Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A focus on 1-azahomocubane: the new kid on the block
Cecile
Elgindy
and
Mark D.
Levin
*
Department of Chemistry, University of Chicago, Chicago, IL 60637, USA. E-mail: marklevin@uchicago.edu
First published on 7th July 2023
Abstract
Strained hydrocarbons have recently regained interest as potential drug candidates. However, the study of their heteroatom analogs has remained limited, despite differing by only a single atom. The first synthesis of 1-azahomocubane by Williams, Eaton and co-workers (T. Fahrenhorst-Jones et al., Chem. Sci., 2023, 14, 2821–2825, https://doi.org/10.1039/D3SC00001J) is discussed within the context of nitrogen scanning of strained hydrocarbons.
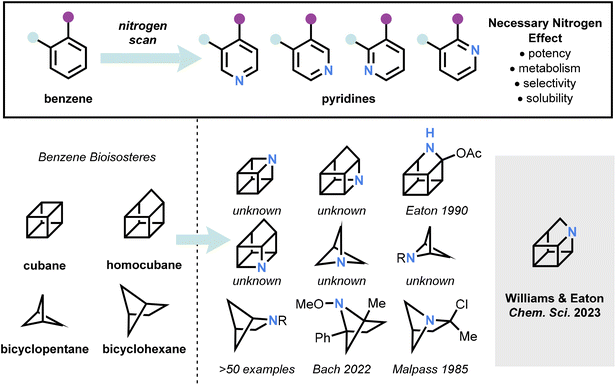
The exchange of a single atom can dramatically alter the properties of a molecule. Reactivity, biological activity, stability, selectivity, physical properties, and synthetic tractability of the molecule can all be impacted, simply by the substitution of one atom for another. Such effects have been observed in natural products and drug candidates, exemplified by the “necessary nitrogen effect” in medicinal chemistry whereby the replacement of a carbon atom for a nitrogen atom in an aromatic system can profoundly alter the molecule’s pharmacological profile.1,2
Meanwhile, renewed interest in strained hydrocarbons has arisen from their potential as benzene bioisosteres, which increase the parent molecule’s three-dimensionality without introducing substantial metabolic hotspots, owing to the high s character of the exocyclic C–H bonds.3 Cubane, bicyclo[1.1.1]pentane, and bicyclo[1.1.2]hexane have all been proposed as attractive substructures for drug candidates along these lines, which has led to a flurry of activity examining the functionalization of these scaffolds, with a particular focus on C–H functionalisation and strain release methods for their synthesis.4–6
One can imagine, in analogy to the necessary nitrogen effect for benzenoid systems, that the corresponding heterocyclic series would be valuable in the strained hydrocarbon family of compounds. Surprisingly, however, very few of the aza-analogs of these hydrocarbons are known, with two azahomocubanes and a small number of substituted azabicyclohexanes previously reported, mostly of the 2-azabicyclohexane class (Fig. 1).7–9
Williams, Eaton, and co-workers disclose the first synthesis of 1-azahomocubane, an aza-analog of homocubane (https://doi.org/10.1039/D3SC00001J).10 The synthesis and study of this surprisingly stable, strained molecule is a rare foray into the azahomocubane series, providing a useful comparison to related structures and enabling insights into this underexplored structural space.
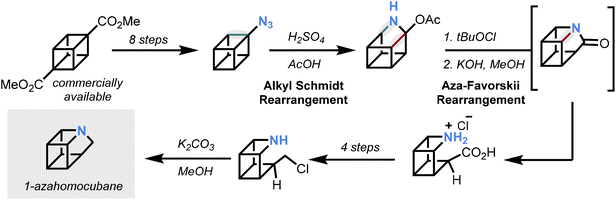
The synthesis pinpointed azidyl cubane as a strategic intermediate that enabled the incorporation of nitrogen into the core of the strained ring framework, building upon previous reports of a substituted 9-azahomocubane.11 A sequence of rearrangements beginning from the azidyl cubane was initiated by strong acid followed by N-chlorination, resulting in nitrogen incorporation into the ring system. To complete the synthesis, functional group manipulation enabled the requisite ring closure via N-alkylation to generate the parent 1-azahomocubane, which was trapped in situ as a salt to simplify its isolation (Fig. 2).
With the target compound in hand, a preliminary survey of chemical and physical properties could be conducted. The free amine was found to be stable as a solution over months, whereas the hydrochloride salt was observed to undergo chloride mediated ring-opening. 1-Azahomocubane was calculated to be more stable than homocubane and was observed to be less reactive towards Ag+ mediated isomerisations, suggesting that nitrogen-atom exchange enhances the stability of the strained ring system. Indeed, an X-ray structure of the bis(azahomocubane) silver(I) perchlorate salt was collected. Finally, azahomocubane was found to be meaningfully less basic than related polycyclic tertiary amines, with the additional strain likely inducing increased lone pair s-character.
This work by Williams, Eaton and co-workers highlights an underexplored region of chemical space. The synthesis of strained polycyclic heterocycles conceptually derived from benzene bioisosteres is not without challenges, but this work will hopefully spur further development in this area.
Author contributions
All authors contributed equally.Conflicts of interest
There are no conflicts to declare.References
- D. L. Boger, J. Org. Chem., 2017, 82, 11961–11980 CrossRef CAS PubMed.
- L. D. Pennington and D. T. Moustakas, J. Med. Chem., 2017, 60, 3552–3579 CrossRef CAS PubMed.
- F. Lovering, J. Bikker and C. Humblet, J. Med. Chem., 2009, 52, 6752–6756 CrossRef CAS PubMed.
- (a) T. A. Reekie, C. M. Williams, L. M. Rendina and M. Kassiou, J. Med. Chem., 2019, 62, 1078–1095 CrossRef CAS PubMed; (b) P. K. Mykhailiuk, Org. Biomol. Chem., 2019, 17, 2839–2849 RSC.
- P. S. Baran, et al. , J. Am. Chem. Soc., 2017, 139, 3209–3226 CrossRef PubMed.
- For some recent examples of BCP and BCH C–H functionalisation: (a) I. F. Yu, J. L. Manske, A. Diéguez-Vázquez, A. Misale, A. E. Pashenko, P. K. Mykhailiuk, S. V. Ryabukhin, D. M. Volochnyuk and J. F. Hartwig, Nature, 2023, 15, 685–693 CAS; (b) O. L. Garry, M. Heilmann, J. Chen, Y. Liang, X. Zhang, X. Ma, C. S. Yeung, D. J. Bennett and D. W. C. MacMillan, J. Am. Chem. Soc., 2023, 145, 3092–3100 CrossRef CAS PubMed.
- (a) P. E. Eaton, A. M. Fisher and R. E. Hormann, Synlett, 1990, 12, 737–738 CrossRef; (b) P. E. Eaton and R. E. Hormann, J. Am. Chem. Soc., 1987, 109, 1268–1269 CrossRef CAS.
- (a) G. R. Krow, Y. B. Lee, W. S. Lester, H. Christian, D. A. Shaw and J. Yuan, J. Org. Chem., 1998, 63, 8558–8560 CrossRef CAS; (b) J. W. Davies, J. R. Malpass and M. P. Walker, J. Chem. Soc., Chem. Commun., 1985, 686–687 RSC; (c) G. Mloston and H. Heimgartner, Helv. Chim. Acta, 2006, 89, 442–449 CrossRef CAS; For a review of substituted azabicyclo[2.1.1]hexanes: (d) G. R. Krow and K. C. Cannon, Heterocycles, 2004, 62, 877–898 CrossRef CAS PubMed; For more recent examples of substituted aza-bicyclo[2.1.1]hexanes: (e) K. Dhake, K. J. Woelk, J. Becica, A. Un, S. E. Jenny and D. C. Leitch, Angew. Chem., Int. Ed., 2022, 61, e202204719 CrossRef CAS PubMed; (f) J. L. Tyler, A. Noble and V. K. Aggarwal, Angew. Chem., Int. Ed., 2022, 61, e202114235 CAS; (g) B. A. Wright, A. Matviitsuk, M. J. Black, P. García-Reynaga, L. E. Hanna, A. T. Herrmann, M. K. Ameriks, R. Sarpong and T. P. Lebold, J. Am. Chem. Soc., 2023, 145, 10960–10966 Search PubMed.
- A 5-aza-bicyclohexane: (a) T. Rigotti and T. Bach, Org. Lett., 2022, 24, 8821–8825 CrossRef CAS PubMed; A benzo-fused analog: (b) K. Takahashi, K. Suenobu, K. Ogura and H. Iida, Chem. Lett., 1985, 14, 1487–1488 CrossRef; Putative characterization in a mixture: (c) A. G. Griesbeck and H. Heckroth, J. Am. Chem. Soc., 2002, 124, 396–403 CrossRef CAS PubMed; Claimed but later reassigned: (d) H. F. Olivo, M. S. Hemenway and M. H. Gezginci, Tetrahedron Lett., 1998, 39, 1309–1312 CrossRef CAS.
- T. Fahrenhorst-Jones, D. L. Marshall, J. M. Burns, G. K. Pierens, R. E. Hormann, A. M. Fisher, P. V. Bernhardt, S. J. Blanksby, G. P. Savage, P. E. Eaton and C. M. Williams, Chem. Sci., 2023, 14, 2821–2825 RSC.
- R. Gilardi, C. George, J. Karle, P. E. Eaton and A. M. Fisher, J. Heterocycl. Chem., 1993, 30, 1385–1388 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |