Smart stimuli-responsive polysaccharide nanohydrogels for drug delivery: a review
Fouad
Damiri
 *ab,
Ahmed
Fatimi
*a,
Ana Cláudia Paiva
Santos
*ab,
Ahmed
Fatimi
*a,
Ana Cláudia Paiva
Santos
 cd,
Rajender S.
Varma
e and
Mohammed
Berrada
*b
cd,
Rajender S.
Varma
e and
Mohammed
Berrada
*b
aChemical Science and Engineering Research Team (ERSIC), Department of Chemistry, Polydisciplinary Faculty of Beni Mellal (FPBM), University Sultan Moulay Slimane (USMS), Beni Mellal 23000, Morocco. E-mail: fouad.damiri@outlook.fr
bLaboratory of Biomolecules and Organic Synthesis (BIOSYNTHO), Department of Chemistry, Faculty of Sciences Ben M’Sick, University Hassan II of Casablanca, Casablanca 20000, Morocco. E-mail: a.fatimi@usms.ma
cDepartment of Pharmaceutical Technology, Faculty of Pharmacy of the University of Coimbra, University of Coimbra, Coimbra, Portugal
dREQUIMTE/LAQV, Group of Pharmaceutical Technology, Faculty of Pharmacy of the University of Coimbra, University of Coimbra, Coimbra, Portugal
eCentre of Excellence for Research in Sustainable Chemistry, Department of Chemistry, Federal University of São Carlos, 13565-905 São Carlos – SP, Brazil. E-mail: berrada_moh@hotmail.com
First published on 1st November 2023
Abstract
Polysaccharides have found extensive utilization as biomaterials in drug delivery systems owing to their remarkable biocompatibility, simple functionalization, and inherent biological properties. Within the array of polysaccharide-based biomaterials, there is a growing fascination for self-assembled polysaccharide nanogels (NG) due to their ease of preparation and enhanced appeal across diverse biomedical appliances. Nanogel (or nanohydrogel), networks of nanoscale dimensions, are created by physically or chemically linking polymers together and have garnered immense interest as potential carriers for delivering drugs due to their favorable attributes. These include biocompatibility, high stability, the ability to adjust particle size, the capacity to load drugs, and their inherent potential to modify their surface to actively target specific cells or tissues via the attachment of ligands that can recognize corresponding receptors. Nanogels can be engineered to respond to specific stimuli, such as pH, temperature, light, or redox conditions, allowing controlled release of the encapsulated drugs. This intelligent targeting capability helps prevent drug accumulation in unintended tissues and reduces the potential side effects. Herein, an overview of nanogels is offered, comprising their methods of preparation and the design of stimulus-responsive nanogels that enable controlled release of drugs in response to specific stimuli.
Dr Ana Cláudia Paiva-Santos obtained her PhD degree in Pharmaceutical Nanotechnology in 2018. She started her pedagogical intervention as a Teaching Assistant during the PhD, and then became an Invited Assistant Professor. Since 2020 she has been an Assistant Professor at the Faculty of Pharmacy of the University of Coimbra (FFUC), where she teaches Pharmaceutical Technology and Nanotechnology. She has published >120 papers and 12 book chapters in nanotechnology and nanomedicine, and submitted one patent application, as a result of work performed with several national and international collaborations (h-index 23). She was among the World's Top 2% of Scientists in 2022 as determined by Stanford University. |
1. Introduction
Each year, a significant portion of the world's population undergoes treatments for cancer, diabetes mellitus,1 neurodegenerative diseases, and cardiovascular conditions. Unfortunately, these treatments often come with side effects that negatively impact the overall well-being of the individuals. Consequently, it is crucial to explore alternative approaches for both diagnosing and treating diseases in order to reduce these adverse effects. Nanotechnologies, encompassing focused administration of drugs, heat treatment, light-based assays, light-induced therapy, and tissue engineering, have been considered to be encouraging methodologies to tackle the drawbacks of existing techniques.2,3 These advancements hold promise in providing potential solutions to surmount these obstacles.Nanohydrogels (NHs), or nanogels, are polymer networks with a size smaller than a micron and comprise hydrogel particles with a nanometer-scale space, exhibiting characteristics of both hydrogels and nanoparticles. In the realm of nanoparticles, which consists of inorganic, lipid, and polymer nanoparticles, nanogels fall into the latter category. To create nanohydrogels, one can either use polymeric precursors or carry out polymerization of monomers under heterogeneous conditions, with cross-linking being a crucial step. Nanohydrogels possess hydrophilic functionalities such as –SO3H, –OH, –CONH2–, and –CONH–, which enable them to absorb considerable quantities of biological fluids or water at the same time as preserving their organizational integrity. However, due to the existence of cross-links, nanogels expand instead of dissolving upon contact with a solvent. This remarkable characteristic makes nanogels highly promising for a wide range of applications. Numerous research studies have showcased the appropriateness of nanogels as carriers for drug delivery.4 This is attributed to their exceptional ability to accommodate a substantial quantity of drugs, maintain a high level of stability, exhibit biocompatibility, and respond to numerous environmental elements, namely ionic strength, pH, and temperature, in a superior manner relative to conventional pharmaceutical nanocarriers.
Extensive research endeavors have focused on investigating stimulus-responsive nanogels in the past decade, resulting in their noteworthy impact on the advancement of drug transport systems (Fig. 1). Nanogels have emerged as targeted nanocarriers that enable precise and controlled release of drugs, thereby improving drug stability in the field of nanomedicine. These nanohydrogels, composed of hydrophilic polymeric systems with a size range below one micron, serve as efficient vehicles for drug delivery. Most nanogels reported previously, however, are not biodegradable, and their synthesis often entails the use of surfactants.5 They possess viscoelastic characteristics and are formed through either non-covalent interactions or covalent bonding between polymer chains. When exposed to an aqueous medium, nanohydrogels tend to absorb water. While their internal structure resembles hydrogels and polyelectrolyte microgels, nanogels differ primarily in size and the kind of reaction deployed in their synthesis. Nanohydrogels possess distinctive qualities that make them ideal for various applications. They exhibit biocompatibility, ensuring compatibility with living systems, and offer exceptional stability.6 Moreover, their particle size can be easily adjusted as needed. Additionally, nanohydrogels are capable of responding to external factors like temperature, pH, light, and ionic strength, thus enhancing their versatility. These exceptional attributes make nanogels well-suited for diverse uses, such as tissue engineering, biomedical implants, gene therapy, and the delivery of medications.7
 | ||
| Fig. 1 A visual representation of nanogels responding to specific stimuli and their application in delivering therapeutic substances (created with https://BioRender.com). | ||
Polysaccharides are sourced from several natural sources, like animals, plants, microbes, and algae.4,8,9 Consequently, nanogels (NG) constructed from biocompatible and biodegradable polymers show immense potential for utilization in drug delivery systems (DDS).4 Moreover, polysaccharide-derived NGs offer excellent biocompatibility, functionality, adjustable size, ample surface area for bioconjugation, and an inner network that allows for precise regulation of the integration and release of bioactive compounds.10 Biopolymeric nanocarriers are created using natural polymers, like cellulose,11 chitosan,12 sodium alginate,13 and hyaluronic acid,14 as well as synthetic polymers, namely polylactic acid (PLA),15 polyacrylamide (PAA),16 poly(lactide-co-glycolide) (PLGA),17 and polyglycolic acid (PGA),18 dendrimers,19 among others.
Intelligent stimuli-responsive polysaccharide nanohydrogels for drug delivery have garnered significant consideration recently. These nanohydrogels, comprising biocompatible and biodegradable polysaccharides, offer promising potential for enhancing drug delivery systems. Herein, a comprehensive overview is offered for the synthesis, characterization, and applications of these intelligent nanohydrogels, besides an exploration of their response to different stimuli like temperature, pH, enzymes, and external triggers.20 Furthermore, the benefits, obstructions, and potential future advancements of utilizing these nanohydrogels in the field of drug delivery are discussed. The advancements in this field are highlighted by exploring the progress made in intelligent stimuli-responsive nanohydrogels derived from polysaccharides, with the ultimate goal of facilitating the growth of these nanohydrogels for effective drug delivery applications.21,22
Nevertheless, the evaluation also highlighted their usage in the transportation of drugs and genes, photodynamic treatment, biological imaging, and biological sensing, with the primary emphasis being on the various types of responsive nanogels, especially those triggered by redox reactions, temperature changes, pH, and light. Simultaneously, it addressed the challenges that need to be circumvented to achieve successful cancer therapy (Fig. 1).
2. Aspects related to the synthesis of stimuli-responsive nanohydrogels
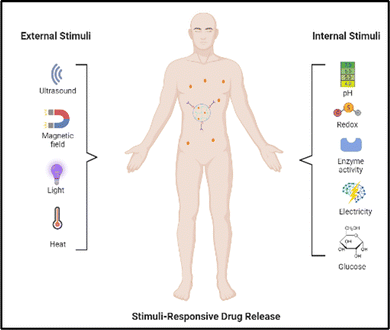
There are two main types of nanoparticle gels (NGs) that respond to various stimuli that can originate from within the body, such as changes in pH, bioreduction, or recognition of specific biomolecules, or can be triggered by external factors like temperature, light, ultrasound, or a magnetic field. The production of “smart” NGs typically involves polymerizing specific functional monomers or utilizing the polymerization of natural polymers that have been modified with functional groups (Table 1). This process is often combined with other synthesis methods and followed by cross-linking.4 NGs can undergo cross-linking via physical or chemical approaches. As mentioned earlier, the physical cross-linking involves non-covalent interactions, and the stability of the resulting physically cross-linked NGs depends on factors such as the composition of the monomer or polymer and the density of cross-linking attained in the course of production. In contrast, chemical cross-linking utilizes “ideal crosslinkable” molecules that form connections with the polymer chains. This method provides accurate manipulation of NG characteristics and represents a promising approach for generating stable NGs without the requirement of supplementary substances like surfactants. Covalent cross-linking has specific advantages in regulating NG strength, structure, and swelling behavior, which are vital for controlled loading and the programmed release of therapeutic and theranostic substances. Over the last ten years, researchers have utilized a range of natural and synthetic polymers, along with cross-linking molecules or macromolecules, to create these dynamic nanogels (NGs). Fig. 2 showcases illustrations of the polymers and cross-linking molecules and macromolecules employed in the manufacturing process.23| Polymer | Nanogel system | Therapeutics | Ref. |
|---|---|---|---|
| Chitosan | CTS-g-PHEMA-maleic acid | Doxorubicin (DOX) | 24 |
| Chitosan/poly(N-isopropylacrylamide) | Gold nanoparticles (AuNPs) | 25 | |
| Trimethyl chitosan (TMC)/poly(2-hydroxyethyl methacrylate) (PHEMA) | Melatonin | 12 | |
| Cellulose | Methacrylated monocarboxylic sugarcane bagasse cellulose (MAMC-SBC)/N-isopropylacrylamide (NIPAM) | Doxorubicin (DOX) | 11 |
| Carboxymethyl nanocellulose (CMNC)/lysozyme | Acyclovir, carbamazepine, and furosemide | 26 | |
| (CDs/DCMC-Gel)-FA | Curcumin (CUR)/doxorubicin (DOX) | 27 | |
| Starch | Carboxymethyl starch-lysozyme | Epigallocatechin gallate (EGCG) | 28 |
| Starch nanocrystals/gum Arabic | Hydroxyurea | 29 | |
| Fe3O4-g-(PNIPAAm-co-PMA)@starch | Doxorubicin (DOX) | 30 | |
| Alginate | Alginate/chitosan (ACC) | Mupirocin | 31 |
| Sodium alginate-chitosan (AO/CHPCS) | Berberine (BBR) | 32 | |
| Alginate | Doxorubicin (DOX) | 33 | |
| Dextran | Dextran (Dex-CHO)/cystamine dihydrochloride (Cys) | Doxorubicin (DOX) | 34 |
| SPI-SA-DX | Curcumin | 35 | |
| (Fe3O4@Dex) | — | 36 | |
| Pectin | Lysozyme-pectin | Methotrexate (MTX) | 37 |
| ALG-g-PHPMA@Et | Etoposide (Et) | 38 | |
| Ovalbumin-pullulan | Curcumin (Cur) | 39 | |
| Hyaluronic acid | Maleoyl-chitosan/poly(aspartic acid) | Amoxicillin (Amox) | 40 |
| (Ce6HANG/DOX) | Doxorubicin (DOX) | 41 | |
| (Lf-DOX/PBNG) | Doxorubicin (DOX) | 42 | |
| Carragenan | (CG or κ-carrageenan) | Rivastigmine tartrate (RIV) | 43 |
| KCAR-NGs | Amoxicillin and iodixanol | 44 | |
| PN-NG@ION PAA-g-κC HG | Levodopa (L-DOPA) | 45 | |
| Heparin | HEP | Doxorubicin (DOX) | 46 |
| Heparin–Pluronic (Hep–Pr) | Paclitaxel and DNase | 47 | |
| HP403 | Cisplatin/curcumin | 48 | |
| Gellan gum | Gellan–cholesterol | Prednisolone (Pred) | 49 |
| Gellan–prednisolone | |||
| Chitosan–gellan gum | Curcumin (Cur) | 50 | |
| Gellan gum (GG)/chitosan (CS) | Polymyxin B (PMB) | 51 | |
| Xanthan gum | Cassava starch (CS)/xanthan gum (XG) | — | 52 |
| Polyethylene glycol/xanthan gum-co-poly(acrylic acid) | Venlafaxine | 53 | |
| Xanthan gum (XG)/poly(AA) | Amoxicillin | 54 |
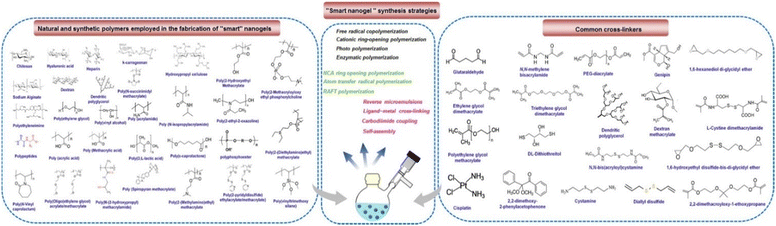 | ||
| Fig. 2 Illustration of various synthetic approaches, natural as well as synthetic polymers and crosslinkers employed in the fabrication of stimuli-responsive polymer nanogels. Reproduced from ref. 23 with permission from Elsevier, copyright 2020. | ||
3. Polysaccharide-based nanogels for drug delivery
Polysaccharides are types of carbohydrates characterized by their extensive polymeric oligosaccharide configurations. These structures are formed through glycosidic linkages, as illustrated in (Fig. 3), which connect several repeating units of monosaccharides. Polysaccharides are naturally occurring compounds that are found in a range of sources, such as plants, as exemplified by pectin, cellulose, and starch.55 Animals also afford polysaccharides such as chitosan, chitin, and glycosaminoglycan.56 The presence of microorganisms is pivotal in the production of diverse polysaccharides, which include notable examples like dextran, pullulan, xanthan gum, and gellan gum. Additionally, algae serve as a valuable source of polysaccharides like agar, alginate, and carrageenan (Fig. 3).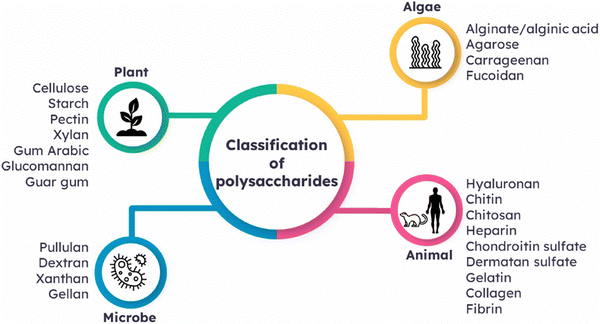 | ||
| Fig. 3 Classification of polysaccharides based on their sources of origin. Reproduced from ref. 4 with permission from Elsevier, copyright 2023. | ||
Natural polymers are favored over synthetic counterparts due to their readily available nature, susceptibility to chemical alterations, renewability, cost-effectiveness, non-toxicity, stability, hydrophilicity, biocompatibility, and biodegradability (Fig. 4). In contrast, synthetic polymers are relatively expensive and pose environmental and toxicity concerns, along with lengthy and involved synthetic processes.
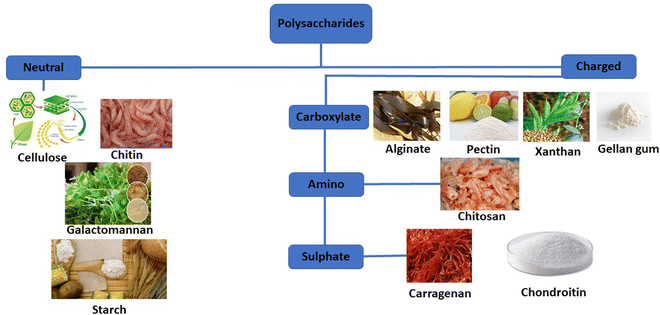 | ||
| Fig. 4 The categorization of common polysaccharides that are widely deployed in the drug delivery field. | ||
3.1. Chitosan-derived nanogels
Chitosan, a cationic polyaminosaccharide, is obtained through the deacetylation process of chitin, which originates from various sources like crustaceans (such as crabs and shrimp), amphineuras (like chiton), archiannelida, fungi, and plants. This naturally derived substance can be dissolved and utilized in numerous appliances, including wound dressings, tissue engineering, drug delivery systems, biosensors, and medical implants.57 Chitosan offers several advantages due to its natural origin, good biocompatibility and biodegradability, bioadhesive properties, non-toxicity, non-immunogenicity, antibacterial and antifungal activities, responsiveness to stimuli, and affordability.4,58,59Yao and colleagues60 introduced an innovative nanogel/gel that exploited chitosan (CS) as a foundation for the oral delivery of myricetin (Myr); CS/β-glycerol phosphate (β-GP) nanogels loaded with Myr of particle sizes ranging from 100 to 300 nm.
3.2. Cellulose-based nanogels
Cellulose is a straight-chain polysaccharide composed of beta D-glucopyranose components arranged in a 4C1 chair configuration and is formed by linking these units together through beta acetal linkages, termed beta-1,4-glycosidic bonds.61 The linear structure of cellulose is a consequence of these linkages. Additionally, cellulose possesses three available hydroxyl groups, which enable the formation of hydrogen bonds within and between the cellulose chains.62,63 Besides, cellulose, the most prevalent biomass material found in nature, possesses remarkable biocompatibility and biodegradability. As a result, cellulose and its derivatives are highly regarded as promising options for fabricating nanogels utilized for drug delivery purposes.11In their study, Pan et al.11 created multi-responsive nanogels by utilizing a modified form of cellulose obtained from sugarcane bagasse (SBC). They deployed cystamine bisacrylamide (CBA) as a crosslinking agent and performed an in situ aqueous copolymerization (free radical) of methacrylated monocarboxylic sugarcane bagasse cellulose (MAMC-SBC) and N-isopropylacrylamide (NIPAM). This synthesis process resulted in the formation of nanogels that exhibit responsiveness to changes in redox conditions, pH levels, and temperature.
3.3. Starch-based nanogels
Due to their affordability, renewability, and biocompatibility, starch and its derivatives have played significant roles in various biomedical applications. Starch is a plentiful polysaccharide and is found in various plant sources such as seeds, beans, and tubers.64 It is made up of two primary components, amylose and amylopectin. These constituents are comprised of glucose molecules interconnected by α-D-(1–6) glycosidic and/or α-D-(1–4) bonds. While amylose primarily possesses a linear structure with minimal branching, amylopectin makes up the majority of starch, accounting for over 75% of its composition.65 Amylopectin consists not only of a chain of α-D-(1–4) glucose units but also of numerous branches that are linked by α-D-(1–6) glucose bonds.66Sousa et al.67 conducted a co-encapsulation synthesis to merge oncocalyxone A (onco A) and magnetite nanoparticles (Fe3O4@citrate) with modified surfaces, forming a unified nanostructure. The nanocapsules obtained displayed a core–shell structure and possessed an average diameter of 143 nm.
3.4. Alginate-derived nanogels
Alginate (ALG) is a linear polysaccharide characterized by non-uniform segments of β-D-mannuronic acid (M) and α-L-guluronic residues (G) linked together through 1–4 bonds.68 Alginate is soluble in water and possesses a block-like structure, where it can exhibit either a homogeneous (poly-G, poly-M) or a heterogeneous pattern (MG).69 The arrangement of monomers and their linkages in the polymer leads to diverse geometries in the M-block regions, G-block regions, and alternating regions.70 The strength or brittleness of ALG gels can be impacted by augmenting the number of G blocks within alginate and increasing the polymer's molecular weight. Although alginic acid is insoluble in water or organic solvents, stable solutions are formed by utilizing monovalent alginate salts. However, if the pH falls below the pKa range of 3.38–3.65, the alginate biopolymer precipitates. Moreover, factors like ionic strength and the presence of gelling ions can affect the solubility of alginate salts.In this context, Suhail et al.71 have created a network of nanogels using polymers to achieve a continuous release of caffeine. To attain this, they employed a free-radical polymerization method to fabricate alginate-based nanogels. To crosslink the alginate polymer, the monomer 2-acrylamido-2-methylpropanesulfonic acid was employed, along with the crosslinker N′,N′-methylene bisacrylamide. Several analyses were performed on the synthesized nanogels, encompassing assessments of the sol–gel fraction, polymer volume fraction, swelling behavior, drug loading capacity, and drug discharge characteristics.
3.5. Dextran-based nanogels
Dextran, derived from microorganisms, is a polysaccharide composed of glucose units connected by α-1,6-glycosidic bonds. In addition, it comprises a minor portion of branches established via α-1,4, α-1,3, and α-1,2 linkages.72,73 This adaptable substance can be readily altered to amplify its reactivity and functionality. In the food sector, dextran has found extensive use due to its stabilizing, emulsifying, viscosity-enhancing, and texturizing characteristics. Apart from its industrial applications, dextran exhibits considerable potential in the realm of biomedicine.34 Dextran is widely utilized in various fields, including drug dispensation and tissue engineering. Specifically, dextran variants with molecular weights of 40, 60, and 70 kDa are commonly employed in biomedical applications owing to their high quality and suitability for clinical use.In their study, Yu et al.34 focused on the generation of dextran-based nanogels termed Dex-SS via a straightforward method that involved the formation of Schiff base bonds containing disulfide between polyaldehyde dextran and cystamine. This process occurred within a water-in-oil inverse microemulsion. The researchers analyzed the morphology of the treated nanogels using SEM imaging and investigated the degradation behavior of these nanogels under both acidic and reductive (GSH) conditions. To achieve controlled drug release, they covalently linked doxorubicin (DOX) to the dextran nanogels through Schiff base connections. As a result, the drug release profiles exhibited sensitivity to both pH and GSH, enabling a dual-responsive drug release mechanism.
3.6. Pectin-based nanogels
Pectin, which is a crucial element found in plant cell walls, is classified as a heteropolysaccharide. It is characterized by the presence of α-(1,4) galacturonic acid units, and this anionic polysaccharide exhibits a substantial molecular weight ranging from 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 130
000 to 130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1.74 Additionally, the pectin backbone also includes neutral sugars such as arabinose, rhamnose, galactose, and smaller quantities of additional sugars.75,76 Pectin is known for its non-toxicity, biocompatibility, and role as a vital component in various biological processes.
000 g mol−1.74 Additionally, the pectin backbone also includes neutral sugars such as arabinose, rhamnose, galactose, and smaller quantities of additional sugars.75,76 Pectin is known for its non-toxicity, biocompatibility, and role as a vital component in various biological processes.
In 2016, Zhou et al.77 created protein/polysaccharide complexes that have captured significant attention due to their potential uses in the food industry, biomedicine, and pharmaceutics. In their research, they focused on developing new nanogels, measuring less than 60 nm, by utilizing a straightforward process involving the complexation of low-density lipoprotein (LDL) from egg yolk with pectin, induced by changes in pH and temperature. The team conducted a detailed examination of the nanostructure of egg yolk LDL under varying pH conditions and thoroughly investigated its interaction with pectin.
3.7. Hyaluronic acid-derived nanogels
Hyaluronic acid (HA) is a glycosaminoglycan naturally present in the human body. It lacks sulfation and is primarily located in skin, connective tissues, and synovial joint fluids. HA is composed of repeating units of the disaccharide β-1,4-D-glucuronic acid – β-1,3 N-acetyl-D-glucosamine.78 HA possesses various physicochemical characteristics, like its viscoelastic properties and remarkable abilities in water retention, making it highly suitable for medical applications due to its biocompatibility and bio-functionality.Luan et al.79 have developed nanogels capable of undergoing degradation in the presence of acidic environments and were synthesized in an aqueous medium using a surfactant-free polymerization technique, employing 2,2-dimethacroyloxy-1-ethoxypropane (DMAEP) as a cross-linker that is sensitive to pH changes. Through the adjustment of cross-linking degrees, nanogels with diverse properties were produced. The researchers successfully loaded the anti-cancer drug doxorubicin (DOX) into the nanogels, achieving drug-loading contents (DLC) ranging from 7.67% to 12.15%. Importantly, when subjected to acidic conditions, the nanogels demonstrated an accelerated release of DOX.
3.8. Carragenan-based nanogels
Carrageenan (CG) is a naturally occurring linear sulfated polysaccharide with a moderate to high molecular weight, typically ranging from 100 to 1000 kDa. It consists of a repetitive pattern of galactose and 3,6-anhydrogalactose, connected by alternating α-(1,3) and β-(1,4)-glycosidic bonds. CG can be classified into six main forms (κ,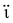 , η, μ, λ, and θ) based on its structural attributes. These forms differ in terms of solubility, the presence and position of sulfate groups (typically ranging from 22% to 35%), as well as the extraction method and source. In the pharmaceutical field, three specific forms of CG, namely κ, λ, and
, η, μ, λ, and θ) based on its structural attributes. These forms differ in terms of solubility, the presence and position of sulfate groups (typically ranging from 22% to 35%), as well as the extraction method and source. In the pharmaceutical field, three specific forms of CG, namely κ, λ, and 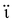 , have particular importance as they have been extensively studied and recognized for their diverse applications. These forms exhibit unique properties and functionalities that make them suitable for pharmaceutical purposes. Therefore, within the pharmaceutical industry, κ, λ, and
, have particular importance as they have been extensively studied and recognized for their diverse applications. These forms exhibit unique properties and functionalities that make them suitable for pharmaceutical purposes. Therefore, within the pharmaceutical industry, κ, λ, and 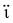 forms of CG are highly regarded and extensively utilized.
forms of CG are highly regarded and extensively utilized.
Rahmani et al.43 developed pH-responsive nanogels that incorporate rivastigmine as a representative drug model. The nanogels were created by the graft polymerizing method, deploying acrylamide and sodium acrylate monomers onto chitosan (CS) and kappa-carrageenan (CG or κ-carrageenan) structures. The synthesis procedure involved the utilization of N,N′-methylenebisacrylamide (MBA) as a cross-linker, along with ammonium persulfate and nitrogen-doped carbon dots (N-CDs) as initiators. Various techniques such as FTIR, FE-SEM, EDX, XRD, and TGA were employed to characterize the nanogels. The swelling performance of the nanogels was affected by factors like monomer content, MBA content, the quantity of CS or CG, and the pH conditions during synthesis. Notably, the nanogels demonstrated notable responsiveness to pH in drug release experiments, with a drug release of less than 61% observed under simulated gastric conditions (pH 1.2) and ∼95% under imitation intestinal conditions (pH 7.4).
3.9. Heparin-based nanogels
Heparin is composed of sulfated disaccharides that repeat and form a polysaccharide, wherein the constituent disaccharides are made up of pyranosyluronic acid and glucosamine residues. Heparin is known for its biological properties and exhibits non-toxic effects besides being capable of undergoing biodegradation.46Nguyen et al.48 developed a nanogel called HP403, which is a combination of amphiphilic heparin and poloxamer P403. The researchers explored a nanogel that possesses the capacity to co-encapsulate cisplatin hydrate (CisOH) and curcuminoid (Cur) through distinct loading mechanisms. Comprehensive analysis of the HP403 nanogels and HP403@CisOH@Cur nanogels was conducted by the researchers using multiple techniques such as FT-IR spectroscopy, 1H-NMR spectroscopy, DLS, and TEM. Through these analyses, it was established that the nanogels maintained their stability effectively and displayed a spherical structure. The drug release process indicated that Cur and CisOH were released at a faster rate under acidic conditions (pH 5.5) compared to under a neutral pH, thus affirming that the nanogels were effective in delivering the loaded compounds to tumor sites.
3.10. Gellan gum-based nanogels
Gellan gum, a type of polysaccharide, is recognized for its linear and negatively charged structure. The composition of the molecule includes a tetrasaccharide segment consisting of two β-D-glucose residues, one α-L-rhamnose residue, and one β-D-glucuronate residue.80 These components make up ∼60% glucose, 20% rhamnose, and 20% glucuronate. Gellan gum can be categorized into two types, high-acyl gellan gum, commonly known as native gellan gum, and low-acyl gellan gum, which is referred to as deacetylated gellan gum.D'Arrigo et al.49 created and characterized nanohydrogels that self-assemble using sonicated chains of gellan gum. They achieved this by chemically linking prednisolone (Pred), an anti-inflammatory drug with limited water solubility, to the carboxylic groups of gellan (Ge-Pred); Ge-Pred played a crucial role as the hydrophobic element accountable for the self-assembly mechanism. The researchers employed 1H-NMR to analyze the Ge-Pred compound, while the cytotoxicity of Ge-Pred on cells was determined using the MTS assay. The self-aggregation characteristics of Ge-Pred in water were examined using the pyrene fluorescence technique, and the resulting nanohydrogels (NHs) were generated through bath sonication in an aqueous medium. Subsequently, these NHs were analyzed utilizing ζ-potential measurements and dynamic light scattering (DLS). The nanohydrogels exhibited an average size of ∼300 nm and demonstrated negative ζ-potential values. The research findings demonstrated that Ge-Pred nanohydrogels were compatible with cells, facilitating the drug's bioavailability. As a result, these nanohydrogels present a promising and innovative carrier for delivering prednisolone.
3.11. Xanthan gum-based nanogels
Xanthan gum (XG) exhibits advantageous properties, including high solubility in water, remarkable biocompatibility, and substantial molecular weight. It is categorized as an exo-polysaccharide due to its structure consisting of branched polymeric chains.81,82 In 1975, the fundamental composition of xanthan gum (XG) was determined, and its constituent units identified as pentasaccharides. XG is composed of D-glucosyl, D-mannosyl, and D-glucuronyl acid residues in a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Additionally, it contains different amounts of pyruvyl and O-acetyl residues. Structurally, XG bears a close resemblance to cellulose, as it exhibits a linear arrangement of D-glucose units linked by (1–4) bonds.83,84
1. Additionally, it contains different amounts of pyruvyl and O-acetyl residues. Structurally, XG bears a close resemblance to cellulose, as it exhibits a linear arrangement of D-glucose units linked by (1–4) bonds.83,84
Ferreira et al.85 conducted a study to investigate the potential anti-tumor effects of polymeric nanocapsules (NC (PhSe)2) loaded with (PhSe)2 on a melanoma cell line called SK-Mel-103, known for its resistance. Additionally, they developed a xanthan gum-based hydrogel for the topical application of NC (PhSe)2. In the in vitro evaluation, the researchers exposed the cells to different concentrations (ranging from 0.7 to 200 μM) of either free (PhSe)2 or NC (PhSe)2. After 48 hours, they conducted the MTT assay to assess cell viability, measured propidium iodide uptake (a marker for necrosis), and evaluated nitrite levels. The hydrogels were prepared by incorporating xanthan gum into the suspension of NC (PhSe)2 or the (PhSe)2 solutions to increase their viscosity. The researchers conducted various characterization tests on the hydrogels, including determination of their average diameter, pH, polydispersity index, spreadability, drug content, and rheological profiles. Additionally, they evaluated the in vitro permeation of the hydrogels through human skin.
4. Different types of stimulus-responsive polysaccharide nanohydrogel
Nanohydrogels have emerged as promising candidates for delivering bioactive substances like drugs, genes, proteins, and enzymes in various drug delivery systems.63 One area of particular interest is the development of stimuli-responsive polymeric nanoparticles, which can detect and respond to internal and external stimuli such as chemical, physical, and biological signals. These nanoparticles, often referred to as “smart” or “intelligent” biomaterials, are highly sought after for their ability to adapt to their surroundings and exhibit environmentally sensitive behavior.4.1. pH-Responsive polysaccharide nanohydrogel
Extensive research has been focused on pH-responsive nanogels, particularly in the realm of targeted drug delivery systems for the treatment of cancer. One distinguishing feature of the tumor microenvironment (TM) is its acidic pH, which sets it apart from healthy tissue.61 Within the tumor microenvironment (TM), both the extracellular and intracellular pH levels, including those in endosomes and lysosomes, exhibit noticeable acidity. The pH ranges for the extracellular environment and intracellular compartments are ∼6.5–6.8 and 4.5–5.5, respectively, in contrast to the normal physiological pH of 7.4. This acidic environment primarily arises from increased glucose conversion into lactic acid during glycolysis, providing additional energy for tumor cell survival. Exploiting this characteristic, pH-sensitive nanogels can be designed to enhance the effectiveness and efficiency of therapeutic interventions. Drug delivery systems that respond to changes in pH provide several benefits, including enhanced drug availability, improved cellular absorption, increased efficiency and stability of drug release, and targeted delivery to specific locations. Nanogels are often designed to exhibit pH responsiveness by incorporating ionizable groups, namely NH2 and –COOH, into the constituent monomers or polymers. Alternatively, they may contain acid-sensitive linkages like ketal, acetal, and hydrazine, among other possibilities. The inclusion of acid-sensitive groups or linkages in nanogels leads to changes in their physicochemical properties. When exposed to an acidic environment, pH-responsive nanogels can swell as a result of electrostatic repulsion among the formed ions or undergo chain dissociation or degradation. These alterations enable the controlled release of drugs. Fig. 5 visually depicts the drug delivery mechanism utilizing acid-sensitive nanogels.86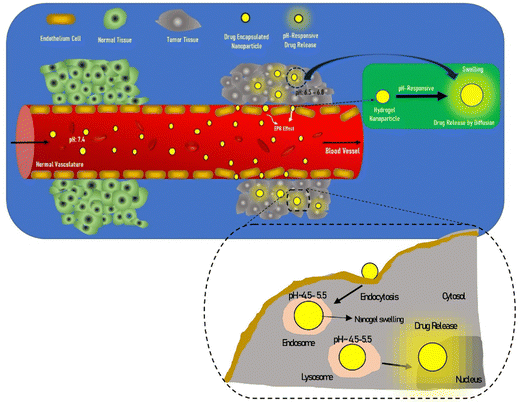 | ||
| Fig. 5 The process of delivering drugs using acid-sensitive nanogels. Reproduced from ref. 86 with permission from Elsevier, copyright 2023. | ||
The pH-responsive degradation of hydrogels is responsible for the targeted discharge of cargo. Zheng et al.87 have developed a hybrid hydrogel that combines micelles and nanocomposites for applications in skin cancer treatment and bacterial extermination, both in vitro and in vivo. They designed a responsive hydrogel system that can respond to multiple stimuli, leading to enhanced photothermal and chemodynamic therapy (CDT). The hydrogel was created by incorporating MoS2@MnFe2O4 nanocomposites into a cross-linked network consisting of chitosan-grafted-dihydrocaffeic acid (CS–DA) and aldehyde Pluronic F127 (F127-CHO) micelles laden with glucose oxidase (GOx). The hydrogel network contained dynamic Schiff-based imine bonds, which allowed for pH responsiveness and controlled degradation of the gel. The hydrogel exhibited a loading capacity of 0.5666 mg g−1 for GOx, and ∼60% of GOx was released over 15 days under a slightly acidic pH of 6.8. Upon release, the GOx enzymatically reacted with glucose, resulting in the generation of hydrogen peroxide and increased acidity in the environment. This acidity allowed iron atoms to participate in the Fenton reaction, producing reactive hydroxyl radicals that induced cell death. The combination of chemodynamic therapy (CDT) with local hyperthermia effectively suppressed tumor growth, achieving a suppression rate of 98.8% in vitro and 97.6% in vivo.
pH-Responsive nanogels find extensive application in drug delivery, and they can be synthesized using both synthetic and natural polymers. Natural polymers like cellulose, chitosan, hyaluronic acid, and dextrin, among others, are highly preferred due to their favorable attributes such as biocompatibility, biodegradability, and non-toxic nature.
In a study conducted by Rahmani et al.,88 a new nanocomposite was created using pH-responsive chitosan (CS), nitrogen-doped carbon quantum dots (NCQDs), and montmorillonite (MMT) (Fig. 6). This nanocomposite was loaded with doxorubicin (DOX) and introduced into a double emulsion system to achieve a controlled and prolonged release of the drug. The incorporation of nitrogen-doped carbon quantum dots (NCQDs) into the CS–MMT hydrogel resulted in notable enhancements in loading and entrapment efficiencies. Additionally, the presence of NCQDs nanoparticles in the CS–MMT hydrogel enabled an extended and pH-responsive release of doxorubicin (DOX) for a duration of 96 hours, surpassing the release profile demonstrated by CS–MMT–DOX nanocarriers at pH 5.4. The release of DOX was precisely controlled and followed the Korsmeyer–Peppas model at pH 5.4, ensuring reduced side effects. On the other hand, no diffusion of DOX was observed at pH 7.4, indicating the potential to minimize the undesirable effects.
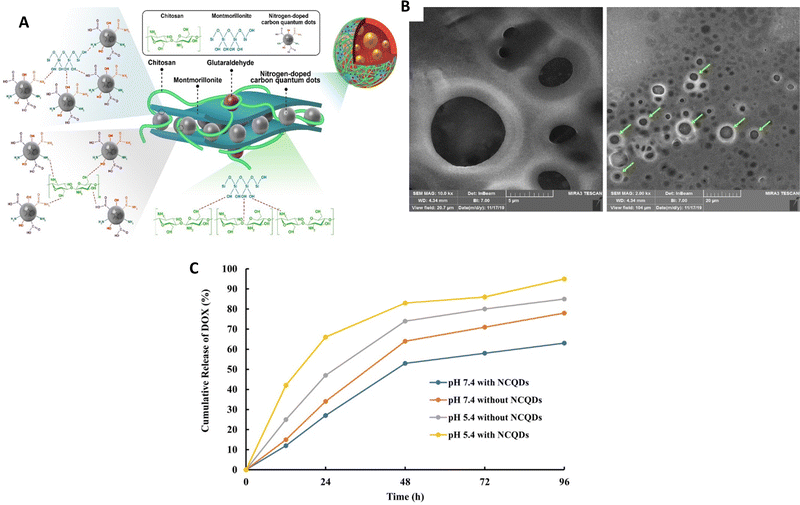 | ||
| Fig. 6 (A) A structural depiction of a hydrogel nanocomposite consisting of cross-linked chitosan (CS), nanocrystalline quantum dots (NCQDs), montmorillonite (MMT) and the drug doxorubicin (DOX) shown as a diagram. (B) An image obtained through field-emission scanning electron microscopy (FESEM) showing the CS–MMT–NCQDs nanocarrier loaded with DOX. (C) The release pattern of DOX from the CS–MMT–NCQDs nanocarrier and CS–MMT without NCQDs in vitro at pH 5.4 and pH 7.4, both at 37 °C, using the dialysis protocol; release profiles measured at specific time intervals. Reproduced from ref. 88 with permission from Wiley, copyright 2022. | ||
An alternative method of developing pH-responsive nanogels involves utilizing polymer components that contain amino functionalities, known as cationic polymers. These polymers have the ability to ionize (protonate) and form NH3+ when exposed to an acidic environment. This ionization process leads to enhanced electrostatic repulsion, triggering a volume-phase transition or swelling of the nanogels. The swelling phenomenon is of paramount importance in the controlled release and regulation of drugs. Furthermore, nanogels with a positive charge have a heightened affinity for negatively charged cell membranes, facilitating their rapid internalization by cells.
Rong et al. conducted a study,89 deploying an injectable composite hydrogel that was obtained by combining hydroxypropyl chitosan (HPCS) and oxidized hyaluronic acid (OHA) through the formation of imine bonds (Fig. 7); functional substances could be delivered using this hydrogel. To enhance its properties, mesoporous polydopamine (MPDA) nanoparticles were integrated into the gel, serving both as an effective photothermal agent and a reservoir for the drug doxorubicin (DOX). This combination allows for effective photothermal conversion and controlled discharge of the drug. In addition, to tackle inflammation resulting from photothermal therapy (PTT), the scientists introduced the curcumin–cyclodextrin host–guest inclusion complex (CUR@NH2-CD) into the hydrogel. In vivo experiments demonstrated that the composite hydrogel effectively inhibited the growth of Hepa1-6 tumors, benefiting from the synergistic effects of MPDA's photothermal properties, DOX's chemotherapy, and CUR's anti-inflammatory activity. These findings indicate that the composite hydrogel has significant potential for comprehensive tumor therapy.
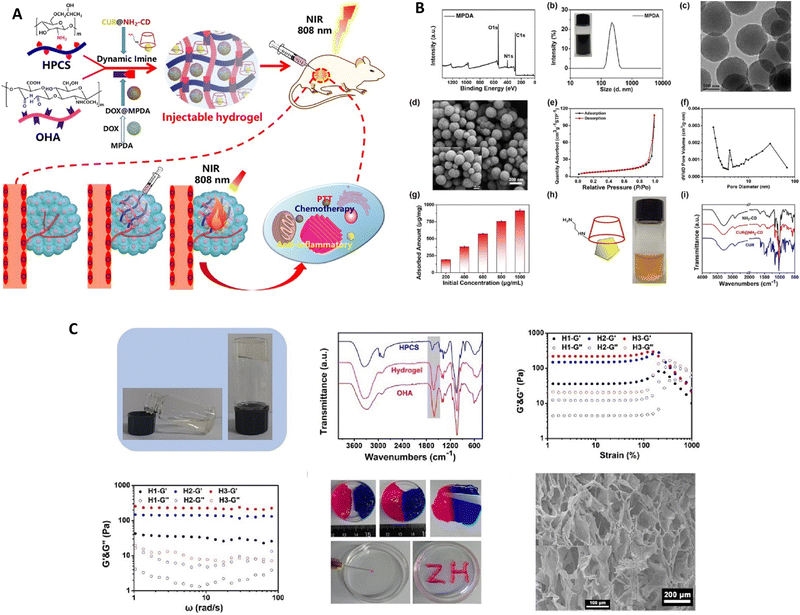 | ||
| Fig. 7 (A) A diagram depicting the conceptual framework of the hydrogel composite intended for tumor treatment utilizing near-infrared (NIR) irradiation. (B) Various analyses were conducted to assess the characteristics of the materials, including the X-ray photoelectron spectroscopy (XPS) wide-scan spectrum, Fourier-transform infrared (FTIR) spectra, size distribution assessment, transmission electron microscopy (TEM) and scanning electron microscopy (SEM) images, nitrogen (N2) sorption isotherm measurement, doxorubicin (DOX) adsorption capacity evaluation, and determination of the corresponding pore size distribution of the MPDA. (C) The process of gelation of the resultant hydrogel, the Fourier-transform infrared (FTIR) spectra of the hydroxypropyl cellulose sulfate (HPCS), octylphosphonic acid (OHA), and the respective hydrogel, the storage modulus (G′) and loss modulus (G′′) of the hydrogel with a gradient composition, as determined through strain sweep and frequency sweep experiments, the images showcasing the self-healing and injecting procedures of the hydrogel, and the scanning electron microscopy (SEM) images of the freeze-dried hydrogel. Reproduced from ref. 89 with permission from Elsevier, copyright 2023. | ||
4.2. Temperature-responsive polysaccharide nanohydrogel
Thermo-responsive polymeric nanoparticles have mainly been used for the precise and controlled delivery of drugs. The temperature in and around diseased areas tends to be higher than the normal body temperature. The distinct properties of tumors have spurred the advancement of temperature-responsive nanogels. At a distinct temperature referred to as the volume-phase transition temperature (VPTT), these nanogels undergo a volume-phase transition, which can manifest as either swelling or deswelling. This transition enables the controlled discharge of drugs within a targeted region. In some instances, external heat is applied to the target site using methods such as light irradiation, electric or magnetic fields, or external heating to trigger the release. Besides polymeric nanogels, nanocarriers based on liposomes, dendrimers, metal nanoparticles, and other materials have also been designed as temperature-responsive nanocarriers for targeted drug delivery.The role of thermoresponsive nanogels as nanocarriers relies on the properties of responsive moieties/polymers, which exhibit a lower critical solution temperature (LCST). At temperatures below the LCST, nanogels maintain sufficient hydration in a water-based environment and form hydrogen bonds with water molecules. However, as the temperature rises above the LCST, nanogels undergo a volume-phase transition, becoming more hydrophobic. This results in the shrinkage of the nanogel structure, enabling the release of drug molecules. The transition is triggered by a reduction in the hydrophilic interactions between the polymer and water molecules, leading to an increase in intra- and inter-hydrophobic interactions among the polymer molecules. The precise lower critical solution temperature (LCST) of a thermosensitive polymer is dictated by its structure and composition, and it can be altered by manipulating the composition of the nanogel. Although PNiPAAm is frequently utilized in nanogel synthesis due to its LCST range of 30–35 °C, its potential toxicity and lack of biodegradability restrict its application as a nanocarrier. To overcome these limitations, efforts have been made to enhance the biodegradability and biocompatibility of nanogels by integrating copolymerization or grafting techniques with biodegradable polymers like cellulose, chitosan, hyaluronic acid, dextran, and similar materials.
Nanogels with temperature responsiveness, derived from hyaluronic acid (HA) and grafted with PNIPAM, have exhibited enhanced drug loading capacity and improved bioavailability when nanogels are utilized for curcumin, which is a hydrophobic anti-cancer medication. The synthesis of HA nanogels grafted with PNIPAM was accomplished using the sonification method, yielding nanogels with a narrow size distribution ranging from 100 to 300 nm and a polydispersity index (PDI) of 0.2. Premature dispensation of the drug payload prior to reaching the intended target sites is a significant challenge in drug delivery systems. To address this issue, the development of nanogels with a hollow-shell structure has been explored, which have the ability to efficiently uptake, store, and subsequently release the cargo, providing a solution to the aforementioned challenge.
Luckanagul et al.90 have developed intelligent material platforms for the delivery of curcumin, via the deployment of nanoscale hydrogel particles derived from the natural polymer chitosan (Fig. 8). In this investigation, chitosan was employed as the fundamental material and subjected to a chemical grafting process with poly-(N-isopropylacrylamide) (pNIPAM) using an EDC/NHS coupling reaction. The resulting conjugated products were analyzed and characterized using TGA and 1H NMR techniques. By employing a sonication method, chitosan-grafted pNIPAM (CS-g-pN) nanogels were prepared, and the loading of curcumin into these nanogels was accomplished through an incubation process. Various analytical techniques, including DLS, TEM, fluorescent spectroscopy, and confocal microscopy, were utilized to investigate the size, morphology, curcumin loading capacity, and cellular uptake of the nanogels, respectively. To evaluate the safety of the CS-g-pN nanogel particles, the CellTiter-Blue® cell viability assay was conducted on NIH-3T3 and HeLa cells. Additionally, an MTT assay was performed on HepG2, Caco-2, MDA-231, and HT-29 cells to assess the cytotoxic effects. The findings indicated that CS-g-pN nanogel particles with submicron sizes could be easily formed by assembling CS-g-pN with a modification degree ranging from 3% to 60%, and the resulting nanogels efficiently encapsulated curcumin. The thermoresponsive properties of the different CS-g-pN nanogel formulations varied based on the density and length of the grafted pNIPAM. Moreover, the CS-g-pN nanogel preparations displayed no toxicity towards HeLa cells or NIH-3T3. Each curcumin-loaded CS-g-pN nanogel formulation was taken up by NIH-3T3 cell lines and exhibited cytotoxicity against the tested cell lines in a dose-dependent manner.
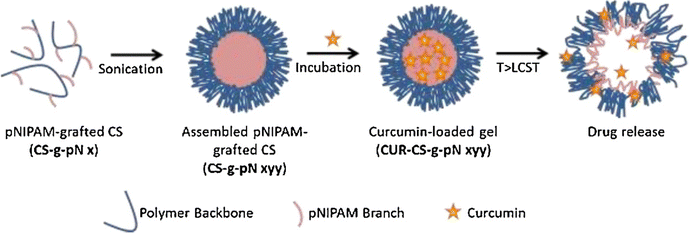 | ||
| Fig. 8 The schematic representation of assembly and drug release of CS-g-pN nanogels. Reproduced from ref. 90 with permission from Elsevier, copyright 2018. | ||
Metawea and his colleagues91 conducted research on the development of poly(N-isopropylacrylamide) (PNIPAM) due to its versatility for chemical modifications, allowing adjustment of its thermoresponsive properties and incorporation of additional stimulus responsiveness (Fig. 9). The main goal of this study was to create a delivery system for resveratrol (RSV), a natural polyphenol with limited bioavailability in breast cancer treatment, that is both targeted and responsive to stimuli. To achieve this, PNIPAM-maltodextrin nanohydrogels (PNIPAM-MD NGs) were synthesized and decorated with folic acid (FA).
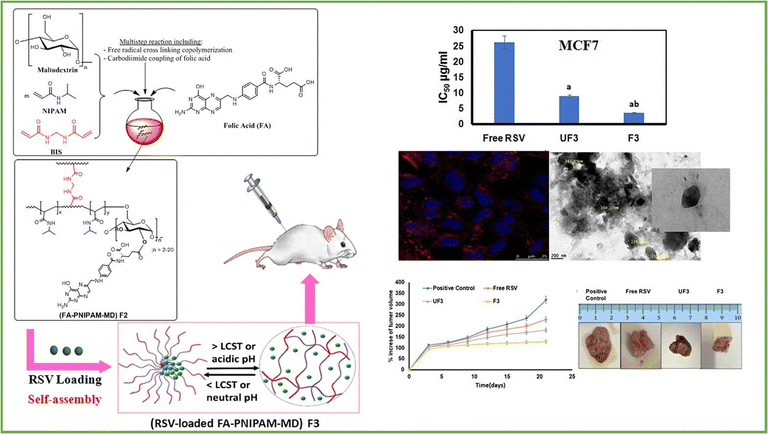 | ||
| Fig. 9 An illustration of the novel thermo-/pH-responsive nanohydrogels composed of folic acid-poly(N-isopropylacrylamide-maltodextrin) for targeted therapy of breast cancer using resveratrol. Reproduced from ref. 91 with permission from Elsevier, copyright 2023. | ||
4.3. Enzyme-responsive polysaccharide nanohydrogel
Enzymes, which are crucial proteins in the human body, play a significant role in facilitating chemical reactions and have great promise for enhancing drug release. They can also accelerate the formation of specific substances, like acids, that can initiate the discharge of drugs. In controlled drug transport, specific enzymes that are found in high levels in tumor cells or the tumor microenvironment can trigger the release of drugs. These enzymes include cathepsin B, MMP-2, MMP-9, and NAD(P)H: quinone oxidoreductase1, among others.Yang et al.92 developed methacrylated hyaluronic acid (HA) nanogels that respond to enzymes for delivering anti-cancer drugs (DOX) to various cancer cells, like NIH3T3, A549, and H22 (Fig. 10). The HA nanogels were synthesized using radical copolymerization with the incorporation of a crosslinker called di(ethylene glycol) diacrylate (DEGDA), which contained enzyme-cleavable ester bonds. The researchers observed the disintegration or breakdown of the enzyme-sensitive nanogel networks by monitoring the change in light scattering intensity (It/I0) of the HA nanogels when exposed to hyaluronidase (Haase) and lipase enzymes. This demonstrated that the nanogels could respond to specific enzymes. The researchers conducted evaluations of the nanogels both in vitro and in vivo to assess their suitability for drug delivery applications.
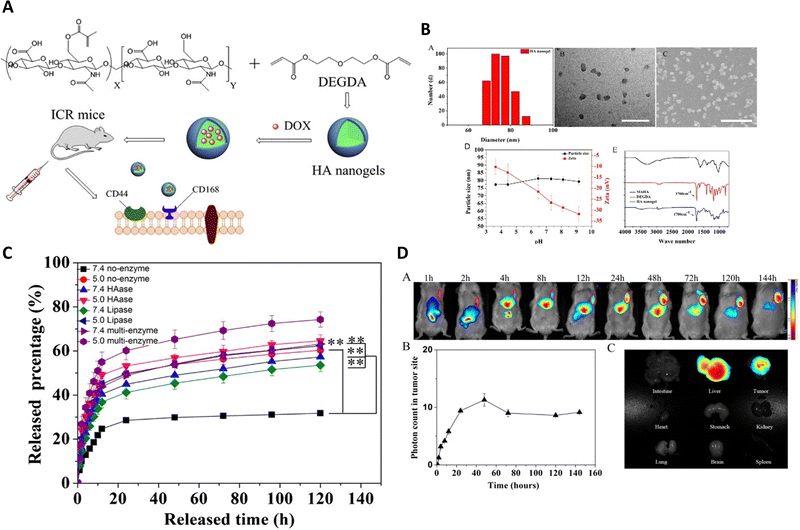 | ||
| Fig. 10 (A) The synthesis pathway for producing HA nanogels is depicted. (B) The hydrodynamic diameter distribution of the HA nanogels is performed using DLS, TEM and SEM images of the HA nanogels, scale bar representing 200 nm with the impact of pH on the particle size and zeta potential of the HA nanogels. The FT-IR spectrum illustrates the comparison between MAHA (black), DEGDA (red), and the HA nanogels (blue). (C) The in vitro release profiles of DOX from the nanogels are evaluated in PBS at pH 5.0 and pH 7.4, at 37 °C, with and without hyaluronidase, lipase, and two other enzymes, respectively. (D) In vivo near-infrared (NIR) fluorescence imaging of H22 tumor-bearing mice after intravenous injection of NIR-797 labeled HA nanogels, and NIR fluorescence intensity of the tumor being measured at different time intervals in mice treated with HA nanogels. Reproduced from ref. 92 with permission from Elsevier, copyright 2015. | ||
Gao et al.93 created a specific nanogel system for treating breast cancer by combining CD44 and biotin receptors, wherein they were loaded with paclitaxel (PTX/Bio-NG). After fabrication using enzyme-sensitive hyaluronic acid, nanogels exhibited a spherical shape with an average particle size of 149.1 ± 1.6 nm (Fig. 11). They demonstrated high entrapment efficiency (90.17 ± 0.52%) and drug loading (15.28± 0.10%). When exposed to hyaluronidase and/or lipase enzymes, the PTX/Bio-NG formulation displayed rapid drug release. Cellular studies verified that the internalization of Bio-NG by 4T1 cells occurred via both the CD44 receptor and the biotin-specific receptor. PTX/Bio-NG, in comparison to PTX-loaded nanogels lacking biotin (PTX/NG), exhibited improved cytotoxicity against breast cancer cells (4T1 cells). Pharmacokinetic analysis conducted in rats demonstrated higher AUC0-t values and lower clearance rates for PTX/NG (6.24 times and 15.96%, respectively) as well as PTX/Bio-NG (6.66 times and 14.89%, respectively) when compared to the control group (Taxol). In vivo studies performed on Balb/c mice with 4T1 tumors demonstrated the excellent therapeutic efficacy of PTX/Bio-NG, indicating its potential as a promising candidate for breast cancer treatment.
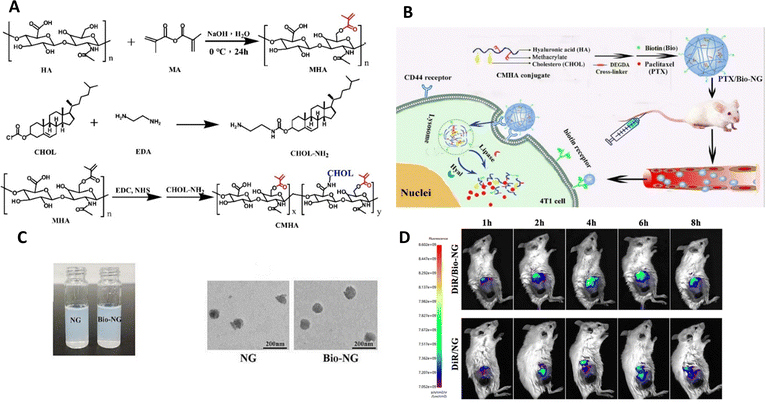 | ||
| Fig. 11 (A) Synthetic schemes for MHA, CHOL-NH2, and CMHA. (B) Schematic illustration showcasing the preparation of PTX/Bio-NG and its in vivo drug transport process. (C) The appearance of PTX/NG and PTX/Bio-NG, along with a TEM image of PTX/NG. (D) Performing in vivo imaging on 4T1 tumor-bearing mice using DiR-loaded formulations. Reproduced from ref. 93 with permission from Elsevier, copyright 2022. | ||
4.4. Glucose-responsive polysaccharide nanohydrogel
Various forms of glucose-responsive hydrogels can be utilized,1,94 including microgels,95 nanogels,96 microneedles,97–99 micelles,100 and mesoporous nanoparticles, which offer diverse options for the application of glucose-responsive nanogels, allowing for flexibility in their use.El Shaarani et al.96 performed a study involving the synthesis of various glucose-responsive nanogels using 4-(1,6-dioxo-2,5-diaza-7-oxamyl) phenylboronic acid (DDOPBA) and N-isopropylacrylamide (NIPAM) (Fig. 12). To serve as the crosslinker, they employed dextran-grafted maleic acid (Dex-MA). The nanogels, referred to as P(NIPAM-co-Dex-co-DDOPBA)s, underwent characterization through various methods, including DLS, TEM, 1H NMR, and X-ray photoelectron spectroscopy (XPS). The incorporation of DDOPBA, which contains an electron-withdrawing group, resulted in significant sensitivity to glucose at physiological pH due to its unique properties. Furthermore, the hydrophilic Dex-MA played a role in modulating the temperature sensitivity of the nanogels near physiological temperatures. The nanogels demonstrated excellent capacity for insulin loading and exhibited a high encapsulation efficiency. In in vitro experiments, the release of insulin from the loaded system was found to be contingent on the concentration of glucose under physiological pH and temperature conditions. This glucose-dependent response allowed for controlled insulin release based on glucose levels. Overall, the study successfully synthesized and characterized glucose-responsive nanogels with desirable properties for drug delivery applications.
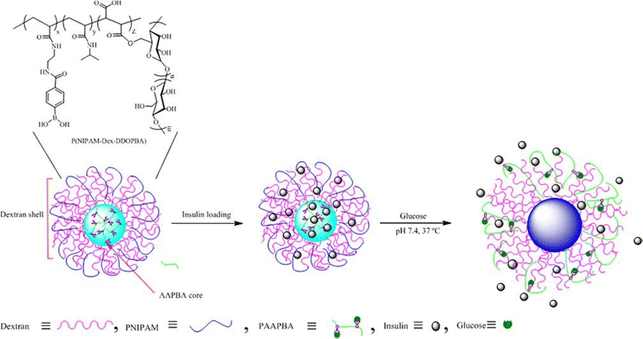 | ||
| Fig. 12 Schematic illustration of glucose-responsive nanogel. Reproduced from ref. 96 with permission from Elsevier, copyright 2020. | ||
Zhang et al.42 developed a novel therapeutic strategy, involving lactoferrin (Lf) and phenylboronic acid (PBA)-functionalized hyaluronic acid nanogels for the treatment of glioma (Fig. 13), and the nanogels were crosslinked using a disulfide-bond crosslinker. This platform, referred to as Lf-DOX/PBNG, is specifically designed to deliver doxorubicin hydrochloride (DOX). The spherical Lf-DOX/PBNG nanogels are endowed with optimized physicochemical properties and exhibit rapid release of DOX under high concentrations of glutathione, which serves as a reduction-sensitive trigger. In cellular investigations, Lf-DOX/PBNG demonstrated enhanced cytotoxicity, increased cellular uptake efficiency, and significantly improved permeability in the brain when compared to neat DOX solution, DOX-loaded PBA functionalized nanogels (DOX/PBNG), and Lf-modified DOX-loaded nanogels (Lf-DOX/NG). Pharmacokinetic analysis indicated that the area under the curve for DOX/PBNG, Lf-DOX/NG, and Lf-DOX/PBNG increased by 8.12, 4.20, and 4.32 times, respectively, in comparison to the DOX solution. Biodistribution studies confirmed that Lf-DOX/PBNG accumulated in the brain at levels 12.37 and 4.67 times higher than neat DOX solution and DOX/PBNG, respectively. These findings emphasize the potential of Lf-DOX/PBNG as an exceptionally effective targeting system for glioma therapy.
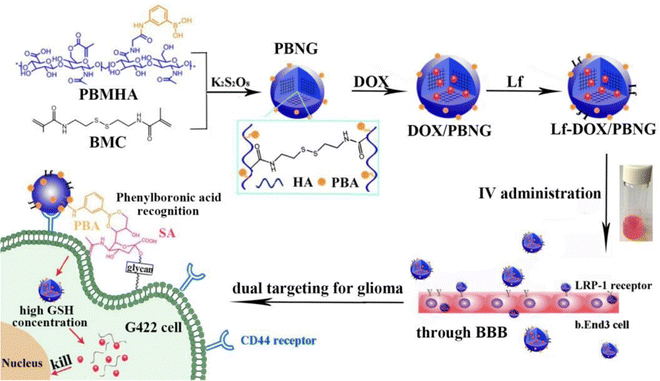 | ||
| Fig. 13 A schematic illustration of Lf-DOX/PBNG for improving BBB penetration and glioma targeting of DOX through receptor-mediated endocytosis. Reproduced from ref. 42 with permission from Elsevier, copyright 2022. | ||
4.5. Light-responsive polysaccharide nanohydrogel
The use of light irradiation encompasses the act of subjecting a sample to light with a specific wavelength, a process that can be readily accomplished. Employing light as a stimulus initiator offers the benefit of toggle-like functionality, enabling precise management of the duration of exposure for a specific system.Extensive research has been conducted on nanoparticles and nanogels that respond to light, during the exploration of their potential applications in targeted drug delivery, diagnostics, and monitoring the progress of healing. These nanocarriers, being sensitive to light, facilitate the transportation of drugs to specific locations through passive, active, and internalization mechanisms. Phototherapy commonly utilizes ultraviolet (UV), visible, and near-infrared (NIR) light. UV light, with a wavelength range of 100–380 nm, has been widely researched for its ability to trigger drug release due to its high sensitivity and energy, which can induce structural changes in materials. However, UV light has limitations such as poor tissue penetrability and phototoxicity, which restrict its use in biomedical applications. On the contrary, visible and infrared lights possess an enhanced capability to penetrate deep tissues as they experience minimal attenuation. The material's reaction to light is contingent upon various factors, including wavelength, intensity/energy, polarity, and length of exposure. While near-infrared light offers advantages in terms of tissue penetration, its limited energy hampers its efficacy in drug transport systems.
The use of light as a trigger for drug transport offers several benefits, including non-invasiveness, precise control over timing and location, and user-friendliness. Nanogels, whether they contain or are attached to bioactive substances or therapeutic medications, experience physical or structural modifications upon exposure to light. These changes enable the targeted discharge of the drug at a specific location. In drug delivery systems activated by light, three main categories of changes in physical, chemical, or structural properties play a crucial role: (i) photochemical alterations, which involve the oxidation, cleavage of bonds, and photopolymerization; (ii) photoisomerization; and (iii) photothermal variations. These modifications in the nanocarriers enable precise and controlled release of the drug when exposed to light stimuli.
Degradable nanogels with the ability to respond to ultraviolet (UV) light and near-infrared (NIR) light have been developed by Hang et al.101 These nanogels utilize hyaluronic acid-g-7-N,N-diethylamino-4-hydroxymethylcoumarin (HA-CM) and are specifically designed for targeted intracellular delivery of doxorubicin (DOX) through CD44 receptors, offering remote control over the drug discharge (Fig. 14). The nanogels, which are of nanometer size, efficiently encapsulate DOX. The nanogels' response to NIR or UV light leads to a significant increase in the release of DOX. This effect is caused by the light-induced breakdown of the urethane bonds that connect CM to HA. When DOX-loaded HA-CM nanogels are combined with NIR irradiation in MTT assays, they exhibit impressive antitumor activity against MCF-7 cells (CD44+), surpassing the activity against U-87MG cells (CD44−) and free HA-pretreated MCF-7 cells. The uptake of DOX-loaded HA-CM nanogels by CD44+ cells was confirmed through observations made using confocal laser scanning microscopy (CLSM). The uptake process occurs via receptor-mediated endocytosis, and the release of DOX inside the cells is induced by NIR light. These HA-CM nanogels possess favorable characteristics for cancer chemotherapy, such as easy preparation, targeting ability for CD44, and light-controlled release of the drug within cells.
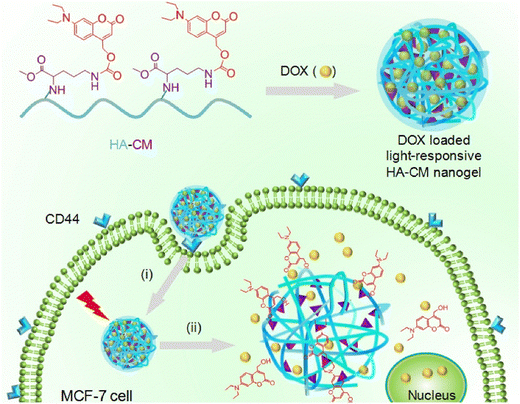 | ||
| Fig. 14 Light-sensitive HA-CM nanogels for CD44 targeted and remotely regulated DOX discharge. (i) Receptor-mediated endocytosis and (ii) nanogel swelling and drug discharge upon exposure to light irradiation. Reproduced from ref. 101 with permission from Elsevier, copyright 2017. | ||
4.6. Magnetic-responsive polysaccharide nanohydrogel
Generally, different types of magnetic nanomaterials, such as iron oxide (Fe3O4, γ-Fe2O3), transition metal ferrites (CoFe2O4, MnFe2O4, etc.),102,103 and transition metal alloys (FePt), can be incorporated into nanogels as magnetic-responsive elements, thus allowing the creation of magnetic hydrogels suitable for biomedical purposes. However, among these potential nanoparticles, Fe3O4 nanoparticles have garnered significant interest and are extensively utilized in biomedical and pharmaceutical fields.104,105 These characteristics contribute to the popularity of superparamagnetic iron oxide nanoparticles (SPIONs), which possess excellent biocompatibility, a high capacity for magnetization, and are relatively easy to prepare and functionalize. They have extensive usage across multiple domains such as magnetic resonance imaging (MRI), blood purification, drug administration, magnetic hyperthermia, in vivo biomarker identification, and cell patterning. In contrast to conventional magnetic particles, SPIONs do not retain their magnetic properties when the external magnetic field is removed. Rather, the magnetic nanoparticles within SPIONs react to variations in a magnetic field, producing heat that plays a vital role in ensuring efficient drug discharge.106 The harmfulness of these nanoparticles is affected by various aspects like their dimensions, form, composition, and surface characteristics. Employing a concentrated magnetic field, magnetically sensitive nanocarriers can be introduced into solid cancer masses to improve the concentration of drugs specifically at the tumor locations.Gao et al.107 have developed a novel type of degradable nanogel known as polymer/Fe3O4 nanocomposite nanogels (NCGs), which possess magnetic, temperature-responsive, and redox-responsive attributes. To produce these NCGs, scientists modified the surface of Fe3O4 magnetic nanoparticles (MNPs) by introducing vinyl groups using 2-isocyanatoethyl methacrylate. Through a process called inverse miniemulsion polymerization, the NCGs were created using poly(N-vinylcaprolactam) (PNVCL) and Fe3O4, employing a cross-linker that contained disulfide. By chemically attaching the Fe3O4 MNPs to the polymer matrix, the structural integrity of the NCGs in water-based environments was ensured. The PNVCL/Fe3O4 NCGs possessed the desirable superparamagnetic properties of Fe3O4 MNPs and demonstrated reversible sensitivity to temperature changes as well as significant responsiveness to redox conditions. The release of a model anticancer drug, 5-fluorouracil, from the PNVCL/Fe3O4 NCGs could be suitably controlled by adjusting the external temperature, the redox state of the surrounding medium, or a combination of both. Moreover, the prepared PNVCL/Fe3O4 NCGs exhibited minimal cytotoxicity, suggesting their potential use as magnetic-guided nanocarriers for precise drug discharge (Fig. 15).
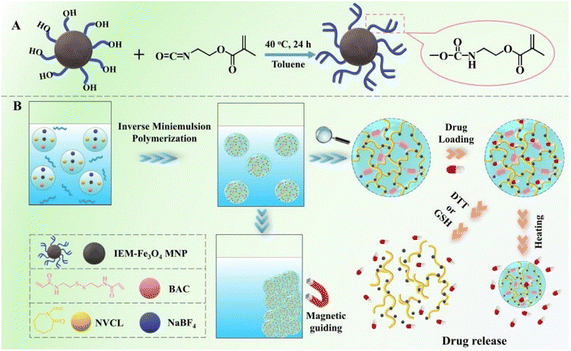 | ||
| Fig. 15 (A) Synthesis process for the surface modification of Fe3O4 magnetic nanoparticles (MNPs) using IEM. (B) Schematic representation of the preparation, multi-responsiveness, as well as the loading and discharge actions of PNVCL/Fe3O4 nanocomposite nanogels (NCGs). Reproduced from ref. 107 with permission from Elsevier, copyright 2020. | ||
DNA nanogels have gained traction as miniature drug carriers in the field of biomedicine and pharmaceuticals due to their exceptional attributes such as biodegradability, biocompatibility, customizable sequence design, and tuning in size.86 The negatively charged nature of DNA facilitates a strong interaction with positively charged drugs like doxorubicin, a potent anticancer medication. This interaction enhances the drug-loading capacity and effectiveness of DNA nanogels.108–111 Recently, Yao et al.,112 devised magnetic nanogels featuring a DNA nanogel shell-core structure incorporating iron oxide (Fe2O4), enabling targeted and triggered release of doxorubicin (DOX) in response to a magnetic field. The magnetic DNA nanogels (M-DNA) with a shell-core structure were created by first generating the DNA shell through a process called rolling circle amplification (RCA), which is an enzymatic DNA polymerization (depicted in Fig. 16A). Subsequently, this DNA shell was coated onto the surface of Fe2O4 nanoparticles modified with amino groups (shown in Fig. 16B). The M-DNA nanogels would accumulate at the tumor site under the influence of an external magnetic field and release the drug in response to pH, temperature, and nuclease as triggers (illustrated in Fig. 16C). Scanning electron microscopy (SEM) imaging revealed the rough surface of the DNA polymer coating on Fe2O4 (shown in Fig. 16D). These meticulously prepared M-DNA nanogels exhibit responsiveness to multiple stimuli, releasing DOX in higher amounts with increasing temperature at pH 7.4 (Fig. 16E) and at lower pH levels (indicating acid sensitivity) (Fig. 16F). Moreover, the application of a magnetic field facilitates the penetration and accumulation of M-DNA nanogels (labeled in green with FTIC) into the tumor, demonstrating enhanced nanoparticle uptake and subsequent release of DOX (in red) into the cytoplasm and nucleus (in blue). This phenomenon becomes more prominent with increased incubation time of nanogels with U87MG cells (as evidenced in Fig. 16G).
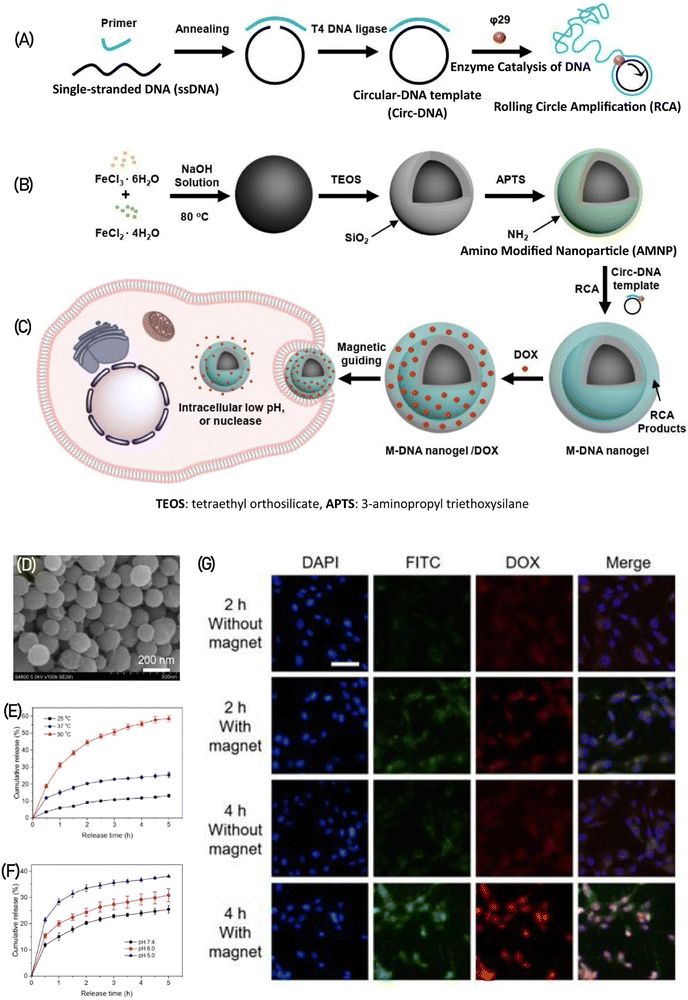 | ||
| Fig. 16 illustrates the production process of M-DNA nanogels. (A) In the first step, a circular DNA template (circ-DNA) is created and subjected to rolling circle amplification (RCA). (B) Concurrently, amino-modified Fe2O4 nanoparticles are prepared. (C) These nanogels are designed to release DOX drug upon exposure to a magnetic field within tumor cells. (D) The SEM image portrays the structure of M-DNA nanogels. The release of DOX from M-DNA nanogels is showcased under various stimuli, including (E) different temperatures at pH 7.4 and (F) different pH levels at 37 °C. (G) Cellular uptake of FITC-stained M-DNA nanogels carrying DOX (depicted in blue) is observed in fluorescence images after incubation with U87MG cells for varying durations of 1, 3, 6, 12, and 14 hours. Reproduced from ref. 112 with permission from ACS, copyright 2022. | ||
4.7. Ultrasound-responsive polysaccharide nanohydrogel
Ultrasounds are produced by applying alternating current and are characterized by high frequency waves. They generate both thermal effects, causing a temperature increase, and non-thermal effects, via the formation of small gas bubbles. The formation and subsequent collapse of these bubbles can locally increase pressure and temperature, leading to changes in the mechanical attributes of the material. Additionally, ultrasounds have the capability to disassemble small vesicles like micelles or nanocarriers, which enables the efficient release of cargo substances, including anti-cancer drugs. However, polymer-based materials that respond to ultrasound are frequently utilized in various applications. These materials encompass polymer-coated bubbles and emulsions,113 such as microbubbles, nanobubbles,114 nanodroplets,115 and nanoemulsions.116 Additionally, polymer vesicles or micelles117 and polymer hydrogels118 are commonly employed. These ultrasound-responsive drug delivery vehicles facilitate the delivery of diverse drugs, including small drug molecules, proteins,119 and DNA.120Maghsoudinia et al.121 investigated the capabilities of theranostic nanoparticles made up of Gd-DOTA/doxorubicin-loaded perfluorohexane (PFH) nanodroplets for both drug delivery and imaging in B16F10 melanoma cancer cells. The internalization of sonicated Gd-DOTA/DOX@PFH nanodroplets by cancer cells was evaluated using inductively coupled plasma optical emission spectrometry (ICP-OES). The analysis indicated a 1.5-fold increase in uptake compared to non-sonicated nanodroplets after a 12 hour period. The biocompatibility of the synthesized nanodroplets was confirmed through in vitro and in vivo toxicity assays, which indicated the absence of organ toxicity. The application of ultrasound notably increased the release of doxorubicin from the nanodroplets, which exhibited strong ultrasound signal intensity and high r1 relaxivity (6.34 mM−1 S−1) for ultrasound echogenicity and T1-MRI relaxometry. The concentration of doxorubicin in the vital organs of mice was significantly lower for Gd-DOTA/DOX nanodroplets compared to free doxorubicin. In the Gd-NDs + US group, which was loaded with doxorubicin, the concentration of doxorubicin in the tumor region reached 14.8 μg g−1 after 150 minutes of sonication, representing a 2.3-fold increase compared to the non-sonicated group. These results emphasize the remarkable diagnostic capabilities (ultrasound/MRI) and therapeutic potential of the synthesized nanodroplets, positioning them as promising theranostic agents for targeted drug discharge in chemotherapy and cancer imaging.
4.8. Redox-responsive polysaccharide nanohydrogel
Apart from the established pH-triggered systems employed in drug delivery, novel strategies utilizing redox-triggered release mechanisms have been developed to achieve targeted drug delivery. The existence of a redox gradient between the intracellular environment with reducing properties and the extracellular environment with oxidizing properties within a tumor serves as the basis for redox-sensitive nanogels.122,123 The capability to regulate and direct drug delivery has attracted significant interest in nanogels as well as other polymeric nanoparticles like micelles, liposomes, and dendrimers. The intracellular microenvironments of cancer cells, comprising the cytoplasm, endosomes, and lysosomes, are characterized by elevated levels of glutathione (GSH), which creates a conducive reducing environment for redox-sensitive nanogels. The variance in redox potential between tumor cells and normal cells offers an ideal stimulus to induce physiochemical alterations in the nanogel's structure, thereby ensuring controlled drug delivery. To impart redox sensitivity, nanogels typically incorporate reduction-sensitive linkages. One frequently utilized linkage among these connections is the disulfide (–S–S–) bond, which can be reduced to thiols (–SH) in reductive environments such as the cancer microenvironment rich in glutathione (GSH). The breakdown of disulfide bonds in nanogels, which are typically stable in extracellular environments, allows for the controlled release of the active drug. Cleavable disulfide linkages in nanogels are attained by deploying crosslinkers bearing disulfide bonds, such as cystamine or dithiodipropionic acid, or through the polymerization of monomers containing disulfides.124Degirmenci et al.125 have developed a method for creating a modular nanogel system that responds to changes in oxidation and reduction and was created by self-assembling dextran-based polymers through host–guest interactions. Nanogel construction involved the intentional utilization of the self-assembly process in a water-based environment, utilizing adamantane (Ada) and β-cyclodextrin (β-CD). By capitalizing on the particular host–guest interactions between β-cyclodextrin (β-CD) and adamantane (Ada), the nanogels were formed through the natural assembly of these molecular constituents. This self-assembly process allowed the fabrication of stable and well-defined nanogels with the desired properties and functionalities. Importantly, they deployed a crosslinker containing disulfide and bis-adamantane, which allowed the nanogels to degrade in response to glutathione (GSH), a naturally occurring reducing agent. In their experiments, the researchers also investigated the potential of loading doxorubicin (DOX) into these nanogels and combining them with a cyclic peptide-based targeting component that contains adamantane, allowing for a non-covalent interaction. Various in vitro studies were performed, focusing on drug release, cytotoxicity, and cellular internalization. The results demonstrated that the nanogels exhibited an enhanced release of the drug in an acidic and glutathione (GSH)-rich environment. Although the empty nanogels demonstrated no toxicity towards cells, the nanogels containing the drug displayed cytotoxic effects against MDA-MB-231 breast cancer cells. The highest level of cytotoxicity was observed in cells with elevated GSH levels when targeted with the drug-loaded nanogels incorporating the targeting component (Fig. 17).
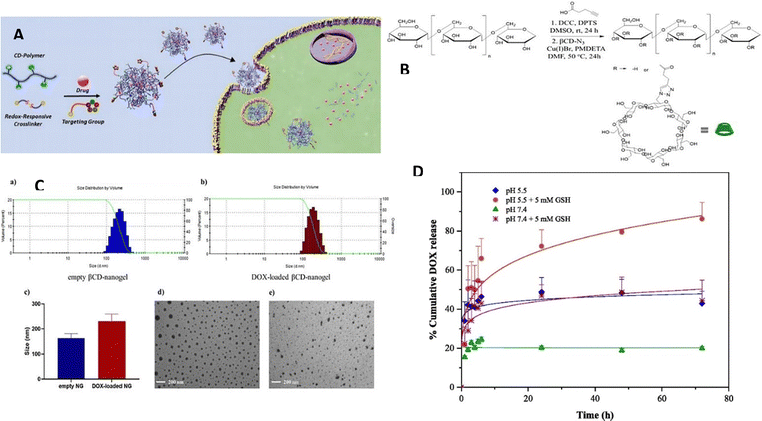 | ||
| Fig. 17 (A) A diagram demonstrating the production process of targeted nanogels containing drugs. (B) The procedure for synthesizing βCD-Dex copolymer using a synthetic route. (C) The range of sizes observed in empty and DOX-loaded βCD-nanogels. (D) The release of DOX in a laboratory setting under different conditions: at pH 5.4 with and without GSH (5 mM), and at pH 7.4 with and without GSH (5 mM). Reproduced from ref. 125 with permission from Elsevier, copyright 2022. | ||
Eskandani et al.126 developed a magnetic hydrogel termed FSRMH, which is a stimuli-responsive system conjugated with folate (FA). This hydrogel has the capability to respond to changes in pH and redox conditions. The researchers utilized tragacanth gum (TG) as the main material for constructing the hydrogel, with the objective of employing it in chemo/hyperthermia therapy of MCF7 cells; the anti-cancer drug doxorubicin hydrochloride (Dox) was loaded into the FSRMH. The hydrogel's porous structure, along with strong hydrogen bonding and ionic interactions between its functional groups and the drug, facilitated efficient drug loading (LE; 8.1 ± 0.25%) and encapsulation (EE; 81 ± 2.50%). In vitro studies on drug release demonstrated that the FSRMH/Dox system exhibited minimal drug release under physiological conditions but displayed pH- and redox-triggered drug release behavior in cancerous conditions characterized by lower pH and higher glutathione (GSH) concentrations (Fig. 18). The biocompatibility of FSRMH was confirmed through an MTT assay, which indicated its cytocompatibility. Hemocompatibility assessment revealed that FSRMH had a hemolysis rate of 2.4 ± 0.17% at 100 μg mL−1, thus affirming the safety of the drug delivery system (DDS). Moreover, the DDS demonstrated a protein adsorption capacity of 59.6 ± 1.70 mg g−1. The cytotoxicity of FSRMH/Dox was evaluated against MCF7 cells through an MTT assay, revealing a synergistic effect between chemotherapy and hyperthermia therapy, with a combination index (CI) of 0.609.
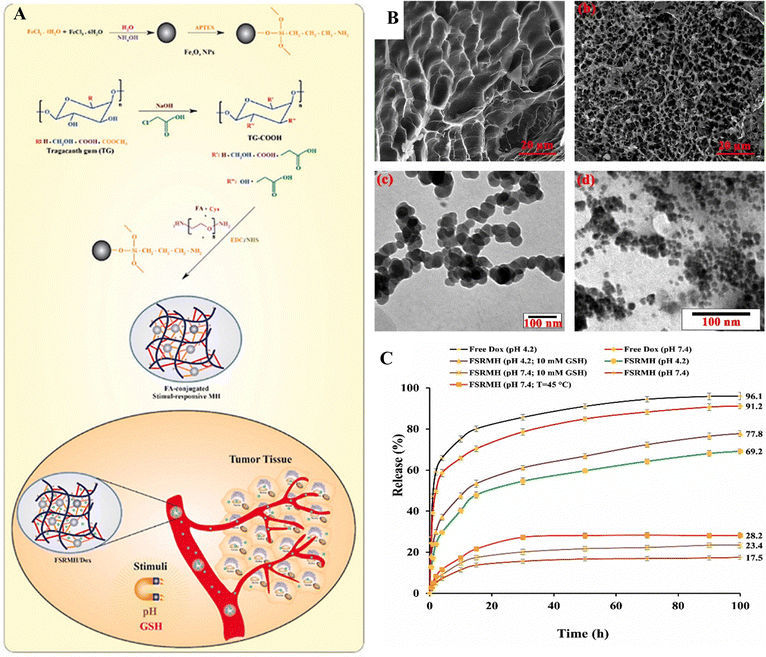 | ||
| Fig. 18 (A) The synthetic pathway for producing FSRMH. (B) SEM images of native TG FSRMH and TEM images of FSRMH and Fe3O4–NH2 nanoparticles. (C) The in vitro drug release profiles of the formulated FSRMH/Dox are evaluated under various conditions; temperature is set at 37 °C, except for FSRMH (pH 7.4; temperature = 45 °C). Reproduced from ref. 126 with permission from Elsevier, copyright 2023. | ||
4.9. Electrical-responsive polysaccharide nanohydrogel
Among all the external stimuli available, an electric field stands out as a convenient and manageable option, particularly in the human microenvironment. Nanogels that respond to electrical stimulation, causing changes in their volume or shape, are typically composed of polyelectrolytes. These electro-responsive nanogels have the ability to convert electrical energy into mechanical energy. Consequently, they find applications as energy conversion devices in assorted fields such as robotics, sensors, controlled drug release systems, and artificial muscles.5. Application of stimulus-responsive polysaccharide nanohydrogels in drug delivery systems
Recently, an increasing body of evidence indicates that the utilization of nanotechnology in the field of medicine has the potential to greatly augment the efficacy of diagnostics and treatments for intricate diseases. This field of research is incessantly advancing, with new breakthroughs occurring almost daily. Among the various drug transport systems available, nanogels have garnered significant attention in view of their unique combination of nanoparticles and hydrogels, as they offer distinct advantages such as targeted and time-controlled delivery of bioactive substances.The drug delivery system based on nanohydrogels has immense promise, primarily attributed to its key characteristics such as stability in encapsulation, intelligent discharge mechanisms, solubility in water, biodegradability, and biocompatibility.127 These properties have paved the way for the advancement of functionalized nanoparticles, serving as carriers for drugs and therapeutics; they allow for targeted and controlled release at precise locations within the body. In recent years, nanotechnology has emerged as a promising avenue for addressing diverse medical conditions, including diabetes, cancer, etc. This section discusses the numerous biomedical applications of nanogels.
5.1. Cancer therapy by nanogels
Irrespective of any nation's level of development, cancer remains a major contributor to illness and mortality on a global scale. In the year 2020, around 19.3 million individuals will have been diagnosed with new cases of cancer, resulting in nearly 10 million deaths worldwide.128 Emphasizing the significance of prevention, early detection, and efficient cancer treatments is of utmost importance. In the field of cancer therapy, targeted drug delivery to precise tumor locations is a crucial strategy, and researchers are exploring various promising approaches. The exploitation of nanogels as drug delivery systems (DDSs) is one such significant strategy. Nanogels, which possess the combined attributes of hydrogels and nanoparticles, have immense potential as targeted DDSs in cancer therapy (Table 2). These versatile systems exhibit a modifiable porous structure and a particle size ranging from 20 to 200 nm, making them well-suited for effective drug delivery.4,129 Nanogels demonstrate excellent stability and possess a significant capacity for drug loading, besides being amenable to customization to actively target specific disease sites, leading to improved drug accumulation. Additionally, nanogels can be designed to respond to several internal or external stimuli, including pH, temperature, light, or redox conditions. This enables the controlled and precise release of the loaded drug.128| NG systems | Active therapeutic agent | NG size (nm) | Stimuli-responsive | Ref. |
|---|---|---|---|---|
| Sugarcane bagasse cellulose | Doxorubicin (DOX) | 90 to 180 | Redox/pH/thermal-responsiveness | 11 |
| Chitosan hydrochloride-carboxymethyl starch | Curcumin (Cu) | 378 | pH responsiveness | 130 |
| Hyaluronic acid (HA) | Doxorubicin (DOX) | 22 to 433 | Redox responsiveness | 131 |
| Carboxymethyl cellulose | Curcumin (Cu) | 120 to 333 | Enzyme responsiveness | 132 |
| Hyaluronic acid-g-7-N,N-diethylamino-4-hydroxymethylcoumarin (HA-CM) | Doxorubicin (DOX) | 147 to 165 | Light responsiveness (irradiated by a UVITRON INTELLI-RAY-400 system operated at 315–400 nm) | 101 |
| Protein/sodium alginate | Curcumin (Cu) | — | pH responsiveness | 13 |
| Poly-N-isopropylacrylamide (PNIPAM) | Indocyanine green (ICG)/5-fluorouracil (5-Fu) | 808 | Thermo responsiveness | 133 |
| Chitin | 5-Fluorouracil (5-Fu) | 120 to 140 | pH responsiveness | 134 |
| 2-(2 methoxyethoxy)ethyl methacrylate (MEO2MA) | Curcumin (Cu) | 30 and 5 | Thermo responsiveness | 135 |
| P(DEAEMA-co-HEMA-g-PEGMA) | SiRNA | 71 to 111 | pH and redox responsiveness | 136 |
| Chitosane-poly(N-isopropylacrylamide-co-acrylamide) | Paclitaxel | — | Thermo responsiveness | 137 |
| O-Carboxymethyl-chitosan/thiolated chitosan | Doxorubicin (DOX) | 123 to 150 | pH and redox responsiveness | 138 |
| Dex-SS | Doxorubicin (DOX) | 592 nm | pH and redox responsiveness | 34 |
| Poly(N-isopropylacrylamide-maltodextrin) | Folic acid (FA)/resveratrol (RSV) | 101 to 159 | Thermo-/pH responsiveness | 91 |
| Chitosan/agarose/graphene oxide | 5-Fluorouracil (5-Fu) | 197 | pH responsiveness | 139 |
| Alginate co-gold nanoparticles | Cisplatin | 20 to 80 | Thermo responsiveness | 140 |
| FCNGL | 5-Fluorouracil (5-Fu) | 100 to 250 | pH responsiveness | 141 |
| 4-Mercaptophenyle boronic acid/oxidized alginate | Doxorubicin (DOX) | 155 | pH and redox responsiveness | 142 |
| PVP | 5-Fluorouracil (5-Fu) | 41 | pH responsiveness | 143 |
| Bovine serum albumin-gum arabic aldehyde (BSA-GAA) | 5-Fluorouracil (5-Fu) | 231 | Thermo-/pH responsiveness | 144 |
5.2. Diabetes therapy by nanogels
In recent years, there has been a significant rise in the worldwide prevalence of diabetes mellitus. The 9th edition of the Diabetes Atlas, published by the International Diabetes Federation (IDF), reported an assessed global population of 463 million with diabetes in 2019. Projections indicate that this number is expected to increase to 578 million by 2030 and reach an astonishing 700 million by 2045. Currently, diabetes mellitus is considered a severe non-communicable disease, ranking closely behind cardiovascular disease and malignant tumors in terms of both mortality and morbidity rates. Diabetes mellitus, one of the most common chronic ailments, is an endocrine and metabolic disorder distinguished by heightened levels of blood glucose (hyperglycemia) and the emergence of various complications.An optimal smart insulin delivery system aims to promptly and accurately respond to fluctuations in blood sugar levels by delivering the appropriate dose of insulin. To fulfill these requirements, these systems must incorporate continuous glucose monitoring sensors and an insulin infusion pump. Consequently, these advanced systems are commonly referred to as glucose-responsive insulin delivery systems (Table 3).
| NG systems | Therapeutics (drugs) | NG size (nm) | Stimuli-responsive | Ref. |
|---|---|---|---|---|
| Kappa-carrageenan/chitosan | Insulin | 18 to 22 | pH responsiveness | 43 |
| Concanavalin A/starch | Insulin | 100 to 300 | Glucose responsiveness | 145 |
| Oxidized starch | Exenatide | 100 to 200 | Glucose responsiveness | 146 |
| Dextran-4-carboxyphenylboronic acid-NIPAM | Insulin | 160 | Glucose responsiveness | 96 |
| 300 | ||||
| Dextran | Insulin | 293 to 340 | Glucose responsiveness | 147 |
| P(NIPAM-co-Dex-co-DDOPBA) | Insulin | 160 to 300 | Glucose responsiveness | 96 |
| Methoxyl poly(ethylene glycol) acrylate and N-acryloyl-3-aminophenylboronic acid | Insulin | 80 | Glucose responsiveness | 148 |
| 107 | ||||
| Carboxymethyl-hexanoly-chitosan-lysozyme | Insulin | 40 | Enzyme responsiveness | 149 |
| Hydroxypropyl methylcellulose methacrylic acid | Insulin | 210 | Thermo responsiveness | 150 |
| 170 | ||||
| Chitosan-based luminescent/magnetic (CLM) | Insulin | 160 | Magnetic responsiveness | 151 |
5.3. Magnetic NG for hyperthermia
Superparamagnetic iron oxide nanoparticles (SPIONs), commonly referred to as magnetic-responsive nanoparticles or NGs, encompass a core comprising magnetite (Fe3O4) or maghemite (c-Fe2O3). These nanoparticles find diverse utility in numerous fields, such as magnetic resonance imaging (MRI), blood purification, pharmaceutical delivery, magnetic hyperthermia, in vivo biomarker detection using biosensors, and cell patterning. Unlike regular magnets, SPIONs lose their magnetism when not exposed to a magnetic field. The magnetic nanoparticles within NGs react to changes in a magnetic field, producing heat that plays a crucial role in pharmaceutical delivery. The toxicity of these products depends on factors such as their shape, size, structure, and surface properties. By utilizing a focused magnetic field, magnetically responsive nanocarriers can be injected into solid tumors to enhance drug accumulation at the tumor sites (Table 4).| NG systems | Therapeutics (drugs) | NG size (nm) | Stimuli-responsive | Ref. |
|---|---|---|---|---|
| Starch | Oncocalyxone A | 143 | Magnetic responsiveness | 67 |
| HA-CysNG@AuNR | Doxorubicin (DOX) | 80 to 250 | Redox responsiveness | 131 |
| Chitosan-g-PNVCL | Doxorubicin (DOX) | 180 to 250 | Thermo-/pH responsiveness | 152 |
| Poly(N-vinylcaprolactam) (PNVCL)/Fe3O4 | 5-Fluorouracil (5-Fu) | 210 | Thermo-/redox responsiveness | 107 |
| 423 | ||||
| (Au/Fe3O4@PEG-b-P(DMAEMA-co-HEMA)-g-PNIPAAm) | Methotrexate (MTX) | 50 | Thermo-/pH responsiveness | 153 |
| 92 | ||||
| MNP@DAS | Doxorubicin (DOX) | 6–10 | pH responsiveness | 154 |
| 42–52 | ||||
| Chitosan-g-N-isopropylacrylamide | Doxorubicin (DOX) | 30 to 50 | Thermo-/pH responsiveness | 155 |
| Poly(N-isopropylacrylamide-co-acrylic acid) | Doxorubicin (DOX) | 60 | Thermo-/pH responsiveness | 156 |
| 380 | ||||
| PLP | Doxorubicin (DOX) | 121 | Thermo responsiveness | 157 |
| 163 | ||||
| Silk fibroin | Curcumin (CUR) | 130 to 210 | Magnetic/pH responsiveness | 158 |
| O-carboxymethylchitosan (O-CMCS) | Maleimides | 4.2 | Magnetic responsiveness | 159 |
| Alginate | Doxorubicin (DOX) | 120 to 320 | Magnet-, pH-, and reduction responsiveness | 160 |
5.4. Photodynamic and photothermal therapy by nanogels
Phototherapies are rapidly advancing methods for treating cancer that utilize different wavelengths of light to create chemical or thermal changes in targeted tissues.161 Photothermal therapy (PTT) and photodynamic therapy (PDT) are the primary categories of phototherapy (Table 5). Both PTT and PDT employ light along with either external or internal absorbers to induce the generation of cytotoxic reactive oxygen species (ROS) or localized temperature increases, respectively. These therapies can complement traditional cancer treatments by acting through unique mechanisms. At the cellular level, both PTT and PDT have displayed the capacity to circumvent chemotherapy resistance and compensate for signaling pathways. PTT and PDT can have effects on various aspects of the tumor microenvironment, including tumor blood flow, vascular permeability, and extracellular matrix permeability. These effects can be leveraged to improve the delivery of cancer medications to tumors. Additionally, PDT and PTT offer superior control over treatment location and timing when compared to systemic therapies, thereby reducing the occurrence of off-target side effects. The latest developments in endoscopic and fiberoptic light delivery methods have enabled the precise targeting of solid tumors, even in anatomically sensitive areas where surgical procedures may not be viable. Significantly, phototherapies utilize nonionizing radiation, thus reducing the risk of secondary cancers compared to radiation therapy.| NG systems | Therapeutics (drugs) | NG size (nm) | Stimuli-responsive | Ref. |
|---|---|---|---|---|
| CS/PNIPAM | Gold | 90–150 | Magnetic and light (green light irradiation at 530 nm) responsiveness | 25 |
| PNIPAM/chitosan | Curcumin (CUR) | 167 | Thermo-/pH responsiveness | 162 |
| CS-g-poly(N-vinylcaprolactam) | Doxorubicin (DOX) | 180 to 250 | pH/thermo responsiveness | 152 |
| Hyaluronic acid/polypyrrole | Doxorubicin (DOX) | 77 | Light responsiveness (808 nm laser irradiation) | 163 |
| CS-GCNCs | 5-Fluorouracil (5-Fu) | 960 | Light responsiveness (microwave irradiation) | 164 |
| Au nanoclusters-Cu2+@sodium alginate/hyaluronic acid | HAuCl4·4H2O | 193 | Light responsiveness (light yellow) | 165 |
| 275 | ||||
| NanoGold-core dendrimeric | Methotrexate (MTTX) | 108 | Light responsiveness (NIR laser-mediated at λ = 808 nm) | 166 |
| 118 | ||||
| Chitosan | Gold | 140 | Light (irradiated alternately with a 655 nm laser light (20 J cm−2) for PDT)/thermo responsiveness | 167 |
| DNA/TB | Cisplatin | 175 | Light responsiveness (irradiated by an LED light source (660 nm, 25 mW cm−2)) | 168 |
| 236 | ||||
| Graphene/hyaluronic acid | Doxorubicin (DOX) | 120 | Light responsiveness (red color light emitted by graphene at 670 nm) | 169 |
| HTCCm-CECm-(HP-β-CD-A) | Paclitaxel (PTX) | 305 | pH responsiveness | 170 |
| 308 | ||||
| 336 | ||||
| ICG/PNIPAM | 5-Fluorouracil (5-Fu) | — | Thermo responsiveness | 133 |
| CS-CD hydrid | Doxorubicin (DOX) | 65 nm | pH responsiveness | 171 |
| (P(NIPAM-co-AAM)) | Doxorubicin (DOX) | — | Light (near-infrared (NIR) light at 700 to 900 nm)/thermo responsiveness | 172 |
5.5. Gene delivery by nanogels
Gene interference technology, centered on gene silencing, has been explored as a potential treatment for genetically inherited diseases (Table 6). Established methods have utilized nanoassemblies, such as stimulus-responsive NGs, to transport siRNA, creating stable polyplexes with improved knockdown capabilities.| NG systems | Therapeutics (drugs) | NG size (nm) | Stimuli-responsive | Ref. |
|---|---|---|---|---|
| Dendritic polyglycerol/polyethylenimine | siRNA | 79 ± 3 | pH responsiveness | 173 |
| GS-AS1411/siRNA | siRNA | 162.3, 216.5, and 234.9 | Redox responsiveness | 174 |
| P(DEAEMA-co-HEMA-g-PEGMA) | siRNA | 71.0 ± 0.7 to 524.6 ± 10.7 | pH and redox responsiveness | 136 |
| 75.1 ± 3.4 to 699.5 ± 20.0 | ||||
| Cationic glycol | siRNA | 750 (∼400 to 500) | Thermo responsiveness | 175 |
| PEI/PNIPAM | microRNA | 50–70 and 20–40 | Redox/pH responsiveness | 176 |
6. Toxicity of the nanohydrogels
The potential harm caused by nanohydrogels deployed in the drug delivery process is a significant issue. The chemical composition of polymer nanogels based on acrylate and methacrylate contains elements that may be harmful, making their routine medical use cumbersome. Furthermore, the presence of unreacted initiators, surfactants, monomers, and oligomers during the production of nanogels introduces additional challenges. To address these issues, purification techniques have been investigated, including thorough rinsing of the end product and adjustments to improve polymerization speed. Additionally, alternative methods like gamma radiation and physical self-assembly have been explored for the preparation of nanogels, thus eliminating the requirement for cross-linking agents and polymerization initiators, strategies that have the potential to decrease toxicity levels. Various methods have been widely employed to evaluate the toxicity of hydrogels or nanogels, namely agar diffusion tests, direct contact, extract dilution, and cell culture techniques. Other tactics employed entail the creation of non-harmful nanogel systems by exploiting biocompatible and abundant natural polymers like pullulan, dextran, alginate, and hyaluronic acid as safer materials. Research findings indicate that cells exposed to these nanogels exhibit elevated rates of survival, thus indicating minimal toxicity.7. Advantages of nanohydrogels
Nanogels possess exceptional features that make them excellent options for co-delivering drugs.177 Their remarkable biocompatibility, stability, significant drug loading capacity, and the ability to release drugs in a controlled manner with environmental triggers render them optimal candidates for drug co-administration,178 primarily due to:1. Exceptional biocompatibility renders nanogels a highly promising avenue for drug delivery systems.179
2. The significant biodegradability of nanogels is vital in preventing the buildup of nanogel material within bodily organs, consequently mitigating toxicity and adverse effects.180
3. In the bloodstream and internal aqueous environment, nanogels remain inert, causing no immunological reactions within the body.181
4. Nanogels can be delivered through diverse methods, such as oral, pulmonary, nasal, parenteral, intra-ocular, and topical routes of administration.182
5. Nanogels are appropriate for delivering both water-loving (hydrophilic) and water-repelling (hydrophobic) medications, as well as charged substances and various diagnostic agents. This characteristic is greatly affected by the kinds of functional groups found in the interconnected polymer chains, the degree of crosslinking, and the specific crosslinking agent integrated into the polymeric structure.183
6. Nanogels exhibit a strong attraction to aqueous solutions, enabling them to expand or contract by absorbing water when placed in certain environments. This quality stands as the most advantageous aspect of nanogels, positioning them as excellent options for absorbing and transporting proteins, peptides, large biomolecules, and voluminous medications.184
7. The drug encapsulation within nanogels surpasses that of other nanocarriers and drug delivery systems, demonstrating notably superiority. This heightened loading capacity can be attributed to the impact of functional groups within the polymer structure. These functionalities create hydrogen bonds or similar weak connections within the polymer network, thus enhancing the capacity to load drugs or proteins by engaging with molecules at the interface.182
8. Integrating drugs into nanogels is a straightforward and natural process, often not dependent on specific chemical reactions. This characteristic streamlines the preparation of nanogels, as the drug doesn't have to be present in the initial stages of manufacturing. Instead, it can be introduced into the nanogel network in subsequent stages, particularly when the nanogels expand with water or aqueous biological fluids.185
9. Nanogels are engineered to release drugs in a controlled and consistent manner at the intended site, ultimately amplifying the drug's therapeutic effectiveness while mitigating any undesirable reactions.186
8. Conclusion and future perspective
Lately, there has been considerable attention directed towards nanocarriers within the realm of drug transport research. These nanocarriers have gained attention due to their diverse structures, which can be customized to package and transport loaded cargo to specific locations. Nanogels are a type of sub-micron hydrophilic polymeric network that exhibits good biocompatibility, high water absorption, and stability, besides offering intriguing possibilities for drug loading with minimal toxicity. The synthesis of nanogels is achievable through diverse methods, and the selection of a particular method influences the ultimate characteristics of the ensuing nanogel.Overall, nanogels exhibit significant potential as vehicles for transporting drugs, offering promising prospects for intelligent drug delivery. The formulation of nanogels as optimal drug carriers should encompass several key attributes, including a high capacity for drug loading, an extended duration of circulation within the body, the presence of specific ligands that can be recognized by target cells, and the ability to degrade in response to specific stimuli. Undoubtedly, drug carriers play a vital role in tumor treatments by minimizing drug toxicity, enhancing therapeutic effectiveness, and improving patient tolerance. While numerous research endeavors have evaluated the effectiveness and safety of nanogel formulations, there is a scarcity of data regarding their long-term buildup and breakdown patterns. Thus, safety remains a principal apprehension for the prospective clinical use of nanogels. It's important to emphasize that a nanogel suitable for clinical use ought to be created using biocompatible and biodegradable materials, ensuring a chemistry that is safe and devoid of toxicity. Innovative design strategies, coupled with thorough in vivo investigations of nanogels, are pivotal in advancing them towards the clinical implementation.
In conclusion, we believe that polysaccharides-based nanocarriers represent a category of transporters that are straightforward to manufacture, feature a broad range of functions, and are economically viable. These carriers have significant promise for advancing precision medicine by enhancing targeted drug delivery to augment the drug absorption and mitigate drug-related toxicity. Additionally, these deliberations may serve as a catalyst for future investigations into the potential secondary effects, including potential side effects of drug-loaded polysaccharide-based nanogels. This exploration is crucial for optimizing this drug delivery system for eventual clinical application, especially with a focus on achieving anti-inflammatory effects.
Author contributions
Conceptualization: F. D., A. F., A. C. P. S., R. S. V., and M. B.; investigation: F. D., A. F., and M. B.; methodology: F. D., A. F., A. C. P. S., R. S. V., and M. B.; supervision: A. F., and M. B.; writing – original draft preparation: F. D., A. F., and M. B.; writing – review and editing: F. D., A. F., A. C. P. S., R. S. V., and M. B.; funding acquisition: A. F., and M. B. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.References
- Y. Liang, M. Li, Y. Yang, L. Qiao, H. Xu and B. Guo, ACS Nano, 2022, 16, 3194–3207 CrossRef CAS PubMed.
- M. Ghovvati, M. Kharaziha, R. Ardehali and N. Annabi, Adv. Healthcare Mater., 2022, 11, 2200055 CrossRef CAS PubMed.
- P. Wang, X. Meng, R. Wang, W. Yang, L. Yang, J. Wang, D. Wang and C. Fan, Adv. Healthcare Mater., 2022, 11(13), 2102818 CrossRef CAS PubMed.
- F. Damiri, S. Rojekar, Y. Bachra, R. S. Varma, S. Andra, S. Balu, C. V. Pardeshi, P. J. Patel, H. M. Patel, A. C. Paiva-Santos, M. Berrada and M. C. García, J. Drug Delivery Sci. Technol., 2023, 104447 CrossRef CAS.
- H. Urakami, J. Hentschel, K. Seetho, H. Zeng, K. Chawla and Z. Guan, Biomacromolecules, 2013, 14, 3682–3688 CrossRef CAS PubMed.
- M. Z. Quazi and N. Park, Int. J. Mol. Sci., 2022, 23, 1943 CrossRef CAS PubMed.
- Nano Hydrogels, ed. J. Jose, S. Thomas and V. K. Thakur, Springer Singapore, Singapore, 2021 Search PubMed.
- R. Mohammadinejad, H. Maleki, E. Larrañeta, A. R. Fajardo, A. B. Nik, A. Shavandi, A. Sheikhi, M. Ghorbanpour, M. Farokhi, P. Govindh, E. Cabane, S. Azizi, A. R. Aref, M. Mozafari, M. Mehrali, S. Thomas, J. F. Mano, Y. K. Mishra and V. K. Thakur, Appl. Mater. Today, 2019, 16, 213–246 CrossRef.
- A. O. Ijaola, D. O. Akamo, F. Damiri, C. J. Akisin, E. A. Bamidele, E. G. Ajiboye, M. Berrada, V. O. Onyenokwe, S.-Y. Yang and E. Asmatulu, J. Biomater. Sci., Polym. Ed., 2022, 1–55 Search PubMed.
- J. K. Oh, D. I. Lee and J. M. Park, Prog. Polym. Sci., 2009, 34, 1261–1282 CrossRef CAS.
- Y. Pan, J. Liu, K. Yang, P. Cai and H. Xiao, Mater. Sci. Eng., C, 2021, 118, 111357 CrossRef CAS PubMed.
- K. Lekjinda and P. Sunintaboon, Carbohydr. Polym., 2023, 304, 120495 CrossRef CAS PubMed.
- S. Shahbazizadeh, S. Naji-Tabasi and M. Shahidi-Noghabi, Chem. Biol. Technol. Agric., 2022, 9, 41 CrossRef CAS.
- T. Fernandes Stefanello, A. Szarpak-Jankowska, F. Appaix, B. Louage, L. Hamard, B. G. De Geest, B. van der Sanden, C. V. Nakamura and R. Auzély-Velty, Acta Biomater., 2014, 10, 4750–4758 CrossRef CAS PubMed.
- A. Kongprayoon, G. Ross, N. Limpeanchob, S. Mahasaranon, W. Punyodom, P. D. Topham and S. Ross, Polym. Chem., 2022, 13, 3343–3357 RSC.
- M. Zhu, D. Lu, A. H. Milani, N. Mahmoudi, S. M. King and B. R. Saunders, J. Colloid Interface Sci., 2022, 608, 378–385 CrossRef CAS PubMed.
- S. Inphonlek, P. Sunintaboon, M. Léonard and A. Durand, Carbohydr. Polym., 2020, 242, 116417 CrossRef CAS PubMed.
- C. Guido, M. Testini, S. D’Amone, B. Cortese, M. Grano, G. Gigli and I. E. Palamà, Mater. Adv., 2021, 2, 310–321 RSC.
- S. L. Mekuria, Z. Ouyang, C. Song, J. Rodrigues, M. Shen and X. Shi, Bioconjugate Chem., 2022, 33, 87–96 CrossRef CAS PubMed.
- N. K. Preman, S. Jain and R. P. Johnson, ACS Omega, 2021, 6, 5075–5090 CrossRef CAS PubMed.
- A. A. Ali, A. Al-Othman and M. H. Al-Sayah, J. Controlled Release, 2022, 351, 476–503 CrossRef CAS PubMed.
- S. Pardeshi, F. Damiri, M. Zehravi, R. Joshi, H. Kapare, M. K. Prajapati, N. Munot, M. Berrada, P. S. Giram, S. Rojekar, F. Ali, M. H. Rahman and H. R. Barai, Polymers, 2022, 14, 3126 CrossRef CAS PubMed.
- N. K. Preman, R. R. Barki, A. Vijayan, S. G. Sanjeeva and R. P. Johnson, Eur. J. Pharm. Biopharm., 2020, 157, 121–153 CrossRef CAS PubMed.
- F. Mahmoodzadeh, M. Ghorbani and B. Jannat, J. Drug Delivery Sci. Technol., 2019, 54, 101315 CrossRef CAS.
- A. Pourjavadi, M. Doroudian, M. Bagherifard and M. Bahmanpour, New J. Chem., 2020, 44, 17302–17312 RSC.
- T. Gabriel, A. Belete, G. Hause, R. H. H. Neubert and T. Gebre-Mariam, J. Drug Delivery Sci. Technol., 2022, 75, 103665 CrossRef CAS.
- M. Pooresmaeil and H. Namazi, Int. J. Biol. Macromol., 2022, 200, 247–262 CrossRef CAS PubMed.
- J. An, M. Liu, Z. Din, F. Xie and J. Cai, Int. J. Biol. Macromol., 2023, 247, 125697 CrossRef CAS PubMed.
- I. M. Alwaan, M. M. R. M. Jafar and Z. S. M. Allebban, Biomed. Phys. Eng. Express, 2019, 5, 025021 CrossRef.
- B. Massoumi, Z. Mozaffari and M. Jaymand, Int. J. Biol. Macromol., 2018, 117, 418–426 CrossRef CAS PubMed.
- M. Hesan, A. Gholipour-Kanani, M. Lotfi and M. Shafiee, Biochem. Eng. J., 2023, 190, 108742 CrossRef CAS.
- D. Qin, F. Wang, W. Sheng, S. Chang, H. Duan and L. Wang, J. Iran. Chem. Soc., 2023, 20, 921–930 CrossRef CAS.
- Y. Xue, X. Xia, B. Yu, X. Luo, N. Cai, S. Long and F. Yu, RSC Adv., 2015, 5, 73416–73423 RSC.
- K. Yu, X. Yang, L. He, R. Zheng, J. Min, H. Su, S. Shan and Q. Jia, Polymer, 2020, 200, 122585 CrossRef CAS.
- M. He, F. Teng, H. Chen, C. Wu, Y. Huang and Y. Li, LWT, 2022, 160, 113259 CrossRef CAS.
- H. Su, X. Han, L. He, L. Deng, K. Yu, H. Jiang, C. Wu, Q. Jia and S. Shan, Int. J. Biol. Macromol., 2019, 128, 768–774 CrossRef CAS PubMed.
- L. Lin, W. Xu, H. Liang, L. He, S. Liu, Y. Li, B. Li and Y. Chen, Colloids Surf., B, 2015, 126, 459–466 CrossRef CAS PubMed.
- G. Geyik, E. Güncüm and N. Işıklan, Int. J. Biol. Macromol., 2023, 250, 126242 CrossRef CAS PubMed.
- Q. Zeng, W. Zeng, Y. Jin and L. Sheng, Food Chem., 2022, 367, 130716 CrossRef CAS PubMed.
- A. G. Rusu, A. P. Chiriac, L. E. Nita, A. Ghilan, D. Rusu, N. Simionescu and L. M. Tartau, Biochem. Eng. J., 2022, 179, 108341 CrossRef CAS.
- Y.-T. Pan, Y.-F. Ding, Z.-H. Han, L. Yuwen, Z. Ye, G. S. P. Mok, S. Li and L.-H. Wang, Carbohydr. Polym., 2021, 268, 118257 CrossRef CAS PubMed.
- M. Zhang, S. Asghar, C. Tian, Z. Hu, Q. Ping, Z. Chen, F. Shao and Y. Xiao, Carbohydr. Polym., 2021, 253, 117194 CrossRef CAS PubMed.
- Z. Rahmani, M. Ghaemy and A. Olad, Polym. Bull., 2021, 78, 2709–2726 CrossRef CAS.
- O. Peleg-Evron, M. Davidovich-Pinhas and H. Bianco-Peled, Int. J. Biol. Macromol., 2023, 227, 654–663 CrossRef CAS PubMed.
- G. R. Bardajee, N. Khamooshi, S. Nasri and C. Vancaeyzeele, Int. J. Biol. Macromol., 2020, 153, 180–189 CrossRef CAS PubMed.
- W. Wu, W. Yao, X. Wang, C. Xie, J. Zhang and X. Jiang, Biomaterials, 2015, 39, 260–268 CrossRef CAS PubMed.
- Y. K. Joung, J. Y. Jang, J. H. Choi, D. K. Han and K. D. Park, Mol. Pharmaceutics, 2013, 10, 685–693 CrossRef CAS PubMed.
- N. T. Nguyen, Q. A. Bui, H. H. N. Nguyen, T. T. Nguyen, K. L. Ly, H. L. B. Tran, V. N. Doan, T. T. Y. Nhi, N. H. Nguyen, N. H. Nguyen, N. Q. Tran and D. T. Nguyen, Gels, 2022, 8, 59 CrossRef CAS PubMed.
- G. D’Arrigo, C. Di Meo, E. Gaucci, S. Chichiarelli, T. Coviello, D. Capitani, F. Alhaique and P. Matricardi, Soft Matter, 2012, 8, 11557 RSC.
- H. S. Mahajan and P. P. Patil, Indian J. Pharm. Educ. Res., 2017, 51, S40–S45 CrossRef CAS.
- V. M. de, O. Cardoso, N. A. P. de Brito, N. N. Ferreira, F. I. Boni, L. M. B. Ferreira, S. G. Carvalho and M. P. D. Gremião, Colloids Surf., A, 2021, 628, 127321 CrossRef.
- W. Zhou, Z. Cai, R. Zhang, K. Hu, F. Wu, Y. Hu, C. Huang and Y. Chen, Food Chem., 2023, 421, 136143 CrossRef CAS PubMed.
- F. Pervaiz, W. Tanveer, H. Shoukat and S. Rehman, Polym. Bull., 2023, 80, 469–493 CrossRef CAS.
- J. Singh, S. Kumar and A. S. Dhaliwal, J. Drug Delivery Sci. Technol., 2020, 55, 101384 CrossRef.
- A. Fatimi, O. V. Okoro, D. Podstawczyk, J. Siminska-Stanny and A. Shavandi, Gels, 2022, 8, 179 CrossRef PubMed.
- F. Damiri, Y. Bachra, C. Bounacir, A. Laaraibi and M. Berrada, J. Chem., 2020, 2020, 1–10 CrossRef.
- A. Fatimi, P. Chabrot, S. Berrahmoune, J.-M. Coutu, G. Soulez and S. Lerouge, Acta Biomater., 2012, 8, 2712–2721 CrossRef CAS PubMed.
- T. Kean and M. Thanou, Adv. Drug Delivery Rev., 2010, 62, 3–11 CrossRef CAS PubMed.
- A. Fatimi, in IOCN 2022, MDPI, Basel Switzerland, 2022, p. 1 Search PubMed.
- Y. Yao, M. Xia, H. Wang, G. Li, H. Shen, G. Ji, Q. Meng and Y. Xie, Eur. J. Pharm. Sci., 2016, 91, 144–153 CrossRef CAS PubMed.
- A. Fatimi, J.-F. Tassin, R. Turczyn, M. A. V. Axelos and P. Weiss, Acta Biomater., 2009, 5, 3423–3432 CrossRef CAS PubMed.
- J. Kushwaha and R. Singh, Inorg. Chem. Commun., 2023, 152, 110721 CrossRef CAS.
- A. Fatimi, J. François Tassin, S. Quillard, M. A. V. Axelos and P. Weiss, Biomaterials, 2008, 29, 533–543 CrossRef CAS PubMed.
- H. Ismail, M. Irani and Z. Ahmad, Int. J. Polym. Mater., 2013, 62, 411–420 CrossRef CAS.
- V. Vamadevan and E. Bertoft, Starch - Stärke, 2015, 67, 55–68 CrossRef CAS.
- P. Guo, Y. Li, J. An, S. Shen and H. Dou, Carbohydr. Polym., 2019, 226, 115330 CrossRef CAS PubMed.
- A. C. C. Sousa, A. I. B. Romo, R. R. Almeida, A. C. C. Silva, L. M. U. Fechine, D. H. A. Brito, R. M. Freire, D. P. Pinheiro, L. M. R. Silva, O. D. L. Pessoa, J. C. Denardin, C. Pessoa and N. M. P. S. Ricardo, Carbohydr. Polym., 2021, 264, 118017 CrossRef CAS PubMed.
- R. Abka-khajouei, L. Tounsi, N. Shahabi, A. K. Patel, S. Abdelkafi and P. Michaud, Mar. Drugs, 2022, 20, 364 CrossRef CAS PubMed.
- J. Sun and H. Tan, Materials, 2013, 6, 1285–1309 CrossRef CAS PubMed.
- V. Urtuvia, N. Maturana, F. Acevedo, C. Peña and A. Díaz-Barrera, World J. Microbiol. Biotechnol., 2017, 33, 198 CrossRef PubMed.
- M. Suhail, C.-W. Fang, I.-H. Chiu, A. Khan, Y.-C. Wu, I.-L. Lin, M.-J. Tsai and P.-C. Wu, ACS Omega, 2023, 8, 23991–24002 CrossRef CAS PubMed.
- Y. Zhao and S. Jalili, Int. J. Biol. Macromol., 2022, 207, 666–682 CrossRef CAS PubMed.
- L. Liang, M. Xu, L. Pan, Z. Zhou and Y. Han, Processes, 2022, 10, 891 CrossRef CAS.
- A. Noreen, Z.-H. Nazli, J. Akram, I. Rasul, A. Mansha, N. Yaqoob, R. Iqbal, S. Tabasum, M. Zuber and K. M. Zia, Int. J. Biol. Macromol., 2017, 101, 254–272 CrossRef CAS PubMed.
- D. Mohnen, Curr. Opin. Plant Biol., 2008, 11, 266–277 CrossRef CAS PubMed.
- B. L. Ridley, M. A. O’Neill and D. Mohnen, Phytochemistry, 2001, 57, 929–967 CrossRef CAS PubMed.
- M. Zhou, T. Wang, Q. Hu and Y. Luo, Food Hydrocolloids, 2016, 57, 20–29 CrossRef CAS.
- L. A. Pérez, R. Hernández, J. M. Alonso, R. Pérez-González and V. Sáez-Martínez, Biomedicines, 2021, 9, 1113 CrossRef PubMed.
- S. Luan, Y. Zhu, X. Wu, Y. Wang, F. Liang and S. Song, ACS Biomater. Sci. Eng., 2017, 3, 2410–2419 CrossRef CAS PubMed.
- D. Kang, H.-B. Zhang, Y. Nitta, Y.-P. Fang and K. Nishinari, Polysaccharides, Springer International Publishing, Cham, 2015, pp. 1–48 Search PubMed.
- X. T. Le and S. L. Turgeon, Soft Matter, 2013, 9, 3063 RSC.
- K. M. Rao, A. Kumar, A. Haider and S. S. Han, Mater. Lett., 2016, 184, 189–192 CrossRef CAS.
- S. Faria, C. L. de Oliveira Petkowicz, S. A. L. de Morais, M. G. H. Terrones, M. M. de Resende, F. P. de França and V. L. Cardoso, Carbohydr. Polym., 2011, 86, 469–476 CrossRef CAS.
- P. Jansson, L. Kenne and B. Lindberg, Carbohydr. Res., 1975, 45, 275–282 CrossRef CAS PubMed.
- L. M. Ferreira, M. H. M. Sari, J. H. Azambuja, E. F. da Silveira, V. F. Cervi, M. C. L. Marchiori, S. S. Maria-Engler, M. R. Wink, J. G. Azevedo, C. W. Nogueira, E. Braganhol and L. Cruz, Invest. New Drugs, 2020, 38, 662–674 CrossRef CAS PubMed.
- S. Bhaladhare and S. Bhattacharjee, Int. J. Biol. Macromol., 2023, 226, 535–553 CrossRef CAS PubMed.
- H. Zheng, S. Wang, L. Zhou, X. He, Z. Cheng, F. Cheng, Z. Liu, X. Wang, Y. Chen and Q. Zhang, Chem. Eng. J., 2021, 404, 126439 CrossRef CAS.
- E. Rahmani, M. Pourmadadi, S. A. Ghorbanian, F. Yazdian, H. Rashedi and M. Navaee, Eng. Life Sci., 2022, 22, 634–649 CrossRef CAS PubMed.
- L. Rong, Y. Liu, Y. Fan, J. Xiao, Y. Su, L. Lu, S. Peng, W. Yuan and M. Zhan, Carbohydr. Polym., 2023, 310, 120721 CrossRef CAS PubMed.
- J. A. Luckanagul, C. Pitakchatwong, P. Ratnatilaka Na Bhuket, C. Muangnoi, P. Rojsitthisak, S. Chirachanchai, Q. Wang and P. Rojsitthisak, Carbohydr. Polym., 2018, 181, 1119–1127 CrossRef CAS PubMed.
- O. R. M. Metawea, M. Teleb, N. S. Haiba, A. O. Elzoghby, A. F. Khafaga, A. E. Noreldin, S. N. Khattab and H. H. Khalil, Eur. Polym. J., 2023, 182, 111721 CrossRef CAS.
- C. Yang, X. Wang, X. Yao, Y. Zhang, W. Wu and X. Jiang, J. Controlled Release, 2015, 205, 206–217 CrossRef CAS PubMed.
- D. Gao, S. Asghar, J. Ye, M. Zhang, R. Hu, Y. Wang, L. Huang, C. Yuan, Z. Chen and Y. Xiao, Carbohydr. Polym., 2022, 294, 119785 CrossRef CAS PubMed.
- F. Damiri, Y. Bachra and M. Berrada, Chin. J. Inorg. Anal. Chem., 2022, 50, 100092 CrossRef.
- T. Ye, X. Bai, X. Jiang, Q. Wu, S. Chen, A. Qu, J. Huang, J. Shen and W. Wu, Polym. Chem., 2016, 7, 2847–2857 RSC.
- T. Elshaarani, H. Yu, L. Wang, L. Lin, N. Wang, K. Ur Rahman Naveed, L. Zhang, Y. Han, S. Fahad and Z. Ni, Eur. Polym. J., 2020, 125, 109505 CrossRef CAS.
- Q. Zong, R. Zhou, Z. Zhao, Y. Wang, C. Liu and P. Zhang, Eur. Polym. J., 2022, 173, 111217 CrossRef CAS.
- F. Damiri, N. Kommineni, S. O. Ebhodaghe, R. Bulusu, V. G. S. Sainaga Jyothi, A. A. Sayed, A. A. Awaji, M. O. Germoush, H. S. Al-malky, M. Z. Nasrullah, M. H. Rahman, M. M. Abdel-Daim and M. Berrada, Pharmaceuticals, 2022, 15, 190 CrossRef CAS PubMed.
- D. Kulkarni, F. Damiri, S. Rojekar, M. Zehravi, S. Ramproshad, D. Dhoke, S. Musale, A. A. Mulani, P. Modak, R. Paradhi, J. Vitore, M. H. Rahman, M. Berrada, P. S. Giram and S. Cavalu, Pharmaceutics, 2022, 14, 1097 CrossRef CAS PubMed.
- N. Wen, S. Lü, C. Gao, X. Xu, X. Bai, C. Wu, P. Ning and M. Liu, Chem. Eng. J., 2018, 335, 52–62 CrossRef CAS.
- C. Hang, Y. Zou, Y. Zhong, Z. Zhong and F. Meng, Colloids Surf., B, 2017, 158, 547–555 CrossRef CAS PubMed.
- R. Messing, N. Frickel, L. Belkoura, R. Strey, H. Rahn, S. Odenbach and A. M. Schmidt, Macromolecules, 2011, 44, 2990–2999 CrossRef CAS.
- B. Hermenegildo, C. Ribeiro, L. Pérez-Álvarez, J. L. Vilas, D. A. Learmonth, R. A. Sousa, P. Martins and S. Lanceros-Méndez, Colloids Surf., B, 2019, 181, 1041–1047 CrossRef CAS PubMed.
- D. Fouad, Y. Bachra, G. Ayoub, A. Ouaket, A. Bennamara, N. Knouzi and M. Berrada, in Chitin and Chitosan - Physicochemical Properties and Industrial Applications, IntechOpen, 2020, pp. 1–24 Search PubMed.
- M. Khalil, A. Fahmi, N. M. Nizardo, Z. Amir and B. Mohamed Jan, Langmuir, 2021, 37, 8855–8865 CrossRef CAS PubMed.
- A. Narmani and S. M. Jafari, Carbohydr. Polym., 2021, 272, 118464 CrossRef CAS PubMed.
- F. Gao, X. Wu, D. Wu, J. Yu, J. Yao, Q. Qi, Z. Cao, Q. Cui and Y. Mi, Colloids Surf., A, 2020, 587, 124363 CrossRef CAS.
- W. Sun, T. Jiang, Y. Lu, M. Reiff, R. Mo and Z. Gu, J. Am. Chem. Soc., 2014, 136, 14722–14725 CrossRef CAS PubMed.
- F. Ding, Q. Mou, Y. Ma, G. Pan, Y. Guo, G. Tong, C. H. J. Choi, X. Zhu and C. Zhang, Angew. Chem., Int. Ed., 2018, 57, 3064–3068 CrossRef CAS PubMed.
- J. Li, C. Zheng, S. Cansiz, C. Wu, J. Xu, C. Cui, Y. Liu, W. Hou, Y. Wang, L. Zhang, I. Teng, H.-H. Yang and W. Tan, J. Am. Chem. Soc., 2015, 137, 1412–1415 CrossRef CAS PubMed.
- K. Lee, T. Kim, Y. M. Kim, K. Yang, I. Choi and Y. H. Roh, Macromol. Rapid Commun., 2021, 42, 2000457 CrossRef CAS PubMed.
- C. Yao, Y. Yuan and D. Yang, ACS Appl. Bio Mater., 2018, 1, 2012–2020 CrossRef CAS PubMed.
- M. C. Cochran, J. Eisenbrey, R. O. Ouma, M. Soulen and M. A. Wheatley, Int. J. Pharm., 2011, 414, 161–170 CrossRef CAS PubMed.
- C. Hernandez, S. Gulati, G. Fioravanti, P. L. Stewart and A. A. Exner, Sci. Rep., 2017, 7, 13517 CrossRef PubMed.
- P. Shende and S. Jain, J. Drug Targeting, 2019, 27, 1035–1045 CrossRef CAS PubMed.
- N. Rapoport, K.-H. Nam, R. Gupta, Z. Gao, P. Mohan, A. Payne, N. Todd, X. Liu, T. Kim, J. Shea, C. Scaife, D. L. Parker, E.-K. Jeong and A. M. Kennedy, J. Controlled Release, 2011, 153, 4–15 CrossRef CAS PubMed.
- P. Wei, M. Sun, B. Yang, J. Xiao and J. Du, J. Controlled Release, 2020, 322, 81–94 CrossRef CAS PubMed.
- N. Huebsch, C. J. Kearney, X. Zhao, J. Kim, C. A. Cezar, Z. Suo and D. J. Mooney, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 9762–9767 CrossRef CAS PubMed.
- S. Yamaguchi, K. Higashi, T. Azuma and A. Okamoto, Biotechnol. J., 2019, 14, 1800530 CrossRef PubMed.
- P. Wei, E. J. Cornel and J. Du, Drug Delivery Transl. Res., 2021, 11, 1323–1339 CrossRef CAS PubMed.
- F. Maghsoudinia, H. Akbari-Zadeh, F. Aminolroayaei, F. F. Birgani, A. Shanei and R. K. Samani, Eur. J. Pharm. Sci., 2022, 174, 106207 CrossRef CAS PubMed.
- F. Q. Schafer and G. R. Buettner, Free Radical Biol. Med., 2001, 30, 1191–1212 CrossRef CAS PubMed.
- H. Sun, F. Meng, R. Cheng, C. Deng and Z. Zhong, Expert Opin. Drug Delivery, 2013, 10, 1109–1122 CrossRef CAS PubMed.
- H. Wen, C. Dong, H. Dong, A. Shen, W. Xia, X. Cai, Y. Song, X. Li, Y. Li and D. Shi, Small, 2012, 8, 760–769 CrossRef CAS PubMed.
- A. Degirmenci, H. Ipek, R. Sanyal and A. Sanyal, Eur. Polym. J., 2022, 181, 111645 CrossRef CAS.
- M. Eskandani, H. Derakhshankhah, R. Jahanban-Esfahlan and M. Jaymand, J. Drug Delivery Sci. Technol., 2023, 84, 104449 CrossRef CAS.
- A. Jha, A. Rama, B. Ladani, N. Verma, S. Kannan and A. Naha, J. Appl. Pharm. Sci., 2021, 11, 001–016 CAS.
- A. A. Attama, P. O. Nnamani, O. B. Onokala, A. A. Ugwu and A. L. Onugwu, Front. Pharmacol., 2022, 13, 1–23 Search PubMed.
- C. I. Idumah, R. S. Odera, E. O. Ezeani, J. H. Low, F. A. Tanjung, F. Damiri and W. S. Luing, Polym.-Plast. Technol. Mater., 2023, 1–26 Search PubMed.
- X.-M. Li, Z.-Z. Wu, B. Zhang, Y. Pan, R. Meng and H.-Q. Chen, Food Chem., 2019, 293, 197–203 CrossRef CAS PubMed.
- B. Li, Q. Xu, X. Li, P. Zhang, X. Zhao and Y. Wang, Carbohydr. Polym., 2019, 203, 378–385 CrossRef CAS PubMed.
- N. Tiwari, L. Nawale, D. Sarkar and M. Badiger, Gels, 2017, 3, 8 CrossRef PubMed.
- S. Yao, X. Jin, C. Wang, A. Cao, J. Hu, B. Chen and B. Wang, J. Biomater. Appl., 2021, 36, 565–578 CrossRef CAS PubMed.
- M. Sabitha, N. Sanoj Rejinold, A. Nair, V.-K. Lakshmanan, S. V. Nair and R. Jayakumar, Carbohydr. Polym., 2013, 91, 48–57 CrossRef CAS PubMed.
- A. Soto-Quintero, N. Guarrotxena, O. García and I. Quijada-Garrido, Sci. Rep., 2019, 9, 18187 CrossRef CAS PubMed.
- M. K. Notabi, E. C. Arnspang, N. A. Peppas and M. Ø. Andersen, J. Drug Delivery Sci. Technol., 2023, 86, 104510 CrossRef CAS.
- Y. Wang, H. Xu, J. Wang, L. Ge and J. Zhu, J. Pharm. Sci., 2014, 103, 2012–2021 CrossRef CAS PubMed.
- Y. Zuo, M. Kong, Y. Mu, C. Feng and X. Chen, Int. J. Biol. Macromol., 2017, 104, 157–164 CrossRef CAS PubMed.
- M. Rajaei, H. Rashedi, F. Yazdian, M. Navaei-Nigjeh, A. Rahdar and A. M. Díez-Pascual, J. Drug Delivery Sci. Technol., 2023, 82, 104307 CrossRef CAS.
- M. Mirrahimi, Z. Abed, J. Beik, I. Shiri, A. Shiralizadeh Dezfuli, V. P. Mahabadi, S. Kamran Kamrava, H. Ghaznavi and A. Shakeri-Zadeh, Pharmacol. Res., 2019, 143, 178–185 CrossRef CAS PubMed.
- P. Sahu, S. K. Kashaw, S. Sau, V. Kushwah, S. Jain, R. K. Agrawal and A. K. Iyer, Colloids Surf., B, 2019, 174, 232–245 CrossRef CAS PubMed.
- S. Pillarisetti, V. Vijayan, J. Rangasamy, R. Bardhan, S. Uthaman and I.-K. Park, J. Ind. Eng. Chem., 2023, 123, 361–370 CrossRef CAS.
- A. Ges Naranjo, H. Viltres Cobas, N. Kumar Gupta, K. Rodríguez López, A. Artimez Peña, D. Sacasas and R. Álvarez Brito, J. Mol. Liq., 2022, 362, 119716 CrossRef CAS.
- G. Bashiri, S. A. Shojaosadati and M. Abdollahi, Int. J. Biol. Macromol., 2021, 170, 222–231 CrossRef CAS PubMed.
- R. Chang, M. Li, S. Ge, J. Yang, Q. Sun and L. Xiong, Ind. Crops Prod., 2018, 112, 98–104 CrossRef CAS.
- J. Bai, H. Zhang, Z. Yang, P. Li, B. Liu, D. Li, S. Liang, Q. Wang, Z. Li, J. Zhang, S. Chen, G. Hou and Y. Li, J. Controlled Release, 2022, 352, 673–684 CrossRef CAS PubMed.
- Z. Gu, A. A. Aimetti, Q. Wang, T. T. Dang, Y. Zhang, O. Veiseh, H. Cheng, R. S. Langer and D. G. Anderson, ACS Nano, 2013, 7, 4194–4201 CrossRef CAS PubMed.
- L. Zhao, C. Xiao, J. Ding, P. He, Z. Tang, X. Pang, X. Zhuang and X. Chen, Acta Biomater., 2013, 9, 6535–6543 CrossRef CAS PubMed.
- H.-S. Chou, M. Larsson, M.-H. Hsiao, Y.-C. Chen, M. Röding, M. Nydén and D.-M. Liu, J. Controlled Release, 2016, 224, 33–42 CrossRef CAS PubMed.
- D. Zhao, X. Shi, T. Liu, X. Lu, G. Qiu and K. J. Shea, Carbohydr. Polym., 2016, 151, 1006–1011 CrossRef CAS PubMed.
- J.-M. Shen, L. Xu, Y. Lu, H.-M. Cao, Z.-G. Xu, T. Chen and H.-X. Zhang, Int. J. Pharm., 2012, 427, 400–409 CrossRef CAS PubMed.
- S. Indulekha, P. Arunkumar, D. Bahadur and R. Srivastava, Colloids Surf., B, 2017, 155, 304–313 CrossRef CAS PubMed.
- M. Ghorbani, H. Hamishehkar, N. Arsalani and A. A. Entezami, Mater. Sci. Eng., C, 2016, 68, 436–444 CrossRef CAS PubMed.
- N. Zohreh, S. Alipour, S. H. Hosseini, M. S. Xaba, R. Meijboom, M. F. Ramandi, N. Gholipour and M. Akhlaghi, ACS Appl. Nano Mater., 2019, 2, 853–866 CrossRef CAS.
- W. Pon-On, T. Tithito, W. Maneeprakorn, T. Phenrat and I.-M. Tang, Mater. Sci. Eng., C, 2019, 97, 23–30 CrossRef CAS PubMed.
- W. Xiong, W. Wang, Y. Wang, Y. Zhao, H. Chen, H. Xu and X. Yang, Colloids Surf., B, 2011, 84, 447–453 CrossRef CAS PubMed.
- S. Seo, C.-S. Lee, Y.-S. Jung and K. Na, Carbohydr. Polym., 2012, 87, 1105–1111 CrossRef CAS.
- W. Song, M. Muthana, J. Mukherjee, R. J. Falconer, C. A. Biggs and X. Zhao, ACS Biomater. Sci. Eng., 2017, 3, 1027–1038 CrossRef CAS PubMed.
- C. A. Demarchi, A. Debrassi, F. de Campos Buzzi, R. Corrêa, V. C. Filho, C. A. Rodrigues, N. Nedelko, P. Demchenko, A. Ślawska-Waniewska, P. Dłużewski and J.-M. Greneche, Soft Matter, 2014, 10, 3441 RSC.
- J. Huang, Y. Xue, N. Cai, H. Zhang, K. Wen, X. Luo, S. Long and F. Yu, Mater. Sci. Eng., C, 2015, 46, 41–51 CrossRef CAS PubMed.
- M. Overchuk, R. A. Weersink, B. C. Wilson and G. Zheng, ACS Nano, 2023, 17, 7979–8003 CrossRef CAS PubMed.
- F. Howaili, E. Özliseli, B. Küçüktürkmen, S. M. Razavi, M. Sadeghizadeh and J. M. Rosenholm, Front. Chem., 2021, 8, 1–17 RSC.
- T. Xiao, W. Hu, Y. Fan, M. Shen and X. Shi, Theranostics, 2021, 11, 7057–7071 CrossRef CAS PubMed.
- Y. Guo, Y. Chen, P. Han, Y. Liu, W. Li, F. Zhu, K. Fu and M. Chu, Acta Biomater., 2020, 103, 237–246 CrossRef CAS PubMed.
- Z. Yang, Z. Zhao, H. Cheng, Y. Shen, A. Xie and M. Zhu, J. Colloid Interface Sci., 2023, 641, 215–228 CrossRef CAS PubMed.
- S. Mahajan, N. Raval, D. Kalyane, N. Anup, R. Maheshwari, V. Tambe, K. Kalia and R. K. Tekade, J. Drug Delivery Sci. Technol., 2020, 58, 101814 CrossRef CAS.
- J.-Y. Kim, W. Il Choi, M. Kim and G. Tae, J. Controlled Release, 2013, 171, 113–121 CrossRef CAS PubMed.
- H. Guo, H. Wang, H. Deng, Y. Zhang, X. Yang and W. Zhang, Front. Bioeng. Biotechnol., 2023, 11, 01–08 Search PubMed.
- Z. Khatun, M. Nurunnabi, M. Nafiujjaman, G. R. Reeck, H. A. Khan, K. J. Cho and Y. Lee, Nanoscale, 2015, 7, 10680–10689 RSC.
- Q. Song, Y. Yin, L. Shang, T. Wu, D. Zhang, M. Kong, Y. Zhao, Y. He, S. Tan, Y. Guo and Z. Zhang, Nano Lett., 2017, 17, 6366–6375 CrossRef CAS PubMed.
- H. Wang, S. Mukherjee, J. Yi, P. Banerjee, Q. Chen and S. Zhou, ACS Appl. Mater. Interfaces, 2017, 9, 18639–18649 CrossRef CAS PubMed.
- L. Yu, A. Dong, R. Guo, M. Yang, L. Deng and J. Zhang, ACS Biomater. Sci. Eng., 2018, 4, 2424–2434 CrossRef CAS PubMed.
- M. Dimde, F. Neumann, F. Reisbeck, S. Ehrmann, J. L. Cuellar-Camacho, D. Steinhilber, N. Ma and R. Haag, Biomater. Sci., 2017, 5, 2328–2336 RSC.
- X. Zhao, Y. Xi, Y. Zhang, Q. Wu, R. Meng, B. Zheng and L. Rei, Nanoscale Res. Lett., 2019, 14, 273 CrossRef PubMed.
- M. Ahmed, P. Wattanaarsakit and R. Narain, Polym. Chem., 2013, 4, 3829 RSC.
- L. Liu, H. Yi, H. He, H. Pan, L. Cai and Y. Ma, Biomaterials, 2017, 134, 166–179 CrossRef CAS PubMed.
- F. Pinelli, M. Saadati, E. N. Zare, P. Makvandi, M. Masi, A. Sacchetti and F. Rossi, Int. Mater. Rev., 2023, 68, 1–25 CrossRef CAS.
- Y. Yin, B. Hu, X. Yuan, L. Cai, H. Gao and Q. Yang, Pharmaceutics, 2020, 12, 290 CrossRef CAS PubMed.
- L. Zha, B. Banik and F. Alexis, Soft Matter, 2011, 7, 5908 RSC.
- W. Xiao, J. Xiong, S. Zhang, Y. Xiong, H. Zhang and H. Gao, Int. J. Pharm., 2018, 538, 105–111 CrossRef CAS PubMed.
- G. D. Lewis Phillips, G. Li, D. L. Dugger, L. M. Crocker, K. L. Parsons, E. Mai, W. A. Blättler, J. M. Lambert, R. V. J. Chari, R. J. Lutz, W. L. T. Wong, F. S. Jacobson, H. Koeppen, R. H. Schwall, S. R. Kenkare-Mitra, S. D. Spencer and M. X. Sliwkowski, Cancer Res., 2008, 68, 9280–9290 CrossRef CAS PubMed.
- E. Anooj, M. Charumathy, V. Sharma, B. V. Vibala, S. T. Gopukumar, S. I. B. Jainab and S. Vallinayagam, J. Mol. Struct., 2021, 1239, 130446 CrossRef CAS.
- R. Ni, R. Feng and Y. Chau, Life, 2019, 9, 59 CrossRef CAS PubMed.
- J. Peng, Q. Yang, Y. Xiao, K. Shi, Q. Liu, Y. Hao, F. Yang, R. Han and Z. Qian, Adv. Funct. Mater., 2019, 29, 1900004 CrossRef.
- Z. Wang, N. Zhang, C. Chen, R. He and X. Ju, J. Agric. Food Chem., 2020, 68, 3607–3614 CrossRef CAS PubMed.
- R.-Q. Li, W. Wu, H.-Q. Song, Y. Ren, M. Yang, J. Li and F.-J. Xu, Acta Biomater., 2016, 41, 282–292 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |




