 Open Access Article
Open Access ArticleRecent advances in supramolecular self-assembly derived materials for high-performance supercapacitors
Honghong
Cheng
 *,
Ruliang
Liu
,
Ruyi
Zhang
,
Lan
Huang
and
Qiaoyi
Yuan
*,
Ruliang
Liu
,
Ruyi
Zhang
,
Lan
Huang
and
Qiaoyi
Yuan
School of Chemistry and Materials Science, Guangdong University of Education, Guangzhou 510800, P.R. China. E-mail: chenghonghong@gdei.edu.cn
First published on 14th April 2023
Abstract
The key preponderance of supramolecular self-assembly strategy is its ability to precisely assemble various functional units at the molecular level through non-covalent bonds to form multifunctional materials. Supramolecular materials have the merits of diverse functional groups, flexible structure, and unique self-healing properties, which make them of great value in the field of energy storage. This paper reviews the latest research progress of the supramolecular self-assembly strategy for the advanced electrode materials and electrolytes for supercapacitors, including supramolecular self-assembly for the preparation of high-performance carbon materials, metal-based materials and conductive polymer materials, and its beneficial effects on the performance of supercapacitors. The preparation of high performance supramolecular polymer electrolytes and their application in flexible wearable devices and high energy density supercapacitors are also discussed in detail. In addition, at the end of this paper, the challenges of the supramolecular self-assembly strategy are summarized and the development of supramolecular-derived materials for supercapacitors is prospected.
1. Introduction
Supercapacitors have gained much attention in the last two decades as a new type of energy storage device. The energy density of supercapacitors is 3–4 times higher than that of traditional physical capacitors. Compared with secondary batteries, supercapacitors possess higher power density (300–5000 W kg−1) and long cycle life (>1 million cycles). In addition, supercapacitors also have the advantages of safety and environmental friendliness.1–4 These characteristics make supercapacitors widely used in electric vehicles, aerospace, smart grids, civil 3C digital and national defense technology, etc.5–7 Generally, according to the energy storage mechanism supercapacitors can be divided into two categories: electrochemical double-layer capacitors (EDLCs) with porous activated carbon as the electrode material, which mainly store energy through the fast and reversible double-layer capacitor at the electrode/electrolyte interface8–10 and Faraday pseudocapacitors with metal-based materials or conducting polymers as electrodes, which mainly store energy through a rapidly reversible Faraday process at the electrode/electrolyte interface.9,11 The electrode material is the core factor that determines the performance of supercapacitors. The most widely studied electrode materials can be classified into three categories: carbon based materials,12–14 metal oxide materials,15,16 and conductive polymer materials,17–19 each of which possesses its inherent advantages and shortcomings. Carbon-based materials, such as porous activated carbon and graphene, possess high conductivity and large specific surface area, and have been commercialized on a large scale.20,21 However, the limitations of their own structures (for example, the mismatch between the diameter of electrolyte ions and the pore size of porous activated carbon, and the unavoidable aggregation of the individual nanosheets of graphene) usually lead to the low energy density of carbon materials. The metal oxide materials have high theoretical capacity but insufficient conductive properties and cycling stability.22,23 Conductive polymers also possess high specific capacitance but insufficient conductive properties and cycling stability.24–26 The above shortcomings seriously limit the application of metal oxides and conductive polymers in the field of supercapacitors. Obtaining high energy density and developing advanced electrode materials with high conductivity and capacitance are key to realizing high performance supercapacitors. Generally, the power and energy density of electrode materials can be significantly improved by combining the EDLC effect and pseudo capacitance effect. Doping heteroatoms into carbon materials can introduce extra pseudocapacitive active sites, which can significantly improve the specific capacitance of carbon-based materials.27–31 Conductive polymers or metal oxides can also be combined with high electrical conductivity carbon materials to achieve the purpose of complementary advantages.32–34 The common methods such as the hydrothermal method, electrodeposition, co-precipitation and sol–gel method used in the preparation of heteroatom doped carbon and composite materials can improve the conductivity and increase the active sites to a certain extent. However, the distribution of components in the composite obtained by these methods is random, and also it is difficult to achieve accurate control of the microstructure of composite materials. Therefore, an accurate structural regulation strategy is urgently needed to achieve molecular-level assembly of specific functional units.Supramolecular chemistry is an interdisciplinary subject involving organic chemistry, material chemistry, and biochemistry. Supramolecular systems are formed through reversible non-covalent bonds (such as hydrogen bonds, electrostatic interactions, halogen bonds, ionic dipoles, and π–π interactions) between several chemical species molecules, which are a high complexity chemical system with certain organization.35–37 It is indispensable to assemble molecular modules through non-covalent interactions in various solvents or bulk liquid or solid systems for the fabrication of supramolecular materials.38,39 Different from the rigid bond (molecular covalent bond/ionic bond), a key advantage of supramolecular materials is to assemble a variety of different functional units to form brand-new function materials by using flexible non-covalent bonds.40,41 These non-covalent reversible bonds allow supramolecular systems to exhibit regeneration/self-healing properties along with structural flexibility and mobility that are highly needed for energy storage devices.42,43 For example, the challenge of designing materials for energy storage and conversion involves combining multiple components through specific interactions to produce truly functional energy storage materials.44 Supramolecular bonding (hydrogen/electrostatic interaction) is characterized by its moderate binding energies compared to covalent bonds.45,46 The moderate bonding strength not only ensures the interaction between the supramolecular solid scaffold and polar solvated electrolyte ions, but also is effective in providing improved ion conductivity in both interface and bulk regions.47,48 In order to realize the rapid response ability of electrodes in charge–discharge processes, the synergistic interaction between electrode materials and electrolyte ions is essential. Among the many possibilities, supramolecular nanostructured materials are best suited to overcome these challenges. Supramolecular materials can be used as electrode materials as well as electrolytes in supercapacitor systems.49,50 Supramolecular interactions not only benefit the solid electrolyte (gel) polymer electrolyte, but can effectively improve the ionic conductivity in the electrolyte/electrode interface, which is an onerous challenge for solid-state SCs.51,52
By precision-oriented self-assembly of different molecules through non-covalent action, large supramolecular aggregates can be formed, which are also known as “supramolecular polymers”. The different components in supramolecular systems are combined at the atomic level, and by using well-designed self-assembly strategies, supramolecular polymers with specific structures can be obtained. In the self-assembly field, a number of systematic methods based on molecular geometry have been developed, such as the Israelachvili rule for surfactants based on molecular shapes.53 In recent years, supramolecular assembly has been widely used to prepare a series of new materials with complex topological structures, for example, mushroom,54 twisted ribbon,55 hairpin,56 hollow tube,57etc. The use of supramolecular assembly strategies to obtain energy materials with excellent charge storage and ion transport capabilities is a hot research point in this field. From this perspective, the non-covalent interaction of components in supramolecular systems can be very useful and versatile. This hybrid design method can achieve functional integration that cannot be achieved by other methods.
Considering these merits of supramolecular materials, it can be considered that supramolecular self-assembly strategies have great research value in the fabrication of advanced supercapacitor electrode materials. This review covers recent advances in supramolecular self-assembly strategies for the preparation of state-of-the-art electrode materials and electrolytes in supercapacitors. Several examples are presented to discuss the strategic concepts of supramolecular self-assembly for the precise structural design, chemical synthesis, and self-assembly of high-performance carbon materials, metal oxide materials, and conductive polymer materials. Meanwhile, the key design concepts of supramolecular self-assembly for the development of integrated functional electrode materials in a multi-component system are summarized from a comprehensive case study. We hope that this review will help transfer knowledge from reported studies and provide new ideas for the application and development of supramolecular self-assembly in electrode materials for supercapacitors.
2. Supramolecular self-assembly method for the preparation of advanced supercapacitor electrode materials
2.1 Carbon-based materials
Carbon-based materials with their high surface area, diverse low-dimensional morphology, and exceptional physical and chemical stability have long been the go-to electrode materials for supercapacitors.58 The family of carbon materials includes graphite, porous activated carbon,59 carbon aerogel,60,61 graphene,62 carbon nanotubes,63,64etc. Carbon-based materials usually have good electrical conductivity and excellent chemical stability, which makes them exhibit good high current charge–discharge performance and cycle stability. Compared with pseudocapacitor materials, the theoretical specific capacity of carbon materials is lower (usually several hundred F g−1). Although there has been a lot of progress in the structural morphology research of carbon-based materials, the large porosity curvature, lack of pseudocapacitive effect, and tendency to aggregate of carbon-based materials can result in low practical capacitance. To address these shortcomings, two approaches have been developed: (1) preparing porous carbon with well-defined porous structures and controllable pore sizes through precise structural regulation strategies, for example, by controlling the precursor structure to obtain pore sizes that match electrolyte ions and improve charge storage capacity; and (2) doping heteroatoms into the structure of carbon materials to induce additional pseudocapacitive active sites and enhance the surface wettability. This paper mainly introduces the application of supramolecular self-assembly strategies in structure regulation and heteroatom doping of carbon-based materials.Yao's73 group prepared a γ-CD/F127 complex by inserting epoxy-polypropylene (PPO) chains of PEO-PO-PEO (F127) into the hydrophobic cavity of γ-CD through host–guest interaction via hydrophobic attraction. The γ-CD/F127 supramolecular complex then self-assembled into mono-micelles. In further hydrothermal treatment process free γ-CDs and γ-CD/F127 supramolecular complex further combined to form larger spheres. After high temperature carbonization and KOH activation of the supramolecular complex, the free γ-CDs on the outer and inner of the polymer formed a large carbon sphere skeleton, while the γ-CD/F127 mono-micelles transformed into carbon quantum dots with a pore diameter of 10 nm. This resulted in a unique ball-in-ball structured HPC-NS with a large specific surface area (765 m2 g−1). Fig. 1a shows the formation mechanism for HPC-NS, where nanopores in macrospheres are interconnected on scales below 10 nm – the lowest pore separation ever reported.73 Good pore connectivity can significantly enhance electrolyte transport in the pore network. The HPC-NS obtained by this method exhibited high specific capacity (405 F g−1 at 1 A g−1 and 71% capacitance retained at 200 A g−1), a wide voltage window (up to 1.6 V in aqueous electrolyte), and high energy as well as power density.73 Additionally, this work developed a method for evaluating pore connectivity, where the size of the main units constituting interconnected 3D-Hpcs, the longest possible pore separation (LPPS), can be used to characterize pore profiling and serve as a guide for designing high-performance HPCs. Cucurbit[6]uril (CB [6]) is a pumpkin-shaped supramolecule with a 5.8 A diameter cavity that can be used as a carbon source to prepare narrowly dispersed subnano/mesoporous materials. Cui74et al. obtained a CB[6] supramolecular complex (SPCC) by selective coordination of the CB[6] and Ba2+. As shown in Fig. 1b the SPCC was post-processed by calcination under an inert atmosphere at high temperature. During the self-pyrolysis process and subsequent washing, the top bond of CB [6] was broken and the cavity opened, leaving a large number of subnano pore cavities in the cucurbit[6]uril carbon materials (CBC-Xs). At the same time, the inner BaCl2 nanocrystals were washed away, resulting in mesoporous and interstitial space in the CBC-Xs. Finally, carbon materials (SCSCS) with subnano/mesoporous structures concentrated in ∼5.9 A were obtained. The formation process and pore size distribution of the porous structure can be controlled by adjusting the temperature of the self-pyrolysis process.74 The centrally distributed pore size of subnanometer-pores in CBC-Xs can smartly match the positive/anion diameter (5.8/2.3 A) of the pure ionic liquid 1-methyl-3-methylimidazolium tetrafluoroborate (MMIMBF4), and the mesoporous pores can provide rapid ion migration channels. As a result, using SCSC as an electrode material in an ionic liquid electrolyte can achieve state-of-the-art performance with a maximum energy density of 117.1 W h kg−1.74 Graphene has very high theoretical surface and electron transport properties, and can reach a theoretical specific capacitance of 550 F g−1.75 However, interlayer van der Waals forces cause graphene sheets to inevitably restack and overlap, greatly reducing their electrochemical properties.76 By using graphene oxide (GO) as a precursor, three-dimensional (3D) graphene with the inherent properties of two-dimensional graphene sheets and porous structure can be obtained by the bottom-up method. Chen et al.77 selected milk as a multifunctional biomass additive, using macromolecular protein emulsions (MPMs) in milk as a pillaring agent. The supramolecular interaction between MPMs and GO effectively prevents reaccumulation and overlap of GO sheets (the synthesis mechanism is shown in Fig. 3c). The size of the MPMs can be adjusted by controlling the concentration of sodium dodecyl sulfate surfactant, thus making the surface area and chemical properties of the synthetic Milk/rGO aerogel adjustable. Nitrogen atoms in MPMs can be doped into GO aerogel efficiently and selectively. After the aerogels were processed, porous hybrid nanocarbon materials (MGPC) were obtained through further graphitization and activation of the Milk/rGO aerogel at 600 °C. This material exhibits excellent supercapacitor performance, with a specific capacitance of up to 518.8 F g−1 at 0.1 A g−1 in 6 M KOH solution. Symmetric supercapacitors with MCP-5 as the electrode show good cycle stability, with the highest energy density reaching 36.7 W h kg−1 in the aqueous Li2SO4 electrolyte.77
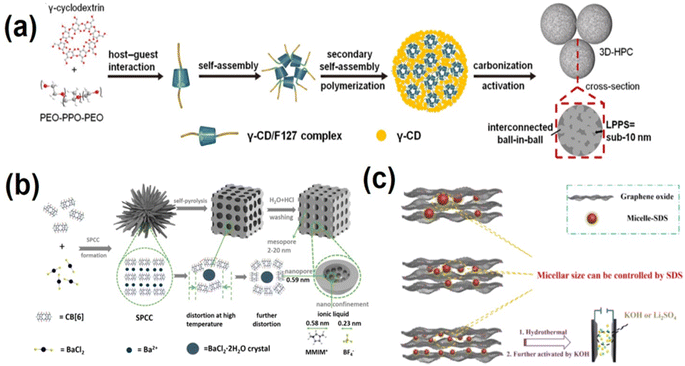 | ||
| Fig. 1 (a) Illustration of the formation mechanism for HPC-NS with a low longest possible pore separation (LPPS) (high interpore connectivity).73 Copyright 2022, Wiley-VCH. (b) Formation and the following self-pyrolysis of SPCC at high temperature that creates both mesopores and subnanopores in the resulting carbon.74 Copyright 2020, American Chemical Society. (c) Schematic illustration of the MGPC-based symmetric supercapacitors and the supramolecular interactions between GO and macromolecule protein micelles.77 Copyright 2020, Elsevier. | ||
2.1.2. Heteroatom doping of carbon-based materials
Heteroatom doping has been shown to be an effective way for enhancing the electrochemical activity of carbon-based materials.78,79 Among these heteroatoms, nitrogen (N) element is the most widely studied due to its ease of doping and the resulting nitrogen-doped carbon has good electrical conductivity, surface wettability and excellent electrochemical performance.80–82 Nitrogen-doped carbon materials can be obtained by using N-containing organic supramolecular polymers as precursors or assembling N-containing supramolecular polymers with a carbon source.83–85 Melamine-cyanuric acid (MCA), a hydrogen bond supramolecular polymer with high nitrogen content, rich functional groups (–NH2 and –COOH), variable morphology and easy decomposition characteristics, can be used as the ideal nitrogen source.86,87 Liu's group used MCA as the template growth agent and nitrogen source; in the presence of glucose, MCA was restructured and an MCA/C aerogel with a one-dimensional rod-like structure was obtained (Fig. 2a).60 Nitrogen-doped nanotube carbon aerogel (N-NTC) was obtained via high temperature carbonization of the MCA/C aerogel. The prepared N-NTC aerogel has a unique one-dimensional nanotube-like structure, high nitrogen content, extremely high specific surface area, large inner diameter and uniform wall thickness. This work systematically analyzed the formation mechanism of the N-NTC aerogel and comprehensively studied the effect of carbonization temperature on the morphology structure and chemical properties of the N-NTC aerogel. When used as an electrode material for supercapacitors (SCs), the N-NTC aerogel exhibited a specific capacitance of 563 F g−1 at 1 A g−1. The corresponding symmetric SC devices exhibit a high energy density of 21 W h kg−1.60 In our previous work,88 melamine-cyanurate supramolecular polymers were connected to –COOH and OH on GO surface by non-covalent bonding force to generate a GO/melamine cyanurate (GO/MC) supramolecular system (Fig. 2b). GO/MC was then hydrothermally treated and calcined at 350 °C to obtain nitrogen-doped graphene with a porous structure and nitrogen doping content up to 10.5%. The introduction of the MC supramolecular polymer acts as a barrier to prevent overlapping of graphene sheets, while also serving as a soft template for pore-making and in situ nitrogen doping. The nitrogen doping level and pore structure of the final product can be manipulated by controlling the relative ratio of GO to MC. The obtained P-NrGO showed a specific capacitance of 355 F g−1 at 1 A g−1, and possessed excellent cycling stability.88 In a similar way, we also carried out supramolecular self-assembly of a melamine-phytic acid (MP) supramolecular polymer,89 which contains nitrogen and phosphorus atoms, with GO to obtain a GO/MP supramolecular system, and further calcined it at high temperature to obtain porous N, P co-doped graphene. L-cysteine is a kind of amino acid containing nitrogen and sulfur elements. L-cysteine molecules were grafted onto the oxygen-containing functional group of GO through supramolecular interaction, followed by hydrothermal and high-temperature treatment to obtain N and S co-doped graphene with excellent supercapacitor performance.90 Zhang et al.91 applied 2, 5-dimercapto-1, 3, 4-thiadiazole (DMTD) as a bifunctional pillared agent and efficient dopant precursor. Fig. 2c illustrates that the planar DMTD molecules were assembled with graphene oxide (GO) through strong supramolecular π–π and hydrogen bond interactions to prevent reaccumulation of graphene sheets. The amount and efficiency of doping are enhanced, which is conducive to improving the performance of the sandwich-like N, S co-doped three-dimensional (3D) porous graphene aerogels (NSGAs).91 To sum up, the molecules or supramolecular polymers containing specific heteroatoms can be combined with amphiphilic GO through non-covalent interaction and then post-treated through processes such as hydrothermal or high-temperature calcination to obtain the corresponding heteroatom-doped graphene. The biggest advantage of this method is its high doping efficiency, more uniform and stable doping, and mild doping conditions. However, the mechanism of supramolecular combination with GO and structural changes during post-treatment processes are not yet well understood and require further study.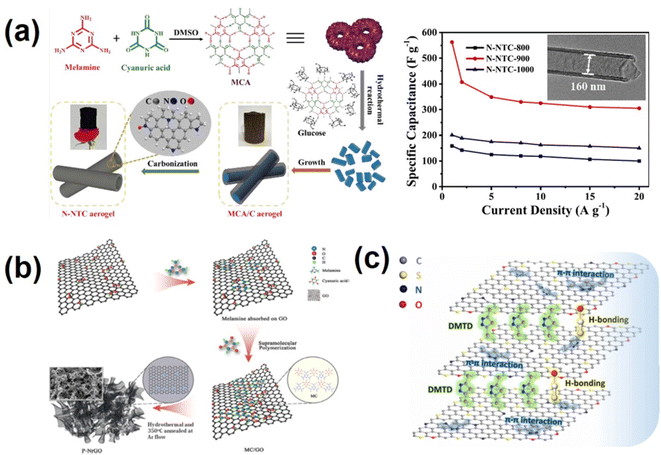 | ||
| Fig. 2 (a) Schematic illustration of the synthesis and rate performance of the N-NTC aerogel.60 Copyright 2021, American Chemical Society. (b) Schematic illustration of the supramolecular polymerization method for preparing P-NrGO materials.88 Copyright 2018, Elsevier. (c) Schematic illustration of the interactions between GO and DMTD molecules.91 Copyright 2020, Elsevier. | ||
To sum up, carbon-based materials prepared by supramolecular self-assembly strategies have the following characteristics:
(1) The porous carbon-based materials obtained by supramolecular self-assembly strategies possess a highly ordered structure and highly controllable pore size. Since the structure of the supramolecular precursor is relatively ordered, the pore structure of the carbon-based material derived from supramolecular self-assembly is more ordered than that obtained by traditional chemical activation methods. By selecting different supramolecular precursors, carbon materials with a hierarchical porous structure can be obtained, and the pore size can be adjusted according to the size of electrolyte ions, so as to improve the surface utilization of porous carbon and increase its actual capacitance.
(2) The heteroatom doped carbon-based materials obtained by supramolecular self-assembly strategies have uniform heteroatom distribution and adjustable doping state and amount. Through supramolecular self-assembly strategies, the doping amount of heteroatoms and the morphology of doped carbon materials can be accurately regulated, and the chemical state of doped heteroatoms can be effectively controlled, resulting in higher activity of heteroatom doped materials. The synergistic effect between excellent structure and highly adjustable doping state results in high energy density, good rate performance and excellent cycle stability of supramolecular-derived heteroatom doped carbon-based materials.
2.2 Structure and property regulation of transition metal-based electrode materials
The specific capacitance of transition metal-based electrode materials, such as transition metal oxides,92 hydroxides93 and sulfides,94 is several times higher than that of carbon materials. However, the high resistance and poor structural stability of these pseudocapacitor materials95,96 result in unsatisfactory rate performance and cycle stability. Coordination supramolecular networks (CSNs) are another type of coordination supramolecular material, in which supramolecular synthons include simple metal–organic complexes (mononuclear, binuclear, etc.) or first formed from metal ions/clusters and organic ligands to form oligomerized coordination entities. These entities are then assembled into coordination supramolecular networks through hydrogen bonding, π–π self-stacking, van der Waals interactions, hydrophobic effects, etc.97,98 CSNs not only have the advantage of adjustable pore size or space of MOF materials, but also, contrary to rigid MOF crystals, the weak interaction between supramolecular synthons makes the structure of CSNs more flexible, and the pore size or space of CSNs can be adjusted to match the size of electrolyte ions, and the steric hindrance to ion insertion is greatly reduced.99,100 However, the preparation of electrodes from powder CSNs requires the addition of insulating polymer binders and conductive additives, which reduce the effective surface area of the CSNs and increase the internal electrode resistance.101 Therefore, direct growth of CSNs on a conductive collector or conductive material (graphene) with a high specific surface are two ways to address these issues.The solvothermal method is a common way to achieve in situ growth of CSNs on conductive substrates. As illustrated in Fig. 3a Yang's group102 used Ni foam as both the nickel ion source and current collector. They first reacted the nickel foam with hydrochloric acid to generate nickel ions, and then the salicylic acid ligand and nickel ions were coordinated on the surface of the Ni foam skeleton to form a mononuclear metal–organic complex. Finally, a large number of mononuclear complexes rapidly self-assembled into a submicron array of Ni-salicylic acid CSNs by intermolecular interaction. Zhang's group103 used the same method, employing Co foam as the Co ion source and substrate and salicylic acid as the ligand, to in situ grow a helical rod new cobalt base coordination supramolecular network (Co-CSN) on the Co base. Helical micro- and nano-structured Co-CSN has a three-dimensional (3D) network formed by hydrogen bonding. Fig. 3b reveals the formation of Co-CSN. This in situ growth method not only takes full advantage of the pseudo capacitance of the coordination supramolecular network, but also enables close contact with the metal substrate without a binder. The unique submicron rod array structure enlarges the specific surface area, increases the electroactive sites, and reduces the diffusion distance between the electrolyte and electrode. Yang's group104 used Ni ions as the coordination center, potassium thiocyanate and isonicotinic acid as ligands, and Mn ions as dopants to grow a Mn-doped nickel CSN material on the nickel foam substrate by a simple one-step solvothermal method (Fig. 3c), and investigated the influence of Mn on the morphology, composition, structure and electrochemical properties of Ni-CSN. The Mn-doped binder-free electrode has an extremely high surface capacity. When the current density increases from 10 mA cm−2 to 50 mA cm−2, the capacitance retention rate is 73.33%. Zhang's group105 first achieved in situ growth of Ni coordination supramolecular nanowires (Ni-CSN) on the current collector by using Ni foam as the nickel ion source. Then by suffixation, a free-standing Ni3S2 nanowire electrode was successfully obtained. The Ni3S2 electrode showed a high specific capacitance of 5.8 F cm−2 at 5 mA cm−2 due to its structural flexibility and abundant open redox reaction sites. Based on these advantages, the self-supported Co-CSN spiral micro nanorods of the material provide a high areal capacitance of 10.19 F cm−2 and good rate performance.
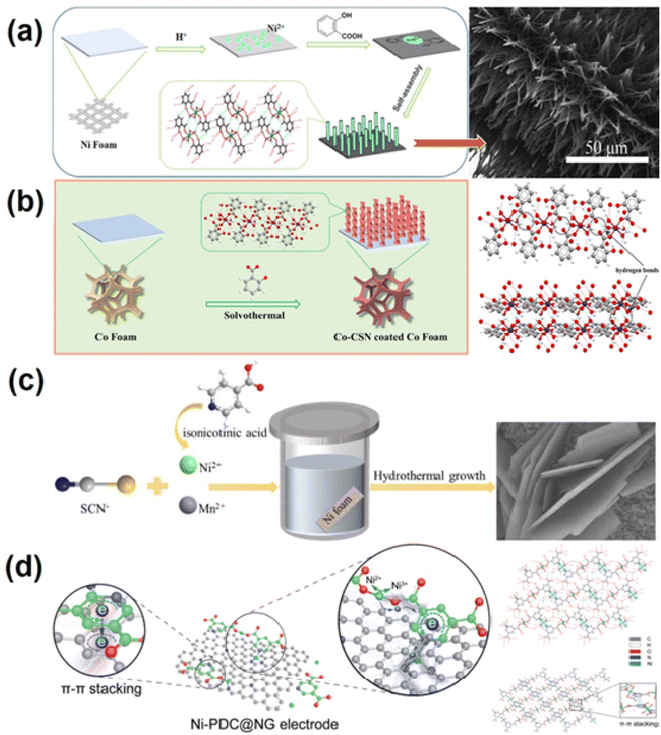 | ||
| Fig. 3 Coordination supramolecular network derived metal-based electrode materials for supercapacitors. (a) Schematic illustrating the possible formation mechanism and SEM images of the Ni-Hsal CSN electrodes.102 Copyright 2018, Elsevier. (b) Schematic illustration of the synthetic process of Co-CSN helical micro-nano rods and three-dimensional networks of Co-CSN formed via hydrogen bonds.103 Copyright 2022 Elsevier. (c) The schematic illustration for the fabrication of Mn-NSNA.104 Copyright 2018, Elsevier. (d) Schematic illustration of the Ni-PDC@NG electrode with continuous electronic conduction pathways and the charge-storage mechanism and the supramolecular network structure of Ni-PDC.96 Copyright 2017 The Royal Society of Chemistry. | ||
CSNs can be closely self-assembled with carbon-based materials (especially graphene with a large specific surface) through intermolecular forces to form composite materials. This prevents them from easily separating even when charged and discharged at high current densities, improving material conductivity and optimizing electrochemical performance. Yang's research group96 reported a method for assembling the novel nickel-coordinated supramolecular network [Ni2(3,5-PDC)2·(H2O)8·(H2O)2]n (labeled as Ni-PDC, 3,5-PDC = 3,5-pyridine dicarboxylic acid) with nitrogen doped graphene (NG) to form an excellent composite electrode material Ni-PDC@NG, which is shown in Fig. 3d. Ni-PDC effectively cooperates with NG to significantly improve the electrochemical performance of SCs. The composite electrode exhibits excellent rate capability, maintaining 53% of the initial capacitance at a high current density of 40 A g−1 and excellent cycle life. Using a similar method, Yang's team106 selected pyridine-2, 3-dicarboxylic acid (H2-pda) as a ligand and successfully constructed a series of Ni-pda@3D reduced GO materials (Ni-pda@3DrGO) by solvothermal reaction of Ni2+ with graphene oxide (GO) in H2O-DMF solution. In this composite, Ni-pda with a special structure is highly coordinated with high conductivity 3DrGO, enabling full use of the active site of pseudocapacitance. The maximum capacitance that the Ni-CSN@3DrGO sample can provide at a current density of 1 A g−1 is 952.85 F g−1.106
Polyoxometalates (POMs) are nanomolecular clusters composed of multiple transition metals and oxygen-richness and are potential pseudocapacitor electroactive materials.107 POMs are prone to rapid multi-electron variable redox reaction and multi-electron and proton transport, meanwhile maintaining their original structure during electrode reactions. However, their water solubility,108 small surface area, and poor conductivity have hindered their practical application in supercapacitors.109 Currently, two methods are adopted to improve the electrochemical performance of POM materials. One approach is to immobilize POMs on appropriate large surface area substrates, such as graphene,110 porous activated carbon,111 carbon nanotubes,112 conductive polymers,113,114etc. Attaching POMs to highly dispersed substrate materials is challenging due to electrostatic repulsion. Previous studies commonly introduced linkers115,116 to provide an integrated or tailored scaffold to capture POM molecules. However, POM-based supercapacitors still exhibit low energy density due to poor connectivity caused by blocked interaction and/or decreased interfacial surface area due to the agglomeration of POMs.116 The key to solving this problem is to improve the dispersion of POM molecules on the substrate, exposing sufficient electrochemical active sites at the molecular level. By using a supramolecular self-assembly strategy, anchoring single PMO molecules by the traditional chemical method has obvious advantages such as simple preparation process, low cost and high yield. Liu's group reported a supramolecular strategy for confining a single polyoxometalate (POM) cluster precisely in a polypyrrole (PPy) hydrogel-wrapped CNT framework with molecular-scale cages.117 As shown in Fig. 4a, a “fishnet” positively charged PPy hydrogel (TCPP) crosslinking agent doped with 5,10,15,20-tetrad (4-carboxyl) porphyrins uniformly coated the outer surface of negatively charged carbon nanotubes, while creating a cage structure with ∼1.8 nm nanopores (DFT calculation). To facilitate the capture of PMo12O40 (about 0.92 nm in diameter), the final PMo12/PPy/CNT ternary hybrid hydrogel active material with a single molecule dispersed PMo12 was obtained. The “cage” effect also activated the PMo12 molecule, enhancing its charging/discharging performance by introducing new proton transfer active sites.117 The well-structured interconnection network enhanced connectivity and flexibility.117 The flexible solid supercapacitor with the PMo12/PPy/CNT electrode had a power density of 700 μW cm−2 and a high energy density of 67.5 μW h cm−2. Another strategy is to combine POMs with a suitable metal–organic complex (MOC), which not only perfectly overcomes the inherent disadvantages of pure POM electrodes, but also provides additional advantages to the electrode material (including but not limited to an ordered crystal structure, modulated conductivity and rate properties, and low solubility in water and common organic solvents).118–121 Additionally, the POM-based MOC electrode material can be obtained by a simple hydrothermal method, and its clear structure facilitates the study of the corresponding relationship between the structure and properties. Hydrogen bond supramolecular scaffolds (HSFs) are a subclass of MOCs. In POM-HSFs, hydrogen bond interactions are important not only in self-assembled supramolecular frameworks but also provide electron transport and/or proton conduction pathways to enhance electrochemical performance.122 Pang's group123 prepared two nitrogen-rich organic ligands 1-imidazol-1-ylmethyl-1H-benzotriazole (imbta) and 1-pyridine-3-ylmethyl-1H-benzotriazole (pybta), and chose three PMo12O403− Keggin POM, CuAc2·H2O and AgNO3 salts to prepare three novel water cluster enclosed polyoxometalate-based hydrogen bond supramolecular frameworks (PHSFs) [Cu2(H2O)4H2(imbta)4](PMo12O40)2·6H2O (1), Cu(H2O)2H4(pybta)4(PMo12O40)2·2H2O (2), and [Ag2H7(pybta)6(PMo12O40)3]·12H2O (3) through a hydrothermal method. The detailed structure of the PHSF is displayed in Fig. 4b. The encapsulation of water clusters into the porous structure of PHSFs improves the conductivity of the electrode material and stabilizes the entire space as a guest. Owing to the unique PHSF structure of (PHSFs) [Cu2(H2O)4H2(imbta)4](PMo12O40)2 with numerous O–H⋯O interactions, the maximum specific capacitance of the compound 1-based electrode is 710 F g−1 at 1 A g−1,123 which is better than that of most of the reported POM-based electrodes.
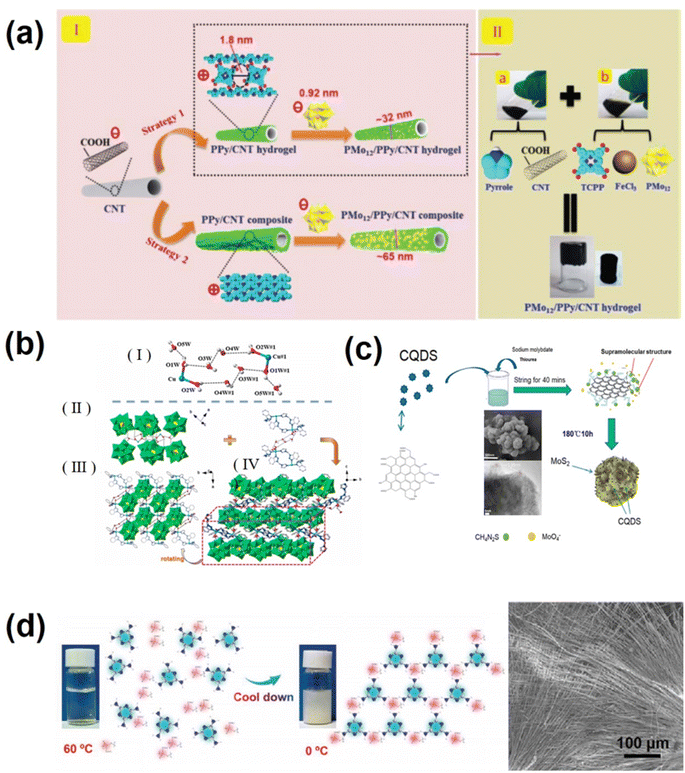 | ||
| Fig. 4 (a) (I) Electrostatic capture of PMo12 molecules by a fishnet-like PPy hydrogel (strategy 1) or blanket-like PPy chain (strategy 2)-wrapped CNT. (II) Fabrication process of the PMo12/PPy/CNT ternary hybrid hydrogel. (Inset) Photographs of the PMo12/PPy/CNT hybrid hydrogel.117 Copyright 2020 The Royal Society of Chemistry. (b) (I) The view of the chair-shaped water-copper cluster. (II) View of the linkages between water clusters and PMo12 POMs. (III) and (IV) Extended PHSF formation view.123 Copyright 2022, Elsevier. (c) Schematic diagram for the synthesis of MoS2/NCQDs. Inset: SEM and high resolution TEM of MoS2/NCQDs-6.125 Copyright 2022, Elsevier. (d) Synthetic mechanism diagram of V-supramolecular nanoribbons and SEM image of V-supramolecules with the mass ratio of NH4VO3 to C3H6N6 of 2.8.127 Copyright 2022 Wiley-VCH. | ||
MoS2 features a two-dimensional (2D) layered structure similar to graphene,124 which exists basically in 2H and 1T crystal phases. Compared to 2H phase MoS2, 1T phase MoS2 has superior electrochemical activity and has attracted widespread attention in the fields of electrochemistry and energy storage. However, it is challenging to synthesize 1T MoS2 by common chemical methods. Shu's group125 synthesized nitrogen-doped carbon quantum dot modified 1T phase molybdenum disulfide (MoS2/NCQDs) using a supramolecular assisted method. As shown in Fig. 4c, citric acid was first reacted with urea to form NCQDs. Oxygen containing functional groups (–OH and –COOH) on the surface of the carbon quantum dots can be connected with thiourea through hydrogen bonding to form supramolecular structures.85 During the hydrothermal process, the thiourea in the supramolecular system combines with sodium molybdate and transforms into MoS2. The formed supramolecules can also act as intercalators to promote the transition from triangular prismatic 2H-MoS2 to triangular antiprismatic 1T-MoS2. As MoS2 nanosheets grow, the carbon quantum dots in the supramolecular system can be anchored to the MoS2 nanosheets and further act as intercalation agents to effectively prevent the aggregation of MoS2 nanosheets.126 The 1T-2H phase MoS2 in the resulting MoS2/NCQD materials features a few layered structure with an ultra-wide space of 0.95 nm, facilitating the diffusion of electrolyte ions. Under the synergistic action of NCQD introduction and the formation of 1T phase MoS2, the conductivity and hydrophilicity of MoS2/NCQD materials have been greatly improved, and the prepared MoS2/NCQDs-6 has excellent supercapacitor performance (379.5 F g−1 at 0.5 A g−1) and excellent magnification performance (269.7 F g−1 at 10 A g−1).126
Vanadium supramolecules (V-supramolecules) and their derivatives are in great demand due to their potential applications in various fields, especially as the electrode for supercapacitors and batteries.127 However, studies on V-supramolecules are still lacking, and there are many shortcomings: (1) the metal centers of V-supramolecules are mainly vanadium trichloride (VCl3), tetravalent vanadium sulfate (IV) hydrate (VOSO4·nH2O), vanadium dioxide (VO2) and vanadium chloride (VCl4), and the prices of these metal centers are relatively expensive. (2) The preparation of vanadium MSPs is a complex, time-consuming and lengthy process; (3) due to the complex synthesis process (high temperature and pressure), key issues related to the nucleation and growth of vanadium MSPs remain unresolved. To address these problems, the C3H6N6–NH4VO3 supramolecular (V-supramolecular) nanoribbons (Fig. 4d)127 with an “aloe vera” shape using cheap and readily available NH4VO3 as the vanadium metal center and C3H6N6 as the organic ligand were fabricated through a rapid cooling self-assembly method by pang et al.118 The highlight of this work is that the nucleation and growth mechanism of V-supramolecules were observed in situ, and the influences of precipitation temperature, cooling rate, stirring and drying methods on nucleation and growth were analyzed. The VN/C nanoribbons derived from C3H6N6–NH4VO3 supramolecules possess high specific capacity. Different from conventional methods, this method is simple, efficient and suitable for large-scale preparation.
2.3 Structure and property regulation of conducting polymers
Conductive polymers (such as polyaniline, polypyrrole, etc.) possess high conductivity, good flexibility, low cost, and extremely high theoretical capacitance. However, the poor electrochemical stability limits their practical application in supercapacitors, making it urgent to improve the cycling stability of conductive polymer materials.128–130 To date, two main strategies have been used to improve polymer cycling performance: (1) nanocomposite strategy (solution blending and in situ growth method). However, the improvement of the cycling performance by the nanocomposite strategy is limited due to the drawbacks such as mechanical expansion during ion insertion and delamination of electrode materials from substrates. Through the precise supramolecular self-assembly strategy, the morphology of conductive polymer nanocomposites can be effectively regulated, the connection between the conductive polymer and substrate can be strengthened, and the molecular dispersion of the conductive polymer on the substrates can be realized. Kuila's group131 reported a simple but efficient route based on the supramolecular approach for large scale synthesis of intercalated polyaniline graphene nanohybrids with three dimensional pillar structures. First, GO was linked with pyrene butyric acid (PBA) in an alkaline medium by supramolecular forces. Then aniline monomers were protonated by PBA; meanwhile, the interfacial polymerization of aniline monomers (chloroform, water interface) was performed in the presence of an oxidizer. During interfacial polymerization, the protonated aniline monomer attached to the graphene surface acts as an initiator and the polymer grows between the graphene interlayer, and after PBA is removed a porous and pillar polyaniline intercalation reduced graphene oxide structure (PANIRGO) is formed. This three-dimensional porous structure can increase electrolyte penetration and thus improve the utilization of the electrode surface. The PANIRGO material exhibits an optimal capacitance value with good electrochemical stability.131 A similar supramolecular self-assembly strategy was used in our previous research study,132 which is shown in Fig. 5a; by supramolecular interaction, β-cyclodextrin (b-CD) was attached to the GO surface, and the hydrophobic inner cavity of β-cyclodextrin was included with polyaniline monomer, inducing in situ polymerization of aniline on the GO surface; after hydrothermal treatment, β-CD was carbonized into carbon nanodots anchored on the surface of RGO, and the reduced GO/carbon nanodot/polyaniline (RGO@CN/PANI) nanocomposites presented a regular polyaniline particle array morphology on the surface of the graphene layer. The specific capacitance of RGO@CN/PANI nanocomposites is up to 871.8 F g−1 at a current density of 0.2 A g−1 and has good cycle performance.132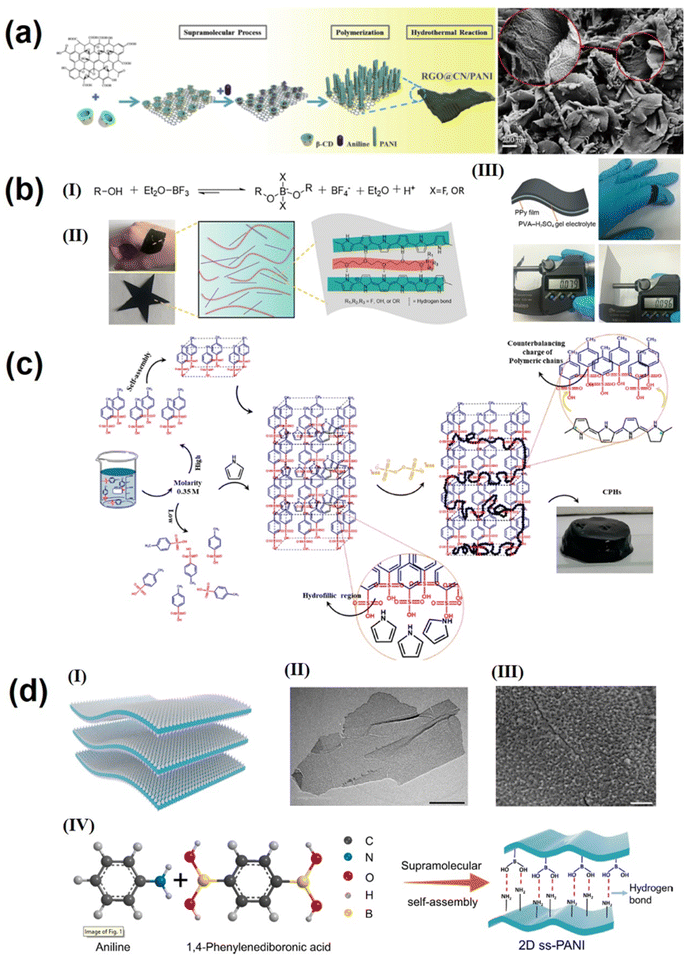 | ||
| Fig. 5 (a) Fabrication process and SEM image of RGO@CN/PANI nanocomposites.132 Copyright 2018 Elsevier. (b) (I) Formation of the dynamic PEG-borate polymers possessing negative charges. (II) Photograph of PEG600-PPy films and the dynamic network constructed by rigid PPy chains and soft PEG chains through electrostatic interactions and hydrogen bonds. (III) Schematic illustration and photograph of the UF-SC.138 Copyright 2020 Elsevier. (c) Schematic representation of PPy-CPH synthesis and hydrogel formation.139 Copyright 2021 American Chemical Society. (d) A supramolecular assembling strategy to construct ss-PANI nanosheets. (a) Schematic illustration of the structure of 2D ss-PANI. (II) TEM and (III) SEM (the scale bars of II and III are 2 μm and 200 nm, respectively). (IV) Supramolecular crosslinking of aniline with 1,4-PDBA to synthesize 2D ss-PANI consisting of numerous hydrogen bonds.128 Copyright 2021 Elsevier. | ||
Conducting polymer hydrogels (CPHs), such as poly(3,4-ethylene dioxythiophene) (PEDOT), polypyrrole (PPy) and polyaniline (PANI) hydrogels, have been widely used in flexible supercapacitors.19,133,134 However, conductive polymer hydrogels generally have shortcomings such as insufficient electron transport abilities and weak mechanical strength; therefore, non-electrochemically active collectors are usually required for mechanical support and to compensate for the lack of mechanical properties and stability of traditional CP materials. However, the existence of these collectors reduces the overall energy density of flexible capacitors.135 Inspired by the dynamic network structure of animal dermis, composed of collagen fibrils (rigid and strong) and elastin fibers (soft and elastic), combining a rigid conductive polymer and soft hydrophilic polymer through appropriate supramolecular interaction can produce a high-strength and stable structure of CPHs.136 This is an effective strategy to construct high strength flexible CPHs. Ma et al.137 used polyvinyl alcohol (PVA) as a soft polymer and polyaniline (PANI) as a rigid polymer, and a polyaniline-polyvinyl alcohol hydrogel (PPH) with a tensile strength of 5.3 MPa (conductivity of 0.1 S cm−1) was formed by cross-linking PVA and PANI with boric acid at the molecular level. Due to the excellent mechanical strength of hydrogels obtained by supramolecular self-assembly, no additional collector is required for structural support. The PVA–PANI hydrogel has excellent capacitive properties (928 F g−1). The energy density of the PVA–PANI hydrogel flexible solid-state supercapacitor reaches 13.6 W h kg−1, and it still has a high capacity retention rate after bending and folding.137 By the same strategy, the rigid polypyrrole (PPY ∼ 90 wt%) and soft polyethylene glycol (PEG, only 10 wt%) were connected through supramolecular interaction to form a dynamic network structure by Ma's research group (Fig. 5b).138 The PEG600 obtained by supramolecular crosslinking between PEG (with a molecular weight of 600) and PPy showed the best supercapacitor performance. Compared to the PVA–PANI hydrogel, the PEG600-PPy hydrogel showed a qualitative improvement in tensile strength (92 MPa) and electrical conductivity (110 S cm−1). The high-strength PEG600-PPy hydrogel, therefore, can be used directly as an electrode for the preparation of full polymers without the use of any substrate or current collector. With a thickness of up to 80 μm (thinner than a piece of A4 paper) (Fig. 6b(III)), the fully polymerized flexible supercapacitor provides a large capacity of 547 F cm −3 with excellent mechanical and electrochemical stability.138
 | ||
| Fig. 6 (a) (I) Schematic diagram of the self-healing mechanism of the supramolecular hydrogels. Supramolecular bonds were formed through host–guest interactions between the grafted β-CD molecules and residual amino acid molecules on the interactive surfaces of the supramolecular hydrogel pieces; (II) self-healing experiments of the supramolecular hydrogels via host–guest interactions. (III) Tensile strain–stress profiles of the supramolecular hydrogel and the comparative PAA-hydrogel; (IV) ionic conductivity of the supramolecular hydrogel after multiple times cutting–healing.148 Copyright 2021 Wiley-VCH. (b) (I) Schematic of the preparation process of the DMAPS–PAA/H2SO4/BAAS hydrogel; (II) schematic illustration of the dynamic bonds among the cross-linked polymer chains; (III) schematic of the self-healing mechanism of the hydrogel electrolyte.149 Copyright 2020 American Chemical Society. | ||
In conclusion, supramolecular self-assembly strategies have significant advantages in the preparation of conductive polymer hybrid materials and conductive polymer hybrid hydrogels. In addition, supramolecular self-assembly strategies can also regulate the conductive properties, morphology and electrochemical properties of monomer conductive polymers at the molecular level. Alcaraz-Espinoza et al.139 used an amphiphilic alkyl sulfonic acid (p-toluene sulfonic acid, p-TSA), a conventional additive dopant that can improve the electronic conductivity and stability of PPy. When the molar ratio of p-TSA to Py monomer was 1, the low water solubility Py monomer was self-assembled by supramolecular self-assembly at the hydrophobic end of p-TSA and fully polymerized, forming a micellar structure. It can be observed from Fig. 5c that p-TSA has a highly conjugated polymer chain after supramolecular assembly, which not only can improve the overall conductivity, but also can be used as a template to reduce the aggregation and the size of the PPy clusters; PPy has a highly connected 3D network structure and exhibits excellent rate performance and cycle stability as a supercapacitor electrode material.139 Yang's group128 used amphiphilic 1,4-phenylenediboronic acid as an additive for supramolecular self-assembly with aniline monomer through electrostatic interaction and hydrogen bonding to form a spherical micelle with 1,4-pdBA outside and aniline inside in solution. With the formation of polyaniline, the spherical micelle was demolished. The growth orientation of PANI was controlled by the hydrogen bond interaction between 1,4-phenylenediboronic acid and PANI, and second superparticle polyaniline (ss-PANI) with a two-dimensional morphology was constructed by the dynamic evolution emulsion polymerization process. Fig. 5d illustrates the structure of 2D ss-PANI; it is indicated that the excellent volume durability and intermolecular hydrogen bonding effect of ss-PANI can effectively inhibit the volume change during repeated charge and discharge. 2D supercapacitors based on ss-PANI have remarkable cycle stability, with a capacitance retention rate of 93.9% over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.128
000 cycles.128
To sum up, supramolecular materials derived from the supramolecular self-assembly strategy are promising electrode materials for high-performance supercapacitors, because the supramolecular self-assembly strategy can realize the self-assembly of various functional structural units at molecular levels and meet the requirements of electrode materials in different application scenarios. Table 1 summarizes and compares the properties of the three kinds of supramolecular derived electrode materials mentioned above.
| Electrode Materials | Electrolyte | SC | Cyclic stability | EDLC/PSC | Energy density | Rate capability | OPW (V) | Ref. |
|---|---|---|---|---|---|---|---|---|
| N-NTC-900 | 6 M KOH | 563 F g−1 (1 A g−1) | 97% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
EDLC&PSC | 21 W h kg−1 | 54.2% (1–20 A g−1) | 0–1.0 | 60 |
| HPC-NS | 6 M KOH | 405 F g−1 (1 A g−1) | 95% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
EDLC&PSC | 24.3 W h kg−1 | 33.3% (1–250 A g−1) | 0–1.2 | 73 |
| NSGAs-2 | 6 M KOH | 321 F g−1 (1 A g−1) | 92.8% after 6000 cycles | EDLC&PSC | 10.52 W h kg−1 | 80.4% (1–20 A g−1) | 0–1.0 | 91 |
| MB@3DrGO | 1 M H2SO4 | 311 F g−1 (1 A g−1) | 96% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
EDLC&PSC | 8.2 W h kg −1 | 84.2% (1–20 A g−1) | 0–1.0 | 140 |
| SPCC | MMIMBF4 | 233.5 F g−1 (0.2 A g−1) | 95.4% after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
EDLC&PSC | 117.1 W h kg−1 | 50.5% (0.2–8 A g−1) | 0–3.8 | 74 |
| N-MM-Cnets | 0.5 M H2SO4 | 537.3 F g−1 (0.5 A g−1) | 98.8% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
EDLC&PSC | 22.6 W h kg−1 | 69.1% (1–10 A g−1) | 0–0.6 | 84 |
| MGPC-5 | 6 M KOH | 351.4 F g−1 (1 A g−1) | 95% after 5000 cycles | EDLC&PSC | 36.7 W h kg−1 (in 2 M Li2SO4) | 71.8% (1–20 A g−1) | 0–1.6 | 77 |
| HPNCs-3 | 1 M H2SO4 | 435.6 F g−1 (0.5 A g−1) | 96.1% after 5000 cycles | EDLC&PSC | — | 78.9% (0.5–10 A g−1) | 0–0.8 | 80 |
| NS-SHC-8:8 | 1 M H2SO4 | 258.5 F g−1 (0.5 A g−1) | 94.4% after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
EDLC&PSC | 10.2 W h kg−1 (in H2SO4/PVA gel electrolyte) | 79.9% (0.5–10 A g−1) | 0–1.0 | 85 |
| NP-CNC | 6 M KOH | 435 F g−1 (0.05 A g−1) | 96.1% after 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
EDLC&PSC | 130.6 W h·kg−1 (as cathode for ZHSC) | 71.5% (0.05–50 A g−1) | 0.2–1.8 | 83 |
| P-NrGO | 6 M KOH | 335 F g−1 (1 A g−1) | 94.3% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
EDLC&PSC | — | 77% (1–5 A g−1) | 0–1.0 | 88 |
| NP-rGO | 6 M KOH | 416 F g−1 (1 A g−1) | 94.6% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
EDLC&PSC | 22.3 W h kg−1 | 72.7% (0.5–10 A g−1) | 0–1.0 | 89 |
| N/S-rGO | 6 M KOH | 416 F g−1 (0.5 A g−1) | 110% after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
EDLC&PSC | — | 30% (0.5–5 A g−1) | 0–1.0 | 90 |
| PMo12/PPy/CNT hybrid hydrogel | 0.5 M H2SO4 | 1170 F g−1 (1 A g−1) | 85.7% after 3000 cycles | PSC | 67.5 μW h cm−2 solid-state supercapacitor | 73.4% (1–5 A g−1) | 0–1.4 | 117 |
| Ni-Hsal CSN | 4 M KOH | 6.04 F cm−2 (10 mA cm−2) | 94% after 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
PSC | 2.39 mW h cm−3 | 76.2% (10–50 mA cm−2) | 0–1.65 | 102 |
| [Cu2(H2O)4H2(imbta)4](PMo12O40)2·6H2O | 0.5 M H2SO4 + 0.5 M Na2SO4 | 710 F g−1 (1 A g−1) | 91.2% after 1000 cycles | PSC | — | 70.2 (1–10 A g−1) | −0.2–0.8 | 123 |
| Ni-PDC@NG | 1 M LiOH | 735 F g−1 (1 A g−1) | 85.3% after 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
PSC&EDLC | 21.7 W h kg−1 | 53% (1–40 A g−1) | 0–1.6 | 96 |
| Ni-pda@3DrGO | 1 M LiOH | 952.85 F g−1 (1 A g−1) | 97% after 1500 cycles | PSC&EDLC | 17.70 W h kg−1 | 57% (1–10 A g−1) | 0–1.5 | 106 |
| Mn-doped CSN | 3 M KOH | 8.12 C cm−2 (10 mA cm−2) | 90.14% after 4000 cycles | PSC | 3.41 mW h cm−3 | 73.33% (10–50 mA cm−2) | 0–1.8V | 104 |
| Ni3S2 nanowires | 2 M KOH | 5.8 F cm−2 (5 mA cm−2) | 83.7% after 5000 cycles | PSC | 2.15 mW h cm−3 | 50% (5–30 mA cm−2) | 0–1.7 | 105 |
| Co-CSN | 2 M KOH | 10.19F cm−2 (3 mA cm−2) | 84.8% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
PSC | 2.65 mW h cm−3 | 76.4% (3–30 mA cm−2) | 0–1.6 | 103 |
| 3DMoS2/C@RGO | 1 M Na2SO4 | 340 F g−1 (1 A g−1) | 90% after 1000 cycles | EDLC | — | 71% (10–100 mV s−1) | −0.8–0.2 | 141 |
| MoS2/NCQDs | 1 M Na2SO4 | 379.5 F g−1 (0.5 A g−1) | 82% after 5000 cycles | PSC&EDLC | 33.5 W h kg−1 | 70.1% (0.5–10 A g−1) | 0–1.8 | 125 |
| MoS2@HCS-17 | 1 M Na2SO4 | 314.5 F g−1 (1 A g−1) | 87% after 4000 cycles | PSC&EDLC | 34.0 W h kg−1 | 62.3% (1–10 A g−1) | 0–1.7 | 124 |
| VN/carbon nanoribbons | 6 M KOH | 266.3 F g−1 (0.5 A g−1) | 71% after 5000 cycles | PSC&EDLC | 25.6 W h kg−1 | 78.3% (0.5–5 A g−1) | 0–1.1 | 127 |
| PPy-CPHs | 1 M H2SO4 | 560 F g−1 (0.75 A g−1) | 82% after 5000 cycles | PSC | 13 W h kg−1 | 72% (0.75–10 A g−1) | 0–0.8 | 139 |
| PEG600-PPy | 1 M H2SO4 | 555 F cm−3 (0.5 A cm−3) | 83% after 2000 cycles | PSC | 9.1 mW h cm−3 | 52.0% (0.3–11 A g−1) | 0–0.7 | 138 |
| P-GH | 1 M H2SO4 | 292 F g−1 (1 A g−1) | 96% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
PSC | 10.07 W h kg−1 | 74% (1–100 A g−1) | 0–1 | 142 |
| PANIRGO | 1 M H2SO4 | 630 F g−1 (0.5 A g−1) | 81% after 5000 cycles | PSC | — | 57.5% (0.5–4 A g−1) | −0.2–0.8 | 131 |
| ss-PANI | PVA/H2SO4 gel | 378 F g−1 | 93.9% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
PSC | 9.93 μW h cm−2 | 66.7% (0.2–5 A g−1) | 0–0.8 | 128 |
| PHMeDOT | 10 mM HMeDOT + 0.1 M LiClO4 | 45.4 ± 0.7 mF cm−2 (100 mV s−1) | 87.5% after 1000 cycles | PSC | — | — | −0.5–1.1 | 143 |
| RGO@CN/PAN | 1 M H2SO4 | 871.8 F g−1 (0.2 A g−1) | 72% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
PSC&EDLC | — | 76.8% (0.2–10 A g−1) | −0.7–0.3 | 132 |
| PANI–PVA hydrogel | 1 M H2SO4 | 928 F g−1 (0.5 A g−1) | 90% after 1000 cycles | PSC | 13.6 W h kg−1 | 84% (1–10 A g−1) | 0–0.8 | 137 |
3. Supramolecular self-assembly strategy for the preparation of advanced electrolytes
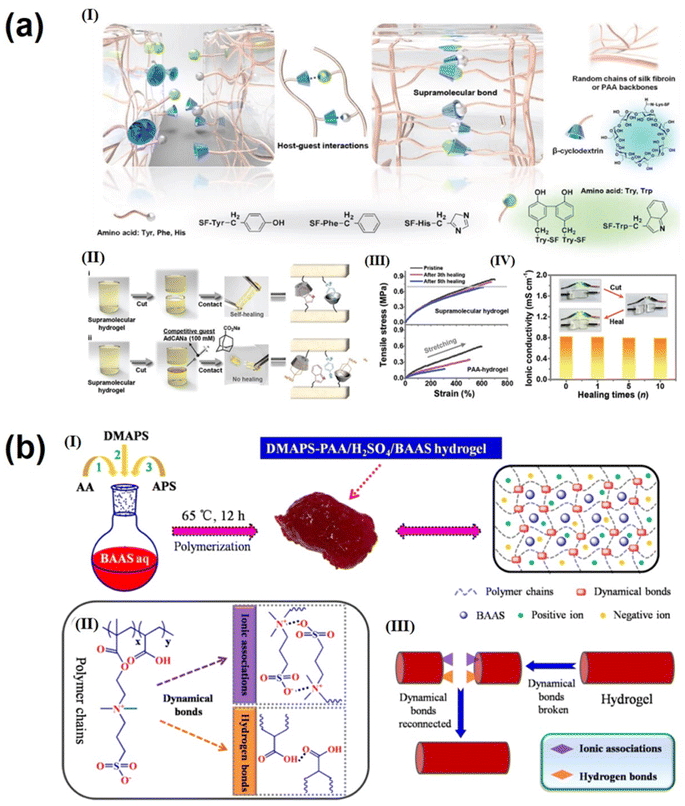
The electrolyte is one of the key components that affect the performance of supercapacitors. The size of the electrolyte ions, ionic conductivity and mobility, and the wettability of the electrode in the electrolyte have a significant impact on the operating voltage, stability, and energy and power density of the supercapacitor.144,145 Gel polymer electrolytes (GPEs) with high ionic conductivity, wide voltage windows, good mechanical properties and adequate safety are attracting increasing attention for use in flexible supercapacitors. Next-generation portable and wearable energy storage devices are often subjected to different mechanical strains and damage; as a result, GPEs with good self-healing properties and excellent tensile and electrochemical properties are essential for realizing advanced flexible wearable supercapacitors. The self-healing properties of traditional gels are achieved through reversible interaction of dynamic covalent and non-covalent bonds, and this involuntary self-healing property severely hinders their application.146,147 In contrast, supramolecular hydrogel electrolytes are formed through non-covalent interactions such as hydrogen bonding, electrostatic interaction and π–π interactions. These electrolytes have several advantages over traditional gel electrolytes, including the absence of initiators or chemical crosslinking agents, environmental friendliness, easy formation and a simple preparation process. Since non-covalent bonds require only a lower energy to form and break, they have excellent reversibility and self-healing properties; this non-covalent design strategy can be applied to GPEs to enable them to self-heal under mild conditions without significant loss of mechanical and electrochemical properties. Considering the high stress of traditional three-layer supercapacitors under mechanical bending, irreversible structural damage can be caused, including interlayer slip, crease formation, and layering of the electrode–electrolyte interface. Wei's group fabricated an all-polymeric, all-elastic and non-laminated supercapacitor with high mechanical reliability and excellent electrochemical performance by in situ integrating a polypyrrole electrode layer into a silk fibroin-based elastic supramolecular hydrogel film with a broad range of hydrogen and covalent bonds.148 The non-laminated structure of the integrated device avoids slip and delamination between interlayer, while the elasticity prevents crease formation. The supramolecular design of the host–guest interaction in the hydrogel matrix (Fig. 6a(I)) ensures that the self-healing efficiency of the supercapacitor remains close to ≈95.8% even after 30 cut/healing cycles and above 85.2% even after 5 cut/healing cycles at −20 °C (Fig. 6a(II–IV)), reaching the highest level available for foldable flexible supercapacitors. Feng et al.149 prepared a stretchable, self-healing, adhesive, redox-active multifunctional supramolecular hydrogel (DMAPS-PAA/H2SO4/BAAS) by free radical copolymerization. In H2SO4 aqueous solution containing BAAS, DMAPS and PAA were physically cross-linked through dynamic bonds between polymer chains using APS as an initiator. These bonds included hydrogen bonds between carbonyl and hydroxyl groups and ion association between sulfonic acid and quaternary ammonium groups. The supramolecular interaction is shown in Fig. 6b;149 in addition, the supramolecular hydrogel showed good self-adhesion on the electrode, preventing relative displacement and delamination between the electrolyte and electrode layer during repeated mechanical deformation and facilitating the lightweight and portability of energy storage devices. In addition, BAAS is introduced as a redox additive to increase the additional pseudocapacitance in this work. Carbon-based supercapacitors with DMAPS–PAA/H2SO4/BAAS hydrogel electrolytes have a specific capacitance of up to 240 F g−1. At the same time, good self-healing ability (Fig. 6b(III)) can withstand more than 400 bends/releases and still retain 96% of the initial specific capacity. In addition, there are many supramolecular gel electrolytes, such as PVA-TA-H3PO4 supramolecular gel electrolyte,51 and PANI-decorated PVA/PHEA;52 both demonstrated excellent mechanical stability and self-healing. For example, good self-healing ability can withstand more than 400 bends/releases while still retaining 96% of the initial specific capacity.There are three main types of electrolytes used for supercapacitors: aqueous-based, organic and ionic liquid electrolytes.150,151 Among these, aqueous electrolytes have attracted much attention due to their high ionic conductivity, low cost, high safety and simple equipment manufacture and operation.152,153 However, the low working potential window (usually around 1.00 V) and narrow working temperature range (0–100 °C) are the two major drawbacks. The use of “water-in-salt” (WIS) electrolytes, such as LiTFSI, can effectively widen the potential window and reduce the operating temperature of energy storage devices. However, the performance of WIS electrolytes decreases rapidly at high temperatures because organic solvents in the electrolyte will inevitably weaken the coordination structure between metal cations and water molecules at high temperatures. Li's group154 developed a new electrolyte of lithium bis(trifluoromethanesulfonyl) imide (LiTFSI) in a dimethyl sulfoxide (DMSO) and water co-solvent system. Through supramolecular interactions (mainly hydrogen bonding and electrostatic) between LiTFSI, DMSO and water (Fig. 7a(I)), the coordination structure of LiTFSI, DMSO and water in the electrolyte can be adjusted to affect the anion pair of H2O, electron density and the reactivity of H atom in the electrolyte. This allows for adjustment of the electrochemical stability window of freezing and volatilization of the electrolyte. The supercapacitor assembled with this new electrolyte has an operating voltage of 2.40 V(Fig. 7a(II)) and an operating temperature range up to 130 °C (−40 to 90 °C) (Fig. 7a(III)). Due to the increase of the operating voltage, the energy density of the assembled supercapacitor (21 W h kg−1) is much higher than that of the conventional water-based supercapacitor (10 W h kg−1).154
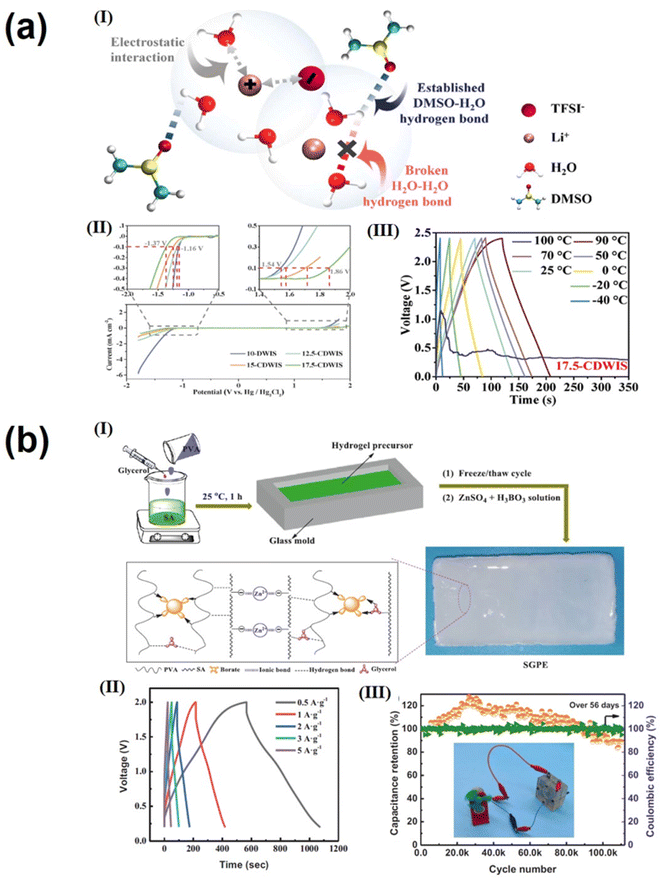 | ||
| Fig. 7 (a) (I) Schematic illustration of the possible supramolecular interactions among LITFSI, DMSO, and water; (II) electrochemical stability windows of 10-DWIS and X-CDWIS at 10 mV s−1; (III) the GCD curves of SCs with 17.5-CDWIS at 1 A g−1 under different operating temperatures.154 Copyright 2021 Elsevier. (b) (I) Schematic illustration of preparing SGPEs; (II) GCD curves at different current densities of ZIHSs using SGPE-3; (III) cycling performance and coulombic efficiency at a current density of 5 A g−1 (digital photo of a mini-fan driven by a ZIHS).159 Copyright 2022 Elsevier. | ||
Zinc ion hybrid supercapacitors (ZIHSs) have the advantages of standard electric potential low (0.76 V vs. SHE), high theoretical capacity (820 mA h g−1), abundant natural resources, double electron transfer reaction, low toxicity in the aqueous solution and inherent safety.155,156 However, ZIHSs still face several challenges, such as the energy density of commonly used activated carbon electrode materials being suboptimal and uncontrolled growth of zinc dendrites leading to poor cycling stability.157,158 Supramolecular gel polymer electrolytes (GPEs) are ideal zinc ionic electrolytes due to their similar ionic liquid conductivity and solid like mechanical properties, friendly environment, easy formation and simple preparation process. In order to inhibit the growth of zinc dendrites and improve the energy density of zinc-ion capacitors, Zhang el ta.159 developed a new type of Zn2+ containing supramolecular gel polymer electrolyte (SGPE) by the classic freeze/thawing method and the simple soaking process; Fig. 7b(I) illustrates the preparation of SGPEs. The optimized SGPE has good mechanical properties and high Zn2+ conductivity (89.7 mS cm−1) due to the interweaving of hydrogen and ionic bond networks. ZIHSs assembled with a SGPE as the electrolyte exhibit a wide voltage window of 0.2–2 V. Due to the inhibition of zinc dendrite growth in SGPEs, the capacitance retention rate is ∼84% after 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1, showing unprecedented cycle durability.159
000 cycles at 5 A g−1, showing unprecedented cycle durability.159
4. Conclusion and perspectives
As the world moves towards renewable and sustainable energy sources, there is an increasing urgency to integrate energy storage devices with high energy density, excellent cycle stability and mechanical flexibility. The supramolecular self-assembly strategy can realize accurately self-assembly of various functional units at the molecular level using non-covalent bonds. The flexibility and maneuverability of non-covalent bonds between different groups enable the supramolecular self-assembly strategy to produce materials with unique self-healing properties, which are particularly important for energy-related applications. Despite significant progress in the field of supramolecular energy materials, several challenges remain when designing supramolecular materials for next-generation high-performance supercapacitors in the future:(1) The high cost of supramolecular derived carbon materials: compared to traditional activated carbon, the cost of raw materials used in the preparation of hierarchical porous carbon materials by the supramolecular self-assembly structure regulation strategy is relatively high, which hindered the application in supercapacitors. Therefore, low-cost supramolecular precursors should be developed to enable large-scale preparation of supramolecular-derived carbon materials.
(2) Improving the electrical conductivity of CSNs remains a challenge: although a variety of coordination supramolecular networks (CSNs) with excellent electrochemical activity can be obtained through the coordination of different metal–organic non-covalent bonds, most CSNs have poor conductivity and are prone to aggregation, which requires the use of conductive additives or conductive substrates to give full play to their supercapacitor performance. Therefore, it is still a challenge in the future to select suitable metal ions and coordination systems to prepare CSN materials with stable dispersion and good conductivity.
(3) The structural stability of conductive polymer materials needs to be further strengthened: conducting polymer supramolecular, including electrode materials and electrolytes, exhibit outstanding electrochemical and mechanical properties. However, their mechanical properties should be improved without sacrificing electrochemical properties and conductivity. At present, the maximum fracture/healing cycle of most conductive polymer supramolecular gel electrolytes is only dozens of times, which is far from the level of practical application. Therefore, improving the structural stability of conductive polymer materials remains a challenge.
(4) Dynamic mechanism of supramolecular structure formation is still indistinct: currently, supramolecular self-assembly is mainly achieved by solvothermal or mixing reactions in solution. Most research focuses on characterizing the formed supramolecular structure, with relatively little attention paid to the dynamic mechanism of supramolecular structure formation. However, understanding this dynamic mechanism can provide strong support for the accurate construction of supramolecular systems. To overcome this challenge, some advanced characterization methods (such as in situ characterization techniques) and theoretical calculations are needed.
(5) The relationship between the structure and properties of supramolecular materials needs further study: although the synthesis of supramolecular materials is relatively simple and convenient, most supramolecular materials have poor crystallinity, and contain defects and crystal boundaries, making it difficult to accurately characterize their physical properties. Therefore, exploring the relationship between the structure and properties of supramolecular materials using experimental and theoretical methods such as DFT calculations and molecular dynamics (MD) simulations has become an active field of research.
Most excitingly, supramolecular materials are supported by a large number of structurally diverse molecules that can be assembled together in a variety of ways to form functionally diverse materials, which makes supramolecular materials an important application prospect in the field of energy storage. In summary, further studies are needed on the formation mechanism of supramolecular structures and the relationship between their structures and electrochemical properties. Systematic theoretical guidance on supramolecular self-assembly strategies is still insufficient. Further in-depth and systematic studies on the above challenges and prospects are needed to fill the gap between laboratory research and industrial applications of supramolecular energy materials.
Conflicts of interest
We declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors are grateful to the following financial support of this work: Key Scientific Research Projects of General Universities in Guangdong Province, Grant Number: 2021KCXTD086; Science and Technology Projects in Guangzhou, grant number: 202204400805; the Quality and Reform Project of Guangdong province undergraduate teaching (No. XQSYS-2222873).References
- S. Bashir, K. Hasan, M. Hina, R. Ali Soomro, M. A. Mujtaba, S. Ramesh, K. Ramesh, N. Duraisamy and R. Manikam, J. Electroanal. Chem., 2021, 898, 115626 CrossRef CAS.
- Y. Shao, M. F. El-Kady, L. J. Wang, Q. Zhang, Y. Li, H. Wang, M. F. Mousavi and R. B. Kaner, Chem. Soc. Rev., 2015, 44, 3639–3665 RSC.
- S. Manoharan, P. Pazhamalai, V. K. Mariappan, K. Murugesan, S. Subramanian, K. Krishnamoorthy and S.-J. Kim, Nano Energy, 2021, 83, 105753 CrossRef CAS.
- P. Xue, C. Guo, L. Li, H. Li, D. Luo, L. Tan and Z. Chen, Adv. Mater., 2022, 34, 2270109 CrossRef.
- C. Kim, D. Y. Kang and J. H. Moon, Nano Energy, 2018, 53, 182–188 CrossRef CAS.
- Y. Zhang, H. Mei, Y. Cao, X. Yan, J. Yan, H. Gao, H. Luo, S. Wang, X. Jia, L. Kachalova, J. Yang, S. Xue, C. Zhou, L. Wang and Y. Gui, Coord. Chem. Rev., 2021, 438, 213910 CrossRef CAS.
- R. Kumar, A. Pérez del Pino, S. Sahoo, R. K. Singh, W. K. Tan, K. K. Kar, A. Matsuda and E. Joanni, Prog. Energy Combust. Sci., 2022, 91, 100981 CrossRef.
- L. Liu, P.-L. Taberna, B. Dunn and P. Simon, ACS Energy Lett., 2021, 6, 4311–4316 CrossRef CAS.
- M. Salanne, B. Rotenberg, K. Naoi, K. Kaneko, P.-L. Taberna, C. P. Grey, B. Dunn and P. Simon, Nat. Energy, 2016, 1, 16070 CrossRef CAS.
- X. Zhang, X. Chen, T. Bai, J. Chai, X. Zhao, M. Ye, Z. Lin and X. Liu, J. Mater. Chem. A, 2020, 8, 11589–11597 RSC.
- F. Zhao, Y. Shi, L. Pan and G. Yu, Acc. Chem. Res., 2017, 50, 1734–1743 CrossRef CAS.
- S. Zhang, J. Zhu, Y. Qing, L. Wang, J. Zhao, J. Li, W. Tian, D. Jia and Z. Fan, Adv. Funct. Mater., 2018, 28, 1805898 CrossRef.
- L. Yan, D. Li, T. Yan, G. Chen, L. Shi, Z. An and D. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 42494–42502 CrossRef CAS.
- J. Mao, J. Iocozzia, J. Huang, K. Meng, Y. Lai and Z. Lin, Energy Environ. Sci., 2018, 11, 772–799 RSC.
- Z. Qu, M. Shi, H. Wu, Y. Liu, J. Jiang and C. Yan, J. Power Sources, 2019, 410–411, 179–187 CrossRef CAS.
- W. Fu, E. Zhao, X. Ren, A. Magasinski and G. Yushin, Adv. Energy Mater., 2018, 8, 1703454 CrossRef.
- Y. Huang, H. Li, Z. Wang, M. Zhu, Z. Pei, Q. Xue, Y. Huang and C. Zhi, Nano Energy, 2016, 22, 422–438 CrossRef CAS.
- Q. Meng, K. Cai, Y. Chen and L. Chen, Nano Energy, 2017, 36, 268–285 CrossRef CAS.
- L. Li, K. Wang, Z. Huang, C. Zhang and T. Liu, Nano Res., 2016, 9, 2938–2949 CrossRef CAS.
- J. Gamby, P. Taberna, P. Simon, J. Fauvarque and M. Chesneau, J. Power Sources, 2001, 101, 109–116 CrossRef CAS.
- H. Nishihara and T. Kyotani, Adv. Mater., 2012, 24, 4466 CrossRef CAS.
- J. Jiang, Y. Li, J. Liu, X. Huang, C. Yuan and X. W. D. Lou, Adv. Mater., 2012, 24, 5166–5180 CrossRef CAS.
- W. Wei, X. Cui, W. Chen and D. G. Ivey, Chem. Soc. Rev., 2011, 40, 1697–1721 RSC.
- T. Lin, I. W. Chen, F. Liu, C. Yang, H. Bi, F. Xu and F. Huang, Science, 2015, 350, 1508–1513 CrossRef CAS PubMed.
- Z. S. Wu, A. Winter, L. Chen, Y. Sun, A. Turchanin, X. Feng and K. Müllen, Adv. Mater., 2012, 24, 5130–5135 CrossRef CAS PubMed.
- M. Liu, B. Li, H. Zhou, C. Chen, Y. Liu and T. Liu, Chem. Commun., 2017, 53, 2810–2813 RSC.
- M. Liu, Y. Song, S. He, W. W. Tjiu, J. Pan, Y. Y. Xia and T. Liu, ACS Appl. Mater. Interfaces, 2014, 6, 4214–4222 CrossRef CAS.
- C. Cui, Y. Gao, J. Li, C. Yang, M. Liu, H. Jin, Z. Xia, L. Dai, Y. Lei, J. Wang and S. Wang, Angew. Chem., 2020, 132, 8002–8007 CrossRef.
- Y. Li, Y. Shan and H. Pang, Chin. Chem. Lett., 2020, 31, 2280–2286 CrossRef CAS.
- S. Ghosh, S. Barg, S. M. Jeong and K. K. Ostrikov, Adv. Energy Mater., 2020, 10, 2001239 CrossRef CAS.
- H. Liu, F. Liu, Z. Qu, J. Chen, H. Liu, Y. Tan, J. Guo, Y. Yan, S. Zhao, X. Zhao, X. Nie, X. Ma, Z. Pei and M. Liu, Nano Res. Energy, 2023, 2, e9120049 CrossRef.
- V. Prabhakaran, B. L. Mehdi, J. J. Ditto, M. H. Engelhard, B. Wang, K. D. D. Gunaratne, D. C. Johnson, N. D. Browning, G. E. Johnson and J. Laskin, Nat. Commun., 2016, 7, 11399 CrossRef CAS PubMed.
- J. Lin, H. Liang, H. Jia, S. Chen, Y. Cai, J. Qi, J. Cao, W. Fei and J. Feng, Inorg. Chem. Front., 2017, 4, 1575–1581 RSC.
- J. Yu, C. Yu, W. Guo, Z. Wang, S. Li, J. Chang, X. Tan, Y. Ding, M. Zhang, L. Yang, Y. Xie, R. Fu and J. Qiu, Nano Energy, 2019, 64, 103921 CrossRef CAS.
- D. B. Amabilino, D. K. Smith and J. W. Steed, Chem. Soc. Rev., 2017, 46, 2404–2420 RSC.
- E. A. Kovalenko, D. Y. Naumov and V. P. Fedin, Polyhedron, 2018, 144, 158–165 CrossRef CAS.
- G. Cavallo, G. Terraneo, A. Monfredini, M. Saccone, A. Priimagi, T. Pilati, G. Resnati, P. Metrangolo and D. W. Bruce, Angew. Chem., Int. Ed., 2016, 55, 6300–6304 CrossRef CAS PubMed.
- T. Aida, E. W. Meijer and S. I. Stupp, Science, 2012, 335, 813–817 CrossRef CAS PubMed.
- J. A. A. W. Elemans, A. E. Rowan and R. J. M. Nolte, J. Mater. Chem., 2003, 13, 2661–2670 RSC.
- K. Liu, Y. Kang, Z. Wang and X. Zhang, Adv. Mater., 2013, 25, 5530–5548 CrossRef CAS PubMed.
- A. S. Weingarten, R. V. Kazantsev, L. C. Palmer, M. McClendon, A. R. Koltonow, A. P. S. Samuel, D. J. Kiebala, M. R. Wasielewski and S. I. Stupp, Nat. Chem., 2014, 6, 964–970 CrossRef CAS PubMed.
- Q. Wang, T. Xia, W. Wu, J. Zhao, X. Xue, C. Ao, J. Zhang, X. Deng, X. Zhang, W. Zhang and C. Lu, Chem. Eng. J., 2022, 431, 133228 CrossRef CAS.
- Z. Wang, Q. Zhang, S. Long, Y. Luo, P. Yu, Z. Tan, J. Bai, B. Qu, Y. Yang, J. Shi, H. Zhou, Z. Y. Xiao, W. Hong and H. Bai, ACS Appl. Mater. Interfaces, 2018, 10, 10437–10444 CrossRef CAS PubMed.
- G. Vantomme and E. W. Meijer, Science, 2019, 363, 1396–1397 CrossRef CAS PubMed.
- O. Dumele, J. Chen, J. V. Passarelli and S. I. Stupp, Adv. Mater., 2020, 32, 1907247 CrossRef CAS PubMed.
- T. Islam, M. M. Hasan, S. S. Akter, N. H. Alharthi, M. R. Karim, M. A. Aziz, M. D. Hossain and A. J. S. Ahammad, ACS Appl. Polym. Mater., 2020, 2, 273–284 CrossRef CAS.
- W. Fang, Y. Zhang, J. Wu, C. Liu, H. Zhu and T. Tu, Chem. - Asian J., 2018, 13, 712–729 CrossRef CAS PubMed.
- D. Zhao, Q. Zhang, W. Chen, X. Yi, S. Liu, Q. Wang, Y. Liu, J. Li, X. Li and H. Yu, ACS Appl. Mater. Interfaces, 2017, 9, 13213–13222 CrossRef CAS PubMed.
- G. Li, H. Yang, D. Zuo, J. Xu and H. Zhang, Int. J. Hydrogen Energy, 2021, 46, 13044–13049 CrossRef CAS.
- D. Das and S. Kurungot, Energy Technol., 2020, 8, 2000061 CrossRef CAS.
- G. Qin, M. Wang, L. Fan, X. Fang, D. Zhang, J. Liu, J. Qin, J. Shi, J. Yang and Q. Chen, J. Power Sources, 2020, 474, 228602 CrossRef CAS.
- J. Yang, X. Yu, X. Sun, Q. Kang, L. Zhu, G. Qin, A. Zhou, G. Sun and Q. Chen, ACS Appl. Mater. Interfaces, 2020, 12, 9736–9745 CrossRef CAS PubMed.
- J. N. Israelachvili, D. J. Mitchell and B. W. Ninham, J. Chem. Soc., Faraday Trans. 2, 1976, 72, 1525 RSC.
- S. I. Stupp, V. LeBonheur, K. Walker, L. S. Li, K. E. Huggins, M. Keser and A. Amstutz, Science, 1997, 276, 384–389 CrossRef CAS PubMed.
- T. F. A. de Greef and E. W. Meijer, Aust. J. Chem., 2010, 63, 596 CrossRef CAS.
- I. D. Tevis, L. C. Palmer, D. J. Herman, I. P. Murray, D. A. Stone and S. I. Stupp, J. Am. Chem. Soc., 2011, 133, 16486–16494 CrossRef CAS PubMed.
- J. P. Hill, W. Jin, A. Kosaka, T. Fukushima, H. Ichihara, T. Shimomura, K. Ito, T. Hashizume, N. Ishii and T. Aida, Science, 2004, 304, 1481–1483 CrossRef CAS PubMed.
- Y. Dong, Y. Wang, X. Zhang, Q. Lai and Y. Yang, Chem. Eng. J., 2022, 449, 137858 CrossRef CAS.
- W. Tian, H. Zhang, X. Duan, H. Sun, G. Shao and S. Wang, Adv. Funct. Mater., 2020, 30, 1909265 CrossRef CAS.
- B. Hu, X. Shang, P. Nie, B. Zhang, K. Xu, J. Yang, J. Qiu and J. Liu, ACS Appl. Energy Mater., 2021, 4, 6991–7001 CrossRef CAS.
- X. Zhang, X. Cui, C. H. Lu, H. Li, Q. Zhang, C. He and Y. Yang, Chem. Eng. J., 2020, 401, 126031 CrossRef CAS.
- X. Xie, X. He, H. Zhang, F. Wei, N. Xiao and J. Qiu, Chem. Eng. J., 2018, 350, 49–56 CrossRef CAS.
- R. Li, X. Li, J. Chen, J. Wang, H. He, B. Huang, Y. Liu, Y. Zhou and G. Yang, Nanoscale, 2018, 10, 3981–3989 RSC.
- R. Ma, X. Cui, X. Xu, Y. Wang, G. Xiang, L. Gao, Z. Lin and Y. Yang, Nano Energy, 2023, 108, 108179 CrossRef CAS.
- Y. Huang, H. Cheng, D. Shu, J. Zhong, X. Song, Z. Guo, A. Gao, J. Hao, C. He and F. Yi, Chem. Eng. J., 2017, 320, 634–643 CrossRef CAS.
- H. Pan, Y. Zhang, Y. Pan, W. Lin, W. Tu and H. Zhang, Chem. Eng. J., 2020, 401, 126083 CrossRef CAS.
- L. Borchardt, D. Leistenschneider, J. Haase and M. Dvoyashkin, Adv. Energy Mater., 2018, 8, 1800892 CrossRef.
- D. W. Wang, F. Li, M. Liu, G. Q. Lu and H. M. Cheng, Angew. Chem., Int. Ed., 2009, 48, 1525 CrossRef CAS.
- Q. Bai, Y. Shen, T. A. Asoh, C. Li, Y. Dan and H. Uyama, Nanoscale, 2020, 12, 15261–15274 RSC.
- O. A. Vasilyev, A. A. Kornyshev and S. Kondrat, ACS Appl. Energy Mater., 2019, 2, 5386–5390 CrossRef CAS.
- J. Min, X. Wen, T. Tang, X. Chen, K. Huo, J. Gong, J. Azadmanjiri, C. He and E. Mijowska, Chem. Commun., 2020, 56, 9142–9145 RSC.
- Y. Li, Y. Du, G. Sun, J. Cheng, G. Song, M. Song, F. Su, F. Yang, L. Xie and C. Chen, EcoMat, 2021, 3, e12091 CAS.
- L. Yao, J. Lin, Y. Chen, X. Li, D. Wang, H. Yang, L. Deng and Z. Zheng, InfoMat, 2022, 4, e12278 CrossRef CAS.
- J. Cui, J. Yin, J. Meng, Y. Liu, M. Liao, T. Wu, M. Dresselhaus, Y. Xie, J. Wu, C. Lu and X. Zhang, Nano Lett., 2021, 21, 2156–2164 CrossRef CAS PubMed.
- X. Cao, Z. Yin and H. Zhang, Energy Environ. Sci., 2014, 7, 1850–1865 RSC.
- H. Cheng, L. Long, D. Shu, J. Wu, Y. Gong, C. He, Z. Kang and X. Zou, Int. J. Hydrogen Energy, 2014, 39, 16151–16161 CrossRef CAS.
- H. Chen, X. Lu, H. Wang, D. Sui, F. Meng and W. Qi, J. Energy Chem., 2020, 49, 348–357 CrossRef.
- X. Cui, L. Gao, S. Lei, S. Liang, J. Zhang, C. D. Sewell, W. Xue, Q. Liu, Z. Lin and Y. Yang, Adv. Funct. Mater., 2021, 31, 2009197 CrossRef CAS.
- X. Liu, X. Zhang, Y. Dou, P. Mei, X. Ma and Y. Yang, Chem. Commun., 2021, 57, 4966–4969 RSC.
- L. Lai, Y. Zhao, S. Ying, L. Li, Z. Ma and L. Pan, RSC Adv., 2018, 8, 18714–18722 RSC.
- Z. Li, Z. Xu, H. Wang, J. Ding, B. Zahiri, C. M. B. Holt, X. Tan and D. Mitlin, Energy Environ. Sci., 2014, 7, 1708–1718 RSC.
- Y. Li, G. Wang, T. Wei, Z. Fan and P. Yan, Nano Energy, 2016, 19, 165–175 CrossRef CAS.
- L. Yang, X. He, Y. Wei, H. Bi, F. Wei, H. Li, C. Yuan and J. Qiu, Nano Res., 2022, 15, 4068–4075 CrossRef CAS.
- L. N. Han, X. Wei, Q. C. Zhu, S. M. Xu, K. X. Wang and J. S. Chen, J. Mater. Chem. A, 2016, 4, 16698–16705 RSC.
- C. Liu, F. Yi, D. Shu, W. Chen, X. Zhou, Z. Zhu, R. Zeng, A. Gao, C. He and X. Li, Electrochim. Acta, 2019, 319, 410–422 CrossRef CAS.
- W. Ai, J. Jiang, J. Zhu, Z. Fan, Y. Wang, H. Zhang, W. Huang and T. Yu, Adv. Energy Mater., 2015, 5, 1500559 CrossRef.
- L. He, J. Liu, Y. Liu, B. Cui, B. Hu, M. Wang, K. Tian, Y. Song, S. Wu, Z. Zhang, Z. Peng and M. Du, Appl. Catal., B, 2019, 248, 366–379 CrossRef CAS.
- H. Cheng, X. Zhou, A. Gao, F. Yi, D. Shu, X. Song, R. Zeng, C. He, S. Li and D. Zeng, Electrochim. Acta, 2018, 292, 20–30 CrossRef CAS.
- H. Cheng, F. Yi, A. Gao, H. Liang, D. Shu, X. Zhou, C. He and Z. Zhu, ACS Appl. Energy Mater., 2019, 2, 4084–4091 CrossRef CAS.
- H. Cheng, B. Li, T. Meng, C. Liu and D. Shu, Int. J. Energy Res., 2022, 46, 18624–18633 CrossRef CAS.
- L. Zhang, H. Chen, X. Lu, Y. Wang, L. Tan, D. Sui and W. Qi, Appl. Surf. Sci., 2020, 529, 147022 CrossRef CAS.
- G. J. H. Lim, X. Liu, C. Guan and J. Wang, Electrochim. Acta, 2018, 291, 177–187 CrossRef CAS.
- L. Tian, K. Xia, S. Wu, Y. Cai, H. Liu, X. Jing, T. Yang, D. Chen, X. Bai, M. Zhou and L. Li, Electrochim. Acta, 2019, 307, 310–317 CrossRef CAS.
- J. Xu, Y. Sun, M. Lu, L. Wang, J. Zhang and X. Liu, Sci. China Mater., 2019, 62, 699–710 CrossRef CAS.
- F. Zhang, H. Yao, T. Chu, G. Zhang, Y. Wang and Y. Yang, Chem. - Eur. J., 2017, 23, 10293–10300 CrossRef CAS PubMed.
- G. Zhang, F. Zhang, H. Yao, Z. Liu and Y. Yang, J. Mater. Chem. A, 2017, 5, 19036–19045 RSC.
- K. Qi, R. Hou, S. Zaman, Y. Qiu, B. Y. Xia and H. Duan, ACS Appl. Mater. Interfaces, 2018, 10, 18021–18028 CrossRef CAS PubMed.
- J. Xie, H. J. Peng, J. Q. Huang, W. T. Xu, X. Chen and Q. Zhang, Angew. Chem., Int. Ed., 2017, 56, 16223–16227 CrossRef CAS PubMed.
- F. Zhang, Y. Wang, T. Chu, Z. Wang, W. Li and Y. Yang, Analyst, 2016, 141, 4502–4510 RSC.
- Z. Wang, H. Yao, F. Zhang, W. Li, Y. Yang and X. Lu, J. Mater. Chem. A, 2016, 4, 16476–16483 RSC.
- Y. Liu, G. Li, Y. Guo, Y. Ying and X. Peng, ACS Appl. Mater. Interfaces, 2017, 9, 14043–14050 CrossRef CAS PubMed.
- F. Zhang, G. Zhang, H. Yao, Z. Gao, X. Chen and Y. Yang, Chem. Eng. J., 2018, 338, 230–239 CrossRef CAS.
- F. Zhang, J. Ma, L. Bai, W. Xie, X. Wang and R. Zhang, Appl. Surf. Sci., 2022, 601, 154219 CrossRef CAS.
- Q. Li, H. Yao, F. Liu, Z. Gao and Y. Yang, Electrochim. Acta, 2019, 321, 134682 CrossRef CAS.
- J. Ma, W. Li, X. Zhang, Y. Cheng and F. Zhang, Appl. Surf. Sci., 2020, 507, 145074 CrossRef CAS.
- H. Yao, G. Zhang, F. Zhang, W. Li, Y. Yang and L. Chen, Mater. Today Energy, 2017, 6, 164–172 CrossRef.
- J. Q. Shen, Y. Zhang, Z. M. Zhang, Y. G. Li, Y. Q. Gao and E. B. Wang, Chem. Commun., 2014, 50, 6017 RSC.
- D. Wang, L. Liu, J. Jiang, L. Chen and J. Zhao, Nanoscale, 2020, 12, 5705–5718 RSC.
- K. Wang, K. Yu, J. Lv, M. Zhang, F. Meng and B. Zhou, Inorg. Chem., 2019, 58, 7947–7957 CrossRef CAS PubMed.
- W. J. Liu, G. Yu, M. Zhang, R. H. Li, L. Z. Dong, H. S. Zhao, Y. J. Chen, Z. F. Xin, S. L. Li and Y. Q. Lan, Small Methods, 2018, 2, 1800154 CrossRef.
- J. J. Zhu, R. Benages-Vilau and P. Gomez-Romero, Electrochim. Acta, 2020, 362, 137007 CrossRef CAS.
- A. Guillén-López, N. D. Espinosa-Torres, A. K. Cuentas-Gallegos, M. Robles and J. Muñiz, Carbon, 2018, 130, 623–635 CrossRef.
- A. Anandan Vannathan, P. R. Chandewar, D. Shee and S. Sankar Mal, J. Electroanal. Chem., 2022, 904, 115856 CrossRef CAS.
- Z. Zheng, M. Li, Q. Zhou, L. Cai, J. F. Yin, Y. Cao and P. Yin, ACS Appl. Nano Mater., 2021, 4, 811–819 CrossRef CAS.
- F. M. Toma, A. Sartorel, M. Iurlo, M. Carraro, P. Parisse, C. Maccato, S. Rapino, B. R. Gonzalez, H. Amenitsch, T. Da Ros, L. Casalis, A. Goldoni, M. Marcaccio, G. Scorrano, G. Scoles, F. Paolucci, M. Prato and M. Bonchio, Nat. Chem., 2010, 2, 826–831 CrossRef CAS PubMed.
- S. Chen, Y. Xiang, M. K. Banks, C. Peng, W. Xu and R. Wu, Nanoscale, 2018, 10, 20043–20052 RSC.
- M. Wang, Y. Zhang, T. Zhang, Y. Li, M. Cui, X. Cao, Y. Lu, D. Peng, W. Liu, X. Liu, T. Wang and Y. Huang, Nanoscale, 2020, 12, 11887–11898 RSC.
- D. Kumar, A. Joshi, G. Singh and R. K. Sharma, Chem. Eng. J., 2022, 431, 134085 CrossRef CAS.
- G. Wang, T. Chen, C. J. Gómez-García, F. Zhang, M. Zhang, H. Ma, H. Pang, X. Wang and L. Tan, Small, 2020, 16, 2001626 CrossRef CAS PubMed.
- L. Cui, K. Yu, J. Lv, C. Guo and B. Zhou, J. Mater. Chem. A, 2020, 8, 22918–22928 RSC.
- S. Hu, K. Li, X. Yu, Z. Jin, B. Xiao, R. Yang, H. Pang, H. Ma, X. Wang, L. Tan and G. Yang, J. Mol. Struct., 2022, 1250, 131753 CrossRef CAS.
- Z. Q. Shi, N. N. Ji, K. M. Guo and G. Li, Appl. Surf. Sci., 2020, 504, 144484 CrossRef CAS.
- X. Yu, S. U. Khan, Z. Jin, Q. Wu, H. Pang, H. Ma, X. Wang, L. Tan and G. Yang, J. Energy Storage, 2022, 53, 105192 CrossRef.
- K. Yang, A. Gao, H. Wu, F. Yi, D. Shu, X. Li and L. Xie, Int. J. Energy Res., 2020, 44, 7082–7092 CrossRef CAS.
- K. Yang, Z. Luo, D. Shu, F. Yi, Z. Zhu and A. Gao, J. Electroanal. Chem., 2022, 908, 116093 CrossRef CAS.
- J. Guo, H. Zhu, Y. Sun, L. Tang and X. Zhang, J. Mater. Chem. A, 2016, 4, 4783–4789 RSC.
- Y. Yang, Y. Wang, L. Zhao, Y. Liu and F. Ran, Adv. Energy Mater., 2022, 12, 2103158 CrossRef CAS.
- Y. Wang, X. Chu, Z. Zhu, D. Xiong, H. Zhang and W. Yang, Chem. Eng. J., 2021, 423, 130203 CrossRef CAS.
- F. Zhao, J. Bae, X. Zhou, Y. Guo and G. Yu, Adv. Mater., 2018, 30, 1801796 CrossRef PubMed.
- Q. Zhang, Y. He, Y. Wang, J. Lu, N. Jiang and Y. Yang, Adv. Funct. Mater., 2023, 33, 2370027 CrossRef.
- P. Kumari, K. Khawas, S. Nandy and B. K. Kuila, Electrochim. Acta, 2016, 190, 596–604 CrossRef CAS.
- S. Li, A. Gao, F. Yi, D. Shu, H. Cheng, X. Zhou, C. He, D. Zeng and F. Zhang, Electrochim. Acta, 2019, 297, 1094–1103 CrossRef CAS.
- Y. Zhao, B. Liu, L. Pan and G. Yu, Energy Environ. Sci., 2013, 6, 2856 RSC.
- Y. Guo, J. Bae, Z. Fang, P. Li, F. Zhao and G. Yu, Chem. Rev., 2020, 120, 7642–7707 CrossRef CAS PubMed.
- S. Naficy, J. M. Razal, G. M. Spinks, G. G. Wallace and P. G. Whitten, Chem. Mater., 2012, 24, 3425–3433 CrossRef CAS.
- M. Ma, L. Guo, D. G. Anderson and R. Langer, Science, 2013, 339, 186–189 CrossRef CAS PubMed.
- W. Li, F. Gao, X. Wang, N. Zhang and M. Ma, Angew. Chem., Int. Ed., 2016, 55, 9196–9201 CrossRef CAS PubMed.
- F. Gao, J. Song, H. Teng, X. Luo and M. Ma, Chem. Eng. J., 2021, 405, 126915 CrossRef CAS.
- J. J. Alcaraz-Espinoza, G. Ramos-Sánchez, J. H. Sierra-Uribe and I. González, ACS Appl. Energy Mater., 2021, 4, 9099–9110 CrossRef CAS.
- X. Zhou, T. Meng, F. Yi, D. Shu, D. Han, Z. Zhu, A. Gao, C. Liu, X. Li, K. Yang and H. Yi, J. Power Sources, 2020, 475, 228554 CrossRef CAS.
- Q. Liu, A. Gao, Y. Huang, F. Yi, H. Cheng, S. Zhao, H. Chen, R. Zeng, Z. Sun, D. Shu and X. Song, J. Alloys Compd., 2019, 777, 1176–1183 CrossRef CAS.
- R. Zhao, K. Li, R. Liu, M. Sarfraz, I. Shakir and Y. Xu, J. Mater. Chem. A, 2017, 5, 19098–19106 RSC.
- M. C. G. Saborío, S. Lanzalaco, G. Fabregat, J. Puiggalí, F. Estrany and C. Alemán, J. Phys. Chem. C, 2018, 122, 1078–1090 CrossRef.
- G. Li, X. Zhang, M. Sang, X. Wang, D. Zuo, J. Xu and H. Zhang, J. Energy Storage, 2021, 33, 101931 CrossRef.
- S. Xu, X. Liang, K. Ge, H. Yuan and G. Liu, ACS Appl. Energy Mater., 2022, 5, 2929–2936 CrossRef CAS.
- Z. Wang, X. Han, Y. Wang, K. Men, L. Cui, J. Wu, G. Meng, Z. Liu and X. Guo, Front. Mater. Sci., 2019, 13, 54–63 CrossRef.
- J. Gačanin, J. Hedrich, S. Sieste, G. Glaßer, I. Lieberwirth, C. Schilling, S. Fischer, H. Barth, B. Knöll, C. V. Synatschke and T. Weil, Adv. Mater., 2019, 31, 1805044 CrossRef PubMed.
- F. Mo, Q. Li, G. Liang, Y. Zhao, D. Wang, Y. Huang, J. Wei and C. Zhi, Adv. Sci., 2021, 8, 2100072 CrossRef CAS PubMed.
- E. Feng, W. Gao, J. Li, J. Wei, Q. Yang, Z. Li, X. Ma, T. Zhang and Z. Yang, ACS Sustainable Chem. Eng., 2020, 8, 3311–3320 CrossRef CAS.
- B. Pal, S. Yang, S. Ramesh, V. Thangadurai and R. Jose, Nanoscale Adv., 2019, 1, 3807–3835 RSC.
- V. Jose, J. M. V. Nsanzimana, H. Hu, J. Choi, X. Wang and J. Lee, Adv. Energy Mater., 2021, 11, 2100157 CrossRef CAS.
- O. Borodin, J. Self, K. A. Persson, C. Wang and K. Xu, Joule, 2020, 4, 69–100 CrossRef CAS.
- H. Zhang, X. Liu, H. Li, I. Hasa and S. Passerini, Angew. Chem., Int. Ed., 2021, 60, 598–616 CrossRef CAS PubMed.
- C. Tang, M. Li, J. Du, Y. Wang, Y. Zhang, G. Wang, X. Shi, Y. Li, J. Liu, C. Lian and L. Li, J. Colloid Interface Sci., 2022, 608, 1162–1172 CrossRef CAS PubMed.
- H. Li, L. Ma, C. Han, Z. Wang, Z. Liu, Z. Tang and C. Zhi, Nano Energy, 2019, 62, 550–587 CrossRef CAS.
- L. Dong, X. Ma, Y. Li, L. Zhao, W. Liu, J. Cheng, C. Xu, B. Li, Q. H. Yang and F. Kang, Energy Storage Mater., 2018, 13, 96–102 CrossRef.
- X. Gong, J. Chen and P. S. Lee, Batteries Supercaps, 2021, 4, 1527–1528 CrossRef.
- Z. Wang, M. Zhang, W. Ma, J. Zhu and W. Song, Small, 2021, 17, 2100219 CrossRef CAS PubMed.
- H. Yang, J. Zhang, J. Yao, D. Zuo, J. Xu and H. Zhang, J. Energy Storage, 2022, 53, 105089 CrossRef.
| This journal is © The Royal Society of Chemistry 2023 |


