Open Access Article
Open Access ArticleField testing of low-cost titania-based photocatalysts for enhanced solar disinfection (SODIS) in rural India†
Victoria
Porley
a,
Efthalia
Chatzisymeon
b,
Bhim Charan
Meikap
c,
Somnath
Ghosal
d and
Neil
Robertson
 *a
*a
aSchool of Chemistry, David Brewster Road, The King's Buildings, Edinburgh, Scotland EH9 3FJ, UK. E-mail: neil.robertson@ed.ac.uk
bSchool of Engineering, Thomas Bayes Road, The King's Building, Edinburgh, Scotland EH9 3FG, UK
cDepartment of Chemical Engineering, Indian Institute of Technology, Kharagpur, West Bengal, India
dRural Development Centre, Indian Institute of Technology, Kharagpur, West Bengal, India
First published on 20th January 2020
Abstract
To widen access to safe potable water in rural areas, many novel photocatalysts have been developed and presented in the literature, with the potential to be used in conjunction with simple solar disinfection (SODIS) techniques, showing successful removal of a range of contaminants. However, it is often the case that investigations into new photocatalytic systems are limited to laboratory tests, which are generally conducted under idealised conditions that do not take into account many practical limitations of real-world conditions. To address this need, we have conducted tests under sunlight using real water sources from rural villages in India to verify the results of previous successful laboratory tests on a novel photocatalyst. It was found that SODIS can be significantly enhanced with the addition of photocatalyst, with an enhanced titania-based material showing better performance under solar irradiation relative to titania alone, consistent with our lab studies. The study also highlights areas for further optimisation, desirable to achieve before the technology can be most-effectively implemented.
Water impactPhotocatalytic water purification is an emerging method by which micropollutants may be removed from drinking water. Despite many hundreds of papers on new materials, there are very few studies testing the real-world performance in rural areas of the developing world. We report our study on solar photocatalysis for the destruction of bacteria in drinking water from villages in West Bengal, and show that it can provide a simple and effective method to destroy bacteria. We also identify areas for further improvement to enable large-scale practical application. |
Introduction
According to the World Health Organisation (WHO), approximately 30% of the global population are currently without a safe source of drinking water,1 which in turn leads to around half a million entirely preventable deaths per year.1,2 Thus, affordable, simple and safe water treatment systems are necessary to meet the United Nation's Sustainable Development Goals (SDGs), specifically Goal 6 to “ensure availability and sustainable management of water and sanitation for all” by 2030.3 However, commonly used methods of water treatment are often energetically and chemically intensive, which in turn leads to them being too expensive for use in less affluent areas that typically have some of the most dangerous water conditions.2 Conventional industrial water treatment technologies usually employ chlorination as the means of disinfection, though UV and ozone treatments are also becoming more widespread.2 For decentralised water treatment in rural areas, chlorination is not always suitable due to the requirement for trained operators, the possibility of toxic by-products, the potential for poor taste, and its ineffectiveness against eggs of certain parasites. Although more effective against a range of biological contaminants, UV technologies can be very energy intensive and require that the mercury-containing lamps be replaced every 6–12 months.4 Despite UV emissive LEDs becoming more widespread, which are less energy intensive and can thus mitigate these issues, UV treatment is not a suitable method for use in rural areas without any reliable source of electricity to power it.In India in particular, where a significant portion of the population live in rural areas (66% in 2018 (ref. 5)), often without access to water from any municipal, centralised water treatment system, this need is particularly apparent. Further, WaterAid reported in 2018 that India is the country with the highest number of people without access to clean water close to home.6 Hence, there is a clear need to introduce a reliable and accessible method of point-of-source water treatment for India's rural villages.
An example of a simple method already employed globally by approximately 4.5 million people7,8 is solar disinfection (SODIS) of water, which was first introduced by Acra in 1984 (ref. 9 and 10) and is now recognized and promoted by the WHO.8 The method involves placing contaminated water in a transparent container, typically a plastic bottle, and exposing it to sunlight, often for at least 6 hours. This is an extremely inexpensive and simple method which, according to a 2009 study by Meierhofer and Landolt,7 is used on a daily basis in rural India, showing it is practically feasible and accessible. A 2010 study by Rai et al.11 indicated that the prevalence of diarrhoea-causing bacteria is significantly reduced in water following exposure to sunlight, although some children still became ill after treatment, implying not all bacteria are completely removed.
Thus, further improvement to this simple technique to enhance and speed up the process would be extremely beneficial. An example of an enhanced SODIS system using a reflective solar concentrator to increase the intensity of light showed that the process still required up to 6 hours to remove all bacteria on days of high light intensity, and for no bacterial regrowth to occur, between 24 to 48 hours was necessary.12 In order to ensure complete safety of water and meet guidelines set by the WHO, supplementary or alternative enhancements will be necessary. Additionally, other chemical contaminants, such as priority organic pollutants, cannot be broken down by photolysis alone, hence SODIS may not be able to remove all hazardous contaminants.
One possible candidate for addressing this issue is photocatalysis,8,13 whereby reactive oxygen species are generated and can initiate decomposition of organic pollutants and deactivation of pathogens. Photocatalysis shows promise for enhancing SODIS because it can also be conducted affordably, be powered directly by sunlight and requires minimal professional training to operate, meaning little additional equipment would be required for a potentially significant improvement in water quality. An important benefit of enhancing SODIS by incorporating photocatalysis is the possibility of removing particularly resistant microorganisms, such as Cryptosporidium. Though SODIS has been found to remove many microorganisms with the potential to cause ill health, it is typically ineffective against Cryptosporidium without enhancements,14,15 where photocatalytic studies have succeeded.16 Further, the photocatalysis process does not produce any carcinogenic, malodorous or mutagenic compounds during the treatment process,17 so would be safe to use on daily basis, rather than the addition of non-catalytic materials such as chlorine. Another benefit of both solar photocatalysis and SODIS is that neither technique depends on an electrical energy supply or chemical addition, making them inexpensive and simple to employ,9 which implies that the two methods would have good compatibility for creating an enhanced water treatment system for such a context.
The field of photocatalysis is extremely active, with new materials and reactor systems constantly developed, however its practical implementation for environmental remediation is sparse. As highlighted by Loeb et al.,18 photocatalysis research is commonly framed in the context of application to water treatment, despite the materials being very rarely tested under the real-world conditions for which they are nominally intended. Thus, conducting field studies on photocatalytic materials which have shown promise under idealised laboratory conditions is a high priority in order to confirm the viable implementation of such a technique.
Examples of field tests that have been conducted under solar illumination exist in the literature and show promising results, but are often conducted with a stirred system of nanoparticles, which would need to be recovered using expensive micro-filters or centrifuges. For example, a ZnO suspension was employed by Vela et al.19 to remove various endocrine disruptors under solar irradiation with water flowing through tubes lined with reflective solar concentrators and a large tank (250 L) for mechanical stirring. Although the pilot plant was successful for removing the studied contaminants, such systems are not practical for the specific context of rural Indian villages, which may lack electrification and the resources necessary for maintenance.
Commercial options are also very limited, though one noteworthy example is the SolarBag by Puralytics (Fig. 1).20 This is a promising example of solar photocatalysis being utilised to remove pollutants from water as a personal, point-of-source method and has won numerous awards for innovation in the field of water treatment. Puralytics claim that “The water is purified in 2–3 hours on a sunny day or 4–6 hours on a cloudy day or if the source water is tea-colored. An indicator tells you when the water is ready”.20 However, the price-per-unit is very high ($80 for one bag, which is quoted to last up to 500 times), limiting its applicability in less affluent, rural areas, with much of its use being part of disaster relief or charitable provision.
In previous studies, we have developed successful photocatalysts based on TiO2, including C–TiCl4–TiO2,21 BiVO4–TiO2,22 BiOI–TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 and Bi4Ti3O12–TiO2.24,25 These offer enhanced photocatalytic activity over un-modified titania and, thus far, the most promising of these under laboratory conditions is the Bi4Ti3O12–TiO2 co-catalyst system (BTO–TiO2), attributed to its good stability and the presence of the heterojunction band architecture, which can reduce the extent of charge recombination. After demonstrating the BTO–TiO2 co-catalyst system as a layer coated on glass beads,24 it was imperative that field tests should be conducted. For this purpose, we report here real-world testing of the photocatalytic beads, conducted using water from rural villages in the vicinity of the Indian Institute of Technology Kharagpur (IIT KGP), where the analysis following treatment took place, in the Indian state of West Bengal, over March and April of 2019. We use photocatalyst immobilised on beads, rather than suspended powder, in our studies to ensure the system is appropriate for rural use without the need for complicated separation processes. Rather than a rigorous quantitative analysis of the comparison between TiO2 and bismuth titanate, which already exists in the literature,25,26 this study provides an assessment of catalyst performance when used outdoors under natural sunlight to treat contaminated water of practically useful volumes, when coupled with a simple and inexpensive SODIS-type reactor set-up, as well as useful insights into how best to conduct such studies.
23 and Bi4Ti3O12–TiO2.24,25 These offer enhanced photocatalytic activity over un-modified titania and, thus far, the most promising of these under laboratory conditions is the Bi4Ti3O12–TiO2 co-catalyst system (BTO–TiO2), attributed to its good stability and the presence of the heterojunction band architecture, which can reduce the extent of charge recombination. After demonstrating the BTO–TiO2 co-catalyst system as a layer coated on glass beads,24 it was imperative that field tests should be conducted. For this purpose, we report here real-world testing of the photocatalytic beads, conducted using water from rural villages in the vicinity of the Indian Institute of Technology Kharagpur (IIT KGP), where the analysis following treatment took place, in the Indian state of West Bengal, over March and April of 2019. We use photocatalyst immobilised on beads, rather than suspended powder, in our studies to ensure the system is appropriate for rural use without the need for complicated separation processes. Rather than a rigorous quantitative analysis of the comparison between TiO2 and bismuth titanate, which already exists in the literature,25,26 this study provides an assessment of catalyst performance when used outdoors under natural sunlight to treat contaminated water of practically useful volumes, when coupled with a simple and inexpensive SODIS-type reactor set-up, as well as useful insights into how best to conduct such studies.
Experimental
Photocatalytic bead preparation
The TiO2 and BTO–TiO2 coated beads were prepared according to the procedures outlined by Odling et al.24 Briefly, soda-lime glass beads were etched using potassium bifluoride before immersion in a P25 titania suspension. This was produced by first obtaining a Ti(OBu)4 sol by adding Ti(OBu)4 (1 mL) to n-butanol (20 mL) and HCl (0.23 mL, 37%) and stirring vigorously. To this sol, P25 TiO2 (0.667 g) was then added and stirred overnight before use. The etched beads were then immersed in the suspension for 5 minutes, before being drained and dried at 150 °C, then annealed at 500 °C for 1 hour. This was repeated three times to build up a suitably thick film. This process allows for approximately 0.2 mg of titania to be deposited onto the surface of each bead. Some of these beads were kept for use as a TiO2 control, while the others were modified to produce BTO–TiO2. This was achieved by employing the sequential ionic layer adsorption reaction (SILAR) procedure, immersing first in a solution of Bi(NO3)3 (1 mM) for 5 minutes, then rinsing in ultrapure water for 5 minutes, before immersing in KBr (1 mM) for a further 5 minutes and finally rinsing in ultrapure water once more. This cycle was repeated 7 times before annealing at 600 °C for 1 hour.Water sample collection
All water samples were collected from villages in the vicinity of IIT KGP, in the Kharagpur subdivision within the district of Paschim Medinipur, West Bengal, that are used daily for drinking purposes. Five samples were identified as being valuable for testing (Fig. 2): | ||
| Fig. 2 Images of the water sources used throughout this investigation. From left to right: W1P, W2B, TW, STW, CW. | ||
• Water from a well in the village of Porapara (W1P), which was situated in an open space with dry ground close to freely roaming livestock. The well was open at ground-level, exposing it to contamination. The walls of the well were not bricked-lined, but the soil was densely packed (depth ca. 15 m, diameter ca. 1 m).
• Well water from a village along the boundary wall of IIT KGP (W2B), also open and close to livestock, situated in a grassy field. The walls of the well were brick-lined (depth ca. 15 m, diameter ca. 2.3 m).
• Water from a tube well (TW), situated near a toilet block, agricultural land (a possible source of pesticides) and a pond (depth ca. 20 m, diameter ca. 0.1 m).
• Water from a government-controlled tap which was only turned on for limited times during the day, known as ‘time’ or ‘social’ tap water (STW). The water supplying the tap is sourced by ground water which is pumped up and stored in overhead water tanks.
• Water from a tap on the campus (CW), which undergoes thorough treatment on the campus itself, though may become contaminated by bacteria growing in old pipes. Treatment includes aeration, filtration and oxidation.
Water from sources W1P, W2B, TW and STW does not receive any treatment before being used for drinking, and as such the consumption of this water is a cause for concern regarding health. Further pictures of the water sources are in the ESI† (Fig. S1–S7).
Photocatalytic testing
Testing involved filling 3 plastic bottles (500 mL capacity polyethylene terephthalate (PET) produced by Indian beverage company Bisleri) with the relevant water sample and exposing to sunlight for 3 hours. The following treatment methods were tested using each of the aforementioned five water samples:• BTO–TiO2 catalyst in 500 mL water.
• TiO2 catalyst in 500 mL water.
• No catalyst, only exposure of the 500 mL of water to the same sunlight and temperature conditions (SODIS) to act as a control.
For the conditions with the catalyst present, 45 g of beads were used in each bottle (each bead weighing approximately 0.04 g, with approximately 0.2 mg of catalyst per bead). This gave approximately 0.2 g of immobilised catalyst in the system. After each set of treatments, the bottles were thoroughly cleaned using water and detergent, with each bottle also being flushed with some of the water sample to be treated before each new test began in order to prevent cross-contamination.
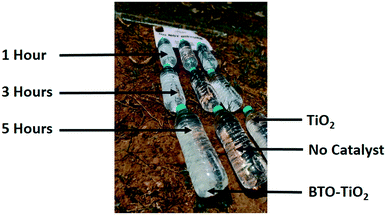
In order to investigate the effects on the duration of sunlight exposure, an additional test was performed using water from W2B (chosen for its high bacterial colony count). This was done following the same set-up as described above for 3 hours, but conducted in triplicate, such that samples could be removed and tested after 1 hour, 3 hours and 5 hours of sunlight exposure (see Fig. 3). Each of the test conditions was repeated at least three times.
Sample analysis and characterisation
All of the water samples were characterised before and after treatment using bacterial culture counts to assess the extent of contamination, as well as measurements of the total dissolved solids (TDS), conductivity and salinity to gain insight into the nature of contamination pathways, and general water quality in different areas. The bacterial culture counts were obtained by taking 40 μL of each sample and spreading it onto a Petri dish containing a non-selective nutrient agar medium (‘Nutrient Agar M001’ produced by HIMEDIA). This was performed a maximum of 1 hour after the treatment was completed and the samples were removed from exposure to sunlight in order to prevent further bacterial growth that could falsely affect the results. The Petri dishes were then covered and sealed with paraffin wrap before placing in an incubator at 37 °C overnight. Following this, the Petri dishes were removed from the incubator and the colonies counted, which indicated the extent of contamination in general, rather than tracking one specific species, since a non-selective nutrient agar medium was used. This number was then converted to colony forming units per mL (CFU mL−1). All equipment that had to be re-used was sterilised using ethanol and also autoclaved (glass inoculation loops were rinsed in ethanol and flame sterilised), with all Petri dishes being single-use. This limited contamination to reduce fluctuations in colony counts due to external factors.For the solid contamination parameters, all measurements were obtained using the Waterproof PCSTestr 35 pH/conductivity/TDS/salinity/temperature Tester by Electric Burst. These measurements were trivial to collect, by simply filling a beaker with the sample to be tested, inserting the multi-meter and taking the reading. The meter was cleaned with distilled water in between measurements.
The analysis was performed three times, once for each of the experimental repeats.
Mass transport limitation
An additional laboratory study was performed to support the findings from the field work in India, which tested the effect of agitating the water to reduce mass transport limitations. This was performed by filling two 500 mL PET bottles with 4-chlorophenol (160 μM) and 45 g of BTO–TiO2 beads. Both bottles were placed on their side and illuminated from above with a 370 nm LED at 0.5 V and 2.5 A. The bottles were illuminated for 5 hours, with UV-vis absorption measurements taken every 30 minutes over a wavelength range of 200–400 nm. One bottle was left stationary for the duration of the treatment, whereas the other bottle was rotated every 15 minutes to mix the water sample.Results and discussion
Solid content parameters – TDS, salt and conductivity
According to the WHO, TDS levels should not exceed 1000 ppm for potable water,27 and are optimum between 300 and 600 ppm,28 above which the water will be too hard and unpalatable, and significantly below this range the content of essential minerals may be too low (e.g. below around 100 ppm), with the extremes of both cases having the possibility of leading to poor health. The TDS, values for all of the water samples fell below the upper limit for causing health risks, though complaints were made that some of the water was too hard and unpleasant to drink. As expected, it was observed throughout this study that the photocatalysis treatment did not significantly alter the TDS, salt or conductivity values for any of the water sources (ESI† – Table S1 and Fig. S8), and though the safety of the water remains the main priority, concerns over palatability are nonetheless useful to bear in mind when considering developing a water treatment system.Bacterial content
One of the most useful and important metrics for monitoring water quality is the bacterial content, specifically the colony forming units per mL (CFU mL−1), as the presence of pathogens has the potential to cause serious illness, with poor sanitation being highlighted as a significant issue in India.29,30 According to the WHO,31 there should be no E. coli present in any 100 mL sample. Their guidelines for safe drinking water state:“Ideally, drinking-water should not contain any microorganisms known to be pathogenic…”.
“The detection of Escherichia coli provides definite evidence of faecal pollution…”.
Indian legislation on water quality follows this guidance, stating that E. coli or thermotolerant coliform bacteria should not be detectable in any 100 mL sample. Further, per 100 mL, treated water in a distribution system should also have the total coliform bacteria present be undetectable.32
Fig. 4 indicates that all five of the water sources studied contain significant bacterial contamination making them unsuitable for human consumption. CW and STW show lower, but still finite, values of CFU mL−1. For CW, this suggests that the pipes supplying water to the campus are likely to contain substantial bacterial growth leading to recontamination, since the water flowing through the pipes has undergone extensive treatment, both before entering the campus and after with aeration, filtration and oxidation employed.
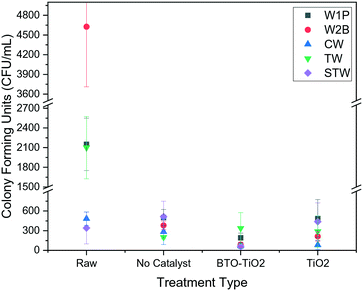 | ||
| Fig. 4 Plot showing the average colony counts for all water sources after 3 hours of each treatment type, compared with the raw, untreated water. | ||
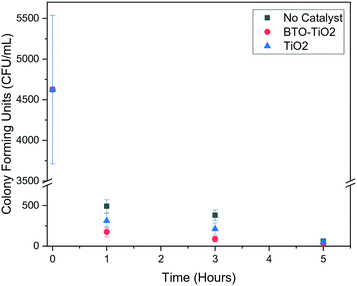
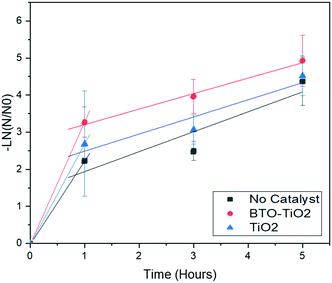
The general efficacy of each treatment type for each water source (Fig. 4, data in Table S2 of ESI†) shows that the modified BTO catalyst is the most effective at removing bacteria on average. This is consistent with our reported laboratory studies, indicating that these provide a good prediction of real-world solar performance. The large error bars in Fig. 4 relate to the impossibility of controlling all external parameters, such as weather, during field studies, however the reproducibility is sufficient for the general trends to still be apparent. These trends can also be seen with the bacterial inactivation over time (Fig. 5 and 6, data in Tables S3 and S4 of ESI,† respectively), where the BTO–TiO2 catalyst is shown to give a faster rate of bacterial removal relative to the other treatment methods.
However, we observed that none of our treatments consistently achieved complete bacterial disinfection, as reflected by the average colony counts never reaching zero. Treatment with the BTO–TiO2 catalyst shows the least amount of fluctuation in results after the 3 hours in sunlight (Fig. 4), as well as a decrease in colony counts for all samples relative to the raw value, which was not found for any other treatment methods. Though this indicates promise for implementation, further enhancement to the materials or the method is desirable to fully remove all remaining bacteria.
We note that since a selective nutrient medium was not used, the colonies formed were not necessarily all E. coli, as other bacteria, pathogenic or otherwise, may also be found in naturally occurring water.
Due to photocatalysis being largely non-selective in its disinfection mechanism, the raw number of bacteria can be used as an indication of the effectiveness of the treatment method employed here in general, without the need to culture only one type of microbe, such as E. coli. Indeed, there is opportunity for further research into selective disinfection methods such that the rate of removal of specific types of bacteria can be increased,33 however this is beyond the scope of the present study.
Mass transport limitation
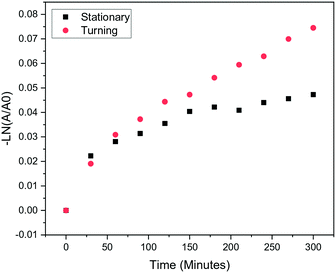
To investigate the rate of bacterial deactivation, we studied W2B water over 1, 3 and 5 hours of treatment. The rates indicated by Fig. 6 deviate from those expected for simple first-order rate kinetics, with a rapid initial drop in bacterial content seen after 1 hour of treatment, before slowing for the remainder of the 5 hours. These results suggest that the deviation from pseudo first-order rate kinetics may be due to mass-transport limitations to the reaction kinetics, caused by the relatively large volume of the water with no agitation. Slow diffusion of living bacteria from the top of the bottle towards the catalyst at the bottom will effectively create a layered system of disinfected and non-disinfected water. To elucidate the mechanism behind these kinetics for the treatment process, an investigation was subsequently conducted under laboratory conditions to compare the degradation of persistent contaminant.4-Chlorophenol (4-CP) via BTO–TiO2 coated glass beads in 500 mL PET bottles under a 370 nm LED (the emission spectrum for the LED is shown in Fig. S9 of the ESI†) when the bottle was turned every 15 minutes and when the bottle was stationary (Fig. 7). It is apparent that regularly turning the bottle enables the first-order rate kinetics to continue with much reduced mass-transport limitations, whereas the stationary bottle led to a plateau of disinfection similar to that seen in the field studies.
Based on these results, it appears that this mass-transport problem could be rectified by turning the bottle over at regular intervals during the real-world treatment period, further enhancing the rate of treatment observed for the photocatalytic process. This is important to develop into further test systems and protocols.
It should also be noted that, because a non-selective growth medium was used throughout this investigation, the treatment may not have been consistently successful for all microorganisms within the water samples, with some species being more resistant to the treatment than others. Indeed, there are plans for more thorough screening of bacterial content and the efficacy of the treatment against certain microorganisms as future work relating to this investigation. However, the increase in rate of degradation shown for 4-CP when turning the bottle does suggest that mass transport limitations do play a significant role, which can be overcome with simple reactor system modifications.
Other practical observations
Another crucial aspect of the study was to highlight areas necessary for further improvement before the treatment method will be ready for implementation in rural villages. Indeed, this study allowed the main limits to practicality associated with photocatalysis as a treatment method for rural villages to be more clearly identified, such that further work can be made in eradicating such barriers.During the study it was clear that the catalyst material was becoming darker in colour (Fig. 8), turning from the original pristine white to a light brown colour, most likely from the build-up of transition metal deposits or carbonaceous material from the water sources. Throughout this study, the same regeneration technique was used as in previous laboratory studies, whereby the beads were rinsed in distilled water and calcinated in a furnace at 500 °C for 1 hour after each use. In doing so, any remaining contaminants could be removed from the photocatalyst's active sites, meaning the beads remained active for the duration of the study and compatibility between runs could be ensured. However, it is unlikely that such a cleaning method could be implemented without also developing the necessary infrastructure to service the villages. This could lead to more rapid deterioration of the catalyst films, resulting in the need to replace the beads more frequently, which would have a negative impact on the required cost-effectiveness of the technology.
 | ||
| Fig. 8 Colour difference between pristine, unused beads, and those which had been used in testing multiple times. | ||
Further, the beads also began to show significant loss of coating as the investigation continued, with some beads appearing completely clean and without a coating at all by the end of the study. Although the treatment process did not notably seem to become slowed relative to the start when the beads were in their optimum condition, this does suggest that improved methods need to be developed for adhering catalyst films to the support for systems that are intended for prolonged use. We regard these stability questions as the largest practical issue raised by this investigation and improving catalyst adhesion and regeneration methods will therefore be an important focus of future studies, which we are separately studying in parallel with the real-world testing reported here.
For successful implementation to be feasible, further chemical enhancements are desirable to improve the visible light efficiency of the catalyst, such that the rate of disinfection can be increased without the need to add more of the catalyst beads, which would increase the cost and weight of the system. Although the glass beads provide a suitable surface area available for the catalytic reaction to occur, the low mass of catalyst compared with that of the bead leads to a bulky and heavy system that would be hard to mass produce. For this reason, different support materials and structures should be investigated to improve the practicality of the system, in tandem with improved adhesion methods to reduce the extent of catalyst flaking.
Conclusions
Through this investigation, it was found that all treatment methods were able to reduce bacterial content to some extent, verifying SODIS and solar photocatalysis as viable techniques for improving the safety of water in rural areas with intense sunlight exposure. It was found throughout this study that the use of our photocatalyst materials provided an enhanced treatment ability relative to the simple SODIS technique and relative to standard titania, as indicated by overall lower bacterial content and faster disinfection rates shown in Fig. 4–6. The improved performance of BTO–TiO2, relative to plain titania, verifies the predictive ability of our lab-based studies used during materials development.Although it was observed that the complete removal of all bacteria could not be achieved after exposure to sunlight for up to 5 hours, we attribute this to mass-transport limitations incurred by using large volumes of water. As the lab investigation into loss of 4-CP subsequently indicated, turning the bottles regularly throughout treatment could be sufficient to mitigate this issue, leading to an improved rate and greater extent of bacterial removal. By coupling the rotation of the bottle during intense sunlight exposure with the chemically enhanced BTO–TiO2 catalyst, we predict that the time required for complete removal of all bacteria could be significantly reduced from a recommended 6 hours for SODIS to as little as 1 hour.
All of the results gained from this investigation provided valuable insights into the viability of photocatalysis as a point-of-source decentralised waste water treatment method, and have identified crucial areas for development that can help to further optimise this method. Though it is clear further work is required, the study supports the notion that photocatalysis can indeed be a practical option for water disinfection when the necessary system optimisation measures are made. The prospect of further investigation is extremely timely as the increase in global population puts even more strain on existing water sources.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the Scottish Government Hydro Nation Scholars Program for the PhD studentship to VP and the funding of this research.References
- WHO, Drinking-Water Fact Sheet, https://www.who.int/en/news-room/fact-sheets/detail/drinking-water, (accessed 12 March 2019) Search PubMed.
- N. Pichel, M. Vivar and M. Fuentes, The problem of drinking water access: A review of disinfection technologies with an emphasis on solar treatment methods, Chemosphere, 2019, 218, 1014–1030 CrossRef CAS PubMed.
- The United Nations, Sustainable Development Goals, https://sustainabledevelopment.un.org/?menu=1300, (accessed 13 March 2019) Search PubMed.
- The Environmental Protection Agency, Waste Water Technology Fact Sheet - Ultraviolet Disinfection, https://www3.epa.gov/npdes/pubs/uv.pdf, (accessed 12 March 2019) Search PubMed.
- World Bank Group, Rural population (% of total) - India, https://data.worldbank.org/indicator/SP.RUR.TOTL.ZS?locations=IN, (accessed 29 July 2019) Search PubMed.
- WaterAid, The Water Gap, https://washmatters.wateraid.org/sites/g/files/jkxoof256/files/The Water Gap State of Water report lr pages.pdf, (accessed 5 August 2019) Search PubMed.
- R. Meierhofer and G. Landolt, Factors supporting the sustained use of solar water disinfection - Experiences from a global promotion and dissemination programme, Desalination, 2009, 248, 144–151 CrossRef CAS.
- J. A. Byrne, P. A. Fernandez-Ibañez, P. S. M. Dunlop, D. M. A. Alrousan and J. W. J. Hamilton, Photocatalytic Enhancement for Solar Disinfection of Water: A Review, Int. J. Photoenergy, 2011, 2011, 1–12 CrossRef.
- A. Acra, Z. Raffoul, Y. Karahagopian and UNICEF, Solar Disinfection of Drinking Water and Oral Rehydration Solutions, Illustrated Publications, Beirut, 1984 Search PubMed.
- A. Acra, M. Jurdi, H. Mu'allem, Y. Karahagopian and Z. Raffoul, Water Disinfection by Solar Radiation: Assessment and Application, International Devopment Research Centre, Ottawa, 1990 Search PubMed.
- R. Pal, S. Kar, D. C. Tseringand and B. Rai, Solar disinfection improves drinking water quality to prevent diarrhea in under-five children in sikkim, India, J. Global Infect. Dis., 2010, 2, 221–225 CrossRef PubMed.
- E. Ubomba-Jaswa, P. Fernández-Ibáñez, C. Navntoft, M. I. Polo-López and K. G. McGuigan, Investigating the microbial inactivation efficiency of a 25 L batch solar disinfection (SODIS) reactor enhanced with a compound parabolic collector (CPC) for household use, J. Chem. Technol. Biotechnol., 2010, 85, 1028–1037 CrossRef CAS.
- S. Malato, P. Fernández-Ibáñez, M. I. Maldonado, J. Blanco and W. Gernjak, Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends, Catal. Today, 2009, 147, 1–59 CrossRef CAS.
- W. Heaselgrave and S. Kilvington, The efficacy of simulated solar disinfection (SODIS) against Ascaris, Giardia, Acanthamoeba, Naegleria, Entamoeba and Cryptosporidium, Acta Trop., 2011, 119, 138–143 CrossRef PubMed.
- H. Gómez-Couso, M. Fontán-Sainz, P. Fernández-Ibáñez and E. Ares-Mazás, Speeding up the solar water disinfection process (SODIS) against Cryptosporidium parvum by using 2.5l static solar reactors fitted with compound parabolic concentrators (CPCs), Acta Trop., 2012, 124, 235–242 CrossRef PubMed.
- J. Shen, R. Steinbach, J. M. Tobin, M. Mouro Nakata, M. Bower, M. R. S. McCoustra, H. Bridle, V. Arrighi and F. Vilela, Photoactive and metal-free polyamide-based polymers for water and wastewater treatment under visible light irradiation, Appl. Catal., B, 2016, 193, 226–233 CrossRef CAS.
- J. A. Ibáñez, M. I. Litter and R. A. Pizarro, Photocatalytic bactericidal effect of TiO2 on Enterobacter cloacae: Comparative study with other Gram (−) bacteria, J. Photochem. Photobiol., A, 2003, 157, 81–85 CrossRef.
- S. K. Loeb, P. J. J. Alvarez, J. A. Brame, E. L. Cates, W. Choi, J. Crittenden, D. D. Dionysiou, Q. Li, G. Li-Puma, X. Quan, D. L. Sedlak, T. David Waite, P. Westerhoff and J.-H. Kim, The Technology Horizon for Photocatalytic Water Treatment: Sunrise or Sunset?, Environ. Sci. Technol., 2019, 53, 2937–2947 CrossRef CAS PubMed.
- N. Vela, M. Calín, M. J. Yáñez-Gascón, I. Garrido, G. Pérez-Lucas, J. Fenoll and S. Navarro, Photocatalytic oxidation of six endocrine disruptor chemicals in wastewater using ZnO at pilot plant scale under natural sunlight, Environ. Sci. Pollut. Res., 2018, 25, 34995–35007 CrossRef CAS PubMed.
- Puralytics, SolarBag, http://www.puralytics.com/html/solarBag.php, (accessed 5 August 2019) Search PubMed.
- G. Odling, A. Ivaturi, E. Chatzisymeon and N. Robertson, Improving Carbon-Coated TiO 2 Films with a TiCl 4 Treatment for Photocatalytic Water Purification, ChemCatChem, 2018, 10, 234–243 CrossRef CAS.
- G. Odling and N. Robertson, BiVO4-TiO2Composite Photocatalysts for Dye Degradation Formed Using the SILAR Method, ChemPhysChem, 2016, 2872–2880 CrossRef CAS PubMed.
- G. Odling and N. Robertson, SILAR BiOI-Sensitized TiO 2 Films for Visible-Light Photocatalytic Degradation of Rhodamine B and 4-Chlorophenol, ChemPhysChem, 2017, 18, 728–735 CrossRef CAS PubMed.
- G. Odling, E. Chatzisymeon and N. Robertson, Sequential ionic layer adsorption and reaction (SILAR) deposition of Bi4Ti3O12 on TiO2: an enhanced and stable photocatalytic system for water purification, Catal. Sci. Technol., 2018, 8, 829–839 RSC.
- G. Odling, Z. Y. Pong, G. Gilfillan, C. R. Pulham and N. Robertson, Bismuth titanate modified and immobilized TiO 2 photocatalysts for water purification: broad pollutant scope, ease of re-use and mechanistic studies, Environ. Sci.: Water Res. Technol., 2018, 4, 2170–2178 RSC.
- G. Odling and N. Robertson, Bridging the gap between laboratory and application in photocatalytic water purification, Catal. Sci. Technol., 2019, 9, 533 RSC.
- World Health Organisation, Guidelines for Drinking-water Quality: Fourth Edition Incorporating the First Addendum, https://apps.who.int/iris/bitstream/handle/10665/254637/9789241549950-eng.pdf;jsessionid=10940A560E4D7D2B87B711CC6D0A2249?sequence=1, (accessed 5 August 2019) Search PubMed.
- World Health Organisation, Total dissolved solids in Drinking-water: Background document for development of WHO Guidelines for Drinking-water Quality, https://www.who.int/water_sanitation_health/dwq/chemicals/tds.pdf, (accessed 5 August 2019) Search PubMed.
- G. S. Kumar, S. S. Kar and A. Jain, Health and environmental sanitation in India: Issues for prioritizing control strategies, Indian J. Occup. Health, 2011, 15, 93–96 Search PubMed.
- K. Nath, Home hygiene and environmental sanitation: a country situation analysis for India, Int. J. Environ. Health Res., 2003, 13, S19–S28 CrossRef PubMed.
- World Health Organisation, Guidelines for drinking-water quality: Second Edition, https://www.who.int/water_sanitation_health/water-quality/small-community-management/2edvol3a.pdf, (accessed 5 August 2019) Search PubMed.
- Bureau of Indian Standards, Indian Standard: Drinking Water - Specification (Second Revision), New Delhi, 2015 Search PubMed.
- E. M. Wurtzler and D. Wendell, Selective Photocatalytic Disinfection by Coupling StrepMiniSog to the Antibody Catalyzed Water Oxidation Pathway, PLoS One, 2016, 11, e0162577 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ew01023h |
| This journal is © The Royal Society of Chemistry 2020 |