Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Naturally derived nano- and micro-drug delivery vehicles: halloysite, vaterite and nanocellulose
Anna
Vikulina
 *a,
Denis
Voronin
*a,
Denis
Voronin
 bc,
Rawil
Fakhrullin
bc,
Rawil
Fakhrullin
 bd,
Vladimir
Vinokurov
bd,
Vladimir
Vinokurov
 b and
Dmitry
Volodkin
b and
Dmitry
Volodkin
 be
be
aFraunhofer Institute for Cell Therapy and Immunology, Branch Bioanalytics and Bioprocesses, Am Mühlenberg 13, 14476 Potsdam-Golm, Germany. E-mail: anna.vikulina@izi-bb.fraunhofer.de; Tel: +49-331-58 187-122
bGubkin Russian State University of Oil and Gas, Department of Physical Chemistry, Leninsky pr. 65-1, Moscow, 119991, Russian Federation
cSaratov State University, Educational and Research Institute of Nanostructures and Biosystems, Astrakhanskaya 83, 410012 Saratov, Russian Federation
dKazan Federal University, Institute of Fundamental Medicine and Biology, Kreml uramı 18, Kazan, Republic of Tatarstan, 420008, Russian Federation
eSchool of Science and Technology, Nottingham Trent University, Clifton Lane, Nottingham NG11 8NS, UK
First published on 18th March 2020
Abstract
Recent advances in drug delivery and controlled release had a great impact on bioscience, medicine and tissue engineering. Consequently, a variety of advanced drug delivery vehicles either have already reached the market or are approaching the phase of commercial production. Progressive growth of the drug delivery market has led to the necessity to earnestly concern about economically viable, up-scalable and sustainable technologies for a large-scale production of drug delivery carriers. We have identified three attractive natural sources of drug carriers: aluminosilicate clays, minerals of calcium carbonate, and cellulose. Three classes of drug delivery carriers derived from these natural materials are halloysite nanotubes, vaterite crystals and nanocellulose. These carriers can be produced using “green” technologies from some of the most abundant sources on the Earth and have extremely high potential to meet all criteria applied for the manufacture of modern delivery carriers. We provide an up-to-date snapshot of these drug delivery vehicles towards their use for bioapplications, in particular for drug delivery and tissue engineering. The following research topics are addressed: (i) the availability, sources and methodologies used for production of these drug delivery vehicles, (ii) the drug loading and release mechanisms of these delivery vehicles, (iii) in vitro, in vivo, and clinical studies on these vehicles, and (iv) employment of these vehicles for tissue engineering. Finally, the prospects for vehicles’ further development and industrialisation are critically assessed, highlighting most attractive future research directions such as the design of third generation active biomaterials.
1. Introduction
During the last few decades, emerging new drug delivery technologies allowed control of the design and fabrication of various materials at the nanoscale, which revolutionised biomedical science and significantly impacted the fields of nanomedicine, diagnostics and tissue engineering. Since drug delivery allows controlled transfer of drugs to the target with a high efficiency and minimised adverse side effects, drug delivery vehicles have progressively become an integral part of modern medications. Besides this, the costs for the development of new clinical therapeutics might be estimated as $100–500 million, while the development of new delivery vehicles is much less expensive and generally gives significantly quicker profit.1 This provides an additional economic reason for the boost of drug delivery technologies.Despite their promising prospects and their success in drug delivery research, new delivery vehicles are making their way from science to the market extremely slow. This is due to multiple factors, and most of them are not related to science and range from rather complicated policies and procedures for Food and Drug Administration (FDA) agency approval to high fabrication costs that largely pose a manufacturing challenge. Indeed, sometimes the costs for manufacturing drug delivery vehicles much exceed the costs for the drug itself. For instance, liposomal doxorubicin Doxil (the drug widely used for the treatment of ovarian cancer, sarcoma and myeloma) costs ca. $130 per unit, which is 40–100 times higher than that of free doxorubicin (ca. $1.3–3 per unit; USA prices). Another complication is a multi-step fabrication of drug delivery carriers that is often challengeable to upscale, at least using modern technologies. For instance, upscaling remains a serious limitation for the commercialisation of polymeric nanoparticles.2 Finally, the use of animal and plant sources for the isolation of substances for delivery carriers (e.g. lipids, biopolymers, proteins) is quite common.3 Low sustainability of delivery carriers production stimulates scientists to think about the possible negative environmental impact that the global production of delivery vehicles may have.4
In view of the above, the quest for alternatives sources for drug delivery vehicles – minerals, bacteria and waste materials – resulted in the rise of a new wave of drug delivery research. Scientists have proposed dozens of novel drug delivery vehicles, the most important examples of which are clays, minerals of calcium salts such as carbonates and hydroxyapatites, and cellulose-based materials. Typical examples of nano- and micro-sized particles made of such materials are halloysite nanotubes (HNTs), vaterite CaCO3 (VCC) and nanocellulose (NC).
All these materials exhibit null to low toxicity, good biocompatibility and/or biodegradability. These delivery vehicles may be loaded with tremendously diverse compounds ranging from small drugs to macromolecules and from highly hydrophilic to solely lipophilic encapsulates. Importantly, material costs required for their production are significantly lower than those for conventional delivery vehicles (Table 1). A variety of fabrication technologies proposed during the last few decades lays a platform for their swift industrialisation.
| Drug delivery vehicles | Price, $ per g of dried weight |
|---|---|
| DPPC – dipalmitoylphosphatidylcholine; PLGA – poly(lactic-co-glycolic acid).a Lab grade chemicals; prices provided by official distributers – Sigma Aldrich; Merck; Fisher Scientific; Polysciences; Cellulose Lab; Avanti Polar Lipids, etc.b Produced from CaCl2·2H2O and Na2CO3. | |
| Liposomes (DPPC) | 90–170 |
PLGA (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 1–2 dL g−1) 50, 1–2 dL g−1) |
30–70 |
| Halloysite nanotubes | 0.3–0.4 |
| Vaterite CaCO3 | 0.2–0.4b |
| Nanocellulose | 0.8–5.5 |
In addition to lower material costs, production of HNTs and NC is commonly reduced to their isolation and purification from abundant sources (Fig. 1), which is generally less expensive compared to sophisticated methods for the fabrication of conventional vehicles such as liposomes and polymer beads. Calcium carbonate is also an abundant natural mineral that can be isolated from natural sources; however, the most common in nature is calcite, a nonporous polymorph of CaCO3, while mesoporous VCC (more suitable for biomedical applications) is not so abundant. Because of low abundance of vaterite in nature, its bottom-up production is economically more profitable. NC growth by bacteria is also widely proposed as a sustainable alternative to plant-derived sources. Moreover, “green” strategies for the production of nanocellulose from wood or paper waste5,6 as well as vaterite from eggshell and seashell waste7,8 are now being widely discussed.
Although these “sustainable” delivery vehicles have not dislodged their conventional analogies yet, they have rapidly gained popularity and have extremely high potential to enter the market of drug delivery vehicles in the nearest future.
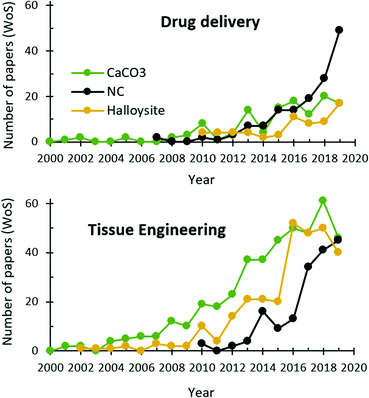
In this paper, we discuss the up-to-date knowledge on and prospects for biomedical use of three naturally derived delivery vehicles: HNTs, VCC, and NC. All of them have been known for ages but discovered as drug delivery vehicles quite recently and witnessed an exponential growth of scientific interest since that time (Fig. 2). This article aims at uncovering key features of HNTs, VCC and NC in a comparative manner, revealing their strong and weak sides for drug delivery and tissue engineering/regeneration applications, and evaluating the prospects for their further study and biomedical use.
2. Clay nanotubes
2.1. Clay-based nanomaterials
Among the variety of different minerals, clay minerals are the most widely used for therapeutic purposes due to their high specific area, chemical and mechanical stability, biocompatibility and low toxicity for humans. Nanoclays are largely abundant in nature, which eliminates the need for their industrial synthesis. The only exception is LAPONITE®, a synthetic equivalent of hectorite produced by Laporte (Netherlands). Other nanoclays exist in nature; these minerals refer to the group of phyllosilicates, also called “sheet silicates”. Nanoclays are hydrous alumina or magnesium silicates (sometimes with variable amounts of other metals) composed of parallel sheets of Si–O tetrahedral and octahedral sheets containing metal atoms (Al, Mg) coordinated with the oxygen from Si–O tetrahedra and OH groups.Nanoclays are very diverse and classified based on the number of tetrahedral and octahedral sheets per clay layer as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 minerals. Thus, kaolinite and halloysite (Fig. 3), the polymorphs of Al2Si2O5(OH)4, are 1
1 minerals. Thus, kaolinite and halloysite (Fig. 3), the polymorphs of Al2Si2O5(OH)4, are 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 clays in which one octahedral sheet is bonded to one tetrahedral sheet, yielding a 7 Å repeat along the z-axis. These layers may have lateral dimensions from tens of nanometres to micrometres.9 Several layers may interact with each other via electrostatics and van der Waals interactions, hydrogen bonding or interlayer cations that ensure the diversity of nanoclays. The mineralogical properties of nanoclays are described in detail in a recent review.10
1 clays in which one octahedral sheet is bonded to one tetrahedral sheet, yielding a 7 Å repeat along the z-axis. These layers may have lateral dimensions from tens of nanometres to micrometres.9 Several layers may interact with each other via electrostatics and van der Waals interactions, hydrogen bonding or interlayer cations that ensure the diversity of nanoclays. The mineralogical properties of nanoclays are described in detail in a recent review.10
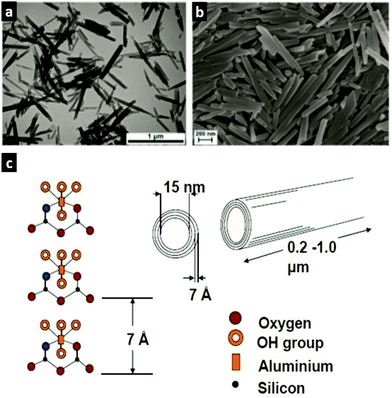 | ||
| Fig. 3 Morphology and crystal structure of halloysite nanotubes: (a) TEM image and (b) SEM image of HNTs. Adopted with the permission from ref. 11, copyright@ 2019 John Wiley & Sons. (c) Crystal structure and dimensions of HNTs. Adopted with the permission from ref. 12, copyright© 2008, American Chemical Society. | ||
Halloysite is a significant component of many kaolinitic clays that commonly exists in a mixture with kaolinite, silica, or other mineral contaminants. Production of pure HNTs requires careful separation of halloysite from accompanying minerals and therefore the economical costs of HNTs directly depend on the initial purity of halloysite deposits. In this respect, two main sources of HNTs are located in Northland, New Zealand, and Utah, USA, where large deposits of high-grade halloysite are found. Smaller or lower-grade deposits are also found in many countries in Asia, South America, Australia, and Europe.13 Annually, about 30 kilotonnes of halloysites are excavated and processed into dispersed nanotubes.14
Although no direct approval of HNTs has been done by the FDA so far, kaolin (a mineralogical group of clays to which halloysite also belongs) is approved as an indirect food substance generally recognised as safe (e.g. as a possible component of paper and paperboard that contact food) under §186.1256. Besides this, colloidal kaolin is approved as a “drug product containing certain active ingredients offered over-the-counter”, §310.545, with the note that there are inadequate data to properly assess its safety and effectiveness. Therein, kaolin is proposed as an antidiarrheal drug product.
The fact that clays are generally recognised as safe for humans makes biomedical use of nanoclays and in particular HNTs highly promising. The next two sections describe advantages, limitations and up-to-date achievements in utilisation of HNTs in drug delivery and tissue engineering.
2.2. HNTs for drug delivery
In general, bare HNTs exhibit weak bonding with drugs. Chemical modification of both the inner lumen and the outer surface is a general strategy to tune the capacity and drug delivery performance of HNTs.16 Multiple Si–OH and Al–OH groups present at the HNT surface provide various ways for this modification. The lumen covered with Al–OH groups can be grafted with organosilane and organophosphonic acid.17 For instance, the functionalisation of HNTs with (3-aminopropyl)triethoxysilane (APTES) introduces hydrogen-binding sites and thus enhances the retention of drugs that are prone to form H-bonds.18 Insights into the surface modification of HNTs are substantially described in reviews 15 and 19. The external surfaces of HNTs can be coated with positively charged polymers. Thus, grafting of HNTs with an amine of polyethylene glycol enhanced cellular uptake and improved the loading of low water-soluble antioxidant quercetin.20
Undoubtedly, the possibility of both loading a drug into the lumen and grafting it to the outer surface offers some advantages for drug delivery. HNTs are suitable for entrapment of various drugs (analgesics,18,21 anticancer drugs,22,23 antibacterials,24etc.) and especially attractive for the entrapment of large macromolecules (genes,25 enzymes26) and nanoparticles (quantum dots,27 metal nanoparticles,28 more examples reviewed in ref. 29), whose sizes correlate well with the diameter of the lumen. Some recent reviews have described the loading of molecules and particles of various natures in more detail.24,30,31
Some recent studies have indicated that HNTs are excellent tablet excipients (e.g. if mixed with passive components such as microcrystalline cellulose32 and starch33 or layered with alginate and chitosan34) due to their powder flow and compressibility properties.
Owing to the fact that HNTs have different sites for drug loading, it is not surprising that the release of the payload directly depends on the way in which the drug was loaded. Thus, the release of drugs from the lumen is mostly controlled by the diffusion of the drug molecule from the nanotube interior that is substantially retarded by the 1D nanotube structure. Thus, the release of analgesics (ibuprofen,18 aspirin,35 sodium salicylate36) from bare HNTs obeys the Fickian diffusion model. Different modifications of the HNT lumen allow adjusting the affinity of the drug to the HNTs and therefore controlling the release rate.18,36 For instance, the release of ibuprofen from APTES-modified HNTs is governed by non-Fickian diffusion due to the additional electrostatic binding of the drug to the APTES-coated lumen that ensures slower release compared to unmodified nanotubes.18 Besides this, the functionalization of HNTs with stimuli-responsive materials (e.g. pH-responsive polymers,22 thermosensitive polymers,37 magnetic nanoparticles38) allows tuning the release profile over a much wider range, e.g. for achieving sustained release for days38 and sometimes weeks.22 Alternatively, clogging of the ends of the tubes with polymers (e.g. dextrin39) allows controlling the release and extending it up to weeks. This strategy and other strategies are reviewed in ref. 40. The release of drugs linked to the external surfaces of HNTs strongly depends on the nature of drug–surface binding. Since this binding often involves strong covalent interactions,41 the release of drugs from the outer surface might be slow, prolonged and sometimes incomplete as was demonstrated for curcumin covalently linked to the outer surfaces of the HNTs.41
Numerous in vitro studies have pointed out no or a rather low toxicity of HNTs.44,45 This was also confirmed in in vivo studies on nematodes46 and zebrafish.47 The effect of HNTs on cells is dose-dependent as has been shown for human cell lines.48 Recently, the size-dependent character of cellular internalisation of HNTs (250–650 nm in length) has been demonstrated in colon cancer cells.49 A toxic effect was reported only when high doses of HNTs were orally administered into mice, while low and moderate doses remain non-toxic.50 Since HNTs are non-toxic even at high doses (concentrations of 1000 μg mL−1 and higher46), they can be suitable for nearly all administration routes. For instance, HNTs loaded with a neurotransmitter (γ-aminobutyric acid) were successfully attempted for delivery to the brain.51 Functionalisation of HNTs with mucoadhesive polymers enhances their interactions with intestinal cells and causes no cytotoxicity.52
Topical administration of HNT coatings on hair surfaces was recently proposed. Inclusion of selected dyes or drugs into HNTs prior to their application allows for hair coloring or medical treatment, respectively.53
Recent in vivo studies confirmed the potential of HNTs as effective drug delivery containers, showing that treatment with curcumin-loaded HNTs suppresses the growth of pathogenic bacteria in C. elegans and completely restores the longevity of infected nematodes.54 An in vivo study of pharmacokinetics revealed that an orally administered suspension of fluorescently labelled HNTs is not absorbed by the intestines and eliminated via feces within one day, while systemically administered nanotubes are eliminated via urine/feces within nearly 72 h.55 Notably, although in this study and some other studies23 HNTs are intravenously injected, HNTs are non-biodegradable in blood. Indeed, recent studies have not recommended direct injections of HNTs that may lead to a thrombosis.45 Besides this, a recent study demonstrated that inhalation of HNTs may cause oxidative stress, inflammatory response, and autophagy and therefore results in sub-chronic toxicity in mice.56 However, this does not hinder the use of HNTs for drug delivery via alternative routes as well as their use in tissue engineering.
2.3. HNTs for tissue engineering
In recent years, functionalisation of natural and synthetic polymer scaffolds with nanoscale dopants has been widely attempted. Among others, HNTs have shown great promise as an additive to polymeric tissue engineering scaffolds due to their excellent biocompatibility and controlled drug delivery behaviour. Importantly, HNTs can be mixed with almost any materials used for scaffold fabrication employing traditional polymer blending methods. It has been verified that the introduction of only a few wt% of HNTs leads to a significant improvement of scaffold materials. At the same time, the incorporation of extremely high contents of HNTs (up to 80 wt%) can be safely used to form hard tissue substitutes.57With respect to tissue engineering applications, the use of HNTs promotes the attachment, viability, proliferation, adhesion, and growth of cells and improves the mechanical and thermal stability of scaffolds, having no negative impact on the biocompatibility of the scaffolds.58,59 The good blood compatibility of HNT-doped scaffolds has also been confirmed by haemolysis tests.59 Later, blood vessel formation around HNT-doped polymer 3D scaffolds has been demonstrated.60 The scaffolds used in this study are shown in Fig. 4.
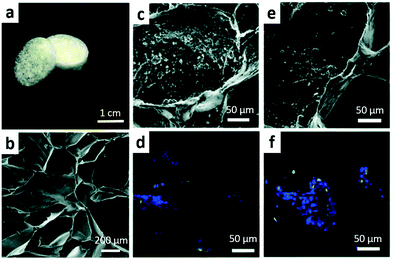 | ||
| Fig. 4 (a) Photograph and (b) SEM image of pillar-shaped 3D porous hydrogel scaffolds functionalised with 6 wt% of HNTs and SEM and confocal images of (c and d) A549 and (e and f) Hep3B cell distributions on them. Adopted with permission from ref. 60, copyright@ 2016, Royal Society of Chemistry. | ||
Besides this, HNT-doped scaffolds benefit from the loading of the nanotubes with various functional compounds such as antimicrobial agents61,62 and the cues essential for tissue regeneration (e.g. growth factors63).
Most of these studies have focused on in vitro evaluation using model cell lines and stem cells. In vivo case studies are still rather limited. Examples include (i) functionalised wound dressing materials like HTNs/chitosan64 and HNTs/poly(L-lactide)61 which demonstrated improved skin reepithelialization; (ii) nanocomposites for bone tissue regeneration65 wherein HNTs themselves stimulated osteogenic differentiation of cells and improved bone repair; (iii) HNT-functionalised alloys with improved corrosion resistance for orthopaedic applications66 and (iv) aligned composites with HNTs for guided nerve regeneration.63
3. Calcium carbonate
3.1. CaCO3 and its polymorphs
Anhydrous calcium carbonates exist in the form of one of the three main polymorphs: calcite, aragonite, or vaterite. All the polymorphs may be found in nature.67 Calcite is the major component of carbonate rocks and hard tissues in marine organisms (seashells) and eggshells. Although traces of vaterite and slightly more abundant amounts of aragonite were found in mollusc pearls, fish otoliths, and ascidians,67 calcite represents the only stable phase of anhydrous CaCO3 and thus much dominates over less stable vaterite and aragonite. Unfortunately, the most common calcite is of least interest for biomedical applications due to its non-porous structure and thus low capacity for drug loading. As opposed to calcite, crystals of aragonite and especially vaterite have a highly developed internal structure suitable for hosting molecules of interest of various natures; this largely determined the use of vaterite in nanomedicine.Unfortunately, the occurrence of vaterite in nature is low. Vaterite can be (bio)mineralised by some calcifying bacteria, yet this approach is rather expensive.68 Therefore, the main source of vaterite remains calcite. Direct transformation of calcite to vaterite or aragonite is thermodynamically restricted;69 nevertheless, vaterite can be synthesized from calcite in a few steps. First, CaCO3 in the form of calcite is degraded by the so-called Solvay process in a reaction with NaCl to produce its soluble salts (e.g. Na2CO3 and CaCl2). In much of the world, this is the major industrial process for the production of sodium carbonate, wherein CaCl2 is the by-product. Second, CaCO3 in the form of vaterite can be precipitated from Na2CO3 and CaCl2 upon mixing solutions of these precursor salts.70 Nowadays, limestone is the primary source of calcite used for the Solvay process. Nevertheless, the use of waste materials – seashells7 and eggshells8 – is an excellent green alternative to rocks, which makes industrially produced vaterite an even more attractive material in future applications.
In view of high interest in VCC, its structure was widely investigated.69,71 VCC comprises of small nanocrystallites aggregated together (Fig. 5). The spaces between these nanocrystalline form interconnected cylindrical pores that are usually in the range of a few tens of nm. Therefore, VCC belongs to mesoporous materials. VCC crystals have typical dimensions ranging from 3 to 20 μm and most often have a spherical shape. A number of recent studies have also proposed novel ways for the fabrication of VCC nanocrystals72–74 indispensable for drug delivery applications. The shape and morphology of these crystals may be tuned over a wide range using organic solvents73 or mild water–urea mixed systems,75 polymer matrices and proteins74 or nanoparticles.76 The porosity of the crystals can be controlled via the variation of preparation temperature with no additives.70
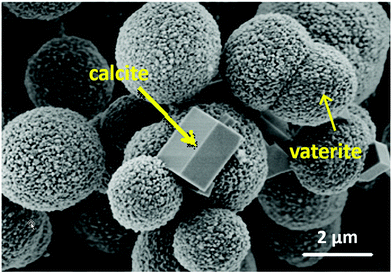 | ||
| Fig. 5 SEM image of CaCO3 crystals demonstrating the mesoporous nature of spherical vaterite and non-porous calcite crystals. Adopted from ref. 77, copyright© 2019, Elsevier. | ||
The FDA has approved the use of CaCO3 (without specifying its polymorphism) as a food additive (§582.1191), a dietary supplement (§582.5191), a direct food substance (§184.1191), and a colour additive for food (§73.70) and drugs (§73.1070). Similar to kaolin clay, calcium carbonate is also mentioned in §310.545, where it is proposed as an antidiarrheal, digestive and weight control drug product. CaCO3 is registered as a food additive (white food colouring) under E170 code. As a dietary supplement, calcium carbonate may be prescribed when calcium taken in the diet is deficient. It comes in the form of tablets, capsules, or suspensions taken orally and sold under such brands as Calel-D®, Calcid®, and Os-Cal 500®. The wide use of CaCO3 in food preparation and the diet premises safe-by-design utilisation of CaCO3 as a drug vehicle in future applications.
3.2. VCC for drug delivery
Typical pores of VCC are in the range of a few tens of nm that in general makes the encapsulation of large molecules/particles more effective when compared to small drugs. In order to overcome the relatively low capacity of crystals to small molecules, the internal volume of the crystals can be filled with a polymer matrix that has high affinity to desired drugs. This strategy to make composite hard–soft vehicles has been widely reported. Examples include cyclodextrin–CaCO3 hybrids designed as carriers for hydrophobic drugs and hormones.89 Carrageenan–CaCO390 and mucin–CaCO3 hybrids91 were produced to host doxorubicin, etc. Besides enhancing loading capacity for small drugs, the inclusion of polymers inside crystals may also stabilise them and slow down the VCC recrystallization to calcite that is important for modulating the drug release profile.91
Loading of molecules into VCC can be performed under mild conditions either during crystal growth (this approach is usually denoted as co-precipitation or co-synthesis76,92) or by means of post-loading into pre-formed VCC crystals (e.g. via adsorption into crystal pores92 or a recently introduced freeze-drying approach93). Among these approaches, adsorption and co-synthesis are two primary methods for drug encapsulation. Adsorption represents the most “mild” method suitable for the encapsulation of labile molecules prone to aggregation, denaturation and the loss of activity in aggressive media.81,82,94 In contrast, for co-precipitation mixing of drug solution with precursor salts results in more effective entrapment and homogeneous distribution of the drug inside the crystals during their growth. However, because of more harsh conditions of crystal growth (high ionic strength and elevated pH), much higher encapsulation efficiencies may be accompanied by a partial loss of activity for sensitive macromolecules,81 while small drugs are usually unaffected. Finally, crystals loaded by means of adsorption are usually prone to faster drug release than crystals loaded by co-synthesis.82
Crystal coating with lipids,95 polymers79 or polymer multilayers76 fabricated via polymer layer-by-layer assembly is often proposed to reduce drug leakage and prolong drug release kinetics. Notably, polymer multilayers are permeable for ions and small molecules, which allows the formation of polymer based capsules when multilayer coated VCC crystals are decomposed by lowering the pH or adding chelating agents (e.g. ethylenediaminetetraacetic or citric acid). CaCO3-templated multilayer capsules are very promising delivery vehicles; their potential for drug delivery has been widely investigated,96–99 this also includes in vitro97 and in vivo studies.98 This article does not consider VCC-templated capsules and beads;100 for these topics the reader can refer to reviews 101 and 102.
The release of drugs from VCC is governed either by VCC dissolution or by VCC recrystallization. Thus, dissolution-mediated release occurs in case of intracellular VCC delivery, when VCC entrapped inside lysosomes is influenced by acidic lysosomal pH (∼4.5–5.0) that promotes VCC dissolution and results in release of the loaded molecules.78 Recent studies of the use of VCC as an anticancer drug delivery vehicle have shown an interesting possibility for employing VCC sensitivity to acidic pH to target the release selectively on a tumor microenvironment that has pH slightly below 7.95,103,104 Later, this approach has shown to be effective not only for in vitro but also for in vivo studies. It is worth noting that targeting an acidic tumor microenvironment is quite a versatile approach for anticancer therapy and has been successfully employed not only for VCC but also for other carriers, e.g. polymeric nanoparticles;105 this supports the potency of this strategy. The pH sensitivity of VCC crystals limits their oral administration (at least if the crystals are not functionalized) due to the swift VCC dissolution and the loss of the payload in the stomach (pH ∼ 1.5–3.5).
Recrystallization-mediated release occurs due to the phase transition of thermodynamically unstable vaterite to more favorable and non-porous calcite with much lower surface area and therefore much lower drug capacity.77,91,106 Recrystallization and, as a result, release kinetics can be effectively controlled via the use of additives that fill the pores of the crystals. For instance, hybrids of VCC with ferromagnetic nanoparticles recrystallize to calcite significantly more rapidly than bare vaterite crystals, while multilayer coating or the co-precipitation of the polymers into VCC crystals may decelerate vaterite → calcite transformation.76,91 Notably, plasma proteins like bovine serum albumin (BSA) may also significantly adsorb to the surfaces of VCC crystals, which is of high importance for intravenous administration. Plasma proteins not only suppress the VCC recrystallization and therefore allow prolonged circulation of the crystals but also minimize the neutrophil activation caused by VCC binding to the membranes of the neutrophils.107
Thus, intravenous injections were applied for the treatment of cancer in mouse models wherein VCC crystals were used as carriers either for doxorubicin employed in chemotherapy95,104 or for cocktails of catalase, fluorescent probes with two-photon excitation, and traditional photosensitizers, i.e. as a platform for photodynamic therapy.112 Both studies have revealed high promise of the use of VCC crystals. In general, anticancer drug delivery seems to be a major biomedical application of VCC attempted so far. Along with the targeting the acidic tumor microenvironment mentioned above, there are some other advantages of VCC. One of them is simple one-step loading of VCC with multiple functional components, e.g. combinations of magnetic nanoparticles and anticancer drugs, which allows external navigation of VCC to tumor sites.113 Similarly, VCC crystals loaded with glucose oxidase and Fe3O4 have been proposed for chemodynamic therapy via the ultrasound-assisted Fenton reaction.114
In view of the high promise of VCC employment in mucosal drug delivery,99,115 a recent report demonstrated an in vivo study of intranasal delivery into the brain using VCC pre-loaded with an anesthetic drug.79 Interestingly, VCC crystals of different sizes ranging from nano- to micro-dimensions have been probed in this study, demonstrating that the micron-sized crystals ensure a more prolonged anesthetic effect than the nano-sized ones, while both are suitable carriers for intranasal delivery. Besides this, transdermal,116 ocular77 and pulmonary delivery117 of VCC crystals have been attempted.
Although the studies described above revealed the high potential of VCC for drug delivery and laid a platform for further clinical studies, VCC-based delivery requires much more investigations and solid basic research prior to translation into practical use. Key milestones of further VCC development as delivery vehicles are highlighted in the last section of this article.
3.3. VCC for tissue engineering
Nowadays, VCC crystals are primarily used for bone tissue engineering and repair which is not surprising considering that CaCO3 is a natural Ca-containing compound and may be used as a mineral storage material or a source of Ca2+ for transformation to hydroxyapatite in bone regeneration.120 VCC has an excellent osteoinductivity, highly developed surface area suitable for tissue growth, suitable mechanical properties and specific binding sites for BMP-2.121 VCC plays a dual role – being a reinforcement component that provides proper mechanical support and a mineral source of Ca2+ essential for bone repair. In addition, VCC may be preloaded with biofactors essential for tissue growth and regeneration for sustained release. This has been shown for both small drugs (e.g. tannic acid releasing for weeks122) and biomacromolecule RNase.123Most of the CaCO3-based scaffolds are composites made of polymer hydrogels with VCC crystals embedded into polymer networks and either homogeneously distributed inside gels123,124 or representing coatings, e.g. on polymeric polycaprolactone fibers (Fig. 6a and b).118,122
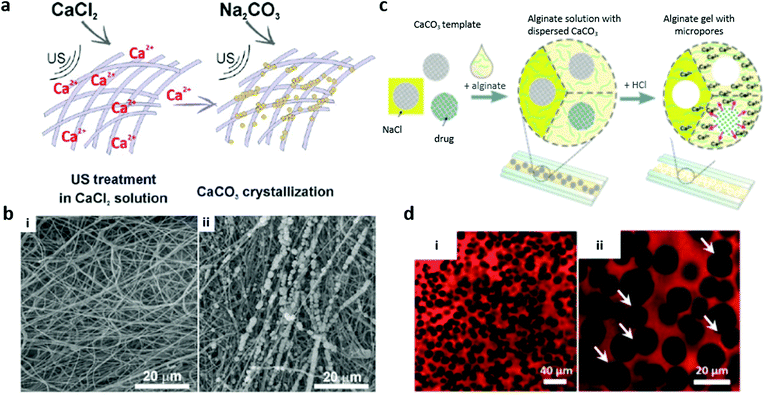 | ||
| Fig. 6 3D polymer scaffolds fabricated using VCC as a coating (a and b) or a sacrificial porogen (c and d). (a) The scheme of fibrous material mineralization under ultrasound treatment and (b) the corresponding SEM images of blank poly(ε-caprolactone) fibrous material (i) and poly(ε-caprolactone) material mineralized with VCC (ii). Adopted from ref. 118, copyright@ 2017 John Wiley & Sons. (c) The scheme of microfluidics-assisted porous alginate hydrogel fabrication and (d) CSLM images of final porous alginate scaffolds (red) formed by compact packing of VCC crystals of different sizes (i and ii) using 5% alginate and HCl. White arrows indicate some interconnected pores. Adopted from ref. 119, copyright@ 2015 John Wiley & Sons. | ||
Scaffolds based on siloxane-containing VCC and biodegradable polymers occupy a separate niche.83,125 VCC doped with siloxane is used as a source for soluble silica that stimulates the proliferation/differentiation of bone-forming cells and may be released for months.83 In view of the topic of this article, it seems interesting to highlight the study on the design of hybrid bacterial cellulose and CaCO3 (that was in the form of a vaterite–calcite mixture).126 The authors of this study highlighted the biodegradability and environmentally friendly fabrication of such scaffolds. In another study,127 scaffolds containing siloxane–polylactic acid–VCC hybrids with hydrated aluminum silicate nanotubes (imogolite) allowed improving the hydrophilicity and enhancing the cellular compatibility of the scaffolds, which is an important issue at the early stage after implantation.
There are multiple examples of in vitro verification of the osteogenic potential of VCC-based scaffolds. The in vivo performance of 1 μm sized VCC crystals functionalised with heparin, loaded with BMP-2, and entrapped into a fibrin based hydrogel was evaluated in repair of rabbit tibia bone. Bone defects almost completely healed after 8 weeks.121 Electrospun scaffolds mineralised with VCC crystals were implanted into rats, showing enhanced cell colonization and tissue vascularization as well as sustained release of tannic acid.122
VCC can serve as a sacrificial template for formulation of polymer-based scaffolds with tailor-made properties such as well-defined internal structures and compositions. Macroporous Ca-alginate scaffolds119,128 (Fig. 6c and d) and those made of interconnected polymer multilayer shells129 have been reported. VCC plays roles of a crosslinker and a porogen agent, allowing the filling of pores with active molecules. Such scaffolds are very soft (a few kPa), which opens new avenues to employ VCC for soft tissue engineering.130
4. Cellulose
4.1. Cellulose-based materials
Cellulose is a natural polysaccharide that is referred to as the most abundant biopolymer on Earth. The cellulose molecule consists of β-D-glucopyranose rings forming monomeric units (Fig. 7a), which in turn are organized in a cellulose network due to intra- and inter-molecular hydrogen bonding. Cellulose molecules possess both hydrophobic and hydrophilic parts, making them insoluble in water.131 The molecular structure gives rise to some beneficial properties of cellulose like biocompatibility, biodegradability, mechanical strength and a number of hydroxyl groups suitable for surface modification.132 Additionally, cellulose is a semicrystalline polymer consisting of crystalline and amorphous parts that are characterized by strong and weak hydrogen bonding, respectively. This opens an avenue for the preparation of various cellulose nano-sized carriers from cellulose nanofibers by multiple processing approaches.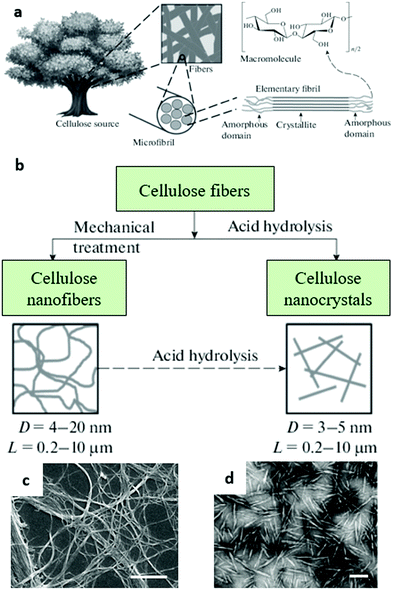 | ||
| Fig. 7 (a) Hierarchical structure of cellulose and (b) synthesis of cellulose nanofibers and cellulose nanocrystals; adopted from ref. 143, copyright© 2018, Springer Nature. (c and d) SEM images of cellulose NFs and CNCs, respectively. (c) Adopted from ref. 144, copyright© 2013, Springer Nature. (d) Adopted from ref. 145, copyright© 2008, Royal Society of Chemistry. | ||
Natural cellulose sources are the biomasses of plants, animals, bacteria, and algae.133 Nowadays, plants (wood, cotton, vegetable fibers) are the most common cellulose sources.134 Besides this, cellulose can be extracellularly produced by some bacteria. Currently, bacterial cellulose attracts significant attention because it is initially free of attendant bioproducts such as hemicelluloses, lignin, and pectin.135 In addition to the above, industrial residues or municipal solid wastes (e.g. waste cotton clothes and cardboard) are currently considered as new environmentally friendly cellulose sources.136
The term “nanocellulose” implies cellulose in the form of structures that do not exceed 100 nm in size at least in one dimension, while in the other dimensions these structures can reach up to a few micrometres.6 Compared to conventional cellulose, NC exhibits enhanced relative surface area along with tuneable mechanical, physical and chemical properties, which makes it a promising material for a wide range of applications including biomedical ones. Generally, depending on the origin and processing conditions three main types of NC can be distinguished: cellulose nanocrystals (CNCs), nanofibers (NFs), and bacterial cellulose (BC) (Fig. 7).
CNCs have a rod-like structure with diameters of 5–60 nm and lengths of 100–500 nm. Typically, they are prepared by acid hydrolysis of cellulose, resulting in the destruction of amorphous regions, whereas the crystalline regions remain intact.137 In turn, NFs have thicknesses up to 100 nm (or even more) and lengths up to several micrometres. These structures are generally prepared by enzymatic hydrolysis or high-pressure homogenization of cellulose,138 although oxidative acid treatment may also be employed.139 BC is also synthesized enzymatically from some bacteria.140 It does not require additional purification steps as it initially possesses a higher purity along with a better crystallinity, degree of polymerization, and mechanical stability compared to other types of NC.
While HNTs and VCC have not yet been approved by the FDA for medical use, cellulosic materials are already widely used in medicine. Mostly, cellulose or its chemical derivatives are approved as materials for surgery devices (e.g. nonabsorbable gauze for internal use, §878.4450; nonresorbable gauze/sponge for external application, §878.4014); another example is cellulose-based contact lens intended to correct vision conditions (§886.5916). Besides this, diluted suspensions of carboxymethylcellulose (CMC), hydroxyethylcellulose (HMC) and methylcelluloses are approved as ophthalmic demulcents under §349.12. Methylcellulose and CMC are substances generally recognised as safe (§582.1480 and §582.1745). A number of cellulose derivatives (ethyl-, hydroxypropyl-, methylethyl-cellulose) are approved as direct additives to food for human consumption for multipurpose use (§172-I). In regard to this, the various types of NC seem to be promising for drug delivery and sustainable release.
4.2. NC for drug delivery
Besides direct use of NC as an excipient, it is often employed as a co-stabilizer to improve the physical–chemical properties of a polymeric matrix that can form beads. Usually, NC has a positive impact on the mechanical properties and the size distribution of the beads and therefore provides a better ability to pack the excipient, e.g. for tablet production. In such formulations, NC plays the role of a passive filler.152
One more biomedical application of CNCs is the preparation of gel-like structures, in particular hydro- and aero-gels. Overall, hydrogels are three-dimensional structures formed of linked polymer chains that are able to absorb and retain water without being dissolved. In fact, NC-based hydrogels differ from most other gels as their constructive elements are insoluble in water. This results in a different mechanism of gel network formation, which includes entanglement of NC particles or fibers followed by physical or chemical crosslinking.153
However, CNCs themselves lack the entanglement ability and more often are employed as fillers in gel composite structures due to their mechanical properties. In these composite hydrogels, the interaction of CNCs with loaded molecules (drugs) usually does not affect the loading and release characteristics but significantly improves the mechanical and chemical stability of the hydrogels. Shojaeiarani et al. suggested the general classification of CNC containing hydrogels into two main groups with respect to their forms; the groups are bulk and injectable hydrogels.154 The bulk hydrogels are prepared by free-radical polymerization of monomeric units with crosslinking agents to form three-dimensional structures. In turn, injectable hydrogels appear as polymer solutions with the ability to gel after injection, for instance, under physiological conditions. Moreover, they can go through reverse sol–gel transition triggered by external stimuli such as pH, temperature, electromagnetic fields, and UV-light. This makes injectable hydrogels promising candidates for drug delivery applications, as they are able to form a depot for sustainable drug release after injection with the possibility of remotely triggering a rapid drug release. While the use of nanoparticulate NC has relatively rarely been reported, NC-based hydrogels in the form of films, membranes and injectable gels have become increasingly popular for drug delivery applications, tissue regeneration and wound healing purposes.
Regardless of the form, the release from nano- and micro-particulate NC is mostly governed by diffusional transport that is often influenced by swelling in the case of the hydrogels.147,155 Depending on chemical modification, the type of NC used (CNCs, NFs or BC), the form of formulation, and the microenvironment, the release can last from tens of minutes to a few months.147 For instance, Li et al. prepared hybrid CNCs functionalised with folate and pH responsive multilayers that did not release the payload at pH 7.4 but released 95% of doxorubicin in 24 h at pH 5.5.156 Integration of drugs into film-like matrix systems composed of NFs ensures their storage for almost three months at acidic pH for itraconazole and beclomethasone dipropionate in water.157 More examples can be found in review 147.
The performance of injectable and bulk CNC hybrid hydrogels was widely investigated in vitro and in vivo. As some recent in vivo examples, de France et al. studied tissue response and biodistribution of CNC/poly(oligoethyleneglycol methacrylate) injectable hydrogels.165 They found that the hydrogel is mainly localized in the injection site and its components did not accumulate in any organ. Additionally, the hydrogel induced moderate inflammatory response and promoted fibroblast proliferation in vivo. Magnetic bulk hydrogel beads for pH-triggered release of anticancer drug dexamethasone in the gastro-intestinal tract have been reported.166 Karzar Jeddi and Mahkam noted that inclusion of 2% (wt) of CNCs improved the swelling degree, drug loading capacity, and drug release behavior of the hydrogel composites. pH sensitive composite dialdehyde CNC/chitosan hydrogels loaded with theophylline were tested in vitro.167
4.3. NC for tissue engineering
Cellulose-based hydrogels are extremely attractive for tissue engineering. In recent years, various methods have been proposed for the processing of hydrogels based on NC. The architecture of NC can be engineered from the nano- to macro-scale that, together with excellent biocompatibility and mechanical properties, allows producing scaffolds particularly suitable for use in tissue regeneration. Generally, NC-based scaffolds are composites wherein NC is employed in combination with other polymers or particles. The role of NC is usually in providing the required mechanical stability and chemical resistance to hydrolytic and enzymatic degradation.Thus, Ghavimi et al. employed CNCs to prepare CNC/chitosan hydrogels with mechanical properties mimicking vertebral bones to treat vertebral compression fractures.168 Silva et al. prepared a composite hyaluronic acid/CNC/tropoelastin hydrogel as a cell growing substrate.169 The incorporation of CNCs improved the hydrogel's stability against hydrolytic and enzymatic degradation due to the chemical bonding of CNCs and hyaluronic acid. The resulting gels were shown to support and promote cell adhesion, viability and growth, which is indispensable for tissue engineering and regeneration applications. Alternatively, for cell growth applications Kumar et al. prepared polysaccharide hydrogels reinforced with halloysites and CNCs.170 The mechanical properties of these hydrogels can be controlled by variation of CNCs and halloysite amount in the gel composition.
The applications of NC-based composite hydrogels in tissue engineering are highly versatile and may cover bone tissue engineering, cornea treatment, heart and vascular muscle regeneration and many other relevant applications as reviewed elsewhere.171
Currently, the development of advanced wound dressing materials is probably one of the most attractive areas of NC-based materials. The advanced healing strategies imply non-invasive monitoring of tissue recovery, pain management and controlled release of drugs accelerating regeneration and minimizing scar formation.172
In regard to this, currently hydrogels prepared with various forms of NC are intensively studied including those which are sensitive to various external stimuli. Xu et al. employed CNCs to improve the mechanical properties of thermo-responsive chitosan/glycerol phosphate sodium injectable hydrogels.173 The resulting hydrogels were loaded with human umbilical cord-mesenchymal stem cells that are able to produce cytokines and growth factors promoting wound healing. The gels were tested for cutaneous wound healing in experiments in vivo and demonstrated accelerated wound closure and skin regeneration. Huang et al. described an injectable hydrogel prepared using carboxymethylchitosan and dialdehyde modified CNCs.174 The gel demonstrated very good biocompatibility, self-healing and on-demand solubilisation under mildly acidic conditions. The gel was tested as a wound-healing agent in a deep partial thickness skin burn model in vivo and was shown to be effective for burn surface covering and treatment. Du et al. reported gel microparticles prepared via Schiff-base reaction between carboxymethylchitosan and oxidized CMC and loaded with BSA or silver sulfadiazine demonstrating alkaline and acid responsive drug release and antibacterial properties against S. aureus and E. coli bacteria when silver sulfadiazine was used.175 In turn, Yan et al. prepared a pH-responsive hydrogel combining CMC, HCM and dopamine. The resulting hydrogel demonstrated a bacteriostatic effect that was maintained for five days.176
Another way for the preparation of a pH-responsive hydrogel via radical polymerization was proposed by Park et al.177 The resulting hydrogel was loaded with flavonoid naringenin to prepare a transdermal delivery system for treatment of atopic skin conditions disbalancing skin pH. The hydrogel effectively wetted the skin, improving drug penetration. An interesting approach for preparation of antimicrobial wound dressing cellulose hydrogels was proposed by Shen et al.178 They employed mesoporous silica nanoparticles SBA-15 coated with CaCO3 to prevent their degradation during the preparation of an SBA-15/cellulose composite. The sustainable antibacterial activity against S. aureus and E. coli was figured out to be 144 h at its longest.
As NC hydrogels may act as both wound dressing materials (Fig. 8) and scaffolds for cell culture, Loh et al. employed bacterial NC/acrylic acid hydrogels to heal wounds requiring exogenous cells for tissue regeneration.179 To do this, human epidermal keratinocytes and dermal fibroblasts were enclosed into a gel matrix. The cells were mostly attached to the hydrogel within 4 h. In turn, the gel was shown to provide cell viability and free transfer to the wound site. The wound healing ability of the cell-loaded hydrogel was estimated in experiments in vivo in a full-thickness wound model. The wound was almost completely closed (about 99%) in two weeks of gel treatment.
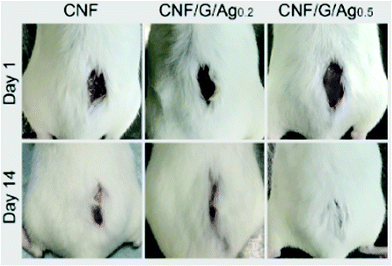 | ||
| Fig. 8 Photographs of mice treated with different wound dressing scaffolds: carboxylated cellulose nanofibers (CNFs) and two composite CNF–gelatine scaffolds (CNF/G/Ag) functionalised with silver nanoparticles (0.2 and 0.5 wt%, respectively). Adopted from ref. 180, copyright© 2018 Elsevier. | ||
Additionally, Pötzinger et al. employed BC as a gene activated matrix for prolonged release of loaded plasmids for site-specific gene delivery.181 Plasmids can be efficiently immobilized in a cellulose matrix providing their prolonged release up to 50 days. The depot for sustainable and targeted delivery of plasmids may be promising for wound healing of soft tissues due to the delivery of plasmids encoding for growth factors of platelets, fibroblasts or brain derived neurotrophic factors.182 The formation of hard tissues like cartilage or bones can be promoted as well by delivering the genes encoding for bone morphogenetic proteins, which control bone differentiation and ossification.183 In addition, wound dressing materials loaded with plasmids encoding for vascular endothelial growth factors or angiogenin triggering the formation of new blood vessels may accelerate angiogenesis.184
5. Summary and prospects
For the convenience of the reader, the key physical–chemical properties that determine the characteristics of vehicles considered for drug delivery and tissue engineering are summarized in Table 2. Each of these three vehicles can be found in nature or synthesized from natural sources using simple and low-cost technologies. In the age when the rapid and effective establishment of large-scale production, which at the same time should necessarily be ecologically friendly, is one of the main requirements for successful translation from science to industry, it is not surprising that research on naturally derived HNTs, VCC and NC is actively developing. Being animal- and plant-free materials that can be found literally beneath your feet or isolated from wastes, HNTs, VCC and NC have high potential to revolutionise drug vehicle production. On the other hand, green and sustainable production is the only thing that unites these vehicles, since each of them exhibits unique properties. Their diverse shapes, sizes, surface charges, and mechanical properties as well as different mechanisms of entrapment and release of molecules give the opportunity to choose the most suitable vehicle depending on the specific biomedical task. This gives the vehicles equal chances for future development and bioapplications and opens broad and new avenues for their utilisation in biomedicine.| Vehicle | Shape | Dimensions | ζ-Potential at pH 7, mV | S (BET), m2 g−1 | Pore ∅, nm | Density, g cm−3 | Young's modulus, GPa | Surface groups for chemical modification |
|---|---|---|---|---|---|---|---|---|
| a Negative charge on the external surface and positive charge on the internal surface over a broad pH range. | ||||||||
| HNTs | Hollow tubes | Length 0.5–2 μm, outer ∅ 20–200 nm | −(13–40)a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 185–187 185–187 |
30–65186,188 | Internal ∅ 10–70 nm31,186 | 2.55–2.65 (Mineralogy Database) | 10–460, average 140189 | Lumen surface: Al–OH groups, suitable for chemical grafting (e.g. organosilanes) |
| Eternal surface: Si–OH groups, chemically inert, suitable for physical adsorption of cationic surfactants and polymers190 | ||||||||
| VCC | Porous spheres | ∅ 50 nm–70 μm | +(11–15)82 | 3–2070,191 | 10–60 nm81,191 | 2.54 (Mineralogy Database) | 60–86192 | Surfaces are not suitable for chemical modification but can be used for physical adsorption, e.g. for polymer multilayer deposition102 |
| CNCs | Rods | Length 100–200 nm, ∅ 3–5 nm | −(70–100)187,193 | 400–500194 | Non-porous | ∼1.5–1.6195 | 100–130195 | Multiple –OH groups suitable for various modifications via oxidation, esterification, fluorescent labelling, click chemistry, etc.196 |
Although the studies of HNTs, VCC and NC toward biomedical applications have significantly advanced in recent years, now it is too premature to speak about the introduction of HNTs, VCC and NC into biomedicine. This is because this research is still relatively new that perhaps has not even fully reached the stage of clinical trials. While low levels of cytotoxicity were verified for HNTs, VCC and NC on various cell lines and bioassays, the number of in vivo studies is still very limited. Some other questions also remain unanswered. For instance, the mechanisms of cellular responses to HNTs, VCC and NC should be better described and understood. This especially concerns VCC which potentially may cause a misbalance in Ca2+ metabolism. Internalization mechanisms for delivery into a cell also should be investigated. These studies should be consistent with the design of new vehicles based on functionalised HNTs, VCC and NC that may can expand the range of their application, enhance cellular uptake and improve drug delivery.
In the next years, we expect the surge of in vitro and in vivo evaluation of the performance of HNTs, VCC and NC as delivery vehicles. Further this research might be smoothly translated to clinical trials that will be a huge step in the development of these naturally derived delivery vehicles.
The fact that kaolin clays, calcium carbonate and cellulose are approved by the FDA for food supplementation and medical uses might significantly simplify the approval of HNTs, VCC and NC for drug delivery purposes. This gives an enormous advantage to HNTs, VCC and NC in contrast to many other delivery vehicles.
Together with the low production costs and availability of the vehicles considered here, they may swiftly find their niche in drug delivery with high potential to outpace their analogues.
Finally, tissue engineering and repair applications of HNTs, VCC and NC deserve specific attention but other fields are expected for future applications.
Herein, HNTs do not form the core of the scaffold but can be used as an additive for scaffold functionalisation. Cosmetic applications of HNTs would be expected due to their low toxicity and ability to host and release materials in a sustained and controlled way.
VCC is an important example for bone tissue engineering; its future applications are expected in intracellular delivery due to its complete degradation as well as in dentistry due to its use as a source of calcium ions that is now achieved mostly using amorphous CaCO3 that has no defined structure. Applications of VCC in cosmetics would also be desirable because of its highly controlled release and ability to encapsulate virtually any molecules.
NC that can serve as the core material for a scaffold for various tissues particularly stands out. At the same time, NC-based scaffolds seem to be promising for orthopaedic and other surgical applications due to their high biocompatibility and an option to make films and membranes with desired characteristics. However, more studies on controlled fabrication and functionalisation are expected in the nearest future.
In addition to the above, new technologies for up-scalable controlled bottom-up production of VCC and NC and new methods for HNT purification/modification and NC isolation from wastes are upcoming. Finally, the combination of these natural materials will be one of the future trends that would allow merging their properties and therefore building a better platform for tissue growth. Scaffolds with unique active properties demonstrating a dynamic crosstalk with a regenerating tissue are expected as a result of hybrid materials made using combinations of HNTs, VCC and NC. These materials belong to the third generation of biomaterials where the dynamics of bio-signals of extracellular matrices is recapitulated within advanced active scaffolds.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
A. V. acknowledges the European Union's Horizon 2020 research and innovation programme (Marie-Curie Individual Fellowship LIGHTOPLEX-747245). This work was supported by a Russian Scientific Fund, Grant no. 19-79-30091.References
- H. He, Q. Liang, M. C. Shin, K. Lee, J. Gong, J. Ye, Q. Liu, J. Wang and V. Yang, Front. Chem. Sci. Eng., 2013, 7, 496–507 CrossRef.
- Y. Zhang, H. F. Chan and K. W. Leong, Adv. Drug Delivery Rev., 2013, 65, 104–120 CrossRef PubMed.
- R. Kanwar, J. Rathee, D. B. Salunke and S. K. Mehta, ACS Omega, 2019, 4, 8804–8815 CrossRef PubMed.
- H. Jahangirian, E. G. Lemraski, T. J. Webster, R. Rafiee-Moghaddam and Y. Abdollahi, Int. J. Nanomed., 2017, 12, 2957–2978 CrossRef PubMed.
- E. J. Cho, L. T. P. Trinh, Y. Song, Y. G. Lee and H.-J. Bae, Bioresour. Technol., 2020, 298, 122386 CrossRef PubMed.
- L. Bacakova, J. Pajorova, M. Bacakova, A. Skogberg, P. Kallio, K. Kolarova and V. Svorcik, Nanomaterials, 2019, 9, e164 CrossRef PubMed.
- M. C. Barros, P. M. Bello, M. Bao and J. J. Torrado, J. Cleaner Prod., 2009, 17, 400–407 CrossRef.
- D. Cree and A. Rutter, ACS Sustainable Chem. Eng., 2015, 3, 941–949 CrossRef.
- G. Cavallaro, G. Lazzara and R. Fakhrullin, Ther. Delivery, 2018, 9, 287–301 CrossRef PubMed.
- R. M. Hazen, D. A. Sverjensky, D. Azzolini, D. L. Bish, S. C. Elmore, L. Hinnov and R. E. Milliken, Am. Mineral., 2013, 98, 2007–2029 CrossRef CAS.
- M. Liu, R. Fakhrullin, A. Novikov, A. Panchal and Y. Lvov, Macromol. Biosci., 2019, 19, e1800419 CrossRef PubMed.
- Y. M. Lvov, D. G. Shchukin, H. Möhwald and R. R. Price, ACS Nano, 2008, 2, 814–820 CrossRef.
- J. Keeling, in Natural Mineral Nanotubes, ed. P. Pasbakhsh and G. Churchman, Apple Academic Press, 2015, vol. 32, pp. 95–116 Search PubMed.
- Y. Lvov and E. Abdullayev, Prog. Polym. Sci., 2013, 38, 1690–1719 CrossRef.
- Y. M. Lvov, M. M. DeVilliers and R. F. Fakhrullin, Expert Opin. Drug Delivery, 2016, 13, 977–986 CrossRef.
- S. Satish, M. Tharmavaram and D. Rawtani, Nanobiomedicine, 2019, 6 DOI:10.1177/1849543519863625.
- Y. Yang, Y. Chen, F. Leng, L. Huang, Z. Wang and W. Tian, Appl. Sci., 2017, 7, 1215 CrossRef.
- D. Tan, P. Yuan, F. Annabi-Bergaya, D. Liu, L. Wang, H. Liu and H. He, Appl. Clay Sci., 2014, 96, 50–55 CrossRef.
- M. Massaro, G. Lazzara, S. Milioto, R. Noto and S. Riela, J. Mater. Chem. B, 2017, 5, 2867–2882 RSC.
- A. M. Yamina, M. Fizir, A. Itatahine, H. He and P. Dramou, Colloids Surf., B, 2018, 170, 322–329 CrossRef.
- H. Li, X. Zhu, J. Xu, W. Peng, S. Zhong and Y. Wang, RSC Adv., 2016, 6, 54463–54470 RSC.
- R. Yendluri, Y. Lvov, M. M. de Villiers, V. Vinokurov, E. Naumenko, E. Tarasova and R. Fakhrullin, J. Pharm. Sci., 2017, 106, 3131–3139 CrossRef.
- Y. Hu, J. Chen, X. Li, Y. Sun, S. Huang, Y. Li, H. Liu, J. Xu and S. Zhong, Nanotechnology, 2017, 28, 375101 CrossRef.
- A. Stavitskaya, S. Batasheva, V. Vinokurov, G. Fakhrullina, V. Sangarov, Y. Lvov and R. Fakhrullin, Nanomaterials, 2019, 9, E708 CrossRef PubMed.
- (a) Y.-F. Shi, Z. Tian, Y. Zhang, H.-B. Shen and N.-Q. Jia, Nanoscale Res. Lett., 2011, 6, 608 CrossRef; (b) Z. Long, J. Zhang, Y. Shen, C. Zhou and M. Liu, Mater. Sci. Eng., C, 2017, 81, 224–235 CrossRef.
- V. Khodzhaeva, A. Makeeva, V. Ulyanova, P. Zelenikhin, V. Evtugyn, M. Hardt, E. Rozhina, Y. Lvov, R. Fakhrullin and O. Ilinskaya, Front. Pharmacol., 2017, 8, 631 CrossRef.
- A. V. Stavitskaya, A. A. Novikov, M. S. Kotelev, D. S. Kopitsyn, E. V. Rozhina, I. R. Ishmukhametov, R. F. Fakhrullin, E. V. Ivanov, Y. M. Lvov and V. A. Vinokurov, Nanomaterials, 2018, 8, E391 CrossRef.
- (a) S. A. Konnova, Y. M. Lvov and R. F. Fakhrullin, Clay Miner., 2016, 51, 429–433 CrossRef; (b) Y. Lvov, A. Panchal, Y. Fu, R. Fakhrullin, M. Kryuchkova, S. Batasheva, A. Stavitskaya, A. Glotov and V. Vinokurov, Langmuir, 2019, 35, 8646–8657 CrossRef.
- V. A. Vinokurov, A. V. Stavitskaya, A. P. Glotov, A. A. Novikov, A. V. Zolotukhina, M. S. Kotelev, P. A. Gushchin, E. V. Ivanov, Y. Darrat and Y. M. Lvov, Chem. Rec., 2018, 18, 858–867 CrossRef.
- G. Lazzara, G. Cavallaro, A. Panchal, R. Fakhrullin, A. Stavitskaya, V. Vinokurov and Y. Lvov, Curr. Opin. Colloid Interface Sci., 2018, 35, 42–50 CrossRef.
- M. Fizir, P. Dramou, N. S. Dahiru, W. Ruya, T. Huang and H. He, Microchim. Acta, 2018, 185, 389 CrossRef PubMed.
- R. Yendluri, D. P. Otto, M. M. de Villiers, V. Vinokurov and Y. M. Lvov, Int. J. Pharm., 2017, 521, 267–273 CrossRef PubMed.
- F. R. Ahmed, M. H. Shoaib, R. I. Yousuf, T. Ali, K. E. Geckeler, F. Siddiqui, K. Ahmed and F. Qazi, Eur. J. Pharm. Sci., 2019, 133, 214–227 CrossRef.
- L. Lisuzzo, G. Cavallaro, S. Milioto and G. Lazzara, New J. Chem., 2019, 43, 10887–10893 RSC.
- H. Lun, J. Ouyang and H. Yang, RSC Adv., 2014, 4, 44197–44202 RSC.
- E. G. Bediako, E. Nyankson, D. Dodoo-Arhin, B. Agyei-Tuffour, D. Łukowiec, B. Tomiczek, A. Yaya and J. K. Efavi, Heliyon, 2018, 4, e00689 CrossRef PubMed.
- G. Cavallaro, G. Lazzara, M. Massaro, S. Milioto, R. Noto, F. Parisi and S. Riela, J. Phys. Chem. C, 2015, 119, 8944–8951 CrossRef.
- P. Dramou, M. Fizir, A. Taleb, A. Itatahine, N. S. Dahiru, Y. A. Mehdi, L. Wei, J. Zhang and H. He, Carbohydr. Polym., 2018, 197, 117–127 CrossRef.
- M. R. Dzamukova, E. A. Naumenko, Y. M. Lvov and R. F. Fakhrullin, Sci. Rep., 2015, 5, 10560 CrossRef PubMed.
- A. C. Santos, C. Ferreira, F. Veiga, A. J. Ribeiro, A. Panchal, Y. Lvov and A. Agarwal, Adv. Colloid Interface Sci., 2018, 257, 58–70 CrossRef PubMed.
- M. Massaro, R. Amorati, G. Cavallaro, S. Guernelli, G. Lazzara, S. Milioto, R. Noto, P. Poma and S. Riela, Colloids Surf., B, 2016, 140, 505–513 CrossRef PubMed.
- H. Liu, Z.-G. Wang, S.-L. Liu, X. Yao, Y. Chen, S. Shen, Y. Wu and W. Tian, J. Mater. Sci., 2019, 54, 693–704 CrossRef.
- E. Rozhina, A. Panchal, F. Akhatova, Y. Lvov and R. Fakhrullin, Appl. Clay Sci., 2019, 105371 Search PubMed.
- (a) S. A. Konnova, I. R. Sharipova, T. A. Demina, Y. N. Osin, D. R. Yarullina, O. N. Ilinskaya, Y. M. Lvov and R. F. Fakhrullin, Chem. Commun., 2013, 49, 4208–4210 RSC; (b) V. Vergaro, E. Abdullayev, Y. M. Lvov, A. Zeitoun, R. Cingolani, R. Rinaldi and S. Leporatti, Biomacromolecules, 2010, 11, 820–826 CrossRef PubMed.
- X. Lai, M. Agarwal, Y. M. Lvov, C. Pachpande, K. Varahramyan and F. A. Witzmann, J. Appl. Toxicol., 2013, 33, 1316–1329 CAS.
- G. I. Fakhrullina, F. S. Akhatova, Y. M. Lvov and R. F. Fakhrullin, Environ. Sci.: Nano, 2015, 2, 54–59 RSC.
- Z. Long, Y.-P. Wu, H.-Y. Gao, J. Zhang, X. Ou, R.-R. He and M. Liu, J. Mater. Chem. B, 2018, 6, 7204–7216 RSC.
- R. F. Kamalieva, I. R. Ishmukhametov, S. N. Batasheva, E. V. Rozhina and R. F. Fakhrullin, Nano-Struct. Nano-Objects, 2018, 15, 54–60 CrossRef CAS.
- J. Liao, S. Peng, M. Long, Y. Zhang, H. Yang, Y. Zhang and J. Huang, Colloids Surf., A, 2020, 586, 124242 CrossRef.
- (a) X. Wang, J. Gong, Z. Gui, T. Hu and X. Xu, Environ. Toxicol., 2018, 33, 623–630 CrossRef CAS; (b) X. Wang, J. Gong, R. Rong, Z. Gui, T. Hu and X. Xu, J. Agric. Food Chem., 2018, 66, 2925–2933 CrossRef CAS.
- G. Y. Kırımlıoğlu, Y. Yazan, K. Erol and Ç. Çengelli Ünel, Int. J. Pharm., 2015, 495, 816–826 CrossRef.
- N. Kerdsakundee, W. Li, J. P. Martins, Z. Liu, F. Zhang, M. Kemell, A. Correia, Y. Ding, M. Airavaara, J. Hirvonen, R. Wiwattanapatapee and H. A. Santos, Adv. Healthcare Mater., 2017, 6, 1700629 CrossRef.
- A. Panchal, G. Fakhrullina, R. Fakhrullin and Y. Lvov, Nanoscale, 2018, 10, 18205–18216 RSC.
- G. Fakhrullina, E. Khakimova, F. Akhatova, G. Lazzara, F. Parisi and R. Fakhrullin, ACS Appl. Mater. Interfaces, 2019, 11, 23050–23064 CrossRef CAS.
- L. Yang, Y.-C. Lee, M. I. Kim, H. G. Park, Y. S. Huh, Y. Shao and H.-K. Han, J. Mater. Chem. B, 2014, 2, 7567–7574 RSC.
- R. Rong, Y. Zhang, Y. Zhang, Y. Hu, W. Yang, X. Hu, L. Wen and Q. Zhang, Nanotoxicology, 2019, 13, 354–368 CrossRef.
- (a) Functional Polymer Composites with Nanoclays, ed. Y. Lvov, B. Guo and R. F. Fakhrullin, Royal Society of Chemistry, Cambridge, UK, 2017, vol. 22 Search PubMed; (b) E. Naumenko and R. Fakhrullin, Biotechnol. J., 2019, 14, e1900055 CrossRef.
- T. S. Gaaz, A. B. Sulong, M. N. Akhtar, A. A. H. Kadhum, A. B. Mohamad and A. A. Al-Amiery, Molecules, 2015, 20, 22833–22847 CrossRef.
- S. S. Suner, S. Demirci, B. Yetiskin, R. Fakhrullin, E. Naumenko, O. Okay, R. S. Ayyala and N. Sahiner, Int. J. Biol. Macromol., 2019, 130, 627–635 CrossRef.
- E. A. Naumenko, I. D. Guryanov, R. Yendluri, Y. M. Lvov and R. F. Fakhrullin, Nanoscale, 2016, 8, 7257–7271 RSC.
- X. Zhang, R. Guo, J. Xu, Y. Lan, Y. Jiao, C. Zhou and Y. Zhao, J. Biomater. Appl., 2015, 30, 512–525 CrossRef.
- W. Wei, E. Abdullayev, A. Hollister, D. Mills and Y. M. Lvov, Macromol. Mater. Eng., 2012, 297, 645–653 CrossRef.
- O. S. Manoukian, M. R. Arul, S. Rudraiah, I. Kalajzic and S. G. Kumbar, J. Controlled Release, 2019, 296, 54–67 CrossRef.
- G. Sandri, C. Aguzzi, S. Rossi, M. C. Bonferoni, G. Bruni, C. Boselli, A. I. Cornaglia, F. Riva, C. Viseras, C. Caramella and F. Ferrari, Acta Biomater., 2017, 57, 216–224 CrossRef.
- Y. Zhang, K. Huang, Q. Yuan, Z. Gu and G. Wu, J. Biomed. Nanotechnol., 2019, 15, 1909–1922 CrossRef.
- M. Chozhanathmisra, D. Govindaraj, P. Karthikeyan, K. Pandian, L. Mitu and R. Rajavel, J. Chem., 2018, 2018, 1–12 CrossRef.
- A. G. Christy, Cryst. Growth Des., 2017, 17, 3567–3578 CrossRef.
- C. Rodriguez-Navarro, C. Jimenez-Lopez, A. Rodriguez-Navarro, M. T. Gonzalez-Muñoz and M. Rodriguez-Gallego, Geochim. Cosmochim. Acta, 2007, 71, 1197–1213 CrossRef.
- P. Bots, L. G. Benning, J.-D. Rodriguez-Blanco, T. Roncal-Herrero and S. Shaw, Cryst. Growth Des., 2012, 12, 3806–3814 CrossRef.
- N. Feoktistova, J. Rose, V. Z. Prokopović, A. S. Vikulina, A. Skirtach and D. Volodkin, Langmuir, 2016, 32, 4229–4238 CrossRef.
- D. Gebauer, A. Völkel and H. Cölfen, Science, 2008, 322, 1819–1822 CrossRef.
- Z. Dong, L. Feng, W. Zhu, X. Sun, M. Gao, H. Zhao, Y. Chao and Z. Liu, Biomaterials, 2016, 110, 60–70 CrossRef.
- D. B. Trushina, T. V. Bukreeva and M. N. Antipina, Cryst. Growth Des., 2016, 16, 1311–1319 CrossRef.
- D. B. Trushina, S. N. Sulyanov, T. V. Bukreeva and M. V. Kovalchuk, Crystallogr. Rep., 2015, 60, 570–577 CrossRef.
- L.-f. Yang, D.-q. Chu, H.-l. Sun and G. Ge, New J. Chem., 2016, 40, 571–577 RSC.
- A. Sergeeva, R. Sergeev, E. Lengert, A. Zakharevich, B. Parakhonskiy, D. Gorin, S. Sergeev and D. Volodkin, ACS Appl. Mater. Interfaces, 2015, 7, 21315–21325 CrossRef.
- P. V. Binevski, N. G. Balabushevich, V. I. Uvarova, A. S. Vikulina and D. Volodkin, Colloids Surf., B, 2019, 181, 437–449 CrossRef.
- S. Maleki Dizaj, M. Barzegar-Jalali, M. H. Zarrintan, K. Adibkia and F. Lotfipour, Expert Opin. Drug Delivery, 2015, 12, 1649–1660 CrossRef.
- D. B. Trushina, T. N. Borodina, S. N. Sulyanov, J. V. Moiseeva, N. V. Gulyaeva and T. V. Bukreeva, Crystallogr. Rep., 2018, 63, 998–1004 CrossRef CAS.
- (a) S. Maleki Dizaj, F. Lotfipour, M. Barzegar-Jalali, M.-H. Zarrintan and K. Adibkia, Artif. Cells, Nanomed., Biotechnol., 2017, 45, 535–543 CrossRef CAS; (b) M. Y. Memar, K. Adibkia, S. Farajnia, H. S. Kafil, S. Maleki Dizaj and R. Ghotaslou, J. Drug Delivery Sci. Technol., 2019, 54, 101307 CrossRef CAS.
- A. S. Vikulina, N. A. Feoktistova, N. G. Balabushevich, A. G. Skirtach and D. Volodkin, Phys. Chem. Chem. Phys., 2018, 20, 8822–8831 RSC.
- N. A. Feoktistova, A. S. Vikulina, N. G. Balabushevich, A. G. Skirtach and D. Volodkin, Mater. Des., 2020, 185, 108223 CrossRef CAS.
- D. W. Green, B. J. R. F. Bolland, J. M. Kanczler, S. A. Lanham, D. Walsh, S. Mann and R. O. C. Oreffo, Biomaterials, 2009, 30, 1918–1927 CrossRef CAS.
- P. Zhao, S. Wu, Y. Cheng, J. You, Y. Chen, M. Li, C. He, X. Zhang, T. Yang, Y. Lu, R. J. Lee, X. He and G. Xiang, Nanomedicine, 2017, 13, 2507–2516 CrossRef CAS.
- C.-Q. Wang, J.-L. Wu, R.-X. Zhuo and S.-X. Cheng, Mol. BioSyst., 2014, 10, 672–678 RSC.
- M. Długosz, M. Bulwan, G. Kania, M. Nowakowska and S. Zapotoczny, J. Nanopart. Res., 2012, 14, 1313 CrossRef.
- T. V. Bukreeva, I. V. Marchenko, T. N. Borodina, I. V. Degtev, S. L. Sitnikov, Y. V. Moiseeva, N. V. Gulyaeva and M. V. Kovalchuk, Dokl. Phys. Chem., 2011, 440, 165–167 CrossRef CAS.
- R. F. Fakhrullin, A. G. Bikmullin and D. K. Nurgaliev, ACS Appl. Mater. Interfaces, 2009, 1, 1847–1851 CrossRef CAS.
- J. R. Lakkakula, R. Kurapati, I. Tynga, H. Abrahamse, A. M. Raichur and R. W. Maçedo Krause, RSC Adv., 2016, 6, 104537–104548 RSC.
- V. E. Bosio, M. L. Cacicedo, B. Calvignac, I. León, T. Beuvier, F. Boury and G. R. Castro, Colloids Surf., B, 2014, 123, 158–169 CrossRef CAS.
- N. G. Balabushevich, E. A. Kovalenko, I. M. Le-Deygen, L. Y. Filatova, D. Volodkin and A. S. Vikulina, Mater. Des., 2019, 182, 108020 CrossRef CAS.
- N. G. Balabushevich, A. V. Lopez de Guerenu, N. A. Feoktistova, A. G. Skirtach and D. Volodkin, Macromol. Biosci., 2016, 16, 95–105 CrossRef CAS.
- S. V. German, M. V. Novoselova, D. N. Bratashov, P. A. Demina, V. S. Atkin, D. V. Voronin, B. N. Khlebtsov, B. V. Parakhonskiy, G. B. Sukhorukov and D. A. Gorin, Sci. Rep., 2018, 8, 17763 CrossRef CAS.
- N. G. Balabushevich, A. V. Lopez de Guerenu, N. A. Feoktistova and D. Volodkin, Phys. Chem. Chem. Phys., 2015, 17, 2523–2530 RSC.
- J. Song, R. Wang, Z. Liu and H. Zhang, Mol. Med. Rep., 2018, 17, 8403–8408 CAS.
- A. S. Sergeeva, E. K. Volkova, D. N. Bratashov, M. I. Shishkin, V. S. Atkin, A. V. Markin, A. A. Skaptsov, D. V. Volodkin and D. A. Gorin, Thin Solid Films, 2015, 583, 60–69 CrossRef CAS.
- D. B. Trushina, R. A. Akasov, A. V. Khovankina, T. N. Borodina, T. V. Bukreeva and E. A. Markvicheva, J. Mol. Liq., 2019, 284, 215–224 CrossRef CAS.
- C. Correa-Paz, M. F. Navarro Poupard, E. Polo, M. Rodríguez-Pérez, P. Taboada, R. Iglesias-Rey, P. Hervella, T. Sobrino, D. Vivien, J. Castillo, P. Del Pino, F. Campos and B. Pelaz, J. Controlled Release, 2019, 308, 162–171 CrossRef CAS.
- N. G. Balabushevich, E. A. Sholina, E. V. Mikhalchik, L. Y. Filatova, A. S. Vikulina and D. Volodkin, Micromachines, 2018, 9, E307 CrossRef PubMed.
- N. Feoktistova, G. Stoychev, N. Puretskiy, L. Ionov and D. Volodkin, Eur. Polym. J., 2015, 68, 650–656 CrossRef.
- (a) D. V. Volodkin, A. I. Petrov, M. Prevot and G. B. Sukhorukov, Langmuir, 2004, 20, 3398–3406 CrossRef CAS; (b) L. L. del Mercato, P. Rivera-Gil, A. Z. Abbasi, M. Ochs, C. Ganas, I. Zins, C. Sönnichsen and W. J. Parak, Nanoscale, 2010, 2, 458–467 RSC; (c) A. S. Timin, D. J. Gould and G. B. Sukhorukov, Expert Opin. Drug Delivery, 2017, 14, 583–587 CrossRef PubMed; (d) M. S. Saveleva, K. Eftekhari, A. Abalymov, T. E. L. Douglas, D. Volodkin, B. V. Parakhonskiy and A. G. Skirtach, Front. Chem., 2019, 7, 179 CrossRef CAS; (e) S. Zhao, F. Caruso, L. Dähne, G. Decher, B. G. de Geest, J. Fan, N. Feliu, Y. Gogotsi, P. T. Hammond, M. C. Hersam, A. Khademhosseini, N. Kotov, S. Leporatti, Y. Li, F. Lisdat, L. M. Liz-Marzán, S. Moya, P. Mulvaney, A. L. Rogach, S. Roy, D. G. Shchukin, A. G. Skirtach, M. M. Stevens, G. B. Sukhorukov, P. S. Weiss, Z. Yue, D. Zhu and W. J. Parak, ACS Nano, 2019, 13, 6151–6169 CrossRef CAS; (f) A. S. Vikulina, A. G. Skirtach and D. Volodkin, Langmuir, 2019, 35, 8565–8573 CrossRef CAS.
- D. Volodkin, Colloid Polym. Sci., 2014, 292, 1249–1259 CrossRef CAS.
- A. Wang, Y. Yang, X. Zhang, X. Liu, W. Cui and J. Li, ChemPlusChem, 2016, 81, 194–201 CrossRef CAS.
- A. Som, R. Raliya, L. Tian, W. Akers, J. E. Ippolito, S. Singamaneni, P. Biswas and S. Achilefu, Nanoscale, 2016, 8, 12639–12647 RSC.
- H. Li, Y. Zhao, Y. Jia, C. Qu and J. Li, Chem. Commun., 2019, 55, 15057–15060 RSC.
- (a) B. V. Parakhonskiy, A. M. Yashchenok, S. Donatan, D. V. Volodkin, F. Tessarolo, R. Antolini, H. Möhwald and A. G. Skirtach, ChemPhysChem, 2014, 15, 2817–2822 CrossRef CAS; (b) A. Hernández-Hernández, A. B. Rodríguez-Navarro, J. Gómez-Morales, C. Jiménez-Lopez, Y. Nys and J. M. García-Ruiz, Cryst. Growth Des., 2008, 8, 1495–1502 CrossRef.
- H. M. Burt, J. K. Jackson, D. R. Taylor and R. S. Crowther, Dig. Dis. Sci., 1997, 42, 1283–1289 CrossRef CAS.
- B. V. Parakhonskiy, C. Foss, E. Carletti, M. Fedel, A. Haase, A. Motta, C. Migliaresi and R. Antolini, Biomater. Sci., 2013, 1, 1273 RSC.
- Y. I. Svenskaya, A. M. Pavlov, D. A. Gorin, D. J. Gould, B. V. Parakhonskiy and G. B. Sukhorukov, Colloids Surf., B, 2016, 146, 171–179 CrossRef CAS.
- S. A. Kamba, M. Ismail, S. H. Hussein-Al-Ali, T. A. T. Ibrahim and Z. A. B. Zakaria, Molecules, 2013, 18, 10580–10598 CrossRef CAS.
- S. Donatan, A. Yashchenok, N. Khan, B. Parakhonskiy, M. Cocquyt, B.-E. Pinchasik, D. Khalenkow, H. Möhwald, M. Konrad and A. Skirtach, ACS Appl. Mater. Interfaces, 2016, 8, 14284–14292 CrossRef CAS PubMed.
- H. Cao, Y. Yang, Y. Qi, Y. Li, B. Sun, Y. Li, W. Cui, J. Li and J. Li, Adv. Healthcare Mater., 2018, 7, e1701357 CrossRef PubMed.
- G. Choukrani, B. Maharjan, C. H. Park, C. S. Kim and A. R. Kurup Sasikala, Mater. Sci. Eng., C, 2020, 106, 110226 CrossRef CAS PubMed.
- J. Chen, X. Wang, Y. Liu, H. Liu, F. Gao, C. Lan, B. Yang, S. Zhang and Y. Gao, Chem. Eng. J., 2019, 369, 394–402 CrossRef CAS.
- (a) T. N. Borodina, D. B. Trushina, I. V. Marchenko and T. V. Bukreeva, J. Bionanosci., 2016, 6, 261–268 CrossRef; (b) N. G. Balabushevich, E. A. Kovalenko, E. V. Mikhalchik, L. Y. Filatova, D. Volodkin and A. S. Vikulina, J. Colloid Interface Sci., 2019, 545, 330–339 CrossRef CAS PubMed.
- Y. I. Svenskaya, E. A. Genina, B. V. Parakhonskiy, E. V. Lengert, E. E. Talnikova, G. S. Terentyuk, S. R. Utz, D. A. Gorin, V. V. Tuchin and G. B. Sukhorukov, ACS Appl. Mater. Interfaces, 2019, 11, 17270–17282 CrossRef CAS PubMed.
- O. Gusliakova, E. N. Atochina-Vasserman, O. Sindeeva, S. Sindeev, S. Pinyaev, N. Pyataev, V. Revin, G. B. Sukhorukov, D. Gorin and A. J. Gow, Front. Pharmacol., 2018, 9, 559 CrossRef PubMed.
- M. S. Savelyeva, A. A. Abalymov, G. P. Lyubun, I. V. Vidyasheva, A. M. Yashchenok, T. E. L. Douglas, D. A. Gorin and B. V. Parakhonskiy, J. Biomed. Mater. Res., Part A, 2017, 105, 94–103 CrossRef CAS PubMed.
- A. Sergeeva, N. Feoktistova, V. Prokopovic, D. Gorin and D. Volodkin, Adv. Mater. Interfaces, 2015, 2, 1500386 CrossRef.
- R. Schröder, H. Pohlit, T. Schüler, M. Panthöfer, R. E. Unger, H. Frey and W. Tremel, J. Mater. Chem. B, 2015, 3, 7079–7089 RSC.
- Y. Gong, Y. Zhang, Z. Cao, F. Ye, Z. Lin and Y. Li, Biomater. Sci., 2019, 7, 3614–3626 RSC.
- M. S. Saveleva, A. N. Ivanov, M. O. Kurtukova, V. S. Atkin, A. G. Ivanova, G. P. Lyubun, A. V. Martyukova, E. I. Cherevko, A. K. Sargsyan, A. S. Fedonnikov, I. A. Norkin, A. G. Skirtach, D. A. Gorin and B. V. Parakhonskiy, Mater. Sci. Eng., C, 2018, 85, 57–67 CrossRef CAS PubMed.
- A. Obata, H. Ozasa, T. Kasuga and J. R. Jones, J. Mater. Sci.: Mater. Med., 2013, 24, 1649–1658 CrossRef CAS PubMed.
- Y. S. Liu, Q. L. Huang, A. Kienzle, W. E. G. Müller and Q. L. Feng, Mater. Sci. Eng., C, 2014, 38, 227–234 CrossRef CAS.
- (a) A. Obata, T. Hotta, T. Wakita, Y. Ota and T. Kasuga, Acta Biomater., 2010, 6, 1248–1257 CrossRef CAS; (b) T. Kasuga, A. Obata, H. Maeda, Y. Ota, X. Yao and K. Oribe, J. Mater. Sci.: Mater. Med., 2012, 23, 2349–2357 CrossRef CAS PubMed; (c) K. Fujikura, S. Lin, J. Nakamura, A. Obata and T. Kasuga, J. Biomed. Mater. Res., Part B, 2013, 101, 1350–1358 CrossRef PubMed.
- A. Stoica-Guzun, M. Stroescu, S. I. Jinga, I. M. Jipa and T. Dobre, Ind. Crops Prod., 2013, 50, 414–422 CrossRef CAS.
- S. Yamazaki, H. Maeda, A. Obata, K. Inukai, K. Kato and T. Kasuga, J. Nanomater., 2012, 2012, 1–7 CrossRef.
- A. S. Sergeeva, D. A. Gorin and D. V. Volodkin, Langmuir, 2015, 31, 10813–10821 CrossRef CAS PubMed.
- T. Paulraj, N. Feoktistova, N. Velk, K. Uhlig, C. Duschl and D. Volodkin, Macromol. Rapid Commun., 2014, 35, 1408–1413 CrossRef CAS PubMed.
- A. Sergeeva, A. S. Vikulina and D. Volodkin, Micromachines, 2019, 10, 357 CrossRef.
- (a) B. Lindman, G. Karlström and L. Stigsson, J. Mol. Liq., 2010, 156, 76–81 CrossRef CAS; (b) B. Medronho and B. Lindman, Adv. Colloid Interface Sci., 2015, 222, 502–508 CrossRef CAS PubMed.
- F. A. Ngwabebhoh and U. Yildiz, J. Appl. Polym. Sci., 2019, 136, 47878 CrossRef.
- S. Gopi, P. Balakrishnan, D. Chandradhara, D. Poovathankandy and S. Thomas, Mater. Today Chem., 2019, 13, 59–78 CrossRef CAS.
- E. Pinho and G. Soares, J. Mater. Chem. B, 2018, 6, 1887–1898 RSC.
- C. Sharma and N. K. Bhardwaj, Mater. Sci. Eng., C, 2019, 104, 109963 CrossRef CAS PubMed.
- Y. W. Chen and H. V. Lee, Int. J. Biol. Macromol., 2018, 107, 78–92 CrossRef CAS PubMed.
- A. Karimian, H. Parsian, M. Majidinia, M. Rahimi, S. M. Mir, H. S. Kafil, V. Shafiei-Irannejad, M. Kheyrollah, H. Ostadi and B. Yousefi, Int. J. Biol. Macromol., 2019, 133, 850–859 CrossRef CAS PubMed.
- S. Salimi, R. Sotudeh-Gharebagh, R. Zarghami, S. Y. Chan and K. H. Yuen, ACS Sustainable Chem. Eng., 2019, 7, 15800–15827 CrossRef CAS.
- A. A. Novikov, B. M. Anikushin, D. A. Petrova, S. A. Konstantinova, V. B. Mel’nikov and V. A. Vinokurov, Chem. Technol. Fuels Oils, 2018, 54, 564–568 CrossRef CAS.
- H. Ullah, H. A. Santos and T. Khan, Cellulose, 2016, 23(2), 291–2314 Search PubMed.
- J. K. Jackson, K. Letchford, B. Z. Wasserman, L. Ye, W. Y. Hamad and H. M. Burt, Int. J. Nanomed., 2011, 6, 321–330 CAS.
- G. Sarkar, J. T. Orasugh, N. R. Saha, I. Roy, A. Bhattacharyya, A. K. Chattopadhyay, D. Rana and D. Chattopadhyay, New J. Chem., 2017, 41, 15312–15319 RSC.
- A. O. Malakhov, T. S. Anokhina, D. A. Petrova, V. A. Vinokurov and A. V. Volkov, Pet. Chem., 2018, 58, 923–933 CrossRef CAS.
- S. Varanasi, R. He and W. Batchelor, Cellulose, 2013, 20(1), 885–1896 CrossRef.
- Y. Habibi, A.-L. Goffin, N. Schiltz, E. Duquesne, P. Dubois and A. Dufresne, J. Mater. Chem., 2008, 18, 5002 RSC.
- J. B. Daud and K.-Y. Lee, in Handbook of Nanocellulose and Cellulose Nanocomposites, ed. H. Kargarzadeh, I. Ahmad, S. Thomas and A. Dufresne, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2017, vol. 280, pp. 101–122 Search PubMed.
- N. Lin and A. Dufresne, Eur. Polym. J., 2014, 59, 302–325 CrossRef CAS.
- S. P. Akhlaghi, R. C. Berry and K. C. Tam, Cellulose, 2013, 20, 1747–1764 CrossRef CAS.
- G. M. A. Ndong Ntoutoume, R. Granet, J. P. Mbakidi, F. Brégier, D. Y. Léger, C. Fidanzi-Dugas, V. Lequart, N. Joly, B. Liagre, V. Chaleix and V. Sol, Bioorg. Med. Chem. Lett., 2016, 26, 941–945 CrossRef CAS PubMed.
- (a) S. Azizi, M. Ahmad, M. Mahdavi and S. Abdolmohammadi, BioResources, 2013, 8, 1841–1851 CrossRef; (b) H. Liu, J. Song, S. Shang, Z. Song and D. Wang, ACS Appl. Mater. Interfaces, 2012, 4, 2413–2419 CrossRef CAS PubMed.
- K. A. Mahmoud, K. B. Male, S. Hrapovic and J. H. T. Luong, ACS Appl. Mater. Interfaces, 2009, 1, 1383–1386 CrossRef CAS PubMed.
- (a) R. Kolakovic, L. Peltonen, T. Laaksonen, K. Putkisto, A. Laukkanen and J. Hirvonen, AAPS PharmSciTech, 2011, 12, 1366–1373 CrossRef CAS PubMed; (b) J. C. O. Villanova, E. Ayres, S. M. Carvalho, P. S. Patrício, F. V. Pereira and R. L. Oréfice, Eur. J. Pharm. Sci., 2011, 42, 406–415 CrossRef CAS PubMed.
- R. Curvello, V. S. Raghuwanshi and G. Garnier, Adv. Colloid Interface Sci., 2019, 267, 47–61 CrossRef CAS PubMed.
- J. Shojaeiarani, D. Bajwa and A. Shirzadifar, Carbohydr. Polym., 2019, 216, 247–259 CrossRef CAS PubMed.
- L. Pachuau, in Nanocellulose and Nanohydrogel Matrices, ed. M. Jawaid and F. Mohammad, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2017, vol. 2, pp. 1–19 Search PubMed.
- N. Li, H. Zhang, Y. Xiao, Y. Huang, M. Xu, D. You, W. Lu and J. Yu, Biomacromolecules, 2019, 20, 937–948 CrossRef CAS PubMed.
- R. Kolakovic, L. Peltonen, A. Laukkanen, J. Hirvonen and T. Laaksonen, Eur. J. Pharm. Biopharm., 2012, 82, 308–315 CrossRef CAS PubMed.
- S. Dong, A. A. Hirani, K. R. Colacino, Y. W. Lee and M. Roman, Nano LIFE, 2012, 02, 1241006 CrossRef.
- S. Dong, H. J. Cho, Y. W. Lee and M. Roman, Biomacromolecules, 2014, 15, 1560–1567 CrossRef CAS PubMed.
- A. S. Jimenez, F. Jaramillo, U. D. Hemraz, Y. Boluk, K. Ckless and R. Sunasee, Nanotechnol., Sci. Appl., 2017, 10, 123–136 CrossRef CAS PubMed.
- K. A. Mahmoud, J. A. Mena, K. B. Male, S. Hrapovic, A. Kamen and J. H. T. Luong, ACS Appl. Mater. Interfaces, 2010, 2, 2924–2932 CrossRef CAS PubMed.
- (a) M. Roman, Ind. Biotechnol., 2015, 11, 25–33 CrossRef CAS; (b) A. B. Seabra, J. S. Bernardes, W. J. Favaro, A. J. Paula and N. Duran, Carbohydr. Polym., 2018, 181, 514–527 CrossRef CAS PubMed.
- Y. Chen, Y.-J. Lin, T. Nagy, F. Kong and T. L. Guo, Carbohydr. Polym., 2020, 229, 115536 CrossRef PubMed.
- C. A. Otuechere, A. Adewuyi, O. L. Adebayo and I. A. Ebigwei, Hum. Exp. Toxicol., 2020, 39, 212–223 CrossRef CAS PubMed.
- K. J. de France, M. Badv, J. Dorogin, E. Siebers, V. Panchal, M. Babi, J. Moran-Mirabal, M. Lawlor, E. D. Cranston and T. Hoare, ACS Biomater. Sci. Eng., 2019, 5, 2235–2246 CrossRef CAS.
- M. Karzar Jeddi and M. Mahkam, Int. J. Biol. Macromol., 2019, 135, 829–838 CrossRef CAS PubMed.
- Q. Xu, Y. Ji, Q. Sun, Y. Fu, Y. Xu and L. Jin, Nanomaterials, 2019, 9, E253 CrossRef PubMed.
- S. A. A. Ghavimi, E. S. Lungren, T. J. Faulkner, M. A. Josselet, Y. Wu, Y. Sun, F. M. Pfeiffer, C. L. Goldstein, C. Wan and B. D. Ulery, Int. J. Biol. Macromol., 2019, 130, 88–98 CrossRef CAS PubMed.
- C. R. Silva, P. S. Babo, S. Mithieux, R. M. Domingues, R. Reis, M. E. Gomes and A. Weiss, J. Biomater. Appl., 2019, 34, 560–572 CrossRef CAS PubMed.
- A. Kumar, I. A. I. Matari, H. Choi, A. Kim, Y. J. Suk, J. Y. Kim and S. S. Han, Mater. Sci. Eng., C, 2019, 104, 109983 CrossRef CAS PubMed.
- (a) S. van Vlierberghe, P. Dubruel and E. Schacht, Biomacromolecules, 2011, 12, 1387–1408 CrossRef CAS PubMed; (b) S. D. Dutta, D. K. Patel and K.-T. Lim, J. Biol. Eng., 2019, 13, 55 CrossRef PubMed.
- A. Gupta, M. Kowalczuk, W. Heaselgrave, S. T. Britland, C. Martin and I. Radecka, Eur. Polym. J., 2019, 111, 134–151 CrossRef CAS.
- H. Xu, S. Huang, J. Wang, Y. Lan, L. Feng, M. Zhu, Y. Xiao, B. Cheng, W. Xue and R. Guo, Int. J. Biol. Macromol., 2019, 137, 433–441 CrossRef CAS PubMed.
- W. Huang, Y. Wang, Z. Huang, X. Wang, L. Chen, Y. Zhang and L. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 41076–41088 CrossRef CAS PubMed.
- S. Du, X. Chen, X. Chen, S. Li, G. Yuan, T. Zhou, J. Li, Y. Jia, D. Xiong and H. Tan, Macromol. Chem. Phys., 2019, 220, 1900399 CrossRef CAS.
- Q. Yan, L. Liu, T. Wang and H. Wang, Colloid Polym. Sci., 2019, 297, 705–717 CrossRef CAS.
- S. H. Park, H. S. Shin and S. N. Park, Carbohydr. Polym., 2018, 200, 341–352 CrossRef CAS PubMed.
- Z. Shen, N. Cai, Y. Xue, V. Chan, B. Yu, J. Wang, H. Song, H. Deng and F. Yu, Polymers, 2019, 11, E808 CrossRef PubMed.
- E. Y. X. Loh, N. Mohamad, M. B. Fauzi, M. H. Ng, S. F. Ng and M. C. I. Mohd Amin, Sci. Rep., 2018, 8, 2875 CrossRef PubMed.
- R. Liu, L. Dai, C. Si and Z. Zeng, Carbohydr. Polym., 2018, 195, 63–70 CrossRef CAS PubMed.
- Y. Poetzinger, L. Rahnfeld, D. Kralisch and D. Fischer, Carbohydr. Polym., 2019, 209, 62–73 CrossRef CAS PubMed.
- H. Storrie and D. J. Mooney, Adv. Drug Delivery Rev., 2006, 58, 500–514 CrossRef CAS PubMed.
- S. D’Mello, K. Atluri, S. M. Geary, L. Hong, S. Elangovan and A. K. Salem, AAPS J., 2017, 19, 43–53 CrossRef PubMed.
- Y. Mo, R. Guo, Y. Zhang, W. Xue, B. Cheng and Y. Zhang, Tissue Eng., Part A, 2017, 23, 597–608 CrossRef CAS PubMed.
- Q. Pan, N. Li, Y. Hong, H. Tang, Z. Zheng, S. Weng, Y. Zheng and L. Huang, RSC Adv., 2017, 7, 21352–21359 RSC.
- Y. Joo, J. H. Sim, Y. Jeon, S. U. Lee and D. Sohn, Chem. Commun., 2013, 49, 4519–4521 RSC.
- V. Vinokurov, A. Novikov, V. Rodnova, B. Anikushin, M. Kotelev, E. Ivanov and Y. Lvov, Polymers, 2019, 11, E919 CrossRef PubMed.
- C. Li, Y. Zhao, T. Zhu, Y. G. Li, J. Ruan and G. Li, RSC Adv., 2018, 8, 14870–14878 RSC.
- B. Lecouvet, J. Horion, C. D’Haese, C. Bailly and B. Nysten, Nanotechnology, 2013, 24, 105704 CrossRef CAS PubMed.
- D. Tan, P. Yuan, D. Liu and P. Du, Nanosized Tubular Clay Minerals – Halloysite and Imogolite, Elsevier, 2016, vol. 7, pp. 167–201 Search PubMed.
- D. B. Trushina, T. V. Bukreeva, M. V. Kovalchuk and M. N. Antipina, Mater. Sci. Eng., C, 2014, 45, 644–658 CrossRef CAS PubMed.
- (a) R. Ševčík, P. Šašek and A. Viani, J. Mater. Sci., 2018, 53, 4022–4033 CrossRef; (b) X. Feng and S. A. T. Redfern, Geochim. Cosmochim. Acta, 2018, 236, 351–360 CrossRef CAS.
- Z. Hosseinidoust, M. N. Alam, G. Sim, N. Tufenkji and T. G. M. van de Ven, Nanoscale, 2015, 7, 16647–16657 RSC.
- A. Brinkmann, M. Chen, M. Couillard, Z. J. Jakubek, T. Leng and L. J. Johnston, Langmuir, 2016, 32, 6105–6114 CrossRef CAS PubMed.
- A. Dufresne, Mater. Today, 2013, 16, 220–227 CrossRef CAS.
- S. Eyley and W. Thielemans, Nanoscale, 2014, 6, 7764–7779 RSC.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2020 |