–2744 CrossRef CAS PubMed.
- S. Li, B. Gu, X. Li, S. Tang, L. Zheng, E. Ruiz-Hitzky, Z. Sun, C. Xu and X. Wang, Adv. Healthcare Mater., 2022, 11, 2102367 CrossRef CAS PubMed.
- Y. Okamoto, K. Kawakami, K. Miyatake, M. Morimoto, Y. Shigemasa and S. Minami, Carbohydr. Polym., 2002, 49, 249–252 CrossRef CAS.
- R. Singh, K. Shitiz and A. Singh, Int. Wound J., 2017, 14, 1276–1289 CrossRef PubMed.
- Y. Shigemasa and S. Minami, Biotechnol. Genet. Eng. Rev., 1996, 13, 383–420 CrossRef CAS PubMed.
- M. G. Mehrabani, R. Karimian, R. Rakhshaei, F. Pakdel, H. Eslami, V. Fakhrzadeh, M. Rahimi, R. Salehi and H. S. Kafil, Int. J. Biol. Macromol., 2018, 116, 966–976 CrossRef CAS PubMed.
- P. Fan, Y. Zeng, D. Zaldivar-Silva, L. Agüero and S. Wang, Molecules, 2023, 28, 1473 CrossRef CAS PubMed.
- A. B. Mohd Hilmi, A. S. Halim, H. Jaafar, A. B. Asiah and A. Hassan, BioMed Res. Int., 2013, 2013, 1–13 CrossRef PubMed.
- F. Wang, S. Hu, Q. Jia and L. Zhang, J. Nanomater., 2020, 2020, 1–14 CAS.
- X. Liu, L. Ma, Z. Mao and C. Gao, in Chitosan for Biomaterials II, ed. R. Jayakumar, M. Prabaharan and R. A. A. Muzzarelli, Springer Berlin Heidelberg, Berlin, Heidelberg, 2011, pp. 81–127 DOI:10.1007/12-2011-118.
- C. Zhang, Q. Zhang, D. Yang, Y. Qiao, B. Wang, J. Yan, Z. Li, Z. Huang, Y. Zhou, K. Hu and Y. Zhang, Front. Bioeng. Biotechnol., 2022, 10, 1002437 CrossRef PubMed.
- R. A. Masud, M. S. Islam, P. Haque, M. N. I. Khan, M. Shahruzzaman, M. Khan, M. Takafuji and M. M. Rahman, Materialia, 2020, 12, 100785 CrossRef CAS.
- Y. Haririan, A. Asefnejad, H. Hamishehkar and M. R. Farahpour, Int. J. Biol. Macromol., 2023, 253, 127081 CrossRef CAS PubMed.
- R. Poonguzhali, S. Khaleel Basha and V. Sugantha Kumari, Int. J. Biol. Macromol., 2018, 112, 1300–1309 CrossRef CAS PubMed.
- K. Varaprasad, T. Jayaramudu, V. Kanikireddy, C. Toro and E. R. Sadiku, Carbohydr. Polym., 2020, 116025 CrossRef CAS PubMed.
- M. Otterlei, K. Østgaard, G. Skjåk-Bræk, O. Smidsrød, P. Soon-Shiong and T. Espevik, J. Immunother., 1991, 10, 286–291 CrossRef CAS PubMed.
- N. Zhou, X. Ma, K. V. Bernaerts, P. Ren, W. Hu and T. Zhang, ACS Biomater. Sci. Eng., 2020, 6, 3310–3326 CrossRef CAS PubMed.
- J. Jia, D. J. Richards, S. Pollard, Y. Tan, J. Rodriguez, R. P. Visconti, T. C. Trusk, M. J. Yost, H. Yao, R. R. Markwald and Y. Mei, Acta Biomater., 2014, 10, 4323–4331 CrossRef CAS PubMed.
- N. Farshidfar, S. Iravani and R. S. Varma, Mar. Drugs, 2023, 21, 189 CrossRef CAS PubMed.
- H. Babavalian, M. A. Shokrgozar, S. Bonakdar, F. Shakeri and H. Tebyanian, Trauma Mon., 2016, 22, e64270 Search PubMed.
- J. P. Junker, R. A. Kamel, E. Caterson and E. Eriksson, Adv. Wound Care, 2013, 2, 348–356 CrossRef PubMed.
- R. Ma, Y. Wang, H. Qi, C. Shi, G. Wei, L. Xiao, Z. Huang, S. Liu, H. Yu, C. Teng, H. Liu, V. Murugadoss, J. Zhang, Y. Wang and Z. Guo, Composites, Part B, 2019, 167, 396–405 CrossRef CAS.
- A. Rezaei and H. Ehtesabi, Mater. Today Chem., 2022, 24, 100910 CrossRef CAS.
- F. G. Torres, S. Commeaux and O. P. Troncoso, Starch/Staerke, 2013, 65, 543–551 CrossRef CAS.
- Y. Ai and J. I. Jane, in Encyclopedia of Food and Health, ed. B. Caballero, P. M. Finglas and F. Toldrá, Academic Press, Oxford, 2016, pp. 165–174, DOI:10.1016/B978-0-12-384947-2.00657-7.
- V. S. Waghmare, P. R. Wadke, S. Dyawanapelly, A. Deshpande, R. Jain and P. Dandekar, Bioact. Mater., 2018, 3, 255–266 Search PubMed.
- M. M. Delavari, I. Ocampo and I. Stiharu, Micromachines, 2022, 13, 2146 CrossRef PubMed.
- A. Tampau, C. González-Martinez and A. Chiralt, J. Food Eng., 2017, 214, 245–256 CrossRef CAS.
- C. Su, H. Zhao, H. Yang and R. Chen, ACS Appl. Bio Mater., 2019, 2, 171–181 CrossRef CAS PubMed.
- S. Nezami, M. Sadeghi and H. Mohajerani, Polym. Degrad. Stab., 2020, 179, 109255 CrossRef CAS.
- G. Yaşayan, E. Alarçin, A. Bal-Öztürk and M. Avci-Adali, Stud. Nat. Prod. Chem., 2022, 74, 367–441 Search PubMed.
- A. Gupta, M. Kowalczuk, W. Heaselgrave, S. T. Britland, C. Martin and I. Radecka, Eur. Polym. J., 2019, 111, 134–151 CrossRef CAS.
- J. D. P. de Amorim, C. J. G. da Silva Junior, A. D. L. M. de Medeiros, H. A. do Nascimento, M. Sarubbo, T. P. M. de Medeiros, A. F. d S. Costa and L. A. Sarubbo, Molecules, 2022, 27, 5580 CrossRef CAS PubMed.
- L. Zheng, S. Li, J. Luo and X. Wang, Front. Bioeng. Biotechnol., 2020, 8, 593768 CrossRef PubMed.
- N. Asadi, A. R. Del Bakhshayesh, S. Davaran and A. Akbarzadeh, Mater. Chem. Phys., 2020, 242, 122528 CrossRef CAS.
- L. Zhang, Y. Yu, S. Zheng, L. Zhong and J. Xue, Carbohydr. Polym., 2021, 257, 117671 CrossRef CAS PubMed.
- M. U. A. Khan, G. M. Stojanović, R. A. Rehman, A.-R. Moradi, M. Rizwan, N. Ashammakhi and A. Hasan, ACS omega, 2023, 8, 40024–40035 CrossRef CAS PubMed.
- Y. Wan, S. Yang, J. Wang, D. Gan, M. Gama, Z. Yang, Y. Zhu, F. Yao and H. Luo, Composites, Part B, 2020, 199, 108259 CrossRef CAS.
- A. Gupta, S. M. Briffa, S. Swingler, H. Gibson, V. Kannappan, G. Adamus, M. Kowalczuk, C. Martin and I. Radecka, Biomacromolecules, 2020, 21, 1802–1811 CrossRef CAS PubMed.
- H. Adelnia, R. Ensandoost, S. S. Moonshi, J. N. Gavgani, E. I. Vasafi and H. T. Ta, Eur. Polym. J., 2022, 164, 110974 CrossRef CAS.
- M. Teodorescu, M. Bercea and S. Morariu, Biotechnol. Adv., 2019, 37, 109–131 CrossRef CAS PubMed.
- M. Kokabi, M. Sirousazar and Z. M. Hassan, Eur. Polym. J., 2007, 43, 773–781 CrossRef CAS.
- E. A. Kamoun, E.-R. S. Kenawy and X. Chen, J. Adv. Res., 2017, 8, 217–233 CrossRef CAS PubMed.
- S. G. Jin, Chem. – Asian J., 2022, 17, e202200595 CrossRef CAS PubMed.
- S. M. Nasef, E. E. Khozemy, E. A. Kamoun and H. El-Gendi, Int. J. Biol. Macromol., 2019, 137, 878–885 CrossRef CAS PubMed.
- S. Song, Z. Liu, M. A. Abubaker, L. Ding, J. Zhang, S. Yang and Z. Fan, Mater. Sci. Eng., C, 2021, 126, 112171 CrossRef CAS PubMed.
- H. Liu, R. Chen, P. Wang, J. Fu, Z. Tang, J. Xie, Y. Ning, J. Gao, Q. Zhong and X. Pan, Carbohydr. Polym., 2023, 121050 CrossRef CAS PubMed.
- G. Nasiri, N. Azarpira, A. Alizadeh, S. M. Zebarjad, M. Aminshahidi, O. Alavi and M. Kamali, Mater. Today Commun., 2022, 33, 104476 CrossRef CAS.
- S. Alippilakkotte, S. Kumar and L. Sreejith, Colloids Surf., A, 2017, 529, 771–782 CrossRef CAS.
- S. F. Gomaa, T. M. Madkour, S. Moghannem and I. M. El-Sherbiny, Int. J. Biol. Macromol., 2017, 105, 1148–1160 CrossRef CAS PubMed.
- B. Kost, M. Svyntkivska, M. Brzeziński, T. Makowski, E. Piorkowska, K. Rajkowska, A. Kunicka-Styczyńska and T. Biela, Colloids Surf., B, 2020, 190, 110949 CrossRef CAS PubMed.
- S. Sun, H. Li, J. Chen and Q. Qian, Physiology, 2017, 32, 453–463 CrossRef CAS PubMed.
- H. T. J. Gilbert, N. Hodson, P. Baird, S. M. Richardson and J. A. Hoyland, Sci. Rep., 2016, 6, 37360 CrossRef CAS PubMed.
- S. Chitrattha and T. Phaechamud, Mater. Sci. Eng., C, 2016, 58, 1122–1130 CrossRef CAS PubMed.
- M. Râpă, L. M. Stefan, T. Zaharescu, A.-M. Seciu, A. A. Turcanu, E. Matei, A. M. Predescu, I. Antoniac and C. Predescu, Appl. Sci., 2020, 10, 2265 CrossRef.
- M. Ranjbar-Mohammadi, P. Shakoori and Z. Arab-Bafrani, Int. J. Biol. Macromol., 2021, 187, 554–565 CrossRef CAS PubMed.
- K. K. Chereddy, G. Vandermeulen and V. Préat, Wound Repair Regen., 2016, 24, 223–236 CrossRef PubMed.
- H. M. E.-S. Azzazy, S. A. Fahmy, N. K. Mahdy, M. R. Meselhy and U. Bakowsky, Nanomaterials, 2021, 11, 2438 CrossRef CAS PubMed.
- T. K. Hunt, W. B. Conolly, S. B. Aronson and P. Goldstein, Am. J. Surg., 1978, 135, 328–332 CrossRef CAS PubMed.
- P. E. Porporato, V. L. Payen, C. J. De Saedeleer, V. Préat, J.-P. Thissen, O. Feron and P. Sonveaux, Angiogenesis, 2012, 15, 581–592 CrossRef CAS PubMed.
- K. Huang, Z. Jinzhong, T. Zhu, Y. Morsi, A. Aldalbahi, M. El-Newehy, X. Yan and X. Mo, J. Mater. Chem. B, 2021, 9, 1452–1465 RSC.
- L. Sutrisno, H. Chen, T. Yoshitomi, N. Kawazoe, Y. Yang and G. Chen, J. Mater. Chem. B, 2022, 10, 204–213 RSC.
- G. El Fawal, H. Hong, X. Mo and H. Wang, J. Drug Delivery Sci. Technol., 2021, 63, 102501 CrossRef CAS.
- A. Bharadwaz and A. C. Jayasuriya, Mater. Sci. Eng., C, 2020, 110, 110698 CrossRef CAS PubMed.
- S. Moeini, M. R. Mohammadi and A. Simchi, Bioact. Mater., 2017, 2, 146–155 Search PubMed.
- H. A. Rather, R. Thakore, R. Singh, D. Jhala, S. Singh and R. Vasita, Bioact. Mater., 2018, 3, 201–211 Search PubMed.
- M. Naseri-Nosar, S. Farzamfar, H. Sahrapeyma, S. Ghorbani, F. Bastami, A. Vaez and M. Salehi, Mater. Sci. Eng., C, 2017, 81, 366–372 CrossRef CAS PubMed.
- S. Kouser, A. Prabhu, S. Sheik, K. Prashantha, G. K. Nagaraja, J. N. D’Souza, K. M. Navada and D. J. Manasa, Int. J. Pharm., 2021, 607, 121048 CrossRef CAS PubMed.
- J. M. Lee, T. Chae, F. A. Sheikh, H. W. Ju, B. M. Moon, H. J. Park, Y. R. Park and C. H. Park, Mater. Sci. Eng., C, 2016, 68, 758–767 CrossRef CAS PubMed.
- A. Keirouz, M. Zakharova, J. Kwon, C. Robert, V. Koutsos, A. Callanan, X. Chen, G. Fortunato and N. Radacsi, Mater. Sci. Eng., C, 2020, 112, 110939 CrossRef CAS PubMed.
- H. Khalid, H. Iqbal, R. Zeeshan, M. Nasir, F. Sharif, M. Akram, M. Irfan, F. A. Khan, A. A. Chaudhry and A. F. Khan, Polym. Bull., 2021, 78, 7199–7218 CrossRef CAS.
- M. Ragothaman, A. K. Villalan, A. Dhanasekaran and T. Palanisamy, Mater. Sci. Eng., C, 2021, 128, 112328 CrossRef CAS PubMed.
- M. Ahmadi, M. Mehdikhani, J. Varshosaz, S. Farsaei and H. Torabi, J. Biomater. Appl., 2021, 35, 958–977 CrossRef CAS PubMed.
- G. Sandri, C. Aguzzi, S. Rossi, M. C. Bonferoni, G. Bruni, C. Boselli, A. I. Cornaglia, F. Riva, C. Viseras, C. Caramella and F. Ferrari, Acta Biomater., 2017, 57, 216–224 CrossRef CAS PubMed.
- D. Archana, B. K. Singh, J. Dutta and P. K. Dutta, Carbohydr. Polym., 2013, 95, 530–539 CrossRef CAS PubMed.
- P. T. S. Kumar, V.-K. Lakshmanan, R. Biswas, S. V. Nair and R. Jayakumar, J. Biomed. Nanotechnol., 2012, 8, 891–900 CrossRef CAS PubMed.
- A. E. Eldeeb, S. Salah, M. S. Amer and N. A. Elkasabgy, J. Drug Delivery Sci. Technol., 2022, 71, 103292 CrossRef CAS.
- Y. Fan, W. Wu, Y. Lei, C. Gaucher, S. Pei, J. Zhang and X. Xia, Mar. Drugs, 2019, 17, 285 CrossRef CAS PubMed.
- M. Ansarizadeh, S. A. Haddadi, M. Amini, M. Hasany and A. Ramazani SaadatAbadi, J. Appl. Polym. Sci., 2020, 137, 48916 CrossRef CAS.
- D. Z. Zmejkoski, Z. M. Marković, D. D. Mitić, N. M. Zdravković, N. O. Kozyrovska, N. Bugárová and B. M. Todorović Marković, J. Biomed. Mater. Res., Part B, 2022, 110, 1796–1805 CrossRef CAS PubMed.
- W. Wentao, Z. Tao, S. Bulei, Z. Tongchang, Z. Qicheng, W. Fan, Z. Ninglin, S. Jian, Z. Ming and S. Yi, Appl. Mater. Today, 2019, 17, 36–44 CrossRef.
- S. Faraji, N. Nowroozi, A. Nouralishahi and J. S. Shayeh, Life Sci., 2020, 257, 118062 CrossRef CAS PubMed.
- A. Góra, M. P. Prabhakaran, G. T. L. Eunice, R. Lakshminarayanan and S. Ramakrishna, J. Appl. Polym. Sci., 2015, 132, 1–11 CrossRef.
- P. Zhu, H. Yin, J. Wei, J. Wu, D. Ping and X. Zhang, Int. J. Biol. Macromol., 2023, 125093 CrossRef CAS PubMed.
- D. Sharma, K. K. Bhardwaj and R. Gupta, in Bionanocomposites in Tissue Engineering and Regenerative Medicine, ed. S. Ahmed and Annu, Woodhead Publishing, 2021, pp.611–630 DOI:10.1016/B978-0-12-821280-6.00001-5.
- J. Chen, Y. Fan, G. Dong, H. Zhou, R. Du, X. Tang, Y. Ying and J. Li, Biomater. Sci., 2023, 11, 3051–3076 RSC.
- F. Trotta, A. Dony, M. Mok, A. Grillo, T. Whitehead-Clarke, S. Homer-Vanniasinkam and A. Kureshi, Nano Select, 2023, 5, 2300087 CrossRef.
- D. Warale, M. Shabeena, A. Prabhu, S. Kouser, D. Manasa and G. Nagaraja, Int. J. Biol. Macromol., 2024, 257, 128628 CrossRef CAS PubMed.
- M. Singh, M. Changmai, T. Ghosh and A. Karwa, Advanced Materials and Manufacturing Techniques for Biomedical Applications, 2023, pp.61–101 Search PubMed.
| This journal is © The Royal Society of Chemistry 2024 |