Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effect of polymer addition on the phase behavior of oil–water–surfactant systems of Winsor III type
Ming
Lu
abcd,
Björn
Lindman
*efg and
Krister
Holmberg
 *h
*h
aQingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, 266101 Qingdao, China
bShandong Energy Institute, 266101 Qingdao, China
cQingdao New Energy Shandong Laboratory, 266101 Qingdao, China
dQingdao GiNZRE Oil & Gas Technology Development Co., Ltd, Qingdao, China
eDivision of Physical Chemistry, Department of Chemistry, Centre for Chemistry and Chemical Engineering, Lund University, SE-221 00, Lund, Sweden
fSchool of Materials Science and Engineering, Nanyang Technological University, 639798 Singapore, Singapore
gCoimbra Chemistry Centre (CQC), Faculty of Sciences and Technology, University of Coimbra, 3004-535 Coimbra, Portugal
hDepartment of Chemistry and Chemical Engineering, Chalmers University of Technology, 41296 Gothenburg, Sweden. E-mail: krister.holmberg@chalmers.se
First published on 31st October 2023
Abstract
Ternary oil–water–surfactant systems can give rise to an O/W microemulsion in equilibrium with excess oil, a W/O microemulsion in equilibrium with excess water, or a bicontinuous microemulsion in equilibrium with excess oil and water. This type of phase behavior has been known for a long time and the three systems are often referred to as Winsor I, Winsor II and Winsor III, respectively after the British scientist P. A. Winsor who pioneered the area. The Winsor systems are technically important and well understood today. It was later found that addition of a polymer to the oil–water–surfactant system can influence the phase behavior considerably. While a hydrophilic polymer will be incorporated in the water phase and a hydrophobic polymer in the oil phase, an amphiphilic polymer with the right hydrophilic–lipophilic balance may expand the middle phase microemulsion in a Winsor III system. Expansion of the middle phase of such a system will lead to a reduction of the oil/microemulsion and the microemulsion/water interfacial tensions. This can be practically important, and the effect is currently of considerable interest for so-called surfactant flooding for enhanced oil recovery (EOR). Boosting the middle phase of the Winsor III system by addition of a polymer to the surfactant system is still not an established procedure and not so well understood from a scientific point of view. In this review we summarize the work done in the field and we demonstrate that the role of the polymer is intimately linked to its interactions with the three other components in the system: the oil, the water, and the surfactant(s).
Introduction and background
Microemulsions are thermodynamically stable mixtures of oil, water, and surfactant, which on the microscopic level consist of domains of oil and water separated by a monolayer of the amphiphile. They were scientifically described by J. H. Schulman in 1943,1 but the concept had appeared in the patent literature before that.2,3 Schulman, who pioneered the area, initially used a fatty acid soap as surfactant and a medium chain alcohol as cosurfactant. He later coined the term “microemulsion”, indicating that such systems were some kind of emulsions but with smaller drops.4 We now know that microemulsions and emulsions are fundamentally different. While microemulsions are thermodynamically stable with structures in the nanometer range that constantly form, disintegrate, and reform, emulsions are not thermodynamically stable, and their structures are not dynamic. Thus, microemulsions are neither micro, nor emulsions but the term is so established that we must live with it. A useful definition of a microemulsion was formulated in 1981: “a system of water, oil and amphiphile which is a single optically isotropic and thermodynamically stable liquid solution”.5When the amount of amphiphile in the formulation is not high enough to make a microemulsion of the whole mixture, two- or three-phase systems will form. With a surfactant that is more soluble in the water than in the oil used in the formulation an oil-in-water microemulsion in equilibrium with excess oil will form and when the surfactant is more soluble in the oil than in the water a water-in-oil microemulsion in equilibrium with excess water will be generated. If the surfactant is “balanced”, i.e., approximately equally soluble in the oil and the water, a three-phase system will form with a middle-phase microemulsion in equilibrium with both excess water and excess oil. The structure of the middle-phase microemulsion was a matter of debate for a long time but it is now established that the structure is bicontinuous, consisting of infinitely long channels of oil and water and with the oil–water interface roughly having a zero mean curvature. The pulsed-gradient spin-echo NMR technique, below referred to as NMR diffusometry, proved to be particularly useful for elucidating the structure.6
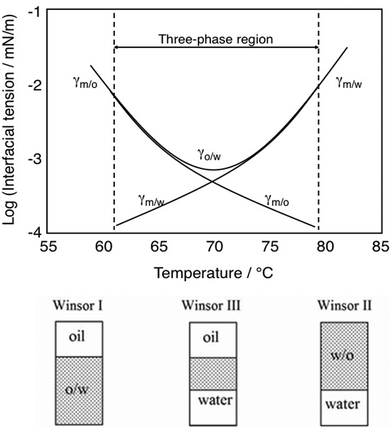
The two- or three-phase systems described above are commonly referred to as Winsor I, Winsor II and Winsor III, respectively, named after P. A. Winsor, who made important contributions in the area in the 1950's and 60's and who described the technology in a widely spread book.7 He showed that by changing parameters, typically the salt concentration for systems based on an ionic surfactant and the temperature for systems based on a nonionic surfactant containing a polyoxyethylene chain as polar head group, one could go from the Winsor I system, via Winsor III to Winsor II. This is illustrated in Fig. 1 for a nonionic surfactant.
The transition Winsor I to Winsor III to Winsor II (and back) is caused by a change in curvature of the oil–water interface from positive (curved towards oil), via zero mean to negative (curved towards water).8,9 This is illustrated in Fig. 2.
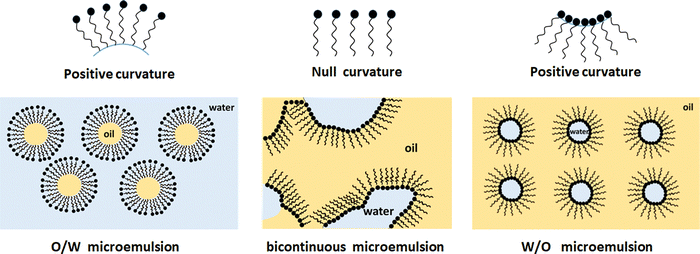 | ||
| Fig. 2 Structure of left: an oil-in-water microemulsion; middle: a bicontinuous microemulsion; and right: a water-in-oil microemulsion. A monolayer of surfactant covers all oil–water interfaces. | ||
In the majority of applications of microemulsions, such as cleaning and personal care products, foods, pharmaceutics and formulations for cleaning of art, as well as industrial niches such as cutting fluids and hard surface degreasing, one-phase microemulsions are needed, i.e., the surfactant is added in an amount sufficient to make a microemulsion of all the water and oil in the formulation.10–12 However, there is one exception to this, and that is an application with a very large potential: enhanced oil recovery, abbreviated EOR. EOR can be subdivided into different technologies, and one is surfactant flooding, sometimes also called microemulsion flooding or surfactant EOR. In surfactant flooding a surfactant, or more often a combination of surfactants, is injected into the reservoir with the aim of increasing the recovery of oil. The surfactant, or surfactants, can be the sole chemical used for the purpose. However, in most cases the surfactant is combined with other chemicals, such as a water-soluble polymer to regulate the viscosity and/or alkali to generate naphthenic acid salts from the crude oil and to increase the negative charge of the surface of the sandstone rock. A variety of surfactants are of interest for the purpose but for sandstone reservoirs, where surfactant flooding is of particular interest, they are all anionic amphiphiles. Alkylaryl sulfonates, propoxylated branched alcohol sulfates, and branched ether carboxylates are examples of classes of surfactants of considerable current interest.13 If this EOR technique will be used in full scale, which is not yet the case, the volumes of surfactants consumed will be of the same magnitude as for the large established applications such as detergents and personal care products.
The main role of the surfactant used for EOR is to reduce the interfacial tension between the oil present in the reservoir and the injected water, γo/w. It has been known since long back that the amount of oil retained in the reservoir after water flooding will depend on the ratio between viscous forces striving to displace the oil and capillary forces, which trap the oil in the pores.14 The relationship between the viscous and the capillary forces is often described by the capillary number, Nc, according to the expression
| Nc = ην/γo/w |
In principle, the Nc value can be raised by either increasing the viscous forces or decreasing the capillary forces. The viscous forces can be increased by thickening the water, e.g., by addition of a polymer, and/or by increasing the water pressure in the pumping. However, there is a limit to what can be done to the viscous forces. Too high viscosity or too high water pressure will damage the reservoir, generating cracks in the porous rock. The injected fluid will then go through the large cracks avoiding the narrow pores where most of the oil is situated.
Consequently, the approach taken to raise the Nc value is to drastically reduce the capillary forces (together with a moderate increase in the viscous forces by adding a water-soluble polymer to the injection water). It has been demonstrated that, at least for water-wet reservoirs, a reduction of the oil–water interfacial tension down to 10−3 mN m−1 or even lower is needed to obtain proper mobilization of the oil in the reservoir.15 This can be achieved with a surfactant that is well balanced, i.e., gives a Winsor III system with the actual oil and water at the reservoir temperature and salinity. The current standard is to use as little as 0.3% surfactant (or surfactants) in the laboratory experiments.16 The middle phase microemulsion then becomes extremely thin but the two interfaces in the system, oil-microemulsion and microemulsion-water, have very low interfacial tension values, γo/m and γm/w. When the system is perfectly balanced, as is the case at a salinity of 2.5% in Fig. 1, the two interfacial tensions are the same.
It has been known for a long time that for a given amount of a balanced surfactant there is a reverse relationship between the size of the middle phase and the interfacial tensions in the system.17 Thus, a method to swell the middle phase would be a way to reduce further the interfacial tensions and consequently improve the oil recovery.
A note on polymer–surfactant interactions
The influence of polymers on microemulsions is, of course, mainly dictated by the interactions of the polymer molecules with the surfactant molecules forming the surfactant film. This will depend on both the surfactant used and the properties of the polymer, like polarity, charge, amphiphilicity, branching, etc. The influence of polymers on the surfactant films in microemulsions, and thus the phase behavior, can be deduced from extensive studies of simple mixed aqueous solutions of polymer and surfactant; there is a broad knowledge of the situation based on studies on association in solution and on phase behavior.18,19A basic aspect is that for amphiphiles based on the same hydrophobic chain nonionic surfactants have a much larger tendency for self-assembly than ionic surfactants, as can be learnt for example from the critical micelle concentrations and from phase diagrams. This is due to the electrostatic penalty in forming aggregates with high charge density; the underlying counterion entropy effect is well described by the Poisson–Boltzmann equation.20–22
Ionic surfactants associate with oppositely charged polymers and also in general with nonionic polymers; this can be understood from a reduction of the electrostatic penalty. Nonionic surfactants on the other hand do not associate with homopolymers; therefore, there is no polymer adsorption on nonionic surfactant films in microemulsions. The different behavior of polymer-loaded nonionic and ionic microemulsions illustrates this.23 If the polymer has hydrophobic groups or segments, i.e. is amphiphilic, there is typically an association with all types of surfactants; thus, such polymers adsorb on surfactant films. However, different amphiphilic polymers form very different aggregates with surfactants. Block copolymers, be they of the AB or ABA type, tend to form mixed micelles whereas hydrophilic polymers with hydrophobic grafts give small aggregates around the hydrophobic groups.
A large fraction of the work on microemulsions in general and on the effect of polymer addition in particular has concerned nonionic surfactants of polyoxyethylene type. Furthermore, many of the investigated polymers have EO blocks. As also noted elsewhere in this treatise the behavior of such compounds is highly sensitive to temperature, which has been attributed to temperature induced conformational changes leading to lower polarity at higher temperature. Therefore, the spontaneous curvature will change from positive (towards oil), to zero and to negative with increasing temperature. Another consequence is that the interaction between a nonionic polymer and the surfactant film will become stronger at higher temperatures.
Amphiphilic polymers can swell the middle phase
In a theoretical paper from 1993 R. Nagarayan postulated that addition of a nonionic polymer, capable of interacting with the self-assembled surfactants at the oil–water interface would lead to a transition from a Winsor II to a Winsor III system and a Winsor III system would transform into a Winsor I system.24 Thus, the effect of the added polymer would be a transition from right to left in Fig. 1.As an extension of Nagarayan's theoretical work, A. Kabalnov and coworkers studied experimentally how a Winsor III system is affected by addition of either water-soluble or oil-soluble polymers.25 Dextran and poly(ethylene glycol) (PEG) with different molecular weights were used as hydrophilic polymer and a high molecular weight polyisobutylene was used as hydrophobic polymer. The microemulsion components were decane–water–C12E5. (C12E5 stands for penta(ethylene glycol)monododecyl ether and this type of nomenclature will be used throughout this paper.) Microemulsions based on this type of nonionic surfactant are very temperature sensitive. The balanced state for this system, i.e., the state where the middle phase microemulsion contains equal amounts of oil and water is at 38.2 °C and all experiments were conducted at that temperature.
The two hydrophilic polymers were mainly found in the lower water phase although they were present also in the middle phase if the molecular weight was low. With respect to the molecular weight cutoff there was a good correlation between the size of water domains in the bicontinuous microemulsion and the macromolecular coil end-to-end distance, which indicates that the low molecular weight polymer coils can be accommodated into these domains. Polyisobutylene is soluble in aliphatic hydrocarbons and insoluble in water. It partitioned into the upper oil phase.
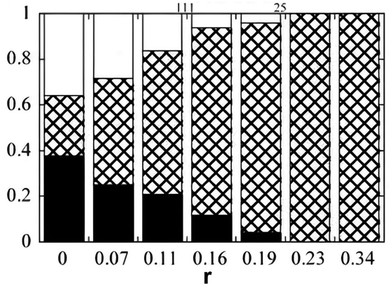
The polymers were added stepwise to the microemulsion up to an added amount of around 2%. For all three polymers the upper and lower phases increased at the expense of the middle phase. For the hydrophilic polymers, dextran and PEG, the effect was more pronounced the higher the molecular weight of the macromolecule. Fig. 3 illustrates the effect for dextran. The shrinkage of the middle phase exerted by a polymer that does not enter the middle phase microemulsion was explained as an osmotic pressure effect. Thus, on dissolution of a polymer in the lower phase the water chemical potential is reduced. Classical thermodynamics as expressed in the Gibbs–Duhem equation demands that there is a balancing change in the oil chemical potential, leading to an expansion of the oil phase as well. A corresponding effect is induced by addition of an oil-soluble polymer lowering the oil chemical potential in the upper phase leading to its swelling; a balancing effect occurs for the lower phase.
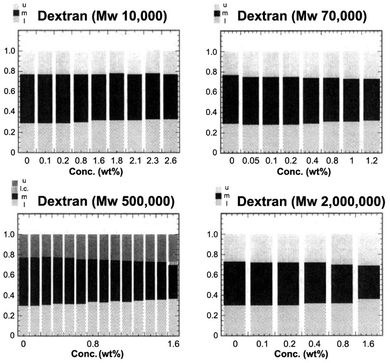 | ||
| Fig. 3 Relative volumes of the phases in a Winsor III system based on decane, water and C12E5 as a function of added dextran of different molecular weights. u, m and l stand for upper phase, middle phase and lower phase, respectively. (Reproduced from ref. 25 with permission from the American Chemical Society, copyright 1994.) | ||
In the analysis of the microemulsion thermodynamics Kabalnov et al. used a monolayer bending approach, according to which the surfactant molecules form an incompressible monolayer between oil and water domains; an estimate of the bending modulus was obtained.
Kabalnov et al. also found that at higher additions of polymer the change in surfactant chemical potential becomes large enough to induce formation of an additional phase, a lamellar liquid crystalline phase. As noted above lamellar phases are common in surfactant–oil–water systems at higher surfactant concentrations.
The same authors showed that the situation is very different for amphiphilic polymers that can enter the middle phase microemulsion. Using the same decane–water–C12E5 microemulsion as before but adding a surface-active polymer, hydrophobically modified ethylhydroxyethylcellulose (HM-EHEC), gave a different pattern.26 As can be seen from Fig. 4, the middle phase increased in volume on increasing the amount of added polymer. This polymer preferentially dissolves in the microemulsion phase and as a response this phase takes up oil and water from the two excess phases. As can be seen, the swelling is not symmetrical; the middle phase takes up more water than oil and at a polymer addition of around 1% the system has almost transformed into a Winsor I system. However, at higher polymer concentration the effect is reversed. The authors relate this reversion to saturation of the oil–water interface of the middle phase with the excess polymer giving rise to a counterpressure from the water phase. The swelling of the middle phase microemulsion when HM-EHEC is added to the Winsor III system is probably the first reported example of a polymer induced boosting of such a system. However, much more efficient examples of boosting of the middle phase would subsequently be described, as is discussed below.
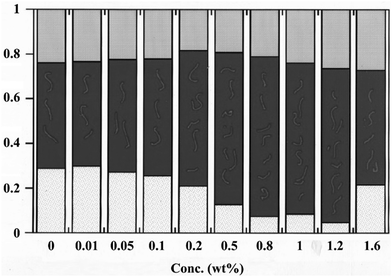 | ||
| Fig. 4 Relative volumes of the phases in a Winsor III system based on decane, water and C12E5 as a function of added HM-EHEC. All vials are composed of an upper phase (grey), a middle phase (black) and a lower phase (white). (Reproduced from ref. 26 with permission from the American Chemical Society, copyright 1994.) | ||
Regular EHEC, without hydrophobic substituents, was also investigated. This is a hydrophilic polymer of rather high molecular weight (around 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and, like dextran, it partitions entirely in the lower water phase. Addition of this polymer to the system leads to contraction of the middle phase.
000) and, like dextran, it partitions entirely in the lower water phase. Addition of this polymer to the system leads to contraction of the middle phase.
The second paper by Kabalnov et al.26 illustrates well the difference between polymers that adsorb at the surfactant film, such as HM-EHEC, and those that are depleted, such as EHEC. The hydrophobic grafts give an association with the surfactant films; as described above there is generally a strong association between HM-polymers and different surfactants, an effect documented mainly in rheological studies. Interestingly, even under highly swollen conditions the middle phase remains bicontinuous as could be demonstrated from studies of the self-diffusion of oil, water, and surfactant by NMR. Adsorption of polymer leads to a positive shift of the spontaneous curvature; by a slight temperature increase a balanced state with zero spontaneous curvature can be recovered. The swelling is associated with an increased rigidity of the surfactant film, but also steric overlap effects may contribute.
The interpretation in terms of a spontaneous curvature effect, rather than effects on the monolayer bending modulus and the saddle splay modulus as suggested by other authors, was strengthened by experiments at temperatures slightly different from that of the balanced state; as noted above nonionic microemulsions are very sensitive to temperature because of large changes in the spontaneous curvature, which in turn is related to changes in the EO–water interaction. Thus, the balanced state of microemulsions loaded with HM-EHEC changes considerably with minor changes in temperature (less than 1 °C) while changes in the bending and saddle splay moduli are expected to be negligible.
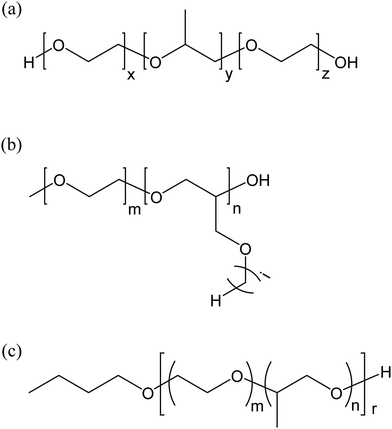
The amphiphilic polymer used in ref. 26 was a commercially available graft copolymer with hydrophobic side chains attached to a hydrophilic backbone. B. Jakobs et al. took the concept one step further by using an amphiphilic polymer tailor-made for the purpose to swell the middle phase microemulsion of a very similar Winsor III system.27 The polymer was poly(ethylene-co-propylene)-co-poly(ethylene oxide), abbreviated PEP–PEO. It was synthesized by hydrogenating a hydroxyl-functionalized polyisoprene segment yielding PEP–OH, which was subsequently reacted with ethylene oxide to yield the block copolymer.28 The degree of ethoxylation could be fine-tuned to optimize the amphiphilic polymer for different oil–water systems. The structure of PEP–PEI is shown in Fig. 5a.
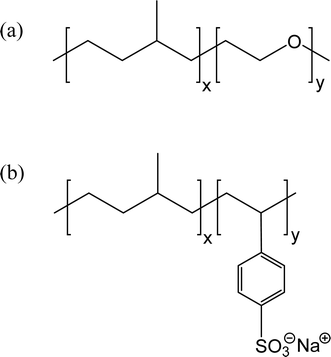 | ||
| Fig. 5 Structure of two related diblock copolymers. (a) Poly(ethylene-co-propylene)-co-poly(ethylene oxide) (PEP–PEO); (b) poly(ethylene-co-propylene)-co-sodium poly(styrene sulfonate). | ||
A very large swelling of the middle phase of a Winsor III system composed of decane–water–C10E4 was obtained with the optimized diblock copolymer. This is illustrated in Fig. 6, which shows the boosting of the middle phase, denoted μE. The sum of surfactant + polymer was kept constant but the ratio of polymer to surfactant increases from left to right. The figure also shows how the middle phase becomes darker as it swells, which is a consequence of the increasing length scale resulting in stronger light scattering.
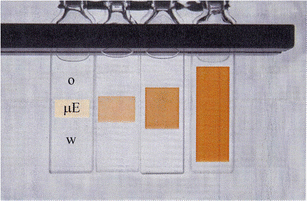 | ||
| Fig. 6 Illustration of the growth of the middle phase of the Winsor III system based on decane–water–C12E4 with different amounts of the amphiphilic polymer PEP–PEO added. The surfactant + polymer content was kept constant, 3 weight%, and the ratio polymer/polymer + surfactant increased from left to right: 0; 0.015; 0.050; 0.115. (Reproduced from ref. 27 with permission from the American Chemical Society, copyright 1999.) | ||
The optimum in molecular weight of the polymer was in the range 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–60
000–60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and not very critical. Somewhat surprisingly the boosting efficiency was also not very dependent on the relative sizes of the hydrophilic and the hydrophobic blocks. An interesting, and practically important, observation was that no lamellar phase in equilibrium with the microemulsion appeared, which is otherwise often the case with very efficient surfactants.
000 and not very critical. Somewhat surprisingly the boosting efficiency was also not very dependent on the relative sizes of the hydrophilic and the hydrophobic blocks. An interesting, and practically important, observation was that no lamellar phase in equilibrium with the microemulsion appeared, which is otherwise often the case with very efficient surfactants.
In several papers following ref. 27 the swelling of the middle phase in the Winsor III system was investigated experimentally and also treated theoretically by the group of R. Strey and also by other groups.29–31 Using a range of scattering techniques, in combination with other methods, the authors were able to demonstrate that the amphiphilic polymer was located at the oil–water interface with the hydrophobic segment protruding into the oil phase and the hydrophilic segment protruding into the aqueous phase. It was also found that an extreme boosting of the middle phase was only obtained when the initial middle phase microemulsion had a zero mean curvature. When the curvature deviated from zero, being either positive or negative, the boosting was much less pronounced. This was attributed to be a polymer induced increase of the rigidity of the surfactant film, as will be discussed in the next section. An interesting observation was that the requirement of a zero mean curvature of the initial microemulsion seems to be more important than the relative sizes of the hydrophilic and the hydrophobic segments of the amphiphilic polymer for efficient boosting of the middle phase. The work presented in ref. 27–31 was later summarized in a paper by T. Sottmann.32
The polymer used in ref. 27–31 is composed of one hydrophilic and one hydrophobic block. If properly balanced such amphiphilic block copolymers will adsorb at the oil–water interface together with the surfactant used to create the microemulsion. Byelov et al. replaced the copolymer with a mixture of the corresponding homopolymers, i.e., the moieties that, when combined, make up the amphiphilic polymer.33 As expected, the homopolymers dissolved entirely in the excess phases and did not partition into the middle phase microemulsion, leading to a contraction of the middle phase. Thus, the homopolymers gave an effect opposite to that of the block copolymer and simultaneous addition of the two homopolymers and the block copolymer resulted in no net effect on the size of the middle phase. A possible application of such an approach could be to be able to adjust the viscosity of the system without affecting the size of the middle phase of a Winsor III system.
The exceptional boosting of the middle phase microemulsion demonstrated in ref. 27 triggered similar work with other polymers. Nilsson et al. created a balanced Winsor III system of octane and water, using n-octyl-β-D-glucoside as surfactant and n-octanol as cosurfactant and studied the effect on the phase behavior when amphiphilic polymers were added.34 Two families of diblock copolymers were investigated: poly(ethylene oxide)-co-poly(dodecene oxide) and poly(ethylene oxide)-co-poly(butylene oxide) and the lengths of both the hydrophilic and the hydrophobic chains were varied. In addition, a comb copolymer with hydrophilic PEG chains and hydrophobic alkyl chains, grafted onto a backbone polymer, was investigated. The structures of the polymers are shown in Fig. 7.
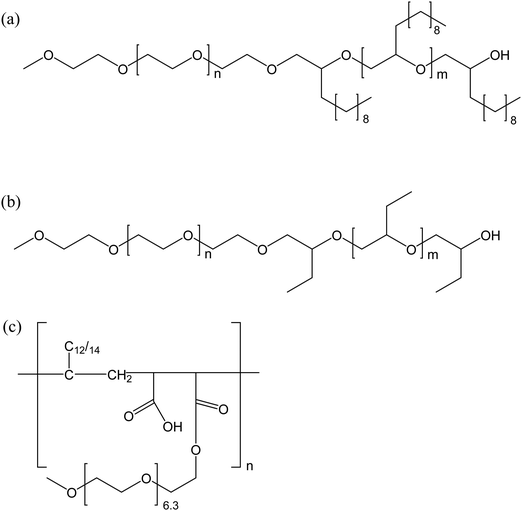 | ||
| Fig. 7 Structure of (a) poly(ethylene oxide)-co-poly(dodecene oxide), (b) poly(ethylene oxide)-co-poly(butylene oxide), and (c) a comb copolymer with grafted alkyl and PEG chains. | ||
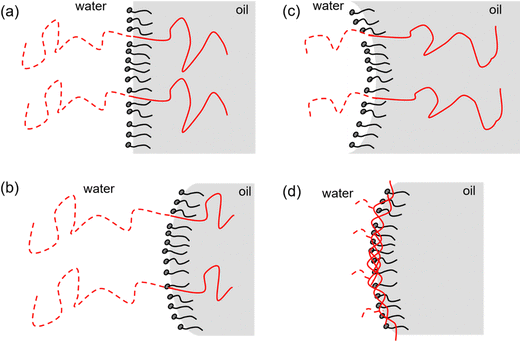
NMR diffusometry was used to study how the polymer addition affected the curvature of the surfactant film. When the film bends away from water (a more positive curvature, see Fig. 2), the self-diffusion of water will increase and the self-diffusion of the hydrocarbon will decrease, and the opposite will be seen when the film bends towards water. It was found that the curvature of the film depended on the relative sizes of the blocks in the block copolymers; the film tended to bend towards the side with the smallest block.
The two diblock copolymers, when optimized with respect to the number of repeating units in the two blocks, were very efficient boosters of the middle phase microemulsion. An example is shown in Fig. 8. As can be seen, the middle phase swells symmetrically as the polymer addition increases and at a certain added amount the whole vial becomes a microemulsion. The NMR measurements indicated that the most efficient polymers were those that gave a planar surfactant film. This finding agrees with what was found when the block copolymer PEP–PEO was added to the decane–water–C12E4 microemulsion, as discussed above.
The amphiphilic graft copolymer did not efficiently swell the middle phase although it is also surface active. This polymer, which contains short PEG chains, which will protrude into the water domain and C12–14 alkyl chains which will point into the oil domain, will most likely be situated along the surfactant film rather than be aligned perpendicular to it. Such an orientation of an amphiphilic polymer is not favorable for boosting the middle phase. NMR self-diffusion measurements showed that the polymer bent the curvature of the surfactant film slightly towards oil. This can be interpreted as the PEG arms (7 EO units) being more effective in swelling water than the C12–14 alkyl chains in swelling oil.
Fig. 9 shows examples of amphiphilic polymers aligned at the surfactant film of a middle phase microemulsion. Block copolymers with one hydrophilic and one hydrophobic segment are situated across the interface and the relative sizes of the segments will govern the curvature of the film. A graft copolymer will be situated along the interface. The most efficient swelling of the middle phase is obtained with block copolymers that give rise to a planar interface, i.e., a zero mean curvature.
Ten years after the work by Nilsson et al. Hoehn and coworkers published a paper on boosting a similar microemulsion using conventional triblock copolymers of EO–PO–EO type, i.e., polymers with a central polyoxypropylene block surrounded by polyoxyethylene blocks, see Fig. 10a.35 These are commercially available polymers in contrast to the tailor-made polymers used in most of the previous works, a fact of considerable practical importance. As expected, the boosting effect depended on the hydrophilic–lipophilic balance of the polymer, a parameter that can easily be adjusted by the relative sizes of the polyoxyethylene and the polyoxypropylene blocks. The more hydrophilic copolymers gave the largest boosting effect. The swelling of the middle phase also depended on the size of the polymer. In general, larger molecular weights gave stronger boosting. The best EO–PO–EO polymers gave a boosting effect comparable with that reported by Jakobs et al. (ref. 27) with tailor-made diblock copolymers.
Triblock copolymers of EO–PO–EO type do not fit into any of the examples shown in Fig. 9. Such polymers are likely to orient the two polyoxyethylene chains into the water domain while the hydrophobic polyoxypropylene segment will form a large loop in the oil domain.
Another type of block copolymer with a PEG chain as hydrophilic segment was investigated with respect to swelling of a bicontinuous microemulsion by K. Schneider et al.36 The hydrophobic segment was poly(alkylglycidyl ethers) with dodecyl and hexadecyl side chains; thus, the hydrophobic part of the diblock copolymer was voluminous. The structure of the polymer is shown in Fig. 10b. The boosting effect was tested on two nonionics-based microemulsions, decane–water–C10E4 and octacosane–water–C16E6. The latter microemulsion with a long very long alkane and a large nonionic surfactant was chosen because of its relevance to EOR. The poly(ethylene oxide)-co-poly(alkylglycidyl ether) type polymer proved to be an efficient microemulsion booster; however, it is not obvious that this somewhat exotic diblock copolymer can compete from a cost-performance point of view with the readily available EO–PO–EO triblock copolymers discussed above.
Also a random copolymer of EO–PO type has been evaluated for boosting efficiency. Takahashi et al. used random polyoxyethylene/polyoxypropylene monobutyl ether, see Fig. 10c, and the microemulsion was decane–water–C12E6.37 It was found that the polymer reduced the amount of surfactant needed to obtain a bicontinuous phase; however, the effect was not impressive compared to other reports. It is likely that the random EO–PO copolymer with a terminal butyl group is not amphiphilic enough to give a strong boosting effect.
The microemulsion-boosting polymers discussed above have all been nonionic, i.e., both the polar segment and the nonpolar segment have been non-charged. In order to study the effect of charged diblock copolymers, Marchal et al. synthesized a copolymer composed of the hydrophobic block poly(ethylene-co-propylene) and the hydrophilic block sodium poly(styrene sulfonate) (PEP–PSS); thus, the hydrophilic segment is in fact an anionic polyelectrolyte.38 The structure is shown in Fig. 5b. The same microemulsion composition as was used in ref. 21 was employed also in this work, i.e., decane–water–C10E4. A very strong swelling of the middle phase was obtained with this polymer; the boosting effect was even higher than what was obtained with the uncharged PEP–PEO used in ref. 27 and discussed above. Evidently, for a Winsor III system based on a nonionic surfactant a powerful boosting effect can be obtained with a polymer that has a charged, as well as an uncharged, hydrophilic segment.
Amphiphilic graft copolymers were tested as microemulsion boosters already in 1994 by Kabalnov et al. and then in 2006 by Nilsson et al., see ref. 26 and 34, respectively. In 2020 Saha et al. revisited the concept and evaluated a number of amphiphilic graft copolymers, also called comb polymers.39 The polymers tested had hydrophilic PEG chains attached to a hydrophobic poly(1,2-butylene oxide) backbone, i.e., the reverse of the polymers that were used by Kabalnov et al., which contained hydrophobic side chains attached to a hydrophilic cellulose backbone. The authors postulated that the PEG chains would anchor in the surfactant film and protrude into the water domain while the backbone would form loops in the oil domain and generate swelling. A range of polymers with different backbone and side chain dimensions were tested. It was found that almost all the polymers became anchored in the surfactant film and that the PEG chains exhibited brush-like characteristics due to high grafting density. However, none of them gave a pronounced boosting effect. Thus, from the results of the three papers reviewed here, ref. 26, 34 and 39, one may conclude that graft copolymers do not bring about a marked swelling of the middle phase of Winsor III systems.
Amphiphilic silicone polymers (also called silicone surfactants) have also been investigated as microemulsion boosters. Kumar et al. obtained a very strong swelling of the middle phase of the dodecane–water–C12E5 system with such amphiphiles.40 The nonpolar segment of these diblock copolymers is extremely hydrophobic, more so than normal hydrocarbon segments. The hydrophilic segment is PEG, like in most regular non-silicone block copolymers. A difference between the system with added amphiphilic silicone polymer and the previously discussed systems with non-silicone polymers is that the lamellar liquid crystalline phase, which often appears at high surfactant concentration, was present with the silicone polymers but suppressed or even non-existent when a non-silicone amphiphilic polymer was used.
S. Maccarrone and coworkers have investigated how the length of the polymer and its architecture affects the swelling of the middle phase microemulsion.41–43 They showed that for diblock copolymers, which are the ones used in most of the works cited above, the boosting effect will only occur if the dimension of the microemulsion, i.e., the distance between the surfactant films, is larger than the end-to-end distance of the polymer. When that is not the case, then there will be no swelling.41 They also investigated a series of so-called sticker polymers with respect to boosting efficiency. Sticker polymers are asymmetric macromolecules that have one or more long hydrophilic arms and a short hydrophobic moiety or vice versa. Thus, they are amphiphilic but they will only be able to influence the membrane from one side. Their effect on the spontaneous curvature of the surfactant film will therefore be large. Sticker polymers of both types and with different geometries were synthesized and evaluated.42 This set of asymmetric polymers is useful for studying the effect of the geometry of the amphiphilic polymer on the spontaneous curvature and on the bending rigidity of the film. However, these polymers seem not to display advantages over the simpler more symmetrical diblock copolymers when it comes to boosting efficiency.
Maccarrone et al. also studied the boosting effect of amphiphilic end-capped polymers, so-called telechelic polymers.43 They used hydrophilic polymers with short hydrophobic chains at both ends. If the end-to-end distance of such polymers is large compared to the microemulsion dimension, the end segments may anchor at different surfactant films, thus acting as bridges across the water domain of the bicontinuous microemulsion. They showed that when the hydrophilic segment was long enough to span the water domain, boosting was achieved but for shorter polymers there was no swelling. However, also these polymers seem not to give an advantage in terms of boosting efficiency over the simpler diblock copolymers.
Several factors determine the effect of polymers on microemulsion swelling
We have above summarized the most important studies on the effect of polymer addition on Winsor III type microemulsions. The polymers investigated are both homopolymers and heteropolymers; of the latter type amphiphilic block copolymers have received the largest attention and have given the largest boosting of bicontinuous microemulsions. The amphiphilic polymers studied range from simple AB structures to more complex ones like ABA structures, end-capped and telechelic polymers, and various graft copolymers. The latter category is of two principal types, those with a hydrophilic backbone and hydrophobic grafts and those with a hydrophobic backbone and hydrophilic grafts. Almost all polymers studied are nonionic and there is little work on charged polymers. The same concerns the surfactant systems chosen. The majority of studies deal with nonionic surfactants, in particular those with a polyoxyethylene polar part, and there are only few studies dealing with swelling of ionic microemulsions. The focus on EO surfactants can be traced to two factors: the phase diagrams are relatively simple since they form microemulsions with only three components, i.e., without cosurfactant, and temperature variations can be used to control the phase behavior and the spontaneous curvature of the surfactant film.The large variety of polymers but also the use of different experimental approaches means that different authors have attributed the observed effects to different mechanisms and emphasized the role of different factors. It is, therefore, not possible at this stage to provide a simple unified picture of the mechanisms; instead, we will briefly mention a few significant points.
The spontaneous mean curvature is zero for maximal swelling of the middle phase. Besides the surfactant chemical structure, the curvature is controlled by temperature for EO-based surfactants and by salinity for ionic surfactants. If a cosurfactant is used, as is usually the case for ionic surfactants, its character and its amount will also affect the curvature. Addition of a polymer adsorbing on the surfactant film may influence the spontaneous curvature strongly. For end-capped polymers or AB block copolymers the size and density of the hydrophilic polymer coils will affect the elasticity and spontaneous curvature and induce a bending towards oil.20 For adsorbing polymers with ionic groups the surfactant film will become charged, and a bending effect due to the counterion entropy will occur.
Microemulsions are thermodynamically stable systems and form under conditions where it has a lower free energy than other phases. Therefore, they will not form if another phase in the surfactant system has a lower energy. For bicontinuous microemulsions there is typically a competition with the formation of a lamellar liquid crystalline phase. Introduction of an amphiphilic polymer disturbs the packing in the surfactant lamellae, destabilizing the lamellar phase. It is, thus, a general observation that addition of amphiphilic polymers increases the stability range of bicontinuous microemulsions.
Besides the effect on the spontaneous curvature a polymer may also increase the rigidity of the surfactant film. A more rigid film gives a larger swelling of the middle phase microemulsion.
Nonadsorbing polymers are known to give segregative phase separation with surfactants. Both hydrophilic and hydrophobic polymers of this type give a deswelling of the middle phase because of a lowered solvent chemical potential.44
Confinement can be expressed as the ratio between the polymer molecular length and the microemulsion domain size. For telechelic polymers it was found that the boosting or antiboosting of a microemulsion depends strongly on confinement.37 If the polymer end-to-end distance is low the polymer coils attach to only one surfactant film whereas with increasing confinement bridging can occur, giving antiboosting. In ref. 41 the role of confinement is discussed for diblock copolymers.
Oil and water self-diffusion has been extensively used to distinguish between different microstructures in surfactant systems since molecular translation is very sensitive to confinement. Nilsson et al.34 could demonstrate that water and oil diffusion in bicontinuous microemulsion allows for a direct insight into the surfactant film curvature, which otherwise is not easily accessible in a direct way. In systems with block copolymer boosting, it is found that the surfactant film bends towards the side of the film with the smallest block.
As mentioned above an ionic block copolymer can have a very large boosting effect on nonionic microemulsions.38 A large effect on the bending modulus can be predicted from the strong anchoring of the charged block on the hydrophilic side of the surfactant film and the electrostatic repulsion between the charged groups. These nonionic microemulsions normally show a change from O/W, to bicontinuous and on to W/O with increasing temperature (see above, Fig. 2) and this applies also in the presence of nonionic block copolymers. However, with an ionic block copolymer such curvature inversion is not possible. This can be understood from the fact that a negative curvature leads to an increased charge density in the surfactant film and a concomitant lowering of the counterion entropy.
Most of the studies discussed above have dealt with adding nonionic polymers to nonionic microemulsions and adding ionic polymers has received little attention. However, ref. 38 and 42 demonstrate that block copolymers with an ionic hydrophilic part can have a large boosting effect on nonionic microemulsions. A large effect on the bending modulus can be predicted from the strong anchoring of the charged block on the hydrophilic side of the surfactant film and the electrostatic repulsion between the charged groups. However, the normal transition from O/W, via bicontinuous, to W/O with increasing temperature (see above) is not found with these block copolymers. This can be understood from the fact that a negative curvature would lead to an increased charge density in the surfactant film and a concomitant lowering of the counterion entropy.
There are many examples of swelling caused by ionic polymers that may be mentioned in this context, all related to the counterion entropy. The extensive swelling with water of polyelectrolyte gels, as used in superabsorbents, is a well-known example. We can also mention the swelling of nonionic lamellar liquid crystals on addition of small amounts of ionic surfactant and of lamellar phases when hydrophobically modified polyelectrolytes are added.45 However, for all ionic systems there is a strong influence of electrolytes which cause a deswelling. With the typical high salt concentrations of oil wells, it is natural that systems based on electrostatic swelling are of less relevance for EOR work.
Implications for EOR
Surfactant flooding has a large potential for improving oil recovery, particularly in mature sandstone reservoirs with relatively light oil. If surfactants are injected together with a viscosity-regulating polymer at a late stage of the field's lifetime, the extra oil recovered can be substantial. The feasibility has been demonstrated both in laboratory experiments and in pilot tests. However, the cost of the surfactant, or surfactants, may be a problem and has forced the engineers to minimize the percentage of amphiphile in the injected water. In the early work on EOR, in the 1980’ and 90's, 2–3% surfactant was the norm; today, the concentration is down to less than 0.5%,16,46 but still the surfactant cost can be prohibitive. If addition of a very small amount of an amphiphilic polymer would be a way to considerably reduce the amount of surfactant needed to arrive at a Winsor III system with very low interfacial tensions, then surfactant flooding would become an even more attractive option.As mentioned above, polymers are normally used today together with the surfactant(s) to obtain the desired viscosity of the injected aqueous surfactant solution.47 Typical polymers used for the purpose are partially hydrolyzed polyacrylamide and xanthan gum.48 These are efficient viscosity modifiers but unfortunately, they are not efficient boosters of the middle phase of Winsor III systems.16 Such polymers can be made more surface active by introducing hydrophobic chains along the polymer backbone, in analogy to what was described above for hydrophobically modified ethylhydroxyethylcellulose (HM-EHEC, see ref. 26 and Fig. 4), but experience has shown that such graft copolymers, although being strongly amphiphilic, are not efficient boosters of middle phase microemulsions. The ideal polymer would be one that aligned in a balanced way at the oil–water interface, see Fig. 9a, and at the same time provided the desired viscosity to the injected water. To the best of our knowledge, such polymers have not yet been reported in the literature.
Conclusions
The observation by Kabalnov and coworkers from 1994 (ref. 25 and 26) that addition of a relatively small amount of an amphiphilic polymer could drastically swell the middle phase of Winsor III systems paved the way for intense research activity along this path. The possibility to make Winsor III systems with a large middle phase without the use of an extensive amount of surfactant was found scientifically interesting and potentially practically useful. Since the size of the middle phase is inversely proportional to the interfacial tensions in the three-phase system, it was realized that microemulsions with extremely low interfacial tensions could be obtained in a cost-efficient way. Among the many applications of such microemulsions, EOR stands out as particularly important. Interfacial tension values of 10−3 mN m−1 or lower are known to be required to obtain successful oil recovery using the surfactant flooding method.The research that followed the seminal work of Kabalnov et al. had a focus on optimizing the structure and the properties of the added polymer. It was found that block copolymers were more efficient than graft copolymers. Amphiphilic block copolymers align at the surfactant film with the hydrophilic segment protruding into the aqueous domain and the hydrophobic segment into the nonpolar domain. It was demonstrated that the highest efficiency was obtained when the hydrophilic and the hydrophobic blocks were comparable in size. Then the surfactant film, i.e., the oil–water interfaces in the middle phase, is roughly planar, which is beneficial for the ability of the middle phase to swell. If the hydrophilic block is larger than the hydrophobic block then the surfactant film becomes curved towards oil, and vice versa for polymers with a large hydrophobic and a small hydrophilic block. Middle phase microemulsions with curved surfactant films do not have a strong ability to swell due to entropic reasons.
An interesting observation is that no lamellar phase was detected in these highly swelled microemulsion systems. This is remarkable because in highly swelled microemulsions based on only surfactants a lamellar phase usually appears in equilibrium with the microemulsion phase and such systems can be difficult to handle. Appearance of a lamellar phase in a microemulsion used for surfactant flooding would likely result in transport problems in the porous rock.
The molecular weight of the copolymer appears not to be critical; however, there is an upper limit to the size of the polymer. If the end-to-end distance of the polymer is large compared to the distance between surfactant films in the microemulsion, the segments may anchor at different surfactant films, thus preventing swelling. The copolymers that have given best results have had a molecular weight in the range 20–70 kDa.
Conflicts of interest
There are no conflicts of interest.Acknowledgements
B. L. acknowledges the Coimbra Chemistry Centre (CQC) FCT through the project UID/QUI/00313/2020. M. L. is supported by Qingdao New Energy Shandong Laboratory Open project (QNESL OP202304).References
- T. P. Hoar and J. H. Schulman, Transparent water-in-oil dispersions: The oleopathic hydro-micelle, Nature, 1943, 152, 102–103 CrossRef CAS.
- V. R. Kokatnur, US Pat., 2111000, 1935.
- D. Bowden and J. Holmstine, US Pat., 2045455, 1936.
- J. H. Schulman, W. Stoeckenius and L. M. Prince, Mechanism of formation and structure of micro emulsions by electron microscopy, J. Phys. Chem., 1959, 63, 1677–1680 CrossRef CAS.
- I. Danielsson and B. Lindman, The definition of microemulsion, Colloids Surf., 1981, 3, 391–392 CrossRef CAS.
- U. Olsson, K. Shinoda and B. Lindman, Change of the structure of microemulsions with the hydrophile-lipophile balance of nonionic surfactant as revealed by NMR self-diffusion studies, J. Phys. Chem., 1986, 90, 4083–4088 CrossRef CAS.
- P. A. Winsor, Solvent properties of amphiphilic compounds, Butterworth, London, 1954 Search PubMed.
- B. Kronberg, K. Holmberg and B. Lindman, Surface chemistry of surfactants and polymers, Wiley, Chichester, 2014, p. 463 Search PubMed.
- R. C. Santana, F. A. Perrechil and R. L. Cunha, High- and low-energy emulsification for food applications: A focus on process parameters, Food Eng. Rev., 2013, 5, 107–122 CrossRef CAS.
- Industrial applications of microemulsions, ed. C. Solans and H. Kunieda, Marcel Dekker, New York, 1997 Search PubMed.
- Handbook of microemulsion science and technology, ed. P. Kumar and K. L. Mittal, Marcel Dekker, New York, 1999 Search PubMed.
- M. Gradzielski, M. Duvail, P. M. de Molina, M. Simon, Y. Talmon and T. Zemb, Using microemulsions: Formulation based on knowledge of their mesostructured, Chem. Rev., 2021, 121, 5671–5740 CrossRef CAS PubMed.
- X. Han, M. Lu, Y. Fan, Y. Li and K. Holmberg, Recent developments on surfactants for enhanced oil recovery, Tenside Surf. Det., 2021, 58, 164–176 CrossRef CAS.
- J. J. Taber, Dynamic and static forces required to remove a discontinuous oil phase from porous media containing both oil and water, SPE J., 1969, 9, 3–12 CAS.
- G. J. Hirasaki, C. A. Miller and M. Puerto, Recent advances in surfactant EOR, SPE J., 2011, 16, 889–907 CrossRef CAS.
- G. Zhang and J. Yu, Effect of commonly used EOR polymers on low concentration surfactant phase behaviors, Fuel, 2021, 286, 119465 CrossRef CAS.
- C. Huh, Interfacial tensions and solubilizing ability of a microemulsion phase that coexists with oil and brine, J. Colloid Interface Sci., 1979, 71, 408–426 CrossRef CAS.
- B. Lindman and K. Thalberg, Polymer–surfactant interactions – recent developments, in Interactions of surfactants with polymers and proteins, ed. E. D. Goddard and K. P. Ananthapadmanabhan, CRC Press, Boca Raton, 1993, pp. 203–276 Search PubMed.
- L. Piculell and B. Lindman, Association and segregation in aqueous polymer/polymer, polymer/surfactant and surfactant/surfactant mixtures: Similarities and differences, Adv. Colloid Interface Sci., 1992, 41, 149–178 CrossRef CAS.
- H. Wennerström and B. Jönsson, Amphiphile-water systems and electrostatic interactions, J. Phys., 1988, 49, 1033–1041 CrossRef.
- J. N. Israelachvili, Intermolecular and surface forces, Academic Press, 1985 Search PubMed.
- D. Fennell Evans and H. Wennerström, The colloidal domain – where physics, chemistry, biology, and technology meet, Wiley-VCH, 2nd edn, 1999 Search PubMed.
- A. Weber and B. Stühn, Structure and phase behavior of polymer loaded non-ionic and anionic microemulsions, J. Chem. Phys., 2016, 144, 144903 CrossRef.
- R. Nagarajan, Polymer-induced structural transitions in microemulsions, Langmuir, 1993, 9, 369–375 CrossRef CAS.
- A. Kabalnov, U. Olsson and H. Wennerström, Polymer effects on the phase equilibrium of a balanced microemulsion, Langmuir, 1994, 10, 2159–2169 CrossRef CAS.
- A. Kabalnov, U. Olsson, K. Thuresson and H. Wennerström, Polymer effects on the phase equilibrium of a balanced microemulsion: Adsorbing versus nonadsorbing polymers, Langmuir, 1994, 10, 4509–4513 CrossRef CAS.
- B. Jakobs, T. Sottmann, R. Strey, J. Allgaier, L. Willner and D. Richter, Amphiphilic block copolymers as efficiency boosters for microemulsions, Langmuir, 1999, 15, 6707–6711 CrossRef CAS.
- J. Allgaier, A. Poppe, L. Willner and D. Richter, Synthesis and characterization of poly(1,4-isoprene-b-(ethylene oxide)) and poly(ethylene-co-propylene-b-(ethylene oxide)) block copolymers, Macromolecules, 1997, 30, 1582–1586 CrossRef CAS.
- H. Endo, J. Allgaier, G. Gompper, B. Jakobs, M. Monkenbusch, D. Richter, T. Sottmann and R. Strey, Membrane decoration of amphiphilic block copolymers in bicontinuous microemulsions, Phys. Rev. Lett., 2000, 85, 102–105 CrossRef CAS.
- H. Endo, M. Mihailescu, M. Monkenbusch, J. Allgaier, G. Gompper, D. Richter, B. Jakobs, T. Sottmann, R. Strey and I. Grillo, Effect of amphiphilic block copolymers on the structure and phase behavior of oil-water surfactant mixtures, J. Chem. Phys., 2001, 115, 580–600 CrossRef CAS.
- M. Mihailescu, M. Monkenbusch, H. Endo, J. Allgaier, G. Gompper, J. Stellbrink, D. Richter, B. Jakobs, T. Sottmann and B. Farago, Dynamics of bicontinuous microemulsion phases with and without amphiphilic block-copolymers, J. Chem. Phys., 2001, 115, 9563–9577 CrossRef CAS.
- T. Sottmann, Solubilization efficiency boosting by amphiphilic block co-polymers in microemulsions, Curr. Opin. Colloid Interface Sci., 2002, 7, 57–65 CrossRef CAS.
- D. Byelov, H. Frielinghaus, O. Holderer, J. Allgaier and D. Richter, Microemulsion efficiency boosting and the complementary effect. 1. Structural properties, Langmuir, 2004, 20, 10433–10443 CrossRef CAS PubMed.
- M. Nilsson, O. Söderman and I. Johansson, The effect of polymers on the phase behavior of balanced microemulsions: diblock-copolymer and comb-polymers, Colloid Polym. Sci., 2006, 284, 1229–1241 CrossRef CAS PubMed.
- S. Hoehn, C. Schulreich and T. Hellweg, Efficiency boosting in technical grade sugar surfactant based microemulsions using Pluronics, Tenside, Surfactants, Deterg., 2014, 51, 32–38 CrossRef CAS.
- K. Schneider, P. Verkoyen, M. Krappel, C. Gardiner, R. Schweins, H. Frey and T. Sottmann, Efficiency boosting of surfactants with poly(ethylene oxide)-poly(alkylglycidyl ether)s: A new class of amphiphilic polymers, Langmuir, 2020, 36, 9849–9866 CrossRef CAS PubMed.
- Y. Takahashi, H. Shirahata, T. Nishimura, M. Murai, K. Wakita and Y. Kondo, Boosting effect of amphiphilic random copolymers for bicontinuous phases, J. Oleo Sci., 2018, 67, 531–537 CrossRef CAS PubMed.
- F. Marchal, P. Guenoun, J. Daillant, D. W. Holley and J. W. Mays, Unprecedented microemulsion boosting effect induced by a charged diblock copolymer: bending modulus and curvature frustration of the surfactant film, Soft Matter, 2009, 5, 4006–4014 RSC.
- D. Saha, K. R. Peddireddy, J. Allgaier, W. Zhang, S. Maccarrone, H. Frielinghaus and D. Richter, Amphiphilic comb polymers as new additives in bicontinuous microemulsions, Nanomaterials, 2020, 10, 2410, DOI:10.3390/nano10122410.
- A. Kumar, M. H. Uddin, H. Kunieda, H. Furukawa and A. Harashima, Solubilization enhancing effect of A-B-type silicone surfactants in microemulsions, J. Disp. Sci. Technol., 2001, 22, 245–253 CrossRef CAS.
- S. Maccarrone, D. V. Byelov, T. Auth, J. Allgaier, H. Frielinghaus, G. Gompper and R. Richter, Confinement effects in block copolymer modified bicontinuous microemulsions, J. Phys. Chem. B, 2013, 117, 5623–5632 CrossRef CAS PubMed.
- M. Brodeck, S. Maccarrone, D. Saha, L. Willner, J. Allgaier, G. Mangiapia, H. Frielinghaus, O. Holderer, A. Faraone and D. Richter, Asymmetric polymers in bicontinuous microemulsions and their accretion to the bending of the membrane, Colloid Polym. Sci., 2015, 293, 1253–1265 CrossRef CAS.
- S. Maccarrone, J. Allgaier, H. Frielinghaus and D. Richter, Anchoring vs. bridging: new findings on polymer additives in bicontinuous microemulsions, Langmuir, 2014, 30, 1500–1505 CrossRef CAS.
- B. Kronberg, K. Holmberg and B. Lindman, Surface chemistry of surfactants and polymers, Wiley, Chichester, 2014, pp. 282–290 Search PubMed.
- H. Bagger-Jörgensen, U. Olsson and I. Iliopoulos, Phase behavior of nonionic microemulsion upon addition of hydrophobically-modified polyelectrolyte, Langmuir, 1995, 11, 1934–1941 CrossRef.
- N. Nadir, S. Shahruddin and J. Othman, Surfactant evaluation for enhanced oil recovery: Phase behavior and interfacial tension, Open Chem., 2022, 20, 1110–1120 CrossRef CAS.
- H. Liao, H. Yu, G. Xu, P. Liu, Y. He and Y. Zhou, Polymer-surfactant flooding in low-permeability reservoirs: An experimental study, ACS Omega, 2022, 7, 4595–4605 CrossRef CAS PubMed.
- J. Machale, S. K. Majumder, P. Ghosh and T. K. Sen, Role of chemical additives and their rheological properties in enhanced oil recovery, Rev. Chem. Eng., 2020, 36, 789–830 CrossRef CAS.
| This journal is © the Owner Societies 2024 |