 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Porous organic cages for gas separations
Wenjing
Wang
 ac,
Kongzhao
Su
ac,
Kongzhao
Su
 *ac and
Daqiang
Yuan
*ac and
Daqiang
Yuan
 *abc
*abc
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, 350002, China. E-mail: skz@fjirsm.ac.cn; ydq@fjirsm.ac.cn
bFujian Science and Technology Innovation Laboratory for Optoelectronic Information of China, Fuzhou 350108, Fujian, P. R. China
cUniversity of the Chinese Academy of Sciences, Beijing, 100049, China
First published on 28th July 2023
Abstract
Gases have played a crucial role in various industries, spanning from manufacturing and medicine to electronics. In these industries, the attainment of pure gases through effective separation methods has been recognized as essential. Porous materials-based adsorption and separation technologies have garnered significant attention due to their advantageous features, including low energy consumption and simplified operational procedures. Among these materials, porous organic cages (POCs) have emerged as a promising class, constructed by individually designed macromolecules possessing inherent cavities that are tailor-made, soluble, easily regenerated, and amenable to precise modifications. Analogous to well-established porous framework materials like zeolites, metal–organic frameworks (MOFs), and covalent organic frameworks (COFs), POCs have exhibited their intrinsic potential for gas separation applications in recent years. In this review, we delve into the progress achieved in the realm of POCs, with a particular focus on their utilization for selective gas separation. Moreover, we present an outlook on the future prospects of this field, along with the existing challenges that demand further attention.
1. Introduction
Separation technology is of paramount importance in contemporary society for various industries to extract pure substances from chemical mixtures. Examples of such applications include the separation of hydrocarbons from crude oil, alkenes from alkanes, greenhouse gases from dilute emissions, as well as the extraction of various derivatives and metals from different sources.1 Gases, in particular, play a fundamental role in the production of chemicals, including fuels, plastics, and polymers, as well as in the domains of petrochemicals, pharmaceuticals, and nuclear industries, where they serve as neutron moderators.2 Traditional methods for gas separation rely on cryogenic distillation, which involves repetitive cycles of evaporation and condensation,3,4 resulting in high energy consumption, environmental concerns, and potential generation of secondary by-products. Consequently, alternative gas purification methods that are both cost- and energy-efficient are urgently needed.In recent years, porous materials have emerged as a promising solution to address gas storage and separation challenges. Notable porous adsorbents encompass a range of materials, including metal-containing framework adsorbents like zeolites and metal–organic frameworks (MOFs),5–10 porous organic adsorbents such as porous organic polymers (POPs),11–13 covalent-organic frameworks (COFs),14–16 and hydrogen-bonded organic frameworks (HOFs),17–19 and molecular cage adsorbents like porous organic cages (POCs)20 and metal–organic cages (MOCs).21 MOFs have witnessed the most rapid development in the field of gas separation due to their remarkable porosity, tunable pore structures, and unsaturated metal sites, leading to their successful applications in various separations such as carbon dioxide (CO2) separation,22,23 alkene purification,24 noble gas separation,25 and isotope separation.26 However, despite their accomplishments, MOFs face challenges concerning cost, regeneration, stability, and selectivity, making it crucial to explore alternative porous materials for gas separation. It is worth noting that the progress in the development of other porous materials has been notably slower compared to MOFs, despite their inherent advantages. For instance, zeolites offer cost-effectiveness and easy availability, while porous organic materials possess a metal-free nature and native nonpolar/inert pore surfaces. POPs exhibit high stability, and HOFs and POCs allow for easy regeneration and tunability in fine-level pore sizes, respectively. Therefore, it is essential to investigate the gas purification capabilities of these materials to foster the advancement of diverse classes of porous materials in the field of gas separation.
Among the porous materials, POCs are constructed through covalent linkages of organic synthons to form discrete macromolecules with intrinsically hollow cavities (Fig. 1).27–34 This distinctive structure provides POCs with several advantages that include solution processing, regeneration, and post-synthesis modification,35–42 compared to other porous framework materials. Organic cage compounds were first reported by the Nobel Prize-winning chemist Jean-Marie Lehn in 1969, who presented the first example of organic cage molecules constructed from crown ether for cation binding.43 However, it was not until 2009 that Cooper's group confirmed the ability of isolated shape-persistent organic cages to serve as porous solids for gas storage.44 Since then, there has been a rapid increase in the number of POCs with different topologies, shapes, and surface areas, attracting significant attention over the past decade.45–73 The common methods used to synthesize POCs can be categorized into two types: irreversible bond formation and reversible bond formation, which mainly include several condensation reactions.20 Both methods have been extensively reviewed in recent publications.
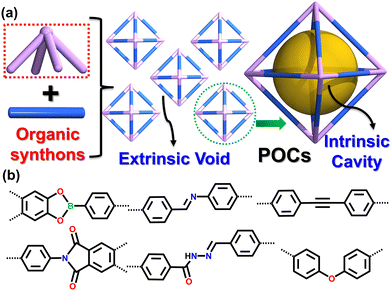 | ||
| Fig. 1 (a) Synthesis and characteristics of POCs. (b) Common covalent linkages in construction of POCs. | ||
POCs, as a relatively new sub-class of porous materials, share many advantages with other porous materials including low density, high surface area and pore volume, tunable window and cavity sizes, and the ability for easy and precise modification. Additionally, POCs show promising potential for numerous applications such as molecular recognition, membranes, catalysis, and separation.74–85 Despite prior reviews covering several applications, there remains an insufficient amount of coverage regarding the efficient gas-selective separation achieved by POCs. Similar to zeolites, MOFs, and COFs, POCs also exhibit gas storage and separation capabilities, fundamental attributes of porous materials. However, POCs are distinct in their construction as discrete molecules held together by weak intermolecular interactions, which results in their porosity arising not only from intrinsic cavities but from extrinsic voids or channels that result from inefficient molecule packing in the solid state. This feature makes POCs vulnerable to structural changes that may significantly impact their porosity and performance in gas storage and separation. Over the last decade, the Brunauer–Emmett–Teller (BET) surface area of POCs has increased from 624 m2 g−1 to 3758 m2 g−1, displaying the ability to selectively store and separate gases such as CO2, hydrocarbons, noble gases, and isotope gases as shown on Fig. 2. Zhang et al. recorded the first shape-persistent prismatic molecular cage capable of high selectivity for CO2 over N2 adsorption in 2010.86 Since then, Mastalerz et al. has developed several shape-persistent POCs that exhibit selective CO2 separation capabilities,87 whereas Cooper et al. reported, in 2014, that an imine-linked tetrahedral POC (CC3) was effective in effectively separating noble gas Xenon (Xe) and Krypton (Kr).88 Moreover, they showed successful separation of greenhouse gas sulfur hexafluoride (SF6) and N2 in 2016,89 and gaseous isotope mixtures (D2/H2) in 2019 by fine-tuning the internal cavities of POCs.90 Furthermore, Zhang et al. achieved efficient separation of binary or ternary C3 hydrocarbon mixtures using a soft imide-based POC (NKPOC-1) with gate-opening behavior in 2019.91 Yuan et al. developed a robust ethane-trapping POC (CPOC-301) in 2021, enabling one-step purification of high-purity ethylene (C2H4) from ethane (C2H6) and C2H4 mixture.92 They also discovered the solvatomorphism, which influenced acetylene (C2H2) and CO2 separation in CPOC-101.93 In 2022, Mastalerz et al. fine-tuned the fluorinated side-chains on the windows of imine-linked POCs,94 subsequently improving the separation of fluorinated alkanes. This review aims to provide a comprehensive overview of the significant progress made by POCs in gas separation. It is divided into distinct sections covering CO2 separation, hydrocarbon purification, rare gas separation, fluorinated greenhouse gas separation, and D2/H2 isotope separation. Additionally, we address remaining challenges and offer an outlook to stimulate further development of POCs in gas separation.
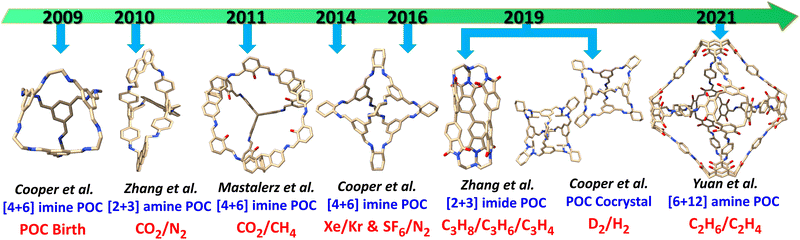 | ||
| Fig. 2 Timeline of POCs for gas separation applications. Carbon is orange, oxygen red, nitrogen blue, and fluorine green. Hydrogen atoms and alkyl groups are omitted for clarity. | ||
2. POCs for gas separations
Gas molecule separation is a challenging technological feat, particularly when the molecules possess similar sizes and shapes. However, highly porous materials utilized for adsorptive separation have become an alternative technology due to the lower energy consumption. POCs, with their exceptional characteristics, offer promising potential for highly efficient gas separation. In this section, we aim to provide an overview of the significant advancements made in POCs regarding the separation of various gases, such as CO2, light hydrocarbons, Xe/Kr, fluorinated greenhouse gases and D2/H2.2.1. CO2 separation
The accelerated increase in atmospheric CO2 concentration caused by climate change has recently received substantial attention. In response, porous materials that use physical adsorption are being considered as promising solutions for CO2 capture. POCs, as a new type of porous material, have also been successfully used in separating CO2/N2.95–98In 2010, Zhang et al. published a study which reported the use of prismatic molecule cage 6 as an effective medium for CO2 and N2 separation.86 They observed that CO2 uptake capacity was 4.46 cm3 g−1, while N2 exhibited negligible adsorption at 0.061 cm3. The CO2/N2 adsorption selectivity was calculated to be 73, highlighting a profound affinity for CO2 over N2 during adsorption. Later, they successfully produced a series of new organic cage compounds (1–4) using a one-pot reversible imine condensation reaction between triamines and dialdehydes,99 followed by hydride reduction of the resulting imine to amine bonds. The triamine moieties functioned as the top and bottom panels of a trigonal prism, while the dialdehyde moieties served as the three lateral edges. The N2 adsorption isotherms at 77 K revealed that these materials had no pores for N2 gas, as indicated by a BET surface area of less than 10 m2 g−1. Cage 4 had the lowest N2 uptake while also displaying a comparable adsorption capacity for CO2, resulting in the highest CO2/N2 adsorption selectivity with a ratio of 138/1. The selectivity in gas adsorption observed could be attributed to the density of amino groups within the cage molecule and the size of the cage cavity. The amino group density predominantly governed the uptake of CO2, whereas the capacity for N2 adsorption related to the dimensions of the molecular prisms.
Cooper et al. reported a new propeller-shaped organic cage known as CC6 in 2011,100 produced via a one-step [2+3] cycloimination reaction between 1,3,5-tri(4-formylphenyl)benzene and 1,5-pentanediamine. The resulting CC6 had a moderate apparent BET surface area of 99 m2 g−1. Examination of the N2 adsorption isotherm at 300 K and 1.2 bar revealed a low gas uptake of 0.08 mmol g−1. Conversely, the CO2 adsorption capacity of CC6 under the same conditions was determined to be 0.90 mmol g−1. Using these isotherms, the ideal selectivity for CO2/N2 at 300 K and 1 bar was calculated to be 11.
In addition to reversible imine condensation, Zhang et al. successfully produced a tricyclooxacalixarene cage, named cage 1, in 2015 via a one-pot SNAr reaction involving tetrahydroxytetraphenylethylene and 2,6-dichloropyridine-3,5-dicarbonitrile (Fig. 3).101 This tricyclic cage had a grid-like porous architecture and demonstrated remarkable adsorption capacity for CO2, accompanied by high selectivity towards CO2/N2. Despite having a moderate surface area (432 m2 g−1), cage 1 could adsorb 84 cm3 g−1 (0.75 wt%) of H2 and 62.7 cm3 g−1 (12.5 wt%) of CO2 at 1.0 bar. The superior CO2 adsorption performance of cage 1 can be attributed to the optimal congruence between its microporous structure and the dimensions of CO2, as well as the electron-rich nature of nitrogen and oxygen atoms within the cage framework, which facilitate local-dipole/quadrupole interactions with CO2. The analysis of the initial slopes of the adsorption isotherms yielded a calculated CO2/N2 selectivity of 80 for cage 1.
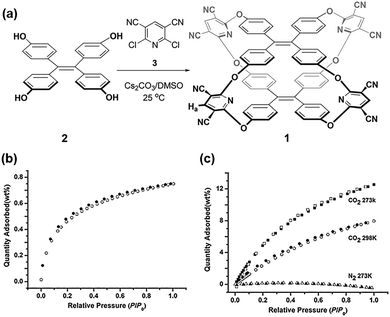 | ||
| Fig. 3 (a) Synthesis of TPE-based tricyclooxacalixarene cage 1. (b) H2 sorption isotherms at 77 K. (c) CO2 and N2 sorption isotherms at 273 K and 298 K. Reproduced with permission from ref. 101. Copyright 2015, Wiley. | ||
In 2018, a new cage (cage 5) was synthesized through a reaction between di(p-methylphenyl)-di(p-aminophenyl)ethylene and 2,6-pyridinediformyl chloride.102Cage 5 has a BET surface area of 347 m2 g−1 and displays considerable CO2 uptake of 39.7 cm3 g−1 at 273 K and 1 bar, equating to approximately six CO2 molecules per cage, with each CO2 molecule binding to one pyridyl ring. Moreover, the N2 adsorption under the same conditions was negligible, resulting in an excellent CO2/N2 selectivity of up to 32.
In 2020, Zhang et al. synthesized a triptycene-based cage (TC) using copper-mediated modified Eglinton-Glaser oxidative coupling reaction.103 The initial structure of TC exhibited limited N2 adsorption at 77 K and a relatively low BET surface area of only 7 m2 g−1. However, by rapidly precipitating TC-rp from a methanol/dichloromethane solution, it transformed into a porous state, increasing the BET surface area to 653 m2 g−1. TC-rp also displayed noteworthy CO2 uptake capacities, with values of 42.3 cm3 g−1 at 273 K and 33.6 cm3 g−1 at 298 K. Based on these measurements, the CO2/N2 selectivity of TC-rp was found to be 4.8 at 273 K.
Although it is primarily composed of methane (CH4), natural gas often contains significant amounts of CO2 that must be extracted to increase its heating value and prevent pipeline corrosion. POCs have also been utilized in the separation of CO2/CH4.104,105 In 2011, Mastalerz et al. examined the separation characteristics of CO2/CH4 using cage 3, which they synthesized through a one-pot Schiff base condensation reaction involving triamine and salicylbisaldehyde (Fig. 4).87Cage 3 had a calculated BET surface area of 1375 m2 g−1 and a Langmuir surface area of 1566 m2 g−1. At 77 K and 1 bar, the H2 adsorption capacity was 5.60 mmol g−1. At the same conditions (273 K and 1 bar), the adsorption capacity of CO2 was 2.10 mmol g−1, while only 0.61 mmol g−1 of CH4 was adsorbed. The marked difference in adsorption capacity between CO2 and CH4 implies the usefulness of cage 3 in removing CO2 from natural gas and enhancing CH4 content. The authors credit the hydroxy groups inside the cages for the high CO2/CH4 selectivity (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) of cage 3. Subsequently, a new exo-functionalized [4+6] cage 5 was reported, with a specific surface area of 919 m2 g−1, which exhibited high selectivity for CO2/CH4. At 273 K and 1 bar, cage 5 adsorbs 3.37 mmol g−1 CO2 and only 0.66 mmol g−1 CH4.96
1 w/w) of cage 3. Subsequently, a new exo-functionalized [4+6] cage 5 was reported, with a specific surface area of 919 m2 g−1, which exhibited high selectivity for CO2/CH4. At 273 K and 1 bar, cage 5 adsorbs 3.37 mmol g−1 CO2 and only 0.66 mmol g−1 CH4.96
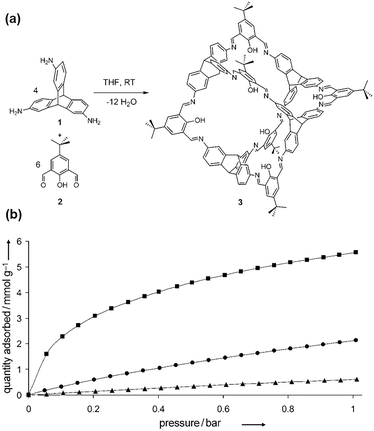 | ||
| Fig. 4 (a) Synthesis of cage 3. (b) Gas adsorption isotherms of H2 (square, 77 K), CO2 (circle, 273 K), and CH4 (triangle, 273 K) for cage compound 3. Reproduced with permission from ref. 87. Copyright 2011, Wiley. | ||
Yuan et al. recently introduced two POCs called CPOC-105 and CPOC-106 constructed using two C4RACHO atoms with four p-xylylenediamine and m-xylylenediamine linkers,106 respectively. The corresponding BET surface areas, calculated using CO2 sorption at 196 K, were 277 and 218 m2 g−1 for CPOC-105 and CPOC-106, respectively. At 298 K and 1 bar, CPOC-105 and CPOC-106 showed higher adsorption capacities for CO2 than CH4, with 44 cm3 g−1vs. 17 cm3 g−1 for CPOC-105 and 37 cm3 g−1vs. 20 cm3 g−1 for CPOC-106. Using Ideal Adsorbed Solution Theory (IAST) results under 1 bar, the CO2/CH4 selectivity values were calculated to be 4.5 for CPOC-105 and 3.1 for CPOC-106. Dynamic breakthrough experiments were used to validate the superior separation performance of CPOC-105.
Amendola et al. presented two novel imide/imine organic cages, named C1 and C2 (Fig. 5),107 synthesized through a [2+3] imine condensation reaction using two distinct polyamines and a novel dialdehyde compound incorporating a rigid bicyclo[2.2.2]oct-7-ene-2,3,5,6-tetracarboxydiimide core. At 298 K, the CO2 adsorption capacities of C1 and C2 were 0.61 mmol g−1 and 0.69 mmol g−1, respectively, while the adsorption capacities of CH4 and N2 were much lower. C1 and C2 demonstrated positive results in terms of CO2/N2 selectivity, with IAST selectivity values of 41 and 32, respectively, for CO2/N2 (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85) mixtures. Moreover, both cages showed potential as fillers for mixed-matrix membranes (MMMs) in combination with polymers such as PEEK-WC and Matrimid® 9725. By incorporating C1 and C2 into Matrimid® 9725, CO2/CH4 selectivity was improved, and inclusion in PEEK-WC enhanced CO2/N2 selectivity.
85) mixtures. Moreover, both cages showed potential as fillers for mixed-matrix membranes (MMMs) in combination with polymers such as PEEK-WC and Matrimid® 9725. By incorporating C1 and C2 into Matrimid® 9725, CO2/CH4 selectivity was improved, and inclusion in PEEK-WC enhanced CO2/N2 selectivity.
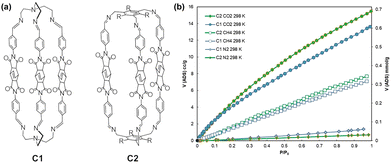 | ||
| Fig. 5 (a) Sketches of C1 and C2 (R = ethyl). (b) Overlay of CO2, CH4, and N2 adsorption isotherms, measured at 298 K. Reproduced with permission from ref. 107. Copyright 2022, Wiley. | ||
In 2021, Mastalerz et al. conducted an investigation on chiral self-sorting of large cubic [8+12] salicylimine cages (Fig. 6).108 The cages formed via the condensation of eight chiral C3-symmetric TBTQ-tris(salicylaldehydes) with 12 p-phenylenediamine. Notably, only three isomers were observed: (P)-4, (M)-4, and (P,M)-4. The comparison between the two enantiomeric cages, (P)-4 and (M)-4, revealed that they had similar BET surface area of 1212 and 1126 m2 g−1, respectively. At 273 K and 1 bar, (P)-4 had an adsorption capacity of 61.1 cm3 g−1 for CO2 and 18.2 cm3 g−1 for CH4, while (M)-4 had an adsorption capacity of 63.6 cm3 g−1 for CO2 and 19.7 cm3 g−1 for CH4. (P)-4 had a CO2/CH4 selectivity of 7.6 and a CO2/N2 selectivity of 24.9, while (M)-4 had a CO2/CH4 selectivity of 7.4 and a CO2/N2 selectivity of 24.7. A comparison between (P,M)-4_1 and (P,M)-4_2 yields intriguing insights. While (P,M)-4_2 has higher crystallinity and a greater specific surface area, it shows lower selectivity. Specifically, it has selectivity values of 5 for CO2/CH4 and 12.3 for CO2/N2, compared to (P,M)-4_1, which has selectivity values of 7.1 for CO2/CH4 and 28.0 for CO2/N2. This finding emphasizes that a higher degree of crystallinity doesn’t always mean superior material properties compared to an amorphous counterpart.
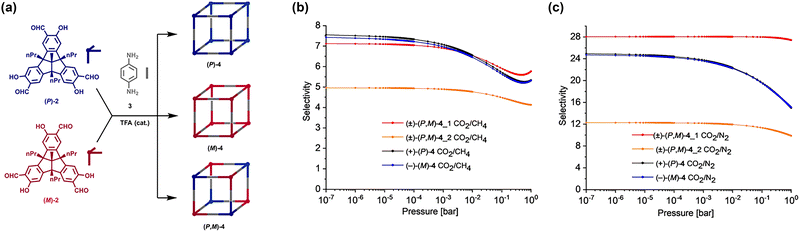 | ||
Fig. 6 (a) Condensation of the tri(salicylaldehyde) 2 with p-phenylenediamine 3 leading to the cubic [8+12] imine cage compounds (P)-4, (M)-4 and (P,M)-4. The cage compounds are represented schematically as cubes with the TBTQ-units at their vertices in blue for the (P)-enantiomer, in red for the (M)-enantiomer and the linear p-phenylenediimine linkers at the edges in grey. (b) IAST selectivity curves of the [8+12] cage compounds for CO2/CH4 (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) at 273 K and (c) for CO2/N2 (20 50) at 273 K and (c) for CO2/N2 (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) at 273 K. Reproduced with permission from ref. 108. Copyright 2021, Wiley. 80) at 273 K. Reproduced with permission from ref. 108. Copyright 2021, Wiley. | ||
The structural stability of POCs is crucial in determining their gas separation performance. A highly acid and base-stable shape-persistent porous carbamate cage 3 was introduced in 2017.109 In addition, cage 3 is capable of stability even in hot 1 M hydrochloric acid and in concentrated hydrochloric acid at room temperature without decomposition. Despite its low specific surface area, cage 3 exhibited good selectivity towards CO2/CH4, adsorbing 58 cm3 g−1 CO2 and 21.8 cm3 g−1 CH4 at 273 K and 1 bar. In 2019, Mastalerz et al. synthesized a large shape-persistent [4+6] amide cage 4. The cages’ chemical robustness enabled post-functionalization under harsh conditions, resulting in the formation of demethylated cage 7, brominated cage 8, and nitrated cage 9.110 Notably, all of these cages demonstrated similar CO2 adsorption capacities, measuring 47.8 cm3 g−1 for cage 4, 45.8 cm3 g−1 for cage 7, 46.3 cm3 g−1 for cage 8, and 45.8 cm3 g−1 for cage 9 at 273 K and 1 bar. Under the same conditions, the cages exhibited similar CH4 adsorption capacities, measuring 13.7 cm3 g−1 for cage 4, 11.2 cm3 g−1 for cage 7, 12.1 cm3 g−1 for cage 8, and 12.0 cm3 g−1 for cage 9. Of these cages, cage 8 demonstrated the greatest selectivity for CO2/CH4, with a value of 28.5.
Yuan et al. recently reported on sp2 carbon-linked POCs (sp2c-POCs) that possess distinctive triangular prism structures through a one-step Knoevenagel reaction (Fig. 7).111 The sp2c-POCs are highly stable, maintaining their structural integrity even in the presence of concentrated HCl, concentrated HNO3, or saturated NaOH solutions. Notably, sp2c-POC1 has the highest BET surface area (350 m2 g−1) among all sp2c-POCs. In addition, sp2c-POC1 can absorb 27.4 cm3 g−1 of CO2 and 6.1 cm3 g−1 of CH4, but has negligible affinity for N2 at 298 K and 1 bar. The observed differences in gas uptake capacities imply that sp2c-POC1 can be useful in separating CO2/N2 and CO2/CH4 mixtures. The IAST selectivities of sp2c-POC1 were calculated, yielding values of 21 and 52 for CO2/CH4 (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) and CO2/N2 (15
50) and CO2/N2 (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85) mixtures, respectively. Breakthrough experiments have substantiated the excellent separation performance of sp2c-POC1 for CO2/CH4 and CO2/N2 gas mixtures. Moreover, the exceptional stability demonstrated by sp2c-POC1 highlights its potential as a solid adsorbent for purifying and separating natural and flue gases.
85) mixtures, respectively. Breakthrough experiments have substantiated the excellent separation performance of sp2c-POC1 for CO2/CH4 and CO2/N2 gas mixtures. Moreover, the exceptional stability demonstrated by sp2c-POC1 highlights its potential as a solid adsorbent for purifying and separating natural and flue gases.
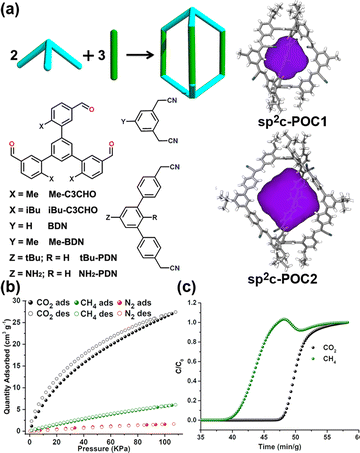 | ||
| Fig. 7 (a) The synthetic route of [2+3] sp2c-POCs and their single-crystal X-ray structures. (b) CO2, CH4 and N2 adsorption isotherms of sp2c-POC1 at 298 K. (c) Experimental breakthrough curves of an equimolar mixture of CO2/CH4 at 298 K and 1 bar over a packed bed of sp2c-POC1. Reproduced with permission from ref. 111. Copyright 2023, Chinese Chemical Society. | ||
The separation of CO2 and C2H2 is crucial in producing high-purity C2H2 by eliminating CO2 impurities and poses significant challenges due to their similar molecular sizes and physical characteristics. It is imperative to study the gas sorption and separation behavior of CO2 and C2H2 in POC with intrinsic porosity, abundant electron-rich π calix[4]-resorcinarene cavities, and oxygen and nitrogen sites. In 2021, Yuan et al. discovered a lantern-shaped calix[4]resorcinarene-based POC (CPOC-101) that exhibits eight distinct solvatomorphs when subjected to crystallization in different solvents,93 presenting a significant breakthrough. Specifically, the CPOC-101α variant obtained through crystallization in toluene/chloroform demonstrated an apparent BET surface area of up to 406 m2 g−1 as determined by nitrogen gas sorption at 77 K, which was much higher than other solvatomorphs of CPOC-101 with BET surface area below 40 m2 g−1. Most importantly, the CPOC-101α variant showed superior adsorption capacities for C2H2 and CO2 and greater ability in C2H2/CO2 separation than CPOC-101β, which served as the representative solvatomorph with low BET surface areas. At 298 K and 1 bar, the activation of CPOC-101α resulted in adsorption capacities of CO2 and C2H2 of up to 63 and 95 cm3 g−1, respectively, while CPOC-101β resulted in lower adsorption capacities of 39 and 60 cm3 g−1 under the same conditions. At ambient conditions, dynamic breakthrough experiments were conducted to verify the practical separation performance of C2H2/CO2 using CPOC-101. The longer separation time of CPOC-101α compared to CPOC-101β indicates that it has a higher preference for trapping C2H2 over CO2. Calculating the separation factors of C2H2/CO2 for CPOC-101α and CPOC-101β using breakthrough experiments yields values of 2.2 and 2.0, respectively. Additionally, the stability of CPOC-101α was confirmed through recycling experiments where the breakthrough time remained consistent after four cycles.
In 2017, Yuan et al. reported the synthesis of seven water-stable hydrazone-linked POCs with four distinct assembly modes: HPOC-101, HPOC-102, HPOC-103, and HPOC-104.112 These cages were synthesized via coupling reaction between C4RACHO and various dihydrazides (Fig. 8). The characterization of the porous organic cages revealed calculated BET surface areas ranging from 373 to 563 m2 g−1. Additionally, the cages’ adsorption properties towards CO2 and C2H2 at room temperature were investigated, resulting in C2H2 capacities ranging from 31 to 47 cm3 g−1 and CO2 capacities ranging from 24 to 33 cm3 g−1. These cages notably exhibited a preference for C2H2 adsorption over CO2. To assess their applicability in C2H2/CO2 separation, breakthrough experiments using a C2H2/CO2 mixture (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), were specifically conducted for HPOC-102 and HPOC-104, which resulted in the effective separation of the C2H2/CO2 mixture. These breakthrough experiments highlight the promising performance of the cages in C2H2/CO2 separation. Furthermore, HPOC-102 and HPOC-104 serve as pioneering examples of hydrazone-linked POC materials successfully achieving C2H2/CO2 gas separation.
50), were specifically conducted for HPOC-102 and HPOC-104, which resulted in the effective separation of the C2H2/CO2 mixture. These breakthrough experiments highlight the promising performance of the cages in C2H2/CO2 separation. Furthermore, HPOC-102 and HPOC-104 serve as pioneering examples of hydrazone-linked POC materials successfully achieving C2H2/CO2 gas separation.
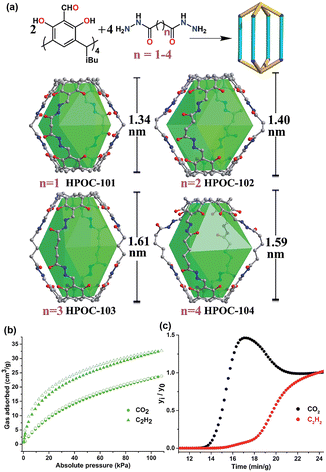 | ||
| Fig. 8 (a) Schematic illustration of the assembly of [2+4] lanterns via hydrazone coupling of C4RACHO and alkanedihydrazides, along with their single-crystal X-ray structures and inner cavity heights. (b) CO2 and C2H2 adsorption isotherms of HPOC-102 at 298 K. (c) Experimental breakthrough curves of an equimolar mixture of CO2/C2H2 at 298 K and 1 bar over a packed bed of HPOC-102. Reproduced with permission from ref. 112. Copyright 2021, Royal Society of Chemistry. | ||
2.2. Light hydrocarbon purification
The separation of light hydrocarbons into individual components with the desired purity remains a significant objective in the petrochemical industry. Cryogenic distillation, a prevailing method, is expensive and energy-intensive, leading to the emergence of adsorption separation with porous materials as a promising alternative technology. Recently, a few POCs have been synthesized to achieve highly efficient separation of light hydrocarbons.In 2018, Mastalerz et al. reported the synthesis of a small series of [4+6] boronic ester cages that varied in the degrees of fluorine content within the diboronic ester struts.113 Of the fluorinated cages, cages 10* and 11* exhibited noteworthy properties. Notably, non-fluorinated cage 9* had an excellent specific surface area of 511 m2 g−1. Encouraged by this result, subsequent investigations concentrated on the adsorption and separation performance of cage 9* for small hydrocarbons. The gas adsorption capacity of cage 9* was found to be 2.52 mmol g−1 for C2H6, 2.50 mmol g−1 for C2H4, and 2.53 mmol g−1 for C2H2. The selectivities for C2H6/C2H4, C2H6/C2H2, and C2H4/C2H2 based on a hypothetical 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture were evaluated to be 1.29, 1.52, and 1.17, respectively.
1 mixture were evaluated to be 1.29, 1.52, and 1.17, respectively.
Zhang et al. subsequently reported the synthesis of a new class of soft porous crystals utilizing an [2+3] imide-based organic cage referred to as NKPOC-1 (Fig. 9).91 One intriguing characteristic of NKPOC-1 is its guest-induced breathing behavior, where it undergoes reversible transformations from a “closed” nonporous phase (α) to two distinct porous “open” phases (β and γ) upon exposure to various gas molecules. Notably, NKPOC-1-α exhibits remarkable selectivity for propyne (C3H4) adsorption over propylene (C3H6) and propane (C3H8) under ambient conditions. To evaluate its separation capabilities, dynamic breakthrough experiments were conducted using binary and ternary gas mixtures consisting of C3H4/C3H6 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), C3H4/C3H8 (2
1, v/v), C3H4/C3H8 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), and C3H4/C3H6/C3H8 (2
1, v/v), and C3H4/C3H6/C3H8 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 : 1, v/v/v) at 298 K. NKPOC-1-α demonstrates exceptional separation efficiency in the case of binary C3H4/C3H6 and C3H4/C3H8 gas mixtures, with C3H4 saturation uptakes and breakthrough retention times of 75.6 cm3 g−1 and 137 min g−1, as well as 55.2 cm3 g−1 and 100 min g−1, respectively. Noteworthy, NKPOC-1-α also effectively removes C3H4 from a ternary gas mixture (C3H4/C3H6/C3H8, 2
1 : 1, v/v/v) at 298 K. NKPOC-1-α demonstrates exceptional separation efficiency in the case of binary C3H4/C3H6 and C3H4/C3H8 gas mixtures, with C3H4 saturation uptakes and breakthrough retention times of 75.6 cm3 g−1 and 137 min g−1, as well as 55.2 cm3 g−1 and 100 min g−1, respectively. Noteworthy, NKPOC-1-α also effectively removes C3H4 from a ternary gas mixture (C3H4/C3H6/C3H8, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v/v) under ambient conditions, based on a C3H4 retention duration of 135 min g−1 and a calculated C3H4 saturation uptake of 67 cm3 g−1. This outstanding separation performance of NKPOC-1-α is attributed to its selective gate-opening effect for C3 hydrocarbons combined with the strength of the interactions once the gate is open.
1, v/v/v) under ambient conditions, based on a C3H4 retention duration of 135 min g−1 and a calculated C3H4 saturation uptake of 67 cm3 g−1. This outstanding separation performance of NKPOC-1-α is attributed to its selective gate-opening effect for C3 hydrocarbons combined with the strength of the interactions once the gate is open.
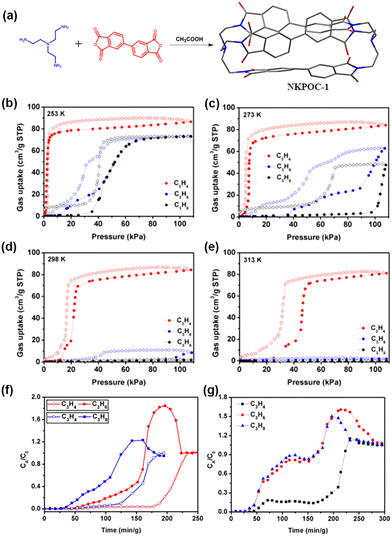 | ||
Fig. 9 (a) Synthesis of NKPOC-1 (C, gray; N, blue; O, red). Sorption isotherms of NKPOC-1-α for C3H4, C3H6, and C3H8 at (b)253 K, (c) 273 K, (d) 298 K, and (e) 313 K. Filled and open symbols represent adsorption and desorption, respectively. (f) Dynamic experimental fixed-bed column breakthrough results of binary (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) C3H4/C3H6 and C3H4/C3H8 gas mixtures in an absorber bed packed with activated NKPOC-1-α. (g) Ternary breakthrough experiment for a C3H4/C3H6/C3H8 (2 1 v/v) C3H4/C3H6 and C3H4/C3H8 gas mixtures in an absorber bed packed with activated NKPOC-1-α. (g) Ternary breakthrough experiment for a C3H4/C3H6/C3H8 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 : 1 v/v/v) gas mixture. Reproduced with permission from ref. 91. Copyright 2019, American Chemical Society. 1 : 1 v/v/v) gas mixture. Reproduced with permission from ref. 91. Copyright 2019, American Chemical Society. | ||
Yuan et al. identified a promising POC, known as CPOC-301, which is composed of a highly porous [6+12] octahedral calix[4]resorcinarene structure (Fig. 10).92 It is important that CPOC-301 shows exceptional ability in selectively capturing C2H6 from mixtures with C2H4, resulting in the direct production of high-purity C2H4. CPOC-301 can adsorb up to 670 cm3 g−1 of N2, a maximal capacity that corresponds to a calculated BET surface area of 1962 m2 g−1. A key finding is that, at 293 K, CPOC-301 selectively binds C2H6 more strongly (87 cm3 g−1) than C2H4 (75 cm3 g−1). The researchers calculated that the heat of adsorption (Qst) at zero coverage for C2H6 and C2H4 is 32.4 and 24.2 kJ mol−1, respectively. This observation suggests that the host–guest interactions between CPOC-301 and C2H6 are much stronger than those with C2H4. The researchers attribute the stronger attraction of CPOC-301 to C2H6 as a result of the creation of multiple C–H···π hydrogen bonds between C2H6 and the resorcin[4]arene cavities, demonstrated by first-principles dispersion-corrected density functional theory (DFT-D) calculations. Additionally, breakthrough experiments have validated the efficient isolation of C2H4 from C2H6/C2H4 mixtures using CPOC-301. The researchers provide the calculated separation factor for an equimolar mixture of C2H6/C2H4, which is 1.3, a value that aligns well with the predicted IAST value. Further, researchers found that the CPOC-301 consistently maintains its separation performance over seven consecutive cycles, which demonstrates its robustness and positions it as a promising candidate for C2H4 purification purposes.
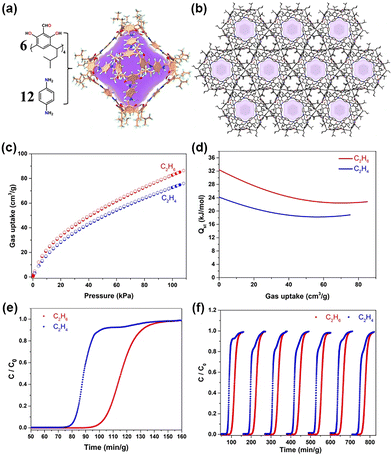 | ||
| Fig. 10 (a) The X-ray crystal structure of CPOC-301. (b) The Solid-state molecular packing of CPOC-301 viewed from [001] direction, where H atoms are committed for clarity. Color codes: phenyl ring; orange, carbon; gray, oxygen; red, nitrogen; blue, and hydrogen; light turquoise. (c) Experimental C2H6 and C2H4 adsorption isotherms of CPOC-301 at 293 K. (d) Isosteric heat of adsorption plots for the adsorption of C2H6 and C2H4 by CPOC-301. (e) Experimental breakthrough curves for equimolar mixture of C2H6/C2H4 at 298 K and 1 bar over a packed bed of CPOC-301. (f) The recyclability of CPOC-301 under multiple mixed gas column breakthrough tests. Reproduced with permission from ref. 92. Copyright 2021, Springer Nature. | ||
Recently, Yuan et al. explored the functionalities of the octahedral calix[4]resorcinarene-based hydrazone-linked porous organic cage (HPOC-401) as a prototype for post-synthetic metalation with different transition metal ions.114 The study demonstrated that HPOC-401 has exceptional properties for this purpose, even under mild conditions. The BET surface areas of HPOC-401 after being metalated with various transition metal ions, namely HPOC-401-V, HPOC-401-Cu, HPOC-401-Zn, and HPOC-401-Mo, were significantly increased compared to pristine HPOC-401. The specific surface areas measured were 1456 m2 g−1 (HPOC-401-V), 1099 m2 g−1 (HPOC-401-Cu), 983 m2 g−1 (HPOC-401-Zn), and 710 m2 g−1 (HPOC-401-Mo), while HPOC-401 had a surface area of 474 m2 g−1. Furthermore, the metalated HPOC-401 showed enhanced CO2, H2, and C2 hydrocarbon uptake capacity along with improved selectivity for C2H6/C2H4, compared to the pure HPOC-401. Specifically, HPOC-401-V and HPOC-401-Cu displayed an interesting preference for C2H6 over C2H4. However, HPOC-401 alone does not exhibit selective adsorption of C2H6. Remarkably, HPOC-401-V demonstrated an outstanding C2H6/C2H4 selectivity of up to 2.3, which surpasses the selectivity values of other POCs. The breakthrough results indicate the potential of HPOC-401-V to achieve efficient one-step separation of C2H4 from an equimolar mixture of C2H6/C2H4.
2.3. Separation for Xe/Kr rare gases
Noble gases, including Kr and Xe, are widely used in diverse industries such as gas lasers, semiconductor, photography lighting, and medical fields.115 However, unstable and hazardous radioisotopes of Kr and Xe, like 133Xe and 85Kr, can be generated by used nuclear fuel reprocessing facilities or nuclear accidents. When these radioisotopes escape into the atmosphere, they pose risks to the environment and living organisms.Cooper et al. developed the first reported POC material for Xe/Kr separation with exceptional performance (Fig. 11).88 The team used a [4+6] tetrahedral POC called CC3, which is an imine-linked structure formed by the Schiff-base reaction of 1,3,5-triformylbenzene and 1,2-diaminocyclohexane in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratio. CC3 was chosen primarily because the largest inclusion sphere in the CC3 cavity measures 4.4 Å, which is close to the size of Xe (4.10 Å). Notably, the window diameter of CC3 observed in its static solid-state crystal structure is only 3.6 Å, which is theoretically too narrow to allow for Xe and Kr diffusion, with diameters of 4.10 Å and 3.69 Å, respectively. However, the flexibility of the cage structure, attributed to cage vibrations, results in temporary enlargement of the pore diameter. Despite being relatively soft since CC3 is assembled from discrete molecules, it exhibits structural deformations, such as window vibrations and packing changes under certain temperature and pressure conditions, allowing many gas molecules to access the pores of CC3. The simulations and experimental gas adsorption isotherms and molecular dynamics revealed that CC3 can accommodate both Xe and Kr, and it demonstrated a strong Xe preferential adsorption over Kr, as evidenced by calculations of the isosteric heat of adsorption. The calculated Xe/Kr selectivity for CC3 reached a value of 20.4, surpassing many MOF materials. To examine the practical separation performance of rare gases at low concentrations in the air, mimicking the spent nuclear fuels reprocessing, the researchers conducted experimental breakthrough measurements. They used gas mixtures containing Xe (400 ppm), Kr (40 ppm), and other elements of air (N2, O2, and CO2), which were passed through a packed column of CC3 crystals. The Kr gas was immediately detected in the effluent, while Xe gas was retained for more than 15 min, which is consistent with the observation of stronger host–guest interactions between Xe and the cage relative to Kr.
3 molar ratio. CC3 was chosen primarily because the largest inclusion sphere in the CC3 cavity measures 4.4 Å, which is close to the size of Xe (4.10 Å). Notably, the window diameter of CC3 observed in its static solid-state crystal structure is only 3.6 Å, which is theoretically too narrow to allow for Xe and Kr diffusion, with diameters of 4.10 Å and 3.69 Å, respectively. However, the flexibility of the cage structure, attributed to cage vibrations, results in temporary enlargement of the pore diameter. Despite being relatively soft since CC3 is assembled from discrete molecules, it exhibits structural deformations, such as window vibrations and packing changes under certain temperature and pressure conditions, allowing many gas molecules to access the pores of CC3. The simulations and experimental gas adsorption isotherms and molecular dynamics revealed that CC3 can accommodate both Xe and Kr, and it demonstrated a strong Xe preferential adsorption over Kr, as evidenced by calculations of the isosteric heat of adsorption. The calculated Xe/Kr selectivity for CC3 reached a value of 20.4, surpassing many MOF materials. To examine the practical separation performance of rare gases at low concentrations in the air, mimicking the spent nuclear fuels reprocessing, the researchers conducted experimental breakthrough measurements. They used gas mixtures containing Xe (400 ppm), Kr (40 ppm), and other elements of air (N2, O2, and CO2), which were passed through a packed column of CC3 crystals. The Kr gas was immediately detected in the effluent, while Xe gas was retained for more than 15 min, which is consistent with the observation of stronger host–guest interactions between Xe and the cage relative to Kr.
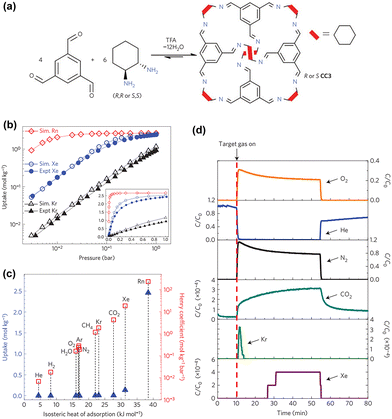 | ||
| Fig. 11 (a) Schematic illustration of the assembly of CC3. (b) Predicted and experimental single-component gas adsorption isotherms for CC3 at 298 K. (c) Isosteric heat of adsorption calculations of noble and other gases at 298 K. (d) Breakthrough curves for CC3 by Xe, Kr, and common gas mixtures. Reproduced with permission from ref. 88. Copyright 2014, Springer Nature. | ||
In 2016, Thallapally et al. reported the selective uptake of Xe by two previously reported POCs,116 namely noria and PgC-noria. These POCs were prepared with an acid-catalyzed condensation reaction between glutaraldehyde and resorcinol (for noria) and pyrogallic acid (for PgC-noria), in the presence of concentrated hydrochloric acid. The isotherms for Xe and Kr adsorption were measured for both noria and PgC-noria. It was observed that both POCs showed selectivity towards Xe over Kr, with noria demonstrating greater Xe adsorption compared to PgC-noria. Furthermore, the IAST calculations demonstrated that noria displayed an exceptional Xe/Kr selectivity of 9.4 under dilute conditions. The remarkable Xe/Kr selectivity, coupled with the high thermal stability of noria implies that it could be a promising candidate for capturing Xe from the off-gas produced during the reprocessing of used nuclear fuel.
2.4. Separation for fluorinated greenhouse gases
Manufactured fluorinated gases, including SF6, perfluorocarbons (PFCs), and nitrogen trifluoride (NF3), are widely utilized in modern industries such as refrigerants, propellants, and blowing agents.117–119 However, it is important to note that fluorinated gases are highly toxic and potent greenhouse gases whose global warming potentials are thousands of times higher than that of CO2. Therefore, the development of porous adsorbents for selective adsorption of fluorinated gases, prioritizing them over other less harmful gases, is critical in both industrial and environmental domains. POCs have undergone development for the separation of fluorinated gases.In 2016, Cooper et al. investigated whether four [4+6] imine-linked tetrahedral POCs (CC2, CC3, CC5, and CC13) could be potentially applied to separate SF6 from N2.89 These POCs were synthesized through a Schiff-base reaction between diamine and tri-aldehyde in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio. Adsorption experiments were conducted to evaluate their capability for SF6 gas uptake, revealing that all four cages demonstrated SF6 adsorption but at varying capacities due to their structural differences. It is important to note that the kinetic diameter of SF6 (5.5 Å) exceeds the window diameters (∼3.6 Å) of CC2, CC3, and CC13, suggesting that they are not capable of encapsulating SF6. However, these POCs possess a flexible nature that enables them to adsorb SF6 despite its larger size than the static window diameter. Further analysis of the adsorption isotherms indicated that CC3α exhibited a typical type I adsorption behavior characterized by sharp increases at low pressures, indicating its higher affinity for SF6. This finding was supported by the calculated Qst values. Furthermore, CC3 showed the highest SF6/N2 selectivity among the four POCs, and activated CC3α achieved selectivities of 178 at 273 K and 74 at 298 K for a 10
2 molar ratio. Adsorption experiments were conducted to evaluate their capability for SF6 gas uptake, revealing that all four cages demonstrated SF6 adsorption but at varying capacities due to their structural differences. It is important to note that the kinetic diameter of SF6 (5.5 Å) exceeds the window diameters (∼3.6 Å) of CC2, CC3, and CC13, suggesting that they are not capable of encapsulating SF6. However, these POCs possess a flexible nature that enables them to adsorb SF6 despite its larger size than the static window diameter. Further analysis of the adsorption isotherms indicated that CC3α exhibited a typical type I adsorption behavior characterized by sharp increases at low pressures, indicating its higher affinity for SF6. This finding was supported by the calculated Qst values. Furthermore, CC3 showed the highest SF6/N2 selectivity among the four POCs, and activated CC3α achieved selectivities of 178 at 273 K and 74 at 298 K for a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 SF6:N2 mixture, which exceeds those of many reported MOFs. Additional simulations suggested that cooperative diffusion and structural rearrangements within CC3 contribute to the near-ideal behavior of the host for SF6 guest molecules. X-ray crystallography analysis provided insight into the location of SF6 within the CC3 cavity, confirming the strong interaction between the CC3 host and SF6 guest.
90 SF6:N2 mixture, which exceeds those of many reported MOFs. Additional simulations suggested that cooperative diffusion and structural rearrangements within CC3 contribute to the near-ideal behavior of the host for SF6 guest molecules. X-ray crystallography analysis provided insight into the location of SF6 within the CC3 cavity, confirming the strong interaction between the CC3 host and SF6 guest.
In 2022, Mastalerz et al. reported three isostructural [2+3] lantern-shaped POCs, including H-cage (with non-fluorinated n-butyl chains), HF-cage (with partially fluorinated n-butyl chains), and F-cage (with perfluorinated n-butyl chains), all with varying degrees of fluorinated side-chains (Fig. 12).94 Triamino triptycene and terphenyl-based bis-salicylaldehydes were used in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratio, catalyzed by trifluoroacetic acid to construct these POCs. The gas adsorption behavior of fluorinated and non-fluorinated alkanes was compared among these isomorphic crystalline states to evaluate the impact of different side chains on gas sorption properties. The study showed that the fluorinated POCs preferentially adsorbed fluorinated gases, whereas the non-fluorinated POCs demonstrated a preference for non-fluorinated gases. Specifically, F-cage exhibited the highest uptakes of PFCs, such as tetrafluoromethane (CF4), hexafluoroethane (C2F6), and octafluoropropane (C3F8), compared to the other two POCs. IAST selectivities of CF4/N2, C2F6/N2, and C3F8/N2 were calculated under different compositions and conditions (273 K and 1 bar), indicating that F-cage had a high selectivity for fluorinated gases over N2. Additionally, F-cage showed the highest selectivity for fluorinated alkanes over non-fluorinated alkanes. Given its advantageous selective adsorption of PFCs, the researchers further investigated the sorption of octafluorocyclobutane (c-C4F8 or PFC-318) in more detail. F-cage demonstrated selectivity for c-C4F8/N2 up to 41475. Experimental breakthrough analyses revealed that F-cage effectively separated c-C4F8 from N2 mixtures with a breakthrough time of 107 minutes, and its separation performance remained consistent over seven consecutive cycles. Furthermore, F-cage demonstrated stability in neutral, acidic, and basic aqueous media as confirmed by 1H and 19F NMR spectroscopy, thermogravimetric analysis (TGA), and N2 sorption at 77 K, indicating that F-cage is robust enough for real-world applications.
3 molar ratio, catalyzed by trifluoroacetic acid to construct these POCs. The gas adsorption behavior of fluorinated and non-fluorinated alkanes was compared among these isomorphic crystalline states to evaluate the impact of different side chains on gas sorption properties. The study showed that the fluorinated POCs preferentially adsorbed fluorinated gases, whereas the non-fluorinated POCs demonstrated a preference for non-fluorinated gases. Specifically, F-cage exhibited the highest uptakes of PFCs, such as tetrafluoromethane (CF4), hexafluoroethane (C2F6), and octafluoropropane (C3F8), compared to the other two POCs. IAST selectivities of CF4/N2, C2F6/N2, and C3F8/N2 were calculated under different compositions and conditions (273 K and 1 bar), indicating that F-cage had a high selectivity for fluorinated gases over N2. Additionally, F-cage showed the highest selectivity for fluorinated alkanes over non-fluorinated alkanes. Given its advantageous selective adsorption of PFCs, the researchers further investigated the sorption of octafluorocyclobutane (c-C4F8 or PFC-318) in more detail. F-cage demonstrated selectivity for c-C4F8/N2 up to 41475. Experimental breakthrough analyses revealed that F-cage effectively separated c-C4F8 from N2 mixtures with a breakthrough time of 107 minutes, and its separation performance remained consistent over seven consecutive cycles. Furthermore, F-cage demonstrated stability in neutral, acidic, and basic aqueous media as confirmed by 1H and 19F NMR spectroscopy, thermogravimetric analysis (TGA), and N2 sorption at 77 K, indicating that F-cage is robust enough for real-world applications.
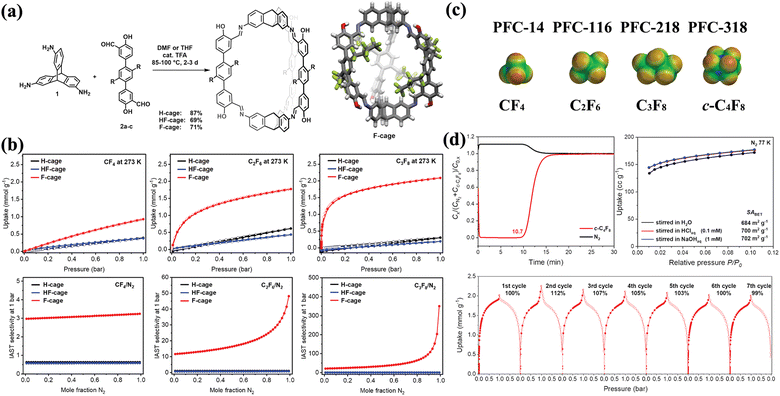 | ||
| Fig. 12 (a) Synthesis of H-cage, HF-cage, and F-cage by imine condensation reactions of triamino triptycene and terphenyl bis-salicylaldehydes, and the single-crystal X-ray structure of F-cage. (b) PFC sorption properties of H-cage, HF-cage, and F-cage in comparison. (c) Space filling models of the fluorinated alkanes, including tetrafluoromethane (PFC-14), hexafluoroethane (PFC-116), octafluoropropane (PFC-218), and octafluorocyclobutane (PFC-318). (d) Applicability investigations of F-cage, including breakthrough curves and stablity tests. Reproduced with permission from ref. 94. Copyright 2022, Wiley. | ||
2.5. Separation for D2/H2 gas isotopes
Deuterium is an irreplaceable raw material that finds widespread use in scientific research, including isotope tracing, neutron scattering, and proton nuclear magnetic resonance spectroscopy, as well as industrial applications, such as fuel in nuclear fusion reactors and for fusion reactions, and in medical uses like imaging and cancer therapy. To meet the growing global demand for deuterium, the development of a cost-effective and large-scale separation method is crucial. Two approaches for separating hydrogen isotopes using porous materials are kinetic quantum sieving (KQS), which utilizes nanoconfined space, and chemical affinity quantum sieving (CAQS), which relies on strong metal adsorption sites. Various porous materials, including activated carbons, zeolites, MOFs, and COFs, have been investigated in attempts to separate D2 from H2.120–127 However, the selectivity of D2/H2 using these materials is often low, typically less than 2, for both separation strategies. Porous materials with ultra-fine pore apertures (∼3 Å) are critical for achieving KQS but are challenging to obtain in activated carbons, zeolites, MOFs, and COFs.Cooper et al. utilized PSM synthesis in 2019, which proved to be effective in modifying POCs' internal cavities and fine-tuning the pore sizes, resulting in outstanding quantum sieves.90 Their work produced six internal functionalized POCs, namely 6ET-RCC3, 1PT-5FT-RCC3, 1AT-5FT-RCC3, 1ET-5FT-RCC3, 5FT-RCC3, and 6FT-RCC3, with PLE values ranging from 1.95 Å to 3.50 Å (Fig. 13). To achieve this, a post-synthetic “tying” and protection-deprotection strategy were utilized based on RCC3, a reduced derivative of CC3. The POCs demonstrated a trade-off behavior, where those with lower PLE values exhibited lower D2 adsorption capacities but higher selectivity while conversely, those with higher PLE values exhibited high D2 adsorption capacities and lower selectivity. The upscaling of the internal functionalized POCs for practical applications presented significant challenges. Nonetheless, combining the small-pore and large-pore functionalized POCs through cocrystallization into a single solid resulted in a material with optimal separation performance, exhibiting excellent D2/H2 selectivity (8.0) and high deuterium uptake (4.7 mmol g−1). In 2022, Vogel et al. used ab initio molecular dynamics (AIMD) simulations to explain how the internal functionalized POCs could manage D2 and H2 separation, leading to the targeted design of novel porous adsorbents for hydrogen isotope separation.128
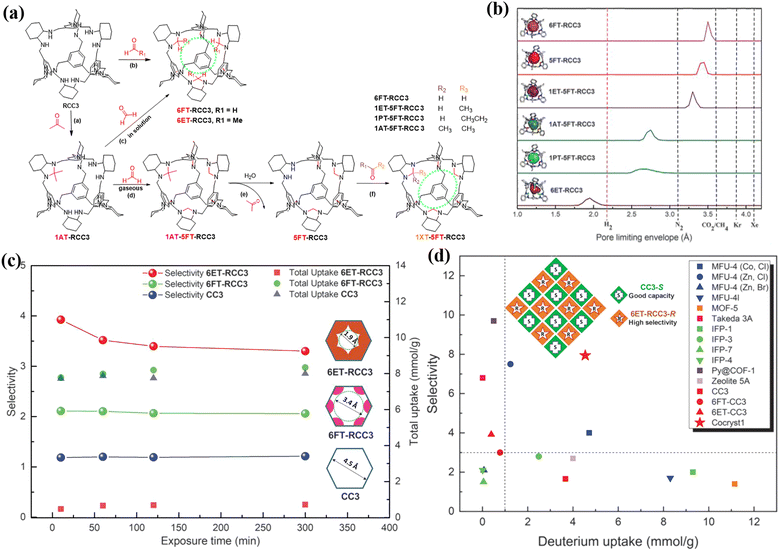 | ||
| Fig. 13 (a) Full synthesis route for the internal functionalized POCs (including 6ET-RCC3, 1PT-5FT-RCC3, 1AT-5FT-RCC3, 1ET-5FT-RCC3, 5FT-RCC3, and 6FT-RCC3). (b) Representative single-crystal structures of the modified cages showing accessible cavities (colored), along with calculated PLEs for each system. (c) D2/H2 selectivities and gas uptakes as a function of exposure time at 30 K for CC3, 6FT-RCC3, and 6ET-RCC3. (d) Summary of hydrogen isotope KQS selectivities and adsorption capacities for various porous materials. Reproduced with permission from ref. 90. Copyright 2019, American Association for the Advancement of Science. | ||
3. Conclusions
In summary, numerous POCs of different shapes, sizes, and functions have been designed and synthesized in the last decade utilizing various organic synthons and covalent condensation reactions. However, only a few of these have been explored for gas separation. To our knowledge, POCs have only been examined in specific gas separation systems such as CO2/N2, CO2/CH4, C2H2/CO2, rare gases Xe/Kr, greenhouse SF6/N2, isotope separation D2/H2, and separation of industrially important C2 and C3 hydrocarbons through mixed gas experiments. These instances have been reviewed thoroughly. However, the gas separation applications of POCs are still in the early stage, and their uses have been limited to the aforementioned gas components in mixed gas experiments. Herein, we present some essential perspectives on the potential applications of POCs in gas separation.(1) Developing stable POCs
Although many POCs have been assembled using dynamically reversible imine and boronate ester bonds, these components are sensitive to hydrolysis under acid, base, or even moisture conditions. This sensitivity often leads to POCs' decomposition, significantly hindering their practical use in gas separation studies considering the presence of acidic, basic, or water vapor gases in gas mixtures. Thus, there is a high demand for the development of robust covalent linkages to build chemically stable POCs using simple and cost-effective procedures.111(2) Separation of industrially important gases and multi-component gas mixtures
POCs exhibit potential for exploring the applications of separating industrially important C4–C6 hydrocarbons as well as multi-component gas mixtures.129–131 POCs may exhibit unexpected and unique separation behavior compared to other extensively studied porous materials such as MOFs, zeolites, and POPs. “Soft” porous materials with flexible and adaptive pore structures have gained attention in recent years because of their capacity to adjust their structures to control gas mobility and diffusivity in their pores for efficient purification.132–134 POCs possess the inherent attribute to serve as “soft” porous materials. They are constructed from small molecules that interact weakly in the solid-state,39,94 allowing gas guests to gain pore access via structural deformations under specified conditions.(3) Efficient and precise separation
Due to their discrete molecules, POCs can be designed with finely-tuned pore sizes at a remarkably precise level with selective interior functionalization. This level of control is challenging to achieve in other porous materials such as COFs and MOFs. Commonly, the strategy of tuning pores in MOFs and COFs involves systematically expanding or reducing the number of phenylene rings in the organic linker, resulting in coarse tuning increments or decrements of approximately 2.8 Å. This tuning method is not suitable for achieving fine-tuning of pores. Therefore, POCs have the potential to achieve high selectivity for the separation of gas mixtures with similar molecule shapes by designing appropriate pores through a fine-tuning approach. This fine-tuning method maintains high gas adsorption capacity while also maintaining the nearly unchanged original cavities.(4) Gas separation using POC-based membranes
Membrane technology has emerged as an efficient and energy-saving alternative for gas separation and purification processes. Leveraging the advantageous solubility and solvent processability of POCs, researchers have successfully developed pure POC-based membranes and mixed matrix membranes. These innovative membranes have demonstrated significant potential across various applications involving gases, ions and small organic molecules.135–138 Therefore, it is imperative to emphasize the promising prospect of achieving high-performance membranes by precisely controlling both the intrinsic and extrinsic pores of POCs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Nature Science Foundation of China (22071244, 22275191, 22275186), Youth Innovation Promotion Association CAS (2022305), Fujian Science & Technology Innovation Laboratory for Optoelectronic Information of China (2021ZZ106) and Natural Science Foundation of Fujian Province of China (2022J01503).References
- D. S. Sholl and R. P. Lively, Seven chemical separations to change the world, Nature, 2016, 532, 435–437 CrossRef PubMed
.
-
N. A. Downie, Industrial gases, Springer Science & Business Media, 2007 Search PubMed
.
- R. Faiz and K. Li, Olefin/paraffin separation using membrane based facilitated transport/chemical absorption techniques, Chem. Eng. Sci., 2012, 73, 261–284 CrossRef CAS
.
- M. Shen, L. Tong, S. Yin, C. Liu, L. Wang, W. Feng and Y. Ding, Cryogenic technology progress for CO2 capture under carbon neutrality goals: A review, Sep. Purif. Technol., 2022, 299, 121734 CrossRef CAS
.
- A. Ebadi Amooghin, H. Sanaeepur, R. Luque, H. Garcia and B. Chen, Fluorinated metal-organic frameworks for gas separation, Chem. Soc. Rev., 2022, 51, 7427–7508 RSC
.
- J.-R. Li, R. J. Kuppler and H.-C. Zhou, Selective gas adsorption and separation in metal-organic frameworks, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC
.
- K. Adil, Y. Belmabkhout, R. S. Pillai, A. Cadiau, P. M. Bhatt, A. H. Assen, G. Maurin and M. Eddaoudi, Gas/vapour separation using ultra-microporous metal-organic frameworks: Insights into the structure/separation relationship, Chem. Soc. Rev., 2017, 46, 3402–3430 RSC
.
- I. C. Medeiros-Costa, E. Dib, N. Nesterenko, J.-P. Dath, J.-P. Gilson and S. Mintova, Silanol defect engineering and healing in zeolites: Opportunities to fine-tune their properties and performances, Chem. Soc. Rev., 2021, 50, 11156–11179 RSC
.
- Y.-X. Tan, F. Wang and J. Zhang, Design and synthesis of multifunctional metal-organic zeolites, Chem. Soc. Rev., 2018, 47, 2130–2144 RSC
.
- T. Lan, L. Li, Y. Chen, X. Wang, J. Yang and J. Li, Opportunities and critical factors of porous metal–organic frameworks for industrial light olefins separation, Mater. Chem. Front., 2020, 4, 1954–1984 RSC
.
- S. Zhang, M. K. Taylor, L. Jiang, H. Ren and G. Zhu, Light hydrocarbon separations using porous organic framework materials, Chem. – Eur. J., 2020, 26, 3205–3221 CrossRef CAS PubMed
.
- J.-S. M. Lee and A. I. Cooper, Advances in conjugated microporous polymers, Chem. Rev., 2020, 120, 2171–2214 CrossRef CAS PubMed
.
- S. Das, P. Heasman, T. Ben and S. Qiu, Porous organic materials: Strategic design and structure-function correlation, Chem. Rev., 2017, 117, 1515–1563 CrossRef CAS
.
- F. Jin, E. Lin, T. Wang, S. Geng, T. Wang, W. Liu, F. Xiong, Z. Wang, Y. Chen, P. Cheng and Z. Zhang, Bottom-up synthesis of 8-connected three-dimensional covalent organic frameworks for highly efficient ethylene/ethane separation, J. Am. Chem. Soc., 2022, 144, 5643–5652 CrossRef CAS PubMed
.
- Z. Zhang, C. Kang, S. B. Peh, D. Shi, F. Yang, Q. Liu and D. Zhao, Efficient adsorption of acetylene over CO2 in bioinspired covalent organic frameworks, J. Am. Chem. Soc., 2022, 144, 14992–14996 CrossRef CAS
.
- C. He, Y. Wang, Y. Chen, X. Wang, J. Yang, L. Li and J. Li, Microregulation of pore channels in covalent-organic frameworks used for the selective and efficient separation of ethane, ACS Appl. Mater. Interfaces, 2020, 12, 52819–52825 CrossRef CAS PubMed
.
- Y. Zhou, C. Chen, R. Krishna, Z. Ji, D. Yuan and M. Wu, Tuning pore polarization to boost ethane/ethylene separation performance in hydrogen-bonded organic frameworks, Angew. Chem., Int. Ed., 2023, 62, e202305041 CrossRef CAS
.
- R.-B. Lin, Y. He, P. Li, H. Wang, W. Zhou and B. Chen, Multifunctional porous hydrogen-bonded organic framework materials, Chem. Soc. Rev., 2019, 48, 1362–1389 RSC
.
- Y. Yang, L. Li, R.-B. Lin, Y. Ye, Z. Yao, L. Yang, F. Xiang, S. Chen, Z. Zhang, S. Xiang and B. Chen, Ethylene/ethane separation in a stable hydrogen-bonded organic framework through a gating mechanism, Nat. Chem., 2021, 13, 933–942 CrossRef CAS PubMed
.
- X. Yang, Z. Ullah, J. F. Stoddart and C. T. Yavuz, Porous organic cages, Chem. Rev., 2023, 123, 4602–4634 CrossRef CAS
.
- E. J. Gosselin, C. A. Rowland and E. D. Bloch, Permanently microporous metal-organic polyhedra, Chem. Rev., 2020, 120, 8987–9014 CrossRef
.
- A. Schoedel, Z. Ji and O. M. Yaghi, The role of metal-organic frameworks in a carbon-neutral energy cycle, Nat. Energy, 2016, 1, 16034 CrossRef CAS
.
- E. González-Zamora and I. A. Ibarra, CO2 capture under humid conditions in metal–organic frameworks, Mater. Chem. Front., 2017, 1, 1471–1484 RSC
.
- T. Wang, E. Lin, Y.-L. Peng, Y. Chen, P. Cheng and Z. Zhang, Rational design and synthesis of ultramicroporous metal-organic frameworks for gas separation, Coord. Chem. Rev., 2020, 423, 213485 CrossRef CAS
.
- Y. Wang, W. Liu, Z. Bai, T. Zheng, M. A. Silver, Y. Li, Y. Wang, X. Wang, J. Diwu, Z. Chai and S. Wang, Employing an unsaturated Th4+ site in a porous thorium-organic framework for Kr/Xe uptake and separation, Angew. Chem., Int. Ed., 2018, 57, 5783–5787 CrossRef CAS PubMed
.
- I. Weinrauch, I. Savchenko, D. Denysenko, S. M. Souliou, H. H. Kim, M. Le Tacon, L. L. Daemen, Y. Cheng, A. Mavrandonakis, A. J. Ramirez-Cuesta, D. Volkmer, G. Schuetz, M. Hirscher and T. Heine, Capture of heavy hydrogen isotopes in a metal-organic framework with active Cu(I) sites, Nat. Commun., 2017, 8, 14496 CrossRef CAS PubMed
.
- T. Hasell and A. I. Cooper, Porous organic cages: Soluble, modular and molecular pores, Nat. Rev. Mater., 2016, 1, 16053 CrossRef CAS
.
- K. Acharyya and P. S. Mukherjee, Organic imine cages: Molecular marriage and applications, Angew. Chem., Int. Ed., 2019, 58, 8640–8653 CrossRef CAS PubMed
.
- M. Mastalerz, Porous shape-persistent organic cage compounds of different size, geometry, and function, Acc. Chem. Res., 2018, 51, 2411–2422 CrossRef CAS PubMed
.
- R. D. Mukhopadhyay, Y. Kim, J. Koo and K. Kim, Porphyrin boxes, Acc. Chem. Res., 2018, 51, 2730–2738 CrossRef CAS
.
- F. Wang, C. Bucher, Q. He, A. Jana and J. L. Sessler, Oligopyrrolic cages: From classic molecular constructs to chemically responsive polytopic receptors, Acc. Chem. Res., 2022, 55, 1646–1658 CrossRef CAS PubMed
.
- S. Huang, Z. Lei, Y. Jin and W. Zhang, By-design molecular architectures via alkyne metathesis, Chem. Sci., 2021, 12, 9591–9606 RSC
.
- F. Beuerle and B. Gole, Covalent organic frameworks and cage compounds: Design and applications of polymeric and discrete organic scaffolds, Angew. Chem., Int. Ed., 2018, 57, 4850–4878 CrossRef CAS PubMed
.
- T. Kunde, T. Pausch and B. M. Schmidt, Porous organic compounds - small pores on the rise, Eur. J. Org. Chem., 2021, 5844–5856 CrossRef CAS
.
- Q. Song, S. Jiang, T. Hasell, M. Liu, S. Sun, A. K. Cheetham, E. Sivaniah and A. I. Cooper, Porous organic cage thin films and molecular-sieving membranes, Adv. Mater., 2016, 28, 2629–2637 CrossRef CAS PubMed
.
- T. Hasell, H. Zhang and A. I. Cooper, Solution-processable molecular cage micropores for hierarchically porous materials, Adv. Mater., 2012, 24, 5732–5737 CrossRef CAS
.
- G. Zhu, F. Zhang, M. P. Rivera, X. Hu, G. Zhang, C. W. Jones and R. P. Lively, Molecularly mixed composite membranes for advanced separation processes, Angew. Chem., Int. Ed., 2019, 58, 2638–2643 CrossRef CAS PubMed
.
- G. Zhu, D. O'Nolan and R. P. Lively, Molecularly mixed composite membranes: Challenges and opportunities, Chem. – Eur. J., 2020, 26, 3464–3473 CrossRef CAS PubMed
.
- M. A. Little and A. I. Cooper, The chemistry of porous organic molecular materials, Adv. Funct. Mater., 2020, 30, 1909842 CrossRef CAS
.
- T. Hasell, J. L. Culshaw, S. Y. Chong, M. Schmidtmann, M. A. Little, K. E. Jelfs, E. O. Pyzer-Knapp, H. Shepherd, D. J. Adams, G. M. Day and A. I. Cooper, Controlling the crystallization of porous organic cages: Molecular analogs of isoreticular frameworks using shape-specific directing solvents, J. Am. Chem. Soc., 2014, 136, 1438–1448 CrossRef CAS PubMed
.
- M. Liu, M. A. Little, K. E. Jelfs, J. T. A. Jones, M. Schmidtmann, S. Y. Chong, T. Hasell and A. I. Cooper, Acid- and base-stable porous organic cages: Shape persistence and ph stability via post-synthetic “tying” of a flexible amine cage, J. Am. Chem. Soc., 2014, 136, 7583–7586 CrossRef CAS
.
- H. Wang, Y. Jin, N. Sun, W. Zhang and J. Jiang, Post-synthetic modification of porous organic cages, Chem. Soc. Rev., 2021, 50, 8874–8886 RSC
.
- B. Dietrich, J. Lehn and J. Sauvage, Diaza-polyoxa-macrocycles and macrobicycles, Tetrahedron Lett., 1969, 10, 2885–2888 CrossRef
.
- T. Tozawa, J. T. A. Jones, S. I. Swamy, S. Jiang, D. J. Adams, S. Shakespeare, R. Clowes, D. Bradshaw, T. Hasell, S. Y. Chong, C. Tang, S. Thompson, J. Parker, A. Trewin, J. Bacsa, A. M. Z. Slawin, A. Steiner and A. I. Cooper, Porous organic cages, Nat. Mater., 2009, 8, 973–978 CrossRef CAS
.
- T. Kunde, E. Nieland, H. V. Schroder, C. A. Schalley and B. M. Schmidt, A porous fluorinated organic [4+4] imine cage showing CO2 and H2 adsorption, Chem. Commun., 2020, 56, 4761–4764 RSC
.
- H. Duan, F. Cao, H. Hao, H. Bian and L. Cao, Efficient photoinduced energy and electron transfers in a tetraphenylethene-based octacationic cage through host-guest complexation, ACS Appl. Mater. Interfaces, 2021, 13, 16837–16845 CrossRef CAS PubMed
.
- H. H. Duan, Y. W. Li, Q. F. Li, P. P. Wang, X. R. Liu, L. Cheng, Y. Yu and L. P. Cao, Host-guest recognition and fluorescence of a tetraphenylethene-based octacationic cage, Angew. Chem., Int. Ed., 2020, 59, 10101–10110 CrossRef CAS PubMed
.
- Y. Chen, G. Wu, B. Chen, H. Qu, T. Jiao, Y. Li, C. Ge, C. Zhang, L. Liang, X. Zeng, X. Cao, Q. Wang and H. Li, Self-assembly of a purely covalent cage with homochirality by imine formation in water, Angew. Chem., Int. Ed., 2021, 60, 18815–18820 CrossRef CAS PubMed
.
- T. Jiao, H. Qu, L. Tong, X. Cao and H. Li, A self-assembled homochiral radical cage with paramagnetic
behaviors, Angew. Chem., Int. Ed., 2021, 60, 9852–9858 CrossRef CAS
.
- Y. Wang, Y. Sun, P. Shi, M. M. Sartin, X. Lin, P. Zhang, H. Fang, P. Peng, Z. Tian and X. Cao, Chaperone-like chiral cages for catalyzing enantioselective supramolecular polymerization, Chem. Sci., 2019, 10, 8076–8082 RSC
.
- H. Qu, Z. Huang, X. Dong, X. Wang, X. Tang, Z. Li, W. Gao, H. Liu, R. Huang, Z. Zhao, H. Zhang, L. Yang, Z. Tian and X. Cao, Truncated face-rotating polyhedra constructed from pentagonal pentaphenylpyrrole through graph theory, J. Am. Chem. Soc., 2020, 142, 16223–16228 CrossRef CAS
.
- S. Ivanova, E. Koester, J. J. Holstein, N. Keller, G. H. Clever, T. Bein and F. Beuerle, Isoreticular crystallization of highly porous cubic covalent organic cage compounds, Angew. Chem., Int. Ed., 2021, 60, 17455–17463 CrossRef CAS PubMed
.
- Y. Shi, K. Cai, H. Xiao, Z. Liu, J. Zhou, D. Shen, Y. Qiu, Q.-H. Guo, C. Stern, M. R. Wasielewski, F. Diederich, W. A. Goddard III and J. F. Stoddart, Selective extraction of C70 by a tetragonal prismatic porphyrin cage, J. Am. Chem. Soc., 2018, 140, 13835–13842 CrossRef CAS PubMed
.
- D. Luo, Y. He, J. Tian, J. L. Sessler and X. Chi, Reversible iodine capture by nonporous adaptive crystals of a bipyridine cage, J. Am. Chem. Soc., 2022, 144, 113–117 CrossRef CAS PubMed
.
- V. W. Liyana Gunawardana, T. J. Finnegan, C. E. Ward, C. E. Moore and J. D. Badjic, Dissipative formation of covalent basket cages, Angew. Chem., Int. Ed., 2022, 61, e202207418 CrossRef CAS
.
- J. Koo, I. Kim, Y. Kim, D. Cho, I.-C. Hwang, R. D. Mukhopadhyay, H. Song, Y. H. Ko, A. Dhamija, H. Lee, W. Hwang, S. Kim, M.-H. Baik and K. Kim, Gigantic porphyrinic cages, Chem, 2020, 6, 3374–3384 CAS
.
- S. Hong, M. R. Rohman, J. Jia, Y. Kim, D. Moon, Y. H. Ko, E. Lee and K. Kim, Porphyrin boxes: Rationally designed porous organic cages, Angew. Chem., Int. Ed., 2015, 54, 13241–13244 CrossRef CAS PubMed
.
- Y. Ding, L. O. Alimi, B. Moosa, C. Maaliki, J. Jacquemin, F. Huang and N. M. Khashab, Selective adsorptive separation of cyclohexane over benzene using thienothiophene cages, Chem. Sci., 2021, 12, 5315–5318 RSC
.
- B. Moosa, L. O. Alimi, A. Shkurenko, A. Fakim, P. M. Bhatt, G. Zhang, M. Eddaoudi and N. M. Khashab, A polymorphic azobenzene cage for energy-efficient and highly selectivep-xylene separation, Angew. Chem., Int. Ed., 2020, 59, 21367–21371 CrossRef CAS PubMed
.
- K. Ono, K. Johmoto, N. Yasuda, H. Uekusa, S. Fujii, M. Kiguchi and N. Iwasawa, Self-assembly of nanometer-sized boroxine cages from diboronic acids, J. Am. Chem. Soc., 2015, 137, 7015–7018 CrossRef CAS
.
- B. Mondal and P. S. Mukherjee, Cage encapsulated gold nanoparticles as heterogeneous photocatalyst for facile and selective reduction of nitroarenes to azo compounds, J. Am. Chem. Soc., 2018, 140, 12592–12601 CrossRef CAS
.
- S. Bera, K. Dey, T. K. Pal, A. Halder, S. Tothadi, S. Karak, M. Addicoat and R. Banerjee, Porosity switching in polymorphic porous organic cages with exceptional chemical stability, Angew. Chem., Int. Ed., 2019, 58, 4243–4247 CrossRef CAS PubMed
.
- S. Bera, A. Basu, S. Tothadi, B. Garai, S. Banerjee, K. Vanka and R. Banerjee, Odd-even alternation in tautomeric porous organic cages with exceptional chemical stability, Angew. Chem., Int. Ed., 2017, 56, 2123–2126 CrossRef CAS PubMed
.
- J. Sun, J. L. Bennett, T. J. Emge and R. Warmuth, Thermodynamically controlled synthesis of a chiral tetra-cavitand nanocapsule and mechanism of enantiomerization, J. Am. Chem. Soc., 2011, 133, 3268–3271 CrossRef CAS PubMed
.
- M. Hua, S. Wang, Y. Gong, J. Wei, Z. Yang and J.-K. Sun, Hierarchically porous organic cages, Angew. Chem., Int. Ed., 2021, 60, 12490–12497 CrossRef CAS
.
- C. Liu, Y. Jin, D. Qi, X. Ding, H. Ren, H. Wang and J. Jiang, Enantioselective assembly and recognition of heterochiral porous organic cages deduced from binary chiral components, Chem. Sci., 2022, 13, 7014–7020 RSC
.
- Q.-P. Hu, H. Zhou, T.-Y. Huang, Y.-F. Ao, D.-X. Wang and Q.-Q. Wang, Chirality gearing in an achiral cage through adaptive binding, J. Am. Chem. Soc., 2022, 144, 6180–6184 CrossRef CAS PubMed
.
- H. Zhou, Y. F. Ao, D. X. Wang and Q. Q. Wang, Inherently chiral cages via hierarchical desymmetrization, J. Am. Chem. Soc., 2022, 144, 16767–16772 CrossRef CAS
.
- S. Wu, Y. Ni, Y. Han, S. Xin, X. Hou, J. Zhu, Z. Li and J. Wu, Aromaticity in fully π-conjugated open-cage molecules, J. Am. Chem. Soc., 2022, 144, 23158–23167 CrossRef CAS PubMed
.
- Y. Ni, T. Y. Gopalakrishna, H. Phan, T. Kim, T. S. Herng, Y. Han, T. Tao, J. Ding, D. Kim and J. Wu, 3D global aromaticity in a fully conjugated diradicaloid cage at different oxidation states, Nat. Chem., 2020, 12, 242–248 CrossRef CAS PubMed
.
- P. Li, S. Xu, C. Yu, Z.-Y. Li, J. Xu, Z.-M. Li, L. Zou, X. Leng, S. Gao, Z. Liu, X. Liu and S. Zhang, De novo construction of catenanes with dissymmetric cages by space-discriminative post-assembly modification, Angew. Chem., Int. Ed., 2020, 59, 7113–7121 CrossRef CAS
.
- X. Liu, G. Zhu, D. He, L. Gu, P. Shen, G. Cui, S. Wang, Z. Shi, D. Miyajima, S. Wang and S. Zhang, Guest-mediated hierarchical self-assembly of dissymmetric organic cages to form supramolecular ferroelectrics, CCS Chem., 2022, 4, 2420–2428 CrossRef CAS
.
- D.-X. Cui, Y. Geng, J.-N. Kou, G.-G. Shan, C.-Y. Sun, K.-H. Zhang, X.-L. Wang and Z.-M. Su, Chiral self-sorting and guest recognition of porous aromatic cages, Nat. Commun., 2022, 13, 4011 CrossRef CAS PubMed
.
- G. Montà-González, F. Sancenón, R. Martínez-Máñez and V. J. C. R. Martí-Centelles, Purely covalent molecular cages and containers for guest encapsulation, Chem. Rev., 2022, 122, 13636–13708 CrossRef
.
- D. Hu, J. Zhang and M. Liu, Recent advances in the applications of porous organic cages, Chem. Commun., 2022, 58, 11333–11346 RSC
.
- P. Bhandari and P. S. Mukherjee, Covalent organic cages in catalysis, ACS Catal., 2023, 13, 6126–6143 CrossRef CAS
.
- W.-T. Dou, C.-Y. Yang, L.-R. Hu, B. Song, T. Jin, P.-P. Jia, X. Ji, F. Zheng, H.-B. Yang and L. Xu, Metallacages and covalent cages for biological imaging and therapeutics, ACS Mater. Lett., 2023, 5, 1061–1082 CrossRef CAS
.
- S. Yu, M. Yang, Y. Liu and M. Liu, Recent advances in separation membranes based on porous organic molecular materials, Mater. Chem. Front., 2023 10.1039/d3qm00217a
.
- D. Chakraborty and P. S. Mukherjee, Recent trends in organic cage synthesis: Push towards water-soluble organic cages, Chem. Commun., 2022, 58, 5558–5573 RSC
.
- L. Tapia, I. Alfonso and J. Sola, Molecular cages for biological applications, Org. Biomol. Chem., 2021, 19, 9527–9540 RSC
.
- K. Jie, Y. Zhou, H. P. Ryan, S. Dai and J. R. Nitschke, Engineering permanent porosity into liquids, Adv. Mater., 2021, 33, 2005745 CrossRef CAS
.
- S. La Cognata, A. Miljkovic, R. Mobili, G. Bergamaschi and V. Amendola, Organic cages as building blocks for mechanically interlocked molecules: Towards molecular machines, ChemPlusChem, 2020, 85, 1145–1155 CrossRef CAS PubMed
.
- H.-H. Huang and T. Solomek, Photochemistry meets porous organic cages, Chimia, 2021, 75, 285–290 CrossRef CAS
.
- Q.-H. Ling, J.-L. Zhu, Y. Qin and L. Xu, Naphthalene diimide- and perylene diimide-based supramolecular cages, Mater. Chem. Front., 2020, 4, 3176–3189 RSC
.
- V. Ramamurthy, Photochemistry within a water-soluble organic capsule, Acc. Chem. Res., 2015, 48, 2904–2917 CrossRef CAS PubMed
.
- Y. Jin, B. A. Voss, R. D. Noble and W. Zhang, A shape-persistent organic molecular cage with high selectivity for the adsorption of CO2 over N2, Angew. Chem., Int. Ed., 2010, 49, 6348–6351 CrossRef CAS PubMed
.
- M. Mastalerz, M. W. Schneider, I. M. Oppel and O. Presly, A salicylbisimine cage compound with high surface area and selective CO2/CH4 adsorption, Angew. Chem., Int. Ed., 2011, 50, 1046–1051 CrossRef CAS
.
- L. Chen, P. S. Reiss, S. Y. Chong, D. Holden, K. E. Jelfs, T. Hasell, M. A. Little, A. Kewley, M. E. Briggs, A. Stephenson, K. M. Thomas, J. A. Armstrong, J. Bell, J. Busto, R. Noel, J. Liu, D. M. Strachan, P. K. Thallapally and A. I. Cooper, Separation of rare gases and chiral molecules by selective binding in porous organic cages, Nat. Mater., 2014, 13, 954–960 CrossRef CAS
.
- T. Hasell, M. Miklitz, A. Stephenson, M. A. Little, S. Y. Chong, R. Clowes, L. J. Chen, D. Holden, G. A. Tribello, K. E. Jelfs and A. I. Cooper, Porous organic cages for sulfur hexafluoride separation, J. Am. Chem. Soc., 2016, 138, 1653–1659 CrossRef CAS PubMed
.
- M. Liu, L. Zhang, M. A. Little, V. Kapil, M. Ceriotti, S. Yang, L. Ding, D. L. Holden, R. Balderas-Xicohtencatl, D. He, R. Clowes, S. Y. Chong, G. Schutz, L. Chen, M. Hirscher and A. I. Cooper, Barely porous organic cages for hydrogen isotope separation, Science, 2019, 366, 613–620 CrossRef CAS PubMed
.
- Z. Wang, N. Sikdar, S.-Q. Wang, X. Li, M. Yu, X.-H. Bu, Z. Chang, X. Zou, Y. Chen, P. Cheng, K. Yu, M. J. Zaworotko and Z. Zhang, Soft
porous crystal based upon organic cages that exhibit guest-induced breathing and selective gas separation, J. Am. Chem. Soc., 2019, 141, 9408–9414 CrossRef CAS PubMed
.
- K. Su, W. Wang, S. Du, C. Ji and D. Yuan, Efficient ethylene purification by a robust ethane-trapping porous organic cage, Nat. Commun., 2021, 12, 3703 CrossRef CAS
.
- W. Wang, K. Su, E.-S. M. El-Sayed, M. Yang and D. Yuan, Solvatomorphism influence of porous organic cage on C2H2/CO2 separation, ACS Appl. Mater. Interfaces, 2021, 13, 24042–24050 CrossRef CAS PubMed
.
- K. Tian, S. M. Elbert, X.-Y. Hu, T. Kirschbaum, W.-S. Zhang, F. Rominger, R. R. Schroeder and M. Mastalerz, Highly selective adsorption of perfluorinated greenhouse gases by porous organic cages, Adv. Mater., 2022, 34, 2202290 CrossRef CAS
.
- D. Beaudoin, F. Rominger and M. Mastalerz, Chiral self-sorting of [2+3] salicylimine cage compounds, Angew. Chem., Int. Ed., 2017, 56, 1244–1248 CrossRef CAS PubMed
.
- M. W. Schneider, H.-J. Siegfried Hauswald, R. Stoll and M. Mastalerz, A shape-persistent exo-functionalized [4+6] imine cage compound with a very high specific surface area, Chem. Commun., 2012, 48, 9861–9863 RSC
.
- J. Tian, P. K. Thallapally, S. J. Dalgarno, P. B. McGrail and J. L. Atwood, Amorphous molecular organic solids for gas adsorption, Angew. Chem., Int. Ed., 2009, 48, 5492–5495 CrossRef CAS PubMed
.
- K. Krishnan, J. M. Crawford, P. K. Thallapally and M. A. Carreon, Porous organic cages CC3 and CC2 as adsorbents for the separation of carbon dioxide from nitrogen and hydrogen, Ind. Eng. Chem. Res., 2022, 61, 10547–10553 CrossRef CAS
.
- Y. Jin, B. A. Voss, A. Jin, H. Long, R. D. Noble and W. Zhang, Highly CO2-selective organic molecular cages: What determines the CO2 selectivity, J. Am. Chem. Soc., 2011, 133, 6650–6658 CrossRef CAS PubMed
.
- S. Jiang, J. Bacsa, X. Wu, J. T. A. Jones, R. Dawson, A. Trewin, D. J. Adams and A. I. Cooper, Selective gas sorption in a 2 + 3 propeller cage crystal, Chem. Commun., 2011, 47, 8919–8921 RSC
.
- C. Zhang, Z. Wang, L. Tan, T.-L. Zhai, S. Wang, B. Tan, Y.-S. Zheng, X.-L. Yang and H.-B. Xu, A porous tricyclooxacalixarene cage based on tetraphenylethylene, Angew. Chem., Int. Ed., 2015, 54, 9244–9248 CrossRef CAS PubMed
.
- J.-B. Xiong, J.-H. Wang, B. Li, C. Zhang, B. Tan and Y.-S. Zheng, Porous interdigitation molecular cage from tetraphenylethylene trimeric macrocycles that showed highly selective adsorption of CO2 and tnt vapor in air, Org. Lett., 2018, 20, 321–324 CrossRef CAS PubMed
.
- H. Ma, T.-L. Zhai, Z. Wang, G. Cheng, B. Tan and C. Zhang, Switching porosity of stable triptycene-based cage via solution-state assembly processes, RSC Adv., 2020, 10, 9088–9092 RSC
.
- F. Wang, E. Sikma, Z. Duan, T. Sarma, C. Lei, Z. Zhang, S. M. Humphrey and J. L. Sessler, Shape-persistent pyrrole-based covalent organic cages: Synthesis, structure and selective gas adsorption properties, Chem. Commun., 2019, 55, 6185–6188 RSC
.
- C. Liu, W. Li, Y. Liu, H. Wang, B. Yu, Z. Bao and J. Jiang, Porous organic cages for efficient gas selective separation and iodine capture, Chem. Eng. J., 2022, 428, 131129 CrossRef CAS
.
- M. Yang, W. Wang, K. Su and D. Yuan, Dimeric calix 4 resorcinarene-based porous organic cages for CO2/CH4 separation, Chem. Res. Chin. Univ., 2022, 38, 428–432 CrossRef CAS
.
- S. La Cognata, R. Mobili, C. Milanese, M. Boiocchi, M. Gaboardi, D. Armentano, J. C. Jansen, M. Monteleone, A. R. Antonangelo, M. Carta and V. Amendola, CO2 separation by imide/imine organic cages, Chem. – Eur. J., 2022, 28, e202201631 CAS
.
- P. Wagner, F. Rominger, W.-S. Zhang, J. H. Gross, S. M. Elbert, R. R. Schroeder and M. Mastalerz, Chiral self-sorting of giant cubic 8 + 12 salicylimine cage compounds, Angew. Chem., Int. Ed., 2021, 60, 8896–8904 CrossRef CAS PubMed
.
- X. Y. Hu, W. S. Zhang, F. Rominger, I. Wacker, R. R. Schroder and M. Mastalerz, Transforming a chemically labile [2 + 3] imine cage into a robust carbamate cage, Chem. Commun., 2017, 53, 8616–8619 RSC
.
- A. S. Bhat, S. M. Elbert, W.-S. Zhang, F. Rominger, M. Dieckmann, R. R. Schroeder and M. Mastalerz, Transformation of a [4 + 6] salicylbisimine cage to chemically robust amide cages, Angew. Chem., Int. Ed., 2019, 58, 8819–8823 CrossRef CAS
.
- F. Qiu, X. Chen, W. Wang, K. Su and D. Yuan, Highly stable sp2 carbon-conjugated porous organic cages, CCS Chem., 2023 DOI:10.31635/ccschem.023.202302903
.
- M. Yang, F. Qiu, E.-S. M. El-Sayed, W. Wang, S. Du, K. Su and D. Yuan, Water-stable hydrazone-linked porous organic cages, Chem. Sci., 2021, 12, 13307–13315 RSC
.
- S. M. Elbert, N. I. Regenauer, D. Schindler, W.-S. Zhang, F. Rominger, R. R. Schroeder and M. Mastalerz, Shape-persistent tetrahedral [4 + 6] boronic ester cages with different degrees of fluoride substitution, Chem. – Eur. J., 2018, 24, 11438–11443 CrossRef CAS PubMed
.
- M. Yang, X. Chen, Y. Xie, E.-S. M. El-Sayed, N. Xu, W. Wang, K. Su and D. Yuan, Post-synthetic metalation of organic cage for enhanced porosity and catalytic performance, Sci. China-Chem., 2023, 66, 1763–1770 CrossRef CAS
.
- Q. Wang, T. Ke, L. Yang, Z. Zhang, X. Cui, Z. Bao, Q. Ren, Q. Yang and H. Xing, Separation of Xe from Kr with record selectivity and productivity in anion-pillared ultramicroporous materials by inverse size-sieving, Angew. Chem., Int. Ed., 2020, 59, 3423–3428 CrossRef CAS
.
- R. S. Patil, D. Banerjee, C. M. Simon, J. L. Atwood and P. K. Thallapally, Noria: A highly Xe-selective nanoporous organic solid, Chem. – Eur. J., 2016, 22, 12618–12623 CrossRef CAS
.
- S.-J. Lee, I.-S. Ryu, S.-G. Jeon and S.-H. Moon, Emission sources and mitigation of fluorinated non-CO2 greenhouse gas in registered cdm projects, Greenhouse Gases: Sci. Technol., 2017, 7, 589–601 CrossRef CAS
.
- A. McCulloch, Fluorocarbons in the global environment: A review of the important interactions with atmospheric chemistry and physics, J. Fluorine Chem., 2003, 123, 21–29 CrossRef CAS
.
- B. K. Sovacool, S. Griffiths, J. Kim and M. Bazilian, Climate change and industrial f-gases: A critical and systematic review of developments, sociotechnical systems and policy options for reducing synthetic greenhouse gas emissions, Renewable Sustainable Energy Rev., 2021, 141, 110759 CrossRef CAS
.
- I. Krkljus, T. Steriotis, G. Charalambopoulou, A. Gotzias and M. Hirscher, H2/D2 adsorption and desorption studies on carbon molecular sieves with different pore structures, Carbon, 2013, 57, 239–247 CrossRef CAS
.
- X. B. Zhao, S. Villar-Rodil, A. J. Fletcher and K. M. Thomas, Kinetic isotope effect for H2 and D2 quantum molecular sieving in adsorption/desorption on porous carbon materials, J. Phys. Chem. B, 2006, 110, 9947–9955 CrossRef CAS
.
- D. Cao, H. Huang, Y. Lan, X. Chen, Q. Yang, D. Liu, Y. Gong, C. Xiao, C. Zhong and S. Peng, Ultrahigh effective H2/D2 separation in an ultramicroporous metal–organic framework material through quantum sieving, J. Mater. Chem. A, 2018, 6, 19954–19959 RSC
.
- L. Zhang, T. Wulf, F. Baum, W. Schmidt, T. Heine and M. Hirscher, Chemical affinity of Ag-exchanged zeolites for efficient hydrogen isotope separation, Inorg. Chem., 2022, 61, 9413–9420 CrossRef CAS
.
- J. Y. Kim, J. Park, J. Ha, M. Jung, D. Wallacher, A. Franz, R. Balderas-Xicohtencatl, M. Hirscher, S. G. Kang, J. T. Park, I. H. Oh, H. R. Moon and H. Oh, Specific isotope-responsive breathing transition in flexible metal-organic frameworks, J. Am. Chem. Soc., 2020, 142, 13278–13282 CrossRef CAS PubMed
.
- J. Y. Kim, H. Oh and H. R. Moon, Hydrogen isotope separation in confined nanospaces: Carbons, zeolites, metal-organic frameworks, and covalent organic frameworks, Adv. Mater., 2019, 31, e1805293 CrossRef PubMed
.
- X. Yan, Y. Song, D. Wang, T. Xia, X. Tan, J. Ba, T. Tang, W. Luo, G. Sang and R. Xiong, Direct observation of highly effective hydrogen isotope separation at active metal sites by in situ drift spectroscopy, Chem. Commun., 2023, 59, 3922–3925 RSC
.
- Y. Si, X. He, J. Jiang, Z. Duan, W. Wang and D. Yuan, Highly effective H2/D2 separation in a stable Cu-based metal-organic framework, Nano Res., 2019, 14, 518–525 CrossRef
.
- D. J. Vogel, T. M. Nenoff and J. M. Rimsza, Design elements for enhanced hydrogen isotope separations in barely porous organic cages, ACS Omega, 2022, 7, 7963–7972 CrossRef CAS
.
- H. Wang and J. Li, Microporous metal-organic frameworks for adsorptive separation of C5-C6 alkane isomers, Acc. Chem. Res., 2019, 52, 1968–1978 CrossRef CAS PubMed
.
- J.-W. Cao, S. Mukherjee, T. Pham, Y. Wang, T. Wang, T. Zhang, X. Jiang, H.-J. Tang, K. A. Forrest, B. Space, M. J. Zaworotko and K.-J. Chen, One-step ethylene production from a four-component gas mixture by a single physisorbent, Nat. Commun., 2021, 12, 6507 CrossRef CAS
.
- P.-Q. Liao, N.-Y. Huang, W.-X. Zhang, J.-P. Zhang and X.-M. Chen, Controlling guest conformation for efficient purification of butadiene, Science, 2017, 356, 1193–1196 CrossRef CAS
.
- H. Zeng, M. Xie, T. Wang, R.-J. Wei, X.-J. Xie, Y. Zhao, W. Lu and D. Li, Orthogonal-array dynamic molecular sieving of propylene/propane mixtures, Nature, 2021, 595, 542–548 CrossRef CAS
.
- R.-B. Lin and B. Chen, A dynamic mof for efficient purification of propylene, Sci. China-Chem., 2021, 64, 2053–2054 CrossRef CAS
.
- S. Horike, S. Shimomura and S. Kitagawa, Soft porous crystals, Nat. Chem., 2009, 1, 695–704 CrossRef CAS
.
- K. Qu, J. Xu, L. Dai, Y. Wang, H. Cao, D. Zhang, Y. Wu, W. Xu, K. Huang, C. Lian, X. Guo, W. Jin and Z. Xu, Electrostatic-induced crystal-rearrangement of porous organic cage membrane for CO2 capture, Angew. Chem., Int. Ed., 2022, 61, e202205481 CAS
.
- T. T. Xu, B. Wu, L. X. Hou, Y. R. Zhu, F. M. Sheng, Z. Zhao, Y. Dong, J. D. Liu, B. J. Ye, X. Y. Li, L. Ge, H. T. Wang and T. W. Xu, Highly ion-permselective porous organic cage membranes with hierarchical channels, J. Am. Chem. Soc., 2022, 144, 10220–10229 CrossRef CAS
.
- Y. D. Yuan, J. Dong, J. Liu, D. Zhao, H. Wu, W. Zhou, H. X. Gan, Y. W. Tong, J. Jiang and D. Zhao, Porous organic cages as synthetic water channels, Nat. Commun., 2020, 11, 4927 CrossRef CAS
.
- A. He, Z. Jiang, Y. Wu, H. Hussain, J. Rawle, M. E. Briggs, M. A. Little, A. G. Livingston and A. I. Cooper, A smart and responsive crystalline porous organic cage membrane with switchable pore apertures for graded molecular sieving, Nat. Mater., 2022, 21, 463–470 CrossRef CAS
.
| This journal is © the Partner Organisations 2023 |



