Open Access Article
Open Access ArticleEvaluation of HOCl-generating anticancer agents by an ultrasensitive dual-mode fluorescent probe†
Donglei
Shi‡
a,
Shuqiang
Chen‡
b,
Biao
Dong‡
 c,
Yanhui
Zhang
a,
Chunquan
Sheng
c,
Yanhui
Zhang
a,
Chunquan
Sheng
 b,
Tony D.
James
b,
Tony D.
James
 d and
Yuan
Guo
d and
Yuan
Guo
 *a
*a
aKey Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education, National Demonstration Center for Experimental Chemistry Education, College of Chemistry and Materials Science, Northwest University, Xi'an 710127, China. E-mail: guoyuan@nwu.edu.cn
bSchool of Pharmacy, Second Military Medical University, 325 Guohe Road, Shanghai 200433, China
cState Key Laboratory on Integrated Optoelectronics, College of Electronic Science and Engineering, Jilin University, Changchun 130012, China
dDepartment of Chemistry, University of Bath, Bath BA2 7AY, UK
First published on 4th March 2019
Abstract
Hypochlorous acid (HOCl), a reactive oxygen species (ROS), plays a crucial role in the process of pathogenic oxidative stress. Some powerful anticancer agents, such as elesclomol, specifically induce cancer cell apoptosis by increasing HOCl levels. However, sensitive tools to monitor subtle changes of biological HOCl in vivo are limited. To achieve this, we herein present rationally designed probes C1–C7 through introducing a bioorthogonal dimethylthiocarbamate receptor. All the probes were shown to sensitively and rapidly detect HOCl in the nanomolar/biologically relevant concentration range with fluorescence turn-on observed in their respective optical regions, resulting in a blue-to-red “fluorescence rainbow” and providing a broad selection of colors for imaging HOCl in vivo. Remarkably, probe C7 exhibited both a turn-on signal at biologically relevant concentrations (LOD1 = 18 nM) and a ratiometric response at the high risk pathogenic concentrations (LOD2 = 0.47 μM), which gives a higher reliability compared to a single signal and avoids cross-talk caused by the combined use of several probes. C7 was used to monitor the oxidative stress process induced by elesclomol in live cancer cells, and using this probe it was further discovered that an evodiamine derivative was capable of generating cancer-cell HOCl.
Introduction
Reactive oxygen species (ROS) are a hallmark of various pathophysiological processes and have close links with many diseases including cancer, aging, inflammation and neuro-degenerative disorders.1–8 They have recently gained enormous attention especially in preclinical medicine and life sciences.9–13 ROS are formed as a normal by-product of aerobic metabolism and are indispensable for cell signaling and immune response, but can be generated in excess under pathophysiological conditions.14 Although the generation of excessive ROS leads to diseases related with oxidative stress damage, some anticancer agents such as elesclomol (a potential drug candidate) specifically induce cancer cell apoptosis by increasing ROS levels.15–23 However, the lack of efficient ROS detection methods for specific concentration ranges in live systems has restricted the pace of progress in the discovery of this type of drug and a deeper understanding of the precise role of ROS in diseases.Endogenous hypochlorous acid (HOCl) is a particularly interesting type of ROS that plays a crucial role in the oxidative stress process induced by anticancer drugs or pathogenic factors.24 Biologically, HOCl is mainly derived from mitochondria in neutrophils and monocytes, because myeloperoxidase (MPO) living in the mitochondria is the catalyst for converting chloride ions (Cl−) and hydrogen peroxide (H2O2) to HOCl.25 Therefore, for the purpose of a pathogenesis investigation or drug discovery, real-time discrimination and quantitative analysis of biological HOCl in live systems, especially mitochondrial HOCl, is of particular interest. To this end, a number of excellent fluorescent probes for the detection and imaging of HOCl have been developed over the past few years.26–40 However, few of them can target live-cell mitochondria, which is essential for real-time and accurate sensing. More importantly, a single fluorescent probe for sensing different concentration ranges of HOCl with distinct modes of fluorescence signals has several advantages, such as a higher reliability compared to a single signal and avoids cross-talk caused by the use of multiple probes.41–43 Nevertheless, to date there are no small-molecule probes to achieve this purpose, resulting in imaging biological HOCl in cells only with single fluorescent signal mode (Fig. 1a).
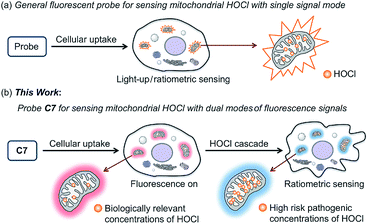 | ||
| Fig. 1 Schematic representation of mitochondrial HOCl sensed by general fluorescent probe (a) and our probe C7 (b) in live cells. | ||
In this work, we present seven novel fluorescent probes C1–C7 for imaging HOCl in vitro and in vivo, all of which can rapidly detect a biologically relevant concentration of HOCl with fluorescence turn-on observed in their respective optical regions and spanning blue to red (453 to 630 nm), resulting in a “fluorescence rainbow”. These probes proved to be highly selective to HOCl among various ROS, and produce a very sensitive fluorescence response to HOCl, thereby ensuring the specific and real-time detection of endogenous HOCl in live cells. Among them, the C6 and C7 can target live-cell mitochondria with high reactivity and very short half-life. Remarkably, probe C7 can for the first time as a single molecule detect two concentration ranges of HOCl (Fig. 1b). Using C7 in live-cell confocal imaging, we obtained a clear and exact view of the cancer cell oxidative stress process induced by elesclomol, an experimental anticancer agent based on promoting ROS,21–23 and discovered that evodiamine derivatives have a similar anticancer mechanism. Furthermore, the red-emitting C6 and C7 were used for the imaging of HOCl in live C. elegans and mice respectively.
Results and discussion
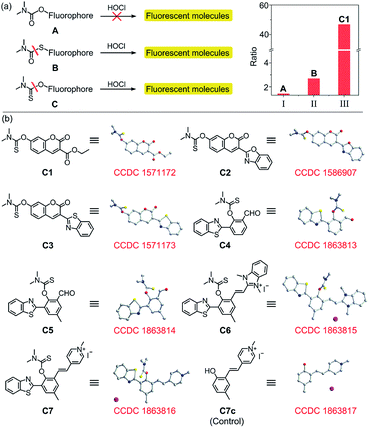
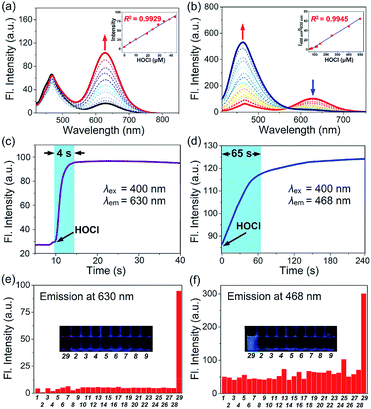
Structurally, probes C1–C7 each consist of a N,N-dimethylthiocarbamate group, which is an ideal entropy-driven receptor for HOCl first reported by Tang in 2016.44 Following the seminal work, a number of groups have developed HOCl probes with this group owing to its fast and ultrasensitive response.45–48 However, none of them constructed thiocarbamate analogues to further systematically investigate the response mechanism. To achieve this aim, we designed two additional types of candidate probe molecules (A and B) by interchanging the S atom and the O atom in the thiocarbamate moiety (Fig. 2a). The results revealed that the S atom was indispensable and could best be located on the double bond, suggesting that C analogs with a thiocarbamate group are the most sensitive among them (Fig. S1†). This could be reasonably explained by their entropy-driven reaction mechanism based on the release of gas molecules (Scheme S1†). Therefore, we designed and synthesized probes C1–C7 by coupling the thiocarbamate group with diverse phenolic fluorophores, including coumarin (C1–C3) and 2-(2-hydroxyphenyl)benzothiazole (HBT) (C4–C7) scaffolds. Furthermore, considering that small molecules containing organic cations are capable of accumulating inside mitochondria under the attraction of the negative potential of the inner membrane thereof,49–51 the mitochondria-targeting probes C6 and C7 were synthesized by introducing benzimidazolium and pyridinium cations as mitochondria-targeting groups, respectively. The synthetic routes of C1–C7 are illustrated in Scheme S2.† All compounds were characterized by 1H NMR, 13C NMR and HRMS and the original spectra are included in the ESI.† Moreover, we obtained the X-ray crystal structures of compound A (Scheme S2†), compound C7c and all probes (C1–C7) (Fig. 2b) to further confirm their chemical structures.The emission and absorption spectra of probes C1–C7 before and after the addition of HOCl were recorded in an aqueous medium buffered to physiological pH (Fig. 3a, b, S2 and S3†). Upon the addition of various low concentration ranges of HOCl, the seven probes all exhibited a significant increase in fluorescence, especially C3 which showed a 223-fold increase in fluorescence (Table S2†). All the probes have a large Stokes shift, which effectively avoids the self-absorption of the fluorescence, with C7 possessing the largest Stokes shift (170 nm, Table 1). The plots of fluorescence intensity of C1–C7 against HOCl at a low concentration all showed a good linear relationship (with a linear correlation coefficient R2 greater than 0.99) (Fig. 3a, b and S3†). Moreover, all these probes have a low limit of detection (LOD) (0.94–23 nM, Table 1). On continued addition of HOCl, C1–C6 exhibited negligible changes in their fluorescence spectra, while C7 showed a notable 48-fold fluorescence ratio (I468/I630) enhancement with a good linear relationship (R2 = 0.9945) and a new limit of detection (0.47 μM). In good agreement, C1–C7 all showed a remarkable red-shift in their absorption spectra upon treatment with HOCl (Fig. S2a–g†). For probe C7, the continued increase in HOCl concentration generated a new absorption peak at 275 nm at the expense of decreasing the absorption peak at 330 nm (Fig. S2h†). Our results indicated that C1–C7 all display an excellent sensitivity in detecting nanomolar/biologically relevant concentrations of HOCl with a fluorometric turn-on signal in response, whereas only C7 exhibited a ratiometric response to micromolar/high risk pathogenic concentrations of HOCl.
| Probes | λ abs (nm) | λ em (nm) | Stokes shift (nm) | Reaction timec (s) | LODd (nM) |
|---|---|---|---|---|---|
| a Maximum absorption wavelength (λabs). b Maximum emission wavelength (λem) of corresponding probes. c The reaction time of our probes to HOCl. d The limit of detection of corresponding probes. e The properties of probe C7 at micromolar level of HOCl. The optical properties of C1 were conducted in pH 7.4 PBS buffer. The optical properties of C2–C4 were conducted in pH 7.4 PBS buffer (10 mM) with 10% MeOH. The optical properties of C5 were conducted in pH 7.4 PBS buffer (10 mM) with 5% EtOH. The optical properties of C6 were conducted in pH 7.4 PBS buffer (10 mM) with 20% EtOH. The optical properties of C7 were conducted in pH 7.4 HEPES buffer (10 mM) with 20% MeOH. | |||||
| C1 | 405 | 454 | 49 | 8 | 6.5 |
| C2 | 427 | 494 | 67 | 6 | 9.1 |
| C3 | 422 | 502 | 80 | 8 | 0.94 |
| C4 | 435 | 532 | 97 | 18 | 7.5 |
| C5 | 440 | 550 | 110 | 10 | 2.7 |
| C6 | 464 | 615 | 151 | 4 | 23 |
| C7 | 460 | 630 | 170 | 4 | 18 |
| C7 | 330 | 468 | 138 | 65 | 0.47 μM |
Subsequently, we evaluated the reaction kinetics of C1–C7 with HOCl (Fig. 3c, d and S4†). Upon the addition of various low concentrations of HOCl, the fluorescence intensity of all probes reached a maximum within 18 s (Table 1), indicating that the probes can rapidly respond to HOCl. Notably, the reaction of probe C7 with HOCl was completed within 4 s. While, titration with a micromolar concentration of HOCl (120 μM) caused that time-dependent fluorescence intensity changes of C7 was completed in ca. 65 s.
Next, the effect of pH changes on the probes to HOCl over a wide pH range from 3–11 was evaluated. As shown in Fig. S5,† on the addition of various low concentrations of HOCl, the fluorescence intensity of probes C1–C7 displayed negligible changes at pH ranging from 6.0 to 7.7, while C7 also exhibited a stable response to a micromolar concentration of HOCl over that pH range. The results indicated that our probes could be applied to detect HOCl at physiological pH. To test the selectivity of C1–C7 for HOCl, the fluorescence response of probes C1–C7 to diverse interfering species including metal ions (Fig. S6†), anion species and biothiols (Fig. 3e, f and S7†) was investigated. The results indicate that none of the interfering species induced any obvious fluorescence changes with any of the probes, even at concentrations as high as 200 μM. Moreover, the fluorescence response of the probes to HOCl in the presence of other interfering species was almost the same as in the absence of these species, suggesting that the presence of other species will not influence the reaction of the probes with HOCl. The results indicated that probes C1–C7 can selectively detect HOCl over other common interfering species.
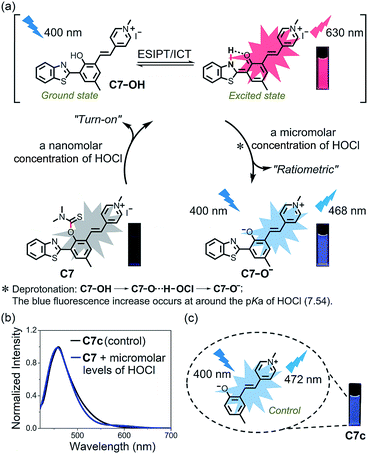
The mechanism for the reaction of C1–C7 with HOCl in the nanomolar concentration range to release fluorophores C1p–C7p was investigated on the basis of the reported literature (Scheme S1†), and can be explained by the contribution of Cl+ from HOCl to the release of the thiocarbamate moiety which acts as a “fluorophore mask”.43 To verify this hypothesis, C1p–C7p were prepared (Scheme S2†) and analyzed by both fluorescence spectroscopy (Fig. S8†) and electrospray ionization (ESI) mass spectrometry (Fig. S9–S15†). As shown in Fig. S8,† the emission wavelength of C1p–C7p is consistent with that of the corresponding probe treated with HOCl in the nanomolar concentration range, and the reaction of each probe with HOCl generates a peak in the ESI mass spectrum which matches the peak position of the respective C1p–C7p. This confirmed the release of the thiocarbamate and the turn-on fluorescent responses. Probe C7 exhibits an obvious red fluorescent turn-on response to HOCl in the nanomolar concentration range due to HOCl-specific activation of the excited state intramolecular proton transfer (ESIPT) and intramolecular charge transfer (ICT) structure (C7p) (Fig. 4a). The optical properties of C7p in various solvents including aqueous solution with different contents of CTAB were studied. As shown in Fig. S16,† the fluorescence emission of C7p changed in different media, confirming the environmental sensitivity of the ESIPT process. More interestingly, C7p can further produce a sensitive ratiometric response to HOCl at a micromolar concentration with a remarkable fluorescence blue shift from 630 to 468 nm. To clarify the second-step response mechanism of C7, 1H NMR and mass titration for HOCl were carried out. Fig. S17 and S18† showed that no obvious changes were observed in the spectra of the products when the concentration of HOCl in the C7/HOCl system was increased from nanomolar to micromolar range. Also considering that the blue fluorescence increase occurs at around the pKa of HOCl (7.54) (Fig. S5h†), we conclude that the molecule of HOCl plays the major function by complexing with C7-OH. Thus, we speculated that this process was attributed to deprotonation (C7-OH → C7-O⋯H-OCl → C7-O−) of the phenol unit occurs to inhibit the ESIPT process at micromolar concentrations of HOCl (Fig. 4a). To test the feasibility of our hypothesis, we designed and prepared a control compound C7c with the same conjugate push–pull system as C7-O− (Fig. 4c) and obtained its crystal structure. The optical properties of C7c were consistent with those of C7-O− (Fig. 4b), verifying the response mechanism.
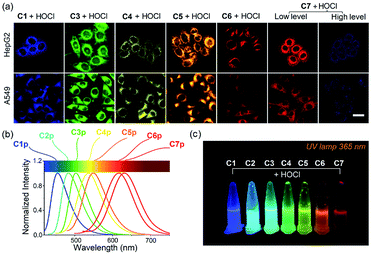
Encouraged by the optical properties of C1–C7 to HOCl in vitro, we next evaluated the capability of the probes to image HOCl in live cells. On the basis of the different emission wavelengths of C1–C7, we selected probes C1, C3–C7 for imaging exogenous HOCl in live cells. They all exhibited low cytotoxicity in live cells (Fig. S19†), which is important for biological applications. HepG2 cells and A549 cells were selected for the following experiments. Fig. 5 shows, the cells which were incubated separately with probes C1 and C3–C7 exhibited negligible fluorescence in their corresponding channels; however, remarkable fluorescence was observed after the addition of a low concentration of HOCl. As expected, with the increase in the concentration of HOCl, the fluorescence intensity of cells treated respectively with C1 and C3–C6 gradually increased (Fig. S20–S24†), while cells incubated with C7 showed an enhanced fluorescence in the red channel upon the addition of a low concentration of HOCl and then exhibited a gradual increase in the fluorescence of the blue channel and a gradually reduced fluorescence in the red channel after the addition of micromolar concentrations of HOCl (Fig. S25†). These results indicate that probes (C1, C3–C7) have excellent cell permeability to live cells and were able to visualize exogenous HOCl by fluorescence, thus offering a good range of choices for imaging HOCl in live cells. To our delight, C7 displayed both a turn-on signal to a low concentration of HOCl and a ratiometric response to a high concentration of HOCl in live cells. Next, the potential for probes C6 and C7 to detect endogenous HOCl in live cells was evaluated. Bacterial endotoxin lipopolysaccharide (LPS) is known to induce cellular apoptosis and release ROS, including HOCl.32–34 HepG2 cells and A549 cells were pretreated with LPS for three hours, and the stimulated cells were then incubated with C6 and C7 (20 μM), respectively. As shown in Fig. S26 and S27,† there was almost no fluorescence in the control group without treatment by LPS, suggesting that the observed changes in fluorescence intensity of the stimulated cells could be attributed to the generation of HOCl induced by LPS. Conversely, 4-aminobenzoic hydrazide (ABH), a specific MPO inhibitor, can decrease the concentration of biological HOCl in cells.36–38 As expected, the introduction of ABH caused an obviously decreased fluorescence in the red channel (Fig S27†), further confirming the specific response of C7 to HOCl. Thus, these data demonstrated that our probes could sensitively detect both exogenous and endogenous HOCl in live cells.
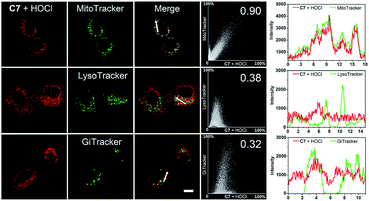
To confirm the mitochondria-targeting capacity of C6 and C7, probes and different commercial staining dyes (MitoTracker Green FM, LysoTracker Green DND-26 and Golgi Tracker Green (NBD C6-ceramide)) were separately coincubated in live HeGp2 cells and then treated with HOCl for another 1 minute. Fig. 6 and S28† indicate that the fluorescence in the red channel of probe C6 or C7 treated with HOCl almost completely overlaps the fluorescence of MitoTracker Green in the green channel. The Pearson's correlation coefficients (PCC) of C6 and C7 were 0.95 and 0.90, respectively. In comparison, the fluorescence in the red channel of probes treated with HOCl partly overlaps that of LysoTracker or Golgi Tracker, with PCC values below 0.6. The correlation mapping of the fluorescence intensity also exhibited excellent colocalization between the probes and MitoTracker Green. The results indicate that C6 and C7 have an excellent targeting ability and are capable of monitoring mitochondrial HOCl in live cells.
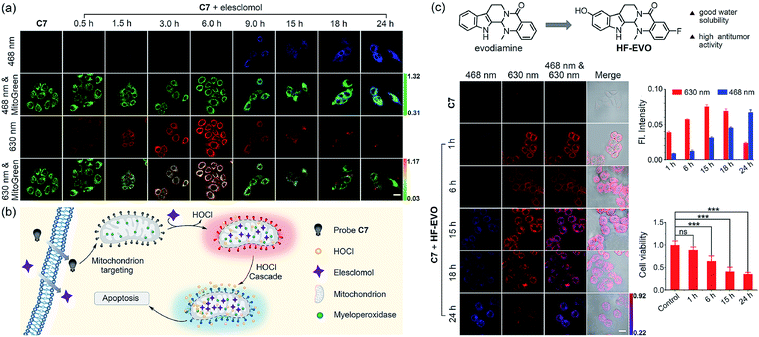
Elesclomol is a known ROS-generating anticancer agent. In 2014, the group of Peng demonstrated for the first time that elesclomol can elevate HOCl levels beyond a threshold, which ultimately triggers apoptosis of cancer cells.24 Therefore, we used elesclomol to assess the feasibility of using C7 to monitor the fluctuation in concentration of intracellular HOCl (Fig. 7b). According to the common method of pharmacochemistry, HepG2 cell samples were treated with elesclomol (0.8 μM) for different times. The elesclomol-stimulated cells were stained with both C7 and MitoTracker Green for 30 min and then imaged by confocal fluorescence microscopy immediately. As shown in Fig. 7a, control cells treated with C7 only displayed negligible fluorescence in the red channel (610–650 nm) and no fluorescence in the blue channel (440–490 nm). When the incubation times of elesclomol were extended from 0 to 6 h, the fluorescence intensity of the red channel gradually enhanced, while there were no changes in the blue channel. However, there was a dramatic fluorescence enhancement of the blue channel and a decreased fluorescence of the red channel when the incubation time was prolonged from 6 h to 24 h. The time-dependent color changes can be observed quite clearly in the merged images. The results above suggested that the concentration of HOCl in elesclomol-stimulated cells increased over time and reached the high risk pathogenic level after 6 hours and confirmed that the concentration of HOCl indeed increased in the apoptosis process of cancer cell induced by the drug.
We then investigated if the probe could be applied to evaluate the underlying mechanism responsible for the action of anticancer molecules isolated from natural products. Within our research group, we have been particularly interested in developing evodiamine-based anticancer drugs/molecules. Among the novel evodiamine derivatives bearing various substitutions or a modified scaffold that we have synthesized, 3-fluoro-10-hydroxy evodiamine (HF-EVO) was screened and has been proven as a powerful anticancer molecule (Fig. 7c).52 However, the underlying mechanism of HF-EVO responsible for the proapoptotic activity has not been elucidated in detail. We therefore employed C7 to monitor the level of HOCl in the HF-EVO-induced cancer cell apoptosis process to determine whether HF-EVO has the same anticancer mechanism as elesclomol. For this purpose, the HepG2 cells were treated with the HF-EVO (0.4 μM) for different times, then incubated with C7 and imaged by fluorescence microscopy. As expected, fluorescence changes were observed in HF-EVO-stimulated cells after incubation with C7. As depicted in Fig. 7d, control cells treated with C7 alone exhibited fairly weak fluorescence in the red channel (610–650 nm) and no fluorescence in the blue channel (440–490 nm), while a bright fluorescence in the red channel was observed upon stimulation by HF-EVO for 6 hours. Then, with prolonging incubation time (6–24 h), the fluorescence intensity of HepG2 cells stimulated by HF-EVO and probe exhibited a clear time-dependent decrease in the red channel (Fig. 7e), accompanied by a gradual time-dependent fluorescence intensity increase in the blue channel. These results demonstrated that HF-EVO gradually increased the HOCl content over time in HepG2 cells and that the concentration of HOCl reached the high risk pathogenic level after 6 hours. Moreover, to evaluate the capacity of HF-EVO to induce HepG2 cell apoptosis, the cytotoxicity of HF-EVO against HepG2 cells was evaluated by CCK8 assay at different time periods. Fig. 7f shows, upon incubation for four different time intervals, a significant increase in the percentage of apoptotic cells was observed over time, suggesting that HF-EVO has a high proapoptotic activity in HepG2 cells. The results revealed that HF-EVO induced the accumulation of HOCl in HepG2 cells to trigger apoptosis. Taken together, these results point to the feasibility of our probe C7 for evaluating HOCl-generating anticancer drugs, and provides a desirable chemical tool for investigating the mechanism of action of these drugs.
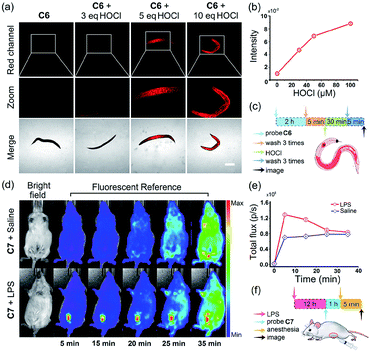
Inspired by the above results, we sought to image exogenous and endogenous HOCl in small laboratory animals. C. elegans were incubated with 490 μL M9 and 10 μL C6 (10 μM) at 20 °C for 2 hours in the dark. Different amounts of HOCl were added to each group and the groups incubated for another 30 min. As shown in Fig. 8a, C. elegans prestained with C6 exhibited very weak fluorescence but exhibited a significant fluorescence after incubating with 3 equiv. HOCl. The fluorescence intensity of C. elegans was enhanced with an increasing concentration of HOCl (Fig. 8b). Moreover, the favorable attributes of C7 encouraged us to evaluate if endogenous HOCl can be monitored in live mice. Kunming mice were divided into an experimental group and a control group to investigate the detection ability of probe C7 for native HOCl in vivo. The experimental group was given an intraperitoneal (i.p.) injection of 1 mL LPS (0.3 mg mL−1) and bred for 12 hours, while the control group was given an intraperitoneal (i.p.) injection of 1 mL saline. After 12 hours, a solution of C7 (30 μM, in 0.2 mL DMSO) was injected into the abdomen of the mice for 5 minutes and then the mice subsequently anesthetized and imaged by an IVIS Spectrum small animal in vivo imaging system. All animal experiments were approved by the Ethics Committee of Northwest University, and were conducted in accordance with European guidelines for the care and use of laboratory animals. As shown in Fig. 8d, the experimental group stimulated with LPS displayed a much higher fluorescence readout (pseudo-color) than the control group in the red channel. A plot of fluorescence intensity measured at the abdomen of the two groups against time is shown in Fig. 8e. The fluorescence intensity of the experimental group gradually increased and displayed the strongest fluorescence at 10 min. Interestingly, the control group also exhibited a slight fluorescence enhancement, suggesting that C7 was sensitive enough to monitor native HOCl in live mice without external stimulation. These results indicate that our probes could detect exogenous and endogenous HOCl in live animals.
Conclusions
In summary, we have designed and prepared three types of potential HOCl fluorescent probes (A, B and C) that were evaluated through their fluorescence response to HOCl under physiological conditions. Initial screening enabled us to delineate the structural determinants for selective recognition of HOCl. The follow-up studies demonstrated that the biocompatible thiocarbamate-based probes C1–C7 with high sensitivity and selectivity are desirable. The various fluorescent dyes emitted over a spectral region spanning blue to red and thus provide a wide range of choices for imaging HOCl in vivo. In particular, the ESIPT and ICT probe C7 can target mitochondria and display different fluorescence responses to both low and high concentrations of HOCl: an obvious turn-on signal to nanomolar concentrations of HOCl (biologically relevant concentrations, LOD1 = 18 nM) and a subsequent ratiometric response to HOCl at the micromolar level (high risk pathogenic concentrations, LOD2 = 0.47 μM) were observed. Better still, we achieved the distinct visualization of elesclomol-induced biological HOCl in HepG2 cells at different time periods and discovered for the first time that 3-fluoro-10-hydroxyevodiamine, a potential anticancer molecule, generated HOCl. The C7-based detection system is unprecedented and offers a promising tool for evaluating ROS-generating anticancer drugs. More broadly, the study will pave the way to evaluate the interrelated roles of HOCl in various physiological and pathological conditions.Conflicts of interest
The authors declare that they have no competing financial interests.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21472148, 21072158 and 81602659), Open Funding Project of the State Key Laboratory of Bioreactor Engineering (2018OPEN12), Overseas Students Science and Technology Activities Project Merit Funding of Shaanxi Province (No. 20151190) and Academic Backbone of Northwest University Outstanding Youth Support Program. We thank Prof. Zhixiang Yu at Peking University and Prof. Sanping Chen at Northwest University for insightful discussions on this work. T. D. J. wishes to thank the Royal Society for a Wolfson Research Merit Award.References
- K. J. Barnham, C. L. Masters and A. I. Bush, Nat. Rev. Drug Discovery, 2004, 3, 205–214 CrossRef CAS PubMed.
- T. Strowig, J. Henao-Mejia, E. Elinav and R. Flavell, Nature, 2012, 481, 278–286 CrossRef CAS PubMed.
- M. P. Murphy, A. Holmgren, N. G. Larsson, B. Halliwell, C. J. Chang, B. Kalyanaraman, S. G. Rhee, P. J. Thornalley, L. Partridge, D. Gems, T. Nystrom, V. Belousov, P. T. Schumacker and C. C. Winterbourn, Cell Metab., 2011, 13, 361–366 CrossRef CAS PubMed.
- N. Branzk, A. Lubojemska, S. E. Hardison, Q. Wang, M. G. Gutierrez, G. D. Brown and V. Papayannopoulos, Nat. Immunol., 2014, 15, 1017–1025 CrossRef CAS PubMed.
- T. Finkel, M. Serrano and M. A. Blasco, Nature, 2007, 448, 767–774 CrossRef CAS PubMed.
- H. J. Kwon, D. Kim, K. Seo, Y. G. Kim, S. I. Han, T. Kang, M. Soh and T. Hyeon, Angew. Chem., Int. Ed., 2018, 57, 9408–9412 CrossRef CAS PubMed.
- R. P. Wu, T. Hayashi, H. B. Cottam, G. Jin, S. Yao, C. C. Wu, M. D. Rosenbach, M. Corr, R. B. Schwab and D. A. Carson, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 7479–7484 CrossRef CAS.
- C. Nathan and A. Cunningham-Bussel, Nat. Rev. Immunol., 2013, 13, 349–361 CrossRef CAS.
- P. Pei, C. Sun, W. Tao, J. Li, X. Yang and J. Wang, Biomaterials, 2018, 188, 74–82 CrossRef PubMed.
- X. Xu, P. E. Saw, W. Tao, Y. Li, X. Ji, S. Bhasin, Y. Liu, D. Ayyash, J. Rasmussen, M. Huo, J. Shi and O. C. Farokhzad, Adv. Mater., 2017, 29, 1700141 CrossRef PubMed.
- K. Mabuchi, H. Maki, T. Itaya, T. Suzuki, M. Nomoto, S. Sakaoka, A. Morikami, T. Higashiyama, Y. Tada, W. Busch and H. Tsukagoshi, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, E4710–E4719 CrossRef CAS PubMed.
- N. Anicic, M. Vukomanovic, T. Koklic and D. Suvorov, Small, 2018, 14, 1800205 CrossRef.
- S. Rodic and M. D. Vincent, Int. J. Cancer, 2018, 142, 440–448 CrossRef CAS PubMed.
- R. S. Balaban, S. Nemoto and T. Finkel, Cell, 2005, 120, 483–495 CrossRef CAS PubMed.
- K. E. Broaders, S. Grandhe and J. M. Frechet, J. Am. Chem. Soc., 2011, 133, 756–758 CrossRef CAS PubMed.
- C. d. G. Lux, S. Joshi-Barr, T. Nguyen, E. Mahmoud, E. Schopf, N. Fomina and A. Almutairi, J. Am. Chem. Soc., 2012, 134, 15758–15764 CrossRef PubMed.
- Y. Zou, D. Zhao, C. Yan, Y. Ji, J. Liu, J. Xu, Y. Lai, J. Tian, Y. Zhang and Z. Huang, J. Med. Chem., 2018, 61, 1821–1832 CrossRef CAS.
- L. Raj, T. Ide, A. U. Gurkar, M. Foley, M. Schenone, X. Li, N. J. Tolliday, T. R. Golub, S. A. Carr, A. F. Shamji, A. M. Stern, A. Mandinova, S. L. Schreiber and S. W. Lee, Nature, 2011, 475, 231–234 CrossRef CAS PubMed.
- J. S. Bair, R. Palchaudhuri and P. J. Hergenrother, J. Am. Chem. Soc., 2010, 132, 5469–5478 CrossRef CAS PubMed.
- J. R. Kirshner, S. He, V. Balasubramanyam, J. Kepros, C. Y. Yang, M. Zhang, Z. Du, J. Barsoum and J. Bertin, Mol. Cancer Ther., 2008, 7, 2319–2327 CrossRef CAS PubMed.
- S. J. O'Day, A. M. Eggermont, V. Chiarion-Sileni, R. Kefford, J. J. Grob, L. Mortier, C. Robert, J. Schachter, A. Testori, J. Mackiewicz, P. Friedlander, C. Garbe, S. Ugurel, F. Collichio, W. Guo, J. Lufkin, S. Bahcall, V. Vukovic and A. Hauschild, J. Clin. Oncol., 2013, 31, 1211–1218 CrossRef PubMed.
- R. K. Blackman, K. Cheung-Ong, M. Gebbia, D. A. Proia, S. He, J. Kepros, A. Jonneaux, P. Marchetti, J. Kluza, P. E. Rao, Y. Wada, G. Giaever and C. Nislow, PLoS One, 2012, 7, e29798 CrossRef CAS PubMed.
- X. Liang, S. Xu, J. Zhang, J. Li and Q. Shen, ACS Appl. Mater. Interfaces, 2018, 10, 38749–38759 CrossRef CAS.
- H. Zhu, J. Fan, J. Wang, H. Mu and X. Peng, J. Am. Chem. Soc., 2014, 136, 12820–12823 CrossRef CAS PubMed.
- J. Zielonka, J. Joseph, A. Sikora, M. Hardy, O. Ouari, J. Vasquez-Vivar, G. Cheng, M. Lopez and B. Kalyanaraman, Chem. Rev., 2017, 117, 10043–10120 CrossRef CAS PubMed.
- Y. Koide, Y. Urano, K. Hanaoka, T. Terai and T. Nagano, J. Am. Chem. Soc., 2011, 133, 5680–5682 CrossRef CAS PubMed.
- L. Yuan, W. Lin, Y. Yang and H. Chen, J. Am. Chem. Soc., 2012, 134, 1200–1211 CrossRef CAS PubMed.
- R. Zhang, J. Zhao, G. Han, Z. Liu, C. Liu, C. Zhang, B. Liu, C. Jiang, R. Liu, T. Zhao, M. Y. Han and Z. Zhang, J. Am. Chem. Soc., 2016, 138, 3769–3778 CrossRef CAS PubMed.
- L. Wu, I. C. Wu, C. C. DuFort, M. A. Carlson, X. Wu, L. Chen, C. T. Kuo, Y. Qin, J. Yu, S. R. Hingorani and D. T. Chiu, J. Am. Chem. Soc., 2017, 139, 6911–6918 CrossRef CAS.
- H. Ma, B. Song, Y. Wang, D. Cong, Y. Jiang and J. Yuan, Chem. Sci., 2017, 8, 150–159 RSC.
- K. Dou, Q. Fu, G. Chen, F. Yu, Y. Liu, Z. Cao, G. Li, X. Zhao, L. Xia, L. Chen, H. Wang and J. You, Biomaterials, 2017, 133, 82–93 CrossRef CAS PubMed.
- X. Jiao, Y. Xiao, Y. Li, M. Liang, X. Xie, X. Wang and B. Tang, Anal. Chem., 2018, 90, 7510–7516 CrossRef CAS PubMed.
- M. Ren, Z. Li, J. Nie, L. Wang and W. Lin, Chem. Commun., 2018, 54, 9238–9241 RSC.
- C. Zhang, Q. Nie, I. Ismail, Z. Xi and L. Yi, Chem. Commun., 2018, 54, 3835–3838 RSC.
- L. Yuan, L. Wang, B. K. Agrawalla, S. J. Park, H. Zhu, B. Sivaraman, J. Peng, Q. H. Xu and Y. T. Chang, J. Am. Chem. Soc., 2015, 137, 5930–5938 CrossRef CAS PubMed.
- P. Wei, W. Yuan, F. Xue, W. Zhou, R. Li, D. Zhang and T. Yi, Chem. Sci., 2018, 9, 495–501 RSC.
- Y. L. Pak, S. J. Park, G. Song, Y. Yim, H. Kang, H. M. Kim, J. Bouffard and J. Yoon, Anal. Chem., 2018, 90, 12937–12943 CrossRef CAS.
- Q. Xu, C. Heo, J. A. Kim, H. S. Lee, Y. Hu, D. Kim, K. M. K. Swamy, G. Kim, S. J. Nam, H. M. Kim and J. Yoon, Anal. Chem., 2016, 88, 6615–6620 CrossRef CAS PubMed.
- Z. Mao, M. Ye, W. Hu, X. Ye, Y. Wang, H. Zhang, C. Li and Z. Liu, Chem. Sci., 2018, 9, 6035–6040 RSC.
- A. C. Sedgwick, L. Wu, H. H. Han, S. D. Bull, X. P. He, T. D. James, J. L. Sessler, B. Z. Tang, H. Tian and J. Yoon, Chem. Soc. Rev., 2018, 47, 8842–8880 RSC.
- H. Chen, Y. Tang, M. Ren and W. Lin, Chem. Sci., 2016, 7, 1896–1903 RSC.
- L. Yuan, W. Lin, Y. Xie, B. Chen and S. Zhu, J. Am. Chem. Soc., 2012, 134, 1305–1315 CrossRef CAS PubMed.
- H. Komatsu, T. Miki, D. Citterio, T. Kubota, Y. Shindo, Y. Kitamura, K. Oka and K. Suzuki, J. Am. Chem. Soc., 2005, 127, 10798–10799 CrossRef CAS PubMed.
- B. Zhu, P. Li, W. Shu, X. Wang, C. Liu, Y. Wang, Z. Wang, Y. Wang and B. Tang, Anal. Chem., 2016, 88, 12532–12538 CrossRef CAS.
- Y. Jiang, G. Zheng, Q. Duan, L. Yang, J. Zhang, H. Zhang, J. He, H. Sun and D. Ho, Chem. Commun., 2018, 54, 7967–7970 RSC.
- L. Wu, Q. Yang, L. Liu, A. C. Sedgwick, A. J. Cresswell, S. D. Bull, C. Huang and T. D. James, Chem. Commun., 2018, 54, 8522–8525 RSC.
- P. Xing, Z. Zhang, Y. Niu, Y. Qi, L. Dong and C. Wang, Chem. Commun., 2018, 54, 9889–9892 RSC.
- J. Sun and F. Feng, Analyst, 2018, 143, 4251–4255 RSC.
- H. Xiao, P. Li, X. Hu, X. Shi, W. Zhang and B. Tang, Chem. Sci., 2016, 7, 6153–6159 RSC.
- J. Zielonka, J. Joseph, A. Sikora, M. Hardy, O. Ouari, J. Vasquez-Vivar, G. Cheng, M. Lopez and B. Kalyanaraman, Chem. Rev., 2017, 117, 10043–10120 CrossRef CAS.
- Y. Huang, G. Zhang, F. Hu, Y. Jin, R. Zhao and D. Zhang, Chem. Sci., 2016, 7, 7013–7019 RSC.
- G. Dong, S. Wang, Z. Miao, J. Yao, Y. Zhang, Z. Guo, W. Zhang and C. Sheng, J. Med. Chem., 2012, 55, 7593–7613 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic procedures, characterisation transcripts for the compounds used in this study and crystallographic data. CCDC 1571172, 1571173, 1586906, 1586907 and 1863813–1863817. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9sc00180h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: