Highly stretchable and self-healing photoswitchable supramolecular fluorescent polymers for underwater anti-counterfeiting†
Haitao
Deng
a,
Hong
Wang
 a,
Yong
Tian
a,
Zhong
Lin
a,
Jiaxi
Cui
a,
Yong
Tian
a,
Zhong
Lin
a,
Jiaxi
Cui
 *b and
Jian
Chen
*b and
Jian
Chen
 *a
*a
aKey Laboratory of Theoretical Organic Chemistry and Functional Molecule of Ministry of Education, Hunan Provincial Key Laboratory of Controllable Preparation and Functional Application of Fine Polymers, Hunan Provincial Key Lab of Advanced Materials for New Energy Storage and Conversion, Hunan Province College Key Laboratory of QSAR/QSPR, School of Chemistry and Chemical Engineering, Hunan University of Science and Technology, Xiangtan, Hunan 411201, China. E-mail: cj0066@gmail.com
bInstitute of Fundamental and Frontier Sciences, University of Electronic Science and Technology of China, Chengdu 610054, China. E-mail: jiaxi.cui@uestc.edu.cn
First published on 15th September 2023
Abstract
Thanks to the non-destructiveness and spatial-controllability of light, photoswitchable fluorescent polymers (PFPs) have been successfully applied in advanced anti-counterfeiting and information encryption. However, most of them are not suitable for use in harsh underwater environments, including high salinity seawater. In this study, by integrating photochromic molecules into a hydrophobic polymer matrix with the fluorine elastomer, including dipole–dipole interactions, we describe a class of novel photoswitchable supramolecular fluorescent polymers (PSFPs) that can adaptively change their fluorescence between none, green and red by the irradiation of different light. The PSFPs not only exhibited excellent photoswitchable properties, including fast photo-responsibility, prominent photo-reversibility, and photostability, but also exhibited some desired properties, including exceptional stretchability, hydrophobicity, antifouling, self-healing ability, simple preparation process, and processability. We thus demonstrated their applications in underwater data encryption and anti-counterfeiting labels.
New conceptsPhotoswitchable fluorescent polymers are often designed for advanced anti-counterfeiting and information encryption. These applications often involve harsh underwater environments (acid, base, or seawater), wherein traditional strategies for mechanical strengthening and self-healing normally fail due to the aqueous conditions. Dipole–dipole interactions are hydrophobic and expected to bring about self-healing ability even in an underwater environment. Herein, we demonstrate this concept by photoswitchable supramolecular fluorescent polymers crosslinked by dipole–dipole interactions. These materials combine the excellent photoswitchable properties of diarylethylene derivatives and the dynamic properties of the supramolecular materials, resulting in an excellent overall performance. We evidence their applicability in underwater data encryption and anti-counterfeiting labels. They may be promising next-generation photoswitchable materials for underwater anti-counterfeiting labels and encryption. |
Introduction
Nowadays, the development of the marine economy has become more and more important, which inevitably involves underwater anti-counterfeiting and encryption in some important or high value-added equipment.1,2 Creating underwater anti-counterfeiting and encryption with high-level safety is very necessary to prevent imitation or reproduction without the permission of the owner and avoid unnecessary economic losses.3,4 By learning from organisms in nature, many stimulus-responsive (e.g., light, temperature, pH, metal ion, mechanical force, and so on) polymer systems with alterable fluorescence emission have been developed to apply in anti-counterfeiting and encryption.5–8 Among them, thanks to the non-destructiveness and spatial-controllability of light, light-driven photochromic molecules were integrated into polymer systems to create photoswitchable fluorescent polymers (PFPs) and applied in photorewritable patterns, anti-counterfeiting, and information encryption.9–16 Recent reported PFPs are mainly based on nanoparticles, films, and hydrogels. However, nanoparticles possess poor mechanical properties due to the independent nanostructure.17–20 Films and hydrogels cannot easily realize self-healing in harsh underwater environments (acid, base, or seawater).21–27 Films containing supramolecular interactions (e.g., H-bond, ionic interaction, host–guest interactions) and hydrogels can also be abnormally swelled or de-swelled in acid, base, or high-concentration salt solutions, resulting in poor stability.28–33 These disadvantages greatly limit their application. Therefore, it is highly desirable to develop PFPs with well-pleasing overall performance, including photoswitchable properties, antifouling, and self-healing ability for extending their application in humid environments and even seawater.Supramolecular materials consisting of supramolecular interactions (e.g., hydrogen bonds, ionic interaction, host–guest interactions, coordination complex, hydrophobic association, π–π stacking interaction) show many eminent characteristics, such as processability, self-healing ability, recyclability, etc.34–43 In the last two decades, these materials have been applied to anti-counterfeiting, information encryption, self-healing, functional coatings, soft robots, etc.44–51 However, these materials are still far away from applications in some special environments, such as underwater anti-counterfeiting and encryption, due to their poor stability and self-healing capability in harsh environments (high salt, strong acid–base environment). To address this issue, Wang et al. developed a new supramolecular system with dipole–dipole interactions, which exhibited remarkable stability in harsh aqueous environments and self-healable capability.52 Therefore, introducing dipole–dipole interactions into polymer systems to create PFPs with self-healing capability is very necessary for meeting the criteria required for underwater anti-counterfeiting labels and encryption.
Herein, we report a class of new photoswitchable supramolecular fluorescent polymers (PSFPs) with excellent stability and self-healable capability in harsh aqueous environments via the facile copolymerization of diarylethylene derivatives (BTBA, SDTE), 2,2,2-trifluoroethyl methacrylate (TFEMA) and 2,2,3,4,4,4-hexafluorobutyl acrylate (HFBA). In our system, PSFPs have unique dipole–dipole interactions based on strong molecular dipoles, ensuring that the PSFPs not only show remarkable photoreversibility, photostability, and easy preparation, but also exhibit stretchability, hydrophobicity, antifouling, exceptional environmental stability including salt, acid and alkali resistance, and self-healing. These characteristics allow us to apply PSFPs in underwater adaptive discoloration, anti-counterfeiting, and information encryption.
Results and discussion
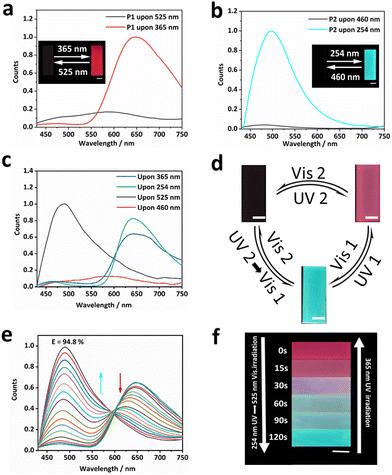
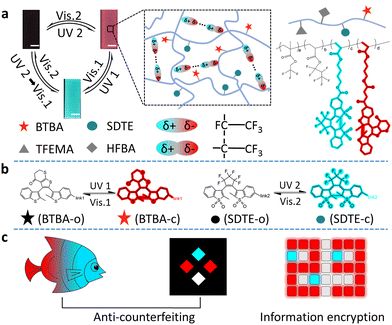
Fig. 1 shows our design. We chose the photoswitchable diarylethylene derivatives as a photochromic molecule and fluorescence resonance energy transfer (FRET) donor (SDTE-c) and acceptor (BTBA-c) to integrate into the hydrophobic fluorine elastomer including dipole–dipole interactions for the development of PSFPs. The fluorescence of PSFPs can be reversibly altered between none, green and red (Fig. 1a and 1b).53 Briefly, UV2 (254 nm) could not only induce the structure transformation of SDTE from non-fluorescence to green fluorescence, but also cause the structure transformation of BTBA from non-fluorescence to red fluorescence. Vis2 (460 nm) could cause an opposite change. However, the BTBA can be reversibly switched between non-fluorescence and red fluorescence using UV1 (365 nm) and Vis1 (525 nm) to stimulate alternately. In addition, dipole–dipole interactions make PSFPs have processability, hydrophobicity, antifouling, and self-healing ability.54–56 Finally, the as-prepared PSFPs possess both photoswitchable properties and a well-pleasing comprehensive performance. Therefore, thanks to the non-destructiveness and spatial-controllability of light, we could use PSFPs as the desired tools to apply for underwater anti-counterfeiting and information encryption (Fig. 1c).The two diarylethylene derivatives (BTBA, SDTE) were first synthesized based on our previous work.53 The ring-closed state of SDTE-c exhibited an obvious absorbance peak (410 nm) and green fluorescence emission (500 nm) in tetrahydrofuran (THF), which was served as the FRET donor. The ring-closed state of BTBA-c exhibited an obvious absorbance peak (564 nm) and red fluorescence emission (655 nm) in THF, which was used as the FRET acceptor. Thereafter, PSFPs (P3) were synthesized by the light-induced free radical polymerization of the two diarylethylene derivatives (BTBA, SDTE), 2,2,2-trifluoroethyl methacrylate (TFEMA), and 2,2,3,4,4,4-hexafluorobutyl acrylate (HFBA, Fig. S4, ESI†). Other copolymers (P0, P1, P2) were synthesized (Fig. S1–S3 and Table S1, ESI†). The absorbance and fluorescence spectra of P1, P2, and P3 indicated the successful copolymerization of TFEMA, HFBA, BTBA, and SDTE. The obtained polymers with moderate molecular weights (P0, Mn = 31.5 kDa; P1, Mn = 32.9 kDa; P2, Mn = 31.7 kDa; P3, Mn = 27.4 kDa, Table S1, ESI†) further demonstrated that the monomers were converted into copolymers. Because of the reversibly photoswitchable performance of SDTE and BTBA and the effective FRET process between ring-closed SDTE-c (donor) and ring-closed BTBA-c (acceptor), the obtained PSFPs (P3) can be reversibly switched among three distinct states (non-emission, green, and red) under different light stimuli (Fig. 1a). In addition, due to the combination of TFEMA and HFBA containing CF3 dipoles (Fig. 1a), the PSFPs existed unique dipole–dipole interactions based on a strong molecular dipole.57 Furthermore, the glass transition temperatures (Tg) of these polymers (P0, Tg = 18.25 °C; P1, Tg = 19.82 °C; P2, Tg = 20.07 °C; P3, Tg = 21.02 °C, Fig. S5, ESI†) illustrated that the introduction of BTBA and SDTE could enhance the Tg. These low Tg endowed the polymer materials with eminent processability.58
With PSFPs in our hands, the optimal excitation wavelength was first investigated. Selecting the optimum wavelength as the excitation light can effectively avoid the test error caused by the excitation light in a photoswitchable material. Therefore, P1 and P2 were exposed to UV light (254 nm) for 5 min to obtain P1 with red fluorescence emission and P2 with green fluorescence emission, respectively. Subsequently, the P1 with red fluorescence and P2 with green fluorescence were excitated by 410 nm light. The results showed that the fluorescence intensity of P1 and P2 exhibited a slight reduction (<10%) even after continuous illumination for 30 min (Fig. S6, ESI†), indicating the photostability of the ring-closed state of BTBA-c and SDTE-c. Hence, 410 nm was chosen as the excitation wavelength for subsequent tests.
Next, we explored the photoswitching properties of PSFPs. As shown in Fig. 2a, the fluorescence of P1 containing BTBA can be reversibly changed between red emission and non-emission states by alternately using 365 nm UV and 525 nm visible light to irradiate samples (Fig. 2a and Fig. S7a, ESI†). P2 containing SDTE also revealed a similar change between green emission and non-emission states under UV (254 nm) and Vis (460 nm) light irradiation (Fig. 2b and Fig. S7b, ESI†). No significant fluorescence attenuation in P1 and P2 was noted during the 20 tests (Fig. S7c and S7d, ESI†), demonstrating that P1 and P2 presented satisfactory photo-reversibility. They also exhibited satisfying thermostability within 24 h (25 °C and 65 °C, Fig. S8a–d, ESI†) and long-term stability within 4 weeks (Fig. S8d and S8e, ESI†). As depicted in Fig. 2c and d and Fig. S9 (ESI†), P3 displayed a reversible change between non-fluorescence, red fluorescence, and green fluorescence. Importantly, any two states are completely reversible (Fig. 2d). In addition, due to the overlap between the emission spectrum of SDTE-c units and the absorption spectrum of BTBA-c units (Fig. S10, ESI†), the obviously photo-controlled FRET process between SDTE-c and BTBA-c was achieved by utilizing Vis (525 nm) and UV (365 nm) to irradiate alternately the sample. As shown in Fig. 2e, when P3 was translated to red fluorescence using UV (254 nm, FRET efficiency: 94.8%), upon the increase of the time of irradiation of Vis1, the red fluorescence was gradually turned to green fluorescence (Fig. 2f, and Fig. S11, ESI†). Subsequently, when green-fluorescent P3 was further exposed to UV1 (365 nm), a decreased fluorescence quantum yield (3.26% to < 0.01%, λex = 410 nm, λem = 430–550 nm, Table S2, ESI†) and fluorescence lifetime (3.6 ns to 2.6 ns, Fig. S12, ESI†) indicated that a FRET process between SDTE-c (donor) and BTBA-c (acceptor) occurred. The multi-wavelength response is very conducive to creating highly secure information encryption and anti-counterfeiting labels.
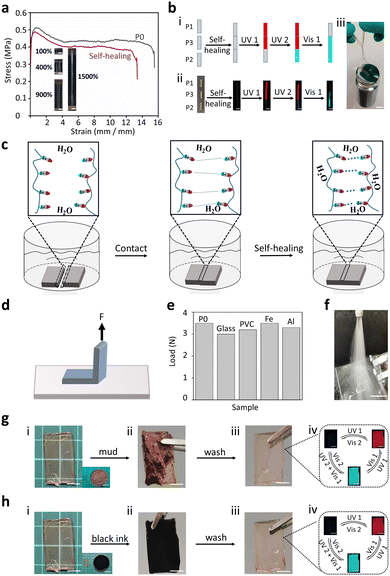
The polymer matrixes exhibited typical supramolecular interactions (dipole–dipole interactions) based on a strong molecular dipole (Fig. 1c). These dynamic interactions endow PSFPs (P0) with high moduli (196 MPa), typical yielding points (0.51 MPa), strength (0.38 MPa), and moderate stretchability (15.5 times strain) (Fig. 3a). In addition, PSFPs can achieve highly efficient self-healing because of the existence of a large number of dynamic bonds (healing efficiency: 79.8% (25 °C), Fig. 3a). The unique dipole–dipole interactions in polymer matrixes thus could on-demand implement self-healing. We thus employed three samples (P1, P2, P3) to create a self-healing sample for evaluating the self-healing ability (Fig. 3b). As revealed in Fig. 3b, when the samples were healed under water for 24 h, the healed sample containing P1, P2 and P3 not only showed outstanding photoswitchable properties but also lifted 200 times its own weight (500 g). This sample could be stretched well without breaking, and exhibited fluorescent properties under UV light (Fig. S13, ESI†). Such excellent underwater self-healing performance should be attributed to the fact that the dipole–dipole interactions can lock the interfaces in contact with each other (Fig. 3c). We also investigated its adhesion ability to different substrates (Glass, PMMA, sheet iron, aluminium sheet) using a stripping method (Fig. 3d). The polymers are polymerized directly to the surface of the corresponding materials. The results exhibited that its adhesion is greater than its fracture energy (>3.5 N, Fig. 3e, and Fig. S14, ESI†), demonstrating the application potential of PSFPs in anti-counterfeiting labels. As shown in Fig. 3f, samples (P0) attached to the glass will not fall off under the impact of high-speed water flow (>16 L min−1, Video S1). In addition, the non-covalent crosslinking supramolecular interaction can endow the material with eminent processability. We thus evaluated the processability of PSFPs (P0) through solvent and heat. The cuboid sample can be transformed into a triangular body by cutting and subsequent reprocessing with solvent (THF, Fig. S15, ESI†). This shape was further transformed into a pentagonal sample by heat treatment (100 °C). These results demonstrated that PSFPs present the desired self-healing and processability. Such features could ensure that PSFPs have good application prospects in information encryption and anti-counterfeiting labels with self-healing ability.
The dipole–dipole interactions ensured that the PSFPs exhibited hydrophobicity (water contact angle (WCA) > 115°, Fig. S16, ESI†) and chemical stability in aqueous conditions because of the weak hydrogen bond acceptance of the C–F bond.57 This feature would make the material have good self-cleaning properties. P3 was first immersed into the mud for a week. As seen in Fig. 3g, when the samples stained by sludge were simply cleaned with water, all the remaining stains were completely removed. The cleaned sample (P3) still showed satisfactory photo-response properties. A similar result could be observed by immersing P3 in black ink for a week (Fig. 3h), indicating that the sample has well-pleasing antifouling ability. Furthermore, the exceptional environmental stability including salt, acid and alkali resistance was also explored. The P3 was soaked into the different solutions (pure water (pH = 7.05), acid solution (HCl, pH = 1), basic solution (NaOH, pH = 14), and salt solution (26.5 wt%, seawater)). Even when stored in these environments for more than a week, no significant change in the photoswitchable ability and weight was observed (Fig. S17–S19, ESI†). In addition, a negligible change in the fluorescence quantum yields of P1, P2, and P3 under ambient conditions and in water also supports this conclusion (Table S2, ESI†). These characteristics enabled us to apply PSFPs in underwater information encryption and anti-counterfeiting labels.
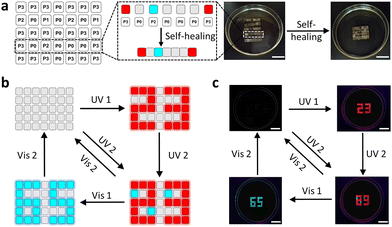
Based on the above-mentioned features, PSFPs were further applied to constructing information encryption systems. We first make the polymer (P0: dye-free; P1: including BTBA units; P2: including SDTE units; P3: including BTBA and SDTE units) into the corresponding information encryption boxes. P0 boxes served as a no information carrier, and P1, P2, and P3 boxes acted as carriers of primary information, secondary information and tertiary information. These boxes are bonded together under water to form a complete rectangle block (Fig. 4a). The on-demand multilevel information could be integrated into the polymer block (Fig. 4a). Among them, P3 boxes were utilized to serve as an independent encryption unit and an information jamming unit. As depicted in Fig. 4b and c, the encrypted information combining with P1, P2, and P3 (“89”) can be decrypted via UV2, and then be re-encrypted using Vis 2. When the UV1 and Vis 1 were further introduced, the encrypted information (“23”, “89”, and “65”) appeared and disappeared one by one form “23” to “89” and “65”, and eventually returning to their original state (Fig. 4c). This result indicated that PSFPs could be utilized to create on-demand multilevel information encryption systems.
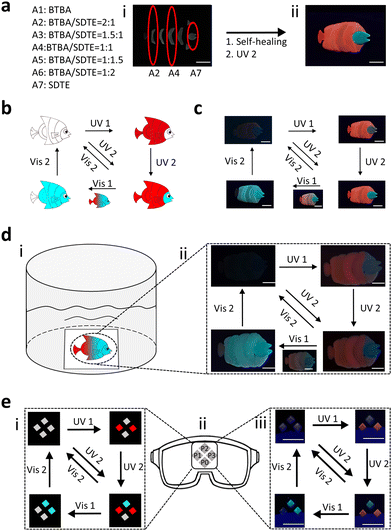
In daily life, anti-counterfeiting labels are a very important anti-counterfeiting means to identify counterfeit and shoddy products.59,60 Multilevel information decrypted by multiple means is necessary to improve information security and prevent copying. Moreover, underwater anti-counterfeiting labels often have to be used in harsh environments such as high salt concentrations, which require materials with better properties. Therefore, thanks to their excellent properties, including fast photo-responsibility, prominent photo-reversibility, antifouling, self-healing ability, simple preparation process, and processability, PSFPs were a desired platform for creating underwater anti-counterfeiting labels. We thus fabricate an artificial butterflyfish as an anti-counterfeiting label to evaluate the underwater applicability of PSFPs. As shown in Fig. 5a, we divided the butterflyfish into seven parts (A1–A7). The seven corresponding polymers with different proportions of dyes (BTBA/SDTE:2/0-0/2) were synthesized (Fig. 5a). Briefly, A1 only contained BTBA, and served as a fish's tail. The content of BTBA was gradually decreased from A2 to A6. A7 only included SDTE. Finally, the as-prepared parts are pieced together, and healed into an artificial butterflyfish under water (Fig. 5a). The artificial butterflyfish showed a distinct gradient of fluorescence (red to green) from the tail to the head. Fig. 5b and c shows the schematic diagram and optical photographs of the photoswitchable discoloration process, respectively. It's non-fluorescent in its initial state. When the artificial butterflyfish were irradiated by 365 nm UV, red fluorescence occurred. Subsequently, UV (254 nm) would trigger a change in green fluorescence. Next, the red and green fluorescence were gradually faded by the stimulation of Vis1 (525 nm) and Vis2 (460 nm), respectively. Finally, the artificial butterflyfish changes to its initial non-fluorescence state. To further demonstrate the spatial-controllability of this process, we simulated an underwater environment to explore this performance (Fig. S20, ESI†). As illustrated in Fig. 5d, the artificial butterflyfish is posted on a glass plate as an anti-counterfeiting label, exhibiting a non-fluorescent state under a 365 nm UV lamp. By employing UV (254 nm and 365 nm) to irradiate the artificial butterflyfish, non-fluorescent butterflyfish were converted into a fluorescent state. Subsequently, Vis light was chosen to illuminate the fluorescent state of the butterflyfish to adapt and blend in with the new environment. This process can be implemented underwater. These results demonstrated that PSFPs with a spatial-controlled photo-response were an ideal platform for creating underwater anti-counterfeiting labels and information encryption systems.
In addition, swimming equipment, especially ocean diving equipment, has higher requirements for anti-counterfeiting labels because of the harsh environment. The anti-counterfeiting labels in diving goggles were fabricated using PSFPs (P0, P1, P2, and P3 and Fig. S21, ESI†). As indicated in Fig. 5e, the non-fluorescence state of the anti-counterfeiting labels could be converted into a different form by combining different light (UV1, UV2, Vis1, and Vis2). Briefly, UV1 drives P1 and P3 to display information, UV2 prompts P2 to exhibit all information, Vis1 erases part of the displayed information from P1 and P3, and Vis2 eliminates all information. In this process, reversible multi-state fluorescence changes can be achieved by using different light to stimulate the anti-counterfeiting labels, indicating the application potential and advantage of PSFPs in underwater anti-counterfeiting labels.
Conclusions
In summary, we have successfully prepared PSFPsvia the copolymerization of diarylethylene derivatives (BTBA, SDTE), and fluorinated acrylate (TFEMA, HFBA). Thanks to the satisfying stability of dipole–dipole interactions based on strong molecular dipole and the photoswitchable performance of diarylethylene derivatives, the as-prepared PSFPs not only displayed outstanding photoreversibility, and eminent photostability, but also showed excellent self-healing ability, stretchability, hydrophobicity, antifouling, and exceptional environmental stability including salt, acid and alkali resistance. We thus successfully implemented PSFPs in underwater light-induced adaptive discoloration like butterflyfish, information encryption and anti-counterfeiting labels. Therefore, we anticipate their potential applications for anti-counterfeiting labels in harsh underwater environments.Experimental
Preparation of PSFPs
Taking P3 as an example: the solution of TFEMA (10 mmol) and HFBA (10 mmol) containing I-819 (0.2 mmol), BTBA (8.18 × 10−2 mmol), and SDTE (6.36 × 10−2 mmol) was irradiated by Vis light (460 nm, 40 mW cm−2) for 1 h in reaction cells consisting of a pair of glass plates with 0.8 mm spacing to get polymers (P3). For other samples containing BTBA or SDTE, detailed sample information can be found in Table S1 (ESI†).Preparation of films
The obtained polymers P0, P1, P2 and P3 were dissolved in dichloromethane, and after ultrasonic treatment for 5 min, a polymer solution of 30 mg mL−1 was obtained. The transparent solution was added dropwise to a clean glass substrate (Piranha solution as washing agent) and dried overnight at room temperature to obtain the fluorescent films.Author contributions
Haitao Deng: conceptualization, methodology, investigation, validation, formal analysis, writing – original draft, writing – review & editing. Hong Wang: conceptualization, methodology, investigation, validation, formal analysis, supervision, writing – original draft, writing – review & editing. Yong Tian: formal analysis, writing – review & editing. Zhong Lin: formal analysis, writing – review & editing. Jiaxi Cui: conceptualization, methodology, formal analysis, supervision, writing – original draft, writing – review & editing, funding acquisition. Jian Chen: conceptualization, methodology, formal analysis, supervision, writing – original draft, writing – review & editing, funding acquisition.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (52273206), Hunan Provincial Natural Science Foundation (2021JJ10029), and Huxiang High-level Talent Gathering Project (2022RC4039).Notes and references
- Q. Zhang, W. Wang, C. Cai, S. Wu, J. Li, F. Li and S. Dong, Mater. Horiz., 2022, 9, 1984–1991 RSC.
- T.-P. Huynh, M. Khatib and H. Haick, Adv. Mater. Technol., 2019, 4, 1900081 CrossRef.
- H. Zhang, J. He and J. Qu, Eur. Polym. J., 2022, 178, 111487 CrossRef CAS.
- J. Liu, X. Li, K. Chen, Y. Li, S. Feng, P. Su, Y. Zou, Y. Li and W. Wang, Macromol. Rapid Commun., 2023 DOI:10.1002/marc.202300282.
- Y. Sun, X. Le, S. Zhou and T. Chen, Adv. Mater., 2022, 34, e2201262 CrossRef PubMed.
- C. Liu, A. K. Steppert, Y. Liu, P. Weis, J. Hu, C. Nie, W. C. Xu, A. J. C. Kuehne and S. Wu, Adv. Mater., 2023, 35, 202303120 Search PubMed.
- J. Ma, Y. Yang, C. Valenzuela, X. Zhang, L. Wang and W. Feng, Angew. Chem., Int. Ed., 2022, 61, e202116219 CrossRef CAS PubMed.
- F. Xu and B. L. Feringa, Adv. Mater., 2023, 35, e2204413 CrossRef PubMed.
- T. Fukaminato, T. Umemoto, Y. Iwata, S. Yokojima, M. Yoneyama, S. Nakamura and M. Irie, J. Am. Chem. Soc., 2007, 129, 5932–5938 CrossRef CAS PubMed.
- L. Kortekaas and W. R. Browne, Chem. Soc. Rev., 2021, 50, 2211 RSC.
- C. Li, H. Yan, L.-X. Zhao, G.-F. Zhang, Z. Hu, Z.-L. Huang and M.-Q. Zhu, Nat. Commun., 2014, 5, 5709 CrossRef CAS PubMed.
- W. Hu, C. Sun, Y. Ren, S. Qin, Y. Shao, L. Zhang, Y. Wu, Q. Wang, H. Yang and D. Yang, Angew. Chem., Int. Ed., 2021, 60, 19406–19412 CrossRef CAS PubMed.
- M. Chen, B. Yao, M. Kappl, S. Liu, J. Yuan, R. Berger, F. Zhang, H. J. Butt, Y. Liu and S. Wu, Adv. Funct. Mater., 2019, 30, 1906752 CrossRef.
- Z. Zhang, W. Wang, P. Jin, J. Xue, L. Sun, J. Huang, J. Zhang and H. Tian, Nat. Commun., 2019, 10, 4232 CrossRef PubMed.
- S. Lin, K. G. Gutierrez-Cuevas, X. Zhang, J. Guo and Q. Li, Adv. Funct. Mater., 2020, 31, 2007957 CrossRef.
- J. Jiang, Q. Chen, M. Xu, J. Chen and S. Wu, Macromol. Rapid Commun., 2023, 44, e2300117 CrossRef PubMed.
- M. C. Baier, J. Huber and S. Mecking, J. Am. Chem. Soc., 2009, 131, 14267–14273 CrossRef CAS PubMed.
- W. R. Algar, M. Massey, K. Rees, R. Higgins, K. D. Krause, G. H. Darwish, W. J. Peveler, Z. Xiao, H.-Y. Tsai, R. Gupta, K. Lix, M. V. Tran and H. Kim, Chem. Rev., 2021, 121, 9243–9358 CrossRef CAS PubMed.
- K. K. Ng and G. Zheng, Chem. Rev., 2015, 115, 11012–11042 CrossRef CAS PubMed.
- Z. Lin, H. Wang, M. Yu, X. Guo, C. Zhang, H. Deng, P. Zhang, S. Chen, R. Zeng, J. Cui and J. Chen, J. Mater. Chem. C, 2019, 7, 11515–11521 RSC.
- X. Xu, V. V. Jerca and R. Hoogenboom, Mater. Horiz., 2021, 8, 1173–1188 RSC.
- Z. Wang, A. Li, Z. Zhao, T. Zhu, Q. Zhang, Y. Zhang, Y. Tan and W. Z. Yuan, Adv. Mater., 2022, 34, e2202182 CrossRef PubMed.
- F. Luo, T. L. Sun, T. Nakajima, T. Kurokawa, Y. Zhao, K. Sato, A. B. Ihsan, X. Li, H. Guo and J. P. Gong, Adv. Mater., 2015, 27, 2722–2727 CrossRef CAS PubMed.
- X. Du, J. Zhou, J. Shi and B. Xu, Chem. Rev., 2015, 115, 13165–13307 CrossRef CAS PubMed.
- H. Wang, C. N. Zhu, H. Zeng, X. Ji, T. Xie, X. Yan, Z. L. Wu and F. Huang, Adv. Mater., 2019, 31, 1807328 CrossRef PubMed.
- Y. Guo, J. Bae, Z. Fang, P. Li, F. Zhao and G. Yu, Chem. Rev., 2020, 120, 7642–7707 CrossRef CAS PubMed.
- H. Wang, X. Xiong, L. Yang, Y. Fang, J. Xue and J. Cui, Adv. Funct. Mater., 2022, 33, 2212402 CrossRef.
- W. Lu, X. Le, J. Zhang, Y. Huang and T. Chen, Chem. Soc. Rev., 2017, 46, 1284–1294 RSC.
- R. Cheng, M. Xu, X. Zhang, J. Jiang, Q. Zhang and Y. Zhao, Angew. Chem., Int. Ed., 2023, 62, e202302900 CrossRef CAS PubMed.
- L. Li, W. Li, X. Wang, X. Zou, S. Zheng, Z. Liu, Q. Li, Q. Xia and F. Yan, Angew. Chem., Int. Ed., 2022, 61, e202212512 CrossRef CAS PubMed.
- B. J. Cafferty, R. R. Avirah, G. B. Schuster and N. V. Hud, Chem. Sci., 2014, 5, 4681–4686 RSC.
- P. R. A. Chivers and D. K. Smith, Nat. Rev. Mater., 2019, 4, 463–478 CrossRef CAS.
- Z. Sun, F. Lv, L. Cao, L. Liu, Y. Zhang and Z. Lu, Angew. Chem., Int. Ed., 2015, 54, 7944–7948 CrossRef CAS PubMed.
- D. B. Amabilino, D. K. Smith and J. W. Steed, Chem. Soc. Rev., 2017, 46, 2404–2420 RSC.
- X.-L. Ni, X. Xiao, H. Cong, Q.-J. Zhu, S.-F. Xue and Z. Tao, Acc. Chem. Res., 2014, 47, 1386–1395 CrossRef CAS PubMed.
- W. Han, W. Xiang, Q. Li, H. Zhang, Y. Yang, J. Shi, Y. Ji, S. Wang, X. Ji, N. M. Khashab and J. L. Sessler, Chem. Soc. Rev., 2021, 50, 10025–10043 RSC.
- H. Chen and J. Fraser Stoddart, Nat. Rev. Mater., 2021, 6, 804–828 CrossRef CAS.
- S. J. D. Lugger, S. J. A. Houben, Y. Foelen, M. G. Debije, A. Schenning and D. J. Mulder, Chem. Rev., 2022, 122, 4946–4975 CrossRef CAS PubMed.
- P. Sun, B. Qin, J.-F. Xu and X. Zhang, Prog. Polym. Sci., 2022, 124, 101486 CrossRef CAS.
- X. N. Zhang, C. Du, Y. J. Wang, L. X. Hou, M. Du, Q. Zheng and Z. L. Wu, Macromolecules, 2022, 55, 7512–7525 CrossRef CAS.
- Y. Liu, J. Wan, X. Zhao, J. Zhao, Y. Guo, R. Bai, Z. Zhang, W. Yu, H. W. Gibson and X. Yan, Angew. Chem., Int. Ed., 2023, 62, e202302370 CrossRef CAS PubMed.
- S. Chen, Z. Li, C. Zhang, X. Wu, W. Wang, Q. Huang, W. Chen, J. Shi and D. Yuan, Small, 2023, 19, e2301063 CrossRef PubMed.
- S. J. D. Lugger, S. J. A. Houben, Y. Foelen, M. G. Debije, A. P. H. J. Schenning and D. J. Mulder, Chem. Rev., 2022, 122, 4946–4975 CrossRef CAS PubMed.
- H. Yang, S. Li, J. Zheng, G. Chen, W. Wang, Y. Miao, N. Zhu, Y. Cong and J. Fu, Adv. Mater., 2023 DOI:10.1002/adma.202301300.
- J. Tang, Y. Tian, Z. Lin, C. Zhang, P. Zhang, R. Zeng, S. Wu, X. Chen and J. Chen, ACS Appl. Mater. Interfaces, 2023, 15, 2237–2245 CrossRef CAS PubMed.
- Z. Qin, Y. Yang, Q. Tian, H.-Y. Mi, H. Li, R. Guo, Y. Wang, C. Liu and C. Shen, Chem. Eng. J., 2023, 467, 143434 CrossRef CAS.
- N. M. Sangeetha and U. Maitra, Chem. Soc. Rev., 2005, 34, 821–836 RSC.
- P. Wang, Q. Liao, B. Yuan and H. Zhang, ACS Appl. Mater. Interfaces, 2023, 15, 32945–32956 CrossRef CAS PubMed.
- A. K. Chau and F. K. Leung, Adv. Colloid Interface Sci., 2023, 315, 102892 CrossRef CAS PubMed.
- D. Li, Z. Feng, Y. Han, C. Chen, Q.-W. Zhang and Y. Tian, Adv. Sci., 2022, 9, 2104790 CrossRef CAS PubMed.
- H. Wang, X. Ji, Z. Li and F. Huang, Adv. Mater., 2017, 29, 1606117 CrossRef PubMed.
- Y. Liu, T. Chen, Z. Jin, M. Li, D. Zhang, L. Duan, Z. Zhao and C. Wang, Nat. Commun., 2022, 13, 1338 CrossRef CAS PubMed.
- J. Jiang, P. Zhang, Y. Tian, Z. Lin, C. Zhang, J. Cui, J. Chen and X. Chen, Sci. China Mater., 2023, 66, 1949–1958 CrossRef CAS.
- S. Wang and M. W. Urban, Adv. Sci., 2021, 8, 2101399 CrossRef CAS PubMed.
- A. M. C. Maan, A. H. Hofman, W. M. Vos and M. Kamperman, Adv. Funct. Mater., 2020, 30, 2000936 CrossRef CAS.
- Y. Jia, L. Zhang, M. Qin, Y. Li, S. Gu, Q. Guan and Z. You, Chem. Eng. J., 2022, 430, 133081 CrossRef CAS.
- S. Huang, Y. Wan, X. Ming, J. Zhou, M. Zhou, H. Chen, Q. Zhang and S. Zhu, ACS Appl. Mater. Interfaces, 2021, 13, 41112–41119 CrossRef CAS PubMed.
- F. Nonque, A. Benlahoues, J. Audourenc, A. Sahut, R. Saint-Loup, P. Woisel and J. Potier, Eur. Polym. J., 2021, 160, 110799 CrossRef CAS.
- A. O. Larin, L. N. Dvoretckaia, A. M. Mozharov, I. S. Mukhin, A. B. Cherepakhin, I. I. Shishkin, E. I. Ageev and D. A. Zuev, Adv. Mater., 2021, 33, e2005886 CrossRef PubMed.
- J. Zhang, Y. Liu, C. Njel, S. Ronneberger, N. V. Tarakina and F. F. Loeffler, Nat. Nanotechnol., 2023, 18, 1027–1035 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3mh01239e |
| This journal is © The Royal Society of Chemistry 2023 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: