Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
John Dalton – the man and the myth
Edwin C.
Constable

University of Basel, Department of Chemistry, BPR 1096, Mattenstrasse 24a, CH-4058 Basel, Switzerland. E-mail: edwin.constable@unibas.ch; Tel: +41 61 207 1001
First published on 23rd December 2021
Abstract
John Dalton is one of the pioneers who transformed chemistry into the science that we enjoy today. His name is irrevocably linked with the atomic theory that underlies our modern understanding of chemical structure. This article summarizes his life and contributions and attempts to place them in the context of the intellectual revolution that was transforming all aspects of science.
Introduction
Everyone knows of John Dalton (Fig. 1) as the man who invented atoms! This article will try to provide an overview of what Dalton did, and also of what he did not, do. The story is written from the viewpoint of a chemist rather than a historian, which allows us to truly understand the influence of one man on the way we understand and conduct science today. It is appropriate that this journal, dedicated to inorganic chemistry in its broadest sense, commemorates his achievements. Fifty years ago, in 1972, the Royal Society of Chemistry renamed its flagship inorganic journal J. Chem. Soc. A to J. Chem. Soc., Dalton Transactions to commemorate the achievements of John Dalton. All dates in this article refer to the common era, CE. | ||
| Fig. 1 John Dalton (1766–1844) by Charles Turner (1773–1857) after James Lonsdale. (https://commons.wikimedia.org/wiki/File:John_Dalton_by_Charles_Turner.jpg). | ||
One can argue that the glory and success of modern inorganic chemistry is the legacy of three of the most influential thinkers of the past quarter of a millennium – Antoine Lavoisier, John Dalton, and Dmitri Mendeleev. Lavoisier delivered the insight and the vocabulary to describe and distinguish elements and compounds, Mendeleev brought order into the chaos of chemical observations, but Dalton provided us with the basic descriptions of atoms and molecules that today define the science of chemistry. The choice of John Dalton as the “face of inorganic chemistry” is not well-documented, but Brian F. G. Johnson recalls “At the time (1972) Mike Lappert owned Dalton's cottage and Geoff Wilkinson was from Dalton's home area. The name for the journal came almost automatically”.
This article aims to provide a short overview of the life and work of John Dalton and will try to set his achievements in the context of the contemporary developments in natural and chemical science. For more information about John Dalton, the reader is referred to the numerous publications dealing with his life and works.1–14
Reading the contemporary literature of the early 19th Century is often a challenge! Dalton used the term atom to describe both modern atoms (an atom of iron), gases which he thought to be monatomic (atoms of N2) and compounds (atoms of water). In contrast, Avogadro used the word molécule to describe atoms (molécule élémentaire), molecules of elements (molécule constituante) and molecules of compounds (molécule intégrante). Caveat usor!
A short biography
John Dalton was born in Eaglesfield, Cumberland on the 5th or 6th September 1766 as the third of six sons to a quaker family. His formal education was at the village school, where he gained an enthusiasm for natural science from a talented schoolmaster Elihu Robinson. From the age of 12 to 14 he taught at the same school after which he moved to teach at a boarding school in Kendal, firstly as an assistant and subsequently as principal. It was at this stage in his life that he began to lecture to the broader public on natural science and in 1793 he became tutor in natural philosophy at Manchester Academy where he remained for six years.After 1799 he relied on private tuition and lecturing for his income. Dalton's earliest scientific endeavours were in meteorology and astronomy, showing the influence of his earliest mentor Robinson, and his first major publication was Meteorological Observations and Essays from 1793.15 Although the first part of the book is concerned primarily with meteorology, the Essays give an indication of his interest in chemistry, heat and gases. The first essay is entitled On the Atmosphere; its Constitution Figure, Height, &c and the fourth On the Relation between Heat and other Bodies. Dalton considered air to be a mixture of “elastic fluids, or gasses” including “dephlogistated air”. That description together with subsequent references to Mr Kirwan (of whom more later) indicates that in 1793 he adhered to the phlogiston theory. This theory postulated that the substance phlogiston was found in all combustible materials and was released upon burning (or oxidation in modern terms). The phlogiston theory either ignored or used elegant circumlocutions to explain the increase in mass upon oxidation.
He joined the Manchester Literary and Philosophical Society in 1794 and many of his ideas were first presented as papers at its meetings. However, the critical period for Dalton and the atomic theory was in the first decade of the 19th Century and he was exposed to the pre-eminent scientists of the day, such as Humphry Davy, when he lectured at the Royal Institution in London in 1803–1804.
After the publication of the two volumes (three parts) of A New System of Chemical Philosophy,16–19 his reputation in the scientific community grew, although he remained in Manchester as a lifelong bachelor. However, honours and recognition began to flow towards this humble quaker. He was elected a Corresponding Member and subsequently Foreign Associate, of the Paris Academy of Sciences, made a Fellow of the Royal Society and awarded an honorary doctorate by the University of Oxford. In 1833 he was awarded a Civil Pension of £150 per annum and in 1834, the University of Edinburgh awarded him an L.L.D. Manchester eventually came to recognize the greatness of its adopted son and a statue of Dalton was commissioned from Francis Leggatt Chantrey for 2000 guineas.
Following a stroke in 1837, Dalton's experimental and lecturing work was much curtailed and after seven years of varying degrees of invalidity, he died on 27th July 1844. In death, his adopted home of Manchester acknowledged him as it had done in his lifetime. A civic funeral was arranged preceded by his body lying in state in the Town Hall for four days. Personally, one of my favourite images of Dalton, which links the earliest studies of gases with his subsequent home in Manchester is the 1887 Ford Madox Brown painting “Dalton Collecting Marsh Fire Gas” which is found in the Manchester Town Hall (Fig. 2).
 | ||
| Fig. 2 “Dalton Collecting Marsh Fire Gas” is an 1887 mural by 1887 Ford Madox Brown in the Manchester Town Hall (https://en.wikipedia.org/wiki/File:BrownManchesterMuralDalton.jpg). | ||
Atoms before 1803
The chemical atom did not spring unannounced onto an unprepared world at the beginning of the 19th Century. Rather, it was the product of a slow evolution of the philosophical atom from classical time.20 By the 18th Century, the late medieval concept of corpuscularianism was being replaced by atomism, based upon a physical atom as the basic building block of matter. Nevertheless, the word atom was used with various meanings and could be related to atoms of the “modern” elements but, in the pre-Lavoisier world, Robert Boyle (1627–1691) also wrote of “little nimble Atoms of Fire” in The Sceptical Chymist.21Atomism was a theme running through science from the late 16th Century onwards. In his 1612 book, De Principiis atque Originibus, Francis Bacon (1561–1626) presents the case for atomism, although in subsequent work he preferred corpuscularianism. Similarly, Daniel Sennert (1572–1637) in his Hypomnemata Physica develops a model in which matter is composed of indivisible atoms although he also subscribed to the Aristotelian four elements—earth, air, fire, and water. Robert Boyle was a convinced atomist, but he also made important contributions to understanding what should be described as an element. He differed from Sennert in rejecting the Aristoteleian elements, in The Sceptical Chymist he wrote “I now mean by Elements … Primitive and Simple, or perfectly unmingled bodies; which not being made of any other bodies, or of one another, are the Ingredients of all those call'd perfectly mixt Bodies…”. Sir Isaac Newton (1627–1691) addressed the question of attraction between atoms in his work Opticks “…Particles attract one another by some Force, which … is exceeding strong, [and] at small distances performs the chymical Operations … and reaches not far from the Particles with any sensible Effect”.
By the end of the 18th Century, the time was right for a broader chemical understanding of the nature of atoms, and the catalyst for the next step was the modern definition of an element in Traité Élementaire de Chimie published by Antoine-Laurent de Lavoisier in 1789.22 In his table of “simple substances” he listed antimony, arsenic, bismuth, boron*, carbon, chlorine*, cobalt, copper, fluorine*, gold, hydrogen, iron, lead, manganese, mercury, molybdenum, nitrogen, oxygen, phosphorus, platinum, silver, sulfur, tin, tungsten, and zinc (all using the modern English names). Although the elements denoted with an asterisk had not been isolated from their compounds in elemental form, Lavoisier knew that they had to exist. Similarly, he listed the “earthy substances”, calcium carbonate, magnesium oxide, barium sulfate, aluminium oxide and silicon dioxide which were only to reveal their hidden elements in the future.
The background laws
The modern definition of an element is an essential pre-requisite for Dalton's atomic theory, but there are several important laws which predate his theory, and which can either be seen as part of the intellectual path to that theory, or as the inevitable consequences of it. These laws are so much a part of modern chemical thought that we tend to forget that these ideas were at the cutting edge of science in the late 18th Century.The first of these is the “law of constant proportions”, “the law of definite proportions” or simply “Proust's law”. This originates in a 1798 publication from Proust which reported some studies on the pigment Prussian Blue and ended with the conclusion that “the principle that I established at the beginning of this memoir; namely, that iron is, like several other metals, subject to that law of nature which governs all true combinations, subject, I say, to two constant proportions of oxygen. It does not therefore differ in this respect from tin, mercury, lead, etc., and finally from almost all known fuels”.23 He subsequently investigated the composition of copper oxide and concluded that copper only combined with oxygen in one fixed ratio. Regardless of the synthetic method used to prepare CuO, the weight ratio of copper to oxygen was constant.24 He extended this to a general statement that all substances could only combine with each other in the same fixed ratio.
The other law to be considered is the “law of reciprocal proportions” (sometimes known as the “law of equivalents”) which dats to 1791 and the work of Jeremias Richter. The original statement of the law is difficult to understand, and it is probably best to formulate it in the modern form “If two different elements combine separately with the same weight of a third element, the ratio of the masses in which they do so are either the same or a simple multiple of the mass ratio in which they combine themselves”.
These laws make the relationships between stoichiometry and weight explicit and, taken with the definition of an element by Lavoisier, set the arena for Dalton to consider the mass of atoms.
From gases to atoms
Dalton was fascinated with gases and with heat. The immediate genesis of his atomic theory can be discerned in papers that he presented to the Manchester Literary and Philosophical Society in 1800, including “Heat and Cold produced by the Mechanical Condensation of Air” and “The Expansion of Elastic Fluids by Heat”. The canonical version of the origins of Dalton's atomic theory is that it arose from his studies of the solubility of gases in water.Dalton's atomism
The exact origin of Dalton's atomic theory continues to be debated. The first attempts to assign relative weights to atoms are to be found in his notebooks in the 1802–1803 period and the first public mention seems to be in a lecture given to the Manchester Literary and Philosophical Society in 1803 (but only published in 1805). This work is entitled On the Absorption of Gases by Water and Other Liquids and contains the comment “Why does water not admit its bulk of every gas alike? … I am nearly persuaded that the circumstance depends upon the weight and number of the ultimate particles of the several gases”. Most importantly, this publication contains the first listing of relative atomic weights entitled Table of the relative weights of the ultimate particles of gaseous and other bodies (Fig. 3). This table is based on H = 1, and has amongst other values of N = 4.2, C = 4.3, ammonia (formulated NH) = 5.2, oxygen (formulated O) = 5.5 and ethene (olefiant gas, formulated CH) = 5.3. It is not known if this table was presented in the 1803 lecture although his 1802/3 notebooks suggest that it might have been. From Dalton's first introduction of atomic weights, the values have been progressively refined and corrected with increasing accuracy, latterly by IUPAC. It is of interest that, although the notion of atomic weights can be attributed to Dalton, of those considered above, only his value for hydrogen has stood the test of time.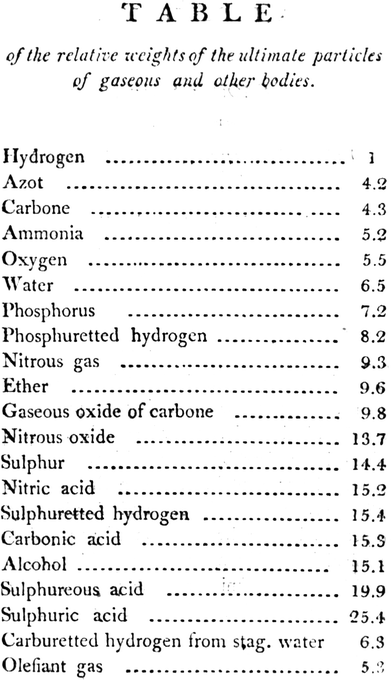 | ||
| Fig. 3 The first published table of relative atomic weights, presented by Dalton at a meeting of the Manchester Literary and Philosophical Society in 1803 (but only published in 1805).25 Image from Biodiversity Heritage Library under CC-BY-NC 3.0. | ||
Popularisation by Thomson
Although Dalton gave lectures in Manchester and across the country, his published work contains no further development of these ideas until the publication of the first part of volume 1 of A new system of chemical philosophy in 1808.16 However, Dalton's book was predated by the third edition of Thomson's System of Chemistry in 1807 in which the theory is presented thus “We have no direct means of ascertaining the density of the atoms of bodies; but Mr Dalton, to whose common ingenuity and sagacity the philosophic world is no stranger, has lately contrived an hypothesis which, if it prove correct, will furnish us with a very simple method of ascertaining that density with great precision” (by density Thomson means weight).26 Thomson also gives a Table of the composition and density of the gases – the second table of relative atomic weights and subsequently went further than Dalton and suggested that the atomic model could be extended beyond gases.A new system of chemical philosophy
The canonical form of Dalton's thinking is to be found in the three-part, two volume A new system of chemical philosophy (1808, 1810 and 1827).16–19 The crucial text comes towards the end of the 1808 book: “Chemical analysis and synthesis go no farther than to the separation of particles one from another, and to their reunion. No new creation or destruction of matter is within the reach of chemical agency. We might as well attempt to introduce a new planet into the Solar System, or to annihilate one already in existence, as to create or destroy a particle of hydrogen. All the changes we can produce, consist in separating particles that are in a state of cohesion or combination, and joining those that were previously at a distance. In all chemical investigations, it has justly been considered an important object to ascertain the relative weights of the simples which constitute a compound. But unfortunately, the enquiry has terminated here; whereas from the relative weights in the mass, the relative weights of the ultimate particles or atoms of the bodies might have been inferred, from which their number and weight in various other compounds would appear, in order to assist and to guide future investigations, and to correct their results. Now it is one great object of this work, to shew the importance and advantage of ascertaining the relative weights of the ultimate particles, both of simple and compound bodies, the number of simple elementary particles which constitute one compound particle, and the number of less compound particles which enter into the formation of one more compound particle”.In modern parlance, “compound particle” is equivalent to molecule and a “simple elementary particle” is an atom. Dalton felt it necessary to clarify his use of the word particle, possibly in response the Bakerian lecture from Sir Humphry Davy in 1810, in an article entitled Inquiries concerning the signification of the word Particle, as used by modern chemical writers, as well as concerning some other terms and phrases.27 This article is unusual for a number of reasons; firstly, Dalton uses it as an opportunity to attack the language of (most of!) his contemporaries and, secondly, he eschews the chance to make a clear and robust definition of his own. Dalton uses the word atom just once, and almost in passing “For, it is obvious, such integrant parts may either be Dr Thomson's particles (of the first order) or Mr. Murray's smallest particles into which a substance can be resolved without decomposition, which I call atoms”. It is interesting to note that Dalton is here using the word atom to mean both atoms and molecules (in modern parlance).
On the following page of A new system of chemical philosophy, Dalton sets out his rules for chemical combination “The following general rules may be adopted as guides in all our investigations respecting chemical synthesis.
1st. When only one combination of two bodies can be obtained, it must be presumed to be a binary one, unless some cause appear to the contrary.
2d. When two combinations are observed, they must be presumed to be a binary and a ternary.
3d. When three combinations are obtained, we may expect one to be a binary, and the other two ternary.
4th. When four combinations are observed, we should expect one binary, two ternary, and one quaternary, &c.
5th. A binary compound should always be specifically heavier than the mere mixture of its two ingredients.
6th. A ternary compound should be specifically heavier than the mixture of a binary and a simple, which would, if combined, constitute it; &c.
7th. The above rules and observations equally apply, when two bodies, such as C and D, D and E, &c. are combined”.
In these three pages, Dalton defines the future course of chemistry! Naturally, the atomic theory presented in A new system of chemical philosophy was neither complete nor correct in all aspects. The seven rules essentially lead to the law of multiple proportions (also known as Dalton's law) which states that if two elements form more than one compound, then the ratios of the masses of the second element which combine with a fixed mass of the first element will always be ratios of small whole number. This law can also be seen as an expansion and consolidation of the laws of constant proportion and reciprical proportion mentioned earlier.
One of his rules, although logical, resulted in untold confusion for the next half Century and almost resulted in the rejection of the atomic model in favour of equivalents in the 1830s and 1840s.28 This is a consequence of the first rule, which meant that water should be formulated as HO, since only one compound of hydrogen and oxygen was known. Similarly, he made the reasonable assumption that oxygen, hydrogen and nitrogen gases were O, H and N respectively.
Dalton was not a man who changed his mind easily. In the 17 years intervening between the 1810 publication of part two of volume one of “A new system of chemical philosophy” and the publication of volume 2 in 1827, the chemical world had advanced significantly. Nevertheless, in the appendix to the 1827 publication, the “new” table of atomic weights still lists hydrogen = 1 and oxygen = 7, and water with a weight of 8, all implying his continued formulation of water as HO, despite the establishment of the formula H2O by Amadeo Avogadro in 1811.
Sir Humphry Davy was an early convert to the law of multiple proportions29 and used Daltonian weights in his classic work on chlorine (oxymuriatic acid).30
Other claims to the atomic model
Over the years there have been heated discussions regarding the priority of Dalton's claim to have developed the atomic theory.2,31–38 It is not my intention to examine those arguments, but rather to place the claims into the context of the intellectual environment in which Dalton was working. It is fair to say that the contemporary writers in the early to mid-19th Century were, in general, kinder to the alternative claims than more modern commentators.The first of the rival claims is attributed to Bryan Higgins (1741–1818), an Irish chemist. Higgins, like Dalton, adhered to the caloric theory which maintains caloric is responsible for the phenomenon of heat. Caloric is a fluid that repels itself and flows from hotter bodies to colder bodies. Higgins proposed that atoms comprised a central particle surrounded by an atmosphere of caloric. Higgins’ ideas on atoms were certainly quite advanced, as seen in his 1775 work A philosophical essay concerning light, Vol. 1 where he states “I consider the smallest parts, into which any mass of matter is ever divided in the processes of Nature or Art, as the ultimate parts of that mass, and as small bodies which are incapable of actual division or diminution. These minute bodies are very aptly called Atoms; and using the word Atom in this sense, I express by it no more nor less, than what really exists. A body consisting of two coherent and heterogenous atoms, I call a Molecule, after the example of modern chemists; and small bodies, composed of an unknown number of cohering, atoms, are by common consent called Particles”.39 He also states that “the atoms of each element are accurately or nearly globular”.39 We need to moderate this seemingly modern view of chemistry with his belief in the “seven distinct elements of matter, viz. Earth, Water, Acid, Alcali, Air, Phlogiston, Light”.39
Based on the evidence, Dalton was probably aware of Bryan's theory and adopted very similar ideas and language, but he never acknowledged Bryan's anticipation of his caloric model. The importance of caloric in the development of Dalton's ideas is seen clearly in A new system of chemical philosophy. Both Higgins and Dalton developed a model based on central, hard, atoms surrounded by caloric. Caloric was self-repulsive and its density decreased with greater distance from the atom. Higgins relates the size and the weight of atoms but does not explicitly extend the ideas to relative atomic weights.40 Higgins appears to have been protective of his intellectual property – he accused Priestley of plagiarism eliciting a written response from the latter41 – but ill-health seems to have prevented his pursuing his case with Dalton.
In contrast, his nephew, William Higgins, pursued his own claim to having originated the atomic theory with considerable energy and enthusiasm.42–48 His claims rest on his 1799 work A Comparative View of the Phlogistic and Antiphlogistic Theories49 and subsequent, post-Daltonian, book Experiments and observations on the atomic theory, and electrical phenomena.42
There is no doubt that A Comparative View of the Phlogistic and Antiphlogistic Theories is a remarkable work, containing implicit understanding of the law of multiple proportions and developing models of chemical bonding in both diatomic and polyatomic systems. The book contains one of the earliest examples of the use of a line between atoms to indicate bonding (Fig. 4), significantly predating the 1861 introduction by Crum Brown50,51 together with the use of letter symbols to indicate atoms. Ultimately, A Comparative View of the Phlogistic and Antiphlogistic Theories is an excellent account of the debate between the “new” chemistry of Lavoisier and the “old” chemistry of the Phlogistonists but it cannot be viewed as a coherent presentation of atomism.
It is interesting to note that after his 1810 endorsement of Dalton's work, Davy gave more credit to William Higgins in his 1811 Bakerian lecture “In my last communication to the Society, I have quoted Mr. Dalton as the original Author of the hypothesis, that water consists of 1 particle of oxygen, and 1 of hydrogen; but I have since found that this opinion is advanced, in a work published in 1789—A Comparative View of the Phlogistic and Antiphlogistic Theories, by William Higgins. In this elaborate and ingenious performance, Mr. Higgins has developed many happy sketches of the manner in which (on the corpuscular hypothesis) the particles or molecules of bodies may be conceived to combine; and some of his views, though formed at this early period of investigation, appear to me to be more defensible, assuming his data, than any which have been since advanced.52 This lecture is also important as it also contains Davy's explicit doubling of Dalton's atomic weight of nitrogen “As hydrogen is the substance which combines with other bodies in the smallest quantity, it is perhaps the most fitted to be represented by unity; and on this idea, the proportions in ammonia will be 3 of hydrogen to 1 of nitrogen, and the number representing the smallest proportion in which nitrogen is known to combine will be 13.4. Mr. Dalton, New System of Chemical Philosophy, pages 323 and 436, has adopted 4.7 or 5.1, as the number representing the weight of the atom of nitrogen; and has quoted my experiments, Researches, Chemical and Philosophical, as authorising these numbers; but all the inquiries on nitric acid, nitrous gas, nitrous oxide, and on the decomposition of nitrate of ammonia stated in that work, conform much more nearly to the number 13.4”.52
Rumblings through the 19th Century
Perhaps it is no surprise that the chemical world, which can be conservative, did not convert overnight to Daltonian atomism. Inherent in Dalton's model was that every element (in the sense defined by Lavoisier) had to be composed of a unique type of atom. As the nature and limits of the periodic system had not been elucidated, this opened up the prospect of a near infinite number of types of atoms.Fleck goes so far as to state “The Daltonian atom was the subject of heated and confused argument for a quarter century, fell into disrepute for another quarter century, and was revived quite convincingly in 1858 by Stanislao Cannizzaro”.53 The resistance can be seen as having two key arguments: firstly, the confusion between atomic weights and equivalent weights was to continue until the resolution by Cannizzaro and, secondly, classical physical chemistry, thermodynamics and statistical mechanics were exceptionally successful and neither predicated nor postulated an atomistic model of matter. One of the stumbling blocks to acceptance was the concept of the atom as a physical object with mass and spatial volume. No less a figure than Ernst Mach stated, “we are not justified in thinking of atoms spatially”.54,55 Equally influential was the French physicist and philosopher Pierre Duhem, who categorically rejected atomism, most specifically in his 1892 publication Notation atomique et hypothèses atomistiques.56–59
Eventually the success of the atomic model in organic chemistry held sway and it became generally accepted, in particular after the Karlsruhe Congress.
Symbols and symbolic wars
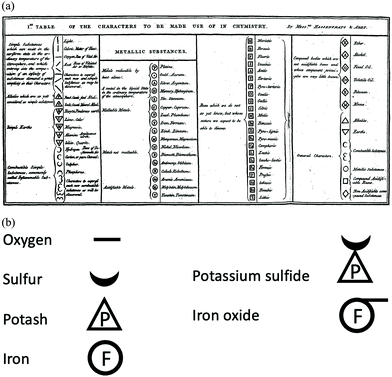
Another of the things that “everyone knows” about John Dalton is that he “invented atomic symbols”. Once again, the whole story is not quite so simple.60 Alchemists, iatrochemists and chemists had used symbols throughout history to describe and denote individual compounds and processes. These were often derived from symbols of the classical civilizations relating to astrology and celestial objects. These symbols were obscure and far from universally used. The bridge from the past to modern chemistry came with the identification of simple substances (elements) and compound substances composed of those elements by Guyton de Morveau, Lavoisier, Berthollet and de Fourcroy in 1787 as published in Méthode de nomenclature chimique, proposée par MM. de Morveau, Lavoisier, Bertholet & Foucroy. On y a joint un systême de caractères chimiques, adaptés à cette nomenclature, par MM. Hassenfratz & Adet.61–63 This provided the intellectual environment in which the elements would be described by symbols, which could, in turn, be combined to describe the elemental composition of compound materials. However, the Méthode went further than this and contained a section written by Hassenfratz and Adet introducing a new notation for simple substances which allowed symbols to be combined to describe the atomic composition of a compound.61–64 In general, metals were represented by the first or first two letters of their name in a circle and bases with the initial letter (P or S) in a triangle. With this publication Hassenfratz and Adet redefined and improved the methods available for denoting stoichiometry. Together with the various translators of the Méthode, in particular by adopting the initial letter of the element in their language. By the first decade of the 19th Century, multiple variants of the “standard” notation for chemical entities introduced in the Méthode had been proposed!This was the environment into which Dalton was to introduce his symbols (Fig. 5). However, there was one very important difference between Dalton's symbols and the subsequent notations. The Hassenfratz and Adet symbols did not represent individual atoms but could be applied to any amount of a substance. In contrast, and in accord with the tenets of his atomic theory, Dalton's symbols represented individual atoms. The bulk of a material was described by a given number of atoms, depending on the atomic weight.
The symbols used by Dalton were not widely adopted in the literature and were considered difficult to write and to print. Trouble was on the horizon and in 1814 Jöns Jacob Berzelius published the fifth part of his Essay on the Cause of Chemical Proportions, and on Some Circumstances Relating to Them; together with a short and easy Method of expressing them.65
Berzelius stated “The chemical signs ought to be letters … I shall take, therefore, for the chemical sign, the initial letter of the Latin name of each elementary substance: but as several have the same initial letter, I shall distinguish them in the following manner: –
1. In the class which I call metalloids, I shall employ the initial letter only, even when this letter is common to the metalloid and to some metal.
2. In the class of metals, I shall distinguish those that have the same initials with another metal, or a metalloid, by writing the first two letters of the word.
3. If the first two letters be common to two metals, I shall, in that case, add to the initial letter the first consonant which they have not in common: for example, S = sulphur, Si = silicium, St = stibium (antimony), Sn = stannum (tin), C = carbonicum, Co = cobaltium (cobalt), Cu = cuprum (copper), O = oxygen, Os = osmium, &c”.
What could be simpler or easier to represent? Well … Dalton, never a man who changed his mind easily, refused to use them and wrote in a letter of 1837 to Thomas Graham “Berzelius's symbols are horrifying; a young student in chemistry might as soon learn Hebrew as make himself acquainted with them. They appear like a chaos of atoms. Why not put them together in some sort of order? … Nothing has surprised me more than that such a system should ever have obtained a footing anywhere”.14 Dalton at his most charming!
Dalton's other contributions
In addition to his chemical work, Dalton was a passionate and lifelong meteorologist and observer of the night skies. His book Meteorological observations and essays was published in 1793 with a second edition in 1834. Throughout his life, he regularly published meteorological observations and scientific papers about meteorological and cosmic phenomena in the Memoirs of the Literary and Philosophical Society, Manchester.His greatest work was probably the three volumes of A new system of chemical philosophy, the first volume of which appeared in a German edition as early as 1812. The primary scientific studies, in particular on gases, were mainly published in Memoirs of the Literary and Philosophical Society, Manchester.
Although we think of Dalton as a scientist and chemist, he had broad interests and published a tome entitled Elements of English grammar: or a new system of grammatical instruction, for the use of schools and academies published in 1801 followed by a second edition in 1803. He also published articles on mathematical questions in both the Gentleman's Diary and the Ladies’ Diary.
Although many of Dalton's scientific publications are difficult to access, a number of the most important has been reprinted in full or in part; particularly useful are the Alembic club publications number 2 and number 4 from 1899,66,67 and David Knight's collection of Classical Scientific Papers: Chemistry.68 The 1968 and 1997 works by Smyth provide the definitive bibliographic listings of Dalton's published and unpublished works, including notebooks and correspondence.7,9
Last words
I hope that this short article has indicated why the choice of John Dalton as “the face of inorganic chemistry” by the Royal Society of Chemistry is so appropriate. I hope that I have not been iconoclastic, but rather have placed the work and accomplishments of John Dalton in the context of the discoveries and ideas of his contemporaries. The body of his work collectively defines the transition into the modern era of chemistry – a chemistry defined by a knowledge of, and the ability to manipulate, atoms and molecules. The identification of Dalton as an individual to represent inorganic chemistry in the title of this journal should also be seen as a collective tribute to the great chemical innovators and thinkers active at the cusp of the 18th and 19th Centuries.Author contributions
All 14 authorship contributions identified by CrediT were fulfilled by the lead and sole author.Conflicts of interest
There are no conflicts to declare.Acknowledgements
As always, I thank the University of Basel for the opportunity to pursue my various interests in chemistry. I also thank the Universitätsbibliothek Basel for responding to my ever more bizarre requests for obscure literature items – and for their enthusiastic interest and support over the years. I would like to take this opportunity to thank Sir Timothy John Berners-Lee – the World Wide Web has made the early chemical literature accessible to all through resources such as the Internet Archive, Google Books and Biodiversity Heritage Library. Most of all, thanks to Catherine for indulging me in these whimsical chemical diversions rather than getting on with the writing of mainstream research papers!References
- D. M. Knight, John Dalton (1766–1844), in 2012, Philosophy of Chemistry, Elsevier, 2012, p. 71 Search PubMed.
- H. E. Roscoe and A. Harden, A New View of the Origin of Dalton's Atomic Theory: A Contribution to Chemical History, Together with Letters and Documents Concerning the Life and Labours of John Dalton, Now for the First Time Published from Manuscript in the Possession of the Literary and Philosophical Society of Manchester, Macmillan and Company, London, UK, 1896 Search PubMed.
- H. Hartley, Proc. R. Soc. London, Ser. B, 1967, 168, 291 Search PubMed.
- H. Hartley, Proc. R. Soc. London, Ser. B, 1967, 168, 335 CAS.
- Wilson, Br. Q. Rev., 1845, 1, 157 Search PubMed.
- R. A. Smith, Mem. Proc. - Manchester Lit. Philos. Soc., 1856, 13, 1 Search PubMed.
- A. L. Smyth, John Dalton, 1766–1844 : a Bibliography of Works by and about him, Manchester University Press, Manchester, UK, 1966 Search PubMed.
- A. Thackray., John Dalton. Critical Assessments of his Life and Science; Harvard University Press: Cambridg, Mass, USA, 1972 Search PubMed.
- A. L. Smyth, John Dalton, 1766–1844 : a Bibliography of Works by and about him, Manchester University Press, Manchester, UK, 2nd edn, 1997 Search PubMed.
- E. C. Patterson, John Dalton and the Atomic Theory; the Biography of a Natural Philosopher, Doubleday, Garden City, NY, USA, 1970 Search PubMed.
- F. Greenaway, John Dalton and the Atom, Heinemann, London, UK, 1966 Search PubMed.
- R. M. Wenley and A. B. Grifiths, John Dalton, Createspace Independent Publishing Platform, 2016 Search PubMed.
- G. Wilson, Religio Chemici, Macmillan and Co., London, 1862, p. 304 Search PubMed.
- W. C. Henry, Memoirs of the Life and Scientific Researches of John Dalton, The Cavendish Society, London, UK, 1854 Search PubMed.
- J. Dalton, Meteorological Observations and Essays, W. Richardson; J. Phillips; W. Pennington, London, and Kendal, UK, 1793 Search PubMed.
- J. Dalton, A New System of Chemical Philosophy. Vol. 1, Part 1, S. Russell; R. Bickerstaff, Manchester, UK; London, UK, 1808 Search PubMed.
- J. Dalton, A New System of Chemical Philosophy. Vol. 1, Part 2, Russell & Allen; R. Bickerstaff, Manchester, UK; London, UK, 1810 Search PubMed.
- J. Dalton, A New System of Chemical Philosophy. Vol. 2, Part 1, S. Russell; George Wilson, Manchester, UK; London, UK, 1827 Search PubMed.
- J. Dalton, A New System of Chemical Philosophy, Cambridge University Press, Cambridge, UK, 2010, vol. 1 Search PubMed.
- E. C. Constable and C. E. Housecroft, Molecules, 2020, 25, E2623 CrossRef PubMed.
- R. Boyle, The Sceptical Chymist: or Chymico-Physical Doubts & Paradoxes, J. Cadwell, London, U.K., 1661 Search PubMed.
- A. L. Lavoisier, Traité élémentaire de chimie, présenté dans un ordre nouveau, et d'après des découvertes modernes, Cuchet, Paris, France, 1st edn, 1789 Search PubMed.
- J. L. Proust, Ann. Chim., 1797, 23, 85–101 Search PubMed.
- J. L. Proust, Ann. Chim., 1799, 32, 26–54 Search PubMed.
- J. Dalton, Mem. Lit. Philos. Soc. Manchester, 2nd. Ser., 1805, 1, 271 Search PubMed.
- T. Thomson, A System of Chemistry. 3rd Ed., 4 volumes, Bell & Bradfute, and E. Balfour; John Murray; Gilbert & Hodges, Edinburgh, UK; London, UK; Dublin, Eire, 1807 Search PubMed.
- J. Dalton, J. Nat. Philos. Chem. Arts, 1811, 28, 81 Search PubMed.
- E. C. Constable, Chimia, 2021, 75, 1052 CrossRef PubMed.
- H. Davy, Philos. Trans. R. Soc. London, 1810, 100, 16 CrossRef.
- H. Davy, Philos. Trans. R. Soc. London, 1810, 100, 231 CrossRef.
- M. I. Grossman, Notes Rec. Roy. Soc. London, 2010, 64, 417 CrossRef.
- M. I. Grossman, Notes Rec. Roy. Soc. London, 2014, 68, 339 CrossRef.
- M. I. Grossman, Br. J. Hist. Sci., 2017, 50, 657 CrossRef PubMed.
- A. J. Rocke, Soc. Res., 2005, 72, 125 Search PubMed.
- A. J. Rocke, Hist. Stud. Phys. Sci., 1978, 9, 225 Search PubMed.
- H. E. Roscoe, John Dalton and the Rise of Modern Chemistry, Macmillan and Company, New York, NY, USA, 1895 Search PubMed.
- T. S. Wheeler and J. R. Partington, The Life and Work of William Higgins, Chemist (1763–1825). Including Reprints of “A Comparative View of the Phlogistic and Antiphlogistic Theories” and “Observations on the Atomic Theory and Electrical Phenomena”, Pergamon Press, Oxford, UK, 1960 Search PubMed.
- T. S. Wheeler, Studies, 1954, 43, 78 Search PubMed.
- B. Higgins, A Philosophical Essay concerning Light, London, UK, 1776, vol. I Search PubMed.
- B. Higgins, Minutes of the Society for Philosophical Experiments and Conversations, T. Cadell, junior, and W. Davies (successors to Mr. Cadell), London, UK, 1795 Search PubMed.
- J. Priestley, Philosophical Empiricism : Containing Remarks on a Charge of Plagiarism Respecting Dr. H––s, Interspersed with Various Observations Relating to Different Kinds of Air, J. Johnson, London, UK, 1775 Search PubMed.
- W. Higgins, Experiments and Observations on the Atomic Theory, and Electrical Phenomena; Graisbury and Campbell, Dublin, Ireland, 1814 Search PubMed.
- W. Higgins, Philos. Mag., 1816, 48, 408 CrossRef.
- W. Higgins, Philos. Mag., 1816, 48, 363 CrossRef.
- W. Higgins, Philos. Mag., 1817, 49, 241 CrossRef.
- W. Higgins, Philos. Mag., 1818, 51, 81 CrossRef.
- W. Higgins, Philos. Mag., 1818, 51, 161 CrossRef.
- W. Higgins, Philos. Mag., 1819, 53, 401 CrossRef.
- W. Higgins, A Comparative View of the Phlogistic and Antiphlogistic Theories with Inductions, to which is Annexed, an Analysis of the Human Calculus with Observation on its Origin,&c, J. Murray, London, UK, 1789 Search PubMed.
- A. Crum Brown, On the Theory of Chemical Combination: A Thesis presented to the Faculty of Medicine of the University of Edinburgh, 1861, Neill and Co., Edinburgh, UK, 1879 Search PubMed.
- D. F. Larder, Ambix, 1967, 14, 112 CrossRef CAS PubMed.
- H. Davy, Philos. Trans. R. Soc. London, 1811, 101, 1 Search PubMed.
- G. M. Fleck, J. Hist. Ideas, 1963, 24, 106 CrossRef.
- F. Seaman, J. Hist. Ideas, 1968, 29, 381 CrossRef.
- E. Mach, History and Root of the Principle of the Conservation of Energy. Translated from the German and annotated by Philip E.B. Jourdain, The Open Court Publishing Co, Chicago, IL, USA, 1911 Search PubMed.
- A. J. Rocke, Hist. Stud. Nat. Sci., 2017, 47, 268 CrossRef.
- P. Duhem, Rev. Quest. Sci., 1892, 31, 391 Search PubMed.
- P. Needham, Found. Chem., 2000, 2, 127 CrossRef CAS.
- M. J. Nye, Stud. Hist. Philos. Sci. A, 1976, 7, 245 CrossRef.
- E. C. Constable, Chimia, 2019, 73, 837 CrossRef CAS PubMed.
- L.-B. Guyton de Morveau, A. L. Lavoisier, C.-L. Berthollet and A.-F. de Fourcroy, Méthode de nomenclature chimique, proposée par MM. de Morveau, Lavoisier, Bertholet & Foucroy. On y a joint un systême de caractères chimiques, adaptés à cette nomenclature, par MM. Hassenfratz & Adet, Cuchet, Paris, France, 1787 Search PubMed.
- L.-B. Guyton de Morveau, A. L. Lavoisier, C.-L. Berthollet and A.-F. de Fourcroy, Method of chymical nomenclature/proposed by Messrs. de Morveau, Lavoisier, Bertholet, and de Fourcroy; to which is added, a new system of chymical characters, adapted to the nomenclature, by Mess. Hassenfratz and Adet; translated from the French, and the new chymical names adapted to the genius of the English language, by James St. John, Cuchet, London, UKG. Kearsley, 1788 Search PubMed.
- L.-B. Guyton de Morveau, A. L. Lavoisier, C.-L. Berthollet, A.-F. de Fourcroy, J. H. Hassenfratz, P. A. Adet and K. Meidinger von Freyherrn, Methode der chemischen Nomenklatur für das antiphlogistische System/von Hrn. de Morveau, Lavoisier, Berthollet und de Fourcroy. Nebst einem neuen Systeme der dieser Nomenklatur angemessenen chemischen Zeichen, von Herrn Hassenfratz und Adet. Aus dem Französischen. von Karl Freyherrn von Meidinger, C.F. Wappler, Vienna, Austria, 1793 Search PubMed.
- J. H. Hassenfratz and P. A. Adet, System der Chemischen Zeichen für die antiphlogistische Chemie und ihre Nomenklatur, C.F. Wappler, Vienna, Austria, 1793 Search PubMed.
- J. J. Berzelius, Philos. Mag., 1814, 3, 443–454 Search PubMed.
- J. Dalton, W. H. Wollaston and T. Thomson, Foundations of the Atomic Theory Comprising Papers & Extracts by Dalton, Wollaston and Thomson, Alembic Club, Edinburgh, UK, 1899 Search PubMed.
- J. Dalton, J.-L. Gay-Lussac and A. Avogadro, Foundations of the Molecular Theory, Alembic Club, Edinburgh, 1899 Search PubMed.
- D. M. Knight, Classical Scientific Papers: Chemistry, Harlequin Mills & Boon, 1968 Search PubMed.
| This journal is © The Royal Society of Chemistry 2022 |