Heavy metal-free visible-to-UV photon upconversion with over 20% efficiency sensitized by a ketocoumarin derivative†
Masanori
Uji
a,
Naoyuki
Harada
a,
Nobuo
Kimizuka
 *a,
Masaki
Saigo
b,
Kiyoshi
Miyata
b,
Ken
Onda
*a,
Masaki
Saigo
b,
Kiyoshi
Miyata
b,
Ken
Onda
 b and
Nobuhiro
Yanai
b and
Nobuhiro
Yanai
 *ac
*ac
aDepartment of Applied Chemistry, Graduate School of Engineering, Center for Molecular Systems (CMS), Kyushu University, 744 Moto-oka, Nishi-ku, Fukuoka 819-0395, Japan. E-mail: yanai@mail.cstm.kyushu-u.ac.jp; n-kimi@mail.cstm.kyushu-u.ac.jp
bDepartment of Chemistry, Kyushu University, 744 Moto-oka, Nishi-ku, Fukuoka 819-0395, Japan
cPRESTO, JST, Honcho 4-1-8, Kawaguchi, Saitama 332-0012, Japan
First published on 10th January 2022
Abstract
Efficient triplet–triplet annihilation-based photon upconversion (TTA-UC) from visible to UV light without using heavy metals is still a challenging task. Here we achieve a record-high TTA-UC efficiency of 20.3% among 100% maximum by employing a ketocoumarin derivative as a triplet sensitizer, which shows strong visible absorption, weak UV absorption, and efficient intersystem crossing.
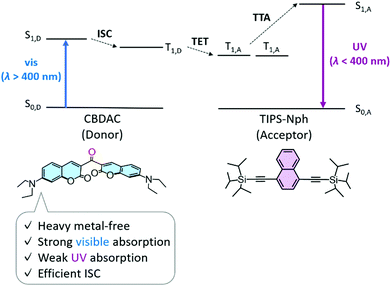
With the necessity to expand the use of renewable energy, sunlight is one of the most powerful energy resources in our environment. Photon upconversion (UC) from visible light (vis, λ > 400 nm) to ultraviolet light (UV, λ < 400 nm) is attracting attention in applications such as photocatalytic fuel production and environmental cleanup. Triplet–triplet annihilation-based UC (TTA-UC) is particularly useful since it works at low excitation intensity.1–10 In the typical TTA-UC mechanism, the donor molecule is photo-excited to an S1 state, followed by intersystem crossing (ISC) from S1 to T1. Triplet energy transfer (TET) from donor to acceptor is followed by annihilation between two acceptor triplets and generation of an acceptor S1 (Fig. 1).
 | ||
| Fig. 1 Energy level diagram of vis-to-UV TTA-UC using a heavy metal-free donor CBDAC and an acceptor TIPS-Nph. | ||
Since the first report of vis-to-UV TTA-UC in 2006,11 various donor–acceptor combinations have been reported, but the efficiency had remained low for a long time.11–27 Our group has achieved the highest TTA-UC efficiency ηUC of 20.5% (theoretical maximum: 100%) in 2020, which was twice as high as the previous record.28 This is due to the use of an Ir-coumarin complex Ir(C6)2(acac) with strong absorption in the visible region and weak absorption in the UV region as a donor, as well as the discovery of an acceptor 1,4-bis((triisopropylsilyl)ethynyl)naphthalene (TIPS-Nph) with high TTA and fluorescence efficiencies and a T1 energy low enough to be sensitized by Ir(C6)2(acac). Subsequently, similar efficiencies have been reported by using semiconductor nanocrystals as donor and 2,5-diphenyloxazole (PPO) as acceptor.29 However, these most efficient vis-to-UV TTA-UC systems use donors containing heavy metal ions such as Ir and Cd, which are not sustainable from the perspective of resource and environmental issues. Several examples of heavy metal-free vis-to-UV TTA-UC have been reported, but even the most efficient one showed a low TTA-UC efficiency of 8.2%.20–27 In addition, the threshold excitation intensity Ith is so high that it exceeds 1 W cm−2 in many cases. It is strongly desired to realize TTA-UC with both high efficiency and low Ith without using heavy metals.
Here, we show the highest ηUC of 20.3% as a heavy metal-free vis-to-UV TTA-UC, which is more than two times higher than the previous record. Since the excellent absorption properties of Ir(C6)2(acac) originate from the ligand coumarin derivative, we searched for a coumarin derivative that does not contain heavy metals and shows efficient ISC, and found 3,3′-carbonylbis(7-diethylaminocoumarin) (CBDAC, Fig. 1), which has been used for TTA-UC in the visible range but not for vis-to-UV TTA-UC.30
It is widely known that chromophores with carbonyl groups such as benzophenone exhibit high ISC efficiency ΦISC even without heavy metals due to the large spin–orbit coupling (SOC) caused by the distinct change in orbital symmetry during the transition from the n–π*/π–π* singlet state to the π–π*/n–π* triplet state.20,30–37 Ketocoumarin derivatives have also been used as heavy metal-free triplet sensitizers with such characteristics.30–33 Among the reported ketocoumarin derivatives, we focused on CBDAC because of its large absorption coefficient in the visible region over 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1 and weak absorption in the UV region (Fig. S1, ESI†),30–33 and it has been reported to have a high ΦISC of 92% in benzene.31
000 M−1 cm−1 and weak absorption in the UV region (Fig. S1, ESI†),30–33 and it has been reported to have a high ΦISC of 92% in benzene.31
To understand the high ΦISC of CBDAC, we performed density functional theory (DFT) calculations. After structural optimization in the ground state, we calculated absorption bands and observed an absorption peak at 447 nm (2.77 eV), which is in good agreement with the experimental result (446 nm, Fig. 2). The energy level of S1 was lower than that of T3 (3.03 eV) and higher than that of T1 and T2 (2.20 eV) (Fig. S2a, ESI†). Focusing on the molecular orbitals involved in the ISC from S1 to T1 or T2, we found the orbital changes from HOMO to HOMO−1 and from LUMO to LUMO+1, both of which involve the significant contribution of the carbonyl groups (Fig. S2b and c, ESI†). It is suggested that the large orbital symmetry changes involving the carbonyl groups lead to the highly efficient ISC.
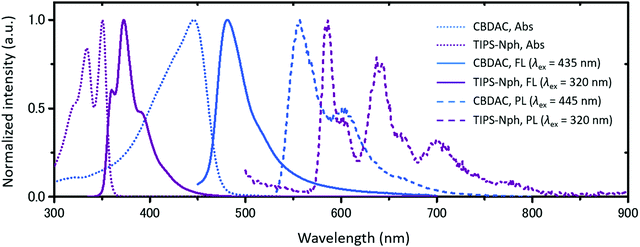
Importantly, CBDAC exhibits a strong and sharp absorption band, which is beneficial to suppress the absorption in the UV region. It was confirmed from the absorption and photoluminescence spectra that CBDAC and TIPS-Nph are a combination with suitable energy levels for vis-to-UV TTA-UC (Fig. 2). A toluene solution of CBDAC (100 μM) showed an absorption peak at 446 nm (2.78 eV) and a fluorescence peak at 481 nm (2.58 eV). The high ΦISC of CBDAC was also supported by its low fluorescence quantum yield ΦFL of 1.6% in toluene (λex = 445 nm). A toluene solution of TIPS-Nph (100 μM) showed an absorption peak at 350 nm (3.54 eV) and a fluorescence peak at 373 nm (3.32 eV), with a ΦFL of 74.8% (λex = 320 nm). The T1 energy levels of CBDAC and TIPS-Nph were estimated from phosphorescence measurements at 77 K in toluene (Fig. 2). CBDAC and TIPS-Nph showed 0–0 emission peaks at 557 nm (2.23 eV) and 586 nm (2.12 eV),38 respectively. Good agreement was found between the DFT calculation and experimental results for the T1 energy level of CBDAC. It is confirmed that the triplet energy level of CBDAC is high enough to sensitize TIPS-Nph.
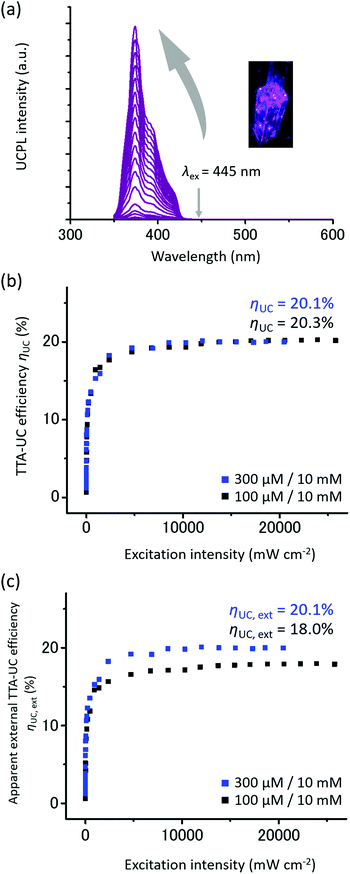
As expected from the energy level matching, an upconverted UV emission was observed by exciting a deaerated toluene solution of CBDAC and TIPS-Nph with a 445 nm laser (Fig. 3a, [CBDAC] = 100 μM, [TIPS-Nph] = 10 mM). This mixed solution showed a remarkably high TTA-UC efficiency ηUC of 20.3%, which was determined by the relative method (Fig. 3b, maximum ηUC = 100%,39 see the ESI† for details). Note that we did not include corrections of inner-filter effect or reabsorption in the relative method in order to evaluate the performance as an UC material. We confirmed the reliability of this high ηUC value by observing a similar value of 21.5% with the absolute method using an integrating sphere (Fig. S3, see the ESI† for details).40,41 The obtained ηUC was more than twice larger than the previous record of 8.2% for the heavy metal-free vis-to-UV TTA-UC.26 The triplet-mediated UC mechanism was confirmed by a millisecond-scale decay of the UC emission (Fig. S4, ESI†). Moreover, the UC emission intensity did not change even after one hour of continuous laser irradiation, demonstrating the high photostability of the current system (Fig. S5, ESI†).
The present heavy metal-free TTA-UC system was compared with the previous most efficient vis-to-UV TTA-UC system, Ir(C6)2(acac) and TIPS-Nph.28 Since the solvents used in the previous and current reports are different, we used toluene as the solvent in this report for comparison. The mixed solution of Ir(C6)2(acac) and TIPS-Nph showed a TTA-UC efficiency ηUC of 21.4% in deaerated toluene (Fig. S6, ESI†). Although CBDAC does not contain any heavy metals, it shows the TTA-UC efficiency comparable to that of the heavy metal-containing Ir(C6)2(acac) when combined with TIPS-Nph. This is reasonable considering that the ΦISC of Ir(C6)2(acac) has been reported to be nearly 100% and that of CBDAC in benzene has been reported to be as high as 92%.28,31 Other parameters that affect the TTA-UC efficiency are expressed as follows,4
| ηUC = fΦISCΦTETΦTTAΦFL | (1) |
Besides the high ηUC, the threshold excitation intensity Ith is also one of the important parameters in evaluating the performance of TTA-UC.42–44 In the typical TTA-UC systems, the UC emission intensity depends on the excitation intensity quadratically in the low intensity region and linearly in the high intensity region. Double logarithmic plots of UC emission intensity of CBDAC and TIPS-Nph against excitation intensity showed a transition from the slope of 2 to 1, and a relatively low Ith value of 38.0 mW cm−2 was obtained. To further reduce the Ith value, we increased the donor concentration from 100 μM to 300 μM, which provided an even lower Ith of 10.8 mW cm−2 (Fig. S10, ESI†). This value is close to the solar irradiance of 1.4 mW cm−2 for 445 ± 5 nm.
While the UC efficiency ηUC is important for evaluating UC properties, high external UC efficiency must be achieved for practical applications. The apparent external UC efficiency ηUC,ext is calculated by multiplying the TTA-UC efficiency by the absorption ratio,
| ηUC,ext = ηUC × (1 − 10−A) | (2) |
In most systems, due to the large absorption of the donor in the UV region, it is necessary to reduce the concentration of the donor to prevent the decrease in the TTA-UC efficiency ηUC by the reabsorption and back energy transfer. Therefore, it has been difficult to obtain a high apparent external UC efficiency due to the small absorbance of the donor. As discussed in our previous report,28 the small absorption of Ir(C6)2(acac) in the UV region allows us to concomitantly achieve both high visible absorbance and high TTA-UC efficiency ηUC at high donor concentration, resulting in a high apparent external UC efficiency ηUC,ext. Similarly, in the present heavy metal-free system, the high ηUC,ext was achieved because CBDAC exhibits strong visible absorption and weak UV absorption, which are notable characteristics of the employed coumarin derivatives.
In conclusion, we showed the highest TTA-UC efficiency ηUC of 20.3% as a heavy metal-free vis-to-UV TTA-UC, which is more than double the previous record. This high value was also confirmed by the absolute method. Inspired by the previous high efficiency using Ir(C6)2(acac),28 we found that CBDAC, the heavy metal-free ketocoumarin derivative, acts as the excellent donor for vis-to-UV TTA-UC. CBDAC exhibits strong visible absorption, weak UV absorption, and high ISC efficiency, which are completely comparable to Ir(C6)2(acac). Therefore, the heavy metal-free CBDAC/TIPS-Nph system successfully showed high TTA-UC efficiency as well as low Ith and high apparent external TTA-UC. This study will provide important guidelines for the future development of heavy metal-free and sustainable UV-generating materials.
Author contributions
N. Y. conceived the project. M. U. and N. H. carried out the experiments and calculations. M. S. and K. M. contributed to the transient absorption measurements. M. U. and N. Y. wrote the manuscript, with the input of N. H., K. O. and N. K.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was partly supported by JSPS KAKENHI (grant numbers JP20H02713, JP20K21211, JP20H05676, JP21J21739), GAP NEXT program, KYUTEC research and development grant, and the Innovation inspired by Nature Program of Sekisui Chemical Co. Ltd.References
- S. Baluschev, T. Miteva, V. Yakutkin, G. Nelles, A. Yasuda and G. Wegner, Phys. Rev. Lett., 2006, 97, 143903 CrossRef CAS PubMed.
- T. N. Singh-Rachford and F. N. Castellano, Coord. Chem. Rev., 2010, 254, 2560–2573 CrossRef CAS.
- J. Zhao, S. Ji and H. Guo, RSC Adv., 2011, 1, 937–950 RSC.
- A. Monguzzi, R. Tubino, S. Hoseinkhani, M. Campione and F. Meinardi, Phys. Chem. Chem. Phys., 2012, 14, 4322–4332 RSC.
- Y. C. Simon and C. Weder, J. Mater. Chem., 2012, 22, 20817–20830 RSC.
- S. P. Hill and K. Hanson, J. Am. Chem. Soc., 2017, 139, 10988–10991 CrossRef CAS PubMed.
- V. Gray, K. Moth-Poulsen, B. Albinsson and M. Abrahamsson, Coord. Chem. Rev., 2018, 362, 54–71 CrossRef CAS.
- B. D. Ravetz, A. B. Pun, E. M. Churchill, D. N. Congreve, T. Rovis and L. M. Campos, Nature, 2019, 565, 343–346 CrossRef CAS PubMed.
- S. Wieghold, A. S. Bieber, Z. A. VanOrman, L. Daley, M. Leger, J. P. Correa-Baena and L. Nienhaus, Matter, 2019, 1, 705–719 CrossRef CAS.
- V. Gray, J. R. Allardice, Z. Zhang and A. Rao, Chem. Phys. Rev., 2021, 2, 031305 CrossRef.
- W. Zhao and F. N. Castellano, J. Phys. Chem. A, 2006, 110, 11440–11445 CrossRef CAS PubMed.
- P. Duan, N. Yanai and N. Kimizuka, Chem. Commun., 2014, 50, 13111–13113 RSC.
- X. Jiang, X. Guo, J. Peng, D. Zhao and Y. Ma, ACS Appl. Mater. Interfaces, 2016, 8, 11441–11449 CrossRef CAS PubMed.
- V. Gray, P. Xia, Z. Huang, E. Moses, A. Fast, D. A. Fishman, V. I. Vullev, M. Abrahamsson, K. Moth-Poulsen and M. L. Tang, Chem. Sci., 2017, 8, 5488–5496 RSC.
- K. Okumura, N. Yanai and N. Kimizuka, Chem. Lett., 2019, 48, 1347–1350 CrossRef CAS.
- S. He, X. Luo, X. Liu, Y. Li and K. Wu, J. Phys. Chem. Lett., 2019, 10, 5036–5040 CrossRef CAS PubMed.
- B. Pfund, D. M. Steffen, M. R. Schreier, M. S. Bertrams, C. Ye, K. Börjesson, O. S. Wenger and C. Kerzig, J. Am. Chem. Soc., 2020, 142, 10468–10476 CrossRef CAS PubMed.
- S. Hisamitsu, J. Miyano, K. Okumura, J. K. H. Hui, N. Yanai and N. Kimizuka, ChemistryOpen, 2020, 9, 14–17 CrossRef CAS PubMed.
- Y. Kawashima, H. Kouno, K. Orihashi, K. Nishimura, N. Yanai and N. Kimizuka, Mol. Syst. Des. Eng., 2020, 5, 792–796 RSC.
- T. N. Singh-Rachford and F. N. Castellano, J. Phys. Chem. A, 2009, 113, 5912–5917 CrossRef CAS PubMed.
- J. Peng, X. Guo, X. Jiang, D. Zhao and Y. Ma, Chem. Sci., 2016, 7, 1233–1237 RSC.
- N. Yanai, M. Kozue, S. Amemori, R. Kabe, C. Adachi and N. Kimizuka, J. Mater. Chem. C, 2016, 4, 6447–6451 RSC.
- Q. Chen, Y. Liu, X. Guo, J. Peng, S. Garakyaraghi, C. M. Papa, F. N. Castellano, D. Zhao and Y. Ma, J. Phys. Chem. A, 2018, 122, 6673–6682 CrossRef CAS PubMed.
- H. L. Lee, M. S. Lee, H. Park, W. S. Han and J. H. Kim, Korean J. Chem. Eng., 2019, 36, 1791–1798 CrossRef CAS.
- D. Han, X. Yang, J. Han, J. Zhou, T. Jiao and P. Duan, Nat. Commun., 2020, 11, 5659 CrossRef CAS PubMed.
- Y. Murakami, A. Motooka, R. Enomoto, K. Niimi, A. Kaiho and N. Kiyoyanagi, Phys. Chem. Chem. Phys., 2020, 22, 27134–27143 RSC.
- T. J. B. Zähringer, M. S. Bertrams and C. Kerzig, J. Mater. Chem. C, 2022 10.1039/d1tc04782e.
- N. Harada, Y. Sasaki, M. Hosoyamada, N. Kimizuka and N. Yanai, Angew. Chem., Int. Ed., 2021, 60, 142–147 CrossRef CAS PubMed.
- L. Hou, A. Olesund, S. Thurakkal, X. Zhang and B. Albinsson, Adv. Funct. Mater., 2021, 2106198 CrossRef CAS.
- D. Huang, J. Sun, L. Ma, C. Zhang and J. Zhao, Photochem. Photobiol. Sci., 2013, 12, 872–882 CrossRef CAS PubMed.
- D. P. Specht, P. A. Martic and S. Farid, Tetrahedron, 1982, 38, 1203–1211 CrossRef CAS.
- J. B. Borak and D. E. Falvey, Photochem. Photobiol. Sci., 2010, 9, 854–860 CrossRef CAS PubMed.
- R. Dong, K. K. Chen, P. Wang, N. Zhang, S. Guo, Z. M. Zhang and T. B. Lu, Dyes Pigm., 2019, 166, 84–91 CrossRef CAS.
- P. S. Song and K. J. Tapley, Jr., Photochem. Photobiol., 1979, 29, 1177–1197 CrossRef CAS PubMed.
- J. J. Serrano-Pérez, M. Merchán and L. Serrano-Andrés, Chem. Phys. Lett., 2007, 434, 107–110 CrossRef.
- S. Aloïse, C. Ruckebusch, L. Blanchet, J. Réhault, G. Buntinx and J. P. Huvenne, J. Phys. Chem. A, 2008, 112, 224–231 CrossRef PubMed.
- M. Hussain, J. Zhao, W. Yang, F. Zhong, A. Karatay, H. G. Yaglioglu, E. A. Yildiz and M. Hayvali, J. Lumin., 2017, 192, 211–217 CrossRef CAS.
- M. Koharagi, N. Harada, K. Okumura, J. Miyano, S. Hisamitsu, N. Kimizuka and N. Yanai, Nanoscale, 2021, 13, 19890–19893 RSC.
- Y. Zhou, F. N. Castellano, T. W. Schmidt and K. Hanson, ACS Energy Lett., 2020, 5, 2322–2326 CrossRef CAS.
- J. C. de Mello, H. F. Wittmann and R. H. Friend, Adv. Mater., 1997, 9, 230–232 CrossRef CAS.
- N. Yanai, K. Suzuki, T. Ogawa, Y. Sasaki, N. Harada and N. Kimizuka, J. Phys. Chem. A, 2019, 123, 10197–10203 CrossRef CAS PubMed.
- A. Monguzzi, J. Mezyk, F. Scotognella, R. Tubino and F. Meinardi, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 195112 CrossRef.
- Y. Y. Cheng, T. Khoury, R. G. C. R. Clady, M. J. Y. Tayebjee, N. J. Ekins-Daukes, M. J. Crossley and T. W. Schmidt, Phys. Chem. Chem. Phys., 2010, 12, 66–71 RSC.
- A. Haefele, J. Blumhoff, R. S. Khnayzer and F. N. Castellano, J. Phys. Chem. Lett., 2012, 3, 299–303 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, DFT calculations, absolute and relative UC efficiencies, UC emission and transient absorption decays, parameters related to UC efficiency, fluorescence spectra, and threshold excitation intensity. See DOI: 10.1039/d1tc05526g |
| This journal is © The Royal Society of Chemistry 2022 |