Facile access to diverse polyethylenes via neutral salicylaldiminato nickel catalysts†
Qiankun
Li
ab,
Hongliang
Mu
 *a and
Zhongbao
Jian
*a and
Zhongbao
Jian
 *ab
*ab
aState Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Renmin Street 5625, Changchun 130022, China. E-mail: muhongliang@ciac.ac.cn; zbjian@ciac.ac.cn
bUniversity of Science and Technology of China, Hefei 230026, China
First published on 19th June 2023
Abstract
A new family of neutral salicylaldiminato nickel(II) catalysts (Ni1–Ni5) is developed with various well-designed ortho substituents to the phenoxy moiety and these show moderate to high catalytic activity for ethylene polymerization as single-component catalysts. Compared to the classical Grubbs-type neutral nickel(II) catalyst Ni-An, the polymer obtained has lower molecular weight and significantly higher (2–5 times, up to 110 branches/1000C) branching degree. Polyethylene materials from solid semi-crystalline plastic (Tm = 108 °C) to polyethylene wax and polyethylene oil can thus be facilely accessed. In particular, through the cooperative work of N-aryl groups and ortho substituents, the upgraded catalysts Ni6 and Ni7 produce polymers with high molecular weight while retaining high branching degree, thus leading to polyethylenes with excellent elastic properties (tensile strength: 4.3 and 18.4 MPa, strain at break: ∼1500%, strain recovery value (SR): 54% and 67%). This work overcomes the established difficulty in simultaneously improving polymer molecular weight and branching degree using neutral nickel(II) catalysts, enlightening new ideas for catalyst design.
Introduction
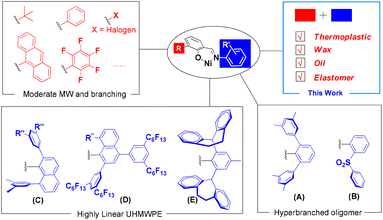
Polyolefins, although very simple in chemical composition, have found applications in every aspect of our daily life and have become the largest class of synthetic polymers since they have various forms such as thermoplastic, elastomer, wax and oil.1 Most commercial polyolefin products are derived from early transition metal catalysts such as titanium, zirconium and chromium catalysts that mediate polymerization in the presence of co-catalysts like alkyl aluminum or borates.1–4 Since Brookhart et al. discovered nickel(II) and palladium(II) catalysts supported by α-diimine ligands,5,6 late transition metal catalysts have received tremendous attention over the past 30 years and have been proved to be very versatile in accessing diverse polyolefins.7–26In contrast to extensive studies on cationic α-diimine nickel(II) catalysts with the activation of co-catalysts, another milestone in catalyst studies, neutral salicylaldiminato nickel(II) catalysts, discovered by Johnson et al.27 and Grubbs et al.,28,29 have better tolerance toward polar media but have attracted much less attention in terms of the production of diverse polyolefins. Unlike the α-diimine nickel(II) catalysts, the active center of this type of catalyst is electrically neutral, and thus it allows ethylene polymerization without the help of Lewis-acidic co-catalysts.30–33 After rigorous modification in recent years, this type of catalyst has been able to generate not only moderately branched polyethylene with moderate molecular weight, but also highly linear or lightly branched ultrahigh-molecular-weight polyethylene and low-molecular-weight hyperbranched polyethylene via the modification of versatile N-aryl motifs (Chart 1).34–49 It is noteworthy that current reports on the effect of structural modification of neutral salicylaldiminato nickel(II) catalysts indicate a simultaneous fluctuation for both polymer molecular weight and branching degree with polymerization conditions. Consequently, even though highly linear high-molecular-weight or highly branched low-molecular-weight polyethylene products can be facilely obtained, it is rather difficult to synthesize high-molecular-weight polyethylene with high branching degree, which is a prerequisite for elastic polyethylene. This balance between chain propagation/chain transfer and chain walking/chain propagation has been extensively achieved by using cationic α-diimine nickel(II) catalysts, and is highly desirable for neutral nickel(II) catalysts such as salicylaldiminato-type catalysts in view of their apparent merits.
 | ||
| Chart 1 Influence of substituents on the properties of neutral salicylaldiminato nickel(II) catalysts. | ||
Although, in general, the N-aryl groups in neutral salicylaldiminato nickel(II) catalysts play the most important role in determining the microstructure of polyethylene, little work has been devoted to systematic study on the ortho substituents to the phenoxy moiety, and the types of substituents are also very limited (Chart 1). In the seminal work of Grubbs et al., a bulky phenolate substituent (e.g., 9-anthracenyl) has been proved to be the most important condition for such catalysts producing high-molecular-weight polymers.28 It is believed that the smaller alkyl or aryl substituent leads to poor activity and catalyst instability due to easy bis-ligation deactivation when 2,6-diisopropryl substituents are used in the imine moiety.50
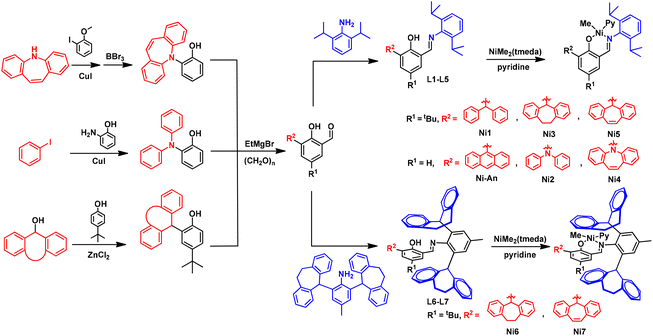
In this contribution, we develop a new substitution pattern adjacent to the phenoxy moiety to prepare a family of new neutral salicylaldiminato nickel(II) catalysts that are able to efficiently promote ethylene polymerization without any co-catalyst. These substituents facilitate the catalytic preparation of polyethylenes with a wide range of branching degrees, so that materials from semi-crystalline solid polyethylene to polyethylene wax and polyethylene oil are available by simply tuning the polymerization conditions. By further cooperation with the appropriate N-aryl group, the ability to elevate polymer branching degree and to generate high-molecular-weight polymer is fully taken advantage of, and polyethylene elastomer with good mechanical properties that was previously difficult to access using this type of catalyst has been obtained using ethylene as the sole feedstock.
Results and discussion
Most of the reported neutral nickel(II) catalysts based on the salicylaldimine framework use conventional ortho substituents (e.g., tert-butyl, halogen, phenyl, anthracenyl, etc.) of the phenoxy moiety in the ligand,30–33,51 and more studies are needed for the design of new substituents to broaden the application of neutral salicylaldiminato nickel(II) catalysts. In this work, we use a series of different types of substituents that had not been applied at this position or even in this catalyst system, for example, dibenzhydryl, diphenyl amine and their analogues with various linkages. These variations exert great influence on catalytic performance and will provide more information that is currently lacking for neutral salicylaldiminato nickel(II) catalysts.Phenol backbones containing amine substituents adjacent to the phenoxy moiety were synthesized via Cu-mediated cross-coupling reactions, while the C-containing substituent was introduced through the reaction promoted by zinc chloride in high yield, in which the presence of a tert-butyl group at the para-position would be required. The resultant phenols reacted with paraformaldehyde in the presence of ethylmagnesium bromide to give target salicylaldehydes, which led to the production of desired ligands (L1–L5) after condensation with 2,6-diisopropylaniline. The nickel(II) complexes Ni1–Ni5 could easily be obtained by the reaction of the corresponding ligands with NiMe2(tmeda) in the presence of excess pyridine (Scheme 1). The occurrence of singlets at −0.76 ppm to −0.60 ppm for Ni–CH3 resonances in 1H NMR spectra signifies successful coordination reactions, which is also supported by 13C NMR spectroscopy (see the ESI† for details).
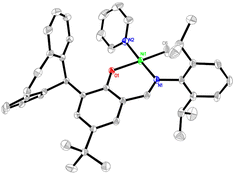
The solid-state structure of the catalyst Ni3 was further determined by X-ray single crystal diffraction analysis (Fig. 1). The bond angles of O1–Ni1–C6 (172.10(10)°) and N1–Ni1–N2 (176.18(9)°) reveal that the complex Ni3 adopts a near square-planar geometry around the nickel centre, and the Ni–C and Ni–N distances are close to those reported for a similar complex.52 The pyridine ligand is situated at the trans position of the bulky N-aryl group, which is similar to that reported for other nickel–methyl pyridine complexes.52,53 This solid-state structure of Ni3 is also consistent with its solution structure, as evidenced by the 1H–1H NOESY NMR spectrum (Fig. S31†), in which only Ni–CH3/iPr–CH3 (−0.69,1.47), Ni–CH3/iPr–CH (−0.69, 4.08) and Ni–CH3/Py–CH (−0.69, 8.92) can be observed. In this structure, the aliphatic linkage between the two phenyl groups and the sp3-hybridized carbon connected to the backbone bring about a distorted conformation for the dibenzosuberyl substituent, which points away from the metal centre. This is in sharp contrast to the conventional rigid and planar 9-anthracenyl substituent in the classical catalyst Ni-An (Scheme 1), which certainly would lead to different catalytic properties.
All these nickel(II) complexes were used as single-component catalysts for ethylene polymerization at 30 °C–90 °C (Table 1), and the classical catalyst Ni-An was chosen for comparison (Table 1, entry 1). In general, catalysts in this work exhibited better or comparable catalytic activities for ethylene polymerization (Table 1, entries 3, 7, 11 and 15), except for Ni5 (Table 1, entry 19). The polymer molecular weights were all lower, and the branching degrees were conspicuously higher (Fig. 2), indicating faster chain transfer and faster chain walking for this family of catalysts. This may be attributed to the reduced steric hindrance of the substituents adjacent to the phenoxy moiety.
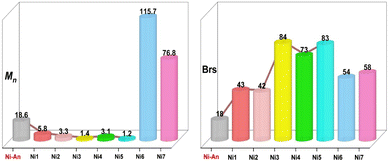 | ||
| Fig. 2 Molecular weight (kDa) and branching degree (brs/1000C) of polyethylenes generated by different nickel(II) catalysts at 50 °C and comparison of Ni-An with Ni1–Ni5. | ||
| Entry | Cat. | T (°C) | Yield (g) | Act.b (105) |
M
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (kDa) c (kDa) |
M
w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (kDa) c (kDa) |
M
w/Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
Brsd |
T
m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e (°C) e (°C) |
|---|---|---|---|---|---|---|---|---|---|
| a Reaction conditions: nickel(II) catalyst (5.0 μmol), toluene (100 mL), ethylene (8 bar), time (30 min). b Activity is in the unit of g mol−1 h−1. c Determined by GPC in 1,2,4-trichlorobenzene at 150 °C using a light scattering detector. d brs = number of branches per 1000C, as determined by 1H NMR spectroscopy. e Determined by DSC. f M n determined by 1H NMR spectroscopy. | |||||||||
| 1 | Ni-An | 50 | 1.97 | 7.9 | 18.6 | 35.8 | 1.9 | 18 | 112 |
| 2 | Ni1 | 30 | 0.53 | 2.1 | 18.4 | 33.6 | 1.8 | 24 | 108 |
| 3 | Ni1 | 50 | 2.19 | 8.8 | 5.8 | 11.5 | 2.0 | 43 | 94 |
| 4 | Ni1 | 70 | 1.49 | 6.0 | 2.6 | 4.8 | 1.9 | 67 | 77 |
| 5 | Ni1 | 90 | 0.81 | 3.2 | (1.8)f | — | — | 91 | — |
| 6 | Ni2 | 30 | 0.61 | 2.4 | 11.2 | 24.5 | 2.2 | 28 | 108 |
| 7 | Ni2 | 50 | 2.68 | 10.7 | 3.3 | 6.6 | 2.0 | 42 | 96 |
| 8 | Ni2 | 70 | 3.27 | 13.1 | 1.5 | 2.9 | 1.9 | 64 | 83 |
| 9 | Ni2 | 90 | 1.81 | 7.2 | (1.4)f | — | — | 75 | — |
| 10 | Ni3 | 30 | 0.43 | 1.7 | 2.1 | 3.3 | 1.6 | 73 | — |
| 11 | Ni3 | 50 | 2.15 | 8.6 | (1.4)f | — | — | 84 | — |
| 12 | Ni3 | 70 | 1.83 | 7.3 | (0.9)f | — | — | 94 | — |
| 13 | Ni3 | 90 | 1.05 | 4.2 | (0.9)f | — | — | 97 | — |
| 14 | Ni4 | 30 | 0.91 | 3.6 | 9.2 | 17.5 | 1.9 | 48 | 89 |
| 15 | Ni4 | 50 | 1.41 | 5.6 | 3.1 | 6.0 | 1.9 | 73 | — |
| 16 | Ni4 | 70 | 2.21 | 8.8 | (1.7)f | — | — | 99 | — |
| 17 | Ni4 | 90 | 1.69 | 6.8 | (1.4)f | — | — | 110 | — |
| 18 | Ni5 | 30 | 0.05 | 0.2 | 1.6 | 2.4 | 1.5 | 66 | 83 |
| 19 | Ni5 | 50 | 0.22 | 0.9 | 1.2 | 1.7 | 1.4 | 83 | 68 |
| 20 | Ni5 | 70 | 0.15 | 0.6 | (1.5)f | — | — | 88 | — |
| 21 | Ni5 | 90 | Trace | — | — | — | — | — | — |
| 22 | Ni6 | 30 | 0.76 | 3.0 | 154.1 | 261.1 | 1.7 | 24 | 98 |
| 23 | Ni6 | 50 | 1.28 | 5.1 | 115.7 | 204.1 | 1.8 | 54 | 69 |
| 24 | Ni6 | 70 | 0.88 | 3.5 | 71.9 | 137.6 | 1.9 | 70 | — |
| 25 | Ni6 | 90 | 0.65 | 2.6 | 29.0 | 61.4 | 2.1 | 94 | — |
| 26 | Ni7 | 30 | 0.65 | 2.6 | 154.2 | 224.2 | 1.5 | 24 | 103 |
| 27 | Ni7 | 50 | 0.81 | 3.2 | 76.8 | 143.7 | 1.9 | 58 | 69 |
| 28 | Ni7 | 70 | 0.93 | 3.7 | 50.2 | 85.4 | 1.7 | 77 | — |
| 29 | Ni7 | 90 | 1.73 | 6.9 | 24.3 | 46.7 | 1.9 | 92 | — |
Although the number of atoms of the substituents in Ni1–Ni5 are similar to that in Ni-An, the sp3-hybridized carbon or nitrogen connected to the phenolate backbone (Ni1 and Ni2) creates a sharp contrast to that for the 9-anthracenyl group in Ni-An. The planar 9-anthracenyl group extends across the salicylaldimine backbone with a certain torsion angle (ca. 65° for the acetonitrile complex50 and 84° for the triphenylphosphine complex29), while the steric hindrance of substituents in Ni1–Ni5 is farther from the nickel centre, which leads to less effective shielding. It is thus reasonable to believe that the additional linkage (Ni3–Ni5) would inevitably exacerbate this situation by restricting the degree of freedom in the substituents so that even less shielding of the metal centre and higher tendency of chain transfer are expected, leading to polymers with lower molecular weights and higher degrees of polymer branching.
Ni1 and Ni2 exhibited comparable catalytic activities (Table 1, entries 2–9) at low temperatures, with Ni2 being more thermally stable. Ni1 reached a maximum catalytic activity at 50 °C, followed by a decreased catalytic activity with increasing temperature, but was still more active (by ca. 1.5 times) at 90 °C than at 30 °C; in comparison, Ni2 demonstrated the highest activity at 70 °C, which was about 1.5 times more than the maximum value for Ni1. The molecular weight of the polymer generated by Ni2 was about half that of Ni1, which was essentially the case at each temperature. The polymer branching degree was similar between Ni1 and Ni2 at lower temperatures, giving semi-crystalline polyethylenes with melting temperatures ranging from 94 to 108 °C (Table 1, entries 2, 3, 6 and 7, e.g., Fig. 3a), which was similar to that using the classical catalyst Ni-An. At 90 °C, Ni1 and Ni2 afforded a wax-like polymer with decreased molecular weight and higher branching degree, which showed a very weak melting peak (e.g., Fig. 3c). Ni1 achieved a much higher polymer branching of 91 brs/1000C (entries 5 vs. 9) at 90 °C to give polyethylene oil. Current state-of-the-art production of lubricant base oil uses α-olefin polymerization promoted by metallocene or non-metallocene systems, and a cationic nickel(II)-promoted process using ethylene as the monomer has also been patented.54 The use of a single-component catalyst and ethylene as the sole monomer is no doubt of great interest as an alternative route.
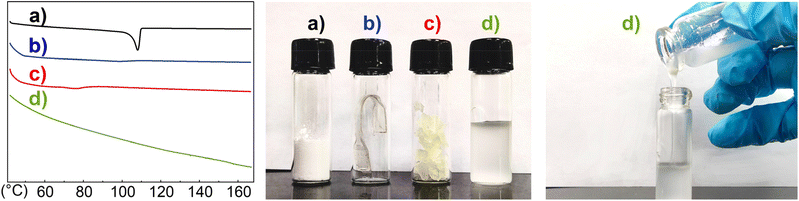 | ||
| Fig. 3 DSC curves (left) and photographs (middle and right) of the representative polyethylene samples with diverse microstructures: (a) (Table 1, entry 2), (b) (Table 1, entry 24), (c) (Table 1, entry 4) and (d) (Table 1, entry 16). | ||
Comparison of catalytic performances using Ni1 and Ni3 (Table 1, entries 2–5 vs. entries 10–13) revealed the effect of the aliphatic linker in Ni3. Similar catalytic activities were observed, coupled with a significant drop in the polymer molecular weight. In the meantime, branching degree was much higher after the introduction of the linker (entries 4 vs. 12, 67 vs. 94 brs/1000C), allowing the production of clear polyethylene oil at 50–90 °C (entries 11–13).
When the linker was further replaced with a conjugated one (Ni5) (Table 1, entries 18–21), the catalytic activity plummeted and the reduced catalyst thermal stability did not even support an active polymerization reaction at 90 °C (Table 1, entry 21). Moreover, similar to that for Ni3, this linking pattern also resulted in a much lower polymer molecular weight and higher branching degree than that for Ni1. In the same way as for Ni1 and Ni5, a significant increase in branching degree was achieved by introducing a linkage when moving from Ni2 to Ni4 (Table 1, entries 6–9 vs. entries 14–17). The 13C NMR spectrum of the polymer in entry 16 (Fig. 3d and 4) indicated extensive chain-walking during polymerization. Methyl branching was the dominant branching pattern, which occupied more than half of the total 91 branches (calculated by 13C NMR), second to which were butyl and longer branches (21 brs/1000C); ethyl branches (7.3 brs/1000C) and sec-butyl branches (10.4 brs/1000C) were also observed.
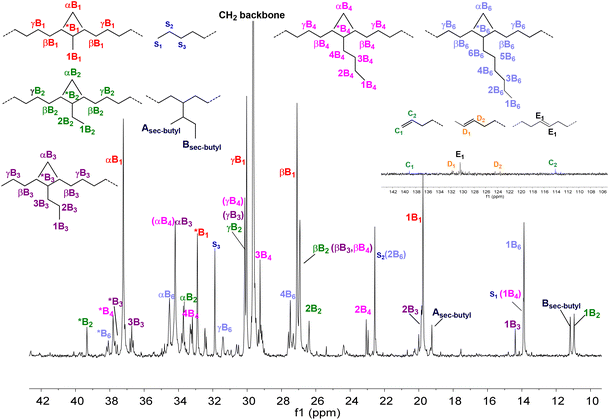 | ||
| Fig. 4 13C NMR spectrum of polyethylene oil in CDCl3 at 25 °C (Table 1, entry 16). | ||
Polyolefin elastomers are typically prepared industrially by copolymerization of ethylene with α-olefins promoted by catalysts based on early transition metal catalysts, such as metallocenes and constrained geometry catalysts (CGC), and representative commercial polyolefin elastomers have been presented by DOW (e.g., Engage™ and Infuse™), LG (e.g., Lucene™), Mitsui (e.g., Tafmer™) and others.55 The advent of late transition metal catalysts has enabled the production of similar materials from ethylene alone via chain walking. In this aspect, α-diimine catalysts based on nickel(II) and palladium(II) have been proved to be the most efficient. Salicylaldiminato nickel(II) catalyst as another famous late transition metal catalyst system, however, has been less successful in this respect owing to the fact that the polymer molecular weight is inextricably bound up with branching degree.
In view of the ability of ortho substituents in catalysts Ni1–Ni5 to effectively elevate polymer branching degree, we are interested in the possibility to construct polyethylene elastomers using this family of catalysts. From the polymerization results using Ni1 to Ni5, however, the dilemma between polymer molecular weight and branching degree still exists. Maintaining the ortho substituents, we then designed Ni6 and Ni7 by introducing to catalysts Ni3 and Ni5 dibenzosuberyl groups, which have been proved to be highly effective in suppressing chain transfer in our previous works.47,48 We aim to achieve the synergistic effect of the substituents at the ortho position and in the arylamine, affording higher molecular weight polymers with high branching degree, which is crucial for polyolefin elastomers.
Ni6 and Ni7 shared the same ortho substituents with Ni3 and Ni5, respectively, while ethylene polymerizations using these two catalysts showed a markedly improved performance in terms of polymer molecular weight. For Ni7vs.Ni5, catalytic activity was obviously another key parameter where progress was made after introducing dibenzosuberyl groups (entries 18–21 vs. 26–29). For these two new catalysts, the polymer branching degree increased with temperature, in which process the polymer molecular weight did decrease but remained at a high level of Mw > 50 kDa. This allowed for a proper balance between polymer molecular weight and branching degree, which gives rise to decent elastic materials. For example, polyethylene with molecular weight of 85.4 kDa and branching degree of 77 brs/1000C was achieved using Ni7 at 70 °C (entry 28). This polymer sample showed high strain at break of 1523%, and its tensile strength was 4.3 MPa. After extending this polymer to 300% strain for 10 cycles, a strain recovery (SR) value of 67% was witnessed (Fig. 5c and d). Under otherwise identical conditions, Ni6 produced polymer with molecular weight of 137.6 kDa and 70 branches per 1000 carbon atoms (entry 24). Much higher tensile strength (18.4 MPa) and similar elongation at break (1484%) were achieved, although the SR value decreased slightly (Fig. 5a and b). For neutral salicylaldiminato nickel(II) catalysts, the polymer molecular weight and branching degree always move together, and it is thus very difficult to obtain high-molecular-weight polyethylene with high branching degree, especially when no activator is used. The polymers in entries 24 and 28 represent a rational combination of properties that had not been previously achieved for this type of catalyst in ethylene polymerization. The results in this work now enable the use of neutral salicylaldiminato nickel(II) catalyst as a promising candidate that generates polyolefin elastomer without the need for α-olefin co-monomers.
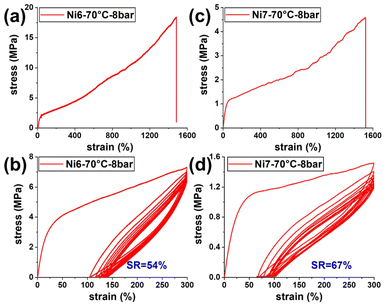 | ||
| Fig. 5 Stress–strain curves and plots of hysteresis experiments of the polyethylene samples generated by Ni6 and Ni7: Table 1, entry 24 (a and b), entry 28 (c and d). | ||
Conclusions
Differing from related pioneering studies, in this contribution a new family of ortho substituents for the phenoxy moiety in the neutral salicylaldiminato nickel(II) catalyst is rationally designed and installed and enables ethylene polymerization without the use of co-catalysts to produce polymers with much higher branching degrees, relative to conventional catalysts bearing the 9-anthracenyl group. Based on this key feature of high branching degree, the other key parameter of high molecular weight for polyethylene is further accessed by installing simple dibenzosuberyl groups into the N-aryl fragment. This generates polyethylene materials with diverse microstructures and properties, including semi-crystalline polyethylene plastic, polyethylene elastomer, polyethylene wax and polyethylene oil, derived from ethylene as the sole feedstock. Adjusting the value of both molecular weight and branching degree in late transition metal-mediated ethylene polymerization is one of the most promising characteristics. This always occurs in a cationic α-diimine system. This strategy of combining the advantages of substituents at two different key positions enables the use of neutral salicylaldiminato nickel(II) catalyst as a new candidate to produce diverse polyethylenes, which is expected to inspire the design of other catalyst systems.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are thankful for financial support from the National Natural Science Foundation of China (22122110) and the Jilin Provincial Science and Technology Department Program (20230101347JC).References
- D. W. Sauter, M. Taoufik and C. Boisson, Polymers, 2017, 9, 185 CrossRef PubMed.
- J. Klosin, P. P. Fontaine and R. Figueroa, Acc. Chem. Res., 2015, 48, 2004–2016 CrossRef CAS PubMed.
- M. C. Baier, M. A. Zuideveld and S. Mecking, Angew. Chem., Int. Ed., 2014, 53, 9722–9744 CrossRef CAS PubMed.
- G. Zhou, H. Mu, X. Ma, X. Kang and Z. Jian, CCS Chem., 2023 DOI:10.31635/ccschem.023.202202621.
- L. K. Johnson, C. M. Killian and M. Brookhart, J. Am. Chem. Soc., 1995, 117, 6414–6415 CrossRef CAS.
- L. K. Johnson, S. Mecking and M. Brookhart, J. Am. Chem. Soc., 1996, 118, 267–268 CrossRef CAS.
- Z. Chen and M. Brookhart, Acc. Chem. Res., 2018, 51, 1831–1839 CrossRef CAS PubMed.
- A. Keyes, H. E. B. Alhan, E. Ordonez, U. Ha, D. B. Beezer, H. Dau, Y.-S. Liu, E. Tsogtgerel, G. R. Jones and E. Harth, Angew. Chem., Int. Ed., 2019, 58, 12370–12391 CrossRef CAS PubMed.
- S. L. J. Luckham and K. Nozaki, Acc. Chem. Res., 2021, 54, 344–355 CrossRef CAS PubMed.
- J. L. Rhinehart, L. A. Brown and B. K. Long, J. Am. Chem. Soc., 2013, 135, 16316–16319 CrossRef CAS PubMed.
- F. Wang and C. Chen, Polym. Chem., 2019, 10, 2354–2369 RSC.
- S. Takano, D. Takeuchi, K. Osakada, N. Akamatsu and A. Shishido, Angew. Chem., Int. Ed., 2014, 53, 9246–9250 CrossRef CAS PubMed.
- L. Zhong, G. Li, G. Liang, H. Gao and Q. Wu, Macromolecules, 2017, 50, 2675–2682 CrossRef CAS.
- Y. Zhang, H. Mu, L. Pan, X. Wang and Y. Li, ACS Catal., 2018, 8, 5963–5976 CrossRef CAS.
- D. Guironnet, P. Roesle, T. Ruenzi, I. Göttker-Schnetmann and S. Mecking, J. Am. Chem. Soc., 2009, 131, 422–423 CrossRef CAS PubMed.
- G. Ji, Z. Chen, X. Wang, X. Ning, C. Xu, X. Zhang, W. Tao, J. Li, Y. Gao, Q. Shen, X. Sun, H. Wang, J. Zhao, B. Zhang, Y. Guo, Y. Zhao, J. Sun, Y. Luo and Y. Tang, Nat. Commun., 2021, 12, 6283 CrossRef CAS PubMed.
- B. K. Long, J. M. Eagan, M. Mulzer and G. W. Coates, Angew. Chem., Int. Ed., 2016, 55, 7106–7110 CrossRef CAS PubMed.
- H. Zhang, C. Zou, H. Zhao, Z. Cai and C. Chen, Angew. Chem., Int. Ed., 2021, 60, 17446–17451 CrossRef CAS PubMed.
- Z. Saki, I. D'Auria, A. Dall'Anese, B. Milani and C. Pellecchia, Macromolecules, 2020, 53, 9294–9305 CrossRef CAS.
- R. Tanaka, K. Sogo, K. Komaguchi, K. Ae, Y. Nakayama and T. Shiono, Organometallics, 2022, 41, 3024–3031 CrossRef CAS.
- Z. Yan, G. Chang, W. Zou, G. Luo and S. Dai, Polym. Chem., 2023, 14, 183–190 RSC.
- Y. Zhang, X. Kang and Z. Jian, Nat. Commun., 2022, 13, 725 CrossRef CAS PubMed.
- X. Hu, X. Kang, Y. Zhang and Z. Jian, CCS Chem., 2022, 4, 1680–1694 CrossRef CAS.
- X. Hu, X. Kang and Z. Jian, Angew. Chem., Int. Ed., 2022, 61, e202207363 CAS.
- C. Wang, J. Xia, Y. Zhang, X. Hu and Z. Jian, Natl. Sci. Rev., 2023 DOI:10.1093/nsr/nwad039.
- G. Zhou, L. Cui, H. Mu and Z. Jian, Polym. Chem., 2021, 12, 3878–3892 RSC.
- L. K. Johnson, A. M. A. Bennett, S. D. Ittel, L. Wang, A. Parthasarathy, E. Hauptman, R. D. Simpson, J. Feldman and E. B. Coughlin, WO1998030609A1, 1998.
- T. R. Younkin, E. F. Conner, J. I. Henderson, S. K. Friedrich, R. H. Grubbs and D. A. Bansleben, Science, 2000, 287, 460–462 CrossRef CAS PubMed.
- C. M. Wang, S. Friedrich, T. R. Younkin, R. T. Li, R. H. Grubbs, D. A. Bansleben and M. W. Day, Organometallics, 1998, 17, 3149–3151 CrossRef CAS.
- H. Mu, G. Zhou, X. Hu and Z. Jian, Coord. Chem. Rev., 2021, 435, 213802 CrossRef CAS.
- S. Mecking and M. Schnitte, Acc. Chem. Res., 2020, 53, 2738–2752 CrossRef CAS PubMed.
- H. Mu, L. Pan, D. Song and Y. Li, Chem. Rev., 2015, 115, 12091–12137 CrossRef CAS PubMed.
- Y. Zhang, Y. Zhang, X. Hu, C. Wang and Z. Jian, ACS Catal., 2022, 12, 14304–14320 CrossRef CAS.
- M. R. Radlauer, A. K. Buckley, L. M. Henling and T. Agapie, J. Am. Chem. Soc., 2013, 135, 3784–3787 CrossRef CAS PubMed.
- D. Takeuchi, Y. Chiba, S. Takano and K. Osakada, Angew. Chem., Int. Ed., 2013, 52, 12536–12540 CrossRef CAS PubMed.
- S. Sujith, D. J. Joe, S. J. Na, Y. W. Park, C. H. Chow and B. Y. Lee, Macromolecules, 2005, 38, 10027–10033 CrossRef.
- F. P. Wimmer, V. Ebel, F. Schmidt and S. Mecking, Polym. Chem., 2021, 12, 3116–3123 RSC.
- M. Schnitte, J. S. Scholliers, K. Riedmiller and S. Mecking, Angew. Chem., Int. Ed., 2020, 59, 3258–3263 CrossRef CAS PubMed.
- M. Schnitte, A. Staiger, L. A. Casper and S. Mecking, Nat. Commun., 2019, 10, 2592 CrossRef PubMed.
- P. Kenyon and S. Mecking, J. Am. Chem. Soc., 2017, 139, 13786–13790 CrossRef CAS PubMed.
- P. Kenyon, M. Wörner and S. Mecking, J. Am. Chem. Soc., 2018, 140, 6685–6689 CrossRef CAS PubMed.
- Z. Chen, M. Mesgar, P. S. White, O. Daugulis and M. Brookhart, ACS Catal., 2015, 5, 631–636 CrossRef CAS.
- T. Wiedemann, G. Voit, A. Tchernook, P. Roesle, I. Göttker-Schnetmann and S. Mecking, J. Am. Chem. Soc., 2014, 136, 2078–2085 CrossRef CAS PubMed.
- C. J. Stephenson, J. P. McInnis, C. Chen, M. P. Weberski Jr., A. Motta, M. Delferro and T. J. Marks, ACS Catal., 2014, 4, 999–1003 CrossRef CAS.
- D. Shu, A. R. Mouat, C. J. Stephenson, A. M. Invergo, M. Delferro and T. J. Marks, ACS Macro Lett., 2015, 4, 1297–1301 CrossRef CAS PubMed.
- C. Wang, X. Kang, H. Mu and Z. Jian, Macromolecules, 2022, 55, 5441–5447 CrossRef CAS.
- Q. Li, C. Wang, H. Mu and Z. Jian, J. Catal., 2021, 400, 332–337 CrossRef CAS.
- C. Wang, X. Kang, S. Dai, F. Cui, Y. Li, H. Mu, S. Mecking and Z. Jian, Angew. Chem., Int. Ed., 2021, 60, 4018–4022 CrossRef CAS PubMed.
- K. Li, H. Mu, X. Kang and Z. Jian, Macromolecules, 2022, 55, 2533–2541 CrossRef CAS.
- E. F. Connor, T. R. Younkin, J. I. Henderson, A. W. Waltman and R. H. Grubbs, Chem. Commun., 2003, 2272–2273 RSC.
- X. Hu, S. Dai and C. Chen, Dalton Trans., 2016, 45, 1496–1503 RSC.
- H. Mu, W. Ye, D. Song and Y. Li, Organometallics, 2010, 29, 6282–6290 CrossRef CAS.
- I. Göttker-Schnetmann, P. Wehrmann, C. Röhr and S. Mecking, Organometallics, 2007, 26, 2348–2362 CrossRef.
- Y. Tang, J. Liu, W. Tao, X. Sun and J. Li, CN105503763A, 2016.
- G. Zanchin and G. Leone, Prog. Polym. Sci., 2021, 113, 101342 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Syntheses of ligands and catalysts, NMR, GPC, DSC and crystallographic data. CCDC 2223362. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3py00450c |
| This journal is © The Royal Society of Chemistry 2023 |