Jan Peter Toennies: an ebullient serendipitous adventurer
Bretislav
Friedrich
 a and
Dudley
Herschbach
a and
Dudley
Herschbach
 b
b
aFritz-Haber-Institut der Max-Planck-Gesellschaft, Faradayweg 4-6, D-14195 Berlin, Germany. E-mail: bretislav.friedrich@fhi-berlin.mpg.de
bDepartment of Chemistry and Chemical Biology, Harvard University, 12 Oxford St, Cambridge, MA 02138, USA. E-mail: dherschbach@gmail.com
Abstract
Our 1. Prologue section applauds some previous celebrations of Peter's inspiring ebullient adventures, including a remarkable new book by Giorgio Benedek and Peter on investigations of surfaces by helium beam scattering. The next sections treat 2. Peter's personal genealogy and 3. Peter's scientific genealogy. Section 4 presents Meanderings and serendipity on the way from Bonn to Göttingen. Section 5 lists Peter's awards and Section 6 is our Epilogue that features an Ode to Peter as well as his response.
1. Prologue
In honor of the 90th birthday (on 3 May 2020) of Professor Jan Peter Toennies, a Festschrift entitled New Horizons in the Dynamics of Molecules: from Gases to Surfaces is being presented to the jubilarian. It is a themed collection of articles residing in the journal Physical Chemistry Chemical Physics (PCCP). This is Peter's second Festschrift!His 80th birthday celebration in 2011 produced a Festschrift carried by a special issue of the Journal of Physical Chemistry A.1 It was comprised of 75 papers written in his honor by colleagues and former students and associates. The Preface had a brisk Autobiography,2 along with lists of his publications, students, and co-workers, as well as his awards. Bretislav Friedrich gave A Toast to Jan Peter Toennies3 that “concentrated on highlighting his research activities, which often took him into uncharted territories and opened up several new research areas of experimental chemical physics. Peter's conscious choice of simple systems for his experiments has stimulated countless theoretical studies. Overall, the work of the Toennies laboratory has been described in nearly 700 [highly cited] papers.”
At Peter's 85th birthday celebration in 2015, Bretislav exhibited a photo album. Much of it will be brought forth herein, showing both Peter's personal and scientific genealogy. “Each picture worth a thousand words!”
In 2018 came another celebration, an exquisite book by Giorgio Benedek and Jan Peter Toennies: Atomic Scale Dynamics at Surfaces: Theory and Experimental Studies with Helium Atom Scattering.4 Its authors are shown at work as they probe a surface in a drawing by Zdenek Herman, Fig. 1. The book has 15 chapters, over 625 pages, with 177 black & white and 51 color figures – about 400 thousand words both literal and proverbial! Researchers and graduate students eager to map dynamical processes at surfaces will greatly benefit from this cornucopia.
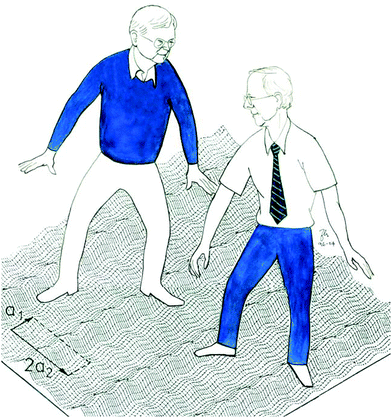 | ||
| Fig. 1 JPT (left) and Giorgio Benedek struggling with the surface lattice dynamics. Drawing by Zdenek Herman, 2004. Reproduced with permission from © Zdenek Herman. | ||
In the present Festschrift, the articles exhibit a wide variety of perspectives. In our own article, we will augment some of Peter's serendipitous adventures, detailed in Peter's superb 2004 review of his scientific career, Serendipitous Meanderings and Adventures with Molecular Beams.5 Exploits from his review and more will burst out in this latest Festschrift.
Bretislav (age 67), as in his Toast and the photo album, ascribes to Peter “bountiful achievements” in science and “greatly enriching the lives of his colleagues and associates.” Dudley (age 88) tells aspects that helped Peter from his PhD in 1957 to reach the wonderful Max Planck Institute in Göttingen in 1969, a “science paradise.”
2. Peter's personal genealogy
Peter's father, Gerrit Toennies (1898–1978), Fig. 2, was drafted at age 18 by the German military and sent to serve on the western front in World War One. He was soon captured by the French and held prisoner until 1920. Among his jobs as a prisoner of war was working at a French gas plant, which aroused his interest in chemistry. And, as Peter put it, determined his (and presumably Peter's) career. After the war, Peter's father studied chemistry at Kiel under Otto Diels, who would receive the 1950 Nobel Prize in chemistry. The post-WWI chaos in Germany spurred Peter's father's desire to emigrate to the New World, which he did in 1924. This is why Peter was born and trained in the U.S. And why he doesn’t have an Umlaut in his name. | ||
| Fig. 2 JPT's father Gerrit Toennies in the laboratory of Fox Chase Cancer Institute in Philadelphia, 1955. Reproduced with permission from © J. Peter Toennies. | ||
Fig. 3 shows Peter's parents about a year before Peter was born, in Philadelphia. This is what he had to say about them in his review:5 “I was fortunate in the choice of my parents. My mother taught me by her example to love nature, work hard, and to stand up for my own ideas. My father aroused my interest in the outside world and the sciences.” Peter's mother was Margerete Christiana (Dita) Toennies (1903–1959), born Jebens. Peter's paternal grandfather was the famous Ferdinand Tönnies (1855–1936), shown in Fig. 4 in an etching by Emil Orlik. He was a founder of sociology, who greatly contributed to the study of social change and of public opinion. He published over 900 works, among them the seminal volume Gemeinschaft und Gesellschaft (1887). According to Ferdinand Tönnies, Gemeinschaft is a family-like grouping, whereas Gesellschaft is impersonal, like a city or state.
 | ||
| Fig. 3 JPT's parents Gerrit and Dita Toennies, about 1929. Reproduced with permission from © J. Peter Toennies. | ||
 | ||
| Fig. 4 JPT's paternal grandfather, Ferdinand Tönnies, in an etching by Emil Orlik. Creative Commons. | ||
Peter's group has surely been a Gemeinschaft, with many personal ties and much individual attention. All that within the benevolent Max-Planck-Gesellschaft that made our joint adventures with Peter possible. Later on we will flash some pictures from the Gemeinschaft's photo album.
3. Peter's scientific genealogy
Peter's genealogical tree, drawn by Ricarda Riechers, a student of Alkwin Slenczka's, is shown in Fig. 5. It is of course also the genealogical tree of all those who did their PhD with Peter. Remarkably, not only Peter and his scientific posterity have scientific ancestors with clearly recognizable names, such as Joseph Louis Gay-Lussac or Claude-Louis Berthollet, not to speak about Antoine Laurent de Lavoisier. Apparently, we all have the same roots.But before we start climbing or rather descending Peter's tree, let us take a step back and start with Peter's college. In 1948, at age 18, Peter was admitted to Amherst College, one of the best liberal arts colleges in the U.S. Getting in was no mean feat, given the fierce competition at that time from the returning veterans of World War Two.
At Amherst, Peter took a physical chemistry course from David C. Grahame (1912–1958), Fig. 6, well known, e.g., for his work on the electric double-layer. Here's what Peter said about the course:5 “[David Grahame, in his physical chemistry lectures] made me aware … for the first time in my studies of the role of molecules in chemical phenomena.” So it was with Grahame's course that molecules entered Peter's life – never to take a leave.
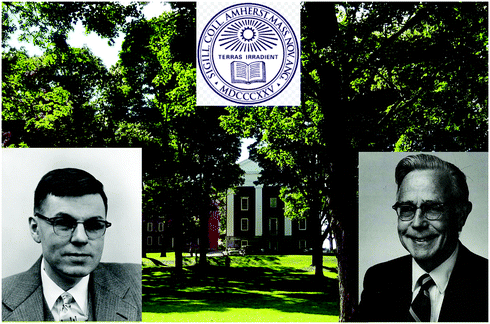 | ||
| Fig. 6 Campus of Amherst College. Lower insets show JPT's college mentors David C. Grahame (left) and William Fairbank (right). Reproduced with permission from © J. Peter Toennies. | ||
Peter's father had a connection to the Sun Oil Company, which provided summer jobs for Peter on its tankers. Fig. 7 shows Peter after his freshman year on a Sun Oil Company tanker, while the tanker was in dry dock for repairs. “We were assigned the job of scraping lime deposits from a boiler that was just large enough that we could crawl into and lie on our backs in it with an open light bulb to see where we were scraping. At one point my perspiring hand fell on the light bulb and it shattered filling the boiler with glass debris. All this at 34 °C (95 F) and 95% humidity. One of many heroic wonderful experiences.” This episode may have led to Peter's later attitude that each graduate student was allowed to break every piece of equipment in the laboratory once in order to learn from it.
 | ||
| Fig. 7 JPT (right) as a wiper after his freshman year in 1949 on a Sun Oil Company tanker in dry dock. Reproduced with permission from © J. Peter Toennies. | ||
In his final year at Amherst, Peter produced his senior thesis under William Fairbank (1917–1989), Fig. 6, who would become well known for his work on liquid helium. Here's what Peter had to say about this experience:5 “I was fortunate in my choice of William Fairbank as my [senior thesis] adviser. He suggested I study paramagnetic resonance in a simple salt using a war-surplus microwave apparatus … Fairbank was always full of enthusiasm, and often when we met he would inform me rather hastily about some new results from the outside world of research physics. Although I really could not grasp or fully understand what he told me, I remember being flattered and inspired by his sharing his excitement with me.”
In 1952, Peter graduated from Amherst and got admitted to Brown University, as a PhD student. Peter also applied to Princeton, but was turned down because he had not taken an organic chemistry course. Brown had other priorities than Princeton, as apparent from the acceptance letter to Peter:5 “We are not so interested in the courses you have taken, but more in your future potential.”
Peter's PhD advisor at Brown was Edward (Ned) Greene (1923–2005), Fig. 8. Peter was Greene's first graduate student. Ned Greene was a shock-wave scientist, trained at Harvard by George Kistiakowsky (1900–1982), Fig. 9, a guru of shock-wave science. Both played a major role in Peter's ontogeny. Peter graduated in 1957, Fig. 10, with a thesis on dissociation energies of small molecules inferred from measurements of the velocities of reflected shock waves. So what was key to Peter's thesis work was the conversion of translational to internal energy via molecular collisions.
 | ||
| Fig. 8 Campus of Brown University, Providence, Rhode Island. The inset shows JPT's PhD advisor Edward (Ned) Greene. Reproduced with permission from © J. Peter Toennies. | ||
 | ||
| Fig. 10 JPT in the laboratory at Brown, in 1957. Reproduced with permission from © J. Peter Toennies. | ||
Although Peter was aware of the unsuccessful attempt of his scientific grandfather George Kistiakowsky & Co. to study collisions by means of molecular beams, he got “bitten by the bug of molecular beams” (in the words of Kistiakowsky) nevertheless. More than that, ever since the eye-opening 1955 experiment by Ellison Taylor and Sheldon Datz,6 Peter has been a molecular beam aficionado (or fascinado, as Peter likes to put it). But before he could do his first molecular beam experiment, Peter had to overcome some hurdles: one of them was the curse cast on molecular beams by George Kistiakowsky, cf.Fig. 9. One of the most influential physical chemists of the time, Kistiakowsky didn’t like molecular beams: In fact he is said to have smashed his molecular beam apparatus with an axe: for, by Kistiakowsky's reasoning, there were no collisions within the beams and there were still no collisions between the beams. Which is kind of true, but not quite, as Peter and others would amply demonstrate later on…
However, Peter had to find a place where to pursue collisional studies. In the spring of 1957, Peter met a kindred spirit visiting the U.S. from Germany – Hans Gerhard Bennewitz (1924–2017), Fig. 11. At that time, Hans Bennewitz had not heard of the Taylor and Datz experiment, but had his own penchant for molecular beams anyway. JPT:5 “[Bennewitz] promised to write to his professor at Bonn, Wolfgang Paul, to get his support [for molecular beam work]. I [was] quite intrigued by the idea of going to Bonn, except for the facts that I had decided … to stay in the United States and, moreover, I had never heard of Paul's laboratory in Bonn.” However he did go to Bonn, in 1957, after receiving this telegram from Wolfgang Paul (1913–1993), Fig. 12:5 “Biete Ihnen Arbeitsplatz und Stipendium an, W. Paul.”† And so Peter started working with molecular beams in the group of Hans Bennewitz. Fig. 13 is from a Christmas party at Bonn with Peter as Santa, for whose impersonation he was uniquely qualified thanks to his beard.
 | ||
| Fig. 11 Hans Bennewitz in his laboratory at Bonn, late 1950s. Reproduced with permission from © J. Peter Toennies. | ||
 | ||
| Fig. 12 Wolfgang Paul, late 1950s. Nobel Prize in Physics in 1989. Reproduced with permission from © J. Peter Toennies. | ||
 | ||
| Fig. 13 JPT as Santa at a Christmas party at Bonn, early 1960s. Reproduced with permission from © J. Peter Toennies. | ||
What is it that Peter is writing, Fig. 14? Perhaps it is his first molecular beam paper, published in 1964.7 In this work, Peter et al. tackled the question of the anisotropy of the atom–molecule potential – what turned out to be just a 1% effect in terms of the measured cross sections. The apparatus, Fig. 15, was built by Peter together with Karl Kramer, a PhD student.
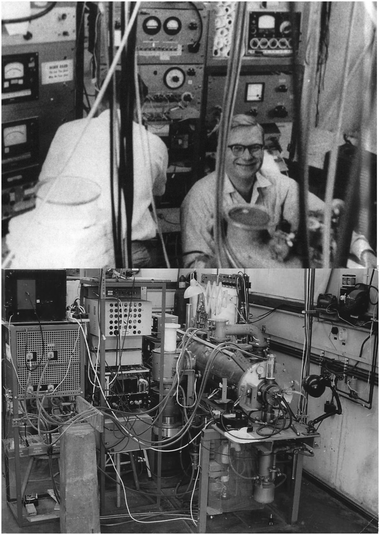 | ||
| Fig. 15 JPT in the lab at Bonn (top); JPT's scattering apparatus (bottom), early 1960s. Reproduced with permission from © J. Peter Toennies. | ||
Or Peter could have been working on his 1965 seminal paper on rotationally inelastic scattering.8 In the ingenious and laborious experiment it describes (it took 4 years to get the first results),2 Peter sent a velocity-selected beam of polar molecules through “polarizer” and “analyzer” quadrupole electric fields, with a scattering center in between. While the polarizer prepared J,M-state-selected molecules, the analyzer would determine in which J′,M′ state the molecules ended up upon scattering by target atoms or molecules in the intermediate region (analogous to Rabi's C field).
Note that apparently during the Bonn period a reversible phase transition was taking place: from “with a beard” to “without a beard.”
As we relay below in Section 4, Peter was appointed director at the Max-Planck-Institut für Strömungsforschung‡ in Göttingen in December 1968 and started his operation there on January 1, 1969. And he has been there happily ever after. A joyful reunion with Peter's Bonn colleagues Hans Pauly, Christoph Schlier, and Dieter Beck in Peter's office in Göttingen (around 1975) is shown in Fig. 16. We note that Peter had made his first acquaintance with Göttingen in 1954 while on a leave of absence from Brown as a Fulbright Fellow, working with Wilhelm Jost and Hans Georg Wagner on spinning detonations.2 The results were written up by the latter, resulting in Peter's first-ever publication.9
In the period from about 1970 to 2000, the Pauly and Toennies departments combined had run about two dozen beam machines and enjoyed the status of a mecca for molecular beam aficionados, much like Otto Stern's laboratory in Hamburg in the 1920s and 30s.
A personal anecdote: When Bretislav§ heard for the first time about Peter's Institute, he didn’t know the word Strömung and inferred from the context that it must mean scattering. For there had been so much scattering work taking place at Peter's institute.
We conclude this part by flashing some photos from the album of the Gemeinschaft that Peter's department was. But firstly, we show Peter as a “conductor of his orchestra,” Fig. 17. We like the orchestra metaphor for it conveys the collaborative character of the work at Peter's department and emphasizes its team spirit and the need for at least a certain degree of harmony. Fig. 18 shows the orchestra, captured by camera – and Peter – at an annual departmental symposium at the Hardehausen Monastery. Fig. 19 is from a trip to Heiligenstadt, in former East Germany, shortly after the German–German border was opened following the Wende (fall of communism and the Berlin Wall). Yuan Lee, an Auswertiges Mitglied of the Max Planck Society, had joined the outing. The happy moment with Manfred Faubel and Christoph Ottinger, Fig. 20, is post-Wende too, likely from 1992, when Andrey Vilesov was an Alexander von Humboldt Fellow with Christoph. Henk Smith and Tim Savas from MIT – the suppliers of the transmission nanogratings – along with those who did some of the wonderful experiments with them, Robert Grisenti, Bodil Holst, and Wieland Schöllkopf, apart from Peter, are shown in Fig. 21. In the background is the spiral tower of the city gate in Duderstadt. Fig. 22 shows Ekkehard Hulpke who was along with Faubel and Ottinger a tenured member of Peter's department, with Wolfgang Sattler, the chief administrator of Peter's institute, whose unbureaucratic approach was much cherished by everyone. Like Yuan Lee, Dudley Herschbach, Fig. 23, is another Auswertiges Mitglied of the Max Planck Society and a big fan of Peter's department, shown here during one of his visits to Göttingen – with Carl Friedrich Gauss and Wilhelm Eduard Weber. Fig. 24 shows a portrait of Peter made by Zdenek Herman, another colleague and friend of Peter's and of his institute.
 | ||
| Fig. 17 JPT addressing his departmental seminar at the MPI für Strömungsforschung in Göttingen, early 1990s. Reproduced with permission from © Bretislav Friedrich. | ||
 | ||
| Fig. 20 Manfred Faubel (left) and Christoph Ottinger (right) with Andrey Vilesov in between. Early 1990s. Reproduced with permission from © Bretislav Friedrich. | ||
 | ||
| Fig. 22 Ekkehard Hulpke (left) and Wolfgang Sattler, in 2010. Reproduced with permission from © Ekkehard Hulpke. | ||
 | ||
| Fig. 23 One of the authors (DH) during a visit to Göttingen, mid-1990s. Reproduced with permission from © Bretislav Friedrich. | ||
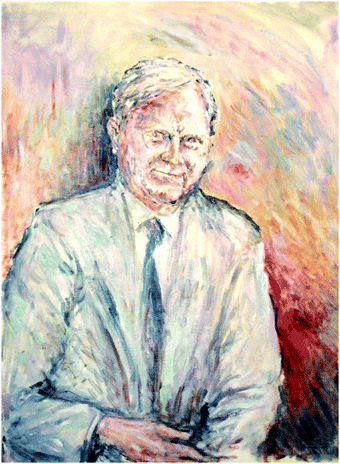 | ||
| Fig. 24 A portrait of JPT by Zdenek Herman (Prague). Oil on canvas, 60 × 45 cm. Reproduced with permission from © Zdenek Herman. | ||
We conclude this section by three quotes by Peter that resonate with the scientific community now and may continue resonating for a long time to come:5
“The lesson I learned was that good science cannot be planned and, moreover, good science can blossom best in a humus of generous support coupled with freedom of ideas and doing.”
“The only solution [of the science funding conundrum] is to reserve substantial funding for off-beat projects for gifted outsiders and to provide them with an environment where there is plenty of room for fruitful serendipity. Perhaps the truly ideal solution was provided by the Max-Planck-Institute system…”
“Working with students and young people has … provided lots of stimulus not only for our research … but also for helping to keep ourselves young, especially in spirit.”
4. More meanderings and serendipity on the way from Bonn to Göttingen
1957 was the year of Peter's PhD from Brown University. His mentors Ned Greene and John Ross had inspired Peter to look for a postdoctoral position to learn about molecular beams. He visited Harvard to ask for advice from Norman Ramsey, who had a brand new book out, Molecular Beams.10 Peter also visited Dudley, then a graduate student doing microwave spectroscopy. Dudley was unprepared for and startled by Peter's billowing red beard, see Fig. 25! But he had fun with Peter discussing molecular beams. | ||
| Fig. 25 JPT during his time in Bonn, about 1960. Reproduced with permission from © J. Peter Toennies. | ||
Peter came when Dudley and Bill Klemperer, then a young instructor, were pursuing ionization of alkali atoms as a function of the surface temperature. Ramsey had kindly lent us one of his beam machines over the Christmas vacation, in 1956. We were gung-ho about beams. Bill used infrared spectroscopy to study alkali halides and was eager to have an electric resonance molecular beam, as described in a chapter in Ramsey's book. J. W. Trischka at Syracuse had such an apparatus and reported a study of lithium chloride, using a tungsten surface ionization filament as a detector. We interpreted Trischka's data such that the detecting reaction depended upon the vibrational state of the incident LiCl molecules. Quickly, we wrote a paper (in February, 1957). Bill wanted to send it to the Proceedings of the National Academy of Sciences because his father, a doctor, read PNAS. Back then, only NAS members could contribute papers, but George Kistiakowsky sent in our paper11 and it was printed (May 1957). Peter must have been surprised; Greene had told him that Kistiakowsky said molecular beams are “a graveyard for good chemists!”
After so many decades, Peter might like to add a few more items to his Review,5 requesting explanations from Dudley (in first person). Along I will skip much. Got my Harvard PhD in May 1958. I moved on to Berkeley and by 1960 and with two graduate students had a rudimentary crossed-beam apparatus that enabled us to measure the angular distributions for the reactants and products. Our first reaction was K + CH3I → KI + CH3. The physics department invited me to give a seminar. After it, I was astonished that Otto Stern, the pioneer of molecular beams, was in the audience! Then met him and heard stories from him.
Another inspiring encounter came in April 1962 at a Faraday Society Discussion on Inelastic Collisions at Cambridge University, UK. This was my first overseas trip. Peter came for the Discussion too. Indeed, he got a room nearby and invited me to sleep on a sofa in the living room! For the Discussion, I was asked to deliver a major paper.12 It was required early (by February) so the participants could read it two months ahead. Peter gave a neat description of his work with Hans Bennewitz in Bonn. They built a superb molecular beam apparatus. It evolved to measure rotational quantum state-to-state inelastic collisions of polar molecules with other atoms or molecules.13 After the Faraday Discussion, I visited Bonn. Peter took me on a long walk in the hills above the Rhine and we discussed research many hours in a hillside café. I remember that Peter asked how many chemical labs will likely be equipped with beam apparatus? My answer was “not many.” Peter urged me to take a Rhine boat to Rüdesheim. It was a lovely Easter Sunday, nine-hour trip, music and dancing. Watching couples, I realized that I worked too much and must have a social life!
Back at Berkeley, in fall 1962, I met Professor Wilhelm Jost, a physical chemist on sabbatical from Göttingen University. I was giving a course on Chemical Kinetics and Molecular Collision Dynamics, and he invited me to give the course as a visiting professor at Göttingen next summer. I gladly accepted, and could visit Peter at Bonn again. But I did not anticipate in spring 1963 that Harvard wanted me to return as a professor. I had great affection for both places, but settled for Harvard. So that summer, my group moved together with all our lab equipment to a new lab at Harvard, while I went to Göttingen. There late June, all July, one week in September, but Germans kept August as vacation (and I went to Greece). I met many interesting people and was glad the audience for my course was sizable. Most intriguing was meeting Manfred Eigen (1927–2019), Fig. 26, and his use of high-frequency sound waves to elucidate rapid chemical reactions. In mid-July, Jost took a trip (at terrific speed in his car) to Bonn for a day, and I went with him to catch up with Peter. In my course, I enjoyed describing some of Peter's results.
My jaunt to Göttingen had happy outcomes. The Harvard Chemistry (years back) had an endowed “visiting professor” that could come for a semester. Each year one of the tenured professors had the choice to pick the visitor. In 1965, I had the choice and luckily Manfred Eigen was glad to come. His lectures were splendid; he drew a wide crowd: physical, organic, and biochemists, together with physicists and biology researchers. Also, Manfred was welcome in social events, often performing piano sometimes along with Bill Lipscomb's clarinet. Manfred was interested in molecular beams, so I took him around the labs of Ramsey, Klemperer, and mine. As well, I told Manfred about Peter's superlative qualities. Up to 1965, Hans Pauly and Peter had a major paper14 (143 pages) about beams in a new journal; I gave a copy to Manfred. With other faculty, I nominated Manfred for an honorary doctorate and it was awarded at the 1966 Harvard commencement. Moreover, in 1967 he was awarded a Nobel Prize.
Manfred sparked a new research direction for the Max Planck Institute at Göttingen, and in July 1968, Hans and Peter were offered to be Members of the Max Planck Society and Directors at the MPI für Strömungsforschung in Göttingen. They were delighted. They had come to a Gordon Research Conference in New Hampshire. On their way back to the airport, I took them to a venerable tobacco shop near Harvard and gave them three presents as foot-long cigars in fine boxes. The third cigar was for Heinz Georg Wagner (1928–2020), a wise and helpful friend, also joining the Göttingen Max Planck Institute. During the summer of 1963, Wagner, then a Privatdozent, often took me to lunch with dessert at the Cron & Lanz, Heisenberg's favorite coffeehouse. Wagner kindly taught me much about German history and culture.
5. Peter received many awards
To our knowledge, Peter is the recipient of the following awards:1964 Physics Prize of the Academy of Sciences, Göttingen
1983 Fellow of the American Physical Society
1988 Alumni Citation, Brown University
1990 Corresponding Member of the Academy of Sciences in Göttingen
1991 Gold Heyrovsky Medal of the Czech Academy of Sciences
1992 Hewlett-Packard Europhysics Prize for solid state physics
1992 Max-Planck-Prize of the German Research Foundation and the Alexander von Humboldt Foundation
1993 Member of the German National Academy “Leopoldina” in Halle, Germany
1996 Recipient of the first MOLEC Conference Award
1999 Honorary Fellow of the International Molecular Beams Symposium
2000 Honorary Doctorate in Philosophy, University of Gothenburg, Sweden
2002 Stern-Gerlach Medal of the German Physical Society
2005 Kolos Medal of the University of Warsaw
2006 Benjamin Franklin Medal in Physics (with Giacinto Scoles)
2007 Honorary Doctorate in Science, Amherst College, MA, USA
2013 Dudley Herschbach Prize in Molecular Collision Dynamics
The last award on the list Peter received at the Conference on the Dynamics of Molecular Collisions. It is named for Dudley Herschbach, who agreed to design the Medal, in 2007. The Medal has two sides, Fig. 27. That for Theory (left) symbolizes, rather whimsically, yearning to attain an exalted, exhilarating comprehension. That for Experiment (right) symbolizes, more realistically, grasping for incisive means to find out what actually happens in encounters of atoms, molecules, and/or photons.
When mounted like a lollypop on a stand, the Medal thus has translational, rotational, and vibrational degrees of freedom, again aptly symbolic of molecular dynamics. The unusual shape of the Medal also has special and specific meaning, left up to the recipients to divine. Criteria for the awards are: “For bold and architectural work, inspiring and empowering. Such work addresses fundamental, challenging, frontier questions; brings forth new perspectives and capabilities; and typically excites evangelical fervor.”
6. EpilogueThe bountiful achievements of Peter's institute were fostered by a convivial and congenial atmosphere. Raucous laughter would often resound in the hallways together with Peter's exclamations “Sehr schön”¶ when he was presented with new exciting results. For half a century, Peter and his group have performed elegant, incisive, and lucid molecular beam experiments that have, in Stern's tradition, resolved numerous outstanding questions and inspired many new ones.Happy birthday, Peter! We thank you for your constant enthusiasm, grace, and friendship – all of which mean so much to so many.
An Ode for Peter Peter, delightful explorer Enters intriguing perspective That begets many experiments! Euphoria bursts at Göttingen, Rapt with serendipitous joy!
Toennies inspires other wizards Obsessed with fresh adventures, Exciting them to find genuine Novelty and reach the occult. Nature celebrates such exploits! In coherent chorus we hail Every gleeful meeting with you; Sagacious, yet most rumbustious!
Peter's response: Your wizardly sagacious ode was celebrated by Peter with gleeful rumbustiousness! It begot genuine euphoric coherent bursts from a gleeful celebrating chorus of Toennies exciting them by the genuine occult novelty and intriguing perspective to reach delightful joy. We all hail the serendipitous explorer!! |
References
- J. Phys. Chem. A, 2011, 115, 6739–7400. J. Peter Toennies Festschrift Special Issue.
- J. P. Toennies, Autobiography of JPT, J. Phys. Chem. A, 2011, 115, 6742–6745 CrossRef CAS PubMed.
- B. Friedrich, A Toast to Jan Peter Toennies, J. Phys. Chem. A, 2011, 115, 6739–6741 CrossRef CAS PubMed.
- G. Benedek and J. P. Toennies, Atomic Scale Dynamics at Surfaces: Theory and Experimental Studies with Helium Atom Scattering, Springer, Heidelberg, 2018 Search PubMed.
- J. P. Toennies, Serendipitous Meanderings and Adventures with Molecular Beams, Annu. Rev. Phys. Chem., 2004, 55, 1–33 CrossRef CAS PubMed.
- E. H. Taylor and S. Datz, Study of Chemical Reaction Mechanisms with Molecular Beams. The Reaction of K with HBr, J. Chem. Phys., 1955, 23, 1711 CrossRef CAS.
- H. G. Bennewitz, K. H. Kramer, W. Paul and J. P. Toennies, Messung der Anisotropie des van der Waals-Potentials durch Streung von Molekülen in definiertem Quantenzustand, Z. Phys., 1964, 177, 84–110 CrossRef CAS.
- J. P. Toennies, Molekularstrahlmessungen von Stossquerschnitten für Übergänge zwischen definierten Rotationszuständen zweiatomiger Moleküle, I Experimentelle Methode, Z. Phys., 1965, 182, 257 CrossRef.
- J. P. Toennies and H. G. Wagner, Photographische Untersuchungen an spinnenden Kohlendioxyd-Sauerstoff-Detonationen, Z. Elektrochem., 1955, 59, 7 CAS.
- N. F. Ramsey, Molecular Beams, Oxford Press, 1956 Search PubMed.
- W. Klemperer and D. Herschbach, Variation of Reaction Rate with Vibrational State, Proc. Natl. Acad. Sci. U. S. A., 1957, 43, 429–435 CrossRef CAS PubMed . See also K. T. Gillen and R. B. Bernstein, Chem. Phys. Lett., 1970, 5, 275–277 CrossRef.
- D. R. Herschbach, Reactive Collisions in Crossed Molecular Beams, Discuss. Faraday Soc., 1962, 33, 149–161 RSC.
- J. P. Toennies, Inelastic Collisions, Discuss. Faraday Soc., 1962, 33, 96–98 Search PubMed.
- H. Pauly and J. P. Toennies, The Study of Intermolecular Potentials with Molecular Beams at Thermal Energies, (305 references), in Advances in Atomic & Molecular Physics, ed. D. R. Bates and I. Estermann, Academic Press, 1965, vol. I Search PubMed.
Footnotes |
| † I offer you a place to work and a stipend, W. Paul. |
| ‡ Its forerunner, the Kaiser-Wilhelm-Institut für Strömungsforschung, was established in 1924 with Ludwig Prandtl (1875–1953) as its founding director and incorporated into the Max Planck Society in 1948. In 2004, after JPT's retirement, the institute changed its name to MPI für Dynamik und Selbstorganisation. |
| § Alexander von Humboldt Fellow at JPT's department in 1986–1987 and 1992. |
| ¶ Very nice! |
| This journal is © the Owner Societies 2021 |