Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Broadening the catalytic region from the cavity to windows by M6L12 nanospheres in cyclizations†
Meiling
Xu
a,
Bin
Sun
b,
David A.
Poole
III
 b,
Eduard O.
Bobylev
b,
Xu
Jing
b,
Eduard O.
Bobylev
b,
Xu
Jing
 *a,
Jinguo
Wu
a,
Cheng
He
*a,
Jinguo
Wu
a,
Cheng
He
 a,
Chunying
Duan
a,
Chunying
Duan
 *a and
Joost N. H.
Reek
*a and
Joost N. H.
Reek
 *b
*b
aState Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian, 116024, P. R. China. E-mail: xjing@dlut.edu.cn; cyduan@dlut.edu.cn
bHomogeneous, Supramolecular and Bio-Inspired Catalysis, Van't Hoff Institute for Molecular Sciences, University of Amsterdam, Science Park 904, Amsterdam 1098 XH, The Netherlands. E-mail: j.n.h.reek@uva.nl
First published on 26th September 2023
Abstract
Supramolecular cages have received tremendous attention as they can contain catalysts that exhibit confinement effects in the cavity, leading to excellent performances. Herein, we report an example wherein the catalytic region is extended from the cage cavity to the windows, and investigate its confinement effect by utilizing the Pd6LAu12 cage that contains rigidly fixed and isolated gold complexes at the windows. Pd6LAu12 exhibit three features of particular interest while assessing their properties in gold-catalyzed cyclization reactions. First, the catalysts experience a cage effect as they display higher reactivity and selectivity compared to the monomeric analogue, as a result of substrate pre-organization at the windows. Second, the metal complexes are physically separated by the cage structure, preventing the formation of less active dinuclear gold complexes making it more stable under hydrous conditions. Third, the cage windows present the characteristics of enzymatic catalysis via Michaelis–Menten-type mechanism analysis. This contribution presents an alternative way to engineer supramolecular catalysts through extending the catalytic region.
Introduction
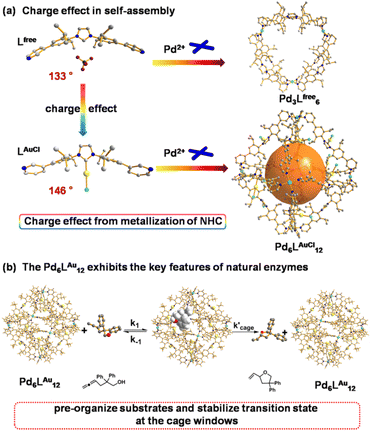
Over the past few decades, supramolecular chemistry has experienced tremendous developments, particularly for potential applications in drug transport,1 recognition and storage,2 sensing,3 and catalysis.4 Inspired by the ingenious protein structures of enzymes in nature providing well-defined pockets around the active sites, supramolecular coordination cages have been explored to emulate the specific microenvironment of enzymes. Several examples have demonstrated that synthetic mimics can provide an ideal reaction container to regulate the reactivity.5 By applying supramolecular strategies, a catalytic system could be formed in which single or multiple active sites reside in a confined micro-environment. In such a micro-environment, the orientation and rotation of substrates and catalysts can be restricted, explaining some of the unusual selectivity displayed by these encaged catalysts. In addition, the restriction of substrate and intermediate conformations within the cavity can be entropically favorable, leading to improved activity.6 Based on these successes, supramolecular strategies in transition metal catalysis developed as a fruitful tool for catalysis tuning, as specific activity and unprecedented selectivity can be achieved.A powerful strategy to produce pyridine-coordinated palladium MnL2n (n = 6, 12, 24) cages was pioneered by Fujita.7 The combination of the ditopic bispyridine building blocks and palladium complexes typically afforded self-assembled structures such as M6L12, M12L24 and M24L48,8,9 depending on the bend angle of the bispyridine building blocks. The M12L24 nanospheres are amongst the most widely studied, as they can be functionalized with facile endohedral or exohedral binding groups, thereby generating a confined microenvironment suited for potential applications.10 We previously reported the utilization of Fujita-type M12L24 nanospheres that were decorated internally with 24 gold complexes to provide systems with extremely high local concentration of gold species.11 It led to d10–d10 aurophilic interaction and, as a result, enhanced the reactivity in cyclization reactions.11 More recently, we reported that an M12L24 nanosphere was able to bind catalysts and substrates via hydrogen bonding interactions.12 In this case, sulfonated gold catalysts were strongly fixed in a guanidinium containing nanocage, and carboxylate-containing substrates were bound more weakly via the remaining guanidine units, providing the ability to pre-organize the substrates and the catalysts within the microenvironment. In the gold-catalyzed cyclization of carboxylate-containing substrates this pre-organization resulted in higher reaction rates. More recently, this guanidinium-functionalized nanosphere was used to accelerate reactions via a dinuclear mechanism, such as dinuclear Cu(I)-catalyzed cyclization13 and ruthenium-catalyzed water oxidation.14 These examples demonstrate that the generation of a high local concentration by encapsulation of multiple transition metal catalysts (and/or substrates) is an interesting new tool to control catalyst properties. Note that these examples have reported the encapsulation of transition metal catalysts that are connected via flexible linkers or via weak non-covalent interactions in the cavity, facilitating the catalysis due to the confinement effect of the cavity. However, similar nanospheres in which the metal catalysts are rigidly fixed and physically isolated on the rims of the cage windows15 and whether the windows could present the confinement effect in catalysis have not been well explored yet.
Recently, Nitschke and co-workers reported a subcomponent tetrahedral cage that was functionalized with N-heterocyclic carbene (NHC) moieties in the middle of each edge.16 They reported a cage that contained NHC–gold complexes as part of the cage backbone and used it as a template to generate gold nanoparticles. Other literature research17,18 reported the construction and host guest studies of cages containing NHC moieties. However, the catalytic properties of these formed cages containing NHC were not explored. In this contribution, we report an example wherein the catalytic region is extended from the cage cavity to the windows on the cage surface area by the Pd6LAuCl12 nanospheres that contain rigidly fixed and physically isolated NHC–AuCl moieties at the cage window, and investigate its confinement effect of the windows and enzymatic catalytic behavior in catalysis (Fig. 1). These types of NHC–gold complexes have previously been explored in cyclization reactions19 of alkynes,20 allenes21 and alkenes,22 and then we set out to assess the catalytic performances of the Pd6LAu12 cage in gold-catalyzed cyclization reactions. Application of Pd6LAu12 nanospheres in the cyclization of allenol and hex-4-ynoic acid shows enhanced activity and selectivity. In addition, the physically separated complexes cannot form dinuclear complexes, making the Pd6LAu12 nanospheres more stable, especially under hydrous conditions. With this cage we have a clear example which shows that catalysis can favorably take place at the cage windows, and the systems display Michaelis–Menten kinetics, a feature also found in enzymatic catalysis.
Results and discussion
Synthesis and characterization of ligands and cages
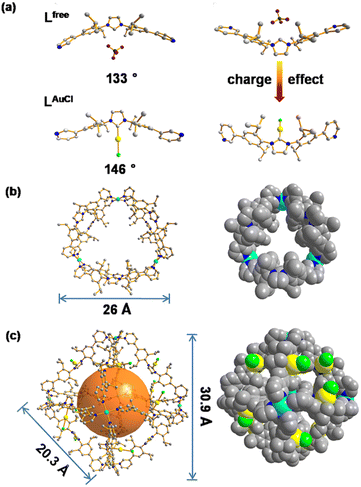
The NHC-functionalized ditopic ligand was designed by embedding the rigid NHC moiety between two 3-pyridyl groups to allow coordination to palladium required for the self-assembly, leading to structures in which the NHC moiety is implemented in a rigid fashion. The pure ditopic pyridyl ligand was obtained in 2 steps with an overall yield of 85% (Fig. S1–S10†) and will be indicated as Lfree in this paper to emphasize that it is metal free. Needle-shaped crystals of Lfree were obtained from vapor diffusion of diethyl ether into a solution of Lfree in acetonitrile over two weeks. The crystal structure of Lfree shows that the ligand adopts a concave bending mode in which the imidazolium ring points inward, and the two neighboring aromatic rings display a bend angle of 133° (Fig. 4a, Tables S4 and S5†).After treating the NHC-based ditopic pyridine compound with a stoichiometric amount of Au(tht)Cl, the gold complex of the ligand was obtained, which is denoted as LAuCl (Fig. S11–S16†). Slow diffusion of isopropyl ether into the acetonitrile solution of LAuCl led to square-shaped crystals. The colorless crystal of LAuCl allowed solving the solid-state structure by SCXRD. The structure shows that pyridine groups were oriented differently when compared to that of Lfree and the bend angle is rather different (146° compared to 133°), as a result of the coordination of gold on the imidazolium ring (Fig. 4a, Tables S6 and S7†). The properties of both Lfree and LAuCl in solution state were investigated by multiple spectroscopic NMR experiments and high-resolution mass spectrometry (HRMS) (all the spectral details can be found in the ESI†).
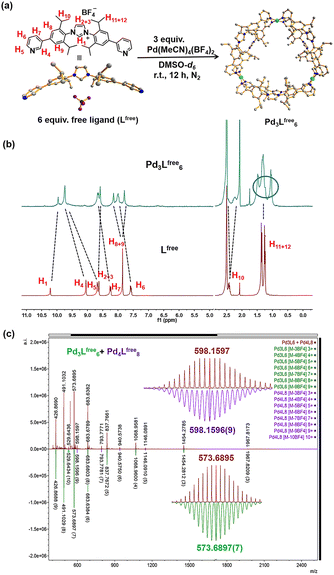
These two ditopic ligands were used to make coordination cages by self-assembly using palladium as the metal source. A solution containing Lfree and Pd(MeCN)4(BF4)2 with a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in DMSO-d6 (Fig. 2a) was stirred in a N2 atmosphere. After stirring vigorously for 12 h at 298 K, the light-yellow solid was collected by precipitation in excess amount of diethyl ether. Analysis of the compound by various techniques showed that the self-assembled Pd3Lfree6 cage was formed. The product was fully characterized by NMR spectroscopy and HR-MS (Fig. S17–S37†).
1 in DMSO-d6 (Fig. 2a) was stirred in a N2 atmosphere. After stirring vigorously for 12 h at 298 K, the light-yellow solid was collected by precipitation in excess amount of diethyl ether. Analysis of the compound by various techniques showed that the self-assembled Pd3Lfree6 cage was formed. The product was fully characterized by NMR spectroscopy and HR-MS (Fig. S17–S37†).
The relative shifts and broadened resonances of the ligand backbone in the 1H NMR spectrum indicated the coordination between Lfree and palladium precursor (Fig. 2b). Compared to that of Lfree, the signals of pyridine protons H4 (Δδ = −0.66 ppm; Fig. 2b) and H5 (Δδ = −1.06 ppm; Fig. 2b) presented typical shifts, indicating the coordination with Pd2+ in line with the cage formation.23 The aromatic protons H8 (Δδ = −0.30 ppm; Fig. 2b) and H9 (Δδ = 0.06 ppm; Fig. 2b) no longer produced an identical chemical shift but split into two singlets and shifted individually, demonstrating that these protons resided in different chemical environments, because the neighboring aromatic rings could not be rotated due to the rigidity of the cage structure. Apparently, the aromatic rings are no longer able to rotate rapidly on the NMR time scale, due to the steric hindrance and rigidity experienced within the structure. Notably, the resonance signals of the isopropyl CH protons H10, H11 and H12 in the 1H NMR spectra of the cage were also split, clearly indicating the distinct chemical environment.
Diffusion-ordered NMR spectra (DOSY) in DMSO-d6 at 298 K exhibited a clear single narrow band around log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D = −10.078 (RH ≈ 2.61 nm, according to the Stokes–Einstein equation) (Fig. S22†). The composition of the Pd3Lfree6 cage was clearly proven by high-resolution cold spray ionization mass spectrometry (HR-CSI-MS) in acetonitrile. A single set of species with various charged states (3+, 4+, 5+, 6+, 7+, 8+ and 9+) was observed (Fig. 2c). All signals were assigned to a structure having the formula Pd3Lfree6(BF4)12 with a progressive loss of BF4− counterions during the MS measurement (Fig. S23†). Clearly, for all of the charged states, the experimental values precisely matched with the calculated isotopic distributions (Fig. S24–S37†).
D = −10.078 (RH ≈ 2.61 nm, according to the Stokes–Einstein equation) (Fig. S22†). The composition of the Pd3Lfree6 cage was clearly proven by high-resolution cold spray ionization mass spectrometry (HR-CSI-MS) in acetonitrile. A single set of species with various charged states (3+, 4+, 5+, 6+, 7+, 8+ and 9+) was observed (Fig. 2c). All signals were assigned to a structure having the formula Pd3Lfree6(BF4)12 with a progressive loss of BF4− counterions during the MS measurement (Fig. S23†). Clearly, for all of the charged states, the experimental values precisely matched with the calculated isotopic distributions (Fig. S24–S37†).
According to the structural information, we propose that this cage holds a double crown ring by combining two rings connected via three Pd2+ metal nodes. The modeled structure shows an average diameter of 26 Å (Fig. 4b), which is in line with that obtained from the DOSY NMR spectra (Fig. S22†). The top view of the structure is displayed showing the rings connected to the palladium nodes (Fig. 4b). The structure consists effectively of three small rings (Pd2Lfree2) connected to one another. The torsion angle of Lfree in the coordination state is 139°, which is larger than its angle in the free state. The structure contained three Pd2Lfree2 rings, showing the symmetry of Pd3Lfree6, which also matched the splitting of isopropyl protons in the NMR results (Fig. 2b). A similar structure of trigonal prismatic Pd3L6 containing PF6− was reported as the basic building block for the formation of crystal mesoporous supramolecular materials.17 The crystals of Pd3L6(PF6)12 crystallize in the monoclinic space group. Its framework contains three Pd2+ metal centers and six ligands. The calculated structure presents a triangular prism skeleton with three small rings of Pd2L2. The structural analysis of Pd3L6(PF6)12 sufficiently verifies our modeling of Pd3Lfree6(BF4)12.
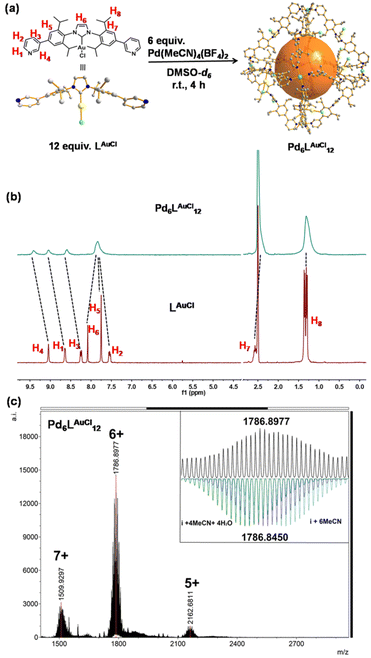
A solution containing LAuCl and palladium salt in DMSO-d6 was stirred at room temperature for 4 hours (Fig. 3a). A single set of slightly broadened signals was observed in the 1H NMR spectra (Fig. 3b and S38†). All the resonance signals were assigned with the help of 2D1H COSY NMR (Fig. S40–S42†). In the1H NMR spectrum, the resonance signals of the pyridine signals were shifted [Δδ (H1) = −0.41 ppm; Δδ (H2) = −0.29 ppm; Δδ (H3) = −0.33 ppm; Δδ (H4) = −0.36 ppm], in line with the coordination with the palladium center23 (Fig. 3b). Moreover, the resonance signals of isopropyl groups [H8] in the cage structure did not shift too much but rather broadened with respect to the signals of LAuCl (Fig. 3b), indicating that a highly symmetric cage had formed. DOSY showed a single band at log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D = −10.10 (RH ≈ 2.75 nm, according to the Stokes–Einstein equation) (Fig. S43†), consistent with the cage size measured from the single crystal structural analyses (Fig. 4c). HR-CSI-MS measurements for the solution of the Pd6LAuCl12 cage in acetonitrile supported the composition of the desired cage, as a set of prominent peaks with different charge states were observed at 2162.6811, 1786.8977, and 1509.9297; this result precisely agreed with the simulated values of Pd6LAuCl12 with the respective amount of counterions (Fig. S44–S46†). Furthermore, a series of peaks with a certain amount of additional coordinated solvent molecules could also be recognized (Fig. S45†). The simulated isotopic distribution of the +6 charged species (Fig. 3c) clearly shows that the cage structure contains some solvent molecules, which results in partly overlapping peaks. By slow diffusion of diethyl ether vapor into an acetonitrile solution of Pd6LAuCl12 over three weeks, rhombus-shaped single crystals of the Pd6LAuCl12 cage were obtained.
D = −10.10 (RH ≈ 2.75 nm, according to the Stokes–Einstein equation) (Fig. S43†), consistent with the cage size measured from the single crystal structural analyses (Fig. 4c). HR-CSI-MS measurements for the solution of the Pd6LAuCl12 cage in acetonitrile supported the composition of the desired cage, as a set of prominent peaks with different charge states were observed at 2162.6811, 1786.8977, and 1509.9297; this result precisely agreed with the simulated values of Pd6LAuCl12 with the respective amount of counterions (Fig. S44–S46†). Furthermore, a series of peaks with a certain amount of additional coordinated solvent molecules could also be recognized (Fig. S45†). The simulated isotopic distribution of the +6 charged species (Fig. 3c) clearly shows that the cage structure contains some solvent molecules, which results in partly overlapping peaks. By slow diffusion of diethyl ether vapor into an acetonitrile solution of Pd6LAuCl12 over three weeks, rhombus-shaped single crystals of the Pd6LAuCl12 cage were obtained.
Single-crystal X-ray diffraction (SCXRD) analysis shows that Pd6LAuCl12 crystallized in a triclinic space group (Fig. 4c, Tables S8 and S9†). The Pd6LAuCl12 cage presents a highly symmetric octahedron geometry, which contains six Pd2+ cations that occupy the vertices and are linked together by twelve LAuCl ligands as the edges. For all the NHC–gold species embedded in Pd6LAuCl12, the bending mode is similar to the free style of LAuCl. However, comparing with the bend angle of LAuCl (146°) in the free state of the structure, this bend in the coordination state has an even larger torsion angle (162°). We hypothesize that this larger torsion angle is derived from the twist of LAuCl during its coordination to palladium. In the solid state, all the gold centers reside at the windows of the Pd6LAuCl12 cage instead of in the cavity. The distance between the gold atoms and the neighboring one is 10 Å. It indicates that the distance of gold–gold in the crystal structure is quite large compared to the actual distance for the aurophilic interactions between two gold atoms (approximately 3 Å), as reported in the literature.24 Therefore, the gold centers here in the Pd6LAuCl12 cage are rather isolated and exposed at the window as observed in the solid-state structure.
A similar structure of self-assembly Pd6L12 (NO3− salt), containing NHC–AuI moieties in ligands, was also reported and applied for anion (PF6− and BF4−) encapsulations.18 The crystals crystallize in a triclinic space group. The highly symmetric octahedral structure contains six Pd2+ metal centers and twelve ligands. The bend angle (174.1°) in the coordination state is closer to linearity than that in the Pd6LAuCl12 cage (162°).
Modeling of the cage structures
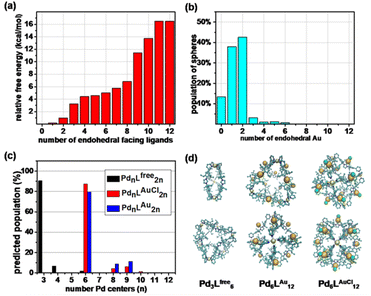
As such, Pd6LAuCl12 contains a unique inversion of exohedral-facing gold catalysts that are fixed on the edges of the windows and exhibits a high symmetry in the solid state (Fig. 4c). While the crystal structure shows a uniform and highly symmetric organization of the gold faces in the solid state, it is known that crystallization preferentially isolates highly symmetric structures.25 To understand the possible solution-state structure of Pd6LAuCl12 we adapted molecular dynamics simulations (MD)26 to assess the number of endohedral (i.e., inward facing) or exohedral (i.e., solvent-facing) facing ligands in Pd6LAuCl12. Model cages featured a varying number of endohedral-facing gold centers, where the relative energy of each configuration (Fig. 5a) could be directly used for computing the distribution of ligand orientations (Fig. 5b). The larger number of configurations accessible by spheres featuring some endo- or exohedrally facing ligands results in an entropic preference for mixed cages (Fig. 5a). Combined with the relatively low energy penalty (<2 kcal mol−1) of including a few endohedral ligands, our model predicts that the plurality of the Pd6LAuCl12 cages features 1–2 endohedral-facing gold centers (Fig. 5a), while a majority of gold sites (87%) (Fig. 5b) are exohedral and remain well separated, as confirmed by UV-vis spectroscopy11 (Fig. S49†) owing to the steric bulk of the cage. This was further confirmed by the crystal structure. Noticeably, gold centers in both the Pd6LAu12 and the Pd6LAuCl12 cages are located on the periphery of the cage windows rather than in the cavity itself, exposing a high concentration of isolated metal centers.As we observed by CSI-HRMS, Lfree forms Pd3Lfree6 double-crown ring assemblies, while gold-containing building blocks afford Pd6LAuCl12 octahedral cages (Fig. 2c and 3c). As reported by Fujita, a small difference in the bend angle of the bidental ligand block may result in the formation of different nanostructures.8Lfree exhibits a concave binding mode with a bend angle of 133°, while LAuCl adopts a convex binding mode with an angle of 146°, probably because pyridine groups in LAuCl are flipped (Fig. 4a). The torsion angle of Lfree (139°) is smaller than that of LAuCl (162°). As both windows in the two cages feature similar triangular geometry, we hypothesize that this torsion angle change drives the divergent outcomes for the self-assembly of these ligands.
Intrigued by these structural differences, we employed MD26 simulations to understand the topological preference of Lfree and gold-containing congeners, LAu and LAuCl (Fig. 5d). While the ligands feature a similar molecular structure, we observed significant differences in the charge distribution between the metalized and non-metalized ligands in their coordination states (Fig. S47†). Surprisingly, Lfree (coordination state) possesses higher charge densities (i.e., increased polarization) on the ortho positions of the pyridine groups compared to its gold-complexed congeners (LAuCl and LAu) in self-assembled structures. This charge difference may account for the divergent self-assembly outcomes we have observed.
In line with our CSI-HRMS measurements (Fig. 2c), our MD26 simulations predict that Lfree favorably self-assembles into double-crowned Pd3Lfree6 assemblies, with a minor population of the Pd4Lfree8 (Fig. 5c). While, the Pd4Lfree8 could not be detected by1H NMR (Fig. 2b) or DOSY experiments (Fig. S22†), the combination of CSI-HRMS and computational evidence supports the Pd4Lfree8 at low concentrations. In contrast, MD simulations of LAuCl and LAu predict the observed Pd6LAuCl12 and Pd6LAu12 octahedral cages as the dominant product of self-assembly (Fig. 5c).
Cyclization of allenol (Pd6LAu12vs. NHC–Au+)
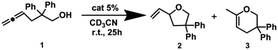
As we have already revealed, Pd6LAuCl12 creates a unique configuration featuring exposed and isolated gold centers on the edges of the cage. We anticipated that these structural characteristics may result in differences in catalysis compared to the mononuclear complex and also compared to previously reported structures in which the complexes are nearer to one another. Inspired by our recent precedents applied in gold-catalyzed cycloisomerizations, in which the gold complexes were furnished inside a well-defined cage via flexible linkers that exhibited aurophilic interactions and led to a highly enhanced reactivity, we started our studies of Pd6LAu12 in the cycloisomerization of allenol (1).27All experiments were precisely carried out under the same benchmark reaction conditions described above and were performed in pre-dried deuterated acetonitrile under argon at room temperature for 25 h (Tables 1 and S1†). In the gold-catalyzed cycloisomerization of allenol (substrate 1), the catalytic result could in principle yield two products, the 5-membered ring (product 2) and the 6-membered ring (product 3). In all reactions, we found that the 5-membered ring (product 2) was the only product formed. With no surprise, the NHC–AuCl complex was found to have no reactivity, and even after pre-activation by AgBF4, the complex only displayed a low reactivity to generate product 2 in 30% yield. As expected, the Pd6LAuCl12 cage was completely inactive, in contrast to our previously reported flexible system, indicating that the gold complexes are well separated at the cage window, to prevent aurophilic interactions that were reported to be important in the flexible system, and the system did not give any conversions of allenol (substrate 1). Gratifyingly, the pre-activated Pd6LAu12 cage displayed a moderate yield of product 2 (57%) compared to the active monomeric NHC–Au+ catalyst, while the gold concentration in these experiments was the same and based on the ligands.
| Entry | Conditions | Conv.b (%) | 2 (%) | 3 (%) |
|---|---|---|---|---|
| a Reaction conditions: [1] = 50 μM, [AgBF4] = 2.5 μM, [NHC–AuCl] = 2.5 μM, [Pd6LAuCl12] = 2.5/12 = 0.21 μM. b The catalytic results were calculated based on 1H NMR by using 1,3,5-trimethoxybenzene as an internal standard. The results are averages of 2 or 3 reactions. | ||||
| 1 | NHC–AuCl | 0 | 0 | 0 |
| 2 | NHC–Au+ | 30 | 30 | 0 |
| 3 | Pd6LAuCl12 | 0 | 0 | 0 |
| 4 | Pd6LAu12 | 57 | 57 | 0 |
Interestingly, when the catalytic reactions were performed in hydrous acetonitrile, we observed an even larger difference in reactivity between NHC–Au+ and Pd6LAu12 (Table S2†). The reactivity of the Pd6LAu12 cage was unaffected in the presence of water, providing a conversion of 57%, whereas catalysis with the NHC–Au+ complex dropped to only 13% (Table S2†). We considered monomeric NHC–Au+ to be deactivated by dimer formation under hydrous conditions. Spectroscopic analysis showed that hydroxy bridge dimeric complexes formed in the presence of water, in line with previous reports.28 The mixture of mononuclear and dinuclear gold species presented a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S53†), as determined via1H NMR, and this ratio remained constant even after the addition of 50 μL of D2O (measured after 24 h, Fig. S54†). In contrast, the NHC–Au+ complexes only present in a monomeric form in an anhydrous acetonitrile system. Notably, the NHC–Au+ units in the Pd6LAu12 cage are restricted to a separated state, effectively blocking the formation of dimeric gold species. This explains the stability of NHC–Au+ units at the window of the cage and the associated higher reactivity in hydrous reaction environments.
1 (Fig. S53†), as determined via1H NMR, and this ratio remained constant even after the addition of 50 μL of D2O (measured after 24 h, Fig. S54†). In contrast, the NHC–Au+ complexes only present in a monomeric form in an anhydrous acetonitrile system. Notably, the NHC–Au+ units in the Pd6LAu12 cage are restricted to a separated state, effectively blocking the formation of dimeric gold species. This explains the stability of NHC–Au+ units at the window of the cage and the associated higher reactivity in hydrous reaction environments.
To further evaluate the difference in the activity and stability between the Pd6LAu12 cage and NHC–Au+ complex, a kinetic analysis of the catalytic reaction was performed in subsequent batch reactions by the addition of a second batch of substrate. This was performed under both hydrous and anhydrous conditions (Fig. S50 and S51†). Under hydrous conditions, in the first reaction cycle, the Pd6LAu12 cage showed a much higher reactivity (TOFini = 1.02 h−1) than the monomeric analog (TOFini = 0.38 h−1), in line with the higher yield of product 2 obtained (Table 1). The curve of NHC–Au+ was flatter after 10 h than that of the Pd6LAu12 as the catalyst (Fig. S50†), in line with dimer formation which decreased the amount of active species. After 49 h, a second batch of substrate was added to test the catalytic performances in the second cycle. Although the rate displayed by the Pd6LAu12 cage (TOFini = 0.80 h−1) slightly decreased compared to that in the first cycle, it was still relatively high. Importantly, this rate was still higher than the rate observed for NHC–Au+ (TOFini = 0.24 h−1). This result demonstrated that the rigidity features of the Pd6LAu12 cage effectively blocked dimer formation and evidently stabilized the gold centers. Under anhydrous conditions, the difference in reactivity in kinetic rates between the Pd6LAu12 cage and NHC–Au+ was smaller (in the first cycle, TOFini = 1.02 h−1 for the Pd6LAu12 cage and TOFini = 0.50 h−1 for NHC–Au+). Additionally, in the experiment when a second batch of substrate was added, the rates for the two catalysts (TOFini = 0.88 h−1 for the Pd6LAu12 cage and TOFini = 0.42 h−1 for monomeric NHC–Au+) were nearly identical to those in the first batch (Fig. S51†). These observations in the experiments demonstrate the excellent stability and activity of the Pd6LAu12 catalyst derived from the rigidity of the cage configuration.
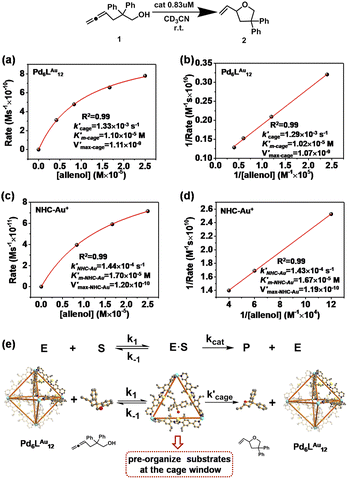
Michaelis–Menten kinetics in the cyclization of allenol
We have demonstrated that this rigid catalytic system (Pd6LAu12) exhibited strong stability and excellent reactivity; thus, to gain a better understanding of its essential role during catalysis, Michaelis–Menten enzymatic rate constant studies29,30 were performed. The kinetic experiments were conducted using the method of initial rates by utilizing Pd6LAu12 or NHC–Au+ as the catalyst (Fig. 6a–d, S55–S57, S69 and S70†). The dependence on the substrate concentration showed a saturation behavior that could be linearized by plotting the double reciprocal of the substrate concentration and the rate. The Michaelis–Menten parameter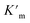 for Pd6LAu12 was determined to be 1.02 × 10−5 M, while the
for Pd6LAu12 was determined to be 1.02 × 10−5 M, while the 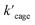 (Michaelis–Menten enzymatic rate constant) was found to be 1.29 × 10−3 s−1 from the Lineweaver–Burk plot (Fig. 6b). These results are consistent with an overall Michaelis–Menten-type mechanism.
(Michaelis–Menten enzymatic rate constant) was found to be 1.29 × 10−3 s−1 from the Lineweaver–Burk plot (Fig. 6b). These results are consistent with an overall Michaelis–Menten-type mechanism.
As expected, Pd6LAu12 shows a higher kcat and a larger Vmax than NHC–Au+ (Fig. 6a–d), which is in line with the higher conversion (Table 1). The Michaelis–Menten parameter Km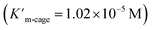 for Pd6LAu12 is lower than that for NHC–Au+
for Pd6LAu12 is lower than that for NHC–Au+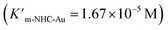 , indicating that the substrate is bound more strongly to Pd6LAu12.
, indicating that the substrate is bound more strongly to Pd6LAu12.
Next, we sought experimental evidence for the binding of the substrate to the cage through NMR experiments. We conducted a set of control experiments to monitor the chemical shifts of Pd6LAu12 upon addition of different equivalents of allenol into the Pd6LAu12 solution (Fig. S73†). It was noted that the NHC–Au(I) five-membered rings in the ligands are placed in-plane of the face of the octahedron (crystal structure in Fig. 4c). In such a case, Au (I) in each NHC–Au (I) five-membered ring has two possibilities of pointing towards the inside of the triangle face, producing stereoisomers. As the rotation of the five-membered ring would be fast, the stereoisomers are not observed by NMR at room temperature. It is consistent with the NMR spectra of the Pd6LAu12 binding substrate (Fig. S73†). In line with the binding of the substrate, the signal of Pd6LAu12 exhibits upfield chemical shifts (approximately 0.01 and 0.02 ppm) in the aromatic region. In addition, the CH proton of the isopropyl group of Pd6LAu12 shows an upfield shift of 0.1 ppm. Importantly, a similar set of NMR experiments using the mononuclear NHC–Au+ complex showed no change in the chemical shifts of the NHC–Au+ in the presence of the substrate (Fig. S74†). It demonstrates that the substrate binds to Pd6LAu12, showing the relevance of the molecular cage structure. Taking all the results together, Pd6LAu12 possesses the ability of substrate pre-organization at the cage windows, which leads to more efficient catalytic conversion. The comparison of 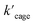 and
and 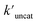 (the rate constant for the uncatalyzed reaction,
(the rate constant for the uncatalyzed reaction, 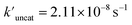 = 2.11 × 10−8 s−1) was also taken into consideration and provided an overall acceleration (6.11 × 104) (Fig. S58†) comparable with the previously reported supramolecular catalysis.30
= 2.11 × 10−8 s−1) was also taken into consideration and provided an overall acceleration (6.11 × 104) (Fig. S58†) comparable with the previously reported supramolecular catalysis.30
Cyclization of hex-4-ynoic acid (Pd6LAu12vs. NHC–Au+)
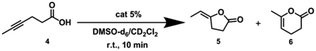
Next, we further extended the catalytic studies in the intramolecular cyclization of hex-4-ynoic acid (substrate 4)31 (Tables 2 and S3†) to probe the cage effect on the selectivity of the reaction. Without pre-activation by AgBF4 to abstract chloride, both the NHC–AuCl (Table 2, entry 1) and the Pd6LAuCl12 cage (Table 2, entry 3) were inactive. When NHC–AuCl was treated with AgBF4, a full conversion of substrate 4 was achieved in 10 minutes. Both the 5-membered ring product 5 (40%) and 6-membered ring product 6 (60%) were formed in a ratio of 5/6 = 0.7 (Table 2, entry 2). Interestingly, after the pre-activation of Pd6LAu12 this catalyst also led to a full conversion of substrate 4 in 10 min. However, the yield of the 5-membered ring product 5 increased to 54%, whereas the yield of product 6 was 45%. In the presence of the Pd6LAu12 cage, the 5/6 ratio was calculated as 1.2 (Table 2, entry 4), compared to 0.7 found for the monomeric catalyst. Clearly, the improved ratio of 5/6 is controlled to some extent by the space constraint of the cage windows.| Entry | Conditions | Conv.b (%) | 5 (%) | 6 (%) |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
|---|---|---|---|---|---|
a Reaction conditions: [4] = 50 μM, [AgBF4] = 2.5 μM, [NHC–AuCl] = 2.5 μM, [Pd6LAuCl12] = 2.5/12 = 0.21 μM, DMSO-d6/CD2Cl2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, the total reaction volume was 0.6 mL, 298 K.
b Catalytic reactions were calculated based on 1H NMR by using durene as an internal standard, and all the reactions were performed for 2–3 runs. 3, the total reaction volume was 0.6 mL, 298 K.
b Catalytic reactions were calculated based on 1H NMR by using durene as an internal standard, and all the reactions were performed for 2–3 runs.
|
|||||
| 1 | NHC–AuCl | 0 | 0 | 0 | — |
| 2 | NHC–Au+ | 100 | 40 | 60 | 0.7 |
| 3 | Pd6LAuCl12 | 0 | 0 | 0 | — |
| 4 | Pd6LAu12 | 100 | 54 | 45 | 1.2 |
Michaelis–Menten kinetics in the cyclization of hex-4-ynoic acid
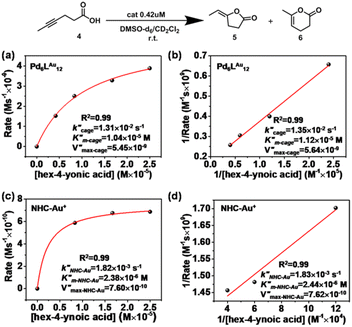
Also for the cyclization of hex-4-ynoic acid, we monitored the reaction kinetics via the method of initial rates and modeled the results by Michaelis–Menten kinetic analysis29,30 (Fig. 7a–d, S59–S67, S71 and S72†). The Pd6LAu12 cage also displayed Michaelis–Menten kinetic behaviour for this substrate (Fig. 7a and b). The corresponding plotting of the double reciprocal of the substrate concentration and the rate afforded an estimation for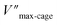 (5.64 × 10−9) and
(5.64 × 10−9) and 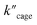 (1.35 × 10−2 s−1)
(1.35 × 10−2 s−1)  (Fig. 7b and Fig. S65–S67†). We observe that the kcat and Vmax for both products 5 and 6 are estimated as the same, whereas the Km for product 5 is lower than that of 6 when utilizing Pd6LAu12 as the catalyst (Fig. S66 and S67†). The data indicate that the cage effect has an influence on the affinity of the intermediate that leads to product 5, which is in line with the selectivity shown in Table 2.
(Fig. 7b and Fig. S65–S67†). We observe that the kcat and Vmax for both products 5 and 6 are estimated as the same, whereas the Km for product 5 is lower than that of 6 when utilizing Pd6LAu12 as the catalyst (Fig. S66 and S67†). The data indicate that the cage effect has an influence on the affinity of the intermediate that leads to product 5, which is in line with the selectivity shown in Table 2.
Pd6LAu12 shows a higher kcat and a larger Vmax than NHC–Au+ (Fig. 7a–d) as shown in the benchmark reaction of allenol cyclization, also in line with the high conversion in Table 2. Interestingly, Pd6LAu12 shows a larger Km (1.12 × 10−5 M) than NHC–Au+ (2.44 × 10−6 M), which indicates that the substrate is more strongly bound to NHC–Au+ than the cage, whereas Pd6LAu12 shows a higher Vmax (5.64 × 10−9) than NHC–Au+ (7.62 × 10−10). The data suggest a transition state stabilization effect by utilizing Pd6LAu12. The comparison of 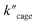 and
and 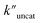 (the rate constant for the uncatalyzed reaction,
(the rate constant for the uncatalyzed reaction, 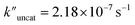 ) was also taken into consideration, showing an enhanced order of 104 (6.19 × 104) (Fig. S68†).
) was also taken into consideration, showing an enhanced order of 104 (6.19 × 104) (Fig. S68†).
Conclusions
In summary, we report an example to extend the catalytic region from the cage cavity to the windows by utilizing Pd6LAu12 cages that contain physically isolated and exposed gold centers fixed rigidly at the windows. Pd6LAu12 displayed enhanced reactivity and high selectivity compared to the monomeric analogue in cyclization reactions. The rigidly fixed gold complexes with the Pd6LAu12 cage cannot interact with each other, thus prohibiting the formation of hydroxy bridged dinuclear complexes, which usually represent a dead end. As a result, the Pd6LAu12 cage is a more stable catalyst under hydrous conditions compared to the monomeric analogue that was demonstrated to form the less active dinuclear complexes. The Pd6LAu12 cage is able to bind and thus pre-organize allenol at the windows of the cage as judged by NMR experiments. Pd6LAu12 shows another key enzymatic feature of transition state stabilization in the cyclization of hex-4-ynoic acid. These lead to Michaelis–Menten-type kinetics when Pd6LAu12 is used as the catalyst, typical features of enzymatic catalysis. This substrate pre-organization and transition state stabilization explain the higher reaction rate displayed by the Pd6LAu12 cage catalyst. This is an example of how a molecular cage presents confinement effects at the windows, and we also report that with these design elements enhanced reactivity, selectivity and stability can be achieved. This contribution extends the catalytic region of the supramolecular cage from the cavity to the windows on the cage surface area, paving a new avenue to innovate bioinspired catalysts, as well as enable deeper understanding of supramolecular catalysis.Author contributions
Meiling Xu, Xu Jing, Chunying Duan and Joost N. H. Reek conceived the project and designed the experiments. Meiling Xu carried out the experiments, collected and interpreted the data. David A. Poole III contributed the calculations. Eduard O. Bobylev contributed the mass experiments. Jinguo Wu and Cheng He solved and refined all the X-ray single-crystal structures. Meiling Xu, Bin Sun, David A. Poole III, Eduard O. Bobylev, Cheng He, Chunying Duan and Joost N. H. Reek cowrote the paper. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support was provided by the National Science Foundation of China (21820102001), the University of Amsterdam and the China Scholarship Council (CSC 201706060163 and 201506140052) for the PhD fellowship.Notes and references
- S. K. Samanta, D. Moncelet, V. Briken and L. Isaacs, J. Am. Chem. Soc., 2016, 138, 14488–14496 CrossRef CAS.
- P. Mal, B. Breiner, K. Rissanen and J. R. Nitschke, Science, 2009, 324, 1697–1699 CrossRef CAS PubMed.
- W. Xuan, M. Zhang, Y. Liu, Z. Chen and Y. Cui, J. Am. Chem. Soc., 2012, 134, 6904–6907 CrossRef CAS PubMed.
- C. J. Brown, F. D. Toste, R. G. Bergman and K. N. Raymond, Chem. Rev., 2015, 115, 3012–3035 CrossRef CAS PubMed.
- (a) T. R. Cook and P. J. Stang, Chem. Rev., 2015, 115, 7001–7045 CrossRef CAS PubMed; (b) J. N. H. Reek, B. de Bruin, S. Pullen, T. J. Mooibroek, A. M. Kluwer and X. Caumes, Chem. Rev., 2022, 122, 12308–12369 CrossRef CAS PubMed; (c) J. Jiao, C. Tan, Z. Li, Y. Liu, X. Han and Y. Cui, J. Am. Chem. Soc., 2018, 140, 2251–2259 CrossRef CAS; (d) M. D. Ward, C. A. Hunter and N. H. Williams, Acc. Chem. Res., 2018, 51, 2073–2082 CrossRef CAS PubMed.
- S. H. A. M. Leenders, R. Gramage-Doria, B. de Bruin and J. N. H. Reek, Chem. Soc. Rev., 2015, 44, 433–448 RSC.
- (a) K. Suzuki, M. Tominaga, M. Kawano and M. Fujita, Chem. Commun., 2009, 1638–1640 RSC; (b) M. Yoneya, S. Tsuzuki, T. Yamaguchi, S. Sato and M. Fujita, ACS Nano, 2014, 8, 1290–1296 CrossRef CAS; (c) D. Fujita, H. Yokoyama, Y. Ueda, S. Sato and M. Fujita, Angew. Chem., Int. Ed., 2014, 53, 1–5 CrossRef; (d) D. Fujita, Y. Ueda, S. Sato, N. Mizuno, T. Kumasaka and M. Fujita, Nature, 2016, 540, 563–566 CrossRef CAS; (e) N. Kamiya, M. Tominaga, S. Sato and M. Fujita, J. Am. Chem. Soc., 2007, 129, 3816–3817 CrossRef CAS PubMed; (f) T. Murase, S. Horiuchi and M. Fujita, J. Am. Chem. Soc., 2010, 132, 2866–2867 CrossRef CAS PubMed; (g) J. Bunzen, J. Iwasa, P. Bonakdarzadeh, E. Numata, K. Rissanen, S. Sato and M. Fujita, Angew. Chem., Int. Ed., 2012, 51, 3161–3163 CrossRef CAS.
- Q.-F. Sun, J. Iwasa, D. Ogawa, Y. Ishido, S. Sato, T. Ozeki, Y. Sei, K. Yamaguchi and M. Fujita, Science, 2010, 328, 1144–1147 CrossRef CAS PubMed.
- H. Yokoyama, Y. Ueda, D. Fujita, S. Sato and M. Fujita, Chem. - Asian J., 2015, 10, 2292–2295 CrossRef CAS PubMed.
- K. Harris, D. Fujita and M. Fujita, Chem. Commun., 2013, 49, 6703–6712 RSC.
- R. Gramage-Doria, J. Hessels, S. H. A. M. Leenders, O. Tröppner, M. Dürr, I. Ivanović-Burmazović and J. N. H. Reek, Angew. Chem., Int. Ed., 2014, 53, 13380–13384 CrossRef CAS PubMed.
- Q.-Q. Wang, S. Gonell, S. H. A. M. Leenders, M. Dürr, I. Ivanović-Burmazović and J. N. H. Reek, Nat. Chem., 2016, 8, 225–230 CrossRef CAS PubMed.
- S. Gonell, X. Caumes, N. Orth, I. Ivanović-Burmazović and J. N. H. Reek, Chem. Sci., 2019, 10, 1316–1321 RSC.
- F. Yu, D. Poole, S. Mathew, N. Yan, J. Hessels, N. Orth, I. Ivanović-Burmazović and J. N. H. Reek, Angew. Chem., Int. Ed., 2018, 57, 11247–11251 CrossRef CAS PubMed.
- J. Guo, Y.-Z. Fan, Y.-L. Lu, S.-P. Zheng and C.-Y. Su, Angew. Chem., Int. Ed., 2020, 59, 8661–8669 CrossRef CAS PubMed.
- W. J. Ramsay, J. A. Foster, K. L. Moore, T. K. Ronson, R. J. Mirgalet, D. A. Jefferson and J. R. Nitschke, Chem. Sci., 2015, 6, 7326–7331 RSC.
- W.-J. Shi, X. Li, P. Li and Y.-F. Han, Nano Res., 2022, 15, 2655–2660 CrossRef CAS.
- L. Zeng, S. Sun, Z.-W. Wen, Y. Xin, L. Liu and J. Zhang, RSC Adv., 2020, 10, 39323–39327 RSC.
- (a) N. Marion and S. P. Nolan, Chem. Soc. Rev., 2008, 37, 1776–1782 RSC; (b) D. Campeau, D. F. León Rayo, A. Mansour, K. Muratov and F. Gagosz, Chem. Rev., 2021, 121, 8756–8867 CrossRef CAS; (c) S. Díez-González, N. Marion and S. P. Nolan, Chem. Rev., 2009, 109, 3612–3676 CrossRef PubMed; (d) P. Nösel, L. Nunes dos Santos Comprido, T. Lauterbach, M. Rudolph, F. Rominger and A. S. K. Hashmi, J. Am. Chem. Soc., 2013, 135, 15662–15666 CrossRef PubMed.
- R. Dorel and A. M. Echavarren, Chem. Rev., 2015, 115, 9028–9072 CrossRef CAS PubMed.
- (a) L.-Y. Mei, Y. Wei, X.-Y. Tang and M. Shi, J. Am. Chem. Soc., 2015, 137, 8131–8137 CrossRef CAS PubMed; (b) D. C. Marcote, I. Varela, J. Fernández-Casado, J. L. Mascareñas and F. López, J. Am. Chem. Soc., 2018, 140, 16821–16833 CrossRef CAS PubMed.
- B. Alcaide, P. Almendros, I. Fernández, R. Martín-Montero, F. Martínez-Peña, M. P. Ruiz and M. R. Torres, ACS Catal., 2015, 5, 4842–4845 CrossRef CAS.
- (a) M. Tominaga, K. Suzuki, T. Murase and M. Fujita, J. Am. Chem. Soc., 2005, 127, 11950–11951 CrossRef CAS PubMed; (b) K. Harris, Q.-F. Sun, S. Sato and M. Fujita, J. Am. Chem. Soc., 2013, 135, 12497–12499 CrossRef CAS PubMed; (c) C. J. Bruns, D. Fujita, M. Hoshino, S. Sato, J. F. Stoddart and M. Fujita, J. Am. Chem. Soc., 2014, 136, 12027–12034 CrossRef CAS.
- S. Cauteruccio, A. Loos, A. Bossi, M. Camila Blanco Jaimes, D. Dova, F. Rominger, S. Prager, A. Dreuw, E. Licandro and A. S. K. Hashmi, Inorg. Chem., 2013, 52, 7995–8004 CrossRef CAS PubMed.
- Y. Tachi, S. Sato, M. Yoneya, M. Fujita and Y. Okamoto, Chem. Phys. Lett., 2019, 714, 185–189 CrossRef CAS.
- D. A. Poole, E. O. Bobylev, S. Mathew and J. N. H. Reek, Chem. Sci., 2020, 11, 12350–12357 RSC.
- (a) Z. Zhang, C. Liu, R. E. Kinder, X. Han, H. Qian and R. A. Widenhoefer, J. Am. Chem. Soc., 2006, 128, 9066–9073 CrossRef CAS; (b) Z. Zhang and R. A. Widenhoefer, Angew. Chem., Int. Ed., 2007, 46, 283–285 CrossRef CAS PubMed.
- R. S. Ramón, S. Gaillard, A. Poater, L. Cavallo, A. M. Z. Slawin and S. P. Nolan, Chem.–Eur. J., 2011, 17, 1238–1246 CrossRef PubMed.
- (a) K. Li, K. Wu, Y.-L. Lu, J. Guo, P. Hu and C.-Y. Su, Angew. Chem., Int. Ed., 2022, 61, e202114070 CrossRef CAS; (b) D. G. Blackmond, Angew. Chem., Int. Ed., 2005, 44, 4302–4320 CrossRef CAS PubMed.
- D. M. Kaphan, M. D. Levin, R. G. Bergman, K. N. Raymond and F. D. Toste, Science, 2015, 350, 1235–1238 CrossRef CAS PubMed.
- (a) A. S. K. Hashmi, Gold Bull., 2003, 36, 3–9 CrossRef CAS; (b) A. S. K. Hashmi, Gold Bull., 2004, 37, 51–65 CrossRef CAS; (c) E. Genin, P. Y. Toullec, S. Antoniotti, C. Brancour, J.-P. Genêt and V. Michelet, J. Am. Chem. Soc., 2006, 128, 3112–3113 CrossRef CAS; (d) N. Nebra, J. Monot, R. Shaw, B. Martin-Vaca and D. Bourissou, ACS Catal., 2013, 3, 2930–2934 CrossRef CAS; (e) H. Harkat, J.-M. Weibel and P. Pale, Tetrahedron Lett., 2006, 47, 6273–6276 CrossRef CAS; (f) L.-C. Lee and Y. Zhao, J. Am. Chem. Soc., 2014, 136, 5579–5582 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2144003–2144005. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3sc02998k |
| This journal is © The Royal Society of Chemistry 2023 |