Open Access Article
Open Access ArticlePOPs in Antarctic ecosystems: is climate change affecting their temporal trends?
Simonetta Corsolini *a and
Nicoletta Ademollob
*a and
Nicoletta Ademollob
aDepartment of Physical, Earth and Environmental Sciences, University of Siena, Via P. A. Mattioli, 4, I-53100 Siena, Italy. E-mail: simonetta.corsolini@unisi.it
bInstitute of Polar Sciences of the Italian National Research Council, (ISP-CNR), Strada Provinciale 35d, km 0.7, 00010 Montelibretti, Roma
First published on 24th August 2022
Abstract
Climate change is affecting Antarctica and the Southern Ocean and effects have been already reported for the abiotic compartments of the ecosystems, e.g. ice loss and iceberg calving. Global warming can alter also the distribution of persistent organic pollutant (POPs) both at a global scale and in the Antarctic Region, due to their physical–chemical characteristics. Effects of climate changes have been already reported on feeding behaviour and reproductive process of organisms. Another consequence for organisms includes the POP bioaccumulation. Here we review the literature reporting the linkage between recorded effects of climate changes and POP bioaccumulation in resident marine Antarctic species (fish and penguins). Notwithstanding Antarctica is a final sink for persistent contaminants due to the extreme cold climate, a general decreasing POP trend has been observed for some POPs. Their concentrations in biota are reported to be linked to ice melting and large iceberg calving; the peculiar marine Antarctic ecosystems and the pelagic-benthic coupling may also contribute to alterations in the bioaccumulation processes. These effects are similar in polar regions, although the comparison with the Arctic biota is not possible due to the lack of data in the Antarctic Region. It remains an open question if the POP amount accumulated in the Antarctic ecosystems is decreasing or not.
Environmental significanceThe climate crisis is affecting the polar regions more than other areas and their ecosystems are particularly vulnerable due to their low resilience. Changes may affect the trophic web structure and functioning with consequences for persistent organic pollutant bioaccumulation. In the Arctic, these events have already been observed and bioaccumulation in organisms has been correlated to climate parameters. Very few data exist for the Antarctic Region; this highlights the urgent need of a pan-Antarctic collection of data to comprehend and predict how the climate crisis may affect these fragile marine ecosystems and their biota in a changing world. Future research and monitoring initiatives for Antarctica should take relevant findings on both polar regions into consideration. |
1. POPs in Antarctica and the Southern Ocean
1.1 Preface
The Arctic Monitoring and Assessment Programme (AMAP) prepared an Assessment (in 2019–2020) titled AMAP Assessment 2020: POPs and Chemicals of Emerging Arctic Concern: Influence of Climate Change, which was published in 2021.1 The aim of this AMAP assessment was to review the recorded and predicted changes in levels and trends of Persistent Organic Pollutants (POPs) and Contaminants of Emergent Arctic Concern (CEAC) in the Arctic environment. The analyses focused on physical and ecological changes occurring under warming conditions in the Arctic and included some additional information from other cold regions. This review on the influence of climate change on POPs in the Antarctic ecosystems originates from this AMAP Assessment. The comparison between observations reported in the Arctic and Antarctic regions was included in the AMAP assessment because it can provide a global perspective on the effects of climate change on contaminant trends in polar regions, that have different remote sources and transport pathways of contaminants.1.2 POPs in Antarctica and the Southern Ocean
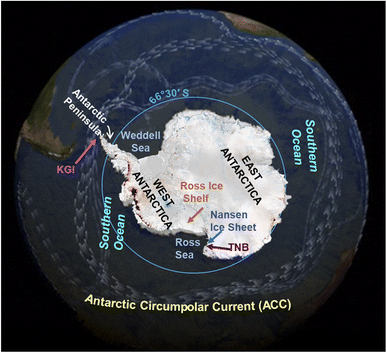
The polar regions are sensitive areas exposed to changes resulting from global warming. The transport of persistent organic pollutant (POPs) to the Arctic and their cycling can be altered by the climate changes2 and the same can be predicted in Antarctica. However, it must be considered that the human impacts on Antarctica and the Southern Ocean are still limited respect to the Arctic due to their remoteness and extreme climate, which make very difficult and challenging to live and work there.The geography and oceanography of Antarctica and the Southern Ocean are responsible of the POP transport in the region. The presence of POPs in the Antarctic continent and Southern Ocean is mainly due to the long range atmospheric transport (LRAT3,4), as the Antarctic Circumpolar Current (ACC; 50°S–60°S, Fig. 1) acts as a natural northern boundary between the cold seawaters of the Southern Ocean and the warmer seawaters of the Pacific, Indian and Atlantic Oceans. At this boundary, the cold Antarctic seawaters sink beneath the warmer sub-Antarctic waters of the other oceans instead of mixing in the upper water column layers, as they show different physical–chemical characteristics (temperature, density). Therefore, seawaters from the other oceans take a long time before reaching and mixing to the Antarctic ones.
The remoteness of the Antarctic Region is supposed to cause a delay in the POP transport and bioaccumulation when compared to the Arctic: while POPs reach the Arctic in a few days or weeks by air and ocean transport,5 they can take months or years before reaching the Antarctic region. Moreover, Antarctica is a remote, inhabited cold desert where only scientific stations are settled and no POP production have never occurred; local emissions are from scientific stations, e.g. flame retardants, building materials, combustion by-products,6,7 electric and electronic parts, hydraulic fluids, textiles. The long distance of the region from any POP production areas and the limited human presence and activity (scientific stations, tourism, fishing) make the long range atmospheric transport (LRAT) the most important contamination source.
The Antarctic Treaty (AT, signed in Washington on 1 December 1959) protects and controls all human activities in the Antarctic continent and the Southern Ocean and aims to guarantee “the interest of all mankind that Antarctica shall continue forever to be used exclusively for peaceful purposes and shall not become the scene or object of international discord” (https://www.ats.aq/). The Protocol on Environmental Protection to the Antarctic Treaty (signed in Madrid on 4 October 1991) designates Antarctica as a “natural reserve, devoted to peace and science”, where all activities are subject to regulations and the exploitation of mineral or other resources is prohibited; only scientific research is permitted (https://www.ats.aq/). The consequence of these international efforts to protect the Antarctic Region have preserved it from POPs contamination, which is recognized to be among the lowest on Earth. However, the detection of a number of contaminants in the Antarctic ecosystems make the region no longer pristine; the human impacts are expected to increase in the near future due to intensifying scientific, touristic, and fishing activities. In the Antarctic Peninsula, the global and local human impacts have transgressed the barriers isolating the continent from the rest of the World and have caused an acceleration of previously observed changes.8
Increasing temperatures and changes in precipitation pattern are among the abiotic parameters that most influence the fate and transport of persistent contaminants: it was reported that the air surface temperature has increased 3–7 °C since the 1950s9–11 in the Antarctic Region, mostly in West Antarctica. The satellite observations have confirmed the retreat,12,13 thinning,14,15 and disintegration16 of ice shelves in Antarctica. The global warming does not affect the continental and marine ice at the same level everywhere and the ice losses in West and East Antarctica is different: it increases dramatically in the first, while it is less evident or show an opposite trend in the second one.17 However, a dramatic and alarming event occurred in March 2022 in East Antarctica: the region was hit by an anomalous heatwave with temperatures reaching a record of −11.8 °C on March 18th at the scientific Concordia Station (average temperatures in March: −48 °C ca.).18 In the same period, the Conger Ice Shelf calved in East Antarctica coast; the cause of this collapse is not clear yet, but global warming is likely a contributing factor.18
The scientific literature on the relationships between global change and contamination was reviewed for instance by Nadal et al.,19 but no specific mention was reported for the Antarctic region; these authors predicted that global change would alter POP concentrations within a factor of 2–3. Other studies estimated the consequences of a temperature increase in Antarctica: for instance, a climate change-related increase of 1 °C in air temperature was estimated to increase the Antarctic atmospheric burden of PCBs by 21–45%. In addition, a concurrent increase of 0.5% of solid organic matter will counteract the influence of warming by reducing the POP fugacity in soil. A 1 °C increase in the Antarctic temperatures would induce a 25% increase of soil-vegetation organic carbon and associated POPs, making it a sink of POPs. Therefore, it is supposed that an amount of up to 70 times more POPs than the amount remobilized to the atmosphere will be trapped.20 Casal et al.21 demonstrated that sea spray aerosols can be an important source of perfluoroalkylated substances (PFASs) to Antarctic marine environments: PFAS scavenged during snow deposition and scavenging sea-salt aerosol were reported as important input of PFCAs to the Maritime Antarctica.
The Antarctic marine ecosystems are fragile, meaning that even small alterations can cause extreme changes;22 they also show low resilience. In this context, the climate crisis can have a devastating impact on the Antarctic ecosystems in a future changes-driven scenario. The overall effects on Antarctic biota are difficult to predict and assess, but consequences on the POP release trapped in the ice, their distribution and bioaccumulation are expected. Then it is important to monitor the POP contamination in relation to measurable changes of climatic parameters.
These observed or predicted changes may affect the POP distribution in the ecosystems and their bioaccumulation. The climate crisis may have an effect on the food web structure and an impact on the availability of food resources; these alterations may shift the trophic position of organisms and consequently the POP bioaccumulation pattern.23 Changes of Antarctic seabird diet, distribution, and population dynamics were already observed in long-term studies24 and these modifications may lead to a shift in foraging or breeding distributions, behaviour or other adaptations, decline or extirpation of populations.24 For instance, it was reported that contaminants are significantly related to hormonal secretion in snow petrels and parental care is affected by hormone levels: the mercury (Hg) concentrations were higher in males that neglected their egg25. These authors suggested that in these Antarctic seabirds, the exposure to legacy POPs and Hg could make birds more susceptible to environmental stressors, despite they were not evaluated or correlated. The effects of changes in the POP distribution in trophic webs could be not easily and promptly detected due to the geographic isolation of the Southern Ocean, where contamination events are delayed respect to the Arctic.23
In other regions of the Earth, studies have shown the effect of some extreme events, such as floods and droughts, on the remobilization and bioaccumulation of POPs.19 In Antarctic terrestrial ecosystems, warming-related increases in vegetation26 and changes in soil chemistry (i.e. increased dissolved organic carbon)20 may increase the capacity of soil to sequester POPs.27 This in turn can result in greater inputs of contaminants into aquatic systems from the re-mobilization of contaminants in soils28 through increased glacial melt projected under future climate scenarios.
The global climate change can also affect the accumulation and release of POPs in polar regions and those trapped in the ice may be released because of ice melting: in the marine environment, the input of POPs can increase with pack ice and glacier increasing melting:29 Geisz et al.29 (2008) speculated that 1–4 kg year−1 of ∑DDTs have been released into the Antarctic marine environment due to glacier ablation. Xavier et al.24 overviewed the latest physical and biological modifications recorded in Antarctica under changing climate with a special focus on Antarctic seabirds. They reported that long-term datasets indicate rapid warming of some regions of Antarctica and Southern Ocean where the area of cold water is reducing its extension because of southward shifts of oceanic fronts; moreover, sea-ice extent and atmospheric conditions are increasing their variability.
Besides spatial differences, exposure scenarios for pelagic and benthic compartments of the marine environment may also vary. The pelagic-benthic coupling of the marine ecosystems includes fundamental processes that allow the exchange of energy and mass between the pelagic and benthic zones.23 The pelagic domain can be affected by POP inputs through ice melting and water–air exchange and the benthic one, which receives nutrients from the upper water column, may depend on those events occurring at the sea surface. These exchanges between the pelagic and benthic ecosystems are also dependent on the seasonal sea-ice dynamics. The POP distribution in the marine pelagic and benthic trophic webs may be difficult to predict under different climatic conditions due to changes in the ecosystem functioning.30
The first detection of POPs in the Antarctic biota dates back to the 1960s when DDT was first documented in Adèlie penguins (Pygoscelis adèliae) and a crabeater sea (Lobodon carcinophaga),31 and PCBs were detected in petrels.32 Since then, an increasing number of articles have reported the presence of POPs in the ecosystems of the Antarctic continent and Ocean.28,33 Unfortunately, only very few studies report also the climate parameters and correlations to contaminant concentrations. For instance, Bhardwaj et al. reviewed the scientific literature on the presence of POPs in the Antarctic environment, but no associations to climate parameters were highlighted.34 Another article reports the annual cycle of POP concentration in an Antarctic planktonic communities, the potential implications for biomagnification, and the direct effects of increasing temperature on the redistribution of HCB under changing climate,35 but no association to climate parameters were assessed.
1.3 Approach
This review was conducted as part of the AMAP Assessment “POPs and Chemicals of Emerging Arctic Concern (CEACs): Influence of climate change”.1 This article is based on peer-reviewed articles, which were known to the authors until April 2022. Many articles reports the concentration of POPs in Antarctic ecosystems, but only very few report time series of POP concentrations in associations with biological and/or physical parameters indicative of climate change. Thus, we selected and discussed those articles relevant to the aims of both the AMAP Assessment and the themed issue of Environmental Science: Processes & Impacts titled POPs and Chemicals of Emerging Arctic Concern: Influence of Climate Change.The presence of POPs in the Antarctic ecosystems have been reported in an increasing number of articles since the 1960s, but their number is still low when compared to the literature on the Arctic contamination. According to Web of Science, since the beginning of polar research on contamination, only 500 articles were published on the contamination in the Antarctic Region, and 10 of them were review articles, while the literature on the Arctic includes more than 2000 articles, with more than 50 reviews among them.
The articles on the presence of POPs, time series and correlation to climate parameters are lacking for Antarctic environments. Thus, we considered for this review, only peer-reviewed articles that linked contaminant concentrations and climate parameters; due to their very limited number, the link to biological parameters was also taken into consideration. We selected articles on marine fish and Adèlie penguins because these species are native to Antarctic marine environment and thus suitable to study temporal changes of contaminant exposure in this region.
2. POP monitoring and time series
2.1 Introductory remarks
Organisms react to climate changes modifying their ecology (e.g. feeding habits and grounds, reproduction timeline and success36–41). For instance, the Colobanthus quitensis and Deschampsia antarctica (phanerogams which only grow in West Antarctica) have increased their seed maturation and germination, and seedling survival in response to a warming trend of air temperatures during Summer; this trend was evident in the region since the late 1940s.42 Regarding the fauna, climate change may affect the structure of trophic webs and the availability of prey, thus they may feed on different items. A shift in the diet may cause different POP bioaccumulation and these variations can be related to climate changes when physical characteristics of the environment and weather conditions are recorded and correlated to POP concentrations. For instance, the POP temporal trend in zooplankton from the Nordic countries was reported to be affected by abiotic factors43 such as an earlier ice breakup or the lack of ice cover.After the calving of the Nansen Ice Shelf (Ross Sea) in 2016, the foraging ground of Adèlie penguins (Fig. 2) changed and penguins fed in this newly accessible sea area.41 A linkage between food item, ice coverage, foraging range, and POP contamination in Adèlie penguins from a rookery in the Ross Sea (Fig. 1) was already reported in the 1990s.44 Here, a sequence of events occurred in the 1995/96 summer season: the ice melted completely very early in the season and penguins during the rearing chick period foraged nearer to the shore to save energy instead of swimming offshore for feeding on krill. The penguin diet during the rearing period is based on krill, which are rich in lipids and thus highly energetic for chicks. During that season, the penguins fed mainly on fish and other food items; higher lipophilic POP concentrations were found in specimens whose stomach contents were richer in krill. This article highlighted how a changed environment affected the functioning of the trophic web and then the POP bioaccumulation;44 unfortunately, concentrations were not directly correlated to the climate parameters.
The sea ice coverage in polar regions is a key issue for those species whose reproduction and feeding behaviour are strictly linked to it.44 Altering oceanographic conditions, climate change affects penguin populations that preferentially use foraging areas where prey is abundant and predictable.45 In particular, altered environmental conditions can affect the arrival, body weight and nutritive status before breeding or moulting, and the timing of egg laying, egg size, breeding success and ultimately the survival of individuals. Starvation due to a modified environment and food resources availability may be another cause of re-contamination from body lipid resources.40 The climate-driven changes do not affect directly contamination in penguins, but the modification of the environment, foraging behaviour, and diet can affect the POP bioaccumulation processes.
Comparisons between observations reported in the Arctic and Antarctic can provide a global perspective on the characteristics of climate change effects on contaminant trends in polar regions, also because the two areas have different remote contaminant sources and transport pathways. Only a few studies have explored correlations between POP temporal trends and climate parameters in Antarctic abiotic compartments27 and biota, such as fish46 and penguins.29 Climate change may affect the structure of Antarctic trophic webs and the availability of prey in the same way as described for the Arctic, and thus could have similar consequences on POP exposure and bioaccumulation in Antarctic ecosystems.
2.2 Terrestrial and freshwater ecosystems
In Antarctica, terrestrial ecosystems cover only 0.34%47 of the continent currently without ice, seasonally or permanently. Limiting factors such as very low temperature, extreme dryness (very limited availability of liquid water), strong winds, and long winter darkness lead to a low level of biodiversity. Organisms (moss, lichens, algae, yeasts, bryophytes, invertebrates48) are extremely adapted to life in environments permanently or seasonally free from ice as soil, freshwater lakes, ponds, streams, and even rocks (cryptoendolithic communities49).Soil and pond sediment samples have been included in several studies on the POP contamination of the Antarctic regions (e.g.6,50–52), although an analysis of a temporal trend in association with climate change still lacks in the literature for these environments. Only an article reports the correlations between climate parameters and contamination in a terrestrial ecosystem of Livingston Island (West Antarctica).27 The authors suggested that the remobilization of PCBs is driven by changes in temperature and soil content of organic matter; they suggested that the current and future POP remobilization and sinks in Antarctica, besides warming, are a function of the close coupling of climate change and carbon cycling.27
Sun et al.53 studied the accumulation of chlorinated pesticides in lake cores collected from Niudu Lake in King George Island (West Antarctica): they analyzed lake sediment cores with and without glacier meltwater input, and found that the DDT accumulation flux showed an anomalous peak in the lake with glacier meltwater input. In the core sediments of the lake without glacier meltwater input, the DDT accumulation flux showed a gradually declining trend after the peak in the 1960s. These authors attributed this difference in DDT flux profiles between the two lake cores to regional climatic warming and the subsequent discharge of DDT stored in the Antarctic ice sheet into lakes.54 The Authors ascribed this increase to the glacier meltwater derived from the regional warming from the early 1970s, which contributed to the release into the lakes of the pesticides trapped in the ice cap. Potapowicza et al.55 reviewed the literature on the influence of permafrost degradation on the contaminant distribution and re-emission in Antarctica; the reviewed data allowed them to assume that the permafrost may constitute a sink for organic and inorganic contaminants. Permafrost in Antarctica is less extended than in the Arctic, but its degradation can contribute to remobilize trapped contaminants. Taking into account that penguins nest in deglaciated areas with permafrost, the release of contaminants may affect directly the terrestrial and marine ecosystems.
Corsolini et al.6 reported correlations with some climate parameters in pond soil from the Victoria Land, although the temporal trend was not investigated. In this study, a trend respect to wind direction was observed, in agreement with data reported for PCBs in air samples from the same area,56 where the highest levels were found in the theoretically less polluted site (Nansen Ice Shelf; Fig. 1), where air masses come mainly from the Antarctic continent.51,56–58 These findings suggest a potential influence of climate on the distribution of POPs in the Antarctic environments.
In the Arctic, available data on temporal trends, or effects of climate change on POP trends, show a significant decrease over time for many POPs in Greenland freshwater fish.59 A comparison to Antarctic freshwater fish is not possible as no fish are present in continental waters.
There is an urgent need to intensify studies on the temporal trend and climate change in these environments, which are then vulnerable to human impacts, as many scientific stations are located in free-ice areas with permafrost.
2.3 Marine environment
In order to compare with and complement observations in the Arctic, the following discussion focuses on POP temporal trends in marine fish and Adèlie penguins. These species are native to Antarctic marine environment and thus suitable to study temporal changes in contaminant exposure in this region. The Tables 1–4 report the concentrations of POPs in the most studied fish and penguin species. Among POPs, we selected PCBs, p,p′-DDT, p,p′-DDE, DDTs, and HCB since other chemicals, including emergent contaminants, were reported only in few articles.| Authors | YoS | Tissue | Site | PCBs | p,p′-DDE | DDTs | HCB |
|---|---|---|---|---|---|---|---|
| a Not specified. | |||||||
| Subramanian et al.79 1983 | 1981 | Whole body | Syowa Station | 0.12–0.24 | 0.5–0.9 | ||
| Subramanian et al.80 1984 | 1981 | Muscle | Syowa Station | 0.25 | 0.63 | ||
| Focardi et al.68 1992 | 1989 | Muscle | TNB | 12.8 | 4 | 0.27 | |
| Focardi et al.68 1992 | 1989 | Liver | TNB | 186 | 67 | 3.4 | |
| Larsson et al.81 1992 | 1989 | Liver | Ross Sea | 0.07* | 0.01* | ||
| Focardi et al.82 | 1992 | Liver | TNB | 21 | |||
| Corsolini et al.83 2003 | 1999 | Muscle | TNB | 6.35 | 0.16 | 2.6 | |
| Corsolini et al.61 2006 | 2001 | Muscle | TNB | 6.35 | 2.53 | 8.6 | 1.44 |
| Borghesi et al.62 2009 | 2005 | Muscle | TNB | 1.65 | |||
| Cincinelli et al. 46 2016 | 2010 | Muscle | TNB | 0.08 | 0.43 | 0.12 | |
| Wolschke et al.84 2015 | 2010 | Musclea | KGI | 102 | |||
| Species | Authors | YoS | Tissue | Site | PCBs | p,p′-DDE | DDTs | HCB |
|---|---|---|---|---|---|---|---|---|
C. gunnari![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
Weber et al. 65 2003 | 1987 | Liver | Elephant Island | 4* | 26 | ||
| Weber et al. 65 2003 | 1996 | Liver | Elephant Island | 5* | 20.4 | |||
| G. gibberifrons | Weber et al. 65 2003 | 1987 | Liver | Elephant Island | 3.7* | 22.6 | ||
| Weber et al. 65 2003 | 1996 | Liver | Elephant Island | 7.5* | 18.6 | |||
| C. aceratus | Weber et al. 65 2003 | 1987 | Liver | Elephant Island | 7.2* | 17 | ||
| Weber et al. 65 2003 | 1996 | Liver | Elephant Island | 14.5* | 15.5 | |||
| C. hamatus | Focardi et al.68 1992 | 1989 | Liver | Ross Sea | 202** | 49** | 8.4** | |
| Focardi et al.68 1992 | 1989 | Muscle | Ross Sea | 9.5** | 3.3** | 0.61** | ||
| Focardi et al.82 1995 | 1992 | Liver | Ross Sea | 36 | ||||
| Kumar et al.85 2002 | 1995 | Muscle | Ross Sea | 0.35 | ||||
| Corsolini et al.86 2002 | 1995 | Muscle | Ross Sea | 8.4 | 0.2 | 1.25 | ||
| Borghesi et al.62 2009 | 2005 | Muscle | TNB | |||||
| Strobel et al.67 2018 | 2015–2016 | Muscle | Weddell Sea | 427 | 73 | 140 |
A study on POP temporal trends in the muscle of emerald rockcod (Trematomus bernacchii) (Fig. 3) from the Ross Sea over a 30 year period from the early 1980s to 2011 observed two elevated concentration peaks within generally decreasing trends of POPs46,60 (Table 1). These concentration peaks occurred in 2001 and 2005 for PCBs, and in 2005 for p,p′-DDE and PBDEs.46,61,62 The iceberg B15 calved from the Ross Ice Shelf at the beginning of 2000. This iceberg broke into several pieces in 2000, 2002 and 2003![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 63 and it was suggested as the possible source of POPs which were trapped in it and then released into seawater contributing to the higher POP concentrations detected in fish.46 These observations provide an example of the potential effects of warming-induced polar ice melt and collapse on POP concentration trends in biota. In the Arctic, observations of relationships between climate and POP trends in fish are limited to freshwater species where associations have been found between POP concentrations and climate oscillation indices, temperature, precipitation and consequences of permafrost thaw. The direct effects of iceberg calving and melting on POP availability to marine fish have not been studied in the Arctic, although such effects are plausible. However, the POP concentrations in the entire trophic web over time were reported to change after the retreatment of tidewater glaciers.64
63 and it was suggested as the possible source of POPs which were trapped in it and then released into seawater contributing to the higher POP concentrations detected in fish.46 These observations provide an example of the potential effects of warming-induced polar ice melt and collapse on POP concentration trends in biota. In the Arctic, observations of relationships between climate and POP trends in fish are limited to freshwater species where associations have been found between POP concentrations and climate oscillation indices, temperature, precipitation and consequences of permafrost thaw. The direct effects of iceberg calving and melting on POP availability to marine fish have not been studied in the Arctic, although such effects are plausible. However, the POP concentrations in the entire trophic web over time were reported to change after the retreatment of tidewater glaciers.64
Disregarding the correlation to climate parameters, stable or slightly increasing POP levels have been reported in benthic feeding Antarctic fish, such as the humped rockcod (Gobionotothen gibberifrons) and blackfin icefish (Chaenocephalus aceratus),33 whereas stable or decreasing concentrations occurred in the more pelagic Antarctic silverfish (Pleuragramma antarctica)44 and mackerel icefish (Champsocephalus gunnari)65,66 (Table 2). Data on PCBs and DDT in icefish (C. aceratus and C. gunnari) collected around the Antarctic Peninsula (Table 2 and Fig. 1) have shown an increasing trend since the 1990s, while other POPs (e.g. hexachlorobenzene, HCB), have shown a stable or slightly decreasing trend.67 Benthic fish collected between 1988 and 1996 from Terra Nova Bay (TNB, Ross Sea) (Fig. 1) also showed increasing concentrations of polychlorobiphenyls (PCBs), p,p′-DDE and HCB.44,68 However, it is not known if these recently increasing trends are related to climate change in any direct or indirect way. Lana et al.69 analyzed POP data from seventeen fish species from four Antarctic regions: Antarctic Peninsula, Weddell Sea, Ross Sea, Adelie Land (Fig. 1). They suggested that PCB burdens in the Antarctic region had not yet reached a steady state, probably because the environmental reservoirs of POPs, that continue to exist after primary emissions, have declined, and may be remobilized by warmer conditions due to climate change.27
The decreasing concentrations of POPs observed in Antarctic pelagic fish over time generally corresponds with declining trends observed in pelagic-feeding Antarctic seabirds, such as Adèlie penguins30 (Fig. 2). This is evident for the development of PCB concentrations since the 1990s, but not equally clear for DDTs, increasing again in the 2000s70 (Tables 3 and 4). These contrasting trends can be ascribed to the exchange of organic contaminants between the pelagic and benthic environments that is especially efficient in the Antarctic region because of its close relation to sea ice dynamics. The transport of organic matter from the water–ice interface and pack ice may be affected by climate change: more ice will melt, contributing to the release of entrapped organic matter to the water column, more lipophilic contaminants will be released to the underlying waters from where they can fall down to the benthic environment and community. Moreover, benthic organisms have limited capability to metabolize these contaminants.68,71
| Authors | YoS | Tissue | Site | PCBs | p,p′-DDT | p,p′-DDE | DDTs | HCB |
|---|---|---|---|---|---|---|---|---|
| Sladen et al.31 1966 | 1964 | Fat | Ross Sea | 23 | 52 | 77 | ||
| George et al.87 1966 | 1964 | Fat | Ross Sea | 0.17 | ||||
| Luke et al.88 1989 | 1981 | Egg | Davis Station | 5 | 20 | |||
| Subramanian et al.79 1986 | 1981 | Fat | Syowa Station | 39.5 | 228 | |||
| Court et al.89 1997 | 1989 | Egg | Cape Bird | 8.8 | 6.3 | 7.1 | 5.6 | |
| Lara et al.90 1990 | 1989 | Blood | KGI | 14 | 1.2 | 0.45 | ||
| Focardi et al.82 1995 | 1992 | Liver | Ross Sea | 101 | ||||
| Wanwimolruk et al.91 1999 | 1993 | Egg | KGI | 0.25 | 0.25 | 4 | 4.5 | 13.5 |
| Corsolini et al.86 2002 | 1995 | Egg | Ross Sea | 2.8 | 0.1 | 0.1 | ||
| Corsolini et al.61 2006 | 1995 | Egg | TNB | 24.9 | 20.7 | 23 | 18.7 | |
| Kumar et al.85 2002 | 1995 | Egg | Ross Sea | 3.3 | ||||
| Corsolini et al. 70 2011 | 1995–2001 | Egg | Ross Sea | 21.99 | 4.74 | |||
| Corsolini et al. 70 2011 | 1995–2001 | Egg | KGI | 12.03 | 0.49 | 18.95 | 7.64 | |
| Corsolini et al.92 2017 | 2001 | Blood | Ross Sea | 2.59 | 0.757 | 0.371 | ||
| Schiavone et al. 200993 | 2004 | Egg | KGI | 12 | 23 | 7.6 | ||
| Cipro et al.94 2010 | 2005 | Egg | KGI | 32.5 | 6.29 | 22.1 | ||
| Rudolph et al.95 2016 | 2009 | Feces | Kopaitic Is | 12.93 | ||||
| Jara-Carrasco et al.96 2017 | 2011 | Feces | Base O'Higgins; KGI | 1.61 | 1.61 | 0.53 |
| Authors | YoS | Tissue | Site | PCBs | p,p′-DDT | p,p′-DDE | DDTs | HCB |
|---|---|---|---|---|---|---|---|---|
| Risebrough et al.32 1968 | 1967 | Egg | Ross Sea | 6 | 0.128 | |||
| Risebrough et al.97 1976 | 1975 | Egg | Doumer Is | 0.07 | 0.126 | |||
| Schneider et al.98 1985 | 1981 | Fat | Weddell Sea | 346 | 38 | 361 | 232 | |
| Court et al.89 1997 | 1989 | Egg | Cape Bird | 37.3 | 26.9 | 29.9 | 22.5 | |
| Focardi et al.99 1993 | 1991–92 | Muscle | TNB | 2281 | 810 | 349 | ||
| van den Brink et al.100 1998 | 1993 | Blood | Davis Station | 286 | 123 | 372 | ||
| Inomata et al.101 1996 | 1991–1993 | Preen oil | AP | 895 | 401 | 1137 | ||
| van den Brink et al.102 1997 | 1994 | Preen oil | Hop Is | 469 | 620 | |||
| van den Brink et al. 30 2011 | 1993/1994 | Preen oil | Hop Island | PCB153 = 69 | 93 | 232 | ||
| van den Brink et al. 30 2011 | 2003/2004 | Preen oil | Hop Island | PCB153 = 2 | 136 | |||
| Geisz et al. 29 2008 | 2004 | Egg | Palmer Station | 170 | ||||
| Geisz et al. 2008 | 2004 | Egg | Cape Royds | 158 | ||||
| Geisz et al. 29 2008 29 | 2005 | Egg | Palmer Station | 177 | ||||
| Kim et al.103 2015 | 2008–2009 | Muscle | KGI | 3.2 | 1.24 | 127 | 134 | |
| Mwangi et al.104 2016 | 2009 | Various tissues | Zhongshan Station | 144 |
Geisz et al.29 explored the potential effects of climate change on DDT levels in Adèlie penguins from the Antarctic Peninsula (Table 4 and Fig. 1). They found that ∑DDT concentrations in Adèlie penguin eggs from the Palmer Archipelago did not decrease from the 1970s to the 2000s, which is in contrast to the observations reported for thick-billed murres, northern fulmars, and black-legged kittiwakes in the Arctic during the same time period.29,72 The detection of p,p′-DDT in the penguins, despite the bans and restrictions on its use in the 1970s, suggests there may be a current source of this chemical to the Antarctic marine environment. A previous study indicated that there had been very little recent DDT deposition in Antarctica,73 although levels in Antarctic glacial meltwater were measurable.74 Thus, glacial meltwater was proposed as a possible source of DDT to the Antarctic marine food web. This hypothesis is supported by measurement-based estimates of the amount of DDT released annually from Antarctic glacial ablation (1–4 kg ∑DDT y−1).29
3. Conclusions and recommendations
Contrary to the Arctic, where the availability of long-term monitoring data allows the study of correlations between climate parameters and POPs in biota over time,75 data on POP concentrations in the Antarctic have been collected less systematically and climate parameters have not been recorded, except very few studies. In some Antarctic fish, POP concentrations have declined over the last two decades; however, these temporal trends appear to be weak and variable. Additionally, concentration peaks of PCBs, DDTs, and PBDEs observed in Antarctic fish were potentially related to the release of POPs after iceberg B15 calved from the Ross Ice Shelf. Comparisons between Arctic and Antarctic data can provide insight into the influence of global change on the distribution of POPs globally, and specifically in polar environments.The lack of data on the POP contamination and the link to climate parameters in the Antarctic make not possible both a comparison to the Arctic and general considerations on a global scale. This situation is due to the logistic difficulty to study in this Region, to the fragmentation of the research due to the lack of a pan-Antarctic research design. Despite the efforts on environment protection and on the study and monitoring of the climate change effects in the region,76 no internationally coordinated programs have been established to monitor the POP contamination in the Antarctic region. This anomaly respect to the Arctic might be due to the absence of resident human populations in the region that might keep low the interest in funding the long-term monitoring of POP contamination and effects in organisms. The need for standardized methods was already highlighted in the 1990s77 because comparable methods and regular monitoring are priority for the assessment of temporal trends. The Action Group on “Input pathways of persistent organic pollutants to Antarctica” (ImPACT, https://www.scar.org/science/impact/home/) of the Scientific Committee on Antarctic Research (SCAR, https://www.scar.org) was established (in 2018) with the aim of facilitating coordinated investigation and chemical monitoring; the United Nations Ocean Decade Action has also endorsed an Antarctic Monitoring & Assessment Program (AnMAP; https://www.oceandecade.org/actions/the-antarctic-monitoring-and-assessment-programme-anmap/). These initiatives aim to facilitate and promote coordinated research across Antarctica and the Southern Ocean, like in the Arctic, in order to collect data following standardized methods, which can allow comparisons and time trend assessment.
In general, data compiled from the Antarctic Region illustrates the potential effect of melting ice and glaciers on POP concentrations in biota; the available data need to be confirmed by further studies that should also include the record of and correlation to climate parameters. Antarctica is still a final sink for POPs due to the extreme cold climate and organisms, as reported previously, are still final receptors.30 The peculiar marine Antarctic ecosystems and the pelagic-benthic coupling leave still open the question whether the POP amount accumulated in the Antarctic ecosystems is decreasing or not, as concentration peaks can still be observed in biota.33,78 Future research and monitoring initiatives for the Arctic and Antarctica should take relevant findings to both polar regions into consideration.
Conflicts of interest
There are no conflicts to declare.References
- AMAP, AMAP Assessment 2020: POPs and Chemicals of Emerging Arctic Concern: Influence of Climate Change, Tromsø, Norway, 2021 Search PubMed.
- R. W. Macdonald, T. Harner and J. Fyfe, Recent climate change in the Arctic and its impact on contaminant pathways and interpretation of temporal trend data, Sci. Total Environ., 2005, 342, 5–86 CrossRef CAS PubMed.
- F. Wania, Assessing the potential of persistent organic chemicals for long-range transport and accumulation in polar regions, Environ. Sci. Technol., 2003, 37, 1344–1351 CrossRef CAS.
- S. M. Bengtson Nash, S. J. Wild, D. W. Hawker, R. A. Cropp, H. Hung, F. Wania, H. Xiao, P. Bohlin-Nizzetto, A. Bignert and S. Broomhall, Persistent Organic Pollutants in the East Antarctic Atmosphere: Inter-Annual Observations from 2010 to 2015 Using High-Flow-Through Passive Sampling, Environ. Sci. Technol., 2017, 51, 13929–13937 CrossRef CAS.
- AMAP, AMAP Assessment 2002: Persistent Organic Pollutants in the Arctic, accessed 22 December 2020, https://www.amap.no/documents/doc/amap-assessment-2002-persistent-organic-pollutants-in-the-arctic/96.
- S. Corsolini, D. Baroni, T. Martellini, N. Pala and A. Cincinelli, PBDEs and PCBs in terrestrial ecosystems of the Victoria Land, Antarctica, Chemosphere, 2019, 231, 233–239 CrossRef CAS.
- S. Corsolini, A. Metzdorff, D. Baroni, J. L. Roscales, B. Jiménez, E. Cerro-Gálvez, J. Dachs, C. Galbán-Malagón, O. Audy, J. Kohoutek, P. Přibylova, M. Poblete-Morales, R. Avendaño-Herrera, E. Bergami and K. Pozo, Legacy and novel flame retardants from indoor dust in Antarctica: Sources and human exposure, Environ. Res., 2021, 196, 110344 CrossRef CAS PubMed.
- A. Znój, K. Chwedorzewska, P. Androsiuk, M. Cuba-Diaz, I. Giełwanowska, J. Koc, M. Korczak-Abshire, J. Grzesiak and A. Zmarz, Rapid environmental changes in the western antarctic peninsula region due to climate change and human activity, Appl. Ecol. Environ. Sci., 2017, 5, 525–539 CrossRef.
- T. H. Jacka, W. F. Budd and A. Holder, A further assessment of surface temperature changes at stations in the Antarctic and Southern Ocean, Ann. Glaciol., 2004, 39, 331–338 CrossRef.
- J. Turner, S. R. Colwell, G. J. Marshall, T. A. Lachlan-Cope, A. M. Carleton, P. D. Jones, V. Lagun, P. A. Reid and S. Iagovkina, Antarctic climate change during the last 50 years, Int. J. Climatol., 2005, 25, 279–294 CrossRef.
- W. L. Chapman and J. E. Walsh, A Synthesis of Antarctic Temperatures, J. Clim., 2007, 20, 4096–4117 CrossRef.
- T. Scambos, H. A. Fricker, C. C. Liu, J. Bohlander, J. Fastook, A. Sargent, R. Massom and A. M. Wu, Ice shelf disintegration by plate bending and hydro-fracture: Satellite observations and model results of the 2008 Wilkins ice shelf break-ups, Earth Planet. Sci. Lett., 2009, 280, 51–60 CrossRef CAS.
- A. J. Cook and D. G. Vaughan, Overview of areal changes of the ice shelves on the Antarctic Peninsula over the past 50 years, Cryosphere, 2010, 4, 77–98 CrossRef.
- F. S. Paolo, H. A. Fricker and L. Padman, Volume loss from Antarctic ice shelves is accelerating, Science, 2015, 348, 327–331 CrossRef CAS PubMed.
- M. McMillan, A. Shepherd, A. Sundal, K. Briggs, A. Muir, A. Ridout, A. Hogg and D. Wingham, Increased ice losses from Antarctica detected by CryoSat-2, Geophys. Res. Lett., 2014, 41, 3899–3905 CrossRef.
- D. Francis, K. S. Mattingly, S. Lhermitte, M. Temimi and P. Heil, Atmospheric extremes caused high oceanward sea surface slope triggering the biggest calving event in more than 50 years at the Amery Ice Shelf, Cryosphere, 2021, 15, 2147–2165 CrossRef.
- A. E. Hogg, L. Gilbert, A. Shepherd, A. S. Muir and M. McMillan, Extending the record of Antarctic ice shelf thickness change, from 1992 to 2017, Adv. Space Res., 2021, 68, 724–731 CrossRef.
- The European Space Agency, ESA - Conger ice shelf collapses, accessed 1 June 2022, https://www.esa.int/ESA_Multimedia/Images/2022/03/Conger_ice_shelf_collapses.
- M. Nadal, M. Marquès, M. Mari and J. L. Domingo, Climate change and environmental concentrations of POPs: A review, Environ. Res., 2015, 143, 177–185 CrossRef CAS.
- P. Roberts, K. K. Newsham, R. D. Bardgett, J. F. Farrar and D. L. Jones, Vegetation cover regulates the quantity, quality and temporal dynamics of dissolved organic carbon and nitrogen in Antarctic soils, Polar Biol., 2009, 32, 999–1008 CrossRef.
- P. Casal, Y. Zhang, J. W. Martin, M. Pizarro, B. Jiménez and J. Dachs, Role of Snow Deposition of Perfluoroalkylated Substances at Coastal Livingston Island (Maritime Antarctica), Environ. Sci. Technol., 2017, 51, 8460–8470 CrossRef CAS PubMed.
- S. L. Hill, E. J. Murphy, K. Reid, P. N. Trathan and A. J. Constable, Modelling Southern Ocean ecosystems: krill, the food-web, and the impacts of harvesting, Biol. Rev. Cambridge Philos. Soc., 2006, 81(4), 581–608 CrossRef CAS PubMed.
- S. Corsolini, Antarctica and NE Greenland: Marine Pollution in a Changing World, in: Life Below Water, Encyclopedia of the UN Sustainable Development Goals, ed. W. Leal Filho, A. M. Azul, L. Brandli, A. Lange Salvia and T. Wall, Springer, Cham, 2021, DOI:10.1007/978-3-319-71064-8_150-1.
- J. C. Xavier, R. A. Phillips and A. Takahashi, Antarctic Seabirds as Indicators of Climate Change, in Seabird Biodiversity and Human Activities, ed. J. A. Ramos and L. Pereira, CRC Press, Boca Raton, 2022, vol. 1, pp. 189–210, DOI:10.1201/9781003047520.
- S. Tartu, F. Angelier, J. C. Wingfield, P. Bustamante, P. Labadie, H. Budzinski, H. Weimerskirch, J. O. Bustnes and O. Chastel, Corticosterone, prolactin and egg neglect behavior in relation to mercury and legacy POPs in a long-lived Antarctic bird, Sci. Total Environ., 2015, 505, 180–188 CrossRef CAS PubMed.
- P. Convey and R. I. L. Smith, Responses of terrestrial Antarctic ecosystems to climate change, in: Plants and Climate Change, Tasks for vegetation science, ed. J. Rozema, R. Aerts and H. Cornelissen, vol 41, Springer, Dordrecht, 2005, DOI:10.1007/978-1-4020-4443-4_1.
- A. Cabrerizo, J. Dachs, D. Barceló and K. C. Jones, Climatic and Biogeochemical Controls on the Remobilization and Reservoirs of Persistent Organic Pollutants in Antarctica, Environ. Sci. Technol., 2013, 47, 4299–4306 CrossRef CAS PubMed.
- R. Bargagli, Environmental contamination in Antarctic ecosystems, Sci. Total Environ., 2008, 400, 212–226 CrossRef CAS.
- H. N. Geisz, R. M. Dickhut, M. A. Cochran, W. R. Fraser and H. W. Ducklow, Melting Glaciers: A Probable Source of DDT to the Antarctic Marine Ecosystem, Environ. Sci. Technol., 2008, 42, 3958–3962 CrossRef CAS.
- N. W. van den Brink, M. J. Riddle, M. van den Heuvel-Greve and J. A. van Franeker, Contrasting time trends of organic contaminants in Antarctic pelagic and benthic food webs, Mar. Pollut. Bull., 2011, 62, 128–132 CrossRef CAS PubMed.
- W. J. L. Sladen, C. M. Menzie and W. L. ReichelL, DDT Residues in Adelie Penguins and A Crabeater Seal from Antarctica, Nature, 1966, 210, 670–673 CrossRef CAS PubMed.
- R. W. Risebrough, P. Rieche, D. B. Peakall, S. G. Herman and M. N. Kirven, Polychlorinated Biphenyls in the Global Ecosystem, Nature, 1968, 220, 1098–1102 CrossRef CAS PubMed.
- S. Corsolini, Industrial contaminants in Antarctic biota, J. Chromatogr. A, 2009, 1216, 598–612 CrossRef CAS PubMed.
- L. Bhardwaj, A. Chauhan, A. Ranjan and T. Jindal, Persistent Organic Pollutants in Biotic and Abiotic Components of Antarctic Pristine Environment, Earth Syst. Environ., 2018, 2, 35–54 CrossRef.
- M. L. Bates, S. M. Bengtson Nash, D. W. Hawker, E. C. Shaw and R. A. Cropp, The distribution of persistent organic pollutants in a trophically complex Antarctic ecosystem model, J. Mar. Syst., 2017, 170, 103–114 CrossRef.
- C. Barbraud and H. Weimerskirch, Antarctic birds breed later in response to climate change, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 6248–6251 CrossRef CAS.
- J. P. Croxall, P. N. Trathan and E. J. Murphy, Environmental change and Antarctic seabird populations, Science, 2002, 297, 1510–1514 CrossRef CAS.
- D. Ainley, J. Russell, S. Jenouvrier, W. R. Fraser, G. L. Kooyman, P. Lyver and E. C. Woehler and in collaboration with World Wildlife Fund, The Fate of Antarctic Penguins when Earth's Tropospheric Temperature Reaches 2°C above Pre-industrial Levels, 2008 Search PubMed.
- C. Barbraud and H. Weimerskirch, Emperor penguins and climate change, Nature, 2001, 411, 183–186 CrossRef CAS.
- J. Forcada and P. N. Trathan, Penguin responses to climate change in the Southern Ocean, Global Change Biol., 2009, 15, 1618–1630 CrossRef.
- S. Park, J. B. Thiebot, J. H. Kim, K. W. Kim, H. Chung and W. Y. Lee, Mare incognita: Adélie penguins foraging in newly exposed habitat after calving of Nansen Ice Shelf, Environ. Res., 2021, 201, 111561 CrossRef CAS PubMed.
- R. I. L. Smith, Vascular plants as bioindicators of regional warming in Antarctica, Oecologia, 1994, 99, 322–328 CrossRef PubMed.
- P. Carlsson, N. A. Warner, I. G. Hallanger, D. Herzke and R. Kallenborn, Spatial and temporal distribution of chiral pesticides in Calanus spp. from three Arctic fjords, Environ. Pollut., 2014, 192, 154–161 CrossRef CAS PubMed.
- S. Corsolini, N. Ademollo, T. Romeo, S. Olmastroni and S. Focardi, Persistent organic pollutants in some species of a Ross Sea pelagic trophic web, Antarct. Sci., 2003, 15, 95–104 CrossRef.
- I. Zimmer, R. P. Wilson, C. Gilbert, M. Beaulieu, A. Ancel and J. Plötz, Foraging movements of emperor penguins at Pointe Géologie, Antarctica, Polar Biol., 2008, 31, 229–243 CrossRef.
- A. Cincinelli, T. Martellini, K. Pozo, P. Kukučka, O. Audy and S. Corsolini, Trematomus bernacchii as an indicator of POP temporal trend in the Antarctic seawaters, Environ. Pollut., 2016, 217, 19–25 CrossRef CAS PubMed.
- P. Convey, Terrestrial biodiversity in Antarctica – Recent advances and future challenges, Polar Sci., 2010, 4, 135–147 CrossRef.
- R. Bargagli, Antarctic Ecosystems, Springer-Verlag, Berlin/Heidelberg, 2005, vol. 175 Search PubMed.
- I. E. Friedmann and, Microorganisms in antarctic desert rocks from dry valleys and Dufek Massif, Antarct. J. United States, 1977, 12, 26–29 Search PubMed.
- F. Borghini, J. O. Grimalt, J. C. Sanchez-Hernandez and R. Bargagli, Organochlorine pollutants in soils and mosses from Victoria Land (Antarctica), Chemosphere, 2005, 58, 271–278 CrossRef CAS PubMed.
- M. Vecchiato, E. Argiriadis, S. Zambon, C. Barbante, G. Toscano, A. Gambaro and R. Piazza, Persistent Organic Pollutants (POPs) in Antarctica: Occurrence in continental and coastal surface snow, Microchem. J., 2015, 119, 75–82 CrossRef CAS.
- S. Giannarelli, A. Ceccarini, C. Tiribilli, R. Spreafico, S. Francesconi and R. Fuoco, Paleo-environmental record of polycyclic aromatic hydrocarbons and polychlorobiphenyls at the peripheral site GV7 in Victoria Land (East Antarctica), Chemosphere, 2017, 174, 390–398 CrossRef CAS PubMed.
- G. Söderström, U. Sellström, C. A. de Wit and M. Tysklind, Photolytic Debromination of Decabromodiphenyl Ether (BDE 209), Environ. Sci. Technol., 2004, 38(1), 127–132 CrossRef PubMed.
- L.-G. Sun, X.-B. Yin, C.-P. Pan and Y.-H. Wang, A 50-years record of dichloro-diphenyl-trichloroethanes and hexachloro-cyclohexanes in lake sediments and penguin droppings on King George Island, Maritime Antarctic, J. Environ. Sci., 2005, 17, 899–905 CAS.
- J. Potapowicz, D. Szumińska, M. Szopińska and Ż. Polkowska, The influence of global climate change on the environmental fate of anthropogenic pollution released from the permafrost: Part I. Case study of Antarctica, Sci. Total Environ., 2019, 651, 1534–1548 CrossRef CAS PubMed.
- K. Pozo, T. Martellini, S. Corsolini, T. Harner, V. Estellano, P. Kukučka, M. D. Mulder, G. Lammel and A. Cincinelli, Persistent organic pollutants (POPs) in the atmosphere of coastal areas of the Ross Sea, Antarctica: Indications for long-term downward trends, Chemosphere, 2017, 178, 458–465 CrossRef CAS PubMed.
- A. Cincinelli, T. Martellini, M. Del Bubba, L. Lepri, S. Corsolini, N. Borghesi, M. D. King and R. M. Dickhut, Organochlorine pesticide air-water exchange and bioconcentration in krill in the Ross Sea, Environ. Pollut., 2009, 157, 2153–2158 CrossRef CAS PubMed.
- M. Vecchiato, S. Zambon, E. Argiriadis, C. Barbante, A. Gambaro and R. Piazza, Polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in Antarctic ice-free areas: Influence of local sources on lakes and soils, Microchem. J., 2015, 120, 26–33 CrossRef CAS.
- F. Rigét, A. Bignert, B. Braune, J. Stow and S. Wilson, Temporal trends of legacy POPs in Arctic biota, an update, Sci. Total Environ., 2010, 408, 2874–2884 CrossRef.
- S. Corsolini, A. Cincinelli, T. Martellini, D. Baroni and D. Randazzo, Have the iceberg B15 affected the POPs bioaccumulation in the Ross Sea? The case of Trematomus bernacchii, Organohalogen Compd., 2016, 78, 1279–1282 CAS.
- S. Corsolini, A. Covaci, N. Ademollo, S. Focardi and P. Schepens, Occurrence of organochlorine pesticides (OCPs) and their enantiomeric signatures, and concentrations of polybrominated diphenyl ethers (PBDEs) in the Adélie penguin food web, Antarctica, Environ. Pollut., 2006, 140, 371–382 CrossRef CAS PubMed.
- N. Borghesi, S. Corsolini, P. Leonards, S. Brandsma, J. de Boer and S. Focardi, Polybrominated diphenyl ether contamination levels in fish from the Antarctic and the Mediterranean Sea, Chemosphere, 2009, 77, 693–698 CrossRef CAS PubMed.
- NIC, World’s Largest Ever Recorded Iceberg Continues to Break Apart Near Antarctica, News Release JW-15-01, 16 September 2014, National Ice Center/Naval Office, NOAA Center - Public Affairs, 2014, 1–3.
- L. Halbach, M. Vihtakari, P. Duarte, A. Everett, M. A. Granskog, H. Hop, H. M. Kauko, S. Kristiansen, P. I. Myhre, A. K. Pavlov, A. Pramanik, A. Tatarek, T. Torsvik, J. M. Wiktor, A. Wold, A. Wulff, H. Steen and P. Assmy, Tidewater Glaciers and Bedrock Characteristics Control the Phytoplankton Growth Environment in a Fjord in the Arctic, Front. Mar. Sci., 2019, 254 CrossRef.
- K. Weber and H. Goerke, Persistent organic pollutants (POPs) in antarctic fish: levels, patterns, changes, Chemosphere, 2003, 53, 667–678 CrossRef CAS PubMed.
- H. Goerke, K. Weber, H. Bornemann, H. S. Ramdohr and J. Plötz, Increasing levels and biomagnification of persistent organic pollutants (POPs) in Antarctic biota, Mar. Pollut. Bull., 2004, 48, 295–302 CrossRef CAS PubMed.
- A. Strobel, P. Schmid, P. Burkhardt-Holm, H. Segner and M. Zennegg, Persistent organic pollutants in red- and white-blooded High-Antarctic notothenioid fish from the remote Weddell Sea, Chemosphere, 2018, 193, 213–222 CrossRef CAS PubMed.
- S. Focardi, L. Lari and L. Marsili, PCB congeners, DDTs and hexachlorobenzene in Antarctic fish from Terra Nova Bay (Ross Sea), Antarct. Sci., 1992, 4, 151–154 CrossRef.
- N. B. Lana, P. Berton, A. Covaci, N. F. Ciocco, E. Barrera-Oro, A. Atencio and J. C. Altamirano, Fingerprint of persistent organic pollutants in tissues of Antarctic notothenioid fish, Sci. Total Environ., 2014, 499, 89–98 CrossRef CAS PubMed.
- S. Corsolini, N. Borghesi, N. Ademollo and S. Focardi, Chlorinated biphenyls and pesticides in migrating and resident seabirds from East and West Antarctica, Environ. Int., 2011, 37, 1329–1335 CrossRef CAS PubMed.
- S. Brockington and L. S. Peck, Seasonality of respiration and ammonia excretion in the Antarctic echinoid Sterechinus neumayeri, Mar. Ecol.: Prog. Ser., 2001, 259, 159–168 CrossRef.
- B. M. Braune, G. M. Donaldson and K. A. Hobson, Contaminant residues in seabird eggs from the Canadian Arctic. Part I. Temporal trends 1975-1998, Environ. Pollut., 2001, 114(1), 39–54 CrossRef CAS PubMed.
- A. Krasnobaev, G. ten Dam, R. Boerrigter-Eenling, F. Peng, S. P. J. van Leeuwen, S. A. Morley, L. S. Peck and N. W. van den Brink, Legacy and Emerging Persistent Organic Pollutants in Antarctic Benthic Invertebrates near Rothera Point, Western Antarctic Peninsula, Environ. Sci. Technol., 2020, 54, 2763–2771 CrossRef CAS PubMed.
- A. L. Chiuchiolo, R. M. Dickhut, M. A. Cochran and H. W. Ducklow, Persistent organic pollutants at the base of the Antarctic marine food web, Environ. Sci. Technol., 2004, 38, 3551–3557 CrossRef CAS PubMed.
- AMAP, POPs and Chemicals of Emerging Arctic Concern (CEACs): Influence of Climate Change. Summary for Policy Makers, Tromsø, Norway, 2021 Search PubMed.
- Antarctic Climate Change and the Environment, Version 1, ed. J. Turner, R. Bindschadler, P. Convey, G. di Prisco, E. Fahrbach, J. Gutt, D. Hodgson, P. Mayewski and C. Summerhayes, Scientific Committee on Antarctic Research, Cambridge, UK, 2009 Search PubMed.
- S. E. Focardi, S. Corsolini and E. Franchi, Protocols for collecting samples for toxicological analysis, Commission for the Conservation of Marine Living Resources, Scientific Committee Working Group on Ecosystem Management Monitoring, WG-EMM-Methods 96/7 Rev. 1, 1996 Search PubMed.
- S. Corsolini, in Global Contamination Trends of Persistent Organic Chemicals, ed. B. G. Loganathan and P. K. S. Lam, CRC Press Taylor & Francis Group, Boca Raton, FL, 2012, pp. 571–592 Search PubMed.
- B. R. Subramanian, S. Tanabe, H. Hidaka and R. Tatsukawa, DDTs and PCB isomers and congeners in antarctic fish, Arch. Environ. Contam. Toxicol., 1983, 12, 621–626 CrossRef CAS PubMed.
- B. R. Subramanian, S. Tanabe and H. Hidaka, DDTs and PCB isomers and congeners in antarctic fish, Arch. Environ. Contam. Toxicol., 1983, 12, 621–626 CrossRef CAS PubMed.
- P. Larsson, C. Järnmark and A. Södergren, PCBs and chlorinated pesticides in the atmosphere and aquatic organisms of Ross Island, Antarctica, Mar. Pollut. Bull., 1992, 25, 281–287 CrossRef CAS.
- S. Focardi, R. Bargagli and S. Corsolini, Isomer-specific analysis and toxic potential evaluation of polychlorinated biphenyls in Antarctic fish, seabirds and Weddell seals from Terra Nova Bay (Ross Sea), Antarct. Sci., 1995, 7, 31–35 CrossRef.
- S. Corsolini, N. Ademollo and S. Focardi, Persistent organic pollutants in selected organisms of an Antarctic benthic community, Organohalogen Compd., 2003, 61, 329–332 CAS.
- H. Wolschke, X.-Z. Meng, Z. Xie, R. Ebinghaus and M. Cai, Novel flame retardants (N-FRs), polybrominated diphenyl ethers (PBDEs) and dioxin-like polychlorinated biphenyls (DL-PCBs) in fish, penguin, and skua from King George Island, Antarctica, Mar. Pollut. Bull., 2015, 96, 513–518 CrossRef CAS PubMed.
- K. S. Kumar, K. Kannan, S. Corsolini, T. Evans, J. P. Giesy, J. Nakanishi and S. Masunaga, Polychlorinated dibenzo-p-dioxins, dibenzofurans and polychlorinated biphenyls in polar bear, penguin and south polar skua, Environ. Pollut., 2002, 119, 151–161 CrossRef CAS.
- S. Corsolini, K. Kannan, T. Imagawa, S. Focardi and J. P. Giesy, Polychloronaphthalenes and Other Dioxin-like Compounds in Arctic and Antarctic Marine Food Webs, Environ. Sci. Technol., 2002, 36, 3490–3496 CrossRef CAS PubMed.
- J. L. George and D. E. H. Frear, Pesticides in the Antarctic, J. Appl. Ecol., 1966, 3, 155 CrossRef.
- B. G. Luke, G. W. Johnstone and E. J. Woehler, Organochlorine pesticides, PCBs and mercury in antarctic and subantarctic seabirds, Chemosphere, 1989, 19, 2007–2021 CrossRef CAS.
- G. S. Court, L. S. Davis, S. Focardi, R. Bargargli, C. Fossi, C. Leonzio and L. Marili, Chlorinated hydrocarbons in the tissues of South Polar Skuas (Catharacta maccormicki) and Adélie Penguins (Pygoscelis adeliea) from Ross Sea, Antarctica, Environ. Pollut., 1997, 97, 295–301 CrossRef CAS PubMed.
- W. H. Lara, H. H. C. Barretto, O. N. K. Inomata, R. Montone and R. R. Weber, Organochlorine residue in antarctic penguins, Pesq. Antart. Bras., 1990, 2, 1–6 Search PubMed.
- S. Wanwimolruk, H. Zhang, P. F. Coville, D. J. Saville and L. S. Davis, In vitro hepatic metabolism of a CYP3A-mediated drug, quinine, in Adélie penguins, Comp. Biochem. Physiol., Part C: Pharmacol., Toxicol. Endocrinol., 1999, 124, 301–307 CAS.
- S. Corsolini, N. Ademollo, T. Martellini, D. Randazzo, M. Vacchi and A. Cincinelli, Legacy persistent organic pollutants including PBDEs in the trophic web of the Ross Sea (Antarctica), Chemosphere, 2017, 185, 699–708 CrossRef CAS PubMed.
- A. Schiavone, S. Corsolini, N. Borghesi and S. Focardi, Contamination profiles of selected PCB congeners, chlorinated pesticides, PCDD/Fs in Antarctic fur seal pups and penguin eggs, Chemosphere, 2009, 76, 264–269 CrossRef CAS PubMed.
- C. V. Z. Cipro, S. Taniguchi and R. C. Montone, Occurrence of organochlorine compounds in Euphausia superba and unhatched eggs of Pygoscelis genus penguins from Admiralty Bay (King George Island, Antarctica) and estimation of biomagnification factors, Chemosphere, 2010, 78, 767–771 CrossRef CAS PubMed.
- I. Rudolph, G. Chiang, C. Galbán-Malagón, R. Mendoza, M. Martinez, C. Gonzalez, J. Becerra, M. R. Servos, K. R. Munkittrick and R. Barra, Persistent organic pollutants and porphyrins biomarkers in penguin faeces from Kopaitic Island and Antarctic Peninsula, Sci. Total Environ., 2016, 573, 1390–1396 CrossRef CAS PubMed.
- S. Jara-Carrasco, R. Barra, W. Espejo, J. E. Celis, D. González-Acunã, G. Chiang and J. Sánchez-Hernández, Persistent organic pollutants and porphyrin levels in excreta of penguin colonies from the Antarctic Peninsula area, Polar Res., 2017, 53, 79–87 CrossRef.
- R. W. Risebrough, W. Walker, T. T. Schmidt, B. W. De Lappe and C. W. Connors, Transfer of chlorinated biphenyls to Antarctica, Nature, 1976, 264, 738–739 CrossRef CAS PubMed.
- R. Schneider, G. Steinhagen-Schneider and H. E. Drescher, in Antarctic Nutrient Cycles and Food Webs, Springer, Berlin, Heidelberg, 1985, pp. 652–655 Search PubMed.
- S. Focardi, R. Bargagli and S. Corsolini, Organochlorines in marine Antarctic food chain at Terranova Bay (Ross Sea), Korean J. Polar Res., 1993, 4, 73–77 Search PubMed.
- N. W. Van den Brink, J. A. Van Franeker and E. M. De Ruiter-Dijkman, Fluctuating concentrations of organochlorine pollutants during a breeding season in two antarctic seabirds: Adelie penguin and southern fulmar, Environ. Toxicol. Chem., 1998, 17, 702–709 CAS.
- O. N. K. Inomata, R. C. Montone, W. H. Lara, R. R. Weber and H. H. B. Toledo, Tissue distribution of organochlorine residues – PCBs and pesticides – in Antarctic penguins, Antarct. Sci., 1996, 8, 253–255 CrossRef.
- N. W. Van den Brink, Directed transport of volatile organochlorine pollutants to polar regions: the effect on the contamination pattern of Antarctic seabirds, Sci. Total Environ., 1997, 198, 43–50 CrossRef CAS.
- J.-T. Kim, M.-H. Son, J.-H. Kang, J.-H. Kim, J.-W. Jung and Y.-S. Chang, Occurrence of Legacy and New Persistent Organic Pollutants in Avian Tissues from King George Island, Antarctica, Environ. Sci. Technol., 2015, 49, 13628–13638 CrossRef CAS PubMed.
- J. K. Mwangi, W.-J. Lee, L.-C. Wang, P.-J. Sung, L.-S. Fang, Y.-Y. Lee and G.-P. Chang-Chien, Persistent organic pollutants in the Antarctic coastal environment and their bioaccumulation in penguins, Environ. Pollut., 2016, 216, 924–934 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |