Enhanced electrochemiluminescence imaging of single cell membrane proteins based on Co3O4 nanozyme catalysis†
Jingjing
Zhang
a,
Lin
Hao
*b,
Jie
Chao
a,
Lianhui
Wang
 c and
Shao
Su
c and
Shao
Su
 *c
*c
aSchool of Geographic and Biologic Information, Nanjing University of Posts and Telecommunications, Nanjing 210023, China
bDepartment of Urology, Xuzhou Central Hospital, Xuzhou 221009, Jiangsu, China. E-mail: haolinxuzhou@163.com
cKey Laboratory for Organic Electronics and Information Displays, Jiangsu Key Laboratory for Biosensors, Institute of Advanced Materials (IAM), Jiangsu National Synergetic Innovation Center for Advanced Materials (SICAM), Nanjing University of Posts and Telecommunications, Nanjing 210023, China. E-mail: iamssu@njupt.edu.cn
First published on 8th September 2023
Abstract
The development of enhanced strategies with excellent biocompatibility is critical for electrochemiluminescence (ECL) imaging of single cells. Here, we report an ECL imaging technique for a single cell membrane protein based on a Co3O4 nanozyme catalytic enhancement strategy. Due to the remarkable catalytic performance of Co3O4 nanozymes, H2O2 can be efficiently decomposed into reactive oxygen radicals, and the reaction with L012 was enhanced, resulting in stronger ECL emission. The anti-carcinoembryonic antigen (CEA) was coupled with nanozyme particles to construct a probe that specifically recognized the overexpressed CEA on the MCF-7 cell membrane. According to the locally enhanced visualized luminescence, the rapid ECL imaging of a single cell membrane protein was eventually realized. Accordingly, Co3O4 nanozymes with highly efficient activity will provide new insights into ECL imaging analysis of more biological small molecules and proteins.
Electrochemiluminescence (ECL) is a kind of optical radiation phenomenon produced by chemical molecules undergoing electrochemical reaction.1–3 ECL does not require an additional excitation light source, since the reaction process depends on the applied voltage to drive the luminescence process, which effectively avoids the interference of light scattering and improves the detection sensitivity. It has become a powerful analytical technique, widely used in biochemical analysis,4 pharmaceutical analysis,5 environmental analysis6 and other fields. In view of the urgent need for high analytical throughput and spatial resolution, ECL imaging was created by coupling ECL technology with optical microscopy. This new imaging method can not only achieve high-throughput and visualization of biomolecules,7 but also be applied to the research of spatiotemporal resolution imaging of single entities such as particles, cells and bacteria.8–12 It can provide more spatial detail information, such as identification of cellular contents and structures. Small molecules in cells or released from cells, (sub-)cellular structures including cell membranes,13,14 proteins,10,15 mitochondria16 and intracellular hierarchical structures such as the nucleolus, nucleus and endoplasmic reticulum17 have been visualized by ECL imaging.18 With the enhancement effects of silica nanochannels on ECL intensity, Su et al. have explored the dynamic variation of cell–matrix adhesions during collective migration using the negative imaging mode.19–21 Despite the significant achievements in ECL imaging, it has the technical limitation of insufficient sensitivity and time resolution due to the weak luminescence signal generated by the luminophore. It is a challenge to further develop a novel biocompatible approach of ECL imaging, and enhance the ECL intensity, thereby improving the sensitivity and temporal resolution.
Nanozymes have sparked widespread research due to their unique properties of nanomaterials and catalytic activity.22–25 They can efficiently catalyze the substrates of enzymes under mild conditions, showing catalytic efficiency and enzymatic reaction kinetics similar to natural enzymes. In view of their advantages such as adjustable catalytic activity, easy large-scale synthesis, higher physiochemical stability, higher durability and lower costs,26 nanozymes have attracted considerable interest and represent great potential applications in biomedicine, disease diagnosis, the environment and biological detection. Numerous studies have indicated that many nanomaterials have been discovered to possess nanozyme properties, such as Fe3O4,26 AuNPs,27,28 carbon dots,29 and so on. Among them, Co3O4 nanoparticles, with catalase-like activity, can be adopted as accelerators of H2O2 for enhancing the ECL emission of the luminol-H2O2 system, because the conversion of the Co2+/Co3+ redox pair could efficiently excite the generation of electron holes, and then react with the molecular orbital of H2O2 for promoting its decomposition.30 Based on this advantage, the Co3O4 nanozyme can be combined with the luminol-H2O2 ECL system, and it is expected to significantly improve the ECL intensity, thereby further improving the sensitivity and time resolution of the imaging technology and achieving rapid single-cell ECL imaging analysis.
Herein, a Co3O4 nanozyme was used as a catalyst of the luminol-H2O2 system for single cell ECL imaging. H2O2 produces reactive oxygen species by the catalysis of Co3O4. Under the condition of applying a suitable voltage, L012 (a luminol analog with high ECL efficiency) undergoes an electrochemical reaction to produce intermediates, which in turn react with reactive oxygen species to produce an ECL signal (Scheme 1). The ECL intensity of the L012-H2O2 system was significantly enhanced and achieved ultra-sensitive visualization of H2O2. Subsequently, a Co3O4 nanozyme-labeled CEA antibody was used as a probe to achieve sensitive and rapid imaging of CEA at the cellular membrane. This work provides a new approach for high-resolution ECL imaging.
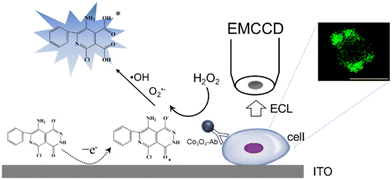 | ||
| Scheme 1 ECL mechanism based on the Co3O4 nanozyme for CEA antigen imaging at the cellular membrane. | ||
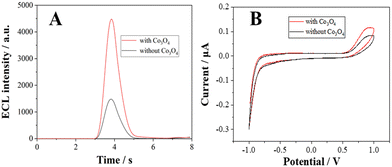
The diameter of the Co3O4 nanoparticles used in this study is ∼30 nm, as characterized in the transmission electron microscopy (TEM) image (Fig. S1, ESI†). To investigate the enhancement effects of the Co3O4 nanozyme, the electrochemical and ECL curves of the L012-H2O2 system on Co3O4 coated ITO electrodes were measured in 10 mM PBS containing 200 μM L012 and 20 μM H2O2. From Fig. 1A, in the presence of Co3O4, the ECL intensity from L012 and H2O2 improved to pprox. 3.0 fold higher than that of the bare ITO electrode. This enhancement should be ascribed to the excellent catalytic performance of Co3O4, which accelerates the decomposition of H2O2 to produce reactive oxygen radicals. The corresponding CV curves show that the peak current of the Co3O4-coated ITO electrode is significantly enhanced compared with the bare ITO electrode (Fig. 1B), indicating that the presence of Co3O4 could promote electron transport. The enhancements of both the ECL and peak current demonstrate that the introduction of Co3O4 served as an accelerator promoting the decomposition of coreactant H2O2 and enhancing the ECL intensity of the L012-H2O2 system. A possible ECL mechanism is proposed (eqn (1)–(4)). L012 anion LH− is oxidized to radical L˙− during the electrochemical scanning. Meanwhile, the Co3O4 catalyzes H2O2 to produce abundant oxygen radicals O2˙− and OH˙ on the ITO surface. In addition, Co3O4 can facilitate the reaction of L˙− and the oxygen radical to generate activated intermediate 3-AP2−*, which goes back to the ground state, generating the ECL emission.
| LH− − e− → LH˙ → L˙− + H+ | (1) |
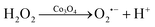 | (2) |
| L˙− + O2˙− → 3-AP2−* | (3) |
| 3-AP2−* → 3-AP2− + hν | (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V results in an increase in the ECL intensity from the Co3O4 particle (Fig. 2B), which is consistent with our previous observation. When the voltage exceeds 1.0 V, the increase in ECL intensity is not significant, therefore 1 V was selected as the ECL imaging voltage.
V results in an increase in the ECL intensity from the Co3O4 particle (Fig. 2B), which is consistent with our previous observation. When the voltage exceeds 1.0 V, the increase in ECL intensity is not significant, therefore 1 V was selected as the ECL imaging voltage.
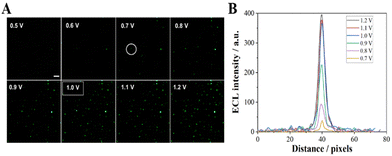 | ||
| Fig. 2 (A) ECL image of Co3O4 at the ITO slides with different applied potentials; the exposure time was 1 s. (B) The luminescence intensity across the particle circle in image A. Scale bar: 5 μm. | ||
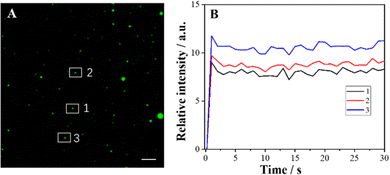
The effect of exposure time on ECL imaging was also explored. Under the condition of applied voltage at 1 V, when the exposure time is 500 ms, all the particles at the ITO interface produce clearly distinguishable luminescence spots (Fig. 3). Compared with the previous ECL imaging analysis,12,31,32 the presence of the Co3O4 nanozyme can shorten the exposure time obviously, indicating that the catalytic activity of the Co3O4 nanozyme can significantly enhance the ECL luminescence of the L012-H2O2 system, endowing it with higher temporal resolution. In addition, the stability of ECL luminescence using the Co3O4 nanozyme as a catalyst was also investigated. As shown in Fig. 4, the intensity maintains good stability (the relative standard deviation is less than 4.4%) during 30 consecutive images, which is extremely important in single-cell ECL imaging.
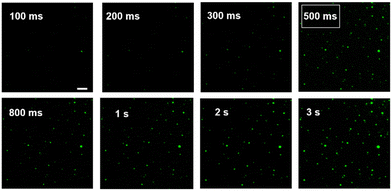 | ||
| Fig. 3 ECL image of Co3O4 at ITO slides with different exposure times; the applied potential was 1 V. Scale bar: 5 μm. | ||
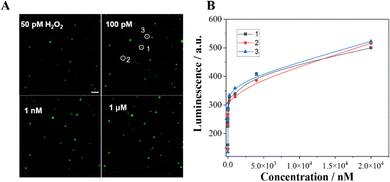
The quantitative visualization of H2O2 at individual Co3O4 nanoparticles can further validate the catalytic performance of the Co3O4 nanozyme. Thus, we investigated the ECL visualization of different concentrations of H2O2 (50 pM–20 μM) at Co3O4 nanoparticles in 10 mM PBS containing 200 μM L012. As shown in Fig. 5 and Fig. S3 (ESI†), as the concentration of H2O2 increases, the ECL intensity of the light spot gradually increases. The spot intensity of three randomly selected nanoparticles shows a good positive correlation with H2O2 concentration. The visualization detection limit is as low as 50 pM, which is much less than the previously reported detection limit (2 μM) under a voltage of 1.0 V.32 The results demonstrate that the Co3O4 nanozyme can effectively catalyze the L012-H2O2 system to generate strong luminescence under a low H2O2 concentration, endowing this strategy with extraordinary visualization sensitivity and ensuring the possibility of further visualization of single cell membrane proteins.
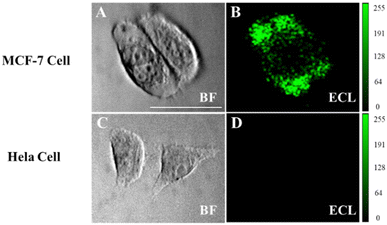
Imaging analysis of low-abundance proteins on the cell surface is crucial for elucidating the molecular mechanisms and related research on disease progression.10,33,34 Since the CEA antibody enables the selective recognition of cells that express CEA, MCF-7 cells with overexpressed CEA and HeLa cells were chosen as the experimental and negative control group, respectively. MTT assay confirms that Co3O4 has a low cytotoxicity to MCF-7 cells (Fig. S4, ESI†). The cells were cultured on the ITO slides to the adherent state and fixed with 4% paraformaldehyde to ensure the interaction between the antigen and the antibody.10 MCF-7 cells show obvious luminescence signals after modifying the functionalized probe Co3O4-antibody, while no obvious signal was generated in the HeLa cell region (Fig. 6). These results confirm that the luminescence observed in MCF-7 cells is attributed to the connection of the functionalized probe to the CEA antigen on the cell membrane, so that L012 and H2O2 in the solution only produced an enhanced ECL signal on the cell surface. We consider that the Co3O4 nanozyme has great potential in imaging other biomarkers on cell membranes owing to its favorable enzyme catalytic activity and biocompatibility.
In summary, we successfully developed a novel Co3O4 nanozyme-based ECL imaging approach for the analysis of single cell membrane proteins for the first time. The excellent catalytic performance of Co3O4 nanozymes can effectively accelerate the decomposition of H2O2 into reactive oxygen species and promote the electrochemical reaction of the L012-H2O2 system, inducing an enhanced ECL signal in a very short period. As a result, our ECL imaging system achieves rapid and sensitive imaging of single cell membrane proteins. Furthermore, our proposed Co3O4 nanozyme-enhanced ECL imaging exhibits good versatility, which sheds new light on the ECL imaging of biological molecules.
This work was supported by the National Nature Science Foundation of China (62101281, 62235008, 21922408 and 22274081), Natural Science Foundation of Jiangsu Province (BK20210584), the Belt and Road Innovation Cooperation Project of Jiangsu (BZ2022011), Natural Science Research Start-up Foundation of Recruiting Talents of Nanjing University of Posts and Telecommunications (Grant No. NY221032), and State Key Laboratory of Analytical Chemistry for Life Science (SKLACLS2205).
Conflicts of interest
There are no conflicts to declare.References
- W. Miao, Chem. Rev., 2008, 108, 2506–2553 CrossRef CAS PubMed.
- M. Richter, Chem. Rev., 2004, 104, 3003–3036 CrossRef CAS PubMed.
- J. Zhang, R. Jin, D. Fang and D. Jiang, Chem. Res. Chin. Univ., 2020, 41, 2421–2425 CAS.
- M. Zhao, W. Zeng, Y. Chai, R. Yuan and Y. Zhuo, Anal. Chem., 2020, 92, 11044–11052 CrossRef CAS PubMed.
- Y. Zhang, R. Zhang, X. Yang, H. Qi and C. Zhang, J. Pharm. Anal., 2019, 9, 9–19 CrossRef PubMed.
- H. Chu, W. Guo, J. Di, Y. Wu and Y. Tu, Electroanalysis, 2009, 21, 1630–1635 CrossRef CAS.
- N. Wang, Z. Wang, L. Chen, W. Chen, Y. Quan, Y. Cheng and H. Ju, Chem. Sci., 2019, 10, 6815–6820 RSC.
- Y. Liu, H. Zhang, B. Li, J. Liu, D. Jiang, B. Liu and N. Sojic, J. Am. Chem. Soc., 2021, 143, 17910–17914 CrossRef CAS PubMed.
- B. Li, X. Huang, Y. Lu, Z. Fan, B. Li, D. Jiang, N. Sojic and B. Liu, Adv. Sci., 2022, 9, 2204715 CrossRef CAS PubMed.
- J. Zhang, R. Jin, D. Jiang and H.-Y. Chen, J. Am. Chem. Soc., 2019, 141, 10294–10299 CrossRef CAS PubMed.
- Y. Wang, D. Jiang and H.-Y. Chen, CCS Chem., 2022, 4, 2221–2227 CrossRef CAS.
- Y. Lu, X. Huang, S. Wang, B. Li and B. Liu, ACS Nano, 2023, 17, 3809–3817 CrossRef CAS PubMed.
- S. Voci, B. Goudeau, G. Valenti, A. Lesch, M. Jovic′, S. Rapino, F. Paolucci, S. Arbault and N. Sojic, J. Am. Chem. Soc., 2018, 140, 14753–14760 CrossRef CAS PubMed.
- C. Ma, M. Wang, H. Wei, S. Wu, J. Zhang, J. Zhu and Z. Chen, Chem. Commun., 2021, 57, 2168–2171 RSC.
- G. Valenti, S. Scarabino, B. Goudeau, A. Lesch, M. Jovic′, E. Villani, M. Sentic, S. Rapino, S. Arbault, F. Paolucci and N. Sojic, J. Am. Chem. Soc., 2017, 139, 16830–16837 CrossRef CAS PubMed.
- S. Li, X. Ma, C. Pang, M. Wang, G. Yin, Z. Xu, J. Li and J. Luo, Biosens. Bioelectron., 2021, 176, 112944 CrossRef CAS PubMed.
- Y. Ma, C. Colin, J. Descamps, S. Arbault and N. Sojic, Angew. Chem., Int. Ed., 2021, 60, 18742–18749 CrossRef CAS PubMed.
- P. Zhou, L. Ding, Y. Yan, Y. Wang and B. Su, Chem. Commun., 2023, 59, 2341–2351 RSC.
- W. Guo, P. Zhou, L. Sun, H. Ding and B. Su, Angew. Chem., Int. Ed., 2021, 60, 2089–2093 CrossRef CAS PubMed.
- H. Ding, W. Guo and B. Su, Angew. Chem., Int. Ed., 2020, 59, 449–456 CrossRef CAS PubMed.
- H. Ding, P. Zhou, W. Fu, L. Ding, W. Guo and B. Su, Angew. Chem., Int. Ed., 2021, 60, 11769–11773 CrossRef CAS PubMed.
- J. Wu, X. Wang, Q. Wang, Z. Lou, S. Li, Y. Zhu, L. Qin and H. Wei, Chem. Soc. Rev., 2019, 48, 1004–1076 RSC.
- S. M. Khoshfetrat, P. Hashemi, A. Afkhami, A. Hajian and H. Bagheri, Sens. Actuators, B, 2021, 348, 130658 CrossRef CAS.
- L. Jiao, H. Y. Yan, Y. Wu, W. L. Gu, C. Z. Zhu, D. Du and Y. H. Lin, Angew. Chem., Int. Ed., 2020, 59, 2565–2576 CrossRef CAS PubMed.
- X. Wang, Y. Xu, N. Cheng, X. Wang, K. Huang and Y. Luo, Catalysts, 2021, 11, 638 CrossRef CAS.
- S. W. Wu, D. Z. Guo, X. C. Xu, J. M. Pan and X. H. Niu, Sens. Actuators, B, 2020, 303, 127225 CrossRef CAS.
- Y. Chen, X. Gou, C. Ma, D. Jiang and J. Zhu, Anal. Chem., 2021, 93, 7682–7689 CrossRef CAS PubMed.
- J. Zhang, Z. Huang, Y. Xie and X. Jiang, Chem. Sci., 2022, 13, 1080–1087 RSC.
- W. Gao, J. He, L. Chen, X. Meng, Y. Ma, L. Cheng, K. Tu, X. Gao, C. Liu, M. Zhang, K. Fan, D. Pang and X. Yan, Nat. Commun., 2023, 14, 160 CrossRef CAS PubMed.
- J. Dong, L. Song, J. Yin, W. He, Y. Wu, N. Gu and Y. Zhang, ACS Appl. Mater. Interfaces, 2014, 6, 1959–1970 CrossRef CAS PubMed.
- G. Valenti, S. Scarabino, B. Goudeau, A. Lesch, M. Jovic, E. Villani, M. Sentic, S. Rapino, S. Arbault, F. Paolucci and N. Sojic, J. Am. Chem. Soc., 2017, 139, 16830–16837 CrossRef CAS PubMed.
- J. Zhang, R. Jin, Y. Chen, D. Fang and D. C. Jiang, Sens. Actuators, B, 2021, 329, 129208 CrossRef CAS.
- T. Gao, B. Wang, L. Shi, X. L. Zhu, Y. Xiang, J.-I. Anzai and G. Li, Anal. Chem., 2017, 89, 10776–10782 CrossRef CAS PubMed.
- Y. Liu, H. Zhang, B. Li, J. Liu, D. Jiang, B. Liu and N. Sojic, J. Am. Chem. Soc., 2021, 143, 17910–17914 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc03484d |
| This journal is © The Royal Society of Chemistry 2023 |