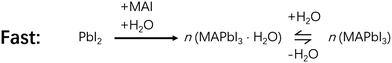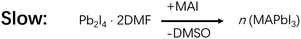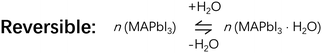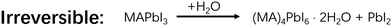Good or evil: what is the role of water in crystallization of organometal halide perovskites?
Shuang
Xiao†
 ,
Kai
Zhang†
,
Shizhao
Zheng
and
Shihe
Yang
,
Kai
Zhang†
,
Shizhao
Zheng
and
Shihe
Yang
 *
*
Guangdong Provincial Key Lab of Nano-Micro Materials Research, School of Chemical Biology and Biotechnology, Shenzhen Graduate School, Peking University, Shenzhen, China. E-mail: chsyang@pku.edu.cn
First published on 5th June 2020
Abstract
Perovskite solar cells (PSCs) have the potential to become one of the most cost-efficient photovoltaic devices. However, current fabrication methods of PSCs still require strict environment control and ultrahigh purity chemicals, which could prevent their large-scale commercialization. To tackle this challenge, the role of water is the first to be thoroughly understood in a perovskite formation process. Until now, there is still controversy about whether water is harmful or beneficial for perovskite formation, not to mention exactly what role water plays therein. In this Focus article, we review recent studies on water involved chemical reactions, solvent interaction, intermediates, and crystal growth in the perovskite film formation process, in order to bring out a full picture about what water does in the perovskite formation process. As our current understanding stands, a suitable amount of water could be of help for growing high quality perovskite films due to the resultant formation of intermediates, such as MAPbI3·H2O, which facilitates the conversion from precursors to perovskites. However, too much water would induce the formation of relatively stable components, such as (MA)4PbI6·2H2O, which are left in the product-films as impurities resulting in degraded device performance. Continual efforts should be made to further understand and develop water-involved strategies for consistent PSC fabrication under ambient conditions.
Introduction
Perovskite solar cells (PSCs) are one of the most promising candidates for the future low-cost photovoltaic industry as a new type of third-generation solar cells.1–3 Even when solution processed, perovskites show long-balanced carrier diffusion length, large light absorption coefficient, tunable bandgaps and low exciton binding energy,4–6 all propitious for developing high-efficiency solar cells. Indeed, within a short time span, the certified efficiency of PSCs has already risen to as high as 25.2%, rapidly approaching the theoretical limit.7,8 However, those highest efficiencies are achieved only with precise processing conditions, strict environmental control and ultra-pure reagents/solvents, which could increase the manufacturing cost and thus hinder the commercialization of PSCs.6,9,10 Robust fabrication methods are deadly sought after to obtain relatively high efficiency and high stability PSCs at an acceptable cost with minimal reliance on stringent control over the fabrication environment and purity of the chemicals involved.Water exists almost everywhere surrounding us: it is in the earth's atmosphere, and it is essentially the solvent of life. Unfortunately, organometal halide perovskites are unstable when exposed to water because they are ionic compounds, and water can act as a base to easily remove cations from the perovskite's lattice.11,12 This poses a serious problem for the fabrication of perovskite-based devices, particularly solar cells. Thus one has to either eliminate water completely in the fabrication environment or design paths to neutralize the etching role of water in the fabrication process. Clearly, in comparison with the former strategy, the latter is more cost-effective and technologically appealing. This underlines the importance of unveiling the role of water in crystallization of perovskite for the development of robust fabrication methods.
There has long been a disagreement about whether water has beneficial or harmful effects on perovskite crystallization and film formation.11,13–19 On the one hand, numerous reports showed that PSCs fabricated in a humid environment yielded poorer power conversion efficiency (PCE) than those fabricated under water free conditions.20,21 Indeed, the highest efficiency PSCs (PCE > 23%) were invariably fabricated in an inert atmosphere with water content less than 1 ppm.22,23 On the other hand, there were also reports that blending water into perovskite precursors could help to form large grains and improve the PSCs’ efficiency.16,24 Most recently, it has been shown that an ideal fabrication method, if well controlled, can be insensitive to the level of humidity for the fabrication of perovskite films.13,15 To shed light on the issue about the role of water in perovskite fabrication, we analyze and discuss the water-included chemical reaction kinetics, intermediates, and solvent properties pertaining to the perovskite fabrication process by summarizing some recent studies in this field. Our objective is to bring out a better picture about the role of water in perovskite fabrication, thereby providing guidelines for future developments of perovskite-based devices.
Solvent dripping method
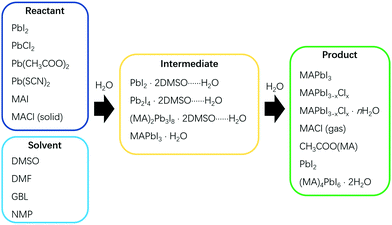
Solvent dripping is an effective method for fabricating perovskite films simply by modulating the solubility of the participating species, most notably the perovskite product.5,10,25 This method was developed by Seok et al. in 2014, and subsequently widely adopted for effectively producing compact perovskite films in an inert environment.10 Briefly, antisolvent is dropped on a spinning wet precursor film to drive out the solvent more rapidly, facilitating the nucleation and growth of perovskites. In previous reports, pinholes were found to form easily during solvent dripping processes of perovskite film fabrication under the influence of water.26,27 Clearly, such pinholes are harmful to PSCs with the consequence of bringing down their performance, and thus should be avoided.21,26,27 However, further studies in our recent work have shown that water can accelerate the formation of perovskite intermediates, and subsequently, fibrous PbI2, before the eventual transformation to perovskite. It is the step of uncontrolled generation of fibrous PbI2 that induced pinholes of perovskite films, which actually could be counteracted by reprogramming the solvent dripping process.13In the solvent dripping method, typical reagents and solvents used for the reactions include Methylammonium iodide (MAI), Lead iodide (PbI2), Dimethyl sulfoxide (DMSO) and N,N-dimethylformamide (DMF), and toluene is commonly used as the dripping solvent (Fig. 1).28 Due to the strong coordination of DMSO to Pb2+, the PbI2·2DMSO intermediate quickly forms just after mixing the reagents and solvents together (Scheme 1). During the fabrication process, PbI2·2DMSO will subsequently convert to Pb2I4·2DMSO, then to (MA)2Pb3I8·2DMSO and finally to MAPbI3, driven by the increasing precursor concentration (Scheme 1).10 In the absence of water, the precursor concentration can be finely controlled by spinning, solvent dripping and annealing, yielding compact and pinhole free perovskite films.
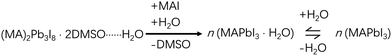
However, the presence of water will change the kinetics of this conversion process dramatically by facilitating DMSO dissociation from these lead complexes.13 Interestingly, the relative electronic energies of PbI2·2DMSO, Pb2I4·2DMSO and (MA)2Pb3I8·2DMSO are modulated by the presence of water in the form of water-intercalated intermediates (PbI2·2DMSO⋯H2O, Pb2I4·2DMSO⋯H2O and (MA)2Pb3I8·2DMSO⋯H2O) in a way that makes the conversion from PbI2·2DMSO⋯H2O to (MA)2Pb3I8·2DMSO⋯H2O much easier than from PbI2·2DMSO to (MA)2Pb3I8·2DMSO, leading to faster precipitation of (MA)2Pb3I8·2DMSO.13 Consequently, the intermediate crystals grow much larger than those grown in a water-free environment (Fig. 2a and b). Since the conversion from one dimensional (1D) (MA)2Pb3I8·2DMSO to three dimensional (3D) MAPbI3 involves a large crystal reconstruction, too large intermediate crystals often make it difficult to smoothly complete the 1D–3D conversion, leaving behind some detrimental pinholes (Fig. 2c and d).25 To forestall this scenario, we recently proposed a prenucleation method, which, by adjusting the nucleation and growth rates, allowed the successful fabrication of high quality and compact perovskite films under ambient conditions (Fig. 2e).13 The corresponding PSCs yielded comparable performance but outstanding performance uniformity compared with those fabricated under water-free conditions. Besides, the stability of these PSCs was also quite good, with only ∼10% efficiency decay after 100-day-storage and only ∼9% decay after 200 h of operation, both in an inert environment at room temperature.
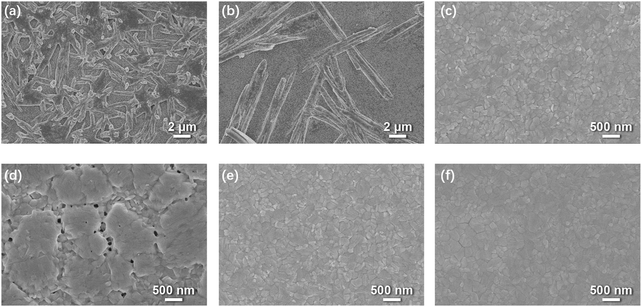 | ||
| Fig. 2 The scanning electron microscopy (SEM) images of (a) intermediates fabricated in a glove box, (b) intermediates fabricated at 40% relative humidity (RH), (c) perovskites fabricated in a glove box using a conventional solvent dripping method, (d) perovskites fabricated at 40% RH using a conventional solvent dripping method, (e) perovskites fabricated at 40% RH using a prenucleation method and annealing treatment, and (f) perovskites fabricated at 40% RH using a prenucleation method and no annealing treatment.13 | ||
Under water-free conditions, annealing is normally required to expedite the conversion from intermediates to MAPbI3.10 However, in a humid environment, MAPbI3 could directly form from the precursor at room temperature due to the water activation.18,29 For example, researchers have shown that after spin-coating, fibrous intermediates grew from the precursor at 40% relative humidity (RH).18 By prolonging the aging time, these fibers grew larger and converted to MAPbI3 in 3 h without annealing, forming a pinhole-rich film. Plausibly, in the presence of water, (MA)2Pb3I8·2DMSO⋯H2O could easily convert to MAPbI3·H2O, a 1D monohydrate intermediate, and then lose its water content to form MAPbI3 (Scheme 2).11,13 This conversion process is fast and can occur at temperatures below 40 °C, much lower than that required for the water-free conversion from (MA)2Pb3I8·2DMSO to MAPbI3.30 With our prenucleation method that inherits the low-temperature advantage, the water assisted conversion is not only fast within 10 min but also controllable owing to the active intervention to prevent the intermediates from growing too large.13 The resulting perovskite film is also compact and uniform giving rise to high performance PSCs (Fig. 2f).
Sequential deposition method
First introduced by Grätzel et al. in 2013, the sequential deposition method is frequently used to fabricate the most highly efficient PSCs (PCE > 23%) in recent years.2 In a sequential deposition method, PbI2 film is first fabricated and then allowed to react with MAI to form MAPbI3, and this makes the intermediates less relevant compared to the solvent dripping method (Fig. 1).2,22 Nevertheless, the water effect is still a very pertinent issue. Reports showed that moisture was pernicious to perovskite formation with the sequential deposition method.17,20,31,32 When fabricated in humidity ≥20% RH, PbI2 films were found to be very rough and have lots of large voids (Fig. 3a).20 After reacting with MAI, part of those voids remained in the perovskite film, obviously boding ill for the performance of PSCs based thereupon (Fig. 3d). However, other reports showed that water in precursors could help to form a high quality perovskite layer by facilitating the reaction between MAI and PbI2 to enlarge the grain size with fewer defects and lower carrier recombination.33,34 PSCs based on these films promoted the PCE from 9.78% to 20.1%.16 To better illustrate the influence of water, we plan to bring out the discussion in 2 processes of a sequential deposition method: (1) the PbI2 formation, (2) the reaction between MAI and PbI2.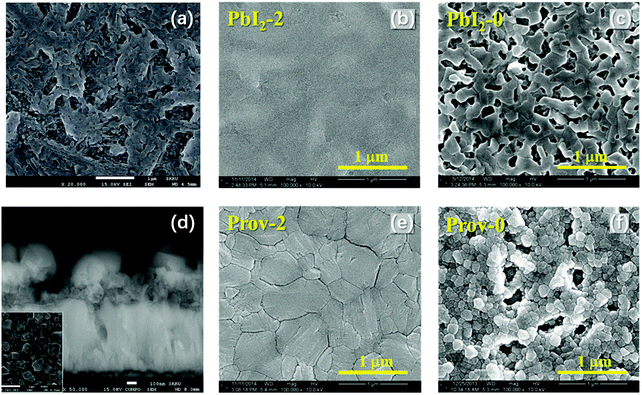 | ||
| Fig. 3 SEM images of (a) PbI2 fabricated at 44% RH, whose amount of water is too much,20 (b) PbI2 fabricated by a precursor with 2 wt% (weight percentage) water in a glove box, whose amount of water is suitable,33 (c) PbI2 fabricated by a precursor without water in a glove box,33 and (d–f) perovskite films of the corresponding PbI2 films, respectively.20,33 | ||
In the PbI2 formation process, the amount of water determines whether it has a noxious or helpful effect on MAPbI3 film formation. As a common solvent for this process, DMF is miscible with water. So, its solution of PbI2 will absorb a great deal of water in humid air.13 Water has a much higher relative polarity (1) than DMF (0.386), thus adding water will decrease the solubility of PbI2 in DMF, resulting in uncontrolled precipitation of PbI2. Thus, the PbI2 films fabricated in humid air are typically rough riddled with pinholes, and the MAPbI3 films from their transformation would be similarly rough (Fig. 3a and d). It was found that >3 wt% (weight percentage) water in PbI2 DMF solution could be easily reached in the spin coating process in humid air, forming rough PbI2 films.13,33 In contrast, a suitable amount of water additive (∼2 wt%) could help PbI2 to form a flat and continuous film, which could then convert to a compact MAPbI3 film after reacting with MAI (Fig. 3b and e). The reason is that ∼2 wt% water in DMF could form a mixture with high solubility of PbI2, which appears to be crucial for the formation of flat PbI2 films.33 Interestingly, PbI2 films fabricated by water-free precursors also exhibited a rough morphology analogous to those prepared with too much water, due perhaps similarly to the low solubility issue (Fig. 3c and f). Together, water mainly serves to tune the solubility of PbI2 and the solubility volcano with a suitable amount of water appears to correlate with the optimum performance of the resultant solvent in forming a smooth pinhole-free PbI2 and then perovskite film for advanced solar cells.
In a bit more detail, for the reaction process of MAI and PbI2, the amount of water in the processing solvent also plays opposite roles. For example, it was found that 0.5 vol% (volume percentage) water in MAI/Isopropanol (IPA) solution helped the grain coarsening of perovskites, which enhanced the conversion ratio and enlarged the grain size from ∼300 nm to 2 μm.16 The higher conversion ratio and larger grain size significantly increased the efficiency of PSCs, lending beneficial effect of water. In this case, water is involved in a key hydrate intermediate, MAPbI3·H2O. The reaction between PbI2 and a water-containing solution of MAI to form MAPbI3·H2O is much more facile than without water to form MAPbI3 (Schemes 3 and 4) due seemingly to the reduced reaction barriers.11 Once formed, MAPbI3·H2O could easily lose its H2O to form MAPbI3 (Scheme 3). Therefore, a suitable amount of water facilitates the formation of MAPbI3 and improves the performance of the corresponding PSCs, with the assistance of MAPbI3·H2O.16,34 However, too much water (>3 vol%) in MAI solution causes the formation of a dihydrate intermediate, (MA)4PbI6·2H2O, accompanied by PbI2 formation, making it difficult to convert to MAPbI3 (Fig. 1).11
Annealing in a humid environment
Annealing a perovskite film in a humid environment is believed to be beneficial for improving film quality and enhancing the PSCs’ efficiency.19,22,35,36 In principle, the annealing process provides heat to activate and facilitate ion migration, thus effectively coarsening grains and reducing defects. Consequently, the PSCs fabricated this way could yield superior performance. More importantly, annealing in a humid environment produces even larger grains than that annealed in an inert atmosphere, leading to even better performance of the corresponding PSCs. Here the key role of water is to generate intermediates at grain boundaries, which lowers the activation barrier to grain boundary migration, and thus eases the process and increases the grain coarsening speed. In particular, an inspiring thing is that this strategy is effective for a wide range of perovskites including MAPbI3, FA1−xMAxPbI3 and FA1−yCsyPbI3−zBrz, foreshadowing its high application potential.19,22,35As a representative example, MAPbI3 polycrystalline films typically absorb water at their grain boundaries, and the annealing process could lead to the formation of a monohydrate intermediate, MAPbI3·H2O (Scheme 5).36 Then the relatively high temperature of annealing (∼100 °C) causes MAPbI3·H2O to easily decompose to MAPbI3 and H2O and establishes a balance between formation and decomposition of this intermediate at grain boundaries. As such, ion exchange at grain boundaries became faster via this chemical reaction than inside the MAPbI3 bulk lattice. In general, surface energy of grains dictates ion migration from small grains to big grains, which ultimately coarsens the grains in perovskite films.37 To maintain the ideal condition for water promoted grain coarsening, the humidity should be kept at 30–40% RH.22 If the humidity is too high (>80% RH), the reaction with H2O will become irreversible with uncontrollable formation of PbI2 and thus deteriorated PSCs’ performance (Scheme 6).11,19 Therefore, only when in a suitable humid environment does annealing enable the water-involved reaction at grain boundaries and help to form large grains to improve the PSCs’ performance. Furthermore, decent stability of PSCs annealed in humid air was demonstrated, with only ∼16% decay in efficiency after 500 h of heating at 85 °C.22
Table 1 summarizes the roles of water in the different perovskite film fabrication processes. One can see from the above discussion that the roles of a suitable amount of water in annealing and sequential deposition are similar: facilitating the reactions or grain boundary migration to enlarge the perovskite grains (Table 1, ①–③). On the other hand, excess water is normally harmful for perovskite crystallization. Next, water could accelerate the growth of PbI2 and (MA)2Pb3I8·2DMSO in sequential deposition and solvent dripping, respectively (Table 1, ④ & ⑤). Too large and rough grains of PbI2 and (MA)2Pb3I8·2DMSO cause pinholes in perovskite films and thus deteriorate the performance of the corresponding PSCs. Furthermore, in the presence of water, (MA)4PbI6·2H2O could be formed in sequential deposition and annealing processes (Table 1, ⑥ & ⑦). Notably, formation of (MA)4PbI6·2H2O is always accompanied by the generation of PbI2 and by perovskite degradation, which is to be avoided in the design and fabrication of high-performance PSCs.
| Method | Condition | Role of water | Effect | |
|---|---|---|---|---|
| ① | Annealing | 30–40% RH22 | Facilitate grain boundary migration | Beneficial: large grains |
| ② | Sequential deposition | ∼0.5 vol% water in MAI solution16 | Facilitate reaction between MAI and PbI2 | Beneficial: large grains |
| ③ | Sequential deposition | ∼2 wt% water in PbI2 solution33 | Facilitate reaction between MAI and PbI2 | Beneficial: large grains |
| ④ | Sequential deposition | >3 wt% water in PbI2 solution33 | Accelerate growth of PbI2 | Harmful: pinholes |
| ⑤ | Solvent dripping | Water in solution or environment13 | Accelerate growth of (MA)2Pb3I8·2DMSO | Harmful: pinholes |
| ⑥ | Sequential deposition | >3 vol% water in MAI solution16 | Formation of (MA)4PbI6·2H2O | Harmful: byproduct |
| ⑦ | Annealing | >80% RH19 | Formation of (MA)4PbI6·2H2O | Harmful: byproduct |
Some computational studies have also been reported on the effect of water on perovskite formation. In general, the studies show that water is highly reactive to perovskites, with large negative formation energy of water in MAPbI3/FAPbI3/CsPbI3 crystals.38 Interestingly, water is more reactive toward the MAI-terminated surface than the PbI2-terminated surface of MAPbI3, leading to the formation of intermediates or decomposition.39 Both computational and experimental studies indicated that water can degrade PSC's stability.6,23–25 As an example, the typical decomposition reaction between water and MAPbI3 is shown in Scheme 6, which eliminates MA and leaves behind PbI2.11,12 Therefore, although PSCs could be fabricated in a humid environment, they have to be strictly encapsulated to avoid the deleterious influence of water.
Interactions between solvents and antisolvents
Properties of solvents and antisolvents are crucial to perovskite formation. The interplay between them and water may further illustrate the role of water in precursors for perovskite fabrication. The most frequently used solvents are DMSO, DMF, n-methyl-2-pyrrolidone (NMP), γ-butyrolactone (GBL), etc., and the most frequently used antisolvents are anisole, chlorobenzene (CB), toluene, ethyl acetate, etc. (Fig. 1).10,21,40,41 In this section, we wish to use Gutmann's Donor Number (DN, or donicity) to put into context the water effect on perovskite crystallization by positively correlating it with the coordination ability of solvents to Pb2+ ions (Table 2).42 DN is defined as the negative value of the molar enthalpy for interaction of a solvent with the strong Lewis acid SbCl5 in dichloroethane.43 In a recent article, Loo et al. compared the DN of DMSO, DMF, and N,N′-dimethylpropyleneurea (DMPU) to show that solvents with high DN help to form compact polycrystalline perovskite films with a suitable grain size, while those with low DN yield a grain size too large for PSCs.42 The purpose of using DN in this paper is to rationalize water's role in perovskite crystallization.| Solvent | DN (kcal mol−1) | Solvent | DN (kcal mol−1) |
|---|---|---|---|
| DMSO | 29.8 | Ethyl acetate | 17.1 |
| NMP | 27.3 | Acetonitrile | 14.1 |
| DMF | 26.6 | Anisole | 9.0 |
| GBL | 18.0 | Chlorobenzene | 3.3 |
| H2O (liquid) | 26.7 | Toluene | 1 |
| H2O (gas) | 18.0 |
For the precursor solvents, the DN order is DMSO > NMP > DMF > GBL in good agreement with their coordinate strengths observed in experiments. This order explains why the complexes of PbI2–DMSO and PbI2–NMP are easier to form than the complexes of PbI2–DMF and PbI2–GBL. The strongly bonded complexes are to be finely controlled in perovskite fabrication since they can retard the formation of perovskites.42 The DN of pure water is among the highest of these solvents, underpinning the high coordination ability of H2O to Pb2+. In addition, the DN of water can be varied when dispersed in an aprotic solvent. Indeed, as shown in Fig. 4, the DN of water increases with the DN of aprotic solvents. As discussed in the sequential deposition section, water could retard perovskite growth and help to form large grains, due probably to its large DN value (>26 kcal mol−1) and thus favorable coordination to Pb2+ in DMF solvent (Fig. 4). However, the situation is different in the antisolvent dripping process. Due to the miscibility and low DN value of antisolvents such as toluene, DMF and DMSO are selectively rinsed away by toluene on dripping the antisolvent, and part of the DMSO as a stronger Lewis base preferentially forms a complex with PbI2. Along with the evaporative removal of the miscible antisolvents and solvents from the wet films, water gradually loses its coordination ability due to its dramatically reduced DN(H2O) approaching 18 kcal mol−1. As a result, the water-containing complex precursors or intermediates will decompose, facilitating the perovskite formation. In all, water has two different functions in precursor solution for perovskite fabrication: (1) forming complexes with lead iodide to retard perovskite growth in relatively high DN value (>26 kcal mol−1) solvents such as DMF; and (2) assisting in perovskite formation in the antisolvent dripping process with a rapidly decreasing DN(H2O) value.
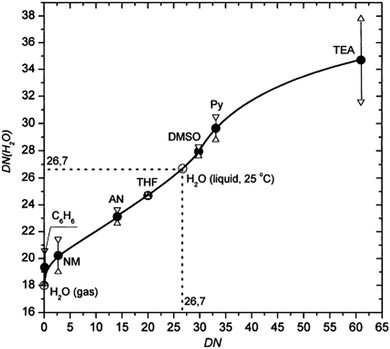 | ||
| Fig. 4 Relationship between the DN value of water in select aprotic solvents. y-Axis is DN (H2O) in different solvents and x-axis is the DN of these solvents.44 | ||
Summary and outlook
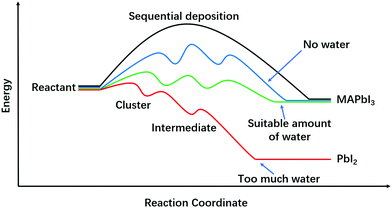
In this focus article, we have reviewed water effects on perovskite fabrication processes, including water promoted lead complex formation (from PbI2·2DMSO to (MA)2Pb3I8·2DMSO), monohydrate (MAPbI3·H2O) facilitated grain growth and interactions between water and solvents relevant in all those processes. In Fig. 5, we round up various perovskite transformation processes from the common reactant precursors. Clearly, direct formation of perovskites from reactants without going through any intermediate steps would have the highest activation energy, and is thus not a viable route for perovskite fabrication (Fig. 5, black curve). However, with the introduction of intermediate steps, the activation energies are much reduced, facilitating the perovskite formation (Fig. 5, blue curve). Moreover, when water is involved in the reactions, the activation energies of perovskite formation and grain boundary migration will be further reduced (Fig. 5, green curve). As we have shown in the various sections above, water could help to form large grains and improve the corresponding PSCs’ performance when its amount is suitable (0.5–3 wt% in precursor and 30–40% RH for annealing). However, too much water (>3 wt% in precursor and >80% RH for annealing) will produce perovskite films full of pinholes and harmful by-products ((MA)4PbI6·2H2O and PbI2), and is thus detrimental to the corresponding PSCs (Fig. 5, red curve). So it is fair to say that water is a bi-functional solvent in that it can be used to retard perovskite formation in high DN (25–30 kcal mol−1) solvents, and it can also assist in perovskite formation in the antisolvent dripping process that rapidly cuts down its DN number.Looking ahead, there is much more to be studied on the water effects in perovskite fabrication. To develop robust fabrication strategies, interaction between water and perovskites (especially the mix ion perovskites with optimal bandgaps) should be more thoroughly understood. Because different ions in a perovskite precursor solution have different interactions with water molecules, new intermediate-compounds may form, which may influence the perovskite formation energetics and kinetics in ways that are still unknown yet. Besides, attempts to exploit the different water/perovskite interactions are certainly meaningful and important for fabrication of PSCs with high performance. Just as we amply discussed in this article, the two prongs of water-involved reactions can be used to full advantage: to suppress and to facilitate perovskite formation in solution deposition and in antisolvent dripping, respectively. Considering the cost-efficiency, we foresee that new strategies that utilize ambient water to improve the quality of perovskites should have great potential in industrial applications of novel perovskite materials.
Author contributions
This manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors acknowledge the support from NSFC (21905006, 21972006, 51961165105 and 51773230), Shenzhen Peacock Plan (KQTD2016053015544057), the Shenzhen & Hong Kong Joint Research Program (SGLH20180622092406130), the Nanshan Pilot Plan (LHTD20170001), and the Guangdong Science and Technology Program (2017B030314002). We thank Dr. Z. Wang for discussion on the simulation of water/perovskite interactions.References
- W. Shockley and H. J. Queisser, J. Appl. Phys., 1961, 32, 510–519 CrossRef CAS.
- J. Burschka, N. Pellet, S. J. Moon, R. Humphry-Baker, P. Gao, M. K. Nazeeruddin and M. Grätzel, Nature, 2013, 499, 316–319 CrossRef CAS PubMed.
- M. Liu, M. B. Johnston and H. J. Snaith, Nature, 2013, 501, 395–398 CrossRef CAS PubMed.
- W. Tress, Adv. Energy Mater., 2017, 7, 1602358 CrossRef.
- H. Tan, A. Jain, O. Voznyy, X. Lan, F. P. García de Arquer, J. Z. Fan, R. Quintero-Bermudez, M. Yuan, B. Zhang, Y. Zhao, F. Fan, P. Li, L. N. Quan, Y. Zhao, Z.-H. Lu, Z. Yang, S. Hoogland and E. H. Sargent, Science, 2017, 355, 722–726 CrossRef CAS PubMed.
- D. Luo, W. Yang, Z. Wang, A. Sadhanala, Q. Hu, R. Su, R. Shivanna, G. F. Trindade, J. F. Watts, Z. Xu, T. Liu, K. Chen, F. Ye, P. Wu, L. Zhao, J. Wu, Y. Tu, Y. Zhang, X. Yang, W. Zhang, R. H. Friend, Q. Gong, H. J. Snaith and R. Zhu, Science, 2018, 360, 1442–1446 CrossRef CAS PubMed.
- Research Cell Efficiency Records, NREL, 2020.
- W. E. I. Sha, X. Ren, L. Chen and W. C. H. Choy, Appl. Phys. Lett., 2015, 106, 221104 CrossRef.
- D. Bi, W. Tress, M. I. Dar, P. Gao, J. Luo, C. Renevier, K. Schenk, A. Abate, F. Giordano, J.-P. C. Baena, J.-D. Decoppet, S. M. Zakeeruddin, M. K. Nazeeruddin, M. Grätzel and A. Hagfeldt, Sci. Adv., 2016, 2, e1501170 CrossRef PubMed.
- N. J. Jeon, J. H. Noh, Y. C. Kim, W. S. Yang, S. Ryu and S. Il Seok, Nat. Mater., 2014, 13, 897–903 CrossRef CAS PubMed.
- K. Shoyama, W. Sato, Y. Guo and E. Nakamura, J. Mater. Chem. A, 2017, 5, 23815–23821 RSC.
- A. M. A. Leguy, Y. Hu, M. Campoy-Quiles, M. I. Alonso, O. J. Weber, P. Azarhoosh, M. V. Schilfgaarde, M. T. Weller, T. Bein, J. Nelson, P. Docampo and P. R. F. Barnes, Chem. Mater., 2015, 27, 3397–3407 CrossRef CAS.
- K. Zhang, Z. Wang, G. Wang, J. Wang, Y. Li, W. Qian, S. Zheng, S. Xiao and S. Yang, Nat. Commun., 2020, 11, 1006 CrossRef CAS PubMed.
- G. Wu, R. Sun, L. Hu, X. Dong, G. Cui, M. Gu, T. B. Tang, Z. Zuo and Y. Liu, Sol. RRL, 2020, 4, 1900406 CrossRef CAS.
- D. Liu, C. J. Traverse, P. Chen, M. Elinski, C. Yang, L. Wang, M. Young and R. R. Lunt, Adv. Sci., 2018, 5, 1700484 CrossRef PubMed.
- C. Chiang, M. K. Nazeeruddin, M. Grätzel and C. Wu, Energy Environ. Sci., 2017, 10, 808–817 RSC.
- Y. Cheng, X. Xu, Y. Xie, H. Li, J. Qing, C. Ma, C. Lee, F. So and S. Tsang, Sol. RRL, 2017, 1, 1700097 CrossRef.
- A. Dubey, N. Kantack, N. Adhikari, K. M. Reza, S. Venkatesan, M. Kumar, D. Khatiwada, S. Darling and Q. Qiao, J. Mater. Chem. A, 2016, 4, 10231–10240 RSC.
- J. You, Y. (Michael) Yang, Z. Hong, T.-B. Song, L. Meng, Y. Liu, C. Jiang, H. Zhou, W.-H. Chang, G. Li and Y. Yang, Appl. Phys. Lett., 2014, 105, 183902 CrossRef.
- H. Ko, J. Lee and N. Park, J. Mater. Chem. A, 2015, 3, 8808–8815 RSC.
- M. Yavari, M. Mazloum-Ardakani, S. Gholipour, M. M. Tavakoli, S.-H. Turren-Cruz, N. Taghavinia, M. Grätzel, A. Hagfeldt and M. Saliba, Adv. Energy Mater., 2018, 8, 1800177 CrossRef.
- Q. Jiang, Y. Zhao, X. Zhang, X. Yang, Y. Chen, Z. Chu, Q. Ye, X. Li, Z. Yin and J. You, Nat. Photonics, 2019, 13, 460–466 CrossRef CAS.
- H. Min, M. Kim, S.-U. Lee, H. Kim, G. Kim, K. Choi, J. H. Lee and S. Il Seok, Science, 2019, 366, 749–753 CrossRef CAS PubMed.
- X. Gong, M. Li, X. Shi, H. Ma, Z. Wang and L. Liao, Adv. Funct. Mater., 2015, 25, 6671–6678 CrossRef CAS.
- S. Xiao, Y. Bai, X. Meng, T. Zhang, H. Chen, X. Zheng, C. Hu, Y. Qu and S. Yang, Adv. Funct. Mater., 2017, 27, 1604944 CrossRef.
- C. Aranda, C. Cristobal, L. Shooshtari, C. Li, S. Huettner and A. Guerrero, Sustainable Energy Fuels, 2017, 1, 540–547 RSC.
- D. Angmo, X. Peng, A. Seeber, C. Zuo, M. Gao, Q. Hou, J. Yuan, Q. Zhang, Y. Cheng and D. Vak, Small, 2019, 15, 1904422 CrossRef CAS PubMed.
- Y. Bai, S. Xiao, C. Hu, T. Zhang, X. Meng, H. Lin, Y. Yang and S. Yang, Adv. Energy Mater., 2017, 7, 1701038 CrossRef.
- J. Li, A. Dobrovolsky, A. Merdasa, E. L. Unger and I. G. Scheblykin, ACS Omega, 2018, 3, 14494–14502 CrossRef CAS PubMed.
- Y. Guo, K. Shoyama, W. Sato, Y. Matsuo, K. Inoue, K. Harano, C. Liu, H. Tanaka and E. Nakamura, J. Am. Chem. Soc., 2015, 137, 15907–15914 CrossRef CAS PubMed.
- W. Wang, S. K. Das and Y. Tai, ACS Appl. Mater. Interfaces, 2017, 9, 10743–10751 CrossRef CAS PubMed.
- Q. Tai, P. You, H. Sang, Z. Liu, C. Hu, H. L. W. Chan and F. Yan, Nat. Commun., 2016, 7, 11105 CrossRef CAS PubMed.
- C.-G. Wu, C.-H. Chiang, Z.-L. Tseng, M. K. Nazeeruddin, A. Hagfeldt and M. Grätzel, Energy Environ. Sci., 2015, 8, 2725–2733 RSC.
- M. K. Gangishetty, R. W. J. Scott and T. L. Kelly, Nanoscale, 2016, 8, 6300–6307 RSC.
- D. P. McMeekin, G. Sadoughi, W. Rehman, G. E. Eperon, M. Saliba, M. T. Horantner, A. Haghighirad, N. Sakai, L. Korte, B. Rech, M. B. Johnston, L. M. Herz and H. J. Snaith, Science, 2016, 351, 151–155 CrossRef CAS PubMed.
- A. Yi, S. Chae, H. Lee and H. J. Kim, J. Mater. Chem. A, 2019, 7, 27267–27277 RSC.
- F. D. Fischer, T. Waitz, D. Vollath and N. K. Simha, Prog. Mater. Sci., 2008, 53, 481–527 CrossRef CAS.
- C. Jiang, Y. Wang, R. Zhou, H. Wang and Q. Chen, J. Appl. Phys., 2018, 124, 085105 CrossRef.
- L. Zhang, M.-G. Ju and W. Liang, Phys. Chem. Chem. Phys., 2016, 18, 23174–23183 RSC.
- Y. Jo, K. S. Oh, M. Kim, K.-H. Kim, H. Lee, C. Lee and D. S. Kim, Adv. Mater. Interfaces, 2016, 3, 1500768 CrossRef.
- S. A. Fateev, A. A. Petrov, V. N. Khrustalev, P. V. Dorovatovskii, Y. V. Zubavichus, E. A. Goodilin and A. B. Tarasov, Chem. Mater., 2018, 30, 5237–5244 CrossRef CAS.
- J. C. Hamill, J. Schwartz and Y. Loo, ACS Energy Lett., 2018, 3, 92–97 CrossRef CAS.
- V. Gutmann, Coord. Chem. Rev., 1976, 18, 225–255 CrossRef CAS.
- J. Stangret, J. Mol. Struct., 2002, 643, 29–35 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |